Abstract
Oral cancer is one of the most prevalent cancers in the world which contains many kinds of malignant neoplasms in the oral cavity. Due to the carcinogenicity of human papillomavirus (HPV) and its prevalence in cancer, including the oral cancer, this study was aimed at investigate the prevalence of HPV and its genotypes in patients suffering from oral tumors using PCR method. In this study, 83 samples of oral lesions were collected in the form of paraffin-embedded tissue. After extracting the DNA using DNA extraction kits, high-risk HPV positive samples were examined using special kits for genotyping, and low-risk types were sequenced after nested PCR. The results showed that 13.2% of samples was HPV positive. The result of PCR using genotyping kit indicated that high-risk types of 18, 31, 16, and 33 appeared in samples with prevalence rate of 27.2, 18.1, 9.09 and 9.09%, respectively. In this manner, the result of sequence indicated that the prevalence of HPV-6 genotype was 36.3% in the samples. The results of this study indicated that both low-risk and high-risk types of HPV are associated with the risk of oral tumors, so that Types 6 and 18 were reported as the most prevalent types in the samples.
Keywords: Human papillomavirus (HPV), Oral tumor, PCR, Genotype
Introduction
Oral cancer is known as the sixth most common cancer in the world, so that about 95% of cases are squamous cell carcinoma (SCC) [17, 24]. Epidemiological and molecular analyses indicated that human papillomavirus (HPV) is involved, as a factor, in oral cancer [15].
HPV is a tiny virus with a diameter of 45–55 nm belonging to papillomaviridae family. The genome of this virus contains a double-stranded DNA molecule with 8 kb size which is located in an uncoated protein capsid with cellular histones [3, 15, 17, 27]. Up till now, more than 200 different types of HPV have been isolated which are classified into high-risk and low-risk groups [15, 19, 22]. Low-risk types of HPV, such as HPV-6 and HPV-11, produce benign warts, and high-risk types, such as HPV-16 and HPV-18, can cause neoplasm precancer in squamous epithelial, that can progress to cancer [22, 23, 25]. About 90% of all HPVs associated with cancer are caused by HPV-16 [10, 11, 14] and can be connected with different kinds of cancers such as breast cancer, cervical cancer, laryngeal cancer, oral cancer and anal cancer [16, 18, 21, 26, 30].
These HPV viruses can enter the body through contacting small wounds of skin and cause hyperproliferation of skin and mucosa epithelial cells [32]. These viruses are one of the etiologic agents in the development of oral cavity cancer by contaminating basal cells of squamous epithelium and oral mucosa, and contribute 25.6% to the development of oral-pharyngeal carcinoma [4, 8, 15]. In the patients suffering from advanced stages of cancers, invasion to surrounding tissues, with lymph nodes and distant metastasis, has been identified, and there is a risk of second malignancy during the patient’s life time [13, 24]. Some of the risk factors for oral cavity cancer include: sexual intercourse behaviors, smoking, chewing areca and drinking alcohol [1, 12, 22, 31, 34].
The HPV prevalence rate varies in different areas from 0 to 91% [6, 29]. Furthermore, the development of oral carcinoma is increasing nowadays, so the aim of this study is to investigate the prevalence rate of HPV, and to appoint its genotypes in patients with oral tumors using PCR method.
Materials and methods
Sampling
In this study, 83 samples of paraffin-embedded tissues, were collected from patients from the beginning of 2006 to the end of 2016 at Bouali Sina hospital in Sari, Iran. In this study, the lesions of SCC, Squamous papilloma, Neoplasia, Dysplasia, Hyperplasia, Leukoplakia, Wart and Keratosis were investigated in the area of mouth anal lips. The patients’ clinical characteristics, such as age, sex, marital status and their occupations, were recorded in their dossier.
