Abstract
The history of wild and cultivated plant diversity in Uganda is reviewed, taking forest species and bananas as examples. Palynological research into past human influences on forests is reassessed. The evidence suggests that crops were first introduced into the country at about 1000 BCE, farming communities practicing slash and burn agriculture started to significantly influence the floristic composition of forests during the 1st millennium BCE and there was a major episode of forest reduction at about 1000 CE related to socio-economic change. Bananas were probably introduced in the early centuries CE. The colonial era from 1894 saw the introduction of new concepts of land ownership and the establishment of forest reserves and agricultural stations. Forests and banana diversity are currently under threat, Uganda having a very high rate of deforestation and endemic banana varieties proving susceptible to introduced pests and diseases. It is suggested that, under these circumstances, conservationists take an opportunistic approach to field engagement, making use of favourable local conditions as they arise. Partnerships should be sought with elements of society concerned with sustainable use, provision of ecosystem services and cultural survival to widen the social base of plant conservation. International organisations involved in conservation of plant genetic resources and wild plant species should collaborate with one another to develop the conceptual basis of plant conservation, to make it more relevant to countries like Uganda.
Keywords: Ecosystem-based plant conservation, Pollen diagrams, Indigenous knowledge, Resource governance
1. Introduction
This paper presents an overview of the history of wild and cultivated plant diversity in Uganda, providing a platform for advancing suggestions for its conservation. Indigenous plant diversity is under great threat in Uganda today, with conservation hampered by many constraints. Uganda shares features with many other countries and hopefully the suggestions offered will be useful for them too.
The Green Revolution of the 1950s and 1960s dramatically increased the yields of some major crops, helping to forestall an anticipated global shortage of food. The introduction of genes resistant to pests and diseases from traditional varieties of crops was critical to this development, which, in turn, drew attention to the rapid rate of decline in the number of such varieties. It is estimated that 75% of the genetic diversity of agricultural crops was lost during the 20th Century (FAO, 1998, Hawkes et al., 2001). The International Board for Plant Genetic Resources (IBPGR) was founded in 1974 to coordinate an international programme to conserve plant genetic resources, concentrating initially on the landraces of major crops and the expansion of gene banks, notably seed banks and field collections (FAO, 1992).
The scope of the conservation movement concerned with plant genetic resources has widened over the years, coming to embrace wild relatives of crops, minor agricultural crops and other uses of plants additional to food (Prescott-Allen and Prescott-Allen, 1988). More emphasis is being placed on in situ conservation. Consequently, this branch of plant conservation has moved closer to the other school of plant conservation that has been developing over the same period, founded on concern about loss of species of wild plants, and associated with tools such as Red Data Books, protected areas and ex situ collections (Given, 1994, Hamilton and Hamilton, 2006). It is estimated that 20% of the world's 380,000 species of plants is threatened with extinction (Kew, 2012). The number of species of actual or potential conservation concern (from the perspective of the plant genetic resource movement) has thus been dramatically expanded, for instance now theoretically including the 50,000–70,000 species of plants estimated to be medicinal (Lange, 1997, Schippmann et al., 2006).
Greater specificity is provided here by paying special attention to species living in one particular type of plant community (rainforest) and to one particular crop (the banana). Forest species and forests (as collective entities) provide a wide range of useful products and ecosystem services in Uganda. The latter include regulatory services (such as climatic amelioration and soil stabilization), provisioning services (such as delivery of water supplies) and cultural services (having significant symbolic value) (Hamilton, 1984, Ray, 1991). Uganda has the highest per capita consumption of bananas in the world and is a secondary centre of genetic diversity for the crop (Daniells and Karamura, 2013-2014, Gold et al., 2002, Karamura et al., 2010, Karamura and Mgenzi, 2004). Bananas in Uganda are eaten steamed, roasted and raw, as well as brewed for beer. Matooke, a dish prepared by steaming and mashing cooking bananas, is the staple food of millions of people (Vernacular terms used here are from Luganda, an indigenous language.).
Uganda is a medium-sized country (area 236,000 km2) straddling the equator in the heart of Africa. It lies within the Great Lakes region of the western part of East Africa, with Lake Victoria to the south and Lakes Albert and Edward in the Albertine Rift to the west (Fig. 1). The population (36 million in 2012) is growing rapidly (3.27% p.a.) and becoming more urbanized (growth rate 4.4% p.a.). Despite urbanization, the primary means of livelihood for most people remains farming (73% of households in 2006–9), complemented by extensive use of wild plants for construction, crafts, fuel, medicines and other purposes. Much of the economy is centrally related to plants. The gross domestic product (GDP) was US $1404 in 2012 with most people financially very poor. Uganda is a culturally diverse country, having 42 indigenous languages classifiable into 4 major language groups. Many types of polity were present prior to the establishment of the Uganda Protectorate by Britain in 1894. English and Swahili are the official languages.
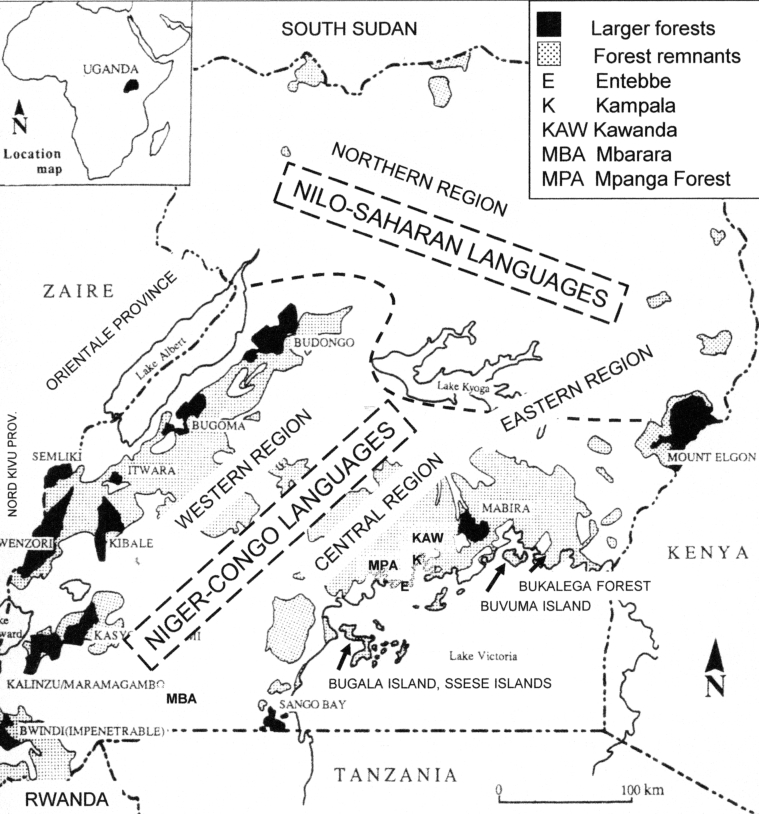
Fig. 1.
Locality map of Uganda.
Uganda is a signatory to the International Treaty on Plant Genetic Resources for Food and Agriculture (2004), the Convention on Biodiversity (1992) and the Global Strategy for Plant Conservation (2002). Official conservation tools include protected areas, ex situ collections and special legislative protection for some species.
