Abstract
In the present study, we carried-out assessment of efficacy of different immunization strategies using two bivalent vaccine formulations containing antigens of inactivated Newcastle disease virus (NDV-genotype VIId) and reassortant highly pathogenic avian influenza virus (H5N1-HPAIV) mixed with Montanide ISA71 and Montanide Gel02 as adjuvants. The efficacy of the prepared vaccines was evaluated by determining the cellular and humoral immune responses. In addition, protection against H5N1-AIV and NDV-genotype VIId challenge viruses post vaccination was assessed when Montanide-Gel02 based vaccine was inoculated in 10-days-old specific pathogen free chicks intraocularly once, twice or once followed by a boost with the Montanide ISA71 based vaccine. The cytokines profile analysis demonstrated that the prime-boost strategy induced the highest up-regulation in interferon-gamma (11.39-fold change) and interleukin-6 (14.12-fold change) genes expression. Also, enhanced lymphocytes proliferation was recorded beside increased antibody titers with protection levels reaching 50 and 60% against H5N1 and NDV challenge; respectively. Immunization with Montanide ISA71 inactivated vaccine induced 80% protection; however, the prime-boost combination afforded complete protection (100%) in the challenged chickens against mortality, clinical signs and virus shedding. Finally, these results highlight the significance of considering not only different vaccine platforms but also vaccination strategies to maximize protection against AIV and NDV with regards to the longevity of the vaccine-induced immune response.
Keywords: Prime-boost, H5N1, Newcastle disease virus, Genotype VIId, Montanide
Introduction
Avian influenza virus (AIV) is a member or Orthomyxoviridae family; genus Influenza virus A. There are 18 known HA (H1-H18) subtypes and 11 known NA (N1–N11) subtypes [1]. Genetic traits and/or severity of the disease in poultry determine if the infection is classified as low pathogenic avian influenza (LPAI) or high pathogenic avian influenza (HPAI) [24]. Infections with HPAI virus are identified as a serious threat to the poultry industry and can cause devastating economic losses due to mortality and morbidity rates that reach up to 100% [15]. Biosecurity practices, education about prevention, preventive culling, early diagnosis, and surveillance to detect the disease and infection are considered the main procedures to combat and prevent further infections and outbreaks [16]. However, this approach is not applicable in developing countries for several reasons [32]. Implementation of extensive vaccination programs in commercial poultry farms is used to help control AI virus and limit losses in areas where the virus is endemic [24]. Inactivated, oil adjuvanted, whole virus vaccines represent the majority of the vaccines available for AIV. Nonetheless, poor quality vaccines and inappropriate application have led to vaccine failures in the field [3]. The widespread and prolonged use of inactivated AI vaccines promotes the emergence of antigenic variants against which the vaccines are ineffective [19]. Attenuated live influenza vaccines in poultry are not recommended due to the potential risk of assortment or mutations [18]. In Egypt, despite regular vaccination of the suspected poultry species, HPAI H5N1 viruses have been endemic in poultry since 2008, following the first wave of the virus outbreak in 2006 [12].
Newcastle disease virus (NDV) belongs to avian paramyxovirus type 1, which is a member of the genus Avulavirus of the Paramyxoviridae family [6, 29]. NDV is as equally important as Avian Influenza, owing to its highly contagious nature and worldwide distribution; it is an economic burden [9, 18]. Based on the severity of the disease symptoms, NDV strains have been classified as lentogenic (avirulent), mesogenic (intermediately virulent) and velogenic (virulent) [29]. Velogenic strains of the virus are responsible for the sudden deaths of the fully susceptible chickens without major clinical signs. Commonly, NDV infection characterizes by respiratory, enteric and/or neurological manifestations with high mortality rates that reaches 100% [8, 39]. Moreover, vaccinated chickens may remain infected while showing no signs [5]. NDV isolates are genetically classified into two broad classes (I and II) with different genotypes. Class I, comprises 9 genotypes that are avirulent and mainly affect wild birds, while Class II, which is currently divided into 18 genotypes with multiple sub-genotypes, including both virulent and avirulent isolates and can be isolated from wild and domestic birds [6]. In Egypt, the sub-genotype VIId is predominant since 2011 causing several ND outbreaks in poultry [11].
Control of Newcastle disease by vaccination is a common strategy in most countries where poultry are raised commercially and where the disease is endemic to keep the disease under control [40]. However, several outbreaks were reported worldwide and in Egypt [4, 11]; despite the fact that vaccination is widely applied and this indicates that the current vaccines and vaccination campaigns are not effective in preventing infection and transmission [27].
After experimental challenge, the ideal vaccine against AIV and/or NDV should prevent and reduce the rates of mortality and morbidity, challenge virus shedding from respiratory and digestive tracts, as well as prevent transmission of the virus between chickens in a flock and, subsequently, the transmission of the virus between flocks [36]. Therefore, in this study, the clinical protection against H5N1 and NDV-VIId strains conferred by different vaccination protocols using an intraocular vaccine containing inactivated antigens of AIV-NDV candidate inoculated in specific pathogen free (SPF) chickens with or without inactivated oil based ISA71 bivalent vaccine boost have been evaluated. Innate, cellular and specific humoral immune responses elicited by these different vaccination regimens were investigated and correlated to the induced AIV and NDV protection levels.
