Abstract
Chikungunya (CHIK) is an arboviral infection having huge global burden affecting the life style of the patient badly due to debilitating polyarthralgia. This study aims to evaluate intracellular reactive oxygen species (ROS) in peripheral blood of patients suffering with persisting polyarthralgia post CHIK infection and the potential of Tinospora cordifolia leaf extract in scavenging those free radicals in peripheral blood mononuclear cells (PBMC) of the patient. Peripheral blood was collected from written informed consented patients and intracellular ROS was measured in PBMC of patients suffering with persisting polyarthralgia 3 months post CHIK infection followed by the study of free radical scavenging by T. cordifolia leaf extract in those cells through flow cytometry. Control population comprising healthy donors were also included in the study. As compared to healthy subjects, twofold higher Intracellular ROS (17.89 ± 1.007 vs. 37.96 ± 1.510, P < 0.0001) was found in patient PBMC. Ex-vivo treatment of those PBMC with ethanolic extract of T. cordifolia leaf (1 μg/mL) decreased intracellular ROS significantly by twofold (P < 0.0001). This study reports that CHIK infection produces high level of intracellular ROS in the patients suffering with persisting polyarthralgia, which was significantly scavenged by ex vivo treatment with T. cordifolia leaf extract.
Keywords: Chikungunya, Polyarthralgia, ROS, Tinospora cordifolia, Flow cytometry
Chikungunya (CHIK) is an arthropod borne viral infection of humans characterized by febrile illness followed by moderate to severe joint pain affecting the life style of the individuals [2]. CHIK infection is caused by a single stranded RNA virus from genus Alphavirus of family Togaviridae. This virus is present in human–mosquito reservoir for long time as evident from ancient history of fevers with acute joint pain, but finally discovered in Southern Tanzania and also named in their Makonde language which means “that which bends up” [18]. A significant proportion of chikungunya patients suffer from recurrent polyarthralgia within a year of the infection. The productivity loss in terms of income due to CHIK associated joint pain in a tropical country like India, was estimated INR 391 million in the year of 2006 [9]. Similarly, in Latin America, it was estimated as USD 73.6 million [3]. In Europe it is estimated as 35,000 Euro per capita [8]. Small scale CHIK outbreak is also reported in China in recent years [10]. So it is evident that not only tropical countries, CHIK have affected Europe and American continents in the last decade heavily, challenging the present healthcare system globally [4]. Currently, the disease pathophysiology is poorly understood and there is lack of CHIK specific therapeutics.
Generation of Reactive Oxygen Species or ROS is reported to be the key player of all kind of disease manifestation. Free radical homeostasis is necessary to maintain the life processes in a healthy individual. Immune system runs on the movement and interactions of millions of immune cells throughout the body and generates ROS in regular fashion [12]. Evolutionary changes have provided better physiological mechanisms to manage these free radicals within the immune system. There are some immunoglobulin-like lectins which are reported to be alter-expressed to counter the generation of excessive ROS and eventually, their expression correlates with the life span of the organism [16].
Management of ROS becomes crucial to minimize the suffering of the patient. Tinospora cordifolia is an herbaceous vine of Menispermaceae family indigenous to Indian subcontinent [17]. Tinospora is mentioned in the ancient texts of ‘Ayurveda’, the Indian system of medicine as ‘Guduchi’. Gulancha is the Bengali name of the climber. In some south Indian languages, this plant is referred as ‘Amrita balli’ or ‘the climber which gives immortality’ signifying its medicinal potential [17]. The word ‘Amrita’ etymologically related to the Greek word ‘Ambrosia’ having the same meaning. Tinospora is mentioned as a reservoir of alkaloids, lactones, glycosides with immunomodulatory potential by Dr. R. N. Chopra from Calcutta School of Tropical Medicine in 1958 [5]. Recently, ministry of AYUSH, Government of India mentioned Tinospora extract for the treatment of fevers with joint pain or ‘Sandhijwara’ (http://www.ccras.nic.in/sites/default/files/viewpdf/Chikunguniya/22092016_MANAGEMENT%20OF%20CHIKUNGUNYA%20THROUGH%20AYURVEDA%20AND%20SIDDHAA%20TECHNICAL%20REPORT.pdf). The etiology and possible management of fever with joint pain was indicated in folklores and fairy tales of India (http://www.anandabazar.com/supplementary/rabibashoriyo/in-bengali-fairy-tales-there-was-strong-indication-about-dengue-and-chikungunya-1.761749). The anti-oxidant activity of T. cordifolia leaf extract has recently been demonstrated [15]. However, till date, there is no scientific study elucidating the potential of scavenging intracellular ROS by T. cordifolia leaf extract on living cells in ex vivo condition.