2. Plant diversity, plant resources and agriculture in Uganda
Uganda offers an exceptionally wide range of habitats available for human exploitation (Schoenbrun, 1998). Rainforest is the natural vegetation in higher rainfall areas (about 20% of the land area), which lie mostly towards the west and north. Lowland rainforest grades into montane forest at higher altitudes, with woodland, bushland and other types of savannah in drier parts (Langdale-Brown et al., 1964). Forest clearance over the years has resulted in once continuous stretches of forest being reduced to scattered remnants embedded within matrices of cropland, secondary vegetation, swamps and urban areas. Much of the country outside forests and settlements is frequently burnt and grazed by livestock. Small-scale farmland covered 43.5% of the land area in 2005, large-scale farmland 4.8% and built-up areas 4.8% (FAO, 2010b).
The indigenous flora contains about 5000 species of higher plants (Davis et al., 1986). Tree floras report many species as having uses (Eggeling, 1952, Hamilton, 1991, Katende et al., 1995) and local inventories of medicinal plants can yield extensive lists (Adia et al., 2014, Galabuzi et al., 2015, Katuura et al., 2007, Lye et al., 2008, Tabuti, 2008, Tabuti et al., 2003). Intensive studies sometimes reveal uses for unsuspected species, suggesting that much ethnobotanical knowledge remains undocumented. The small forest trees Rytigynia kigeziensis Verdc. in Bwindi Impenetrable Forest and Belanophora coffeoides Hook. f. in Mpanga Forest were apparently unknown to scientists to have specific uses before research revealed that the first yielded a vital medicine used as a dewormer (“without this we will die”) (Cunningham, 1996) and the second to have been harvested methodically (carefully differentiated from several similar-looking species) for construction purposes (Taylor et al., 2008).
Many systems of plant use and management exist, embracing both cultivated and wild plants, and varying according to location, ethnicity, household wealth, and ownership of land and livestock. A farming household in the Central Region might, for instance, rely on a home garden (lusuku) to supply its staple food of cooking bananas, outfields (emisiri) for sweet potatoes, forest (ekibira) for firewood, taller grassland for fodder (essubi), swamp (ekisenyi) for papyrus (used in making mats) and sandy valley areas for the many products obtained from the wild date palm, including termite-resistant poles. Wild plants are often collected to sell. Unsustainable harvesting of wild plants is frequently reported, the most obvious problem being the cutting of trees to supply the Kampala market with fuel (firewood and charcoal). At least 90% of people in Uganda rely on woodfuel, 90% of trees cut for products being harvested for this purpose (Kabogozza, 2011).
Tasks in the supply and management of plant resources tend to be gender-related, with men more involved where money is to be made or when the end-product is alcoholic (Karamura et al., 2004). The collection of firewood for home use is overwhelmingly by women and children, but men dominate the commercial trade in charcoal. The lusuku, which serves mainly to supply subsistence products to the household, is almost exclusively the preserve of women (Karamura et al., 2004). Women also take the lead in the provision of food for the family and in maintaining its health, and are responsible for most craft-making, so are the main holders of indigenous knowledge of plants. A continuing interest in indigenous botanical knowledge is apparent from the retention of a diversity of local varieties of crops by some farmers (Mulumba et al., 2004, Zawedde et al., 2014) and the widespread use of herbal medicine (Cunningham, 1993, Hamilton and Aumeeruddy-Thomas, 2013, Lwanga, 1992). Anecdotal reports suggest that an interest in indigenous botanical knowledge is declining among the young, especially those from richer families.
The lusuku (commonly translated as ‘banana garden’ in English) is a type of indigenous agroforestry system that forms a key component of farms in the Central Region. It typically covers 22% of the 0.7 ha of cultivated land on an average farm (area 1.4 ha) (Edmeades et al., 2007). Bananas form a key component, but many other species can be present, having a variety of life forms, uses, degrees of domestication and intensities of management. Types of plants can include: (1) large trees, such as Albizia coriaria Welw. ex Oliv. (omugavu, timber, wood used to smoke barkcloth); (2) medium-sized trees, such as Spathodea campanulata P. Beauv. (kifabakazi, decorative and medicinal); (3) small trees and bushes, such as Coffea canephora Pierre ex A. Froehner (mumwanyi, producing coffee beans for the market); annual crops, such as kidney bean Phaseolus vulgaris L. (ebijanjaalo); (4) perennial crops, such as cocoyam Colocasia esculenta (L.) Schott (ejjuuni); (5) herbaceous plants, some semi-cultivated, such as spinach Amaranthus dubius Mart. ex Thell. (doodo) and (6) climbing and scrambling plants, such as Dioscorea yams (balugu, kyetutumula, etc.). The lusuku can have horizontal as well as vertical structure, for instance with types of bananas containing the B genome typically placed around the periphery and newly acquired varieties of bananas planted near the homestead, where the soil tends to be more fertile and performance easier to monitor (Karamura et al., 2004).
Bananas are classified scientifically into genome groups (based on the contributions of genes from two wild diploid species) and on ploidy. One genome (designated A) is from Musa acuminata Colla, native to western Melanesia and Southeast Asia, and the other (B) from Musa balbisiana Colla, native to Southeast Asia and China (Davey et al., 2013, Perrier et al., 2011). The earliest record of cultivation of bananas globally is from New Guinea (cultivated by at least 5000–4500 BCE) (Denham et al., 2003) Although the banana is an introduced crop in Africa, there are two genome groups that are indigenous to central or west equatorial Africa and that have diversified extensively. These are the East African Highland genome group (AAA), geographically centred on Uganda, and the Plantain genome group (AAB), centred in the rainforest zone of the Congo basin and represented in Uganda by roasting bananas known as gonja. The East African Highland genome group is conventionally labelled AAA-AE to distinguish it from other AAA groups. The total number of AAA-EA varieties in Africa has been estimated at ca. 60 and of Plantains ca. 120 (De Langhe et al., 1994-5). Conservation of the germplasm of the AAA-EA genome group is a particular concern for Uganda. A number of other genome groups (AA, AAA, AB and ABB), collectively known as the ‘Indian Ocean complex’, are thought to have been present in the coastal fringes of East Africa for some centuries, but did not penetrate into the Great Lakes area before 1900 (De Langhe et al., 1994-5).
Surveys of banana diversity on farms in Uganda have revealed the presence of a very large number of locally named varieties of AAA-EA bananas, though few of Plantain (possibly under-recorded) (Table 1) (Edmeades and Karamura, 2007, Gold et al., 2002). High levels of varietal diversity among AAA-EA bananas were recorded at both farm and village levels, the mean number of varieties per farm being 7.0 and 12.3 (in the two surveys respectively) and varieties per village 23 and 26. Some AAA-EA varieties were found to be present on many farms, but others narrowly confined. Thirty-five percent of the varieties recorded in one survey were present at only one or two sites (Gold et al., 2002). Local people have their own ways of classifying bananas, using traits such as the size and shape of various parts of the plant, the texture, flavour and colour of the food, and agronomic and commercial attributes (Karamura et al., 2011). Some varieties receive recognition for their medicinal or aesthetic properties, or have other special uses or meanings (Gold et al., 2002, Nantale et al., 2008).
Table 1.