DNA extraction and electrophoresis
To extract DNA from the paraffin-embedded tissues; at first, 10 micron slices were prepared from the blocks, then they were transferred to microtubes, and to remove paraffin from the tissues, 1 ml of xylene was added to each sample. After removing paraffin, the samples were washed with 1 ml of ethanol 100%. They were placed in Thermo-mixer at 37 °C for 10–20 min to be dried. Then, Qiagen kit (made in Germany) was used to extract DNA from the tissues and the amount of OD of the extracted DNA was determined with spectrophotometry. Two pairs of primers (from Takapouzist Company) were used for PCR and the amplification of piece of the gene. A pair of PCO4.B.globin and GH2O.B.globin primers were used to ensure the presence of DNA that generated a PCR product with the length of 268 bp. The MY11 and MY09 primer pairs were also used to replicate a part of L1 piece of HPV genome. The replicated pieces were used to detect papillomavirus, which produced a PCR product with 454 bp length. After performing PCR, its product was investigated on the 1.5% agarose gel.
Extraction of gel and sequencing
To determine the type of low-risk HPVs, samples were sequenced. Nested PCR was used For HPV positive samples. In this method, MY-11 and MY-09 primers were used in the first PCR, and MY-09 and GP-5 primers (to increase the sensitivity of PCR) were used in the second PCR, which produced a 407 bp length band. The sequence of primers are given in Table 1. Then, the nested PCR product was examined on 1.5% gel. After cutting the gel, it was extracted by YTA kit (Yekta Tajhiz Azma, Iran), and was sent to Bioneer Company in Korea for sequencing.
Table 1.
Features of primers used
| Size | Sequences(5′-3′) | Primers | |
|---|---|---|---|
| 268 bp | 5′-CAACTTCATCCACGTTCACC-3′ | PCO4 (F) | β globin |
| 5′-GAAGAGCCAAGGACAGGTAC-3′ | GH2O (R) | ||
| 454 bp | 5′-GCMCAGGGWCATAAYAATGG-3′ | MY11 (F) | HPV |
| 5′-CGTCCMARRGGAWACTGATC-3′ | MY09 (R) | ||
| 407 bp | 5′-CGTCCMARRGGAWACTGATC-3′ | MY09 (F) | HPV |
| 5′-TTTGTTACTGTGGTAGATAC-3′ | GP5 (R) | ||
Genotyping and electrophoresis
The HPV positive samples were investigated by amplisens genotyping kit (made in Russia) which could identify high risk types (16,18,31,33,35,39,45,52,56,58,59,66). The kit contents which contained PCR-mix, PCR-buffer and Polymerase, were combined with HPV-DNA in a volume of 25 μl mixture and were prepared for PCR. The PCR was performed in a suitable condition and temperature program; initial denaturation at 95 °C for 15 min, followed by 42 cycles of denaturation at 95 °C for 30 s, annealing at 63 °C for 40 s, extension at 72 °C for 50 s with a final extension at 72 °C for 1 min. All these processes were performed with positive and negative controls existed in the kit. After all, the results were examined on 2% gel.
Result and discussion
Oral HPV detection
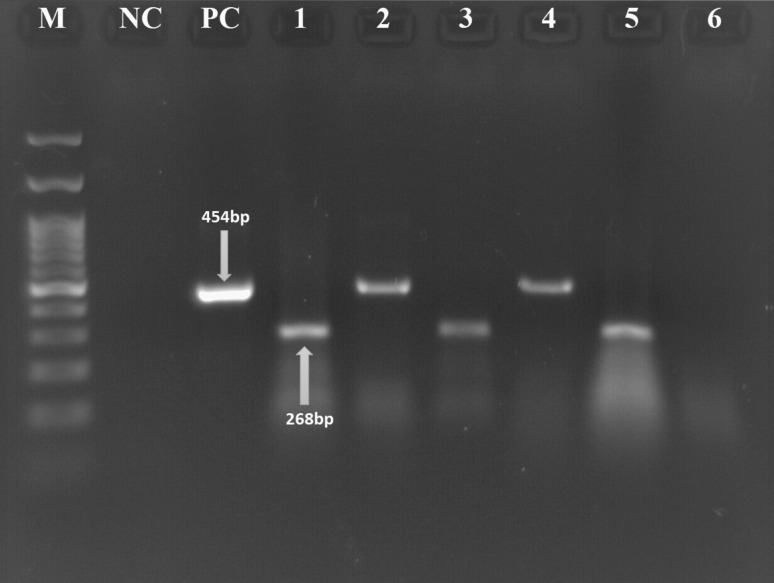
After extraction of the DNA and performing PCR for 83 samples, 40(48.1% female) and 43(51% men) it was indicated that all of the samples (100%) were positive for beta-globin gene, and 11 samples (13.2%) had HPV gene (Fig. 1), among these patients it is found that 7 (5.81%) and 4(3.32%) of them were married and single positive HPV respectively.of which five samples were SCC (45.4%), three samples were hyperplasia (27.2%), one sample was squamous papilloma (9.09%), one sample was neoplasia (9.09%) and one sample was both neoplasia and dysplasia (9.09%).
