Abstract
Objective
The purpose of this study was to review previously published meta-analyses on the effectiveness of dietary fiber on reducing the incidence of cancer.
Methods
An umbrella review of all published meta-analyses was performed. A PubMed search from January 1, 1980 to June 30, 2017 was conducted using the following search strategy: (fiber OR fibre) AND (meta-analysis OR systematic review) AND (cancer OR carcinoma). Only English-language publications that provided quantitative statistical analysis on cancer were retrieved.
Results
Nineteen meta-analyses comparing highest vs lowest dietary fiber intake were retrieved for inclusion in this umbrella review. There was a statistically significant reduction in the relative risk (RR) of colorectal, esophageal, gastric, and pancreatic cancer (RR = 0.52-0.88); however, statistically significant heterogeneity was observed in the meta-analyses on esophageal, gastric, and pancreatic cancer. There was a statistically significant reduction in the RR of breast cancer (RR = 0.85-0.93).
Conclusion
This review suggests that those consuming the highest amounts of dietary fiber may benefit from a reduction in the incidence of developing colorectal cancer, and there also appears to be a small reduction in the incidence of breast cancer.
Key Indexing Terms: Dietary Fiber, Meta-analysis, Neoplasms
Introduction
In 2017, in the United States, it is estimated that there will be approximately 1.67 million new cases of cancer diagnosed, and 600 000 deaths from this disease are projected to occur.1 Epidemiologic studies show that dietary factors are believed to play an important role in the prevention of cancer, among which dietary fiber has received considerable interest.2 Increased intake of dietary fibers has been associated with decreased risk of several cancers, such as colorectal and breast cancer.3, 4, 5, 6, 7 However, the results of many epidemiologic studies have shown conflicting results, with some showing a weak or null association.8, 9, 10, 11, 12 Furthermore, the 2007 World Cancer Research Fund’s second expert report concluded that the data were too inconsistent to draw a conclusion on the association between dietary fiber and cancer risk.13
Given the inconsistency of the existing literature, a pooling of information from individual trials could provide a more precise and accurate estimate of dietary fibers role in reducing the incidence of cancer. To achieve this result, many investigators have turned to performing a powerful statistical method known as meta-analysis. Meta-analyses are fundamental to provide the highest level of evidence to best inform health care decision making. Because of the current inconsistency in the literature on the benefits of dietary fiber’s ability to reduce cancer incidence, the purpose and objective of this paper is to summarize the evidence from previously published meta-analyses regarding the effectiveness of dietary fiber in reducing the incidence of cancer.
Methods
An umbrella review was selected for this study. An umbrella review provides a summary of existing published meta-analyses and systematic reviews and determines whether authors addressing similar review questions independently observe similar results and arrive at similar conclusions.14 Inclusion criteria for assessing the effectiveness of dietary fiber to reduce cancer incidence will have to include meta-analyses that surveyed cancer incidence within normal populations (with no geographic, race, or sex restrictions) while comparing the relative rates (RRs) or odds ratios (ORs) of those with the highest vs lowest dietary fiber intakes.
As meta-analyses started appearing in medical literature in the early 1980s, a systematic literature search of PubMed and CINAHL from January 1, 1980 to June 30, 2017 was conducted using the following search strategy: (fiber OR fibre) AND (meta-analysis OR systematic review) AND (cancer OR carcinoma OR adenoma).
Abstracts, conference proceedings, and gray literature were not included as the focus of this umbrella review; it was restricted to peer-reviewed, full-length papers indexed only in PubMed and CINAHL. The titles and abstracts from the literature search were scanned, and only English-language publications that provided quantitative statistical analysis (RRs and ORs) on cancer incidence were retrieved. Meta-analyses or systematic reviews that did not present study-specific summary data using a minimum of 4 randomized controlled trials were excluded.
For the published meta-analyses that were accepted into this review, the following information was extracted and entered into a Microsoft Excel spreadsheet: number of publications included in the meta-analysis, number of total participants, and pooled treatment effects for RRs or ORs. Although not always present, the meta-analyses were also analyzed for their disclosure of quality assessment, statistical heterogeneity (Cochran’s Q test and I2 statistic), and publication bias (visual inspection of funnel plots and Egger or Begg regression test). A methodological quality appraisal was conducted for all meta-analyses using the Critical Appraisal Checklist for Systematic Reviews, which was developed by the Umbrella Review Methodology Working Group.14 This checklist consists of 10 items, in which each item within the instrument can receive 1 point for an overall quality score that could range from 0 to 10. Meta-analyses with quality scores ranging from 0 to 4 were labeled as low quality, those with scores between 5 and 7 were labeled as medium quality, and those with scores of 8 to 10 were labeled as high quality. Because this is a descriptive summary review of meta-analyses, no statistical analyses were performed.