Types of bananas found on farms in Uganda according to two surveys.
| Genome group or type | Genomea | Survey 1 (1993–1994)b |
Survey 2 (2004–2005)b |
Main uses | Origin and history | |||
|---|---|---|---|---|---|---|---|---|
| Frequency (% farms) | Proportion of plantsc | Commoner varieties (% farms) | Number of varieties identified | Commoner varieties (% farms) | ||||
| Tetraploid hybrids | AAAA AAAB AABB |
4 | Kawanda (4)d | All bred outside Uganda. | ||||
| Triploid hybrid | AAA | 1 | Received from IPGRI.e | |||||
| Gros Michel | AAA | 63 | 2 | Bogoya (60) | 2 | Bogoya (41)f | Dessert | Gros Michel (= Bugoya) introduced to Entebbe Botanical Gardens after 1900; first noted on Martinique (Caribbean) in 1830s. |
| Kamaramasengeg | AAB | 92 | 12 | Ndiizi (85)h | 1 | Ndiizi (61) | Dessert | Ndiizi introduced Entebbe Botanical Gardens after 1900; place of origin unknown. |
| Ney Poovan | AB | Kisubi (40) | 1 | Kisubi (28) | Brewing | Kusubi introduced Entebbe Botanical Gardens; probably originated in India. | ||
| Pisang Awak | ABB | 67 | 8 | Kayinja (63) | 2 |
Musa (16) Kayinja (14) |
Brewing | Pisang Awak and Bluggoe probably brought to East African coast from India by Arab traders possibly ca. 500 CE; probably introduced into Uganda by British in colonial times. |
| Bluggoe | ABB | 2 |
Kivuvu (14) Kidhozi (9) |
Multiuse | ||||
| East African Highland | AAA-EA | 100 | 76 |
Nakabululu (58) Mbwazirume (50) Nakitembe (49) Musakala (42) Enyeru (39) |
82i |
Nakyetengu (58) Nakubululu (44) Mbwazirume (37) Musakala (33) Kibuzi (33) |
Cooking (to make matooke) and brewing | Long present in Great Lakes region of East Africa (many endemic varieties); probably brought to East African coast by Indonesians (possibly later than ancestor of Plantains). |
| Plantain | AAB | 43 | 2 | Gonja (43) | 3j | Gonja (14) | Roasting | Long present in Central and West Africa (many endemic varieties); probably brought to East African coast by Indonesians. |
The wild species contributing the genomes are M. acuminata Colla (AA diploid) and M. balbisiana Colla (BB diploid).
Survey 1 (Gold et al., 2002); Survey 2 (Edmeades and Karamura, 2007).
This is the percentage of plants assigned to genome groups or types, averaged across farms.
Farmers call all these tetraploid hybrids Kawanda.
This triploid hybrid (Yangambi Km5) was received from the International Plant Genetic Research Institute (IPGRI) (Kikulwe et al., 2007).
The second Gros Michel type encountered in the survey was Bogoya Omumyufu (Red Bugoya), found on 3% of farms.
Taxonomy after (Onyango et al., 2011).
Assignment of Ndiizi to the Kamaramesenge subgroup is after (Pillay et al., 2003).
The number of varieties of AAA-EA bananas noted in the other survey (Survey 1) was 120.
The 3 types recognised in Survey 2 were Gonja, Majaga and Manjaya. Most Ugandans do not distinguish between different varieties of Plantains.
The AAA-EA genome group does not normally reproduce sexually. Therefore, its varietal diversification and geographical dissemination are attributed to other processes, including somatic mutation, the excision of suckers carrying the mutations by people, the planting of these suckers at new sites and (at some stage) recognition that the types are distinctive and worthy of being maintained (and named) in their own right (Karamura et al., 2010). Conservationists interested in finding ways to conserve the on-farm diversity of AAA-EA bananas need to understand how people interact with them. Research has revealed that new varieties introduced onto farms are typically sourced from family or friends, living in the same or other villages, and given freely without charge (Karamura and Mgenzi, 2004, Karamura et al., 2004). Criteria considered when selecting new varieties can include end-use attributes, resistance to pests and diseases, and suitability for specific environments. Special efforts can be made to retain rare varieties, even those especially susceptible to pests and diseases, have weak rooting systems or have limited ability to sucker (Mulumba et al., 2004). Measures include manuring, dusting the soil with ash, loosening the soil around the stools (to increase the infiltration of water into the soil) and continuous relocation (to reduce losses through weevils and nematodes).
Taxonomists working in Uganda have tried to develop a standardised system of nomenclature for bananas, assigning a unique name to each cultivar, thus bridging across languages and dialects and hopefully eliminating synonymy (Edmeades and Karamura, 2007, Karamura et al., 2011). This makes it easier to compare banana growing in different parts of the country and devise national strategies for the conservation of banana germplasm. The application of standard taxonomic approaches (based mainly on morphological features) has shown that AAA-EA bananas can be grouped into five major categories (‘clone sets’) (Karamura and Pickersgill, 1999), which, in turn, can be distinguished from bananas belonging to other genome groups (AB, AAB, etc.) (Karamura and Mgenzi, 2004). The names given to the clone sets are Mbidde, Musakala, Nakabululu, Nakitembe and Nfuuka. Of these, Mbidde and Nakitembe are particularly distinctive from a standard taxonomic perspective, which, it has been suggested, is because they have been recognised and deliberately propagated for exceptionally long periods of time. However, DNA analysis has shown that the various varieties included in the Mbidde clone set are not all closely related genetically, their distinctiveness being rather due to mutations at a single locus giving them the ability to synthesise tannins and anthocyanins (and thus produce astringent sap, the quality appealing to beer brewers) (Tugume et al., 2002). Among the other four clone sets, Musakala is particularly distinct genetically, but Nfuuka, Nakitembe and Nakabululu are closely related. A characteristic of the Nfuuka clone set is high susceptibility to morphological change (hence the name in Luganda, which means ‘I change’).
3. Origin and history of plant diversity in Uganda to 1900
3.1. Forests and forest plants before agriculture
Comparison of DNA sequences has demonstrated that some African forest-dwelling species of Afromomum, Begonia and Erythrophleum originated in forest refugia that were isolated from one another during arid periods of the Quaternary (Duminil et al., 2015, Harris et al., 2000, Sosef, 1994). Speciation in these cases occurred relatively recently by geological standards, estimated to have been during the last tens of thousands to hundreds of thousands of years. Other DNA analyses, carried out on groups of related species in the families Annonaceae and Rhamnaceae, have revealed that their last common ancestors lived 4–28 and 19–33 million years ago respectively (Richardson et al., 2004).
The diversity of plant germplasm found at any place today is the product of many processes, among which climatic change is typically a major contributor. Some of the main climatic events believed to have been influential in moulding the composition of the modern forest flora of Uganda are listed on Table 2. Greater detail is given towards the present, since relatively minor climatic events become increasingly important for determining modern distributions as the present approaches. Tropical Africa had a much wetter climate than now at the beginning of the Miocene Period (23 My BP), with rainforest very extensive. Progressive desiccation through the Miocene and Pliocene (23–2.6 Myr BP) led to forest retreat and the extinction of species, contributing to the relative poverty of the modern flora of tropical Africa relative to South America and Asia (deMenocal, 2014, Plana, 2004). A consequence of the progressively drying climate of tropical Africa was a rise to prominence of grasses in the flora, with savannah expanding at the expense of forest. Grasses have been present in the flora of tropical Africa since the Palaeocene (66–56 Myr BP), but only began to increase in abundance after 16 Myr BP and more so from 8 Myr (Jacobs, 2004), with extensive areas of grassland, as found today in Serengeti (Tanzania), only existing after 3 Myr BP (deMenocal, 2014).
Table 2.