Fig. 1.
M: DNA marker (100 bp), NC negative control, PC positive control, 1 and 3: β.globin gene for positive samples, 5: β.globin gene for negative sample, 2 and 4: HPV positive samples, 6: HPV negative sample
Six of these samples were detected on the tongue (54.5%), three of them on the lips (27.2%), and the other two samples were observed on the other parts of oral cavity (18.1%). Six samples (54.5%) of all positive HPV-DNA were observed in females, and five of them (45.5%) were observed in males. The age of these people ranged between 8 and 83 years old with the average age of 46.2. The characteristics of patients are presented in the Tables 2, 3.
Table 2.
Distribution of HPV according to age group of patients
| HPV negative (72) | HPV positive (11) | Total (83) | ||||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Age | ||||||
| 6.02 | 5 | 4.1 | 3 | 18.1 | 2 | < 15 |
| 8.4 | 7 | 8.3 | 6 | 9.09 | 1 | 16–31 |
| 20.4 | 17 | 18.05 | 13 | 36.3 | 4 | 32–47 |
| 34.9 | 29 | 40.2 | 29 | 0 | 0 | 48–63 |
| 19.2 | 16 | 18.05 | 13 | 27.2 | 3 | 64–79 |
| 10.8 | 9 | 11.1 | 8 | 9.09 | 1 | 80 < |
Table 3.
Distribution of HPV according to occupation group of patients
| HPV negative (72) | HPV positive (11) | Total (83) | ||||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Occupation | ||||||
| Housewife | 3 | 27.2 | 20 | 27.7 | 23 | 27.7 |
| Free | 4 | 36.3 | 27 | 37.5 | 31 | 37.3 |
| Employee | 2 | 18.1 | 17 | 23.6 | 19 | 22.8 |
| Student | 2 | 18.1 | 8 | 11.1 | 10 | 12.04 |
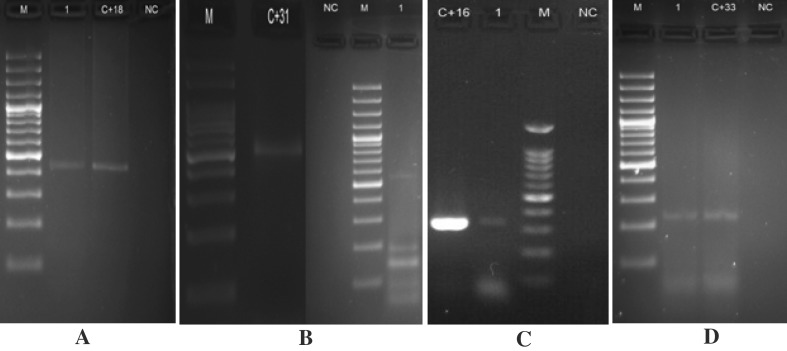
The results of PCR with genotyping kit (Fig. 2), and the results of sequence (Fig. 3) are shown in Table 4.
Fig. 2.
