Abstract
The nephropathogenic infectious bronchitis virus (IBV) strain IS-1494 like (variant-2; GI-23) was first isolated in the Middle East (1998). Despite intensive vaccinations, IS-1494 like IBVs are still circulating in Iran (the dominant genotype) and spread to other countries. Here, the full-length genome of this Iranian IS-1494 like IBV was (Mahed) determined to understand its evolutionary relationships. The genome consists of 27,652 nucleotides, with mutations in most of the structural genes. Thirteen open reading frames (ORFs) were predicted in the Mahed isolate (5′ UTR-1a-1b-S-3a-3b-E-M-4b-4c-5a-5b-N-6b-3′ UTR). ORFs 4b, 4c, and 6b, which has rarely been reported, were present in the Mahed genome. According to phylogenetic analysis of the full-length genome, 1a, S2, M, E, N protein, Mahed isolate clustered with the QX type strain. Based on the partial 1b, S1, Mahed clustered with the Q1 strain. The full-length genome of Mahed isolate shared the highest sequence homology with Gray and JMK (90.06–90.07%) and was least related to the Vic-s (86.21%). These data show that evolutionary variation because of recombination in IBV plays a major role in the adaptation and origin of IBV leading to new genetic and types of the virus strain.
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0462-4) contains supplementary material, which is available to authorized users.
Keywords: Complete genome, Iran, IS-1494 like, Avian infectious bronchitis virus, Phylogenetic study
Infectious bronchitis (IB), caused by infectious bronchitis virus (IBV) is one of the most economically important viral diseases of poultry. The IBV belongs to the genus Gammacorona virus within the Coronaviridae family. The positive-sense, single-stranded, 5′-capped genomic RNA (27 kb) codes for the replicase complex (open reading frame 1 [ORF1]) and five structural proteins on the remaining: spike (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N), besides the accessory proteins 3a, 3b, 5a, and 5b, with an untranslated region (UTR) at both the 5′ and 3′ ends [4, 8]. Two-thirds of the 5′ region in the IBV genome encodes the 1ab or 1b polyproteins, which are proteolytically cleaved by two virus-encoded replicase proteins (the papain-like and 3C-like proteinases) into 15 nonstructural proteins (nsp2–nsp16). The 5′ and 3′ untranslated regions (UTRs) usually harbor important structural elements that are involved in replication and translation [22]. Mutations and recombination in the IBV genome have resulted in viruses that have different tissue tropism, increased virulence, and an increased ability to persist in the chicken host [13]. Currently, more than 60 IBV serotypes have been reported because of mutation in its genome [10]. The isolation of IS/1494/06, one of the Israeli variant-2 (GI-23) isolates, and information on its S1 gene sequence in GenBank (Accession number: EU780077) has first been reported by Meier & Maher from Israel [12]. Strains of this lineage were first identified in Israel in 1998. The IS-1494 like IBVs is the main genotype in the Middle East, and African countries such as Iran, Jordan, Egypt, Turkey and recently reported from Europe [9]. The IS-1494 like IBV is known to be nephropathogenic, and it also affects the respiratory system [15]. Till now, seven genotypes of IBV (Massachusetts (Mass), 793/B, IS-1494, IS-720, QX, IR-1, and IR-2) are reported in Iran [16]. Contrary to the application of various IB vaccines (Mass & 793/B types) in Iran, regular outbreaks have been reported and a recent study showed IS-1494 like is the dominant (~ 70%) IBV genotype in Iran [6]. This study aimed to determine the complete genome analysis of an Iranian IS-1494 like IBV isolate (Mahed;).