Materials and methods
Chickens
One-day-old SPF chicks were purchased from the specific pathogen-free egg project, Kom Oshim, El Fayoum Governorate (n = 125), were used in the challenge trial. All animal experiments were carried out according to the recommendations and guidelines of the “European Communities Council Directive 1986 (86/609/EEC)” and conducted in isolators of the Veterinary Serum and Vaccine Research Institute. The use of animals and protocols were approved by the Animal Care and Use Committee of Veterinary Serum and Vaccine Research Institute, Egypt.
Viruses
Vaccine seed viruses
A reassortant Avian Influenza Virus Strain A/Chicken/Egypt/Q1995D/2010 (H5N1) was provided to VSVRI, Newcastle disease unit, Abbasia, Cairo by National Research Center and used for vaccine preparation. The velogenic NDV (NDV-B7-RLQP-CH-EG-12), was kindly provided by the Reference Laboratory for Quality Control on Poultry Production, Animal Health Research Institute-Egypt (RLQP-AHRI). For propagation; the viruses were inoculated into the allantoic sac of 10-day-old SPF embryonated chicken eggs; titration of the propagated viruses revealed 109/0.1 ml EID50 and 1010/0.1 ml EID50 for AI and NDV; respectively. Both infected fluids were tested for extraneous contaminants then inactivated with binary ethylamine (BEI) [21]. AIV was inactivated after treatment with BEI (0.01 M) for 24 h at 25 °C. while NDV was inactivated after treatment with BEI (0.001 M) for 18 h at 25 °C.
Challenge viruses
The local Egyptian isolate; HPAI-H5N1 (A/chicken/Egypt/VSVRI/2009(H5N1)); (kindly provided by veterinary serum and vaccine research institute (VSVRI)); this is the official strain that is used for imported and locally prepared vaccines evaluation in the central laboratory for evaluation of veterinary biologics and the velogenic NDV (NDV-B7-RLQP-CH-EG-12) were used as challenge viruses.
Adjuvants and vaccine formulation
Two adjuvants provided by (SEPPIC, Puteaux, France) were used for vaccine formulation; Gel 02, an innovative, ready-to-disperse polymeric adjuvant designed to improve the safety and efficacy of aqueous vaccines and Montanide ISA71 Ready-to-use oily vaccine adjuvant for water-in-oil (W/O) emulsion based on specific enriched light mineral oil. A bivalent vaccine was prepared by emulsifying inactivated reassortant H5N1 (107.5 EID50/dose) and inactivated NDV-VIId (106.0 EID50/dose) strains with Montanide Gel02 at a ratio of 20:80 (v/v) and with Montanide ISA71 at a ratio of 30:70 (v/v).
Vaccination scheme and challenge trial
A total of 125 chicks were divided into 5 groups; (25/each), Group (1), chicks were intraocularly vaccinated with 0.2 ml of the mucosal Gel02 AIV/NDV vaccine (1× mucosal) at 10 days of age. Group (2), chicks were primed and boosted intraocularly with the same dose of the mucosal Gel02 AIV/NDV vaccine at 10 and 20 days of age (2× mucosal vaccine). Group (3), chicks were primed intraocularly with 0.2 ml of the mucosal Gel02 AIV/NDV vaccine at 10 days of age and boosted subcutaneously (s/c) with 0.5 ml of the inactivated ISA71 oil-based AIV/NDV vaccine at 20 days (mucosal-parenteral). Group (4), chicks were vaccinated subcutaneously (s/c) with 0.5 ml of the inactivated ISA71 oil-based AIV/NDV vaccine. Group (5), chicks were kept unvaccinated as controls.
Twenty chickens from each group were challenged 21 days’ post last vaccination (DPV), by oculonasal route with a (106.0 egg infectious dose: EID50) of each of the challenge H5N1 and NDV viruses. On 3rd, 7th, 10th, and 14th days post challenge (DPC), oropharyngeal swabs were collected to quantify the virus shedding by quantitative reverse transcriptase PCR (qRT-PCR). Survival was monitored for 14 DPC to determine the percentage of protection. Serum samples were collected on a weekly basis until 12 weeks’ post-vaccination (WPV) from the non-challenged birds (n = 5) in each group for detection of serum antibodies by hemagglutination inhibition (HI) test. Heparinized blood samples were drawn from chickens of each of the five groups at the first and second WPV for quantification of cytokines IFN-γ and IL-6, mRNA expression by qRT-PCR, and measurement of lymphocyte proliferation.
Hemagglutination inhibition test
Two-folds serial dilution of the serum samples were tested weekly by a hemagglutination inhibition (HI) test according to standard procedures described in the OIE (2015) [31]. Inactivated antigens from A/Chicken/Egypt/Q1995D/2010 (H5N1) and NDV (NDV-B7-RLQP-CH-EG-12) were used.
qRT-PCR assay
RNA extraction
Cytokines (IFN-γ and IL-6) total RNA was isolated from peripheral blood mononuclear cells (PBMCs) using an RNeasy mini-RNA Purification Kit (QIAGEN-RNA-catalogue No. 74104). Viral RNA was extracted from the oropharyngeal swabs using QIAamp Viral RNA Mini Kit (QIAGEN-catalog No. 52904). Extraction was done following the manufacturer instruction.
qRT-PCR
qRT-PCR was performed using Quantitect probe RT-PCR Catalog No. 204443 (Qiagen, Inc. Valencia, CA, USA) according to the manufacturer recommendations. Primers and probes were selected for amplification of IL-6 and IFN-γ [35]; AIV-H5 gene [25] and NDV-M gene [42]. qRT-PCR runs were performed using StratageneMX3000 thermocycler. PCR cycling profile for cytokines detection consisted of RT step at 50 °C for 30 min and 94 °C for 10 min, followed by the PCR step 40 cycles of amplification at 94 °C for 10 s and 60 °C for 1 min. For detection of NDV (M) and AIV (H5), qRT-PCR amplification cycles were 30 min at 50 °C and 95 °C for 10 min, followed by 40 cycles of 94 °C for 15 s and 60 °C for 10 s.