The results of PCR with genotyping kit. a M: DNA marker (100 bp), 1: HPV-DNA positive (type 18 (425 bp)), C + 18: positive control of HPV-DNA typing 18 (425 bp), NC negative control. b M: DNA marker (100 bp), C + 31: Positive control of HPV-DNA Type 31 (520 bp), NC negative control, 1: HPV-DNA Positive Sample (Type 31 (520 bp)) c C + 16: Positive control of HPV-DNA typing 16 (325 bp), 1: HPV-DNA positive (Typing 16 (325 bp)), M: DNA marker (100 bp), NC negative control d M: DNA marker (100 bp), 1: HPV-DNA Positive sample (Type 33 (227 bp)), C + 33: Positive control of HPV-DNA Type 33 (227 bp), NC negative control
Fig. 3.
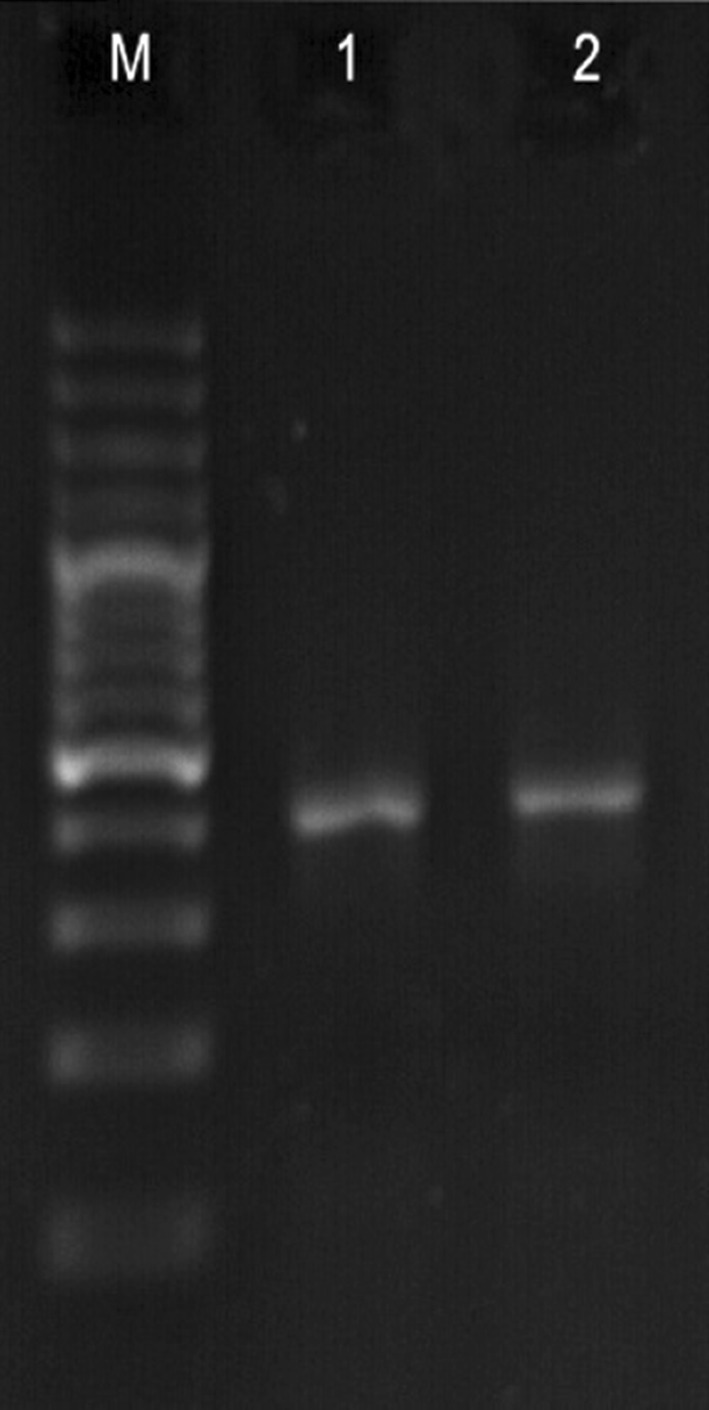
M: DNA marker (100 bp), 1 and 2: nested PCR for HPV-DNA positive (407 bp)
Table 4.