The Iranian Is-1494 like IBV lineage (Mahed) was identified in Iran in December 2015 and occurred in a commercial Ross broiler flock vaccinated with IBV Mass type at day old one. Virus isolation has been done according to OIE manual. For genotyping, positive samples were submitted to a nested PCR reaction amplifying of S1 gene (Data has not been shown). To extract viral genomes from allantoic fluid (5th SF Egg passage) RNAeasy mini kit (Qiagen) was used according to manufacturer’s instructions. For cDNA synthesis, random hexamer primer (SinaClon, Iran) was used and did according previous jobs [19]. Twenty-five primer pairs were synthesized based on the published LX4 strain genome (AY338732) [23]. PCR was performed with 3 ul cDNA as template in a total of 25 ul reaction volume containing 0.5 ul of each primer and 12.5 ul 2× PCR Mix (Sinaclon, Iran). To determine the complete genomic sequence, the extreme 5′ and 3′ termini of the isolates were identified by amplification of cDNA ends, using a 3′/5′ RACE kit (Takara Bio Inc., Shiga, Japan). PCR products were inserted into a pTG19-T vector (Sinaclon, Iran) and transformed into a Top10 (Sinaclon, Iran) competent cell according to the TA cloning kit (Sinaclon, Iran) manufacturer’s instructions.The positive clone have been selected based on blue white screening teat and clocny PCR. The nucleotide (nt) sequences of the positive clones were determined using T7 primers on a commercial service (Bioneer Co., Korea). Chromatograms were evaluated with ChromasPro (ChromasPro Version 1.5). Multiple alignments of different genes were achieved using Clustal W using the MEGA 7 software. The phylogenetic tree was constructed by using the same software with the neighbor-joining method (bootstrap replicates: 1000) [20]. The Mahed strain has submitted to gene bank under Accession number: MG233398.
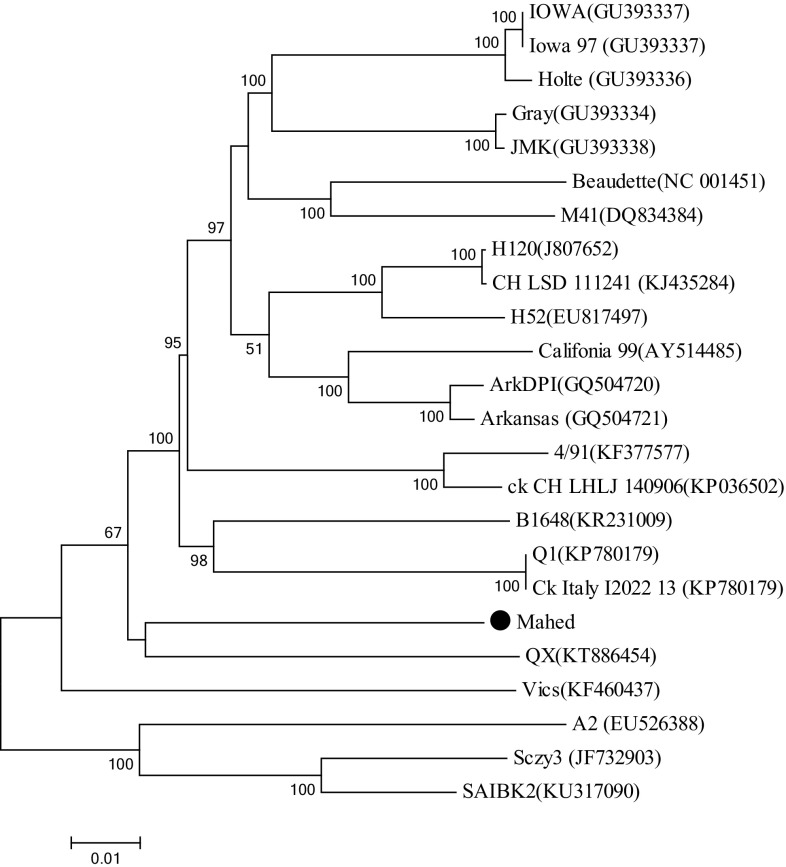
The complete genome sequence of the Mahed isolate is 27,652 nucleotides (nt) in length, excluding the poly (A) tail, and includes 10 open reading frames (ORFs). Thirteen ORFs (5′ -1a-1b-S-3a-3b-E-M-4b-4c-5a-5b-N-6b-3′) were predicted in the Mahed genome (Supplementary material 1). ORFs 4b, 4c, and 6b were predicted in the Mahed genome and most of the GenBank IBV genomes. The length of nucleotide acid, amino acid and ORFs (Start and End) has been shown in (Supplementary material 1). Mahed Strain had the highest nucleotide sequence identity (90.07%) to Gray strain and the lowest nucleotide sequence identity (86.21%) to Vic-S strain. The nucleotide identity among strain Mahed and the H120 and 4/91 vaccine strain 89.41 and 88.39%, respectively (Table 1). The phylogenetic tree showed the genetic relationships of the full-length sequences of strain Mahed with other strains representing the majority of IBV genotypes showed that Mahed strain was closely related to QX (Fig. 1). Based on the phylogenetic analysis of the replicase genes, Mahed clustered with Q1 and B1648 (1a) and QX (1b). Among the 16 NSPs