Abstract
Introduction:
A survey of FDA-approved biologicals focused upon the development of immunotherapies over time to gain insight on the challenges and trends of vaccine development today.
Areas covered:
A total of 135 different immune-based therapies could be broadly divided into passive or active immunotherapies. Whereas just over half of passive immunotherapies targeted infectious diseases, the vast majority of active immunotherapy products (vaccines) were directed against a handful of viral and bacterial pathogens. We also analyze changes in vaccine strategy, including the use of viable antigens and subunit approaches.
Expert Commentary:
An analysis of vaccine innovators revealed an ever-increasing presence of the private sector and a relatively diminishing role for the public sector. Whereas North American companies have contributed to the approval of two-thirds of vaccines, European companies have regained parity in terms of hosting innovators of vaccine research and development.
Keywords: pathogens, vaccine development, vaccine innovation, passive immunization, active immunization
1. Introduction
According to a 2014 report from the United States Centers for Disease Control and Prevention (CDC), a 20-year program of vaccination (1994–2013) was estimated to prevent more than 21 million hospitalizations and avoid almost three quarters of a million premature deaths [1]. Despite and perhaps because of the extraordinary public health benefits afforded by vaccines, they are now largely taken for granted by the public. In recent years, diminishing recollection of the impact of scourges such as smallpox, diphtheria and polio has propagated the spread of misinformation [2] and fueled an anti-vaccine movement. Often forgotten are estimates that more than 15,000 Americans died in 1921 from diphtheria, an infection of oral mucosa by the bacterium Corynebacterium diphtheria.[1] Because of the availability of vaccines targeting this pathogen, only a single case of diphtheria has been reported to the CDC since 2004.[3]
Much is known about individual vaccines and the diseases they prevent [4]. However, there remains an opportunity to assess larger trends in the development of innovative vaccines. Many of the earliest treatments for infectious diseases consisted of passive immunotherapies (immune globulins) derived from immunized horses and other livestock. One of these new ‘wonder drugs’ unintentionally revealed the need for regulatory oversight of vaccines and antibodies. Returning to the history of diphtheria, a retired St. Louis milk wagon horse by the name of “Jim” was immunized with diphtheria toxin and had provided a powerful antitoxin for three years [5]. In 1901, Jim unknowingly became infected with tetanus and the tainted blood from the horse poisoned dozens of St. Louis children, causing at least 13 deaths. At roughly the same time, tetanus-contaminated smallpox vaccine killed nine children in Camden, NJ [6].
The disasters resulting from insufficient standardization in generating antitoxins motivated the United States Congress to adopt the Biologics Control Act of 1902. Among the provisions of the act, oversight of biological medicines was tasked to the Hygienic Laboratory, which had been founded in 1887 by Dr. Joseph J. Kinyoun. Following a series of legislative actions that increased its mandate, the Hygienic Laboratory evolved into the National Institute of Health (NIH) following passage of the 1930 Ransdell Act [7]. Regulatory oversight of biological therapeutics remained within the NIH through both the passage of the 1906 Pure Food and Drugs Acts and 1938 Federal Food Drug and Cosmetic Act, which tasked the Food and Drug Administration (FDA; formerly known as the Bureau of Chemistry within the United States Department of Agriculture) with oversight of most medicines [8]. Over time, vaccine oversight within NIH was conducted by the Division of Biologics Control (created in 1937) and amalgamated into the newly-created National Microbiological Institute (later known as the National Institute of Allergy and Infectious Diseases; NIAID) in 1948.
A more dramatic change occurred when biologics oversight was transferred in 1972 to the FDA, which formed the Center for Drugs and Biologics, which was divided in 1987 to form the Centers for Biologics Evaluation and Research (CBER) and its counterpart, the Center for Drug Evaluation and Research (CDER). A final change arose in 2002 when oversight of recombinant monoclonal antibodies and some other biologicals, was transferred from CBER to CDER, which added these technologies to the portfolio of conventional small molecule drugs that it had historically overseen. Perhaps because of these many changes in regulatory oversight, a thorough synthesis of innovative vaccines and their creators, had presented considerable challenges.
In recent years, much of our work at the Center for Research Innovation at Washington University in St. Louis has analyzed the sources and trends in therapeutics (new molecular entities; or NMEs). A series of reports have documented long-term trends in how medicines are discovered [9]. Among other findings, our work has confirmed a declining efficiency in research and development of new medicines, which in turn has presaged migration away from earlystage research activities by many pharmaceutical companies.[10, 11]. The abandonment of basic and then early-stage drug discovery by the private sector slowed the growth of biotechnology and a parallel trend in industry consolidation began to disassemble new efforts by the private sector to address early drug development. For example, the number of biopharmaceutical companies that had contributed to the development of at least one new medicine has now shrunk to a level not seen since the end of the Second World War.[12] Such findings identified the erasure of industry gains made during both the “Golden Age of Pharmaceuticals (generally defined as 1950–1970 as well as the “Biotechnology Revolution” (1971–2000). Such studies have raised fundamental questions about the sustainability of the pharmaceutical industry and are motivating discussion of potential responses to ensure the continuation of the discovery of new medicines.
Utilizing similar techniques as those used to assess the origins and fate of FDA-approved medicines [9], we report herein an analysis of passive and active immunotherapies. We report trends in scientific and medical approaches such as the breadth of diseases and pathogens targeted by vaccines over time as well as changes in the scientific approaches used for vaccinebased targeting. Such findings may have utility for anticipating unmet medical needs that improve public health.
2. Materials and methods
2.1. Data sources and compilation.
These studies focused upon passive and active immunotherapy. Of note, the term immunotherapy as used in this manuscript is limited to vaccines including immune globulin products, active vaccindation products, and antibodies and is not meant to include other immune-based products such as interferons, interleukins, colony-stimulating factors, and immune receptor antagonists. The definition utilized herein include both passive immunotherapies (polyclonal antibodies and a single monoclonal antibody) and vaccines that elicit a response to one or more antigens to trigger active immunity. This particular study did not include desensitizers of the immune system, namely conventional allergens. Likewise, our desire to focus on therapeutic and prophylactic vaccines precluded the inclusion of diagnostic agents such as the various forms of tuberculin. For those readers interested in vaccine adjuvants, we would direct them towards excellent and relatively recent reviews of this key subject [13, 14]. Importantly, we limited the analysis herein to the first product introduced the United States market and did not include those products that are essentially identical, including bioequivalents, changes in pathogenic strain (such as the yearly influenza vaccine), simple combinations of established vaccines or products withdrawn and re-introduced at a later date (e.g., anthrax immune globulin or adenovirus vaccines). In the latter case, the first introduction of the product is included, but not the later re-introduction. Vaccine products introduced with additional valencies were included in the analysis.
The studies conducted herein utilized the same procedures we have employed previously to study FDA-approved medicines.[9] The Center for Research Innovation in Biotechnology at Washington University (crib.wustl.edu) compiled a database of all innovative vaccines utilized in the United States. The compiled information included all FDA-approved vaccines as well as vaccines utilized in the United States prior to the creation of the modern FDA. Our definition of an innovative vaccine was restricted to any advance in the improvement of a vaccine through the introduction of a different antigen or other substantive improvements and did not include, for example, the combination of previously-used vaccines into a new product or generically equivalent follow-on vaccines. Specifically, publically-available information from the Food and Drug Administration (http://www.fda.gov/BiologicsBloodVaccines/) provided an initial source of information for the creation of a database of all currently-available biological products. We also evaluated publically available information about currently-available biologicals as of the end of the first quarter of the year 2016 (http://www.fda.gov/Drugs/InformationOnDrugs/ucm079750.htm). As these results did not include products that are no longer accessible due to obsolescence or toxicity or market-based withdrawals, these data were supplemented by analyses of additional information provided by the United States Centers for Disease Control and Prevention (http://www.cdc.gov/) and additional information provided by the US Department of Health and Human Services (www.vaccines.gov). the Immunization Action Coalition (www.immunize.org) and additional information provided by the US Department of Health and Human Services (www.vaccines.gov), the Immunization Action Coalition (www.immunize.org) and the College of Physicians of Philadelphia (www.historyofvaccines.org).