Results
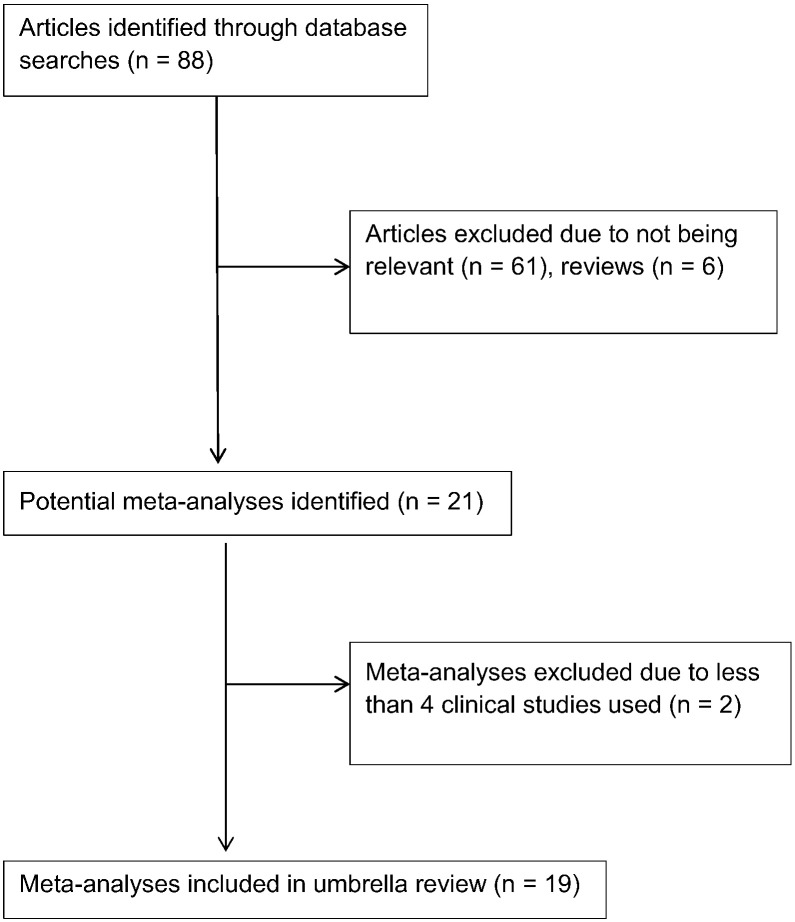
The initial search strategy identified 88 articles; after careful review, 19 meta-analyses were retrieved for inclusion into this umbrella review.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 A flow chart of the meta-analyses selection process is provided in Figure 1. The 2 meta-analyses by Hajishafiee et al34 and Kim and Je35 were not included in the umbrella review because they used only 2 and 3 clinical studies, respectively, to calculate their effect size on cancer mortality RR.
Fig 1.
Flow chart of meta-analysis selection.
In regard to the methodological quality of the 19 meta-analyses in this umbrella review, the mean quality appraisal score was 8 of 10, where 14 (74%) meta-analyses scored as high quality; 2 (10%) satisfied medium quality; and 3 (16%) satisfied low quality. These 3 low quality meta-analyses included a paper by Trock et al15 and 2 papers by Howe et al.16, 25 Although these 3 meta-analyses have been deemed lower quality, they were still included in this umbrella review because they provide useful information regarding the role dietary fiber has for reducing the incidence of colorectal cancer and breast cancer.
The meta-analyses presented in Table 1, Table 2 are based on dietary surveys, which compare the highest vs lowest daily dietary fiber consumption on the incidence of developing gastrointestinal related cancers (Table 1) and nongastrointestinal cancers, such as breast, prostate, endometrial, and renal cancer (Table 2). However, the meta-analysis by Liu et al33 was not entered into either table because this was the only meta-analysis that investigated the impact of fiber consumption on cancer mortality and not cancer incidence. This particular meta-analysis assessed 5 clinical studies with a total population of 640,482 participants and provided a hazard ratio of 0.83, which was statistically significant (P < .05). There was no statistically significant observation of either heterogeneity or publication bias in this particular meta-analysis.