Some major climatic events since 23 Myr BP that have moulded the floristic diversity of modern forests in Uganda.
| Time perioda | Climate | Rainforest |
|---|---|---|
| Last few centuriesb | Many climatic fluctuations, some geographically widespread, others apparently more local. | The influence of climatic events on rainforest in Uganda is difficult to discern against strong human influence on the vegetation. |
| From 4000 to 3500 BPc to the present | Drier than previously (but still wet compared with ice age aridity). | There was a transition to drier forest types at ∼4000–3500 BP in Uganda, experienced at all altitudes. |
| From 12,500 to 10,000 BPd to the present | Warmer and much wetter than previously. | The extent of forest expanded greatly in Uganda at ∼12,500–10,000 BP. |
| From 2.6 Myr BPe to the present | Marked climatic fluctuations in Africa, especially after 800,000 BP. | There were major contractions and expansions of forest in tropical Africa driven by the fluctuating climate. Differentiation and extinction of populations of forest species. |
| 23–2.6 Myr BPf | Climate initially much wetter than now across tropical Africa, becoming progressively drier. | Forest was initially more extensive than now in tropical Africa, then retreating with species being lost. |
Dates are in years before present (BP), those based largely on radiocarbon dating (younger than ∼40,000 BP) being in 14C years before 1950 CE. Dates given in calendar years (BCE or CE) elsewhere in this paper are in calendar years, transposed from 14C years where necessary (Reimer et al., 2009).
The most detailed climatic records for the last ∼1000 years reveal fluctuations in climate of short to medium term duration (decades to centuries) (Ryves et al., 2011, Ssemmanda et al., 2005). A dry phase at ∼1750–1850 CE has been detected widely across East Africa. Similar short-term climatic fluctuations are likely to have occurred at all times.
A mid-Holocene shift to a drier climate has been widely recorded across equatorial and northern Africa, with the abruptness of the transition debated (McGlynn et al., 2013, Tierney et al., 2011). The date of ∼4000–3500 BP given here (equivalent to ∼2050–1850 BCE in calendar years) is one quoted in regional reviews (Hamilton, 1982, Hamilton, 1992, Jolly et al., 1997, Kiage and Liu, 2006). A notable feature seen in many pollen diagrams from Uganda is a rise in the very well dispersed pollen type Podocarpus (produced by the gymnosperm genera Afrocarpus and Podocarpus).
There is much evidence for a major transition from a relatively cool dry climate prevailing across equatorial Africa during the last global ice age (peaking at 18,000 BP) to warmer and much wetter conditions thereafter (the postglacial). The date of this transition given here is based on assessments of the pollen evidence for East Africa as a whole or parts thereof (Hamilton, 1982, Hamilton, 1992, Jolly et al., 1997, Kiage and Liu, 2006).
This is the Quaternary Period, marked by a series of ice ages in temperate parts of the world.
Several publications discuss climatic change during this period and its effects on the flora (Hamilton and Taylor, 1991, Harris et al., 2000, Jacobs, 2004, Plana, 2004, Sosef, 1994).
The spread of savannah at the expense of rainforest was significant for the evolution of the human, whose ancestors adopted an upright stance and started to live more on the ground and less in the trees from about 4 Myr BP (Tattersall, 2014). Human antecedents and their bipedal cousins shared the expanding savannah with a number of other animals, such as bovids, possibly influencing them in their courses of evolution, which, in turn, could have had knock-on influences on the evolution of some plants. The controlled use of fire by human ancestors, probably accomplished prior to 400,000 BP, is likely to have led to more fires in the savannah, increasing the relative abundance of more fire-resistant plants.
The Quaternary Period (2.6 Myr to the present) has been a time of major climatic fluctuations in many parts of the world, a series of glaciations in temperate regions being generally marked by relatively cool arid times in tropical Africa (Rossignol-Strick, 1983, Rossignol-Strick et al., 1982). A consequence for Africa has been the repeated contraction and expansion of forest, resulting in the isolation of populations of some forest species in two or more forest remnants during the drier periods. Such populations have sometimes become evolutionarily differentiated, speciation sometimes resulting (Diamond and Hamilton, 1980, Hamilton, 1988, Plana, 2004). The peak of the last global ice age (18,000 BP) saw very little forest remaining in Uganda (Hamilton, 1982) with Lakes Victoria and Albert at much lower levels than today (Beuning et al., 1997, Kendall, 1969). The transition to the warmer and wetter postglacial climate occurred over the period 12,500–10,000 BP, since when the climate has been relatively wet by ice age standards, though somewhat drier from 4000 to 3500 BP (equivalent to 2050–1850 BCE in calendar years; see caption to Table 2 on how dates are expressed in the present paper). The wetter climate since 12,500–10,000 BP caused a great expansion in the area of Uganda climatically suitable for rainforest, many rainforest species responding by expanding their ranges eastwards from a former ice-age refuge centred in Kivu in the Democratic Republic of Congo (DR Congo). Increased evaporation from an enlarged Lake Victoria would have helped sustain precipitation in a zone to the north of the lake, as is the case today. A consequence of this forest expansion was the development of a gradient of decreasing number of forest species from west to east, related to their differential abilities to spread. Birds, primates, butterflies and trees all share this pattern (Hamilton, 1975, Hamilton, 1976, Howard, 1991). Other factors being equal (such as climate and altitude), the forests with the greatest number of species in Uganda are those nearer to the former Kivu refugium, such as Bwindi-Impenetrable and Semliki.
Until about 1000 BCE, the people living in the Great Lakes region lacked agriculture or domestic stock and relied on hunting, fishing and the gathering of wild foodstuffs for their subsistence. These people were likely speakers of Khoisan languages, which are today spoken mainly in Botswana and Namibia, with relict outliers in Tanzania (Ehret, 1998). The knowledge of wild plants of these people is likely to have been exceptionally great, judging by analogy with modern-day hunter-gatherers, and it is possible that some of their knowledge has passed down through cultural strands to the present-day inhabitants of the region. The Batwa (‘pygmies’) living near Bwindi-Impenetrable forest, the people most orientated towards the forest environment in Uganda, have an exceptional knowledge of nature (Byarugaba, 2008). The Batwa in Uganda speak Bantu languages.
3.2. Agriculture and crops
Linguistic research has demonstrated that speakers of at least two language families, Nilo-Saharan and Niger-Congo, contributed to the development of early agriculture in Uganda (Ehret, 1998, Ehret, 2011, Schoenbrun, 1993b, Schoenbrun, 1998). Speakers of yet another language family (Afroasian) may have been present in the Great Lakes region at the relevant time and may have had agriculture. The Nilo-Saharan and Niger-Congo speakers (represented by its Bantu sub-group) practiced different forms of agriculture. The former, living in drier parts, grew the cereals sorghum (Sorghum bicolor (L.) Moench), pearl millet (Pennisetum glaucum (L.) Br.) and finger millet (Eleusine coracana Gaertn.), all earlier domesticated further north in Africa, as well as sesame (Sesamum indicum L.), bambara groundnut (Vigna subterranea (L.) Verdc.) and cowpea (Vigna unguiculata (L.) Walp.). Their domestic animals included cattle, goats and sheep (all earlier domesticated in the Middle East or northeast Africa) and they knew how to work iron. In contrast, the Bantu speakers used a planting (contrasted with sowing) form of agriculture, more suited to growing crops in clearings made in rainforest, just as their ancestors had been doing for generations. The presence of loan words of Nilo-Saharan origin in the modern Bantu languages of the Great Lakes region shows that the Bantu-speaking people acquired knowledge of the growing of cereals, the keeping of cattle and how to work iron from Nilo-Saharan speakers. Linguistic research further indicates that the localities of these cultural transfers lay to the southwest (later, within the southwest) of Uganda and their timing to the first millennium BCE (Ehret, 1998).