Two particularly useful resources provided insight into active and passive therapeutics approved or otherwise utilized in the United States during the early twentieth century. The first was provided by the American Medical Association, which archived the introduction of new therapeutic options via a series of regular reports in the Journal of the American Medical Association (JAMA) and titled “New and Nonofficial Remedies” from 1909 through the mid- 1940s and later in an annual book form titled “New and Nonofficial Drugs.” Additional resources included the biographies and obituaries of key pioneers in vaccine development, which tended to provide contemporary descriptions of their subjects’ work. Second, information compiled by Kendall Hoyt of Dartmouth University and published in the appendices of her 2012 book, “Long Shot”, provided a comprehensive list of vaccines formally approved by the government since the implementation of the 1902 Biologics Control Act through the end of the century [15]. Notably, these two data sets were not always consistent with one another. Specifically, the availability of medicines, as indicated in “New and Nonofficial Drugs” generally pre-dated their inclusion as a licensed drug. Indeed, the AMA’s decision to initiate “New and Nonofficial Remedies” was based in part on the frustration of the organization and its members with the marketing and availability of non-approved medicines and vaccines [16].
Given the complexity and inconsistent nature of compiling early information, all data were independently verified by searches of public databases, including but not necessarily limited to published literature (https://www.ncbi.nlm.nih.gov/pubmed/) and searches of patent and trademarks (www.uspto.gov). For unapproved vaccines and antibodies or ones that have been withdrawn from the United States market, general web searches were conducted and information verified by at least one additional source from either PubMed or Google Scholar.
The information collected included the generic and trade names of each product as well as the date of FDA approval (at least the year, and ideally, the month and day) as well as the organization receiving the first approval. For approvals in which the reviews are publically available from the FDA (generally products approved on or after the mid-1990s), the reviews were analyzed to identify organizations (public and private sector) that contributed to product development prior to approval. In particular, the clinical review of the biologics license application (BLA) often conveyed the organizations involved in the regulatory life cycle, including submission of the investigational new drug (IND) application or its equivalent, any changes in custody as a result of licensing or mergers and acquisitions, end of phase one and/or two meetings as well as correspondence associated with the approval of the product. For those products, where FDA documentation is not readily available, the organization receiving approval and the date of the final approval was documented and literature-based searches of Federal sites were conducted to identify objective evidence (e.g., information on the CDC website, patent or trademark assignees) of those organizations that may have contributed to the clinical development of the specific product at any point from the initial IND submission through the final approval. To identify any missing information, priority was placed upon information from scientific journals (identified utilizing PubMed searches (see above). If not available or conclusive, press releases from the FDA or private sector organizations provided the sources of the dates and innovators. Lacking this, a general web-based search was conducted, with emphasis upon reliable sources (information from academic organizations such as www.historyofvaccines.org).
2.2. Data analysis and manipulation
The work herein sought to identify sources of innovation in vaccine development. Thus, for all products identified, the earliest example was captured. Many vaccines were developed for use in wartime and often introduced to the military before the general public. To avoid confusion, we report the year of introduction to the general public unless the vaccine is or was only utilized in the military. This was considered to be the equivalent of a “new molecular entity” (NME) as it applies to conventional FDA-approved therapeutics. Thus, other follow-on products and bioequivalents were not analyzed. As a specific example, the “Salk” vaccine for polio was attributed to the University of Pittsburgh as the inventor but the five companies that manufactured and distributed the product (Eli Lilly, Parke-Davis, Wyeth, Pitman-Moore and Cutter) were not considered innovators and their individual products were not included in the analyses herein. In no cases, were data manipulated to exclude any product unless it was a duplicate, i.e. was bioequivalent, of an existing product.
Many innovative vaccines have been removed from the market and are no longer available and thus the list of currently-available vaccines was supplemented with an inventory of prior therapies that have been used over the years but are no longer available. Likewise, some products predated the FDA or never received formal approval. One example is diphtheria antitoxin (DAT), which was first licensed by the United States government in 1903. However, decreased need for DAT caused manufacturers to abandon the license for DAT, which was then transitioned to an investigational product that is currently managed by the Centers for Disease Control and Prevention (CDC) and available by special request.[17]
Due to changes in the structure of the Food and Drug Administration (FDA), the approval process for some products has changed over time. For example, monoclonal antibody products such as, palivizumab (Synagis), were originally approved by the Center for Biologics Evaluation and Research (CBER) but were later governed by the Center for Drug Evaluation and Research (CDER). Thus, records from both organizations were evaluated in the course of the studies herein.
2.3. Identification of pathogen species
The analysis of vaccines targeting infectious disease agents was focused upon identifying innovative vaccines targeting pathogen species and not necessarily serotypes. The example of the pneumococcal vaccine, Prevnar, provides an illustrative example of the complexity involved in our analyses. The original Prevnar (approved in 2000) targeted seven different serotypes and this novel vaccine was considered to have targeted a single pathogen species (Streptococcus pneumoniae). The subsequent approval of a vaccine targeting thirteen different serotypes (Prevnar-13; approved in 2010) was considered a distinct vaccine but did not alter number of pathogen species. The same strategy was applied throughout the study for other bacterial species and viruses (e.g., human papillomavirus).
Another important distinction pertains to the strategy utilized for influenza virus vaccines. Whereas the composition of the influenza vaccine varies annually and each is technically a novel vaccine, we catalogued as innovative only those vaccines with more substantial differences in complexity, such as the unique pandemic influenza vaccines approved in the late 2000s for H1N1 and H5N1 pandemic strains. Likewise, we did not include the move from trivalent to quadrivalent strains as now deployed for many seasonal influenza vaccines.
2.4. Analysis of product withdrawals
To assess the number of approved vaccines that have been withdrawn over time, the full list of vaccines was compared with vaccines licensed for immunization and distribution in the United States as of the end of the first quarter of 2016 as indicated on the FDA website: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts. A product was considered withdrawn only if a licensed product could not be obtained in the United States, regardless of whether the product was from the original innovator or a generic competitor. For products that are no longer available, the reason for withdrawal was assessed by web-based searches for evidence of toxicity, lack of sales, lack of efficacy or obsolescence (i.e., replacement by an improved product). Most of this information was readily available from searches within the FDA website. Given the sensitivities associated with vaccine toxicity, every effort was made to refer only to independently-verifiable information such as FDA announcement or published information from peer-reviewed sources (e.g., from searches of the PubMed database). Please note that the term ‘withdrawal,’ as used herein, refers to discontinuing an entire product line and not the withdrawal of particular manufacturing lots.
2.5. Identification of innovator organizations
The source of innovative products was generally assessed using an algorithm based on information provided on publically-accessible FDA documents. In general, data identifies the current distributor of the product. In light of extensive industry consolidation, it was necessary to work backwards by asking if any predecessor organizations had originated or contributed to the product prior to FDA approval. This was accomplished, when possible, by assessing the prior regulatory interactions with FDA as indicated in the supporting documents associated with each BLA approval. In particular, the clinical review tended to provide the most comprehensive overview of any changes in custody of the product during its development. Due to inconsistencies in reporting, this work was often supplemented by assessments of press releases by companies associated with the product, with emphasis upon announcements of clinical trials initiations or outcomes. Tertiary priority in identifying contributors to the product development was assigned to documentation available from the United States Patent and Trademark Office for search of both granted and published patent and trademark applications (both current and expired in the case of trademarks). Due to our focus on the clinical research and development of vaccines, the individuals and organizations responsible for the early stage basic research on understanding the biology, pathogenesis, and immune response to the agent are generally neglected in this analysis.
2.6. Data availability
All data analyzed herein have been made available to the scientific community and general public on the website of the Center for Research Innovation in Biotechnology (crib.wustl.edu). We actively encourage all interested parties to explore the data and identify any improvements or additions that might be of use for interested investigators.