Table 1.
High vs Low Dietary Fiber Intake on the Incidence of Developing Gastrointestinal Related Cancers
| Meta-analysis Authors and Date | Cancer Type | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Main Findings of Meta-analysis | Q Test P Value |
I2 Statistic | Egger or Begg Test P Value | Quality Assessment and Outcome |
|---|---|---|---|---|---|---|---|---|
| Trock et al. 199015 | Colorectal | 16 | 15 379 | OR = 0.57, P < .001 | P < .001 | |||
| Howe et al. 199216 | Colorectal | 13 | 15 574 | RR = 0.53, P < .001 | ||||
| Asano and McLeod 200217 | Colorectal | 5 | 3641 | RR = 1.04, NS | NS | 5 | Cochrane 5/5 high quality |
|
| Aune et al. 201118 | Colorectal | 18 | 200 066 | RR = 0.88, P < .05 | NS | 0 | NS | |
| Ben et al. 201419 | Colorectal | 20 | 132 102 | RR = 0.72, P < .05 | P = .002 | 55 | NS | NOS 14/20 high quality |
| Coleman et al. 201320 | Esophageal | 8 | 9688 | OR = 0.66, P < .05 | P < .001 | 83 | NS | |
| Sun et al. 201521 | Esophageal | 15 | 16 885 | OR = 0.52, P < .05 | P < .001 | 72 | NS | NOS 9/15 high quality |
| Zhang et al. 201322 | Gastric | 21 | 580 064 | OR = 0.58, P < .05 | P = .001 | 62 | NS | NOS 14/21 high quality |
| Wang et al. 201523 | Pancreatic | 14 | 38 141 | OR = 0.54, P < .05 | P = .043 | 41 | NS | NOS NR |
| Mao et al. 201724 | Pancreatic | 14 | 38 141 | OR = 0.52, P < .05 | NS | 7 | NS | NOS 10/14 high quality |
NOS, Newcastle-Ottawa Scale; NR, not reported; NS, not significant; OR, odds ratio; RR, relative risk.
Table 2.
High vs Low Dietary Fiber Intake on the Incidence of Developing Nongastrointestinal Cancers
| Meta-analysis Authors and Date | Cancer Type | No. of Studies in Meta-analysis | No. of Participants in Meta-analysis | Main Findings of Meta-analysis | Q Test P Value | I2 Statistic | Egger or Begg Test P Value | Quality Assessment and Outcome |
|---|---|---|---|---|---|---|---|---|
| Howe et al. 199025 | Breast | 12 | 10 522 | RR = 0.85, P = .001 | ||||
| Dong et al. 201126 | Breast | 10 | 712 195 | RR = 0.89, P < .05 | NS | 0 | NS | |
| Aune et al. 201227 | Breast | 16 | 999 271 | RR = 0.93, P < .05 | NS | 0 | NS | |
| Chen et al. 201628 | Breast | 24 | 3 662 421 | RR = 0.88, P < .05 | P = .001 | 59 | NS | Jadad 18/24 high quality |
| Bandera et al. 200729 | Endometrial | 8 | 12 312 | OR = 0.71, P < .05 | NS | 21 | Intentionally not performed | |
| Sheng et al. 201530 | Prostate | 17 | 140 179 | OR = 0.89, NS | P = .005 | 54 | NS | NOS 9/17 high quality |
| Wang et al. 201531 | Prostate | 16 | 136 979 | RR = 0.94, NS | NS | 40 | NS | NOS 5/16 high quality |
| Huang et al. 201432 | Renal Cell | 6 | 938 664 | RR = 0.84, P < .05 | NS | 24 | NS | NOS 2/6 high quality |
NOS, Newcastle-Ottawa Scale; NS, not significant; OR, odds ratio; RR, relative risk.
For populations that consumed the highest dietary fiber intake, the incidence of colorectal cancer was significantly reduced in 4 of the 5 meta-analyses, with the RR ranging between 0.53 and 0.88 for those that were statistically significant (Table 1). However, for 2 of these 4 meta-analyses, statistically significant heterogeneity was observed. The only nonsignificant meta-analysis, by Asano and McLeod,17 had an RR of 1.04.