This study aims to evaluate intracellular ROS in patients suffering with persisting polyarthralgia post CHIK infection and the potential of T. cordifolia leaf extract in scavenging those free radicals in peripheral blood mononuclear cells of the patient.
Experimentally, patients were enrolled along with healthy controls. To know the status of intracellular ROS and the free radical scavenging potential of T. cordifolia leaf extract, a simple flow cytometry based technique was followed using 2′,7′-Dichlorofluorescin diacetate or DCFDA as probe. At the first instance, intracellular ROS was measured just after collecting peripheral blood from the study subjects and documented. Further, similar cells were isolated from blood and undergone brief incubation with the plant formulation and intracellular ROS was measured by using the same DCFDA assay. Thus, intracellular ROS is measured directly in the patient blood cells before and after treatment with the plant formulation. This provides a clear idea about the amount of ROS generated in the patient suffering with persistent polyarthralgia along with the effect of T. cordifolia leaf extract in lowering the amount of ROS in the patient.
Total 550 individuals were screened in this study during the period of April 2015 to December 2017. Among them 230 and 92 cases were diagnosed as dengue and chikungunya respectively. Rest of the screened patients was not diagnosed with any of the arboviral infection. After initial clinical evaluation, CHIK diagnosis was confirmed by MAC ELISA performed by a WHO recommended kit provided by ICMR-NIV Pune and/or Real Time PCR by genesig® chikungunya standard kit obtained from Primerdesign®Ltd UK. Further, among these 92 confirmed chikungunya cases, only 23 patients were suffering with persisting polyarthralgia. Thus the present study was initiated with 21 such patients (2 subjects, one each of pediatric and geriatric group has been excluded) along with age and sex matched 20 healthy controls. Adhering to the Helsinki protocol, this study was approved by Institutional ethical committee (IEC) and peripheral blood was collected in EDTA coated vials from informed and written consented individuals. Peripheral Blood Mononuclear cells(PBMC) were obtained by layering cells from whole blood using Lymphoprep™ obtained from Axis-Shield, Oslo, Norway.
Tinospora cordifolia leaves were collected from Nursery of West Bengal State Medicinal Plants Board, Department of Health & Family Welfare, Government of West Bengal (Memo No. 8861). The leaves were air dried, powdered and crude ethanolic extract was prepared as described previously [1]. The final extract was dissolved in propylene glycol and stored at 4 °C.
Intracellular ROS was measured using DCFDA as a probe, in PBMC of the study subjects. DCFDA or 2′,7′-Dichlorofluorescin diacetate is a non-fluorescent probe which is naturally permeable to cell. When de-esterified due to oxidation within a cell, 2′,7′-Dichlorofluorescin diacetate turns into highly fluorescent 2′,7′-Dichlorofluorescin [6]. To determine the optimal concentration of DCFDA, PBMC of 106 order was mixed with various concentrations of DCFDA (0.5–2.5 μM) at 37 °C for 30 min in dark condition. A concentration of 1 μM was found to be optimal and used throughout this study.
The intracellular ROS measurement assay was performed within 2 h of collection of the peripheral blood sample. PBMC of 106 order collected from study subjects were suspended in Phosphate buffered saline of physiological pH and treated with 1 μM 2′,7′-Dichlorofluorescin diacetate obtained from Sigma-Aldrich® in polystyrene tubes suitable for flow cytometry (obtained from BD Biosciences®) maintaining dark condition at room temperature for 30 min. Hydrogen peroxide was used as positive control. After incubation, samples were acquired through a BD FACSCalibur™ flow Cytometer (Becton–Dickinson, San Jose, CA, USA) in FL 1 channel. Geo Mean fluorescence was taken for determining the presence of intracellular ROS in this study. Thus the level of intracellular ROS was evaluated in all study subjects by this experiment.
To determine the free radical scavenging potential of T. cordifolia leaf extract, peripheral blood mononuclear cells isolated from the enrolled subjects, were briefly incubated with the plant formulation. PBMC of 106 order was treated with ethanolic extract of T. cordifolia leaves in concentrations of 0.5 and 1 μg/mL for 1 h at room temperature. After incubation, intracellular ROS was again measured by the same DCFDA assay adding 1 μM DCFDA to each tube followed by 30 min incubation and flow cytometry. Analysis was done and presence of ROS was documented. Thus, the intracellular ROS level before and after treatment with plant formulation helped in determining the free radical scavenging potential of T. cordifolia leaf extract.