3. Results
3.1. Immune Therapies
The goal of the present study was to assess the sources contributing to the development of first-in-class (hereafter referred to as “innovative”) immune-based therapies. Using the criteria defined in the Methods section, 37 passive and 98 active immune-based therapies were identified (Fig. 1a). The earliest introduction was a vaccine for smallpox and its discovery is widely attributed to Edward Jenner. The attenuated cowpox vaccine (being less pathogenic than smallpox) was first tested in 1796 in the United Kingdom and reached Benjamin Waterhouse at Harvard by 1800 [18]. Waterhouse quickly leveraged his prominence in the former colonies and gained a champion for a safer alternative to variolation (the use of antigen retrieved from pustules on patients suffering from smallpox) in the person of Thomas Jefferson, who was transitioning at the time to become the third president of the United States [18, 19].
Figure 1.
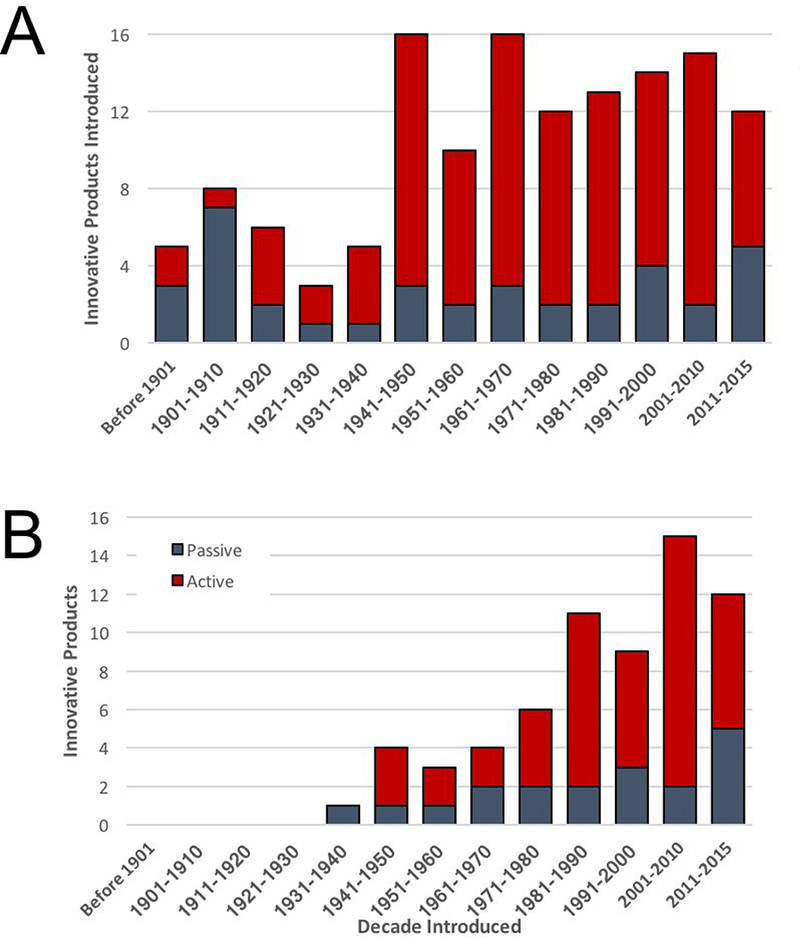
FDA-Approved Biologicals. (a) The accumulation of innovative passive (grey) and active (red) immune-based therapies is shown over time. (b) The accumulation of innovative passive and active immune product that were available for use in the United States as of the end of 2015 are shown.
Despite this extraordinary achievement in the opening years of the nineteenth century, only five immune-based therapies were broadly available in the United States by the close of that century. These products included three passive, animal-based sera (directed against pneumococcal disease, tuberculosis and Salmonella) and one additional active immunotherapy (for typhoid fever), all of which were introduced between 1891 and 1900. The pace of innovation increased in the twentieth century, with 22 additional products (11 each of passive and active immunotherapies) introduced between 1901 and 1940. Spurred by the looming threat of a second worldwide conflict, the rate of innovative vaccines increased further thereafter, with an average of no fewer than one new vaccine introduced each year since 1941. If one narrows the analysis to products still available for use in the United States as of the end of 2015, a total of 66 innovative agents for immunization were identified (Fig. 1b). The earliest product still in use is an equine-derived antivenin first introduced by Merck in 1936 and indicated for the treatment of black widow spider (Latrodectus mactans) bites.
3.2. Passive immunotherapy
A total of 37 passive immunotherapies have ever been approved or otherwise utilized (if predating regulation) in the United States. These products varied from crude sera, semipurified, or purified immune globulins and derivatives thereof. The immune globulins were further distinguished by the species of origin, with 25 non-human and 12 human products identified (Fig 2a). Among animal-based immune globulins, most (20 of 25) were derived from equine sources. The remaining five sera were derived from immunized ovine (3 of 25) or rabbit (2 of 25) sources.
Figure 2.
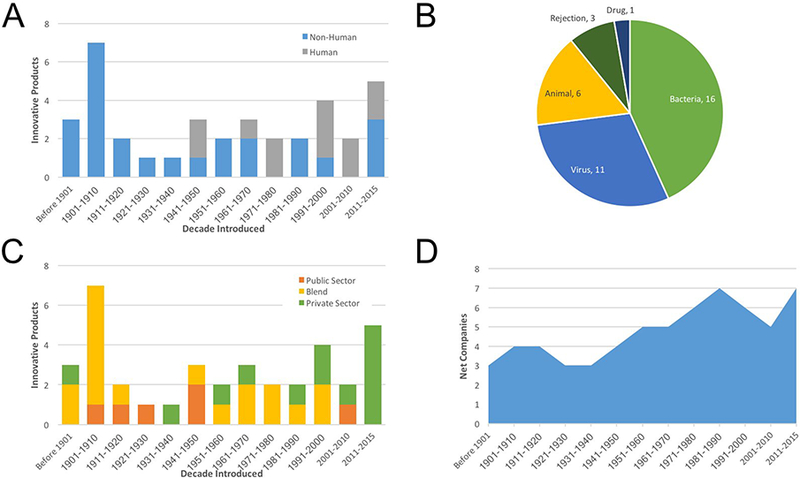
Passive Immunotherapy. (a) The species source of passive immunotherapies are indicated introduced into the United States over time is indicated. (b) The therapeutic application for passive immunotherapies is divided into five pathogen or disease types. Please note that animal diseases include bites from spiders and snakes. (c) The organizations that contributed to the clinical development for passive immunotherapy products are shown over time. Please note “blend” refers to products, who approvals involved a private-public partnership. (d) The net number of private sector companies that contributed to the development of at least one passive immunotherapeutic is indicated over time.
Whereas all immune globulin therapies introduced up until 1940 were isolated from horses and other domesticated mammals, newer products included purified human immunoglobulins. The first use of human immunoglobulin arose from studies of fractionated plasma by Dr. Edwin Cohn at Harvard University. The resulting product, intravenous immunoglobulin (commonly known as IVIG) was introduced in 1944 for wartime use to manage infection and has remained useful for the treatment of immune deficiencies [20]. Since that initial breakthrough, an additional 11 human serum-based immunoglobulin products have been introduced.
With one exception, the analyses herein did not include monoclonal antibodies and other recombinant immunoglobulin products. Our rationale was that animal-derived serum products and vaccines have been largely governed by the Center for Biologics Evaluation and Research (CBER) within the Food and Drug Administration (FDA). In contrast, monoclonal antibodies have been overseen by the Center for Drug Evaluation and Research (CDER) at FDA since 2003. An additional reason is that only a single monoclonal antibody product, palivizumab (Trade name: Synagis, approved in 1998 and developed by the United States Army and MedImmune, Inc.) is directed against an infectious agent. To be thorough, we have included palivizumab in this analysis and imperfectly categorized this humanized mouse monoclonal antibody as a “human” product.