Esophageal, gastric, and pancreatic cancers were also significantly reduced in all 5 meta-analyses, with the OR ranging between 0.52 and 0.66 (Table 1). However, statistically significant heterogeneity was observed in 4 of the 5 of these meta-analyses.
The incidence of breast cancer was significantly reduced in all 3 meta-analyses, with the RR ranging between 0.85 and 0.93 (Table 2). Although the incidence of endometrial cancer was significantly reduced (OR = 0.71), the reduction in the incidence of prostate cancer was not statistically significant. Finally, the incidence of renal cell cancer was significantly reduced with an RR of 0.84.
Discussion
When comparing participants with the highest intakes of total dietary fiber to those with the lowest intakes relative to the incidence of developing colorectal cancer, 4 of the 4 meta-analyses in this umbrella review presented statistically significant reductions that ranged between 12% and 47%.15, 16, 18, 19 However, we must appreciate these positive results with some caution because statistically significant heterogeneity was observed in 2 of these 4 meta-analyses.15, 19 There was 1 meta-analysis published by Asano and McLeod that did not report a statistical significant reduction but instead reported an RR of 1.04.17 This finding may be accounted for by the fact that the 5 clinical trials used in this particular meta-analysis were solely randomized clinical controlled trials, whereas the 4 previous meta-analyses, which observed statistically significant reductions, used clinical studies that were observational case-controlled or cohort studies. This paradoxical finding has been noted in many other dietary interventions, in which randomized controlled trials of diet-related factors have not yet shown any conclusive associations between diet and cancer incidence.2
In regard to the gastrointestinal system beyond the colon and rectum, 5 separate meta-analyses found statistically significant reductions in the RR for developing esophageal, gastric, and pancreatic cancer with reductions ranging between 34% and 48%.20, 21, 22, 23, 24 However, we must appreciate these positive results with some caution because statistically significant heterogeneity was observed in 4 of these 5 meta-analyses.24 Curiously, the I2 statistic and the Q-test’s P values were very different for the pancreatic cancer meta-analyses, considering they were designed using the same 14 clinical trials and observed nearly identical OR (0.54 vs 0.52).23, 24
In 2017, in the United States, it is estimated that colorectal cancer will be only the fourth most common cancer diagnosis in men and women combined, but unfortunately it will be the second most common cause of cancer mortality, second only to lung cancer in cancer-related deaths.1 In regard to the mechanism of action for reducing the incidence of colorectal cancer, it is possible that dietary fiber increases stool bulk, and this dilutes carcinogen concentrations in the colonic lumen. Coupled with a shortened fecal transit time, the time during which luminal carcinogens may be in contact with gastrointestinal epithelial cells decreases.36 Dietary fiber may also bind to both carcinogens and primary and secondary bile acids to promote their excretion in the feces. Bacterial fermentation of fibers to short chain fatty acids such as acetate, propionate and butyrate decreases luminal pH, which helps decrease the conversion of primary bile acids to carcinogenic secondary bile acids.37 Butyrate, a 4-carbon short chain fatty acid, also provides 70% of energy for healthy normal colonic epithelial cells and has been shown to have antineoplastic actions by inhibiting cancer cell proliferation and inducing apoptosis and cell cycle arrest, as well as increasing cell differentiation in colon cancer tissue.36, 37 It is well established that inflammation is directly associated with cancer progression, and it has been observed that butyrate also plays an anti-inflammatory role by inhibiting the transcription factor NF-κB, which results in a reduced concentration of the proinflammatory cytokines interleukin-6 and tumor necrosis factor-α.38, 39 Butyrate’s beneficial effects are mediated at the epigenetic level through the inhibition of histone deacetylases (HDACs), which consequently regulates the expression of downstream genes such as NF-κB and p53.39, 40 The HDACs are important for gene expression, and the levels of these enzymes are increased in tumor cells; thus, a decrease in HDAC activity is associated with the suppression of tumor cell growth.41 In regard to esophageal and gastric cancer, dietary fiber has been shown to scavenge nitrites, which are the precursors of the gastrointestinal cancer causing N-nitroso compounds.20