Today there are about 300 Bantu languages in Africa spread throughout much of its east, centre and south. All are descended from a common ancestral language (Proto-Bantu), spoken at ∼3000 BCE close to the present-day border between Nigeria and Cameroon (Derek and Philippson, 2003). Comparative linguistics has allowed some reconstruction of the way of life of the Proto-Bantu, suggesting that they practiced agriculture in small clearings made in rainforest, interspersed with long periods of forest fallow (Ehret, 1982, Ehret, 1998). Yams (Dioscorea) constituted their staple food and they also grew bambara groundnut, another legume (probably cowpea), castor bean (Ricinus communis L.) and gourds (Lagenaria siceraria (Molina) Standl.). The oil palm (Elaeis guineensis L.) and the raffia palm (Raphia monbuttorum Drude) were planted or wild-nurtured.
Crops grown today in Uganda have diverse origins. Several were first domesticated in Africa, such as those mentioned above. Domesticates originally from Asia include the banana, cocoyam (C. esculenta (L.) Schott.), sugarcane (Saccharum officinarum L.), water yam (Dioscorea alata L.) and Asian rice (Oryza sativa L.). America has contributed sweet potato (Ipomoea batatas (L.) Lam.), kidney bean (P. vulgaris L.), cassava (Manihot esculenta Crantz), peanut (Arachis hypogaea L.) and maize (Zea mays L.). Linguistic studies provide evidence for the dates of arrival in the Great Lakes region of some of these crops (Ehret, 2011). Maize, kidney bean and peanut were present by ∼1800 CE, but Asian rice and cassava came later, the former possibly with Swahili trading caravans (from ∼1840) and the latter possibly not until colonial times, when its adoption was promoted as a famine crop. The introduction of new crops has caused the extensive displacement of indigenous crops of comparable dietary or ecological properties. Thus, the banana has substantially replaced Dioscorea yams, peanut the bambara groundnut, and kidney bean the cowpea. Substitution is continuing today with maize extensively replacing finger millet (which is labour-demanding).
Fossils provide sporadic insights into the history of crops. Impressions of pseudostems (‘trunks’) of the family Musaceae are preserved in slag associated with iron furnaces of Later Iron date, mostly dating to the 18th or 19th centuries (Fig. 2) (Iles, 2009). They are thought likely to be from banana plants, but Ensete is a possibility (see below). A phytolith of maize has been identified in sediments dated 1780 CE at Munsa (Lejja et al., 2005). Pollen of the castor bean has been found in sediments dating from 1300 CE onwards in Lake Wandakara and the same sediments contain a rise in abundance of pollen of the wild date palm (Phoenix reclinata Jacq.) from 1750 CE (Ssemmanda et al., 2005). The castor bean is a plant long cultivated (or at least wild tended) in Africa, while the wild date palm, though not cultivated in Uganda today, is nevertheless a plant of great economic value, supplying many products. Possibly Phoenix was encouraged through removing competing plants. Pollen grains of sorghum and finger millet have been identified from secure (i.e. non-contaminated) Early Iron Age archaeological sites in Rwanda, dated respectively to the 3rd–4th and 5th–6th centuries AD (Van Grunderbeek and Roche, 2007).
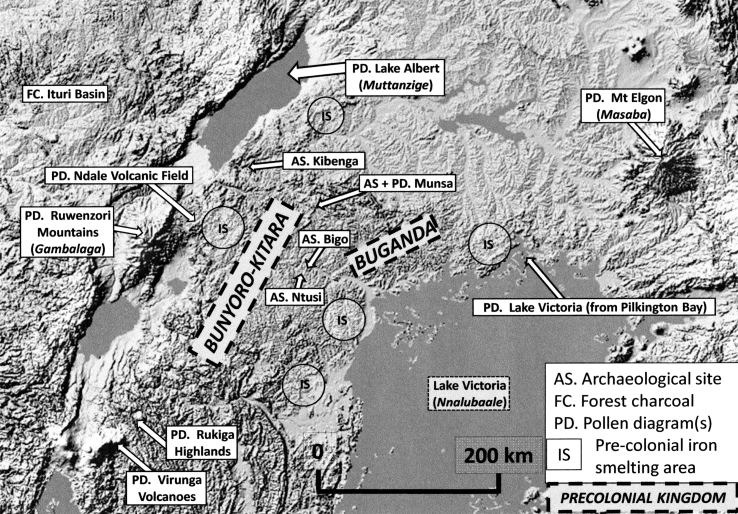
Fig. 2.
Archaeological and other sites in Uganda yielding evidence of pre-1900 AD influences of people on forests. Iron smelting areas after (Iles, 2009). The Luganda names of some geographical features are given in italics.
The conventional story of the banana in Africa is that it was one of a suite of crops introduced from Southeast Asia to the coastal fringes of East Africa during the first millennium CE, later carried inland and then widely dispersed. People from Southeast Asia were certainly capable of trans-oceanic travel at the time, given that the language of Madagascar (Malagasy) has its closest modern relative in Borneo and the earliest human occupancy of Madagascar dates to the first few centuries CE. Types of bananas may have arrived in waves, first the ancestral stock of Plantains, then that of the AAA-EA bananas and finally the varied members of the Indian Ocean Complex (De Langhe et al., 1994-5). The country inland of the East African coast is too dry for moisture-loving crops like the banana, but there are passages of easier penetration, such as up the Ruvuma valley to Malawi and then up the highlands along the western Rift Valley to Uganda (Wrigley, 1989). It has been suggested that the banana may have become known to Bantu-speaking farmers in the Great Lakes region by 500–900 CE (Schoenbrun, 1993a), possibly becoming popular as a nutritional and ecological replacement for Ensete (De Langhe et al., 1994–5). Ensete is a banana-like plant that, unlike the banana, is indigenous to Africa and can be eaten. However, it is not the fruit of Ensete that is eaten, but rather the root-stock, stem of the fruit bunch, the young tender leaves inside the pseudostem and sometimes the seeds (ground into flour). Although apparently not eaten in Uganda today, Ensete ventricosum (Welw.) Cheesman (ekitembe), the locally occurring species, is reported as being eaten historically (Thomas, 1940). Ekitembe is valued in modern Uganda as a source of medicine and for its seeds (empiki, used as counters in the game of omweso), and as a decorative addition to courtyards. Elsewhere in eastern Africa, E. ventricosum provides the staple food in parts of Ethiopia.
There is an alternative hypothesis about the history of the banana in Africa, proposing a much earlier presence. Evidence quoted includes the existence of a large number of varieties of Plantain in the forest zone of central Africa, a reconstructed root word (*kondo) for Plantain in Proto-Bantu and phytoliths of bananas (Musa) found in archaeological settings at Nkanga in Cameroon (dating to the 1st millennium BCE) and Munsa in Uganda (dating to the 4th millennium BCE) (Blench, 2009, De Langhe, 2007, Lejju et al., 2006, Mbida et al., 2001). Objections have been raised on several accounts. The existence of a large number of named varieties of a crop does not necessarily indicate great antiquity, as shown by the morphologically mutable Nfuuka clone set of AAA-EA bananas in Uganda (Tugume et al., 2002). No detailed analyses of the historical implications of banana terminology are included in authoritative works on comparative Bantu linguistics (Ehret, 1982, Ehret, 1998, Schoenbrun, 1998). The identification of the phytoliths has been questioned, as well as the dating in the case of Munsa (Newmann and Hildebrand, 2009).
