Abstract
Peste des petits ruminants (PPR) has been recognized as a globally distributed disease affecting the small ruminant population. The disease results in severe economic losses mainly to small land holders and low input farming systems. The control of PPR is mainly achieved through vaccination with available live attenuated vaccines. The thermo labile nature of PPR virus poses a major constraint in production of quality vaccines which often results in vaccine failures. The lack of quality vaccine production jeopardize the wide vaccination coverage especially in countries with poor infrastructure due to which PPR persists endemically. The vaccine production system may require augmentation to attain consistent and quality vaccines through efforts of process intensification integrated with suitable stabilizer formulations with appropriate freeze drying cycles for improved thermo tolerance. Manufacturing of live attenuated PPR vaccines during batch cultures might introduce defective interfering particles (DIPs) as a result of high multiplicity of infection (MOI) of inoculums, which has a huge impact on virus dynamics and yield. Accumulation of DIPs adversely affects the quality of the manufactured vaccines which can be avoided through use of appropriate MOI of virus inoculums and quality control of working seed viruses. Therefore, adherence to critical manufacturing standard operating procedures in vaccine production and ongoing efforts on development of thermo tolerant vaccine will help a long way in PPR control and eradication programme globally. The present review focuses on the way forward to achieve the objectives of quality vaccine production and easy upscaling to help the global PPR control and eradication by mass vaccination as an important tool.
Keywords: PPR, Process intensification, Defective interfering (DI) particles, Thermo stability, Upscaling
Introduction
Amongst the diverse sub-sectors of agriculture, livestock resource is an important component of the agricultural economy of developing nations in terms of livelihood and food security. Sheep and goats are the most important economic and social assets of marginal and landless agricultural labourers to meet their livelihood and income, thereby contributing to the national economic development. However, a number of infectious diseases pose a significant economic threat to the small land holders by both direct and indirect means which include effects on productivity, vaccination and other disease-control costs and constraints on livestock management. Among the infectious diseases, Peste des petits ruminants (PPR) is a disease with highest economic impact on small ruminants in Africa, Middle East and Asia causing up to 100% morbidity as well as a decline in productive performances [64]. About 80% of the global small ruminant population is at risk due to PPR threatening more than 1.7 billion of the total global population of 2.1 billion sheep and goats [20]. As a rough estimate, PPR causes a global economic loss of US$ 1.2–1.7 billion annually [20]. Meanwhile, the World Organization for Animal Health (OIE) and FAO of the United Nations have estimated the current expenditure on PPR vaccination to be between US$ 270 and 380 million per year [20].
Vaccination is considered to be the most effective way to control PPR throughout the endemic regions. The earlier practice to control the disease was to vaccinate the animals using Plowright’s tissue culture rinderpest vaccine (TCRV), as there was no homologous vaccine available [61]. However, the widespread use of rinderpest vaccine was later discontinued as it would have compromised with the sero-surveillance of Rinderpest during its post-eradication phases [61]. The first successful homologous PPR vaccine was developed using an African strain of PPRV designated Nigeria 75/1 strain, which is now widely used in African countries and elsewhere [14, 72]. In India, a homologous live attenuated vaccine against PPR was developed at ICAR-Indian Veterinary Research Institute, Mukteswar using the PPRV Sungri/96 strain [80]. Vaccine based on Sungri/96 strain is used extensively to control the disease in the Indian subcontinent [78] and some of the countries in the Middle East and South-Asia [53]. Both the strains, PPRV Nigeria 75/1 and PPRV Sungri/96, adapted to grow in vero cells have been later commercialized by manufacturers for production of PPR vaccines [72] in addition to several other isolates or strains which are not commonly used in the field.
Quality of cell culture based vaccines rely on batch cultivations under constant control of key parameters such as process intensification for upscaling of vaccines, interference of virus propagation by defective interfering particles (DIPs) and thermo stabilization etc. Process intensification includes strategies for optimization of batch cultivations to improve cell growth, viability and modifications in the process of virus propagation resulting in a higher virus yield [24]. One of the serious challenges in batch cultivations of vaccines includes accumulation of truncated form of viruses known as defective interfering particles (DIPs) [84]. These particles are deletion mutants and arise as a result of repeated passages at high multiplicity of infection (MOI) leading to slowing down of the parent virus population [56, 62]. Generation of such defective particles has been observed in closely related Morbilliviruses like Rinderpest [15] and Measles [90]. Experiences with PPR vaccine manufacturing also shows instances of low titres when the virus is not propagated using the recommended MOI. Both the homologous strains, PPRV Nigeria 75/1 and PPRV Sungri/96 are presently been extensively used by several commercial firms and government establishments for production of PPR vaccines in African and Asian countries. In such a scenario, a comprehensive understanding of process intensification for upscaling of vaccines as well as investigations related to generation and detection of DIPs during viral vaccine propagation is crucial. A detailed study on formation of DIPs will provide the vaccine manufacturers a clue for consistent, uniform and effective vaccine production methods. We are in a process of a detailed investigation on generation of DIPs in cell culture using an Indian PPRV vaccine strain (PPRV Sungri/96) which has been characterized extensively at genomic and antigenic level [78].
Viral vaccines are more stable in solid state and depending upon the method of drying, freezing or drying stresses may affect the structural integrity of the viruses [51]. Even though production of a large volume of liquid vaccine is easy and simple using roller culture bottles and cell factories, freeze drying remains an important bottleneck in upscaling of live attenuated vaccines like PPR. Due to thermo labile nature of these viruses, lyophilization in the presence of suitable stabilizers is required for preservation of the intact virion in vaccine samples. Investigations on thermo stability have indicated that the combination of stabilizers possess synergistic or additive effects on the stability of vaccines [42, 75]. Identification of a formulation conferring more stability will significantly enhance the utility of the vaccine in order to support manufacturing, shipping and storage (throughout the entire value chain). Analysis of residual moisture is one of the crucial parameters of quality control of such freeze-dried biological products. Higher percentage of residual moisture induces instability and thus reduces the shelf life of vaccines [40]. In view of this, approaches to improve thermo tolerance of freeze-dried vaccine have been achieved by extending the secondary drying cycle, in order to minimize the residual moisture (RM) levels [41]. Besides, preparation of the vaccine virus samples before freeze-drying is also critical as well as crucial for the quality of the end products. A controlled rate of freezing and use of small volumes of very high-titered virus in each aliquot/vial can significantly improve freeze drying quality and easy upscaling of such live vaccines [54].
The current vaccine strains, PPRV Nigeria 75/1 and PPRV Sungri/96, used for control of PPR are safe, effective and provide long lasting immunity [65, 80]. However, instability of such live attenuated vaccines loses potency over time and emerges as a key challenge for vaccine manufacturers and to end users [66]. The current vaccines require continuous cold chain for storage and transport and undergo accelerated degradation on exposure to higher temperatures [53]. Incomplete or unreliable cold chain has contributed to vaccination failures. Sometimes vaccines have been blamed from the point of view of efficacy that outbreak occurred despite being the animals or flocks were vaccinated, although the main reason was the failure to maintain the cold chain. As a result, the cost of production and transportation under cold chain are high and user’s expenses are unavoidable [58]. Therefore, development of a suitable combination of thermo stabilizers and improved lyophilization protocol that would deliver maximum retention of viral infectivity titre is of significant importance in upscaling of the existing PPR vaccines. Availability of a thermo tolerant PPR vaccine which would sustain high ambient temperatures may help in the effective control and represents a critical step in the Global Strategy for the eradication of PPR.
This article is therefore intended to give the readers an overview of process intensification, strategies to avoid generation of DIPs in cell culture, approaches for upscaling of PPR vaccines through innovative freeze drying processes and improved thermo stabilization.