When comparing participants with the highest intakes of total dietary fiber to those with the lowest intakes relative to the incidence of developing breast cancer, 4 of the 4 meta-analyses in this umbrella review presented with statistically significant reductions that ranged between 7% and 15%.25, 26, 27, 28 The meta-analysis on endometrial cancer also observed a statistically significant reduction of 29%.29 However, although the range in reduction for prostate cancer was between 6% and 11%, the observations from both of these 2 meta-analyses were not statistically significant.30, 31
In 2017, it is estimated that, in the United States, breast cancer will be the most common cancer diagnosis overall and the second leading cause of death from cancer in women.1 Dietary fiber effects on reducing the incidence of breast cancer was not as strong as compared to effects observed with colorectal cancer, and this may be attributed to dietary fibers’ different mechanisms of action at these 2 very different sites. Prolonged exposure to estrogen is a strong risk factor for breast and endometrial cancer, and reductions in circulating estrogens have been observed in participants who are consuming larger quantities of dietary fiber.42 It has been proposed that dietary fibers can bind to estrogens in the lumen of colon and increase their fecal excretion.27 In addition, dietary fiber may also reduce intestinal enzymatic activity of β-glucuronidase, which is responsible for the hydrolysis of conjugated estrogens prior to their absorption by colonic epithelial cells.43 Other components in dietary fiber, such as antioxidants, phenolic acids, and lignans, may also be protective against breast and endometrial cancer. Lignans such as enterodiol and enterolactone are phytoestrogens that are derived from the noncarbohydrate dietary fibers called lignins, and they possess weak estrogenic-inhibiting effects.44 Dietary fiber may also promote weight loss, and because adipocytes produce estrogen at a proportional amount relative to their size, there would be a subsequent reduction in the levels of estrogen.27
The Dietary Guidelines for Americans states that the adequate intake value of dietary fiber consumption is 25 to 38 g/day, but the 2009 to 2010 National Health and Nutrition Examination Survey shows that the daily intake of fiber in the United States is only 17 g/d.45 Therefore, emphasizing fiber consumption for health promotion and disease prevention is a critical public health goal, and by aggressively promoting the Dietary Guidelines for Americans recommendation of at least 25 to 38 g/d of total dietary fiber, this may prevent a significant number of new cancer cases. Although the evidence in this umbrella review supports the beneficial association of dietary fiber on reducing the incidence of colorectal and breast cancer, too few long-term, large-population, randomized controlled trials have undertaken the goal to analyze this potential causal relationship between dietary fiber and cancer incidence. Finally, while no Tolerable Upper Intake Level has been established for total fiber intake, it should be noted that minor side effects have been observed, such as flatulence, abdominal bloating, loose stools or diarrhea, and abdominal cramping.46
Limitations
This umbrella review has several limitations that should be acknowledged. First, confounding factors are always a potential threat to the validity of any meta-analysis. For instance, people who have high dietary fiber intakes tend to have other healthy behaviors, such as being more physically active, having lower dietary intakes of saturated fat and processed meats, and avoiding smoking and excessive alcohol intake. Fortunately, the majority of studies included in the meta-analyses that were involved in this umbrella review did adjust for potential confounding factors, but the possibility of residual confounders cannot be excluded. Second, self-reported dietary fiber intake is most often assessed using food frequency questionnaires; because these dietary assessment tools were not specifically developed for dietary fiber intake, it is possible that misclassifications and measurement errors regarding fiber doses and types are quite likely. This problem may also be compounded by the fact that dietary fiber may be defined differently by the various food frequency questionnaire databases in use.46 A third limitation is that the meta-analyses reviewed here represent a heterogeneous group of clinical studies composed from a diverse group of participants of different ages, sexes, races, and ethnic groups: therefore, readers are cautioned against specifying these results to any specific sociodemographic group. Finally, as in all literature reviews, the quality of this umbrella review is directly related to the quality of the included meta-analyses, which are dependent upon the design and reporting quality of the individual meta-analysis itself, as well as on the quality of the individual studies used to conduct the meta-analysis. Fortunately, the majority (84%) of the meta-analyses in this umbrella review were apprised as having moderate to high methodological quality.