An assessment of clinical applications of passive immunotherapy reveals microorganisms (viruses and bacteria) as the targets for more than half of immunoglobulin therapies (Fig. 2b). Human (e.g., Rh factor and transplantation applications) and animal (e.g., venomous snakes and spiders) targets encompass the majority of the remaining products. The one exception, DigiBind, was approved in 1986 to ameliorate overdose with the drugs digoxin or digitoxin.
Our studies then identified the organizations that contributed to the development of passive immunotherapies. Contributing organizations were broadly divided into public (academic and governmental entities) and private sector organizations (Fig. 2c). Almost half (18 of 37) of immunoglobulin products resulted from partnerships involving both private and public sector organizations. Of the remaining immunoglobulins, 13 were developed by private sector companies and six by public sector groups. Multiple organizations contributed to the approval of two (American Cyanamid, American Home Products, Bayer, Harvard University, Merck, United States Army) or three (Burroughs Wellcome, MedImmune, H.K. Mulford, Emergent BioSolutions) different immunoglobulin products. However, only a single organization, Parke- Davis Pharmaceuticals, contributed to the approval of four different immunoglobulin products.
Parke-Davis also provided an example in terms of industry consolidation. Parke-Davis was acquired by Warner-Lambert in 1970, which in turn was subsumed into Pfizer in 2000. Similarly, seven other private sector contributors to immunoglobulin products have been subject to industry consolidation. To put this in perspective, fifteen different private sector organizations have contributed to the approval of at least one immune globulin product. As a result of industry consolidation, just over half were eliminated, leaving seven independent organizations as of the end of 2015 (Fig. 2d).
3.3. Active immunotherapy
Turning to active immunotherapies, ninety-eight (98) vaccines have been introduced or approved for use in the United States (Fig. 1a). When assessed over time, smallpox (Variola major) was the target for the creation of three innovative vaccines; most recently the 2007 FDA approval of ACAM2000 for the prevention of smallpox virus infection. However, the number of vaccines against smallpox has been eclipsed by those targeting influenza virus, for which no fewer than ten innovative products have been introduced (not shown). Based on new technologies and concerns about pandemic influenza, six of the seven innovative vaccines brought to market in the current decade target influenza virus. This reflects a larger trend in which viruses in general have consistently been the most popular targets for vaccines, accounting for 57 different innovations (Fig 3a).
Figure 3.
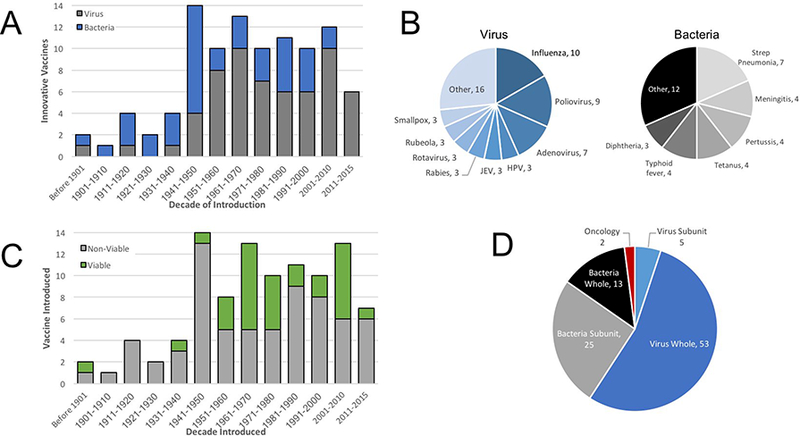
Therapeutic Applications of FDA-Approved Vaccines. (a) The etiology of diseases for which vaccines have been utilized are indicated over time, with broad divisions to indicate viral (blue) or bacterial (black) pathogens. (b) The specific viruses (shades of blue) or bacteria (shades of black) targeted by vaccines is indicated. (c) The composition of vaccines was broadly divided into non-viable (killed or subunit) or viable (attenuated) vaccines. (d) Active immunotherapeutics were broadly divided into either whole or subunit forms of the pathogen targeted and further distinguished between viral or bacterial targets. Also note that two vaccine, shown in red, were directed towards cancer antigens.
Bacterial infections are the next most popular targets for vaccines, with 38 innovative products. When viewed over time, targeting of bacterial pathogens was more common from 1901 through 1950, capturing 19 vaccines as compared to six vaccines directed against viral pathogens (Fig 3a). Starting in the second half of the twentieth century, the ratio of viral to bacterial pathogens reversed, with 52 innovative vaccines approved against viral pathogens as compared with 19 targeting bacteria. The individual pathogen type was also analyzed, revealing that influenza, poliovirus and adenoviruses accounted for just under one half of all innovative vaccines (Fig 3b). Likewise, four bacterial diseases, pneumococcal disease, bacterial meningitis, pertussis, and tetanus captured half of innovative vaccines directed against prokaryotic human pathogens.
Having catalogued the diseases targeted by vaccines over time, we began to ask about the mechanistic bases of innovative immunotherapies. Most vaccines (67 of 98) utilized non-viable antigens, generally killed pathogens or subunits (Fig. 3c). The remaining 31 vaccines were attenuated pathogens. Looking deeper, attenuated vaccines (27 of 31) generally were directed against viral pathogens whereas only two were directed against bacterial pathogens (the remaining two targeted cancer). We also compared whole pathogens with subunit vaccines (Fig. 3d). Whereas five vaccines represented viral subunits, the largest group of 52 virusdirected vaccines consisted of whole viruses (including attenuated vaccines). In contrast to the situation with viruses, subunits predominated for vaccine targeting of bacterial pathogens, accounting for twenty-five subunit vaccines as compared with thirteen whole bacterial vaccines.
3.4. Organizations contributing to vaccine development
These studies of vaccine innovation then assessed the organizations involved in the research and development process. To this end, we focused upon the establishments that initiated or controlled the product from the beginning of clinical trials through regulatory approval (or introduction into the American market if this occurred prior to the onset of Federal oversight). These organizations were broadly grouped into either the private (i.e., for-profit companies) or public (e.g., universities, governmental and non-profit organizations) sector.
Public sector organizations participated in the clinical development of all vaccines introduced into the United States through 1940 (Fig. 4a). This period also witnessed a rise in public/private “blended” partnerships. The four decades corresponding with the beginning of the Second World War through 1980 saw a continuation of this blended strategy as well as a further rise of private sector participation, including the first vaccines with clinical development driven entirely by the private sector. The growing influence of the private sector is emphasized by the increasing absence of public sector participation in the development of only two of the twenty vaccines approved since 2001.
Figure 4.
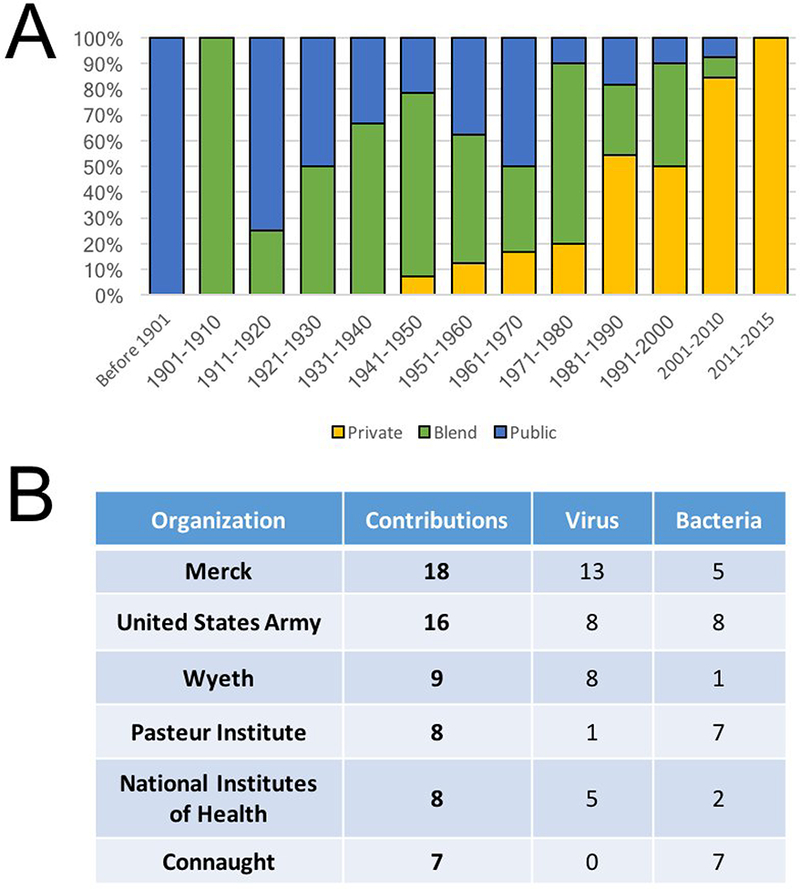
Public and Private Sector Contributions to Vaccine Research and Development. (a) The sources of vaccines developed in the years indicated reveals dynamic changes in the balance of organizations contributing the launch of innovative vaccines. Public sector organization include both academic and governmental laboratories. Please note that “Blend” reflects vaccines, whose late preclinical and/or clinical development involved both private and public sector organizations. (b) The leading organizations that have contributed to vaccine research and development is indicated, along with the number of contributions and the types of pathogens targeted.