3.3. Human influences on forests before 1900
Charcoal is common in soils under rainforests in tropical Africa, mostly interpretable as originating through small-scale slash and burn agriculture (Vleminckx et al., 2014). One site investigated, 200 km to the west of Uganda, is the Ituri basin (DR Congo), where studies of the anatomy of the charcoal have revealed remarkable differences between the modern flora and that which the charcoal represents (Hart et al., 1996). Only one species (Cynometra alexandri C.H. Wright) is abundant in both cases, 14 of the 36 species identified in the charcoal are absent from the forest today and one of the modern forest dominants (Gilbertiodendron dewevrei (De Wild.) J. Leonard) is completely absent from the charcoal record. Radiocarbon dating of 28 samples of the charcoal gave a spread of dates between 2850 BCE and recent, though with only 3 dates older than 200 BCE. The period 200 BCE to the present is fairly evenly covered by the radiocarbon dates, which, together with their pattern of geographical distribution, suggests that burning was a continuing phenomenon, though of limited extent on each occasion. This is just as would be expected for the type of agriculture reconstructed for the early Bantu. Analyses of forest charcoal from the western side of the central African rainforest support the hypothesis of slash and burn agriculture for much of its origin, but here there are hiatuses in agriculture not found at Ituri, at 450–1200 CE in Gabon and 550–1650 CE in Cameroon (Oslisly and White, 2007, Vleminckx et al., 2014).
The evidence from Ituri suggests that people caused major changes to the floristic composition of the rainforest. A similar conclusion has been reached from studies of botanical remains at archaeological sites in Gabon, the researchers further commenting that the people may have influenced the floristic composition of the forest in three ways, the inadvertent encouragement of species adapted to establishment in gaps following agriculture, the nurturing of valued species and possibly planting (Oslisly and White, 2007). Endocarps of oil palm (Elaeis guineensis Jacq.) and Canarium schweinfurthii Engl. are common at archaeological sites in Cameroon and Gabon showing that they were prized by the early Bantu. Canarium (omuwafu) is a large tree held in high esteem in the Central Region of Uganda today, yielding edible fruits (empafu), resin used as incense (obubaane) and valued for its statuesque form and gentle shade-giving qualities. It is common in banana gardens, but rarely seen in dense forest.
The identification and counting of fossil pollen contained within sediments of Upper Quaternary age (last 40,000 years) have allowed the reconstruction of the environmental history of parts of Uganda. This involves the reconstruction of past vegetation from the pollen spectra and then the reconstruction of past environmental variables from the reconstructed vegetation, processes aided by knowledge of the modern pollen rain and the ecology of the parent taxa of the pollen types (Hamilton, 1972, Hamilton and Perrott, 1980, Hamilton and Perrott, 1981). Considerable uncertainties remain and therefore new interpretations may arise as new information comes to light. Distinguishing between the influences of past climates and people on the vegetation can be problematic, especially during those earlier years when relatively low-impact agriculture was (or might have been) practiced (Jolly et al., 1997, Kiage and Liu, 2006). Linguistic evidence indicates that agriculture could have been in Uganda before 1000 BCE.
Fig. 2 shows the localities for which pollen diagrams of Upper Quaternary age are available. Sites yielding pollen diagrams showing (or possibly showing) past human influence on forest are listed on Table 3 with notes in Appendix 1 explaining how this has been inferred. Sites are organised on Table 3 according to their geographical distribution and interpretive qualities. Sites at high altitudes and large lakes can be especially useful for reconstructing landscape-level changes in climate and human influence, since they sample the pollen rain originating from extensive areas (Hamilton, 1972, Hamilton and Perrott, 1980). Pollen diagrams from closely-spaced localities can be useful for differentiating local factors influencing past vegetation from those that were more widespread (Lejja et al., 2005, Ryves et al., 2011).
Table 3.
Sites of pollen diagrams from Uganda providing evidence (or possible evidence) of past influence of people on forest. Dates in calendar years, calibrated where necessary from radiocarbon dates (Reimer et al., 2009). Further explanatory notes in Appendix 1.
| Site and altitude (m) | Type of site | Immediate surrounding landscape | Past human influences |
|---|---|---|---|
| |||
| Muhavura, Virunga Volcanoes, 4127 ma | Summit crater lake | Afroalpine vegetation | Widespread forest clearance around mountains, very well dated at ∼1000 CE. Peaks in charcoal in the sediments accompany the palynological indicators of forest clearance. Floristic composition of montane forest or woodland altered at ∼1000 CE. The same results for the two sites inspires confidence. |
| Gahinga, Virunga Volcanoes, 3474 ma | Summit crater bog | ||
| Kitandara, Rwenzori 3990 mb | Lakes | Afroalpine vegetation | Widespread forest clearance in lowlands around mountains at ∼1000 CE (the date is interpolated from considerably older radiocarbon dates). Forest clearance greater to the east than the west. |
| Bujuku, Rwenzori 3920 mb | |||
| Mahoma, Rwenzori 2960 mb | Bamboo forest | ||
| |||
| Muchoya, 2260 mc,d | Valley swamps and a lake (Bunyonyi) | Bamboo forest | Signs of forest disturbance from ∼225 BCE, more so from ∼1250 CE. Some forest always remaining. Change in swamp vegetation during first millennium CE. |
| Mubwindi, 2100 me | Broadleaved forest | Slopes around swamp forested throughout. Signs of human disturbance from ∼1650 CE. | |
| Katenga, 1980 mf | Small-scale farming, scrub, pasture | Major forest clearance with soil erosion and swamp siltation. Two episodes of forest disturbance at Ahakakyezi, ∼1700–750 BCE and ∼700 CE-present; soil erosion with second episode; final clearance of ridge forest at ∼1150 CE. | |
| Bunyonyi, 1950 mf | |||
| Ahakagyezi, 1830 md,g,h | |||
| |||
| Kabata, 1370 mi | Crater swamp | Small-scale farming, grassland; forest nearby | Some opening up of forest at ∼1600 CE; possible human disturbance earlier (sometime between 1350 BCE and 400 CE). Spread of papyrus over a lake sometime between 400 and 1400 CE. |
| Kasenda, 1260 mj | Crater lakes | Forest around sites replaced by grassland, well dated at ∼900–1000 CE. Some shrub and forest tree regrowth from ∼1700 to 1750 CE (especially from late 1800s). | |
| Wandakara, 1170 mj | |||
| |||
| Munsa, 1220 mk | Papyrus swamp | Small-scale farming, grass, forest patches | Forest clearance well dated at ∼1100 CE with soil erosion and swamp siltation. Economy established with cereal cultivation, cattle-keeping, iron-smelting. Some forest recovery from ∼1780 CE. |
| |||
| Pilkington Bay, Lake Victoria, 1134 ml | Huge lake | Farmland, savannah, forest | Major forest clearance, well dated at ∼1 CE, shown by a major decrease in the ratio of forest to grass pollen. Accompanied by changes in wetland vegetation. Absolute decline in forest tree pollen starts earlier, sometime between 1750 and 1250 BCE. |
The most striking evidence of past human influence on forests, evident in several pollen diagrams, is an episode of major clearance of forest at ∼1000 CE (range 700–1250 CE). All the pollen diagrams showing this event are from sites in mid- to southwest Uganda, a geography that reflects the distribution of sites studied and not necessarily the area to which forest clearance was confined. The pollen spectra, combined with other characteristics of the sediments, show that soil erosion sometimes accompanied the forest clearance, influencing wetland vegetation through siltation.