Characteristics of HPV types
| Lesion location | Type of lesion | Percentage | Number | Type the virus |
|---|---|---|---|---|
| Tongue, lip, oral cavity | Hyperplasia, neoplasia | 36.3 | 4 | HPV-6 |
| Tongue, lip | SCC, Squamous papilloma | 27.2 | 3 | HPV-18 |
| Tongue | SCC, Neoplasia, dysplasia | 18.1 | 2 | HPV-31 |
| Tongue | SCC | 9.09 | 1 | HPV-16 |
| Oral cavity | SCC | 9.09 | 1 | HPV-33 |
HPV prevalence and HPV types
The results of the investigation proved the relation between human papillomavirus (HPV) and squamous cell carcinoma (SCC), so that, the low-risk type (HPV6) had the highest number, and the high-risk types (HPV16 and HPV33) had the lowest numbers.
The prevalence rate of oral diseases varies in different areas. According to a research conducted in 2013 with PCR-MA method in Michigan, the HPV prevalence rate in oral cavity (108 samples) was 9.6%, and the amounts of HPV in oropharynx and nasopharynx were 83% and 44% respectively, so that, the 16 type was reported as the dominant type. The HPV prevalence rate in oral cavity is partly similar to the current study, but the HPV prevalence rate in oropharynx and nasopharynx is more than the current study [33]. According to a study on paraffin-embedded tissue of SCC on the tongue of 50 people from Iran in 2017, the HPV prevalence rate was reported 14%, and none of the 16 and 18 types were reported. The HPV prevalence rate was similar to the current study [2].
According to another study that was performed in 2017 in Iran on 50 oral samples, the HPV prevalence rate was reported 36% that was more than the current study. And the prevalence rate of Types 18 and 11 were respectively reported 55.56 and 44.44% [28]. According to an experiment in the United States in 2014 on laryngeal cancer tissue (148 samples), it was indicated that 20.9% of samples were HPV positive, that was more than the current study. HPV16 (6.1%) and HPV33 (6.1%) were the dominant types in that experiment [20]. Another study was performed on sinus tissue (161 samples) in 2013 in the United States, using in situ hybridization method, HPV-DNA was detected 21% and types 16 (17%), 31/33 (2%), 18 (1%) were also detected [5]. In the experiment carried out in 2016 on formalin-fixed, paraffin-embedded (FFPE) on the head and neck in 29 countries of Europe, United States, Africa and Asia, the HPV-DNA prevalence rate in oral cavity was 7.4% (93 samples of 1264), that was less than the current study, and HPV16 (68.8%) was known as the most dominant type. Moreover, HPV-DNA was detected in oropharynx (24.9%), pharynx (21.4%), nose (7.9%), larynx (5.7%) and hypopharynx (3.9%), and the dominant genotype was HPV16 with the ratio of 75.2% [7]. In 2015 in the United States, the prevalence of oral HPV infection was reported 6.8% using PCR method. Although the 16 type was reported as the dominant type, it was still less than the current study. Most of the oncogenic HPV infections are related to sexual behaviors of men and women respectively 95.9 and 87.5%. The amounts of infection in men and women without sexual behavior were reported 0.3 and 0.2% respectively [9].
Risk factors for HPV infection
The difference in the rate of HPV prevalence in different studies can be due to the sensitivity of laboratory techniques and the type of diagnostic method. In addition, the prevalence rate of this virus is varies in different parts of the head and neck. It seems that the type of the selected sample is also an important agent for the inconsistency between the results. The difference between the results of the current study and the findings of other studies may be due to smoking and various sexual behaviors. The HPV prevalence rate varies in different countries, that may be due to geographical differences or the differences in environmental factors. The prevalence rate in Iran is less than most other countries, may be due to the greater awareness of the people about their sexual health, observance of the principles of prevention and well-timed referral to health centers, and also the existence of precise prevention programs and disease controls. Unlike most studies, in the current study, the prevalence rate of low-risk types are more than high-risk types, which can be due to the regional differences.
In conclusion, the presence of HPV in the samples suggest that there is correlation between HPV and oral tumors. This study show a high prevalence of HPV6 in oral lesions. The presence of high risk HPV16,33 detected at very low, that it might be related to geographic areas and sexual behaviors. however further examination is necessary to declare of clinical implication of these types of HPV.