Epidemiology and global control strategy for PPR
PPRV, though exists as a single serotype, at genetic level, it has been differentiated into four distinct lineages (I–IV), based on the genetic characterization of either F gene [74] or N gene [34]. Lineages I to III were considered as African and Middle East origin whereas lineage IV was reported exclusively from Asian countries. However, over the past decade, lineage IV has been reported from several countries of Africa despite being still prevalent in Asia [6, 48]. Since the first identification of PPR virus in Ivory Coast, West Africa in 1942, it has expanded its geographical distribution beyond its original endemic region and spread steadily to areas of sub-Saharan Africa, Middle East, Central Asia, South Asia, People’s Republic of China and most recently to Mongolia indicating its huge transboundary potential. Uncontrolled cross border animal movements and circulation of animal products between endemic countries through livestock trade makes this disease a global priority. Having devastating effects on the agricultural economy of developing countries, the disease is one of the priorities of FAO’s Emergency Prevention System for Animal Health (EMPRES) Programme and included in the Global Framework for the progressive control of Transboundary Animal Diseases (GF-TADs). Based on the endorsement of the GF-TADs Global Steering Committee, the World Organization for Animal Health (OIE) and the Food and Agriculture Organization (FAO) jointly embark upon the control of PPR on global scale and launched ‘PPR Global Control and Eradication Strategy’ in 2015 with three key objectives (i) to eradicate PPR by the year 2030, (ii) to strengthen the veterinary services and (iii) prevention and control of other major diseases of small ruminants [20]. The first-five year (2017–2021) programme of the PPR Global Eradication (PPR GEP) has laid the foundation to strengthen the implementation models and is complementary to achieving the targeted goals by 2030.
Economic importance of PPR disease, PPR vaccine and vaccination
After the successful global eradication of Rinderpest in 2011, eradication of PPR seems appealing as both the viruses share similarities in terms of protective immune responses. High morbidity and mortality rates of up to 80–90% in affected herds make PPR a potential killer disease for small ruminant populations causing a severe economic impact that affects rural economies, reduces genetic resources and endangers breeding policies. The direct economic losses caused by the disease are aggravated by the sanitary measures imposed by authorities to control animal movement and by trade restrictions on animal by-products [17]. Collectively, it was estimated that PPR causes a loss of US$ 1.5 million annually in Nigeria [27], US$ 39 million in India [77], at least US$ 1.5 million in Iran [8] and US$ 15 million in Kenya [6] and similar loses to other PPR endemic countries. These estimates were based on previous studies carried out at national level by different investigators. As per global strategy for the control and eradication of PPR by FAO/OIE, the disease causes an estimated global economic loss of US$ 1.2–1.7 billion annually [20]. However, all these losses can be avoided if an effective thermo tolerant PPR vaccine is made available and applied under field settings. Even though, development and production of thermo tolerant vaccine will require an additional expenditure on the part of stabilizers and critical freeze drying protocols, once a thermo tolerant vaccine is developed the production cost of such products can be made economical with the technological advancements in respect of state of the art self freeze-driers and miniaturization of freeze-drying protocols (high virus titre, reduced volume etc.). With the availability of stable vaccine preparations more number of vaccine doses will be available at the time of vaccination as compared to conventional vaccine. Further, availability of a thermo tolerant vaccine will reduce the dependence of vaccine for cold chain maintenance during transportation, which adds an additional cost to producers and also to users when the vaccine is stored locally and transported during field applications. As such, with the technological advancements for such products, donors need not to spend additional money on development and use of thermo tolerant vaccine.
Current vaccines against PPR
With the purpose of developing a PPR vaccine, the first successful adaptation of PPRV in vero cells has been achieved by continuous passage of Nigeria 75/1 strain which is now commonly used in African countries. Phylogenetic analysis revealed that the strain belongs to lineage II and the vaccine was also used worldwide to control the disease in different endemic zones with different lineages of PPRV. Anti-PPRV antibodies generated against this vaccine last for at least 3 years, which is the effective economic life of sheep and goats [72]. The vaccine is effective in providing long-term immunity and protects goats against virulent RPV. The effective dose was calculated to be 100.8 TCID50/animal; however, a dose of 103 TCID50 also proved to be safe [43].
Since the Nigeria 75/1 strain belonging to lineage II is restricted to African countries, its use in Asian countries may increase the likelihood of mixing up of lineages. Therefore, in order to develop a specific vaccine for use in Asian countries, the second successful PPR vaccine PPR Sungri/96 strain was developed in ICAR-Indian Veterinary Research Institute (IVRI), Mukteswar. The vaccine was isolated from goats that died with PPRV in Sungri area in Himachal Pradesh, India, during 1994 [80]. This isolate was passaged in B95a (marmoset lymphoblastoid) cells, followed by serial passages in vero cells and was attenuated completely after 56 passages in vero cells [66]. The vaccine was found to be safe in pregnant animals and confer solid protection to PPRV challenge [80]. A single dose of a PPR vaccine contains 103 TCID50 of vero cell-attenuated PPRV and is believed to provide protective immunity in sheep and goats for more than 5 years following vaccination [65]. Two other live attenuated PPR vaccine viruses were also developed in TANUVAS, India, namely Arasur/87 (sheep origin) and Coimbatore/97 (goat origin) and were serially passaged on vero cells to obtain avirulent strains at passage level 75 [72].
At present, Nigeria 75/1 and Sungri/96 strains have been commercialized by private industries and government institutions for mass immunization campaigns [78]. However, the live attenuated PPR vaccines are thermo sensitive and easily degraded in tropical and sub-tropical climates. In addition, poor infrastructure in remote areas and transportation of vaccines under field conditions results in an interrupted cold chain and thus loss of vaccine potency.
Possible reasons for vaccine failures
It is estimated that nearly 50% of the lyophilized and 25% of liquid vaccines are wasted each year due to disruption of the cold chain [67]. Due to inadequate electrical power and refrigeration in developing countries, storage, handling and heat stability of vaccines are matters of concern. A break in the cold chain can cause a substantial drop in the efficacy or potency of a vaccine [67]. Maintaining the cold chain during shipment to remote areas remains a challenge to the vaccine distributors. Most common deficiencies contributing to the instability of vaccines in developing countries with tropical climates include high temperature during storage and transport, failure to record temperature readings regularly and failure to discard the unused vaccines after sessions left at ambient temperature [23]. Besides, instances of immunization with vaccines having no or low live virus counts have been observed in several state veterinary biologicals (unpublished data) which might be due to use of lower MOIs than recommended or possible generation of DIPs during batch cultivation of PPR vaccines. Thus, the manufactured vaccines containing substantial amount DIPs will be of sub-standard quality and such low quality vaccines if distributed for field application would pose a serious risk of vaccine failures despite being used repeatedly.
Batch cultivation of vaccine virus
Cell culture based processes for the production of antigens or vaccines rely on batch cultivations. These are characterized by stepwise scaling up of cells, followed by virus infection at high cell densities in the final production volume, and the subsequent harvest which terminates the process [21]. Under constant control of key parameters, the product quality can be improved thereby avoiding batch to batch variation. With an increasing demand for efficacious, safe and affordable vaccines for animal use, process intensification in cell culture-based viral vaccine production demands advanced process strategies to overcome the limitations of conventional batch cultivations [81]. Another crucial component in the batch cultivation of vaccine is the accumulation of defective interfering particles (DIPs). The generation of these particles should be avoided which can be achieved by quality control of working seed viruses [22] and use of the recommended MOIs throughout the standard operations during vaccine manufacturing.
Process intensification for upscaling of vaccine production
The production of viral vaccines typically requires a cell growth phase followed by a virus replication phase (both typically operated in batch mode) as most viruses propagate in a complex process that requires the internalization of their genetic material into the host cell, the synthesis of viral RNA/DNA and viral proteins as well as the release of progeny virus particles [4]. Furthermore, it has to be taken into account that the replication process of lytic viruses results in cell death due to apoptosis followed by cell degradation and release of contaminants such as cellular DNA and host cell proteins [81]. In a static culture, this process results in the change of pH to acidic, that needs to be taken care of during upscaling in order to maintain the virus titre.