Conclusion
This umbrella review suggests that those consuming the highest amounts of dietary fiber may benefit from a reduction in the incidence of developing colorectal cancer, as well as a small reduction in developing breast cancer. These findings have important public health implications, especially in light of the finding by Liu et al,33 who determined that individuals with the highest dietary fiber consumption reduced their cancer mortality by 17%. Unfortunately, the mean dietary fiber intake in the United States is 17 g/d, which is considerably less than the recommended intake of 25 to 38 g/d.45 Future well-designed, large, multicenter, randomized controlled studies are required to confirm these associations.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): M.P.M.
Design (planned the methods to generate the results): M.P.M.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): M.P.M.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): M.P.M.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): M.P.M.
Literature search (performed the literature search): M.P.M.
Writing (responsible for writing a substantive part of the manuscript): M.P.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): M.P.M.
Practical Applications
-
•
Dietary fiber consumption has been postulated to reduce the incidence of cancer, but unfortunately there is much discrepancy when it comes to individual cohort and case-controlled studies.
-
•
By combining the meta-analyses on these clinical outcomes as an umbrella review, we can show that increased dietary fiber intake may help reduce the incidence of gastrointestinal and breast cancers. More high-quality research is needed regarding the association between use of chiropractic services and risk of adverse drug events.
Alt-text: Unlabelled Box
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18(8):e457–e471. doi: 10.1016/S1470-2045(17)30411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cade JE, Burley VJ, Greenwood DC. Dietary fibre and risk of breast cancer in the UK Women's Cohort Study. Int J Epidemiol. 2007;36(2):431–438. doi: 10.1093/ije/dyl295. [DOI] [PubMed] [Google Scholar]
- 4.Park Y, Brinton LA, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr. 2009;90(3):664–671. doi: 10.3945/ajcn.2009.27758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Holford TR, Zhang Y. Dietary fiber intake and risk of breast cancer by menopausal and estrogen receptor status. Eur J Nutr. 2013;52(1):217–223. doi: 10.1007/s00394-012-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters U, Sinha R, Chatterjee N. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361(9368):1491–1495. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- 7.Tantamango YM, Knutsen SF, Beeson L, Fraser G, Sabate J. Association between dietary fiber and incident cases of colon polyps: the adventist health study. Gastrointest Cancer Res. 2011;4(5-6):161–167. [PMC free article] [PubMed] [Google Scholar]
- 8.Wen W, Shu XO, Li H. Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. Am J Clin Nutr. 2009;89(1):283–289. doi: 10.3945/ajcn.2008.26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaineddin AK, Buck K, Vrieling A. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64(5):652–665. doi: 10.1080/01635581.2012.683227. [DOI] [PubMed] [Google Scholar]
- 10.Deschasaux M, Zelek L, Pouchieu C. Prospective association between dietary fiber intake and breast cancer risk. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platz EA, Giovannucci E, Rimm EB. Dietary fiber and distal colorectal adenoma in men. Cancer Epidemiol Biomarkers Prev. 1997;6(9):661–670. [PubMed] [Google Scholar]
- 12.Fuchs CS, Giovannucci EL, Colditz GA. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med. 1999;340(3):169–176. doi: 10.1056/NEJM199901213400301. [DOI] [PubMed] [Google Scholar]
- 13.Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014;159:35–50. doi: 10.1007/978-3-642-38007-5_3. [DOI] [PubMed] [Google Scholar]
- 14.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 15.Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst. 1990;82(8):650–661. doi: 10.1093/jnci/82.8.650. [DOI] [PubMed] [Google Scholar]
- 16.Howe GR, Benito E, Castelleto R. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992;84(24):1887–1896. doi: 10.1093/jnci/84.24.1887. [DOI] [PubMed] [Google Scholar]
- 17.Asano T, McLeod RS. Dietary fibre for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2002;2 doi: 10.1002/14651858.CD003430. [DOI] [PubMed] [Google Scholar]