Lymphocyte proliferation assay
Lymphocyte proliferation assay was carried out using Cell Proliferation Kit II (XTT) provided by Sigma-Aldrich (catalog No. TOX2). The test was conducted according to the manufacturer’s instructions.
Statistical analysis
Data were presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to assess the significance of the mean difference between groups after testing normality using Shapiro–Wilk test. Least significant difference (LSD) post hoc test was performed when ANOVA showed significant differences. The significance level was set at p value ≤ 0.05 significant. Data analysis was performed using MS Excel and CoStat version 6.400. Graphs were created using GraphPad Prism 7.0 software.
Results
HI titers of the collected sera samples
The average serological titers of chickens in the unvaccinated and Gel02 AIV/NDV (group 1) vaccinated groups were below the threshold of positivity (≤ 3 log2), while serum NDV-specific and AIV-specific HI antibody titer reached 4.6 log2 against NDV and 4.2 log2 against H5N1 at 3 WPV after a second vaccination of the Gel02 AIV/NDV (group 2). Vaccination with inactivated ISA71 oil-based AI/ND vaccine (group 4) showed protective antibody titers (5.7 log2 against NDV; 5 log2 against AIV), at 2 WPV. However, the prime-boost immunized chickens (group 3), elicited the highest significant level of HI titer that reached 8.7 log2 against NDV and 6.8 log2 against H5N1 at 2 WPV (Figs. 1, 2). Statistical analysis of the HI results showed that the increased levels of antibody for group (3) samples are significant (p value ≤ 0.05) compared to other vaccinated groups.
Fig. 1.
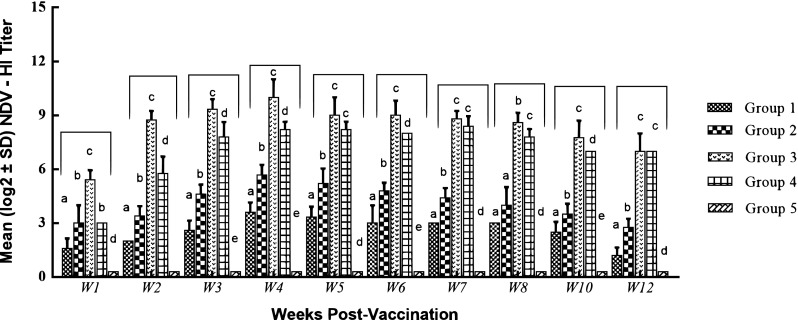
Serum NDV antibody titers post vaccination. Antibody titers were evaluated by hemagglutination inhibition (HI) assay. Plot represents the mean log2 antibody titer obtained at various weeks post vaccination. Different letters within the same week are significantly different at p value (≤ 0.05)
Fig. 2.
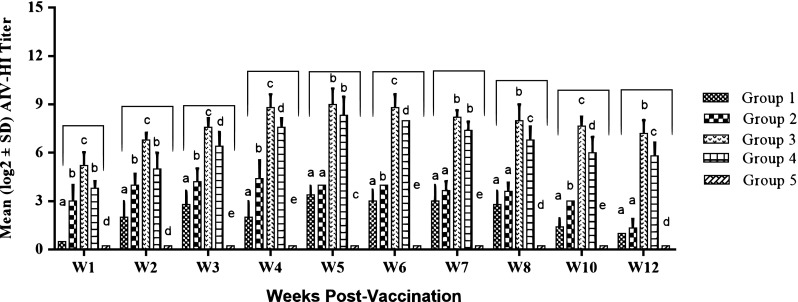
Serum AIV antibody titers post vaccination. Antibody titers were evaluated by hemagglutination inhibition (HI) assay. Plot represents the mean log2 antibody titer obtained at various weeks post vaccination. Different letters within the same week are significantly different at p value (≤ 0.05)
Cytokines’ mRNA genes expression
IFN-γ gene expression levels
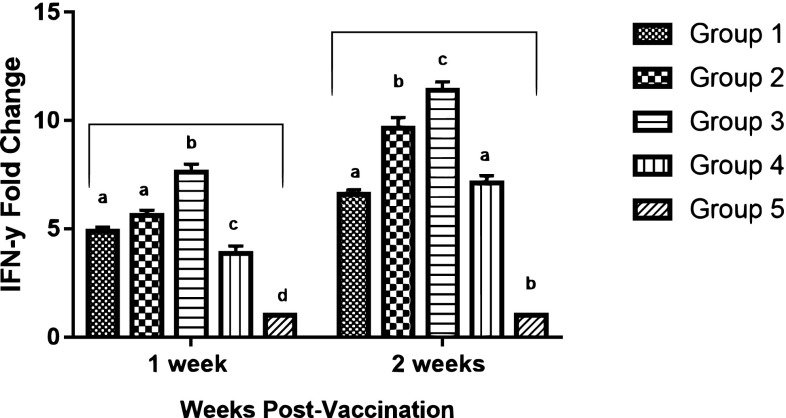
All vaccinated groups induced a significant up-regulation of IFN-γ in comparison with the unvaccinated group. However, group 3; (mucosal–parenteral) vaccinated chickens, demonstrated significantly higher (p value ≤ 0.05) levels of IFN-γ mRNA expression than the other groups, recording 7.62–11.39-fold change at 1 and 2 WPV; respectively (Fig. 3).