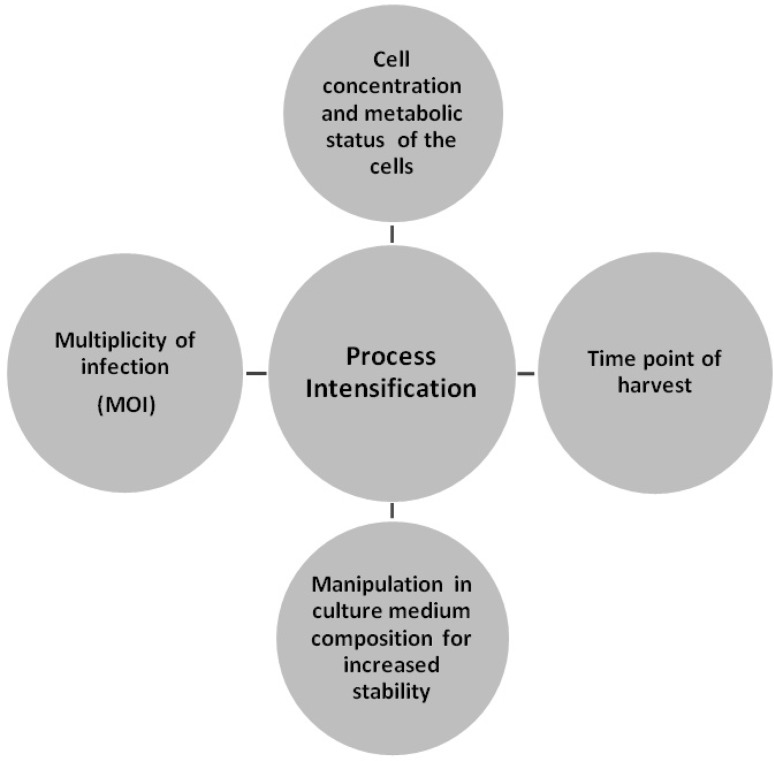
Upscaling of vaccines through process intensification require constant control of key factors—(a) cell concentration and metabolic/physiological status of the cells at the time of infection (toi), (b) ratio of infectious particles to viable cells, i.e. multiplicity of infection (MOI), (c) time point of harvest [81] and (d) manipulation in culture medium composition for enhanced stability [75] (Fig. 1). So far cell concentration and status of the cells are concerned, infection should be performed on healthy cells and infected cells should not be inhibited by accumulated by products. Another crucial component in upscaling of vaccines is the multiplicity of infection (MOI) which influences virus growth dynamics and yield [85]. An optimum or recommended MOI, if not introduced per cell, will result in either low virus titers or promote the generation of defective interfering particles (DIPs). Further, the harvesting time of respective virus samples should be carefully determined in order to achieve maximum titres and should be a part of standard operating procedures. Approaches for upscaling of vaccines can also be made by enriching the growth media with various components which supports the growth of the virus and stabilizes the buffering system of the growth medium. Trabelsi et al. [85] worked on process intensification of Sheep pox vaccine virus (RM 65 strain) by optimizing the production process, the effects of MOI, time of infection (toi) and the culture medium used for propagation of Sheep pox virus. Supplementation of 5 mM fructose, 25 mM sucrose and 5 mM glucose into the growth medium resulted in higher titre of Sheep pox vaccine virus [85]. The stability of PPRV vaccine (Nigeria 75/1) has also been evaluated by addition of 25 mM fructose which resulted in higher virus production with higher stability [75].
Fig. 1.
Key components of process intensification in upscaling of vaccines
Most of live attenuated vaccines in stationary culture systems are scaled up using roller bottles and cell factories which are labour intensive and susceptible to contamination [3, 10]. Commercially relevant process intensification can be achieved through use of bioreactors [13]. Batch processes in bioreactors refer to a partially closed system in which most of the materials are loaded aseptically onto the reactors and removed at the end of operation [79]. Selection of bioreactor system is defined by the growth characteristics of host cells and virus infection kinetics [25]. Upscaling of vaccines through use of bioreactors requires shorter time as compared to conventional batch cultures, [21] which significantly increases the vaccine doses with each of the manufactured batches. Intensification of modified Vaccinia Ankara virus and Influenza virus have been achieved through high cell density cultivation approaches in bioreactors using suspension culture [87]. Trabelsi et al. [86] optimized measles virus production by growing cells on Cytodex 1 microcarriers in stirred bioreactors for easy scale up of measles vaccine by particularly investigating the effect of MOI, glucose regulation and culture medium. In context of continuous production of vaccines in bioreactors, reproducible high titer processes will require appropriate process control systems including the established parameters of MOI, pH, temperature and oxygen concentration [18, 25]. Innovative approaches for large scale production of PPR vaccines will be important to enable effective responses and meet the global demands of vaccine doses. Such consistent studies should be carried out in order to optimize the intensification studies for PPR vaccine batches (Sungri/96 strain), which is readily commercialized by several firms and animal husbandry departments throughout the Asian and Indian territory [78]. Nevertheless, investigations are still required to understand the virus–host cell systems in order to develop an appropriate production process for PPR vaccines which reproducibly produces sufficient yields of high quality virus particles.
PPRV replication and formation of defective interfering particles
PPR virus and its replication strategy
PPR virions are pleomorphic particles which vary in size from 150 to 700 nm containing a host cell-derived lipid bilayer envelope [9]. The linear, single-stranded genomic RNA is packaged by nucleoprotein ‘N’ to form nucleocapsid along with phosphoprotein ‘P’ and polymerase protein ‘L’, togetherly comprising the ribonucleoprotein (RNP) complex. Mostly, the length of the entire genome of PPRV is 15,948 nucleotides, which is the second longest among all morbilliviruses next to feline morbillivirus [5, 15]. The genome of PPR virions is organized into six transcriptional units and each encodes at least one non-overlapping protein: the nucleocapsid ‘N’, the matrix ‘M’, the polymerase or large ‘L’, the phosphoprotein ‘P’, and two envelope proteins haemagglutinin ‘H’ and fusion ‘F’ [49]. The PPRV genome carries six transcriptional units, each encodes for an adjoining and non-overlapping protein except for the phosphoprotein ‘P’ gene, which also expresses ‘C’ and ‘V’ non-structural proteins by an alternative ORF and RNA editing mechanism respectively [38]. The genes are arranged in an order of 3′-N–P/C/V–M–F–H–L–5′ each gene being separated by intergenic (IG) regions of variable length [5, 39].
During virus replication, PPRV ‘H’ protein interacts with sialic acid receptor on the host cell membrane. The virus–host interaction is followed by ‘F’ protein mediated fusion releasing the nucleocapsid into the cytoplasm [45]. The large protein ‘L’ then acts as a RNA dependent RNA polymerase (RdRp) and initiates transcription of messenger RNA (mRNA) in the cytoplasm. The RdRp of all Paramyxoviruses is believed to attach at the genome promoter (GP) on genomic RNA from where the transcription initiates [5]. The individual transcriptional unit, composed of an intergenic region (IG), coding sequences and conserved non-coding sequences flanking the coding region, is synthesized in the ‘start–stop’ fashion [46]. The RdRp may detach from the template during transcription at IG and may reinitiate the transcription at GP, a mechanism of controlling the amount of individual proteins being produced. The ‘N’ protein located closely to the GP is most abundantly transcribed [47]. In contrast, the ‘L’ protein is located farthest from the GP and hence transcribed in the least amount. Each mRNA transcript in Paramyxoviruses is transcribed as naked RNA, which undergoes capping at their 5′ end and polyadenylation at 3′ end by the virus-encoded polymerase and hence is stable and can be efficiently translated by the host ribosomes [7]. After synthesis of the mRNA, the RdRp switches to synthesize full-length positive-sense RNA (antigenome RNA or complementary RNA, cRNA) which is encapsidated by the N protein [26]. It has been hypothesized that the accumulation of unassembled N protein in the cytoplasm plays a major role in switching the RdRp function from mRNA to cRNA synthesis [89]. Paramyxoviruses form virus particles when all the structural components of the virus, including viral glycoproteins and viral RNPs, have assembled at selected sites on the membranes where virions bud, then pinch off to achieve particle release, allowing the transmission of infections to new cells and hosts [28]. Incorporation of the genomic RNA into budding virions is driven by interactions between M and nucleocapsid at virus assembly sites. Paramyxovirus RNPs interact with the M protein under the plasma membrane and buds via the Endosomal sorting complexes required for transport (ESCRT) [73, 88].