- 18.Aune D, Chan DS, Lau R. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Q, Sun Y, Chai R, Qian A, Xu B, Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology. 2014;146(3):689–699. doi: 10.1053/j.gastro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Coleman HG, Murray LJ, Hicks B. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutr Rev. 2013;71(7):474–482. doi: 10.1111/nure.12032. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Zhang Z, Xu J, Xu G, Liu X. Dietary Fiber Intake Reduces Risk for Barrett's Esophagus and Esophageal Cancer. Crit Rev Food Sci Nutr. 2017;57(13):2749–2757. doi: 10.1080/10408398.2015.1067596. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Xu G, Ma M, Yang J, Liu X. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology. 2013;145(1):113–120. doi: 10.1053/j.gastro.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang CH, Qiao C, Wang RC, Zhou WP. Dietary fiber intake and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Sci Rep. 2015;5:10834. doi: 10.1038/srep10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao QQ, Lin YW, Chen H. Dietary fiber intake is inversely associated with risk of pancreatic cancer: a meta-analysis. Asia Pac J Clin Nutr. 2017;26(1):89–96. doi: 10.6133/apjcn.102015.03. [DOI] [PubMed] [Google Scholar]
- 25.Howe GR, Hirohata T, Hislop TG. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82(7):561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 26.Dong JY, He K, Wang P, Qin LQ. Dietary fiber intake and risk of breast cancer: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;94(3):900–905. doi: 10.3945/ajcn.111.015578. [DOI] [PubMed] [Google Scholar]
- 27.Aune D, Chan DS, Greenwood DC. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23(6):1394–1402. doi: 10.1093/annonc/mdr589. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Chen Y, Ma S. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget. 2016;7(49):80980–80989. doi: 10.18632/oncotarget.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Association between dietary fiber and endometrial cancer: a dose-response meta-analysis. Am J Clin Nutr. 2007;86(6):1730–1737. doi: 10.1093/ajcn/86.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng T, Shen RL, Shao H, Ma TH. No association between fiber intake and prostate cancer risk: a meta-analysis of epidemiological studies. World J Surg Oncol. 2015;13:264. doi: 10.1186/s12957-015-0681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang RJ, Tang JE, Chen Y, Gao JG. Dietary fiber, whole grains, carbohydrate, glycemic index, and glycemic load in relation to risk of prostate cancer. Onco Targets Ther. 2015;8:2415–2426. doi: 10.2147/OTT.S88528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang TB, Ding PP, Chen JF. Dietary fiber intake and risk of renal cell carcinoma: evidence from a meta-analysis. Med Oncol. 2014;31(8):125. doi: 10.1007/s12032-014-0125-2. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Wang S, Liu J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2015;59(1):139–146. doi: 10.1002/mnfr.201400449. [DOI] [PubMed] [Google Scholar]
- 34.Hajishafiee M, Saneei P, Benisi-Kohansal S, Esmaillzadeh A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2016;116(2):343–352. doi: 10.1017/S0007114516001938. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. 2016;109(1):39–54. doi: 10.1016/j.acvd.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6(2):41–51. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung KY, Cosgrove L, Lockett T, Head R, Topping DL. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108(5):820–831. doi: 10.1017/S0007114512001948. [DOI] [PubMed] [Google Scholar]
- 38.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52(9):2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Encarnação JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34(3):465–478. doi: 10.1007/s10555-015-9578-9. [DOI] [PubMed] [Google Scholar]
- 40.Bhat MI, Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev. 2017;75(5):374–389. doi: 10.1093/nutrit/nux001. [DOI] [PubMed] [Google Scholar]
- 41.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 42.Key TJ, Allen NE, Spencer EA, Travis RC. Nutrition and breast cancer. Breast. 2003;12(6):412–416. doi: 10.1016/s0960-9776(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 43.Manoj G, Thampi BS, Leelamma S, Menon PV. Effect of dietary fiber on the activity of intestinal and fecal beta-glucuronidase activity during 1,2-dimethylhydrazine induced colon carcinogenesis. Plant Foods Hum Nutr. 2001;56(1):13–21. doi: 10.1023/a:1008188009174. [DOI] [PubMed] [Google Scholar]
- 44.Cohen LA. Dietary fiber and breast cancer. Anticancer Res. 1999;19(5A):3685–3688. [PubMed] [Google Scholar]
- 45.McGill CR, Birkett A, Fulgonii Iii VL. Healthy Eating Index-2010 and food groups consumed by US adults who meet or exceed fiber intake recommendations NHANES 2001-2010. Food Nutr Res. 2016;60:29977. doi: 10.3402/fnr.v60.29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahl WJ, Stewart ML. Position of the academy of nutrition and dietetics: health implications of dietary fiber. J Acad Nutr Diet. 2015;115(11):1861–1870. doi: 10.1016/j.jand.2015.09.003. [DOI] [PubMed] [Google Scholar]