of polyprotein 1ab, NSP15 (85.8–96.85%) and NSP16 (84.07–96.32%) were most variable, whereas the other NSPs (Especially NSP 4, 7, 13) were generally more conserved. Phylogenetic trees were constructed from the S1, and S2 genes of Mahed shows that the virus related to Q1 and QX, respectively (Supplementary material 2). We observed 81% nucleotide sequence identity (The highest) for the S1 gene between our isolate with Q1 strain. Based on the S2 analysis, the Mahed has high homology with QX (93.62%) (Table 1). The amino acid (aa) sequence of cleavage site on the spike was RRTRR. The S2 fusion aa site in S2 of mahed is PSGRS that unique to QX and Vics strain. According to a phylogenetic study of the full-length genome, 1a, M, E, N protein, Mahed strain clustered with the QX type strain. Based on the partial 1b, Mahed clustered with the Q1 strain (Supplementary material 2). There were 7 probable accessory proteins in the Mahed strain, such as 3a (57 aa), 3b (64 aa), 4b (94 aa), 4c (56 aa) 5a (65 aa), 5b (82 aa) and 6b (74 aa).
Table 1.
Percentage of sequence identities of different regions of Iranian IS-1494 like IBV isolate (Mahed strain) compared with other IBV strains
| Genes | Whole Genome | orf1a | orf1b | S1 | S2 | 3a | 3b | E | M | 4b | 4c | 5a | 5b | N | 6b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gray (GU393334) | 90.07 | 91.20 | 91.51 | 75.13 | 85.09 | 88.68 | 95.14 | 90.07 | 90.63 | 88.59 | 92.00 | 85.42 | 92.31 | 90.90 | 94.29 |
| JMK (GU393338) | 90.06 | 91.15 | 91.42 | 75.06 | 85.15 | 88.68 | 94.59 | 90.07 | 91.16 | 88.59 | 92.00 | 85.42 | 92.31 | 91.36 | |
| B1648(KR231009) | 89.96 | 90.44 | 91.66 | 78.08 | 83.38 | 88.02 | 94.03 | 85.46 | 89.72 | 96.46 | 96.83 | 94.22 | 93.67 | 91.90 | 96.48 |
| Q1(KP780179) | 89.76 | 90.25 | 91.86 | 81.00 | 84.20 | 82.19 | 93.44 | 84.75 | 87.15 | 90.75 | 89.18 | 82.84 | 93.22 | 90.41 | 21.78 |
| QX (KT886454) | 89.72 | 87.56 | 93.96 | 73.16 | 93.62 | 86.70 | 73.99 | 90.07 | 90.42 | 88.18 | 94.84 | 96.89 | 94.15 | 89.75 | 62.68 |
| H120 (J807652) | 89.41 | 89.66 | 92.55 | 76.07 | 83.66 | 76.40 | 81.81 | 86.17 | 89.35 | 86.50 | 84.59 | 87.86 | 93.20 | 90.12 | 21.78 |
| IOWA (GU393337) | 89.29 | 89.70 | 91.51 | 75.74 | 83.93 | 83.78 | 92.87 | 90.07 | 88.71 | 87.06 | 89.93 | 86.65 | 92.74 | 90.43 | 91.94 |
| Ark DPI (GQ504720) | 89.25 | 89.61 | 90.88 | 75.44 | 84.61 | 89.34 | 94.03 | 90.43 | 90.64 | 88.12 | 92.00 | 85.42 | 94.10 | 91.72 | 93.56 |
| Beaudette (NC_001451) | 88.45 | 88.79 | 90.73 | 75.87 | 83.31 | 82.12 | 81.11 | 87.59 | 88.08 | 89.19 | 89.85 | 84.77 | 95.00 | 90.13 | 93.54 |
| M41 (DQ834384) | 88.40 | 88.69 | 90.62 | 76.22 | 83.81 | 80.60 | 82.47 | 87.23 | 88.13 | 88.81 | 87.83 | 83.46 | 91.87 | 89.66 | |
| 4/91 (KF377577) | 88.39 | 88.19 | 90.48 | 75.16 | 83.91 | 87.98 | 90.51 | 84.40 | 91.00 | 90.34 | 89.97 | 89.10 | 94.12 | 90.96 | 96.48 |
| Vics (KF460437) | 86.21 | 84.08 | 90.56 | 77.35 | 83.49 | 83.08 | 84.24 | 84.40 | 85.65 | 83.40 | 81.59 | 85.49 | 90.53 | 88.81 | 76.00 |
Fig. 1.