Defective interfering particles and their effects on virus propagation process
Defective interfering particles (DIPs) are generated due to high MOIs in cell culture probably because of exhaustion of cellular machinery at a point when replication is incomplete. These particles get accumulated over a period of time when more and more undiluted (high MOIs) passages are made. Such a phenomenon may not be occurring in natural infections when new cells, new hosts are available for further replication or may be occurring that have not been noticed, which needs to be explored for a possible carrier state in susceptible hosts. Generation of DIPs in continuous production processes or batch cultivations of vaccines represents a serious challenge for vaccine manufacturers if due precautions are not taken while making virus passages. DIPs are deletion mutants and emerge during high MOIs in suitable cells [83]. DIPs are called “defective” because they have lost the capacity to code for all the necessary viral proteins required for independent replication and require the coexistence of the parent virus [56]. In contrast, they are referred as “interfering” because they can attenuate the symptoms or cytopathic effects caused by the helper virus [56]. DIPs have unique properties and have been characterized by their genome length and sequences [19], particle sizes and morphologies [29, 57] and their biological activities [82]. The frequency of their generation is influenced by cell type, virus strain, passage history [90] and the method of infection [83]. Population dynamic models of DIP-virus interaction presumes that co-infection of a cell with at least one DIP will prevent that cell from making any standard virus particles and the co-infected cells will produce only DIPs [44, 83]. Thus, DIPs will readily evolve in natural virus populations under frequent conditions of co-infection which can lower the fitness of full length viruses within the cell [91].
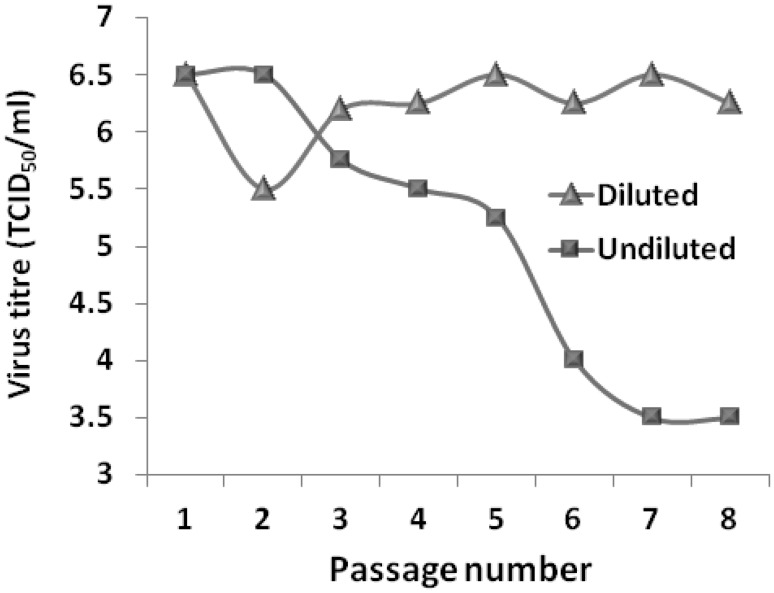
The evolution of DIPs in culture is MOI dependent [32]. Higher MOIs attribute to earlier and faster decline in virus levels. Generation of DIPs also depends on the replication events and the generation intervals [83]. Higher the MOI, more virus and DIPs are transferred from one generation to the next leading to rapid accumulation of DIPs. Moreover, the DI genomes contain packaging sequences and hence become packaged within the usual viral structural proteins and antigens [68]. Investigations on generation of DIPs has been widely documented during propagation of several RNA viruses such as Sendai virus [92], Murray valley encephalitis virus [36], Infectious haematopoiesis necrosis virus [31], Vesicular stomatitis virus [82], Dengue virus [37], Influenza virus [21, 70] and Japanese Encephalitis virus [96]. DIPs have also been documented in live attenuated vaccine of Morbilliviruses such as Measles [90] and Rinderpest which suggests that these particles may resist a desirable humoral immune response by interfering with the standard viral replication [15]. Even though information on generation of DIPs of closely related vaccine viruses is available, the significance of PPRV genome specific DIPs are yet to be addressed. Experiences of the authors, while working on PPRV Sungri/96 virus showed evidences of generation of DIPs. Such experiences were also noticed from some other manufacturers while we were the part of quality assurance of PPR vaccines (unpublished data). Therefore, we studied the pattern of generation of DIPs by making regular passages at low as well as high MOIs using PPRV Sungri/96 vaccine strain in vero cells. The findings clearly indicated the formation of such interfering particles as is evidenced from Fig. 2. Detailed study and characterization of PPRV specific DIPs are in progress.
Fig. 2.
Graph showing PPRV Sungri/96 vaccine virus titre (TCID50/ml) over different passages using undiluted (high MOI) and diluted (low MOI) virus inoculums. Note that the gradual decline in virus titre with undiluted inoculums indicates formation of defective interfering particles (DIPs)
Possible mechanism of generation of DI genomes
The DI genomes of paramyxoviruses arise as a result of replicative errors. The RNA polymerase carrying the nascent genome or antigenome chain leaves its template and falls back on the nascent chain and start synthesizing in opposite direction to create an inverted terminal repeat forming copy back DI genomes which retains 5′ end of the genome and 3′ end of the antigenome [35, 90] (Fig. 3). In other cases, it may rejoin the same template further downstream to finish the chain, thus creating an internal deletion having similar 5′ and 3′ ends of the genome.
Fig. 3.
Possible mechanism of generation of DIPs in Paramyxoviruses.
Image modified from Dhar and Bandyopadhyay (1999)
In case of Paramyxoviruses, the mechanism of synthesis of DIPs is by copy-back mechanism and the DI RNAs originate from the 5′ end of the standard genome and contains terminal inverted repeats of 50–200 nucleotides [90]. The RNA polymerase complex in the middle of replication detached from an original strand and switches over to the growing nascent daughter strand and copies back towards the terminus from which the replication has started [15]. The error occurs while a plus strand is copied resulting in the preservation of the 5′ end terminus of the minus strand and the generation of a 3′ terminus complementary to it in a newly generated sub-genomic RNA. The resulting DI genome is then deleted of all sequences downstream of the cross over point and added the complements of its own 5′ end to the 3′ end. The complementary end of these chains (50–200 nucleotides long) is thus responsible for the circular ‘panhandle’ structures [11].
PPR Sungri/96 vaccine virus is used extensively by several commercial firms and state biologicals for production of PPR vaccine throughout India. These laboratories and establishments have human resource with variable expertise and experiences. These man powers sometimes are transferred due to policy decisions. Often, these laboratories do not follow clear recommendations or standard operating procedures developed by parent laboratories. As a result, on several occasions they experience insufficient infectious virus particles in the end products. Therefore, an extensive understanding of generation of PPRV genome specific DIPs as well as its detection and characterization is a requisite to find out and suggest a safe MOI for the propagation of vaccine viruses in cell culture based systems.