The date of ∼1000 CE marks a significant transition in the archaeological record of the Great Lakes region, from the Early to Later Iron Age and with the pottery type changing from Urewe to rouletted ware. The onset of the Later Iron Age is dated archaeologically to the 9th century CE in north-east Rwanda and to between the 11th and 14th centuries at Ntusi, Munsa and Bigo, sites of large-scale earthworks constructed during the Later Iron Age in western Uganda (Fig. 2) (Schoenbrun, 1998). New types of political structures with greater social stratification are believed to have emerged (Lejja et al., 2005). Oral tradition in Buganda traces the present Kintu dynasty back to a founding monarch (Kabaka Kato Kintu), who is estimated to have reigned at 1200–1230 CE (Nuwagaba, 2014). A contributing factor to social and political change may have been the acquisition of more productive forms of food production, based on the development of intensive banana gardening around Lake Victoria and large-scale cattle-based pastoralism in the region that later became the kingdom of Bunyoro-Kitara to the west (Schoenbrun, 1993a, Schoenbrun, 1998). Oral tradition in Buganda further states that the people who previously occupied the land that became Buganda were known as the Balasangeye (meaning ‘they shoot at colobus monkeys’) and that they were wanderers lacking fixed abode (Nuwagaba, 2014). This seems a reasonable description of a people practicing shifting agriculture and hunting animals in rainforest.
In addition to the major forest clearance event at ∼1000 CE, some earlier and later human influences on forests can be detected in the pollen diagrams. Taking a fresh look at the evidence (Appendix 1), it is suggested that the most persuasive signs of forest disturbance before ∼1000 CE are in pollen diagrams from Lake Victoria (forest clearance from ∼1 CE), Muchoya (forest disturbance from ∼225 BCE) and Ahakagyezi (forest disturbance phase ∼1700–750 BCE). These early human influences on forests were relatively subdued compared with the obvious ∼1000 CE event and seemingly do not warrant the degree of detailed reconstruction of past human influences previously made (Schoenbrun, 1994, Taylor et al., 1999). On the other hand, the pollen evidence broadly substantiates the story of the influence of early agriculturalists on forests reconstructed from linguistic and charcoal analyses, that is, that their long-fallow type of forest agriculture caused rather little disruption to the overall physical structure of the forests.
The introduction of intensive banana gardening is unlikely to be the reason for early forest disturbance at Muchoya and Ahakagyezi or the obvious major episodes of forest clearance seen at these and other sides in the Rukiga Highlands later (Table 3). This is because temperatures would probably have been too low. Bananas are grown around some of the lower altitude of these sites today, but such altitudes were considered too cold for bananas in the 1950s (Edel, 1957). The upper altitudinal limit of banana growing has risen in the Rukiga Highlands since 1970, similar to the upward movement of crops recorded for the East Usambara Mountains, Tanzania (Hamilton and Bensted Smith, 1989). Another biological change since the 1970s common to the Rukiga Highlands and the East Usambaras, is an upward movement of malaria. Possibly the Rukiga Highlands were attractive to early farmers because they were malaria-free.
Regarding events after ∼1000 CE, the pollen diagrams show that the more open landscape that had been created was generally maintained, though with some forest regrowth at Kasenda and Wandakara (from ∼1700 to 1750 CE, especially after 1800 CE) and Munsa (from ∼1780 CE) (Lejja et al., 2005, Ryves et al., 2011, Ssemmanda et al., 2005). Various factors may have been causative, including socio-economic change (Lejja et al., 2005). One factor known to have contributed to the spread of forest historically is disease. Large areas of southern Busoga and elsewhere were depopulated by a sleeping sickness epidemic at the beginning of the 20th century, leading to the substantial spread of colonising forest (Hamilton, 1984).
4. Plant diversity and plant resource management since 1900
4.1. New concepts of land ownership and resource rights
The relationship between people and plants in Uganda has changed greatly since 1900, consequent to major changes in society, economy and culture. The country came into existence through British colonialism, the first European visitors arriving in 1862, a Uganda Protectorate declared in 1894 and the boundaries of the modern state fixed by about 1914. A new concept of private ownership of land was introduced, contrasting sharply with the many types of customary tenure prevailing beforehand. Rights to land in Buganda were traditionally vested in the king (Kabaka) or heads of clans (Ab'akasolya), who then allocated it to their subjects or clan members (Lunyiigo, 2011, West, 1965). Land was not regarded as private property in the exclusive European sense. Rather, political and economic power was considered to be fundamentally related to people, not land, as summed up in the Luganda proverb: ‘Omwami tafuga ttaka afuga bantu’. (‘A chief does not rule land, he rules people.’).
The principal law governing land issues today is the Land Act (1998), which recognises four forms of land tenure – customary, leasehold, freehold and mailo (the latter applying only to Buganda – see below). The government also holds land in trust on behalf of the people of Uganda as a whole, including forest reserves and national parks. About 75%–80% of land holdings are unregistered customary properties, while most freehold land lies in the former administrative districts of Ankole, Toro, Kigezi and Bugisu (USAID, 2015). Provisions in the Land Act allow customary and leasehold land held by Ugandans to be converted into freehold, though, in reality, little customary land has been so converted because the procedures to obtain the required Certificates of Customary Ownership are so cumbersome (FOE, 2012). Holders of customary land can be vulnerable to losing their holdings.
A seminal event was the Uganda Agreement (1900) reached between the colonial administration and the Kingdom of Buganda. This parcelled up all the land of Buganda among several beneficiaries, the principal winners being the British Protectorate Government and a number of ‘chiefs and private landowners’ (whose land became known as mailo land) (Table 4). The losers were the traditional clan chiefs (abataka), whose claims to their customary land holdings were brushed aside, and the common people (abakopi), who became (and may remain) liable to summary eviction at the whim of legal landowners. However, the Uganda Agreement did contain some provisions permitting customary access on Protectorate Land, stating that, once forestry regulations had become defined, “the claims of the Baganda people to obtain timber for building purposes, firewood, and other products of the forests or uncultivated lands, shall be taken into account, and arrangements made by which under due safeguards against abuse these rights may be exercised gratis”. The Uganda Agreement was a hastily drawn-up document which has led to many subsequent complexities and disputes (Lunyiigo, 2011, West, 1965). It is likely that the British official responsible for its framing (Sir Harry Johnston) was motivated mainly by a desire to gain access to ‘unoccupied’ land for large-scale British settlement (which, in the event, never materialised), a goal achieved through granting excessive land privileges to the ruling classes.
Table 4.
Allocation of land in Buganda under the Uganda Agreement of 1900 (West, 1965).
| Beneficiary | Total area |
Components |
|||
|---|---|---|---|---|---|
| sq. mi. | km2 | sq. mi. | km2 | ||
| Protectorate Government | 10,550 | 27,324 | Forestsa | 1500 | 3885 |
| Waste and uncultivated landb | 9000 | 23,310 | |||
| Government stations | 50 | 129 | |||
| One thousand chiefs and private landownersc | 8000 | 20,720 | |||
| The Kabaka and other dignitaries | 958 | 2481 | Private property | 750 | 1942 |
| Land attached to their offices | 208 | 539 | |||
| Mission societies | 92 | 238 | |||
| Estimated total area of Buganda | 19,600d | 50,764 | |||
The Uganda Memorandum of Agreement (Forest) of 25th October 1907 defined the extent to which forest could be included in mailo land (the part allocated to ‘chiefs and private landowners’). In general, all patches of forest over 0.5 sq. mi. (1.3 km2) were to be assigned to the Protectorate Government.