GraphPad Prism software version 5.0 (Graph Pad Software Inc., La Jolla, CA, USA) was used for all statistical analyses. P < 0.05 was taken significant in all cases. t test was done to determine statistical significance among two groups of data and One way ANOVA was done for the same within three groups. All data are represented as Mean ± SEM.
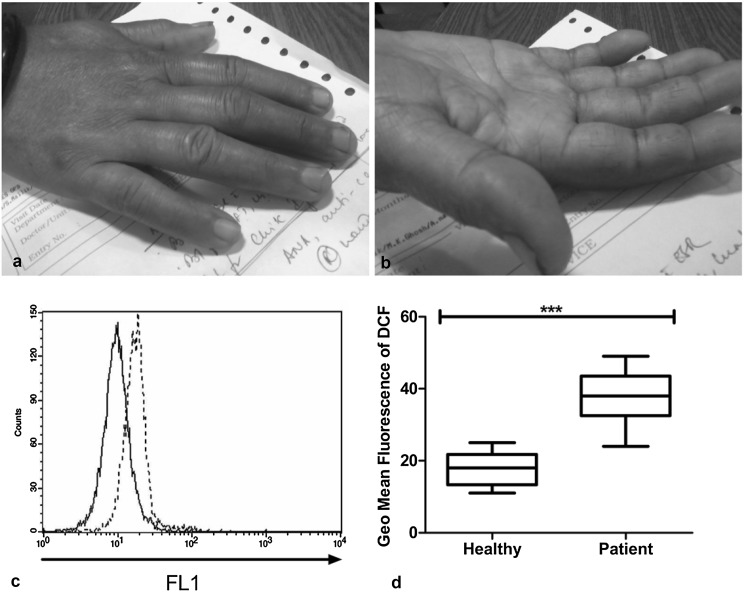
In this present study, prevalence rate of CHIK infection was calculated comprising the positive cases (n = 92) among screened population (n = 550) and found to be 16.72%. Patients who suffered from polyarthralgia 3 months post infection were studied and mentioned as ‘CHIK Patient’ in this work. All CHIK patients were presented with fever; rash and polyarthralgia (Fig. 1). Persisting polyarthralgia associated with CHIK infection after 3 months was the key clinical determinant of this study. Figure 1 clearly shows joint involvement in a patient after 3 months of CHIK infection.
Fig. 1.
Joint inflammation and intracellular ROS post 3 months chikungunya infection: a, b Shows inflamed joints in the right hand of a female patient. c is a representative histogram plot of Dichlorofluorescin or DCF intensity in Healthy controls (solid line) and CHIK patients (dotted line). GMF intensity of DCF in all the study subjects is analyzed statistically and represented as d. Statistical significance was obtained by t test and P < 0.05 was taken as significant
Arboviral infection like CHIK elicits intense immune reaction within the host. Lymphocytes and monocytes are first line of defense within the host comprising the innate immune system. Several immune pathways are switched on and off to combat the consequences of the viral infection. These abrupt immune reactions develop huge free radicals like reactive oxygen species within the patients’ blood and other tissues where generally the virus travels during the fever. Joint inflammation and pain is reported [11] to be caused by the damage of synovial joint tissue which leads to acute and chronic arthropathy.
DCFDA assay revealed 2.12 fold increased intracellular Reactive oxygen species in PBMC from CHIK patients, when compared to healthy individuals (Table 1). The increased value of ROS is found to be significant (P < 0.0001) after statistical analysis (Fig. 1). This intracellular assay directly enumerates ROS within PBMC. More than twofold increase of intracellular ROS gives an insight about the CHIK pathophysiology. It becomes evident that ROS might play a crucial role in the development of joint tissue damage as reported in previous studies [6]. Scavenging of ROS is very important from the beginning of the disease progression and this might control further damage which otherwise can eventually develop into debilitating joint pain.
Table 1.