An analysis of the individual organizations involved in new vaccine introductions confirms the idea of dynamic changes in the contributions of private and public sector organizations. The largest single contributor to vaccines was Merck & Co, which participated in the research or development of at least 18 different vaccines (13 directed at viruses and 5 targeting bacterial pathogens Fig 4b). This figure does not include companies acquired by Merck, including H.K. Mulford & Co. and Sharp and Dohme, each of which contributed additional innovations (not shown). Other major private sector players included Wyeth, which contributed nine innovative vaccines and Connaught, which gained approval for seven.
The Paris-based Pasteur Institute was a major public sector backer of early vaccine research, contributing to the introduction of seven of the first ten vaccines (spanning the period from 1879 through 1931 Fig. 4b). Even this impressive feat was surpassed by the United States Army, which contributed to the introduction of at least 16 different vaccines. These vaccines, as well as the seven products involving the National Institutes of Health (NIH) may actually underrepresent each organization’s contributions as their work was often intermingled, particularly during the formative years for the vaccine enterprise during and immediately following the Second World War. Thus, we assessed contributions from a United States federal or state organizations and found at least 28 vaccines with active participation by a United States governmental agency. This figure yet again may be an underestimation since much of the work conducted by private and public sector organizations was supported by or at the behest of state or federal governments.
In the course of our investigation, we identified the first vaccine (hereafter known as a landmark) for each of the major infectious diseases targeted with active immunotherapy (Fig. 5). We postulated the public sector would play a disproportionate role in the earliest stages of landmark vaccine research and development. Consistent with this idea, public sector institutions (academic or governmental) contributed to at least 25 landmark vaccines. Public sector organizations contributed to 18 and similar to our analyses of vaccines in general, the private sector increasingly plays a role in more recent landmark vaccines as evidenced by private sector participation in all eight landmark vaccines introduced since the mid-1960s and the absence of public sector participation in four of these landmark vaccines (HPV, Lyme disease, Varicella zoster virus and HBV).
Figure 5.

Landmark Vaccines for Infectious Diseases. The pathogens targeted by landmark vaccines (defined herein as the first vaccine approved for the pathogen) are shown in order of vaccine introduction, including the year when each was first use as well as the sources of organizations that contributed to the development of each vaccine. Many vaccines predated the formation of the FDA and/or its mandate to regulate vaccines. Not all vaccines shown were commercially or medically successful and some have been replaced or withdrawn for reasons of safety, efficacy, obsolescence or commercial success.
As indicated above, European organizations such as the Pasteur Institute, dominated the early years of vaccine development (Fig 6a). In the early years of the twentieth century, North American organizations first emerged and then came to dominate vaccine research for the rest of the century. The predominance of North American organizations has seemingly declined in recent years. For example, North American companies contributed to the approval of half of the vaccines approved in the twenty-first century and only two of seven vaccines approved from 2011 through the end of 2015. In large part, this trend reflected the impact of industry consolidation and this in turn led us to assess the impact of mergers and acquisitions over time.
Figure 6.
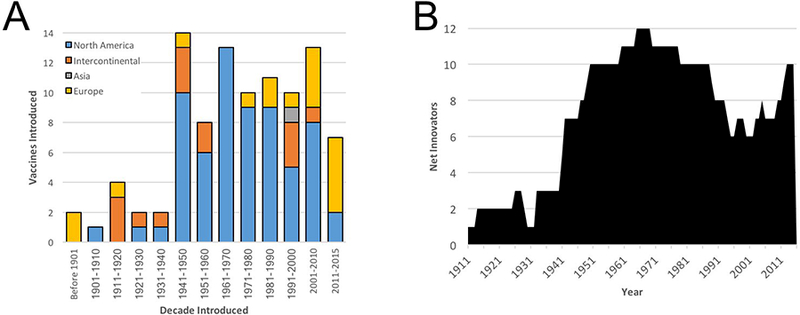
Geographic Sources of Innovative Vaccines. (a) The sources of research and/or development of vaccines are shown based on the site where each organization is headquartered on a decade-by-decade basis. Please note that “intercontinental” reflects vaccines that were developed by multiple organizations from more than one continent. (b) The net number of private sector companies that have contributed to the approval of at least one active immunotherapy (vaccine) and that remain active in vaccine research is shown over time. Please note that whereas Figure 2D focused on antibody products, this figure specifies vaccines.
To assess the dynamics of industry consolidation in terms of vaccine research and development, we considered the fate of those companies that have contributed to innovative vaccine development (Fig 6b). A roster of companies that controlled the vaccine candidate at the time of late-preclinical (i.e., submitting the investigational new drug application or its equivalent) or during clinical investigation was tracked over time. For each organization, we captured the year of their first approval (in effect, the time of their entry into the list of “successful” vaccine developers). An exit point was defined if and when the company was acquired by another organization and only if the acquisition event resulted in a suspension of further vaccine research or development. Using these criteria, thirty private sector companies and twenty-seven public sector organizations have contributed to the research or development of at least one innovative vaccine used in the United States (not shown). Virtually all the public sector organizations remain extant and active in research and development. In contrast, two thirds (20 of 30) of the private sector organizations had been acquired as of the end of 2015. When viewed over time, the number of private sector contributors to vaccine innovation increased steadily from the 1930s onwards, peaking at twelve by 1970 (Fig. 6b). Over the remainder of the twentieth century, industry consolidation reduced the number of active and independent companies by half to six different companies. Notably, the number of these “successful” organizations involved in vaccine research appeared to be rebounding in the past fifteen years and now stands at ten companies.
3.5. Withdrawal & Obsolescence
As detailed above, the range of vaccines available for use by the end of 2015 had shrunk by more than half. According to information available from by the Food and Drug Administration, the current armamentum stands at 19 passive antibody products and 46 active vaccines (Fig. 1b). These reductions led us to ask why the other 18 passive and 51 active immunotherapies had been withdrawn. We reasoned that the explanations could include toxicity, lack of efficacy, poor commercial performance or obsolescence (replacement by an improved product).
An analysis of product discontinuation revealed the majority (42 of 51) of vaccines had been rendered obsolescent due to the introduction of more efficacious replacements (Fig. 7a). Withdrawals attributed to a lack of efficacy accounts for the withdrawal of four additional vaccines: A staphylococcal toxoid introduced in 1933, a poliomyelitis vaccine introduced in 1960 and shown to be contaminated with SV40 virus, an early pneumococcal approved in 1977 and a Hib vaccine approved in 1985. Unacceptable levels of vaccine-mediated toxicity led to the withdrawal of the remaining four vaccines: a Rubeola (measles) virus vaccine approved in 1960; a poliovrus vaccine introduced in 1961, the Lyme disease vaccine (LYMErix) approved in 1998; and a rotavirus vaccine also approved in 1998. Poor sales performance was cited in the withdrawal of two further vaccines (a pneumococcal vaccine approved in 1947 and a typhus vaccine approved in 1967). GlaxoSmithKline reportedly withdrew LYMErix voluntarily for market reasons, however, this coincided with fears of vaccine side-effects were suggested to be the true reason for withdrawl [21].