Thermo stability issues and way forward
Preservation of vaccines, storage and handling is one of the tedious tasks to ensure its optimal potency. All vaccines lose potency over time and the rate of potency loss is temperature-dependent [94]. Viruses exhibit varying degree of thermo lability at different storage temperatures and stabilization conditions. Preservation of live vaccines by freeze drying has become the accepted method for long term preservation and maintenance of stability [1]. The stability of the vaccine depends upon the thermo stability of the virus, the pH of culture system in which the virus is grown, property of the chemical stabilizer, freeze drying conditions optimal for a virus and reconstitution buffers for long-term storage, transportation and use of viral vaccine under field conditions or other viral preparations under extended time of handling in laboratory settings [71]. Further, preparation of the infectious virus samples before and during freeze drying is critical as well as crucial for the quality of the end product [54]. One of the important quality attributes of freeze dried product is the ‘cake appearance’. However, freeze drying of a small volume of samples may result in a non-ideal cake which has no impact on product quality (safety and efficacy) and is an inherent characteristic of the product either due to formulation, drug product presentation and/or freeze-drying process [55]. Therefore, freeze-drying of small volume of high titered vaccine virus following an extended/normal freeze drying protocol may result in a final product with low moisture content. A short freeze drying protocol may also be developed without any compromise in the finished product. This may significantly add to the upscaling of live attenuated vaccines where freeze drying is considered to be a major bottleneck.
Stabilizers for thermo tolerant profile of vaccines
Biologically active macromolecules including vaccines are more stable in the solid state. In general, live attenuated vaccines often require complex formulations of excipients or stabilizers to obtain adequate storage stability [33]. The earliest reports on the thermo stability of the cell culture adapted Rinderpest virus (RPV) were of Plowright and Ferris [59] and Johnson [30]. Both studied the stability of cultured RPV stored in maintainence medium containing a different concentration of normal serum. Therefore, appropriate stabilizers are required for preservation of these properties. It was reported that defibrinated blood [12, 69] and peptone water [50] can increase the half life of attenuated RPV. A study by Plowright et al. [60] indicated that combination of 5% lactalbumin hydrolysate (LAH) and 10% sucrose possesses superior additive effects on thermostabilization of rinderpest vaccine. A comparative study of various chemical stabilizers and lyophilization cycles on the thermostability of a vero-cell adapted Rinderpest vaccine was made by Mariner et al, using three different formulations of stabilizers viz., 5% lactalbumin hydrolysate and 10% sucrose (LS), hydrolysed gelatine and sorbitol, lactobionic acid and buffered hydrolysed gelatine and sorbitol (BUGS) [41]. Vaccine stabilizers are the most studied agents as they play a crucial role to help retain the quality of the vaccines during storage and transit. Of late, the stabilizing effect of various chemicals on the stability of PPR vaccine have been studied and published by several workers. Chemical stabilizers such as trehalose dihydrate [42, 75, 93], lactalbumin hydrolysate–sucrose (LS) [42], weybridge medium (WBM) [16, 75, 76], buffered hydrolysed gelatin and sorbitol (BUGS) [2, 95], polyvinyl pyrolidone [16], deuterium oxide (Heavy water) [71] have been used for studying their stabilizing properties on different strains of PPR vaccine around the globe. In contrast, reports on thermostabilization of PPR vaccine using a controlled freeze drying cycle are little. Evaluation of thermostability of PPRV Sungri/96 of lineage IV origin has been done by Sarkar et al. [66] and Sen [71]. Investigations on thermostability of PPRV Sungri/96 vaccine has shown that the half life of the freeze dried vaccine is only 30 days on storage at 4 °C with the OIE recommended stabilizer weybridge medium, which is perhaps a major drawback to use the vaccine in tropical climates [66]. Several other workers studied thermostability of different other strains of PPRV including PPRV Jhansi/2003 [63], PPRV Revati/2006 [63], thermo adapted PPRV MIB 187T and MIB 197T [52] using different chemical stabilizers. An attempt to enhance thermo stabilization using the synergistic effect of stabilizers, with low volume of high titered virus could be an important approach for enhanced thermo stability of PPR vaccine to be used in tropical countries like India and several other countries of Asia and Africa, looking at global PPR eradication by the year 2030.
Synergistic combinations of stabilizers
Combination of different stabilizers is another approach for the enhanced thermo stability of vaccines. Studies on the chemical effect of stabilizers on PPR vaccines have shown that lactalbumin hydrolysate sucrose (LS) and trehalose dihydrate (TD) allowed higher stability of lyophilized PPR vaccine [66, 75]. A thermostable presentation of PPR vaccine (Nigeria 75/1) was adopted using a combination of TD and LS stabilizers by Mariner and co workers. However, the result indicated that the use of TD along with LS added no additive effect in the manufacture of thermostable PPR vaccine [42]. Because PPR virus grows to a high titre, and on several occasions, the manufacturers dilute the vaccine virus to adjust to a particular dose/TCID50. It would be a good idea to go with low volumes of vaccines for freeze drying to enhance its efficacy or alternately setting up a short freeze drying cycle in order to upscale the vaccine with no compromise in the quality.
Freeze drying cycles
Improved thermotolerance of freeze dried vaccine has been achieved by extending the secondary drying cycle, in order to minimize the residual moisture (RM) levels to around 1–2% [41]. An ultra rapid method (Xerovac) was developed for dehydration and preservation of live attenuated Rinderpest and Peste des petits ruminants vaccines using trehalose dihydrate (TD) as the stabilizer [93]. Xerovac employes the method of dehydration consisting two main components primary drying and secondary drying to develop a thermostable vaccine within 18 h with a residual moisture level of less than 2% [93]. Silva et al. [75] showed the improvement of PPR vaccine (Nigeria 75/1) by using the dehydration method Xerovac in presence of a formulation containing TD which resulted in a vaccine with minimal loss of potency. Mariner et al. [42] evaluated the thermostability of PPR vaccine (Nigeria 75/1) stability using the Xerovac presentation originally developed for lyophilization of RP vaccine by Worrall et al. Short cycles of freeze drying (FD) has also been developed for several other vaccine strains such as Human Herpesvirus 1, Human enterovirus B, Chikungunya viruses and Human Adenovirus type C using a FD cycle of 12 h with a lesser quantity of viral preparations [54]. Therefore, modification of the freeze drying cycle could be significant step to improve the upscaling and thermostable profile of a vaccine which would help in controlling the disease in endemic areas followed by eradication as has been visualized by FAO and OIE. Due to high fecundity and short generation time of small ruminants, such a vaccine is likely to be applied for long time as compared to rinderpest vaccine in cattle and buffalo which have low fecundity as well as long generation time.
Concluding remarks
The global strategy for eradication of PPR will significantly contribute to food security and reducing poverty in the world’s most vulnerable pastoral and rural communities [20]. In such a scenario, vaccines and vaccination strategy plays a crucial role in the sustainability of the global socio-economic status of the livestock owners. Improvement in freeze drying technologies with selected stabilizers, process intensification and automation will help in the development and production of live attenuated PPR vaccines with higher stability. It may also be possible to upscale PPR vaccines by use of inoculums at known multiplicity of infections to avoid formation of defective interfering particles in continuous production systems. Virus harvest with high yield may be possible to freeze dry at low volumes adopting a short freeze drying cycle without compromise in the quality of the end products. Efforts are being made to identify vaccine-stabilizer formulations that would retain the biological activity of the vaccines starting from the manufacturers, shipping to subsequent storage in field conditions.
Acknowledgements
The authors would like to acknowledge the Director, ICAR-IVRI, Joint Director Research and Joint Director (Academic) for the facilities to carry out this work. The authors would like to acknowledge the funding from the Department of Biotechnology (Grant No. BT/IM/Indo-UK/FADH/50/GDR/2013).
References
- 1.Alkeev N, Averin S, von Gratowski S. New method for monitoring the process of freeze drying of biological materials. AAPS PharmSciTech. 2015;16(6):1474–1479. doi: 10.1208/s12249-015-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asim M, Rashid A, Chaudhary AH. Effect of various stabilizers on titre of lyophilized live-attenuated peste des petits ruminants (PPR) vaccine. Pak Vet J. 2008;28(4):203–204. [Google Scholar]
- 3.Aunins JG, Bader B, Caola A, Griffiths J, Katz M, Licari P, Ram K, Ranucci CS, Zhou W. Fluid mechanisms, cell distribution and environment in cellcube bioreactors. Biotechnol Prog. 2003;19:2–8. doi: 10.1021/bp0256521. [DOI] [PubMed] [Google Scholar]
- 4.Aunins JG. Viral vaccine production in cell culture. In: Encyclopedia of cell technology. 2003.