Fig. 3.
qRT-PCR analysis of interferon-γ (IFN-γ) mRNA expression in chickens’ peripheral blood mononuclear cells (PBMCs) at 1 and 2 weeks post vaccination. Different letters within the same week are significantly different at p value (≤ 0.05)
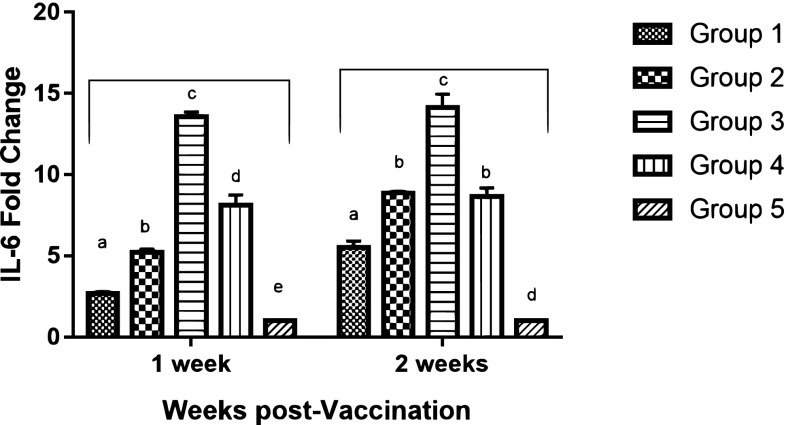
IL-6 gene expression levels
The mRNA expression levels of proinflammatory cytokine (IL-6) of all vaccinated groups demonstrated a significant increase in IL-6 expression compared to the unvaccinated group. Meanwhile, significant differences were recorded among the vaccinated groups (p value ≤ 0.05). The highest expressions levels for IL-6 was induced by group (3) chickens with, 13.55- and 14.12-fold change, 1 and 2 WPV; respectively (Fig. 4).
Fig. 4.
qRT-PCR analysis of interleukin-6 (IL-6) mRNA expression in chickens’ peripheral blood mononuclear cells (PBMCs) at 1 and 2 weeks post vaccination. Different letters within the same week are significantly different at p value (≤ 0.05)
Lymphocyte proliferation
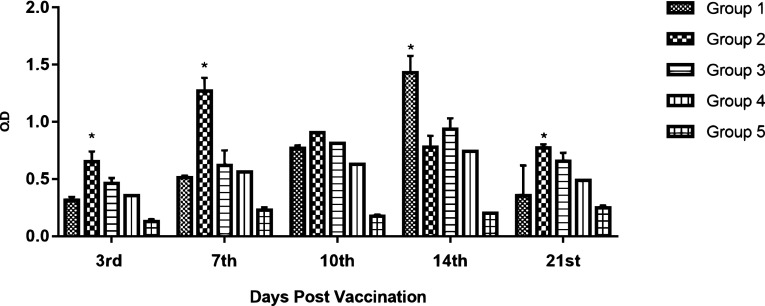
XTT-lymphocyte proliferation assay showed waves of discrepancies differed from group to group, as shown in (Fig. 5) Significant increases in lymphocyte proliferation on the 3rd dpv was detected in all vaccinated groups compared to the unvaccinated group. At 7th DPV, group (2) results were significantly higher when compared to other vaccinated groups. At 14th DPV; group (1), induced the highest significant lymphocyte proliferation.
Fig. 5.
Lymphocytes proliferation assay expressed by O.D. *Significant p value (≤ 0.05)
Protection efficacy
The challenged chicken groups with velogenic NDV genotype VIId and HPAI-H5N1 revealed that single dose of the mucosal vaccine couldn’t afford any protection against challenge viruses (group 1). Even though, the vaccine induced 60 and 50% against NDV and AIV, respectively; when the mucosal vaccine was delivered in two successive doses (group 2). Single s/c injection with the ISA71 AIV/NDV vaccine conferred only 80% protection against both challenge viruses (group 4). The protection percent reached 100% against both challenge viruses when the prepared vaccines were used as prime-boost vaccination strategy (group 3). The survival rate for each group is shown in (Fig. 6a, b).
Fig. 6.
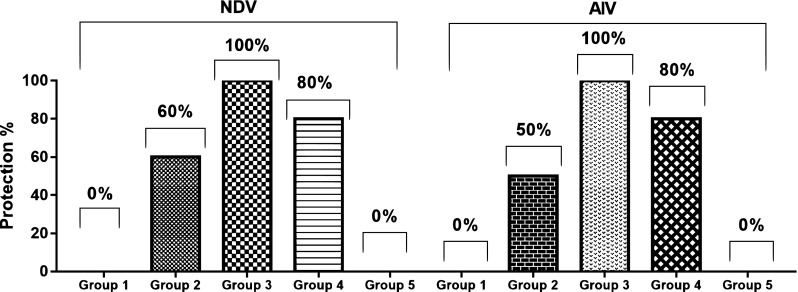
Protection percent post NDV and AIV challenge in the different vaccinated groups
Shedding of the challenge viruses
Quantification of shedding amount of the challenge viruses revealed slight reduction (1 and 2 logs) in case of single or doubles doses of the mucosal vaccine (group 1 and 2) compared to the positive control group. Only group 3, (mucosal-parenteral) showed no NDV or AIV viral shedding as shown in (Tables 1, 2).