Figure 7.

Vaccine Withdrawals from the Market. (a) The total number of innovative vaccines that have been removed from the American market was broadly divided among the four categories shown, revealing a preponderance of obsolescence as the underlying rationale. (b) The same analysis was performed on passive immune therapies to assess the reasoning for the 18 innovative products that have been withdrawn. “Unkown” refers to the fact that these four products were removed from the market in the early part of the 20th century for reasons that could be not substantiated.
For immune globulin therapeutics, 13 of 17 withdrawals could be attributed to obsolescence (Fig 7b). In particular, the development of safer and more powerful antibiotics rendered many passive antibodies treatments uncompetitive. The rationale for the withdrawal of the remaining four products was unknown. Notably, these four witdrawals were for products introduced in the early twentieth century and each indicated targeted was addressable by antibiotics (tuberculosis, gonorrhea, dysentery and Rocky Mountain spotted fever), suggesting either obsolescence and/or relative lack of efficacy ultimately led to their withdrawal. There was no evidence of toxicity-based withdrawals identified in our analyses of passive immunotherapies.
The fact most vaccines were withdrawn as a result of obsolescence due to a superior product identified an unexpected trend. Whereas scientific and technological advances have increased the quality of innovative vaccines, the growth in the number of infectious pathogens targeted by vaccines has stagnated (Fig. 8a). When viewed over the decades, the number of diseases that could be targeted with vaccines grew from one (in 1900) to 26 by the end of the 1990s (Fig. 8b). Excluding vaccines approved for oncology indications since 2000, the net number of infectious indications targeted by vaccines in the past quarter century has grown by only one (the approvals of vaccines for Japanese encephalitis virus and human papillomaviruses were offset by the withdrawal of LYMErix). Looking deeper, the breadth of bacterial pathogens targeted by vaccines has remained largely stagnant for a half century. This contrasts with the situation observed with viral pathogens, where the number of pathogens targeted more than doubled from 1950 through 2015.
Figure 8.
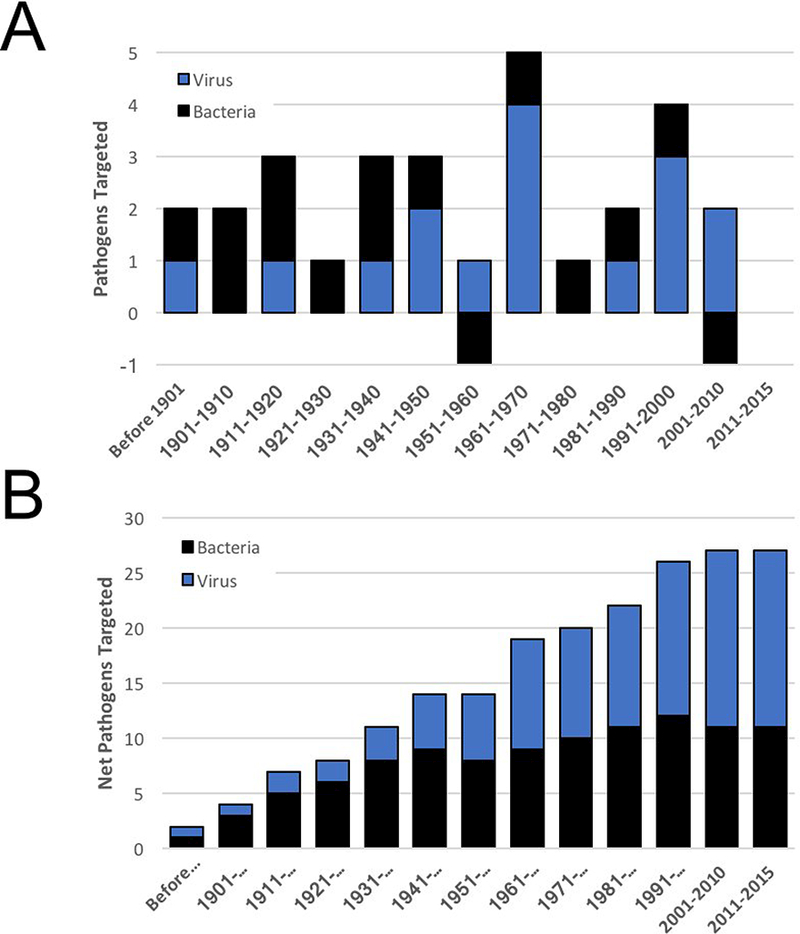
The Breadth of Pathogens Targeted by Vaccines Over Time (a) The number of different pathogens targeted with vaccines was assessed over time and broadly divided into viral or bacterial pathogens. Note that two indications were “lost” as a result of vaccine withdrawal: a staphylococcal toxoid introduced in 1933 and withdrawn in the 1954 due to lack of efficacy and a Lyme disease vaccine introduced in 1998 and withdrawn in 2002 amidst questions of toxicity and poor sales. (b) The overall accumulation of infectious disease indications targeted by vaccines is indicated revealing a consistent growth in the targeting of viral pathogens (blue) as compared with a plateau in vaccines targeting bacterial infections (black bars).
4. Discussion
This analysis provided a comprehensive assessment of immontherapies of the past (earliest analyzed vaccine was made available in 1796) to present day. Active immunotherapies (vaccines) comprise the largest subset of immune modulators with 97 different innovative products; followed by 37 passive immunotherapies. Whereas bacteria were most commonly the targets of vaccine introduced up until 1950, the trend has reversed and viruses now predominate. These studies also revealed a predominance of whole cell (or virus) vaccines (over their subunit counterparts) and that the majority of attenuated vaccines are directed at viral pathogens. The breadth of pathogens addressed by vaccines increased steadily throughout most of the twentieth century but has largely stalled. The primary drivers of vaccine innovation gradually transitioned from the public to the private sector. Whereas the development of innovative vaccines began in Europe, the activities transitioned for a time to North America, and has seemingly been re-balanced between North America and Europe, largely as a result of industry consolidation.
The vaccine credited by many to British physician Edward Jenner (and by others to Benjamin Jesty or John Fewster) set the standard for subsequent vaccine development. The Jenner vaccine for smallpox had demonstrated its usefulness by 1796 [19, 22] and supplanted variolation in the early years of the nineteenth century [22, 23]. As described by Hoyt in his outstanding book “Long Shot” [15], the Jenner vaccine, and variations thereof, comprised three of the first four first biologicals formally approved following the passage of the 1902 Biologics Control Act.
Most active vaccines (85 of 88) prevent or treat viral or bacterial diseases. In contrast, fewer passive immunotherapies, just over one half of immunoglobulins, target microorganisms. The remaining passive therapies antagonize animal venoms or are used to control immune rejection. The finding with passive immunotherapy also differs from the situation with monoclonal antibody therapeutics, in which oncology and autoimmune/inflammation indications are the primary indications addressed. Whereas 96% of biologicals are used to treat infectious diseases, only 6% (2 of 34) of monoclonal antibody-based biologics fall into this category. This difference is intriguing given that most (8 of 10) organizations with biological immunoglobulin products have or had active monoclonal antibody research programs (data not shown). Nonetheless, the monoclonal antibody products from these same companies are rarely directed infectious indications and instead target cancer and other indications.[24]
Since the beginning of the new millennium, only two innovative vaccines have been introduced against a previously-unaddressed bacterial pathogen. To put this into perspective, 16 innovative vaccines targeting viruses and two therapeutic vaccines for cancer were introduced in this same timeframe. More than five innovative vaccines targeting bacteria were approved in each decade from 1970 through 2000. These trends mirror a similar decrease in the introduction of anti-bacterial antibiotic drugs and a substantial uptick in the withdrawal of antibiotics due to obsolescence and drug resistance. In light of increasing recognition of drugresistant and pathogenic bacterial strains, compounded by unmet medical needs and market opportunities, it will be interesting to assess whether the future will witness an uptick in the number of vaccines that target bacterial pathogens. Changing perceptions of the regulatory and commercial opportunities afforded by vaccine targeting of bacterial pathogens may increase their attractiveness in the coming years.