- 5.Bailey D, Banyard A, Dash P, Ozkul A, Barrett T. Full genome sequence of peste des petits ruminants virus, a member of the Morbillivirus genus. Virus Res. 2005;110(1):119–124. doi: 10.1016/j.virusres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91(12):2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 7.Barrett T, Banyard, AC, Diallo A. Rinderpest and peste des petits ruminants virus: plague of large and small ruminants. In: Barrett T, Pastoret P-P, Taylor WP, editors, Molecular biology of the morbillivirus: biology of animal infections. Academic Press: London; 2006. p. 31–67.
- 8.Bazarghani TT, Charkhkar S, Doroudi J, Bani Hassan E. A review on peste des petits ruminants (PPR) with special reference to PPR in Iran. Zoonoses Public Health. 2006;53(1):17–18. [Google Scholar]
- 9.Bourdin P, Laurent Vautier A. Note sur la structure du virus de la peste des petits ruminants. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 1967;20(3):383–386. doi: 10.19182/remvt.7469. [DOI] [PubMed] [Google Scholar]
- 10.Butler M. Growth limitations on microcarriers. Adv Biochem Eng Biotechnol. 1987;34:57–84. doi: 10.1007/BFb0000673. [DOI] [PubMed] [Google Scholar]
- 11.Calain PH, Roux LA. Generation of measles virus defective interfering particles and their presence in a preparation of attenuated live-virus vaccine. J Virol. 1988;62(8):2859–2866. doi: 10.1128/jvi.62.8.2859-2866.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng SC, Fischman HR. Lapinized Rinderpest virus and its use as a vaccine. In: Kesteven KVL, editor. Rinderpest Vaccines their production and use in the field. Washington: Food and Agriculture Organization of the United Nations; 1949. p. 47.
- 13.Chisti Y, Moo-Young M. Bioprocess intensification through bioreactor engineering. Chem Eng Res Des. 1996;74:575–583. [Google Scholar]
- 14.Diallo A, Barrett T, Lefevre PC, Taylor WP. Comparison of proteins induced in cells infected with rinderpest and peste des petits ruminants viruses. J Gen Virol. 1987;68(7):2033–2038. doi: 10.1099/0022-1317-68-7-2033. [DOI] [PubMed] [Google Scholar]
- 15.Dhar P, Bandyopadhyay SK. Defective interfering particles of morbillivirus: a review. Indian J Virol. 1999;15(1):1–5. [Google Scholar]
- 16.El-Bagoury GF, El-Nahas EM, Hussein AM, Mohamed AM. Assesment of two stabilizers used for lyophilized live attenuated peste des petits ruminants (PPR) vaccine. Behna Vet Med J. 2015;29(1):183–188. [Google Scholar]
- 17.EMPRES Transboundary Animal Diseases Bulletin. Peste des petits ruminants. FAO Animal Production and Health Division; 2012.
- 18.Enden G, Zhang YH, Merchuk JC. A model of the dynamics of insect cell infection at low multiplicity of infection. J Theor Biol. 2005;237(3):257–264. doi: 10.1016/j.jtbi.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Epstein DA, Herman RC, Chien I, Lazzarini RA. Defective interfering particle generated by internal deletion of the vesicular stomatitis virus genome. J Virol. 1980;33(2):818–829. doi: 10.1128/jvi.33.2.818-829.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FAO O. Global strategy for the control and eradication of PPR. FAO and OIE. 2015.
- 21.Frensing T, Heldt FS, Pflugmacher A, Behrendt I, Jordan I, Flockerzi D, Genzel Y, Reichl U. Continuous influenza virus production in cell culture shows a periodic accumulation of defective interfering particles. PLoS ONE. 2013;8(9):e72288. doi: 10.1371/journal.pone.0072288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frensing T, Pflugmacher A, Bachmann M, Peschel B, Reichl U. Impact of defective interfering particles on virus replication and antiviral host response in cell culture-based influenza vaccine production. Appl Microbiol Biotechnol. 2014;98(21):8999–9008. doi: 10.1007/s00253-014-5933-y. [DOI] [PubMed] [Google Scholar]
- 23.Galazka A, Milstien J, Zaffran M. Thermostability of vaccines. In: Global programmer for vaccines and inmunization. Geneva: WHO; 1998.
- 24.Gallo-Ramirez LE, Nikolay A, Genzel Y, Reichl U. Bioreactor concepts for cell culture-based viral vaccine production. Expert Rev Vaccines. 2015;14(9):1181–1195. doi: 10.1586/14760584.2015.1067144. [DOI] [PubMed] [Google Scholar]
- 25.Grein TA, Weidner T, Czermak P. Concepts for the production of viruses and viral vectors in cell culture. In: GowderSJT, editor. New Insights into Cell Culture Technology. 2017. 10.5772/62590. ISBN: 978-953-51-3134-2.
- 26.Gubbay O, Curran J, Kolakofsky D. Sendai virus genome synthesis and assembly are coupled: a possible mechanism to promote viral RNA polymerase processivity. J Gen Virol. 2001;82(12):2895–2903. doi: 10.1099/0022-1317-82-12-2895. [DOI] [PubMed] [Google Scholar]
- 27.Hamdy FM, Dardiri AH. Response of white-tailed deer to infection with peste des petits ruminants virus. J Wildl Dis. 1976;4:516–522. doi: 10.7589/0090-3558-12.4.516. [DOI] [PubMed] [Google Scholar]
- 28.Harrison MS, Sakaguchi T, Schmitt AP. Paramyxovirus assembly and budding: building particles that transmit infections. Int J Biochem Cell Biol. 2010;42(9):1416–1429. doi: 10.1016/j.biocel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang AS, Greenawalt JW, Wagner RR. Defective T particles of vesicular stomatitis virus: preparation, morphology, and some biologic properties. Virology. 1966;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RH. Rinderpest in tissue culture. III: use of the attenuated strain as a vaccine for cattle. Br Vet J. 1962;118(4):141–150. doi: 10.1016/S0007-1935(17)43103-4. [DOI] [Google Scholar]
- 31.Kim CH, Dummer DM, Chiou PP, Leong JA. Truncated particles produced in fish surviving infectious hematopoietic necrosis virus infection: mediators of persistence? J Virol. 1999;73(1):843–849. doi: 10.1128/jvi.73.1.843-849.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkwood TB, Bangham CR. Cycles, chaos, and evolution in virus cultures: a model of defective interfering particles. Proc Natl Acad Sci. 1994;91(18):8685–8689. doi: 10.1073/pnas.91.18.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatek O, Minet C, Grillet C, Hurard C, Carlsson E, Karimov B, Albina E, Diallo A, Libeau G. Peste des petits ruminants (PPR) outbreak in Tajikistan. J Comp Pathol. 2007;136(2):111–119. doi: 10.1016/j.jcpa.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, Fields BN, editors. Fields virology. Philadelphia: Lippincott-Raven Press; 1996. [Google Scholar]
- 36.Lancaster MU, Hodgetts SI, Mackenzie JS, Urosevic N. Characterization of defective viral RNA produced during persistent infection of Vero cells with Murray Valley encephalitis virus. J Virol. 1998;72(3):2474–2482. doi: 10.1128/jvi.72.3.2474-2482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li D, Lott WB, Lowry K, Jones A, Thu HM, Aaskov J. Defective interfering viral particles in acute dengue infections. PLoS ONE. 2011;6(4):e19447. doi: 10.1371/journal.pone.0019447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahapatra M, Parida S, Egziabher BG, Diallo A, Barrett T. Sequence analysis of the phosphoprotein gene of peste des petits ruminants (PPR) virus: editing of the gene transcript. Virus Res. 2003;96(1):85–98. doi: 10.1016/S0168-1702(03)00176-X. [DOI] [PubMed] [Google Scholar]
- 39.Mahapatra M, Parida S, Baron MD, Barrett T. Matrix protein and glycoproteins F and H of Peste-des-petits-ruminants virus function better as a homologous complex. J Gen Virol. 2006;87(7):2021–2029. doi: 10.1099/vir.0.81721-0. [DOI] [PubMed] [Google Scholar]