Table 1.
Viral load in oropharyngeal swabs post challenge with NDV-titration by qRT-PCR
| Challenge groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| DPC | Conc. (EID50/0.2 ml) | ||||
| 3 | 5.107 × 104 | 4.621 × 104 | Not detectable | 5.401 × 102 | 3.269 × 105 |
| 7 | 1.811 × 105 | 7.084 × 104 | Not detectable | 3.145 × 101 | 6.053 × 105 |
| 10 | 2.161 × 105 | 9.697 × 103 | Not detectable | Not detectable | Dead |
| 14 | Dead | 7.079 × 103 | Not detectable | Not detectable | Dead |
DPC days post challenge, EID50 embryo infective dose 50%
Table 2.
Viral load in oropharyngeal swabs post challenge with AIV-titration by qRT-PCR
| Challenge groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| DPCa | Conc. (EID50/0.2 ml)b | ||||
| 3 | 3.815 × 103 | 2.262 × 103 | Not detectable | 1.478 × 101 | 5.198 × 105 |
| 7 | Dead | 5.198 × 102 | Not detectable | 4.72 | Dead |
| 10 | Dead | 3.266 × 101 | Not detectable | 4.17 | Dead |
| 14 | Dead | 3.262 × 101 | Not detectable | Not detectable | Dead |
DPC days post challenge, EID50 embryo infective dose 50%
Discussion
In the last period, the widespread use of different types of vaccines against avian influenza (AI) and Newcastle disease (ND) viruses failed to solve such major threats in the poultry industry in some countries where the viruses are endemic [8], beside that AI viruses have a major public health concern [30]. Routine vaccination strategy has reduced the disease, but repeated outbreaks of velogenic NDV and HPAIV in domestic poultry highlight the importance of maintaining research on vaccine efficacy against newly isolated strains; therefore, there is a need to develop a vaccine(s) and/or vaccination strategies that provide a broader and effective immunity and prevent transmission of these viruses [15, 27].
Newcastle disease virus has been widely used as a vaccine vector to express the H5 gene from the H5N1-HPAI virus [23, 30]. These recombinant vaccines were proved to induce robust immunity against vaccine antigens and have shown to be effective in reducing virus shedding, even though, safety concerns regarding the use of NDV live virus and the possibility of reversion to virulence are considered [20]. This could be avoided by developing vaccines with inactivated antigens [24].
In this study, we prepared two inactivated bivalent vaccines against NDV-genotype VIId and H5N1 virus using Montanide Gel02 as a mucosal adjuvant and Montanide ISA71 as a parenteral adjuvant. We evaluated humoral and cellular immune responses, protection percent and the effect on challenge viruses shedding of the prepared vaccines when the mucosal vaccine was inoculated intraocularly once, twice or once followed by a boost with the Montanide ISA71 oil-based vaccine.
Based on HI assay results, chickens in group (1), primed intraocularly with the mucosal-vaccine induced no or little ND-specific and AI-specific systemic antibodies. Similarly, other experiments reported that no AI-specific antibodies were induced after single intraocular immunization of chickens [9, 10]. Meanwhile, after the second mucosal vaccination, Hikono et al. [10] recorded a significant increase in serum antibody responses was detected 3 weeks’ post-vaccination, same observation. Higher antibody titers were induced after a single dose of ISA71 inactivated vaccine in-group 4. However, the HI results of the chickens that were vaccinated with the mucosal-parenteral vaccination regimen were remarkably higher in comparison to the other vaccinated groups. Our results are in line with Mccluskie et al. [26] who pointed-out that the application of mucosal/parenteral vaccination strategy induces strong mucosal (secretory IgA) responses as well as strong systemic (cell-mediated immunity, neutralizing antibodies) immune responses and second exposure to the antigen determines the final location of the effector lymphocytes that were generated following primary exposure, thus explaining the high antibody levels in serum of chickens in group 3 following the s/c parenteral boost in comparison to group 2 which received mucosal boost.
Assessment of the protective efficacy indicated that single-mucosal vaccination could not provide protection against any of the challenged viruses, as disease and mortality were reported in 100% of vaccinated–challenged birds. Whereas, two applications of the intraocular vaccine provided 50–60% protection against AIV and NDV challenge; respectively. The obtained results are in parallel with the results obtained by others [8]. The vaccination with inactivated-ISA71 based vaccine alone conferred 80% protection against challenge viruses. On the contrary, when the vaccines were used in the prime-boost vaccination protocol they were able to induce a level of humoral immunity that was sufficient to provide full protection (100%) against the challenge viruses with no clinical signs of HPAI nor NDV infections. The enhanced protection induced by the mucosal–parenteral vaccination strategy emphasize that there is a positive correlation between the presence of antibody titers at the day of challenge and protection from infection [28, 34].