The balance between public and private sector contributions to vaccine research and development have changed over time. Public sector organizations (universities and government laboratories) were the primary contributors in the early decades of vaccine research and development. As pointed out by Galambos and Sewell in their book about vaccine development at Merck and its predecessors at Sharp & Dohme and the H.K. Mulford Company, the first half of the twentieth century gave rise to public-private partnerships that led to the creation of many antitoxins and vaccines [25]. Private sector activities in vaccine development continually increased and by the 1980s, the private sector had become the dominant source of innovative vaccine research and development.
In an effort to to identify innovative vaccines and their developers, we utilized information that was readily accessible from the FDA and other objective sources. Although we were able to assemble a list of immune-based therapies and innovators, we cannot preclude other products or contributors might exist in records that are unfortunately no longer accessible. Our data and approach vary somewhat from an earlier study [15] in that we utilized information from the American Medical Association and others to identify immune-based therapies that were utilized in the United States prior to the 1902 Biologics Control Act. We also include antivenins as well as more recent approvals and analyze the fate of the organizations that contributed to vaccines over time.
While we strove to identify the organizations involved in the clinical investigation of innovative vaccines, the documentation available from the Food and Drug Administration and others did not necessarily reveal contributions from early-stage foundational research or preclinical investigation. Much of this information was either lost to time or incomplete, particularly for products deployed before the 1950s. We necessarily limited our analyses to information for which multiple sources were available, it is impossible to say with absolute certainty that our studies identified all relevant contributors. Yet within the imperfect criteria used for our studies, we were able to identify trends that characterized changes in the pathogens targeted, the approaches used for development and the organizations participating in vaccine research and development.
Whereas innovative vaccine research was dominated by Europe throughout the nineteenth century, most vaccines were developed in North America and during the mid-twentieth century. This hemispheric dominance may be waning as European organizations have reemerged since the beginning of the new millennium. In part, the rebalancing reflects industry consolidation, in which European companies such as Sanofi, Novartis, AstraZeneca and GlaxoSmithKline have acquired organizations involved in vaccine research and development. Further consolidation occurred as Novartis divested its vaccine business to GlaxoSmithKline and CSL [26]. Such trends might also reflect the fact that biotechnology organizations have been seemingly reticent to embrace vaccine research. North America has historically been the domicile to the majority of biotechnology companies [11, 12]. Given the relative absence of biotechnology in vaccine development, it is perhaps unsurprising that vaccine research has rebalanced towards Europe and its more conventional pharmaceutical industry in recent years.
Industry consolidation also reveals a feature that distinguishes vaccine research organizations from their biopharmaceutical counterparts. A relatively small number of companies have successfully conducted vaccine research since the beginning of the twentieth century. The number of private sector organizations actively involved in the research and development of new vaccines grew to roughly a dozen until 1970, dropped sharply over the following two decades, and more recently, has begun to rebound. Although the number of organizations is relatively modest as compared to their pharmaceutical counterparts, the trends observed are both similar and different. The decline in the number of vaccine developers predates by three decades the analogous situation with biopharmaceutical innovators, whose decline began in earnest in the early years of the twenty-first century. A second difference is that the net number of vaccine innovators, though admittedly modest, rebounded and is up by two-thirds since the early 1990s. Given similarities and timelines associated with vaccine and drug developers, it will be interesting to see whether the rebounding vaccine enterprise might again predict a trend that is later mimicked by the larger biopharmaceutical industry.
In analyzing the vaccine industry, it was impossible to ignore the strong sentiment that has surrounded the field over the past few years. Such skepticism is not new as similar mistrust of vaccines plagued variolation, the Jenner vaccine and many other vaccine innovations over the years [27]. Irrational concerns about the safety of vaccines was propagated in recent years by an irresponsible and fraudulent report linking vaccination with childhood developmental diseases and misinformation persists despite overwhelming evidence refuting the retracted report [28–30]. Some critics have cited the withdrawal of vaccines, such as those for Lyme disease and rotavirus infection as indirect evidence of harm done [31, 32]. However, our results revealed that only four vaccines have been withdrawn from the American market as a result of toxicity concerns. Indeed, even this analysis may be overstated as one of the vaccines (RotaShield) we grouped into this category was officially discontinued by its manufacturer after citing poor sales.
Such findings nonetheless emphasize the importance of establishing the risk to benefit ratio of vaccines within the public health community. The experience with the early Salk polio vaccine illustrates an example of how a landmark vaccine changed the disease landscape but also demonstrated the importance of quickly pivoting to improve the standard. Despite the extraordinary reaction to the positive results obtained in clinical trials of inactivated vaccines, the “Cutter incident” precipitated an equally momentous change. Specifically, the incomplete inactivation of certain batches of “killed” vaccines was the root cause of new waves of disease and led the field to adopt live, attenuated oral polio vaccines (OPV) vaccine as an alternative. Ultimately, the choice between killed and live would come full circle with the recent introduction of the inactivated polio vaccines (IPV), which demonstrated further potency and safety and is anticipated to be the final tool needed to eradicate the disease for all time. Given the experiences with polio, smallpox and other vaccines, a goal should be to develop vaccines in the future where such risk-benefits tradeoffs are no longer necessary.
Our present study also noted what may be the beginning of an uptick in the study and use of therapeutic vaccines for oncology. Retroviral links to cancer provided an early basis for identifying oncogenes such as v-src and v-myc.[33] The recognition of such connections motivated the creation of vaccines targeting oncogenic viruses such as human papillomavirus (HPV) and hepatitis B, which are linked with cervical and liver cancers, respectively.[34, 35] However, the newer trend stands out in that rather than targeting an underlying mechanism in tumor initiation, these new therapeutic vaccines are invoked in the treatment of established tumors and metastases. In light of the considerable exuberance within the biopharmaceutical community towards immune-based therapies for cancer, it seems likely that the early signs of interest in oncology vaccines will blossom into a more sustainable trend.
One of the more unexpected findings of our present report is a demonstration that many advances in vaccinology have been focused upon creating improved products that target a relatively narrow breadth of microbial pathogens. In particular, we identified multiple generations of vaccines against relatively few pathogens (mostly bacterial and viral causes of respiratory or neuropathic diseases). While incremental improvements in the prevention of demonstrated killers such as influenza virus and poliovirus have conveyed immeasurable improvements in public health,[36] it raises the question of whether we might similarly apply the lessons learned to positively impact public health by broadening the number of pathogens targeted by future vaccines. For example, the same deep expertise and technoloigies utilized for these viruses might be useful to combat emerging infectious agents in the future.
A caveat is that our present study evaluated only those vaccines already in use in the United States and did not include investigational products. Indeed, there is much work on agents such as HIV, tuberculosis, Zika and malaria that could substantially widen the net of vaccinepreventable pathogens and we are now expanding our investigation to identify all investigational vaccines. However, this opportunity has not yet been reached and is not a new phenomenon as in 2012, Kendall Hoyt has raised questions about declining innovation in vaccine research over the past half century in his rigorously-researched book [15]. In light of emerging infectious diseases associated with climate change and drug resistance, increased emphasis upon vaccines could provide a means to convey orthogonal changes in the future management of infectious diseases.
5. Key issues:
A comprehensive assessment of innovative vaccines was performed and include technologies and products from 1796 to present day.
Accompanying an analysis of the vaccines themselves, we also catalogued and evaluated the firms responsible for their development.
Whereas bacteria were once the most common targets for vaccines introduced until 1950 where present day vaccine development has been focused on viruses.
Since the beginning of the new millennium, only two innovative vaccines have been introduced against a previously-unaddressed bacterial pathogen.