- 40.Malik KP, Duru C, Ahmed M, Matejtschuk P. LYO/TITRATION: analytical options for the measurement of residual moisture content in lyophilized biological materials. Am Pharm Rev. 2010;13(5):42–47. [Google Scholar]
- 41.Mariner JC, House JA, Sollod AE, Stem C, Van den Ende M, Mebus CA. Comparison of the effect of various chemical stabilizers and lyophilization cycles on the thermostability of a Vero cell-adapted rinderpest vaccine. Vet Microbiol. 1990;21(3):195–209. doi: 10.1016/0378-1135(90)90032-Q. [DOI] [PubMed] [Google Scholar]
- 42.Mariner JC, Gachanja J, Tindih SH, Toye P. A thermostable presentation of the live, attenuated peste des petits ruminants vaccine in use in Africa and Asia. Vaccine. 2017;35(30):3773–3779. doi: 10.1016/j.vaccine.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 43.Martrenchar A, Zoyem N, Diallo A. Experimental study of a mixed vaccine against peste des petits ruminants and capripox infection in goats in northern Cameroon. Small Rumin Res. 1997;26(1–2):39–44. doi: 10.1016/S0921-4488(96)00989-3. [DOI] [Google Scholar]
- 44.McLain L, Armstrong SJ, Dimmock NJ. One defective interfering particle per cell prevents influenza virus-mediated cytopathology: an efficient assay system. J Gen Virol. 1988;69(6):1415–1419. doi: 10.1099/0022-1317-69-6-1415. [DOI] [PubMed] [Google Scholar]
- 45.Mühlebach MD, Leonard VH, Cattaneo R. The measles virus fusion protein transmembrane region modulates availability of an active glycoprotein complex and fusion efficiency. J Virol. 2008;82(22):11437–11445. doi: 10.1128/JVI.00779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munir M, editor. Mononegaviruses of veterinary importance, volume 1: pathobiology and molecular diagnosis. Wallingford: CABI; 2013. [Google Scholar]
- 47.Munir M, Zohari S, Berg M. Molecular biology and pathogenesis of peste des petits ruminants virus. Berlin: Springer; 2012. [Google Scholar]
- 48.Muniraju M, Munir M, Parthiban AR, Banyard AC, Bao J, Wang Z, Ayebazibwe C, Ayelet G, El Harrak M, Mahapatra M, Libeau G. Molecular evolution of peste des petits ruminants virus. Emerg Infect Dis. 2014;20(12):2023–2033. doi: 10.3201/eid2012.140684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muthuchelvan D, Rajak KK, Ramakrishnan MA, Choudhary D, Bhadouriya S, Saravanan P, Pandey AB, Singh RK. Peste-des-petits-ruminants: an Indian perspective. Adv Anim Vet Sci. 2015;3(8):422–429. doi: 10.14737/journal.aavs/2015/3.8.422.429. [DOI] [Google Scholar]
- 50.Nguyen-Ba-Luong DN, Vu-Thien-Thai Contribution to the study of the virus-vaccine against rinderpest, strain Nakamura III. Bull Off Int Epiz. 1958;50:559–563. [Google Scholar]
- 51.Ohtake S, Lechuga‐Ballesteros D, Truong‐Le V, Patzer EJ. Strategies for heat-stable vaccines. In: Wen EP, Ellis R, Pujar NS, editors. Vaccine Development and Manufacturing. 2015. p. 287–318.
- 52.Palaniswami KS, Thangavelu A, Velmurugan R. Development of thermostable peste des petits ruminants (PPR) virus vaccine and assessment of molecular changes in the F gene. In: Applications of gene-based technologies for improving animal production and health in developing countries. 2005. p. 673–678.
- 53.Parida S, Muniraju M, Mahapatra M, Muthuchelvan D, Buczkowski H, Banyard AC. Peste des petits ruminants. Vet Microbiol. 2015;181(1):90–106. doi: 10.1016/j.vetmic.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pastorino B, Baronti C, Gould EA, Charrel RN, De Lamballerie X. Effect of chemical stabilizers on the thermostability and infectivity of a representative panel of freeze dried viruses. PLoS ONE. 2015;10(4):e0118963. doi: 10.1371/journal.pone.0118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel SM, Nail SL, Pikal MJ, Geidobler R, Winter G, Hawe A, Davagnino J, Gupta SR. Lyophilized drug product cake appearance: what is acceptable? J Pharm Sci. 2017;106(7):1706–1721. doi: 10.1016/j.xphs.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Pathak KB, Nagy PD. Defective interfering RNAs: foes of viruses and friends of virologists. Viruses. 2009;1(3):895–919. doi: 10.3390/v1030895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petric M, Prevec L. Vesicular stomatitis virus-A new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- 58.Plotkin S, Robinson JM, Cunningham G, Iqbal R, Larsen S. The complexity and cost of vaccine manufacturing—An overview. Vaccine. 2017;35(33):4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plowright W, Ferris RD. Studies with rinderpest virus in tissue culture. III. The stability of cultured virus and its use in virus neutralization tests. Archiv fur die gesamte Virusforschung. 1962;11:516–533. doi: 10.1007/BF01241304. [DOI] [PubMed] [Google Scholar]
- 60.Plowright W, Rampton CS, Taylor WP, Herniman KA. Studies on rinderpest culture vaccine. III. Stability of the lyophilised product. Res Vet Sci. 1970;11:71–81. [PubMed] [Google Scholar]
- 61.Roeder P, Mariner J, Kock R. Rinderpest: the veterinary perspective on eradication. Philos Trans R Soc B. 2013;368(1623):20120139. doi: 10.1098/rstb.2012.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rima BK, Davidson WB, Martin SJ. The role of defective interfering particles in persistent infection of Vero cells by measles virus. J Gen Virol. 1977;35(1):89–97. doi: 10.1099/0022-1317-35-1-89. [DOI] [PubMed] [Google Scholar]
- 63.Riyesh T, Balamurugan V, Sen A, Bhanuprakash V, Venkatesan G, Yadav V, Singh RK. Evaluation of efficacy of stabilizers on the thermostability of live attenuated thermo-adapted Peste des petits ruminants vaccines. Virol Sin. 2011;26(5):324–337. doi: 10.1007/s12250-011-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santhamani R, Singh RP, Njeumi F. Peste des petits ruminants diagnosis and diagnostic tools at a glance: perspectives on global control and eradication. Arch Virol. 2016;161(11):2953–2967. doi: 10.1007/s00705-016-3009-2. [DOI] [PubMed] [Google Scholar]
- 65.Saravanan P, Sen A, Balamurugan V, Rajak KK, Bhanuprakash V, Palaniswami KS, Nachimuthu K, Thangavelu A, Dhinakarraj G, Hegde R, Singh RK. Comparative efficacy of peste des petits ruminants (PPR) vaccines. Biologicals. 2010;38(4):479–485. doi: 10.1016/j.biologicals.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Sarkar J, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK. Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated Peste des petits ruminants (PPR) vaccine. Vaccine. 2003;21(32):4728–4735. doi: 10.1016/S0264-410X(03)00512-7. [DOI] [PubMed] [Google Scholar]
- 67.Schlehuber LD, McFadyena IJ, Shua Y, Carignana J, Paul Duprexa W, Forsytha WR, Hoa JH, Kitsos CM, Lee GY, Levinsona DA, Lucier SC, Moorea CB, Nguyena NT, Ramos J, André Weinstocka B, Zhang J, Monaglea JA, Gardner CR, Alvarez JC. Towards ambient temperature-stable vaccines: the identification of thermally stabilizing liquid formulations for measles virus using an innovative high-throughput infectivity assay. Vaccine. 2011;29:5031–5039. doi: 10.1016/j.vaccine.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 68.Schlesinger S. Retroviruses, Viroids, and RNA recombination. In: Domingo E, Holland JJ, Ahlquist P, editors. RNA Genetics. Vol II. Boca Raton: CRC Press; 1987, p. 167–185.