Viral shedding from infected chickens or even vaccinated chickens that are apparently healthy, is a constant source of virus transmission [11, 28]. Thus, reduced virus shedding from vaccinated chickens post-challenge significantly reflects the efficacy of the vaccine [15]. Hikono et al. [10] found that chickens vaccinated intraocularly with adjuvanted AIV-inactivated vaccine developed strong AI-specific immune response which was sufficient to prevent shedding of the challenge virus.
Likewise, in our study, two doses of Gel02-mucosal vaccine induced reduction in ND and AI challenge viruses shedding compared to the control unvaccinated group. Whereas, mucosal-parenteral combination afforded complete stoppage of the viral shedding (Tables 1, 2). Indeed, a very recent study published by El-Naggar et al. [7] reported that an inactivated, intranasal, bivalent Montanide-based vaccine against H9N2 and NDV induced complete inhibition in H9N2 virus shedding post challenge. Another study conducted by Lee et al. [24] showed that bivalent vaccine containing inactivated Lasota strain of NDV and reassortant highly pathogenic AI H5N1 virus at 106 EID50 and 107.5 EID50, respectively, induced high HI titers and afforded complete clinical and shedding protection after 3 weeks’ post-vaccination at 6 weeks of age in SPF chickens.
Cytokines and chemokines produced by T-lymphocytes after antigen stimulation are major mediators of the host immune responses during infection [33]. Interferon type II (IFN-γ) and other avian proinflammatory cytokines (IL-6), were reported to play an important role in both adaptive and innate immune responses [2, 13]. The Th1 cytokine IFN-γ is crucial for macrophage activation; augments expression of major histocompatibility class I and II antigens (MHC-I and MHC-II) and class switching if immunoglobulins [22], limits and contains viral replication [14].
Up-regulation of IFN-γ in PBMCs of vaccinated and challenged chicken of the present study was observed. All vaccinated groups showed a significant up-regulation in IFN-γ and reduced viral shedding in comparison to the unvaccinated chicken group which suggests that IFN-γ may have an effect on viral clearance. Interestingly, the chicken’s group that received the mucosal-parenteral vaccines, expressed the highest levels of IFN-γ up-regulation at all tested time points with no viral shedding. Indicating the potentiality of the prime-boost vaccination strategy regarding the reduction of shedding.
Previous studies by Rauw et al. [33] showed that oculonasal administration of adjuvanted NDV vaccine promoted the Th1 orientation of the immune response in chickens by improving the specific ChIFN-γ production. Also, a recent study conducted by Khalifeh et al. [17]; employed the prime-boost strategy against NDV and their results revealed that liposomal-based vaccines are able to elicit high IFN-γ expression levels post vaccination.
As a member of the interleukin family, IL-6 has the capability to promote the proliferation of B-lymphocytes [41]. In addition, as an important cytokine in the mucosa, IL-6 is strongly correlated with IgA antibody responses in the respiratory tract [13]. Our results demonstrated that the expression of the proinflammatory IL-6 was enhanced especially after booster vaccination peaking at 2 weeks P.V. in the chickens primed with the mucosal and boostered with the parenteral vaccines, indicating that cellular immunity was induced and may play a role in protection from clinical signs and shedding of ND and AI challenge viruses.
The obtained results from lymphocytes proliferation assay indicated that all vaccines were able to elicit cellular immune response post vaccination when compared to the unvaccinated chickens; it’s believed that the proliferation recorded in group (1) is due to the Montanide gel adjuvant effect where Montanide gel adjuvants have been demonstrated to induce a short term strong inflammatory response needed to trigger an efficacious immune response: including lymphocytes activation and stimulation [38]. In addition, the results showed an inverse correlation between the cellular and humoral immune response, where levels of lymphocytes proliferation started to decline with the increase in the antibodies titers. This finding is consistent with previously published reports which observed that once the humoral immunity becomes established, there is a corresponding decrease in the cellular immune response [37]. Indeed, our results suggest that hat the use of prime-boost vaccination strategy with bivalent vaccines against HPAI and NDV in poultry species is a promising strategy for controlling both HPAI H5N1 and virulent NDV infections.
Acknowledgements
The authors would like to thank the Veterinary Serum and Vaccine Research Institute (VSVRI) Cairo, Egypt, for providing all kinds of help and support.