The primary drivers of vaccine innovation has transitioned from the public to the private sector.
Acknowledgements
The authors would like to thank Dr. George Kemble for expert advice and guidance.
Funding
The manuscript was supported by a National Institutes of Health, National Center for Advancing Translational Sciences grant (TL1TR002344).
Footnotes
Declaration of interest
RH Griesenauer is supported by an NIH Ruth L. Kirschstein National Research Service Award. (TL1 TR000449). MS Kinch is supported by the Center for Research Innovation and Biotechnology and Washington University, St. Louis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- [1].Whitney C, Zhou F, Singleton J, et al. , BENEFITS FROM IMMUNIZATION DURNG THE VACCINES FOR CHILDREN PROGRAM ERA--UNITED STATES, 1994–2013, Morbidity and Mortality Weekly Report 63(16) (2014) 352–355. [PMC free article] [PubMed] [Google Scholar]
- [2].Scannell JW, Blanckley A, Boldon H, et al. , Diagnosing the decline in pharmaceutical R&D efficiency, Nat Rev Drug Discov 11(3) (2012) 191–200. [DOI] [PubMed] [Google Scholar]
- [3].Adams DA, Jajosky RA, Ajani U, et al. , Summary of notifiable diseases--United States, 2012, MMWR Morb Mortal Wkly Rep 61(53) (2014) 1–121. [PubMed] [Google Scholar]
- [4].Waldmann TA, Immunotherapy: past, present and future, Nat Med 9(3) (2003) 269–277. [DOI] [PubMed] [Google Scholar]
- [5]**.DeHovitz RE, The 1901 St Louis Incident: The First Modern Medical Disaster, Pediatrics 133(6) (2014) 964–965. An outstanding review of the incident that led to the regulation of vaccines in the United States. [DOI] [PubMed] [Google Scholar]
- [6].Dixon ME, Why Nine Camden Children Died from Smallpox Vaccines in 1901, Main Line Today, http://www.mainlinetoday.com/Main-Line-Today/September-2016/Why-Nine-Camden-Children-Died-from-Smallpox-Vaccines-in-1901/, 2016.
- [7]*.Kinch MS, A Prescription For Change: The Looming Crisis in Drug Discovery, UNC Press, Chapel HIll, NC, 2016. An overview of pharmaceutical innovation and the challenges for the fugure. [Google Scholar]
- [8].Greeley AP, The Food and Drugs Act, June 30, 1906: A Study with Text of the Act, Annotated, the Rules and Regulations for the Enforcement of the Act, Food Inspection, Decisions and Official Food Standards, J. Byrne 1907. [Google Scholar]
- [9].Kinch MS, Haynesworth A, Kinch SL, et al. , An overview of FDA-approved new molecular entities: 1827–2013, Drug discovery today (2014). [DOI] [PubMed] [Google Scholar]
- [10].Munos B, Nat. Rev. Drug Discovery 8 (2009) 959. [DOI] [PubMed] [Google Scholar]
- [11].Kinch MS, The Rise (and Decline?) of Biotechnology, Drug Discovery Today (2014). [DOI] [PubMed] [Google Scholar]
- [12].Kinch MS, Moore R, Innovator Organizations in New Drug Development: Assessing the Sustainability of the Biopharmaceutical Industry, Cell chemical biology 23(6) (2016) 644–53. [DOI] [PubMed] [Google Scholar]
- [13].Mbow ML, De Gregorio E, Valiante NM, et al. , New adjuvants for human vaccines, Current opinion in immunology 22(3) (2010) 411–416. [DOI] [PubMed] [Google Scholar]
- [14].Marrack P, McKee AS, Munks MW, Towards an understanding of the adjuvant action of aluminium, Nature Reviews Immunology 9(4) (2009) 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]**.Hoyt K, Long Shot: Vaccines for National Defense, Harvard University Press, Cambridge, MA, 2012. An overview of vaccine research conducted since the Second World War, suggesting declining innovation [Google Scholar]
- [16].Tomes N, Remaking the American Patient: How Madison Avenue and Modern Medicine Turned Patients Into Consumers, UNC Press Books; 2016. [Google Scholar]
- [17].Wagner K, Stickings P, White J, et al. , A review of the international issues surrounding the availability and demand for diphtheria antitoxin for therapeutic use, Vaccine 28(1) (2009) 14–20. [DOI] [PubMed] [Google Scholar]
- [18].Underwood EA, Edward Jenner, Benjamin Waterhouse, and the Introduction of Vaccination into the United States, Nature 163 (1949) 823–828. [Google Scholar]
- [19].Riedel S, Edward Jenner and the history of smallpox and vaccination, Proceedings (Baylor University. Medical Center) 18(1) (2005) 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Starr D, Blood: An epic history of medicine and commerce, Knopf; 2012. [Google Scholar]
- [21].Hitt E, Poor sales trigger vaccine withdrawal, Nature Publishing Group, 2002. [DOI] [PubMed] [Google Scholar]
- [22].Morgan AJ, Parker S, Translational Mini-Review Series on Vaccines: The Edward Jenner Museum and the history of vaccination, Clinical and Experimental Immunology 147(3) (2007) 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Smith KA, Edward Jenner and the Small Pox Vaccine, Frontiers in Immunology 2 (2011) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kinch MS, An overview of FDA-approved biologics medicines, Drug Discov Today (2014). [DOI] [PubMed] [Google Scholar]
- [25]*.Galambos L, Sewell JE, Networks of Innovation: Vaccine Development at Merck, Sharp and Dohme, and Mulford, 1895–1995, Cambridge University Press; 1997. A thorough review of one of the most prolific set of innovators in vaccine development [Google Scholar]
- [26].Cawein A, Emini E, Watson M, et al. , Human capital gaps in vaccine development: an issue for global vaccine development and global health, Annals of the New York Academy of Sciences (2017). [DOI] [PubMed] [Google Scholar]
- [27].Allen A, Vaccine: the controversial story of medicine’s greatest lifesaver, WW Norton & Company; 2007. [Google Scholar]
- [28].Harrison JA, Wrong About Vaccine Safety: A Review of Andrew Wakefield’s “Callous Disregard”, Open Vaccine Journal 6 (2013) 9–25. [Google Scholar]
- [29].Godlee F, The fraud behind the MMR scare, BMJ 342 (2011) d22. [Google Scholar]
- [30]**.Mnookin S, The panic virus: a true story of medicine, science, and fear, Simon and Schuster 2011. An outstanding review of the rise of the recent MMR vaccine denier movement. [Google Scholar]
- [31].McPhillips HA, Davis RL, Marcuse EK, et al. , The rotavirus vaccine’s withdrawal and physicians’; trust in vaccine safety mechanisms, Archives of Pediatrics & Adolescent Medicine 155(9) (2001) 1051–1056. [DOI] [PubMed] [Google Scholar]
- [32].Nigrovic LE, Thompson KM, The Lyme vaccine: a cautionary tale, Epidemiology and Infection 135(1) (2007) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bishop JM, Nobel Lecture. Retroviruses and oncogenes II, Bioscience reports 10(6) (1990) 473–91. [DOI] [PubMed] [Google Scholar]
- [34].Bryan JT, Buckland B, Hammond J, et al. , Prevention of cervical cancer: journey to develop the first human papillomavirus virus-like particle vaccine and the next generation vaccine, Current opinion in chemical biology 32 (2016) 34–47. [DOI] [PubMed] [Google Scholar]
- [35].Beasley RP, Hepatitis B virus. The major etiology of hepatocellular carcinoma, Cancer 61(10) (1988) 1942–1956. [DOI] [PubMed] [Google Scholar]
- [36].C.f.D. Control, Prevention, Ten great public health achievements--United States, 1900–1999, MMWR. Morbidity and mortality weekly report 48(12) (1999) 241. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed herein have been made available to the scientific community and general public on the website of the Center for Research Innovation in Biotechnology (crib.wustl.edu). We actively encourage all interested parties to explore the data and identify any improvements or additions that might be of use for interested investigators.