Basic and oxidative parameters studied in all the study subjects
| Parameters | Healthy controls (n = 20) | Chikungunya patients with persisting polyarthralgia (n = 21) |
|---|---|---|
| Male:female | 1:1 | 1:1.1 |
| Age in years [Median with IQR] |
27 [21.5–34.5] |
36 [30.5–44.5] |
| Intracellular ROSa [GMF of DCF intensity] |
17.89 ± 1.007 | 37.96 ± 1.510 |
aMean ± SEM
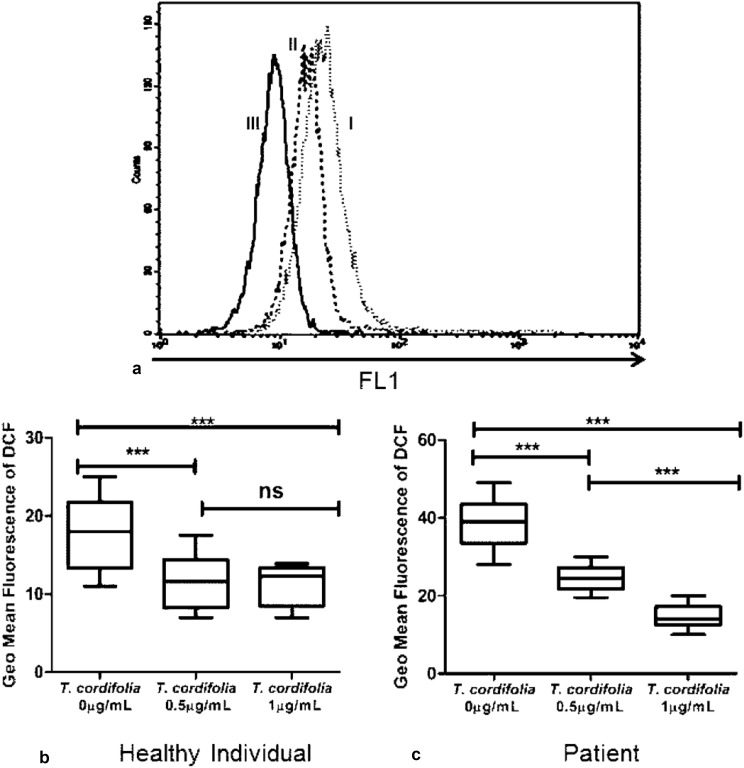
Tinospora cordifolia is reported to have anti-oxidant property but not earlier evaluated in CHIK or similar diseases [14, 15]. Lymphocytes are important players of immune system having crucial role in disease pathophysiology of CHIK. Excessive generation of ROS damages those cells, as well as the surrounding milieu where those lymphocytes reside. Interestingly, this extract decreased intracellular reactive oxygen species in both healthy controls and CHIK patients significantly (Fig. 2). Two concentrations of T. cordifolia leaf extract were taken to know the appropriate dose in healthy controls and patients; as the minimal dose required for healthy control may not be suitable for patient. In healthy individuals, treatment with 0.5 µg/mL Tinospora extract decreased ROS by 1.56 fold whereas with an increased dose of 1 µg/mL, there was no significant change in ROS level. Interestingly, in PBMC isolated from CHIK patients suffering with persisting polyarthralgia, intracellular ROS decreased from 37.96 ± 1.51 to 24.48 ± 0.67 after treatment with 0.5 µg/mL plant extract whereas ROS decreased from 37.96 ± 1.51 to 14.75 ± 0.66 after treatment with 1 µg/mL extract. Both the changes of Geometric Mean Fluorescence of DCFDA were found to be statistically significant having P value < 0.0001. So, it was determined that a dose of 1 µg/mL extract of T. cordifolia leaf extract is required to decrease the intracellular ROS in CHIK patients suffering with persisting polyarthralgia comparable with the basal level of intracellular ROS in healthy individuals.
Fig. 2.
Scavenging of Intracellular ROS by T. cordifolia leaf extract: a is a representative histogram plot of DCF intensity after treatment of Peripheral Blood Mononuclear Cells or PBMC with T. cordifolia leaf extract in various concentrations (I = 0 μg/mL, II = 0.5 μg/mL, III = 1 μg/mL) in a CHIK patient. b, c describes the effect of T. cordifolia treatment in Healthy and CHIK Patients respectively. Statistical significance was obtained by t test and P < 0.05 was taken as significant
Tinospora cordifolia leaf extract was found to scavenge intracellular ROS in PBMC of CHIK patients suffering with persisting polyarthralgia. Treatment with 0.5 and 1 µg/mL of Tinospora leaf extract also decreased ROS in healthy individuals (Fig. 2). Earlier studies and use in folk medicine reports that T. cordifolia extract is non-toxic for humans and rich in alkaloids which might be the possible cause behind scavenging of intracellular ROS (http://www.ccras.nic.in/sites/default/files/viewpdf/Chikunguniya/22092016_MANAGEMENT%20OF%20CHIKUNGUNYA%20THROUGH%20AYURVEDA%20AND%20SIDDHAA%20TECHNICAL%20REPORT.pdf) [5, 11, 14, 17].