Acknowledgements
Hereby, the authors would like to thank Mazandaran University of Medical Sciences for financial support and provision of required facilities to carry out the current project.
References
- 1.Antonsson A, Cornford M, Perry S, Davis M, Dunne MP, Whiteman DC. Prevalence and risk factors for oral HPV infection in young Australians. PLoS ONE. 2014;9(3):e91761. doi: 10.1371/journal.pone.0091761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashraf MJ, Hosseini S, Monabati A, Valibeigi B, Khademi B, Abedi E, et al. The prevalence of human papilloma virus in squamous cell carcinoma of oral tongue. Iran J Pathol. 2017;12(2):144–149. [PMC free article] [PubMed] [Google Scholar]
- 3.Ashrafi GH, Haghshenas MR, Marchetti B, O’Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113(2):276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafi GH, Haghshenas M, Marchetti B, Campo MS. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int J Cancer. 2006;119(9):2105–2112. doi: 10.1002/ijc.22089. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouda M, Gorgoulis VG, Kastrinakis NG, Giannoudis A, Tsoli E, Danassi-Afentaki D, et al. “ High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol. 2000;13(6):644. doi: 10.1038/modpathol.3880113. [DOI] [PubMed] [Google Scholar]
- 7.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. JNCI J Natl Cancer Inst. 2016;108(6):djv403. [DOI] [PubMed]
- 8.Chai RC, Lambie D, Verma M, Punyadeera C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015;4(4):596–607. doi: 10.1002/cam4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Graubard BI, Broutian T, Pickard RK, Tong Z-y, Xiao W, et al. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in US men. Cancer research. 2015;canres. 2843.014 [DOI] [PMC free article] [PubMed]
- 10.Cook RL, Thompson EL, Kelso NE, Friary J, Hosford J, Barkley P, et al. Sexual behaviors and other risk factors for oral human papillomavirus infections in young women. Sex Transm Dis. 2014;41(8):486–492. doi: 10.1097/OLQ.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza G, Gross ND, Pai SI, Haddad R, Anderson KS, Rajan S, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32(23):2408–2415. doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmaelbeigi F, Harirchi I, Omranipour RV, Rajabpour M. Factors affecting professional delay in diagnosis and treatment of oral cancer in Iran. Arch Iran Med. 2014;17(4):253. [PubMed] [Google Scholar]
- 13.Feller L, Lemmer J. Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Ther. 2012;3(04):263. doi: 10.4236/jct.2012.34037. [DOI] [Google Scholar]
- 14.Gillison ML, Broutian T, Pickard RK, Z-y Tong, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Losa MdR, Barrera ES, Herrera-Pech V, Conde-Ferráez L, Puerto-Solís M, Ayora-Talavera G. Epidemiology of oral HPV in the oral mucosa in women without signs of oral disease from Yucatan, Mexico. Braz J Microbiol. 2015;46(1):301–306. doi: 10.1590/S1517-838246120130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guimarães SJA, Vidal FCB, Soares JMC, Nascimento MDDSB, Villa LL, Brito LMO. Prevalence of human papillomavirus infection in squamous cell carcinoma of the anal canal in a Northeast City in Brazil: viral genotyping and clinical aspects. Appl Can Res. 2017;37(1):19. doi: 10.1186/s41241-017-0024-x. [DOI] [Google Scholar]
- 17.Gupta S, Gupta S. Role of human papillomavirus in oral squamous cell carcinoma and oral potentially malignant disorders: a review of the literature. Indian J Dent. 2015;6(2):91. doi: 10.4103/0975-962X.155877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haghshenas M, Golini-Moghaddam T, Rafiei A, Emadeian O, Shykhpour A, Ashrafi GH. Prevalence and type distribution of high-risk human papillomavirus in patients with cervical cancer: a population-based study. Infect Agents Cancer. 2013;8(1):20. doi: 10.1186/1750-9378-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haghshenas MR, Mousavi T, Kheradmand M, Afshari M, Moosazadeh M (2017) Efficacy of human papillomavirus l1 protein vaccines (cervarix and gardasil) in reducing the risk of cervical intraepithelial neoplasia: a meta-analysis. Int J Prev Med 8. [DOI] [PMC free article] [PubMed]