Phylogenetic tree constructed with whole genome of avian infectious bronchitis virus reference strains. The analysis was done with the Neighbor-Joining method, Kimura 2 parameters (MEGA 7). Numbers at nodes correspond to bootstrap values (1000 replications). Mahed strain is marked with a black circle
Over the past 15 years, the nephropathogenic IBV strains have been emerging as most predominant IBV strains in the poultry industry, especially in Asian and Middle East countries [17]. Hosseini et al. [7] reported IS-1494 like the virus with the frequency of 17.2% during their molecular surveillance of IBV genotypes involved in outbreaks between “2010–2014”. In the study done by Najafi et al. (2015), IS-1494 like IBV with the total prevalence of 34% in Iranian chicken flocks. Regarding the pathogenicity of Iranian IS-1494 like a virus, Najdfi et al. [14] demonstrated variant-2 has high tropism to the respiratory tract, digestive system, and renal tissue that is due to its epitheliotropic nature. The Mahed genome organization was: (5′-1a-1b-S-3a-3b-E-M-4b-4c-5a-5b-N-6b-3′). 4b, 4c, and 6b were additional ORFs present in the Mahed genome. However, 4b, 4c, and 6b were present in the most of the IBV genomes, they have sometimes been reported. ORFs 4b, 4c, and 6b have also been reported in a turkey coronavirus. The detailed reason for the infrequent reports of these ORFs (4b, 4c, and 6b) in most of the IBV genomes is not known. It could be that 4b, 4c, and 6b ORFs are present in most of the IBV genomes, but that the success of their identification depends on the algorithms of ORF prediction software that was used [17]. Lately, Bentley et al. [1] have confirmed and indicated 4b as a 5th secondary protein in IBV, as well 3a, 3b, 5a and 5b. The 4b homolog of Middle East respiratory syndrome coronavirus (MERS-CoV) and the 6b homolog of SARS coronavirus were found to be an interferon antagonist and apoptosis inducer [21] but for IBV, additional research is necessary to show the production of 4b, 4c, and 6b proteins and to recognize their actions in pathogenesis [17].