References
- 1.Bouwstra R, Gonzales JL, de Wit S, Stahl J, Fouchier R, Elbers A, et al. Risk for low pathogenicity avian influenza virus on poultry farms, the Netherlands, 2007–2013. Emerg Infect Dis. 2017;23:1510–1516. doi: 10.3201/eid2309.170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardenas-Garcia S, Dunwoody RP, Marcano V, Diel DG, Williams RJ, Gogal RM, Brown CC, Miller PJ, Afonso CL. Effects of chicken interferon gamma on Newcastle disease virus vaccine immunogenicity. PLoS ONE. 2016;11(7):e0159153. doi: 10.1371/journal.pone.0159153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattoli G, Fusaro A, Monne I, Coven F, Joannis T, El-Hamid HSA, et al. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine. 2011;29:9368–9375. doi: 10.1016/j.vaccine.2011.09.127. [DOI] [PubMed] [Google Scholar]
- 4.Cattoli G, Fusaro A, Monne I, Molia S, Le Menach A, et al. Emergence of a new genetic lineage of Newcastle disease virus in West and Central Africa—implications for diagnosis and control. Vet Microbiol. 2010;142(3–4):168. doi: 10.1016/j.vetmic.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Degefa T, Dadi L, Yami AG, Mariam K, Nassir M. Technical and economic evaluation of different methods of Newcastle disease vaccine administration. J Vet Med A. 2004;51:365–369. doi: 10.1111/j.1439-0442.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 6.Dimitrov KM, Lee DH, Coplin DW, Olivier TL, Miller PJ, Afonso CL. Newcastle disease viruses causing recent outbreaks worldwide show unexpectedly high genetic similarity to historical virulent isolates from the 1940s. J Clin Microbiol. 2016;54(5):1228–1235. doi: 10.1128/JCM.03044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Naggar HM, Madkour MS, Hussein HA. Preparation of mucosal nanoparticles and polymer-based inactivated vaccine for Newcastle disease and H9N2 AI viruses. Vet World. 2017;10(2):187–193. doi: 10.14202/vetworld.2017.187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fentie T, Dadi K, Kassa T, Sahle M, Cattoli G. Effect of vaccination on transmission characteristics of highly virulent Newcastle disease virus in experimentally infected chickens. Avian Pathol. 2014;43(5):420–426. doi: 10.1080/03079457.2014.951832. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira HL, Rauw F, Pirlot JF, Reynard F, van den Berg T, Bublot M, Lambrecht B. Comparison of single 1-day-old chick vaccination using a Newcastle disease virus vector with a prime/boost vaccination scheme against a highly pathogenic avian influenza H5N1 challenge. Avian Pathol. 2014;43(1):68–77. doi: 10.1080/03079457.2013.873111. [DOI] [PubMed] [Google Scholar]
- 10.Hikono H, Mase M, Aya M, Nakayama M, Saito T. Intraocular vaccination with an inactivated highly pathogenic avian influenza virus induces protective antibody responses in chickens. Vet Immunol Immunopathol. 2013;151:83–89. doi: 10.1016/j.vetimm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Hussein HA, Emara MM, Rohaim MA. Molecular characterization of Newcastle disease virus genotype VIId in avian influenza H5N1 infected broiler flock in Egypt. Int J Virol. 2014;10(1):46–54. doi: 10.3923/ijv.2014.46.54. [DOI] [Google Scholar]
- 12.Kandeil A, Mostafa A, El-Shesheny R, El-Taweel AN, Gomaa M, Galal H, Kayali G, Ali MA. Avian influenza H5N1 vaccination efficacy in Egyptian backyard poultry. Vaccine. 2017;35(45):6195–6201. doi: 10.1016/j.vaccine.2017.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H, Wang H, Yu Q, Yang Q. Effect of intranasal immunization with inactivated avian influenza virus on local and systemic immune responses in ducks. Poult Sci. 2012;91:1074–1080. doi: 10.3382/ps.2011-01817. [DOI] [PubMed] [Google Scholar]
- 14.Kapczynski DR, Afonso CL, Miller PJ. Immune responses of poultry to Newcastle disease virus. Dev Comp Immunol. 2013;41:447–453. doi: 10.1016/j.dci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Kapczynski DR, Esaki M, Dorsey KM, Jiang H, Jackwood M, Gardin M. Vaccine protection of chickens against antigenically diverse H5 highly pathogenic avian influenza isolates with a live HVT vector vaccine expressing the influenza hemagglutinin gene derived from a clade 2.2avian influenza virus. Vaccine. 2015;33:1197–1205. doi: 10.1016/j.vaccine.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Maatouq AM, Cai Z, McKenzie PP, Webby RJ, El Refaey S, Kandeel A, Ali MA. Avian influenza A(H5N1) virus in Egypt. Emerg Infect Dis. 2016;22(3):379–388. doi: 10.3201/eid2203.150593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalifeh M, Assaf S, Al-Saleh W, Gharaibeh M. Immune response assessment of inactivated Newcastle disease virus liposomal-based vaccine. Vet Sci Dev. 2014;4:5514. [Google Scholar]
- 18.Kim HS, Samal SK. Heterologous prime-boost immunization of Newcastle disease virus vectored vaccines protected broiler chickens against highly pathogenic avian influenza and Newcastle disease viruses. Vaccine. 2017;35(33):4133–4139. doi: 10.1016/j.vaccine.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Kayali G, Walker D, Forrest HL, Ellebedy AH, Griffin YS, Rubrum A, Bahgat M, Kutkat MA, Ali MA, Aldridge JR, Negovetich NJ, Krauss S, Webby RJ, Webster RG. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proc Natl Acad Sci USA. 2010;107:11044–11049. doi: 10.1073/pnas.1006419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Paldurai A, Xiao S, Collins PL, Samal SK. Modified Newcastle disease virus vectors expressing the H5 hemagglutinin induce enhanced protection against highly pathogenic H5N1 avian influenza virus in chickens. Vaccine. 2014;32:4428–4435. doi: 10.1016/j.vaccine.2014.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.King DJ. Evaluation of different methods of inactivation of Newcastle disease virus and avian influenza virus in egg fluids and serum. Avian Dis. 1991;35:505–514. doi: 10.2307/1591214. [DOI] [PubMed] [Google Scholar]