Free radicals are established mediators of joint destruction, as these radicals destroy cartilage and bone tissue and degrade synovial fluid. As a result, bone thickness decreases leading to joint inflammation and pain [7]. Chikungunya is a viral infection and elicits high amount of free radicals which eventually degrades synovial joints in some patients to cause persisting polyarthralgia. Though this is a multifactorial process, still ROS is one of the key players in development of joint pain. T. cordifolia leaf extract contains several potential anti-oxidant molecules as described earlier [13] and can easily ameliorate excess ROS without any further toxic effect. Plant formulations are popular and easy to use. Thus using those formulations are helpful in such multifaceted manifestation of a viral infection like chikungunya.
Taken together, CHIK infection develops intracellular ROS within the host, which might cause tissue damage leading to severe and persisting joint pain among some patients. Scavenging such high amount of ROS possibly is helpful to decrease the oxidative damage. T. cordifolia leaf extract is found to scavenge intracellular ROS in CHIK peripheral blood mononuclear cells and provide insights for further study.
Acknowledgements
NB received Junior Research fellowship from Department of Biotechnology, Government of West Bengal. SM acknowledge funding from Department of Biotechnology, Government of West Bengal, India (File No.: 232/BT(Estt)/RD-24/2014). Authors are thankful to all the patients, their family members and healthy donors for participating in this scientific study. The authors acknowledge the helpful guidance of Prof. (Dr.) Nandita Basu, Director, School of Tropical Medicine, Kolkata, India towards the planning and execution of this study.
References
- 1.Ahmed OM, Fahim HI, Boules MW, Ahmed HY. Cardiac and testicular toxicity effects of the latex and ethanolic leaf extract of Calotropisprocera on male albino rats in comparison to abamectin. SpringerPlus. 2016;5(1):1644. doi: 10.1186/s40064-016-3326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee N, Mukhopadhyay S. Viral glycoproteins: biological role and application in diagnosis. VirusDisease. 2016;27(1):1–11. doi: 10.1007/s13337-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardona-Ospina JA, Villamil-Gómez WE, Jimenez-Canizales CE, Castañeda-Hernández DM, Rodríguez-Morales AJ. Estimating the burden of disease and the economic cost attributable to chikungunya, Colombia, 2014. Trans R Soc Trop Med Hyg. 2015;109(12):793–802. doi: 10.1093/trstmh/trv094. [DOI] [PubMed] [Google Scholar]
- 4.Charrel RN. Chikungunya outbreaks—the globalization of vectorborne diseases. N Engl J Med. 2007;356(8):769. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 5.Chopra RN. Chopra’s indigenous drugs of India. Academic publishers, Kolkata, India. 1958.
- 6.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. In: Advanced protocols in oxidative stress II. Humana Press, Totowa, NJ. 2010. p. 57–72. [DOI] [PubMed]
- 7.Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. ClinExpImmunol. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzzetta G, Trentini F, Poletti P, Baldacchino FA, Montarsi F, Capelli G, Rizzoli A, Rosà R, Merler S, Melegaro A. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Negl Trop Dis. 2017;11(9):e0005918. doi: 10.1371/journal.pntd.0005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamoorthy K, Harichandrakumar KT, Kumari AK, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46(1):26. [PubMed] [Google Scholar]
- 10.Lu X, Li X, Mo Z, Jin F, Wang B, Huang J, Huang J, Zhao H, Shi L. Chikungunya emergency in China: microevolution and genetic analysis for a local outbreak. Virus Genes. 2014;48(1):15–22. doi: 10.1007/s11262-013-0991-2. [DOI] [PubMed] [Google Scholar]
- 11.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr. 1997;127(5):962S–965S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 13.Praveen N, Thiruvengadam M, Kim HJ, Kumar JP, Chung IM. Antioxidant activity of Tinospora cordifolia leaf extracts through non-enzymatic method. J Med Plants Res. 2012;6(33):4790–4795. [Google Scholar]
- 14.Saha S, Ghosh S. Tinospora cordifolia: one plant, many roles. Anc Sci Life. 2012;31(4):151–159. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarala M, Velu V, Anandharamakrishnan C, Singh RP. Spray drying of Tinospora cordifolia leaf and stem extract and evaluation of antioxidant activity. J Food Sci Technol. 2012;49(1):119–122. doi: 10.1007/s13197-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz F, Pearce OM, Wang X, Samraj AN, Läubli H, Garcia JO, Lin H, Fu X, Garcia-Bingman A, Secrest P, Romanoski CE. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 2015;7(4):e06184. doi: 10.7554/eLife.06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spandana U, Ali SL, Nirmala T, Santhi M, Babu SS. A review on Tinospora cordifolia. Int J Curr Pharm Rev Res. 2013;4(2):61–68. [Google Scholar]
- 18.Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;31(120):32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]