The S1 gene of Mahed IBV isolate with published sequences reference strains in GenBank, the isolate shared 95.12% nucleotide identity with attenuated IBVAR2-06, 95.22% homology with both IS/1494/06, Eg/CLEVB-2/IBV/012 and 95.42% nucleotide similarity with TR8 (Turkish IBV) strains. Mahed shared amino acid sequence similarities of 92.16% with Attenuated IBVAR2-06 and 92.36% with both IS/1494/06 and Eg/CLEVB/2/IBV_012. Three hypervariable regions (HVR) of S1 were determined. The most variation was seen in HVR2. The S glycoprotein of IBV is translated as a precursor protein (S0), and then cleaved into two subunits (S1 and S2). The studies reveal that the cleavage recognition site sequence, which consists of five basic amino acid residues, does not appear to correlate with increased cleavability, host cell range and increased virulence as it does with envelope glycoproteins in orthomyxoviruses and paramyxoviruses, but correlates with IBVs in different geographic regions. In this study, the S1 cleavage site of strain Mahed was R-R-T-R-R (Arg- Arg- Thr- Arg- Arg). The proteolytic cleavages of the spike protein are essential for virus infection of host cells. The cleavage site between S1 and S2 contained the furin-recognition motif RRTRRS and was shared by all of the compared viruses [3].The 3c gene encodes the envelope protein that has a critical role in assembly and budding of the virus [2]. Mahed IBV based on 3c gene is located in QX cluster in the phylogenetic tree based on E gene and has 90.07% homology to QX-like IB virus.The Matrix proteins are diverged considerably across genera but moderately well conserved within each CoV genus and are the most abundant component of CoV virions [11]. Variant-2 and Mass strains shared 99.69% homologies based on M gene sequences which contrast with the approximately 20% difference of the S1 gene. Our results agree with published data by Ren et al. [18] who characterized the M gene of IBV Chinese strain HH06. The M gene and protein share 83.9–97.9 and 83.6–96.5% homologous identities compared with IBV reference strains derived from different regions or countries, respectively. In our study, Mahed IBV had high homology with 4/91 (91.00%) and JMK (91.16%) IBVs. The effects of the single amino acid insertions in the ER motif of E and ectodomain of M proteins are unknown. However, the different amino acid sequences of the M protein ectodomains, identified when examining recurrent viruses, encourages further study of a role for IBV antigenic variation [5]. Finally, based on Nucleoprotein gene, Mahed is cluster with QX and 4/91.
The close phylogenetic relationship of Mahed IBV (Iranian IBV) and QX are consistent with the hypothesis that their ancestors shared hosts during their evolutionary history and thus with the hypothesis of recombination between these two genotypes. Phylogenic analysis, it was shown that Mahed was a chimeric strain. The data obtained from this study suggests that this variant of IBV may have arisen through genetic modifications, which conferred changes in pathogenicity and antigenicity. In conclusion, our data suggested that both mutation and recombination were involving the emergence of IBV variants in the field. So, periodic monitoring is really necessary to track the emergence of new variants and is required to develop an effective vaccination plan to control IB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was conducted under a grant from research council, University of Tehran under the Grant (No. 28692/6/16). The authors gratefully acknowledge Ghalyanchilab expert especially Mr. Behrooz Asadi for his extensive technical support.
References
- 1.Bentley K, Keep SM, Armesto M, Britton P. Identification of a noncanonically transcribed subgenomic mRNA of infectious bronchitis virus and other gammacoronaviruses. J Virol. 2013;87(4):2128–2136. doi: 10.1128/JVI.02967-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos EC, Luytjes W, van der Meulen HV, Koerten HK, Spaan WJ. The production of recombinant infectious DI-particles of a murine coronavirus in the absence of helper virus. Virology. 1996;218(1):52–60. doi: 10.1006/viro.1996.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch BJ, Martina BE, van der Zee R, Lepault J, Haijema BJ, Versluis C, et al. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci USA. 2004;101(22):8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandao PE, Ayres GR, Torres CA, Villarreal LY, Hora AS, Taniwaki SA. Complete genome sequence of a Brazil-type Avian coronavirus detected in a chicken. Genome Announc. 