- 22.Lalsiamthara J, Lee JH. Immunization with Salmonella Enteritidis secreting mucosal adjuvant labile toxin confers protection against wild type challenge via augmentation of CD3 + CD4 + T-cell proliferation and enhancement of IFNc, IL-6 and IL-10 expressions in chicken. Vaccine. 2017;35:767–773. doi: 10.1016/j.vaccine.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Lardinois A, Steensels M, Lambrecht B, Desloges N, Rahaus M, Rebeski D, van den Berg T. Potency of a recombinant NDVH5 vaccine against various HPAI H5N1 virus challenges in SPF chickens. Avian Dis. 2012;56:928–936. doi: 10.1637/10173-041012-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Park JK, Kwon JH, Yuk SS, Erdene-Ochir TO, Jang YH, Seong BL, Lee JB, Park SY, Choi IS, Song CS. Efficacy of single dose of a bivalent vaccine containing inactivated Newcastle disease virus and reassortant highly pathogenic avian influenza H5N1 virus against lethal HPAI and NDV infection in chickens. PLoS ONE. 2013;8(3):e58186. doi: 10.1371/journal.pone.0058186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löndt BZ, Nunez N, Banks J, Nili H, Johnson LK, Alexander DJ. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol. 2008;37(6):619–627. doi: 10.1080/03079450802499126. [DOI] [PubMed] [Google Scholar]
- 26.Mccluskie MJ, Weeratna RD, Payette PJ, Davis HL. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol Med Microbiol. 2002;32:179–185. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller PJ, Decanini EL, Afonso CL. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect Genet Evol. 2010;10:26–35. doi: 10.1016/j.meegid.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Miller PJ, Estevez C, Yu Q, Suarez DL, King DJ. Comparison of viral shedding following vaccination with inactivated and live Newcastle disease vaccines formulated with wild-type and recombinant viruses. Avian Dis. 2009;53:39–49. doi: 10.1637/8407-071208-Reg.1. [DOI] [PubMed] [Google Scholar]
- 29.Miller PJ, Koch G. Newcastle disease. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair VL, editors. Diseases of poultry. 13. Wiley-Blackwell: Hoboken, NJ, USA; 2013. pp. 89–138. [Google Scholar]
- 30.Nayak B, Rout SN, Kumar S, Khalil MS, Fouda MM, Ahmed LE, Earhart KC, Perez DR, Collins PL, Samal SK. Immunization of chickens with Newcastle disease virus expressing H5 hemagglutinin protects against highly pathogenic H5N1 avian influenza viruses. PLoS ONE. 2009;4:e6509. doi: 10.1371/journal.pone.0006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.OIE. Avian influenza OIE manual of diagnostic tests and vaccines for terrestrial animals, Chap. 2.3.4. 2015.
- 32.Peeters B, Tonnis WF, Murugappan S, Rottier B, Koch G, Frijlink HW, Huckriede A, Hinrichs WL. Pulmonary immunization of chickens using non-adjuvanted spray-freeze dried whole inactivated virus vaccine completely protects against highly pathogenic H5N1 avian influenza virus. Vaccine. 2014;32:6445–6450. doi: 10.1016/j.vaccine.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Rauw F, Gardin Y, Palya V, Anbari S, Gonze M, Lemaire S, van den Berg T, Lambrecht B. The positive adjuvant effect of chitosan on antigen-specific cell-mediated immunity after chicken’s vaccination with live Newcastle disease vaccine. Vet Immunol Immunopathol. 2010;134:249–258. doi: 10.1016/j.vetimm.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Steensels M, Bublot M, Van Borm S, De Vriese J, Lambrecht B, Richard-Mazet A, Chanavat-Bizzini S, Duboeuf M, Le Gros FX, van den Berg T. Prime-boost vaccination with a fowlpox vector and an inactivated avian influenza vaccine is highly immunogenic in Pekin ducks challenged with Asian H5N1 HPAI. Vaccine. 2009;27:646–654. doi: 10.1016/j.vaccine.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Okada HI, Tada T, Mase T, Nakamura M, Kubo K, Tsukamoto M. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J Virol. 2009;83:7475–7486. doi: 10.1128/JVI.01434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swayne DE, Kapczynski DR. Vaccines, vaccination, and immunology for avian influenza viruses in poultry. In: Swayne D, editor. Avian influenza. Ames: Blackwell Publishing; 2008. pp. 407–451. [Google Scholar]
- 37.Timms LM, Bracemell CD. Cell mediated and humeral immune response of chicken s to inactivated oil emulsion infectious bronchitis vaccine. Res Vet Sci. 1983;34:224–230. [PubMed] [Google Scholar]
- 38.Vialle R, Dupuis L, Deville S, Bertrand F, Gaucheron J, Aucouturier J. Microgel particulate adjuvant: characterization and mechanisms of action. Procedia Vaccinol. 2010;2:12–16. doi: 10.1016/j.provac.2010.03.003. [DOI] [Google Scholar]
- 39.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, Nabel GJ. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 40.Wen G, Li L, Yu Q, Wang H, Luo Q, Zhang T, et al. Evaluation of a thermostable Newcastle disease virus strain TS09-C as an in-ovo vaccine for chickens. PLoS ONE. 2017;12(2):e0172812. doi: 10.1371/journal.pone.0172812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wigley P, Kaiser P. Avian cytokines in health and disease. Rev Bras Cienc Avic. 2003;5:1. doi: 10.1590/S1516-635X2003000100001. [DOI] [Google Scholar]
- 42.Wise MG, Suarez DL, Seal BS, Pedersen JC, Senne DA, King DJ, Kapczynski DR, Erica SE. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J Clin Microbiol. 2004;42(1):329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]