- 20.Hernandez BY, Goodman MT, Lynch CF, Cozen W, Unger ER, Steinau M, et al. Human papillomavirus prevalence in invasive laryngeal cancer in the United States. PLoS ONE. 2014;9(12):e115931. doi: 10.1371/journal.pone.0115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini SZ, Makvandi M, Samarbafzade A, Timori A, Ranjbar N, Saki N, et al. Frequency of human papillomavirus (HPV) 16 and 18 detection in paraffin-embedded laryngeal carcinoma tissue. Asian Pac J Cancer Prev APJCP. 2017;18(4):889. doi: 10.22034/APJCP.2017.18.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hübbers CU, Akgül B. HPV and cancer of the oral cavity. Virulence. 2015;6(3):244–248. doi: 10.1080/21505594.2014.999570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminagakura E, Villa LL, Andreoli MA, Sobrinho JS, Vartanian JG, Soares FA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer. 2012;130(8):1726–1732. doi: 10.1002/ijc.26185. [DOI] [PubMed] [Google Scholar]
- 24.Noguti J, De Moura CFG, De Jesus GPP, Da Silva VHP, Hossaka TA, Oshima CTF, et al. Metastasis from oral cancer: an overview. Cancer Genomics Proteomics. 2012;9(5):329–335. [PubMed] [Google Scholar]
- 25.Nosrati A, Naghshvar F, Torabizadeh Z, Haghshenas M, Sangsefidi H. Relationship between human papilloma virus and colorectal cancer in Northern Iran. Middle East J Cancer. 2015;6(4):237–241. [Google Scholar]
- 26.Poelman MR, Brand HS, Forouzanfar T, Daley EM, Jager DHJ. Prevention of HPV-related oral cancer by dentists: assessing the opinion of dutch dental students. J Cancer Educ 2017:1–8. [DOI] [PMC free article] [PubMed]
- 27.Ramqvist T, Grün N, Dalianis T. Human papillomavirus and tonsillar and base of tongue cancer. Viruses. 2015;7(3):1332–1343. doi: 10.3390/v7031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikoo HR, Ardebili A, Ravanshad M, Rezaei F, Teimoori A, Khanizadeh S, et al. E6-specific detection and typing of human papillomaviruses in oral cavity specimens from Iranian patients. Iran Biomed J. 2017;21(6):411. doi: 10.18869/acadpub.ibj.21.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivero ERC, Nunes FD. HPV in oral squamous cell carcinomas of a Brazilian population: amplification by PCR. Braz Oral Res. 2006;20(1):21–24. doi: 10.1590/S1806-83242006000100005. [DOI] [PubMed] [Google Scholar]
- 30.Salman NA, Davies G, Majidy F, Shakir F, Akinrinade H, Perumal D, et al. Association of high risk human papillomavirus and breast cancer: a UK based study. Sci Rep. 2017;7:43591. doi: 10.1038/srep43591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sand L, Wallström M, Hirsch J-M. Smokeless tobacco, viruses and oral cancer. Oral Health Dent Manag. 2014;13(2):372–378. [PubMed] [Google Scholar]
- 32.Syverton JT. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952;54(1):1126–1140. doi: 10.1111/j.1749-6632.1952.tb39983.x. [DOI] [PubMed] [Google Scholar]
- 33.Walline HM, Komarck C, McHugh JB, Byrd SA, Spector ME, Hauff SJ, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: comparison of multiple methods. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1320–1327. doi: 10.1001/jamaoto.2013.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandberg DP, Liu S, Goloubeva OG, Schumaker LM, Cullen KJ. Emergence of HPV16 positive oropharyngeal cancer in black patients over time: University of Maryland 1992–2007. Cancer Prev Res 2014;canprevres. 0089.2013. [DOI] [PMC free article] [PubMed]