2016 doi: 10.1128/genomeA.01135-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming JO, Shubin RA, Sussman MA, Casteel N, Stohlman SA. Monoclonal antibodies to the matrix (El) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology. 1989;168(1):162–167. doi: 10.1016/0042-6822(89)90415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamadan AM, Ghalyanchilangeroudi A, Hashemzadeh M, Hosseini H, Karimi V, Yahyaraeyat R, et al. Genotyping of Avian infectious bronchitis viruses in Iran (2015–2017) reveals domination of IS-1494 like virus. Virus Res. 2017;240:101–106. doi: 10.1016/j.virusres.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini H, Bozorgmehri Fard MH, Charkhkar S, Morshed R. Epidemiology of avian infectious bronchitis virus genotypes in Iran (2010–2014) Avian Dis. 2015;59(3):431–435. doi: 10.1637/11091-041515-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 8.Jackwood MW, Hall D, Handel A. Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol. 2012;12(6):1305–1311. doi: 10.1016/j.meegid.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisowska A, Sajewicz-Krukowska J, Fusaro A, Pikula A, Domanska-Blicharz K. First characterization of a Middle-East GI-23 lineage (Var2-like) of infectious bronchitis virus in Europe. Virus Res. 2017;242:43–48. doi: 10.1016/j.virusres.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X-l Su, J-l Zhao J-x, G-z Zhang. Complete genome sequence analysis of a predominant infectious bronchitis virus (IBV) strain in China. Virus Genes. 2009;38(1):56–65. doi: 10.1007/s11262-008-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masters PS, Perlman S. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 825–858. [Google Scholar]
- 12.Meir R, Rosenblut E, Perl S, Kass N, Ayali G, Hemsani E, et al. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48(3):635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- 13.Mondal SP, Cardona CJ. Genotypic and phenotypic characterization of the California 99 (Cal99) variant of infectious bronchitis virus. Virus Genes. 2007;34(3):327–341. doi: 10.1007/s11262-006-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Najafi H, Ghalyanchi LA, Hashemzadeh M, Madadgar O, Karimi V, Farahani R, et al. Pathogenicity characteristics of an Iranian variant-2 (IS-1494) like infectious bronchitis virus in experimentally infected SPF chickens. Acta Virol. 2016;60(4):393–399. doi: 10.4149/av_2016_04_393. [DOI] [PubMed] [Google Scholar]
- 15.Najafi H, Ghalyanchi LA, Hashemzadeh M, Madadgar O, Karimi V, Farahani R, et al. Pathogenicity characteristics of an Iranian variant-2 (IS-1494) like infectious bronchitis virus in experimentally infected SPF chickens. Acta Virol. 2016;60(4):393. doi: 10.4149/av_2016_04_393. [DOI] [PubMed] [Google Scholar]
- 16.Najafi H, Langeroudi AG, Hashemzadeh M, Karimi V, Madadgar O, Ghafouri SA, et al. Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014–2015. Adv Virol. 2016;161(1):53–62. doi: 10.1007/s00705-015-2636-3. [DOI] [PubMed] [Google Scholar]
- 17.Reddy VR, Theuns S, Roukaerts ID, Zeller M, Matthijnssens J, Nauwynck HJ. Genetic characterization of the Belgian nephropathogenic infectious bronchitis virus (NIBV) reference strain B1648. Viruses. 2015;7(8):4488–4506. doi: 10.3390/v7082827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren X, Yin J, Ma D, Li G. Characterization and membrane gene-based phylogenetic analysis of avian infectious bronchitis virus Chinese strain HH06. Virus Genes. 2009;38(1):39–45. doi: 10.1007/s11262-008-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seger W, GhalyanchiLangeroudi A, Karimi V, Madadgar O, Marandi MV, Hashemzadeh M. Genotyping of infectious bronchitis viruses from broiler farms in Iraq during 2014-2015. Adv Virol. 2016;161(5):1229–1237. doi: 10.1007/s00705-016-2790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Z, Wong CK, Li P, Xie Y. A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochim Biophys Acta (BBA)-Gen Subj. 2008;1780(12):1383–1387. doi: 10.1016/j.bbagen.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Han Z, Xu Q, Wang Q, Gao M, Wu W, et al. Serotype shift of a 793/B genotype infectious bronchitis coronavirus by natural recombination. Infect Genet Evol. 2015;32:377–387. doi: 10.1016/j.meegid.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao F, Zou N, Wang F, Guo M, Liu P, Wen X, et al. Analysis of a QX-like avian infectious bronchitis virus genome identified recombination in the region containing the ORF 5a, ORF 5b, and nucleocapsid protein gene sequences. Virus Genes. 2013;46(3):454–464. doi: 10.1007/s11262-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.