- 69.Scott GR. Thermal reactions of kenya cattle vaccinated with lapinized rinderpest virus. Nature. 1954;174(4418):44. doi: 10.1038/174044a0. [DOI] [PubMed] [Google Scholar]
- 70.Scott PD, Meng B, Marriott AC, Easton AJ, Dimmock NJ. Defective interfering influenza virus confers only short-lived protection against influenza virus disease: evidence for a role for adaptive immunity in DI virus-mediated protection in vivo. Vaccine. 2011;29(38):6584–6591. doi: 10.1016/j.vaccine.2011.06.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sen A. Development and evaluation of a thermostable Peste des petits ruminants (PPR) vaccine using heavy water (D2O). Ph.D. Thesis. Division of Virology, Indian Veterinary Research Institute (IVRI); 2009.
- 72.Sen A, Saravanan P, Balamurugan V, Rajak KK, Sudhakar SB, Bhanuprakash V, Parida S, Singh RK. Vaccines against peste des petits ruminants virus. Expert Rev Vaccines. 2010;9(7):785–796. doi: 10.1586/erv.10.74. [DOI] [PubMed] [Google Scholar]
- 73.Shaikh FY, Crowe JE., Jr Molecular mechanisms driving respiratory syncytial virus assembly. Futur Microbiol. 2013;1:123–131. doi: 10.2217/fmb.12.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, Barrett T. Geographic distribution and epidemiology of Peste des petits ruminants viruses. Virus Res. 1996;143(2):149–153. doi: 10.1016/0168-1702(96)01312-3. [DOI] [PubMed] [Google Scholar]
- 75.Silva AC, Carrondo MJ, Alves PM. Strategies for improved stability of Peste des petits ruminants vaccine. Vaccine. 2011;29(31):4983–4991. doi: 10.1016/j.vaccine.2011.04.102. [DOI] [PubMed] [Google Scholar]
- 76.Silva AC, Yami M, Libeau G, Carrondo MJ, Alves PM. Testing a new formulation for Peste des petits ruminants vaccine in Ethiopia. Vaccine. 2014;32(24):2878–2881. doi: 10.1016/j.vaccine.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 77.Singh RP, Saravanan P, Sreenivasa BP, Singh RK, Bandyopadhyay SK. Prevalence and distribution of Peste des petits ruminants virus infection in small ruminants in India. Rev Sci Tech. 2004;23(3):807–819. doi: 10.20506/rst.23.3.1522. [DOI] [PubMed] [Google Scholar]
- 78.Singh RP, Bandyopadhyay SK. Peste des petits ruminants vaccine and vaccination in India: sharing experience with disease endemic countries. VirusDisease. 2015;26(4):215–224. doi: 10.1007/s13337-015-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spier MR, de Vandenberghe LPS, Medeiros ABP, Soccol CR. Application of different types of bioreactors in bioprocesses. In: Antolli PG, Liu Z, editors. Bioreactors: design, properties and applications. Nova Science Publishers Inc: New York; 2011. pp. 55–90. [Google Scholar]
- 80.Sreenivasa BP, Dhar P, Singh RP. Bandyopadhyay SK. Evaluation of an indigenously developed homologous live attenuated cell culture vaccine against Peste-des-petits-ruminants infection of small ruminants. In: Proceedings of XX annual conference of indian association of veterinary microbiologists, immunologists and specialists in infectious diseases (IAVMI), Pantnagar, Uttaranchal, India. 2000. p.84.
- 81.Tapia F, Vázquez-Ramírez D, Genzel Y, Reichl U. Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl Microbiol Biotechnol. 2016;100(5):2121–2132. doi: 10.1007/s00253-015-7267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson KA, Rempala GA, Yin J. Multiple-hit inhibition of infection by defective interfering particles. J Gen Virol. 2009;90(4):888–899. doi: 10.1099/vir.0.005249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson KAS, Yin J. Population dynamics of an RNA virus and its defective interfering particles in passage cultures. Virology. 2010;7:257. doi: 10.1186/1743-422X-7-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Timm C, Akpinar F, Yin J. Quantitative characterization of defective virus emergence by deep sequencing. J Virol. 2014;88(5):2623–2632. doi: 10.1128/JVI.02675-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trabelsi K, Majoul S, Rourou S, Kallel H. Process intensification for an enhanced replication of a newly adapted RM-65 sheep pox virus strain in Vero cells grown in stirred bioreactor. Biochem Eng J. 2014;90:131–139. doi: 10.1016/j.bej.2014.06.001. [DOI] [Google Scholar]
- 86.Trablesi K, Majoul S, Rourou S, Kallel H. Development of a measles vaccine production process in MRC-5 cells grown on Cytodex1 microcarriers and in a stirred bioreactor. Appl Microbiol Biotechnol. 2012;93:1031–1040. doi: 10.1007/s00253-011-3574-y. [DOI] [PubMed] [Google Scholar]
- 87.Vazquez D, Genzel Y, Pieler MM, Jordan I, Sandig V, Reichl U. Intensification of MVA and influenza virus production through high-cell-density cultivation approaches. In: Vaccine Technology VI, Laura Palomares, UNAM, Mexico Manon Cox, Protein Sciences Corporation, USA Tarit Mukhopadhyay, University College London, UK Nathalie Garçon, BIOASTER Technology Research Institute, FR Eds, ECI Symposium Series. 2016.
- 88.Vincent S, Gerlier D, Manié SN. Measles virus assembly within membrane rafts. J Virol. 2000;74(21):9911–9915. doi: 10.1128/JVI.74.21.9911-9915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wertz GW, Perepelitsa VP, Ball LA. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc Natl Acad Sci. 1998;95(7):3501–3506. doi: 10.1073/pnas.95.7.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whistler T, Bellini WJ, Rota PA. Generation of defective interfering particles by two vaccine strains of measles virus. Virology. 1996;220:480–484. doi: 10.1006/viro.1996.0335. [DOI] [PubMed] [Google Scholar]
- 91.Williams ES, Morales NM, Wasik BR, Brusic V, Whelan SP, Turner PE. Repeatable population dynamics among vesicular stomatitis virus lineages evolved under high co-infection. Front Microbiol. 2016;7(370):1–10. doi: 10.3389/fmicb.2016.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willenbrink W, Neubert WJ. Long-term replication of Sendai virus defective interfering particle nucleocapsids in stable helper cell lines. J Virol. 1994;68(12):8413–8417. doi: 10.1128/jvi.68.12.8413-8417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Worrall EE, Litamoi JK, Seck BM, Ayelet G. Xerovac: an ultra rapid method for the dehydration and preservation of live attenuated Rinderpest and Peste des Petits ruminants vaccines. Vaccine. 2000;19(7):834–839. doi: 10.1016/S0264-410X(00)00229-2. [DOI] [PubMed] [Google Scholar]
- 94.Xue H, Yang B, Kristensen DD, Chen D. A freeze-stable formulation for DTwP and DTaP vaccines. Hum Vaccines Immunother. 2014;10(12):3607–3610. doi: 10.4161/21645515.2014.980195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yaqub T, Shahid MF, Munir M, Ali M, Mukhtar N, Adid M. Comparative efficacy of stabilizers on the thermostability of Peste des petits Ruminants vaccine. J Vaccines Vaccin. 2016;7:6. doi: 10.4172/2157-7560.1000344. [DOI] [Google Scholar]
- 96.Yoon SW, Lee SY, Won SY, Park SH, Park SY, Jeong YS. Characterization of homologous defective interfering RNA during persistent infection of Vero cells with Japanese encephalitis virus. Mol Cells. 2006;21(1):112–120. [PubMed] [Google Scholar]