The total area of ‘waste and uncultivated land’ became reduced to an estimated 8307 sq. mi. (21,515 km2) once the actual area of Buganda became better known and was reduced again to 6804 sq. mi. (17,622 km2) when the counties of Buyaga and Bugangazi (Bugangaizi) were transferred from Buganda to Bunyoro following a referendum in 1964. This land is sometimes known as mailo akenda (‘the nine thousand mailo land’) (Mutengesa, 2012).
The land distributed to ‘chiefs and landowners’ came to be known as mailo land (from English ‘mile’). It appears that the original intention of the British was that there would be about 1000–1030 recipients. However, distribution of mailo land was made the responsibility of the Buganda parliament (lukiiko) under the Uganda Agreement and land came to be distributed to many more people than the British had apparently intended. The first Allotment List had 3650 names, then, with yet more added, the total number of allotees rose to 4138 in the first authoritative list (1905). Many of these claims had little or nothing to justify them. Numerous transactions in mailo land then followed (many not officially documented), resulting in an estimated total of 100,000 holders of mailo land by 1963.
The total area of Buganda at the completion of the original mailo survey in 1936 was found to be 17,310 sq. mi. (44,833 km2). This resulted in a reduction in the area of land assigned to the Protectorate Government (in its position as recipient of the residue of the estate).
Lunyiigo has commented on the use of the words ‘waste and uncultivated land’ in the Uganda Agreement (Table 4) (Lunyiigo, 2011): “In a hunting and shifting cultivation economy it is not possible to describe land as either wasteland or unoccupied. These so-called waste and unoccupied lands were either hunting grounds or areas preserved to move into when land is exhausted elsewhere”. The idea that much of the land was ‘wild’, an adjective historically often applied to Africa by Europeans (Adams and McShane, 1992), is not born out by the ways that natural resources are regarded today (with plants from many types of habitat being used and managed, though with varying degrees of intensity) nor by the historical evidence (which shows that the extent and floristic composition of forests have long been influenced by people). Judging by analogy with other parts of the world (Pei, 2010, Pei et al., 2009), people would likely have practiced ‘indigenous conservation’, that is, systems of belief and related practices that would have tended to maintain the vital natural resources upon which they depended. Sacred groves, totemic animals and retention of particular specimens of trees have been noted in Uganda (Nuwagaba, 2014, Osmaston, 1968, Sembajjwe, 1995), but otherwise indigenous conservation has been little documented.
The concept of mailo land was not extended to other parts of Uganda, though two components of the Uganda Agreement were, namely the possibility of registering land as private property and the bringing of larger areas of forest under government control. The actual registration of forests in Buganda as the property of the Protectorate Government was slow, partly because unexpectedly large numbers of small forest patches were found to have come into private ownership. It had originally been estimated that the total area of forest on land assigned to ‘chiefs and private landowners’ was 260 km2, but a survey carried out in 1956–1960 revealed the true figure to be about 1550 km2 (Webster and Osmaston, 2003). Out of a total of 3885 km2 of forest intended to come under government control in 1900 (Table 4), only 1373 km2 had actually been registered by 1965.
The gaining of political independence by Uganda (1962) was accompanied, shortly before and after, by major changes relating to the disposition of land. A new agreement (the ‘new Buganda Agreement’) was forged between the British Government and the Kingdom of Buganda in 1961, transferring almost all land in Buganda then classified as Crown (or Public) Land out of the control of the Protectorate Government to the Kingdom of Buganda (Colonial Office, 1961). Some exceptions were made for the wider national interest, for example relating to control over land in towns. In essence, the land that became transferred was that which had been classified as ‘waste and uncultivated’ in the Uganda Agreement, plus all government-owned forests (except for those that lay within the Municipality of Kampala). Later, a political coup (1966) led to the abolition of the federal system of government prevailing beforehand (during both colonial and independence times) and the centralisation of all political power. Advisory services in agricultural and forestry were transferred to the central government and Local Forest Reserves (LFRs, see next section) became absorbed into the central forest estate.
Land issues remain contentious and there are numerous uncertainties and disputes (Lunyiigo, 2011, Rugadya, 1999, USAID, 2015). The expulsion of ‘Asians’ from Uganda in 1972 was accompanied by the declaration of an Economic War, an announcement interpreted by some that they were now free to settle anywhere they liked, including in forest reserves (Hamilton, 1984). The Land Act of 1998 granted responsibility for managing land resources to the districts, a step that resulted in many government forests becoming converted to agriculture or destructively harvested for charcoal (Kabogozza, 2011). The same act makes it possible to convert leasehold land to freehold. It was concern about this that reportedly led to the use of the word ‘license’ rather than ‘lease’ in agreements reached between forestry authorities and private sector operators following a change in policy in 2001 encouraging the outsourcing of forestry activities. However, legalistic caution seems to have arrived too late or to have been too ineffective to prevent the effective transfer of a substantial area of former forest reserves into private hands, as suggested by the virtual disappearance of Local Forest Reserves (LFRs) and a great expansion in the area of non-mailo private forest (see next section). ‘Elites’ (as they can be called) can be disproportionately advantaged over ordinary people in bargaining over land issues, having better access to finance, information, legal advice and decision-makers (Twongyirwe et al., 2015).
A growing trend has been the lease of land by the government to foreign investors, estimated to amount to 4%–8% of the country by 2011 (FOE, 2012). This is part of a global movement in which land, agriculture and forestry have come to be regarded as secure sectors for long-term investment. Land that is (or was until recently) covered by natural forest has been acquired by investors, as on Bugala Island and Mt Elgon, and in Bukaleba Central Forest Reserve (CFR). Disputes with local people are common (FOE, 2012, Lyons et al., 2014).
4.2. Forests and forestry
Colonial forestry was initiated in 1898 with the creation of a Scientific and Forestry Department, followed by establishment of a Forest Department (1917) and adoption of a formal forestry policy (1929) (Hamilton, 1984). This policy stressed the need to press ahead with the reservation of forests, so that they could be properly managed for the production of timber and the protection of ecosystem services, with climatic moderation and provision of water supplies specifically mentioned (Nicholson, 1929). Mountain forests were recognised as especially important for environmental protection and efforts were made to maintain all forests on mountains above an altitude of ca. 2150 m. A two-tier system of forest administration was developed, with larger blocks of forest declared Central Forest Reserves (CFRs), coming under the direct authority of the central government, and smaller forests made Local Forest Reserves (LFRs), falling under local governments and aimed at meeting local needs.
After Uganda gained political independence (1962), the local government of Buganda was active in enlarging its forestry estate, declaring 1373 km2 of new forest reserves during the period 1964–1966 alone (Hamilton, 1984). The total area of forest reserves (of all types) probably reached its maximum extent in 1966, at which time all larger blocks of rainforest were included in reserves, as well as sizable areas of wooded savannah. Many small plantations, mostly of introduced species, had been established.
Several species of trees have been introduced into Uganda for forestry purposes, notably eucalyptus (especially Eucalyptus grandis W. Hill ex Maiden, introduced 1912), cypress (Cupressus lusitanica Mill., used in the first conifer plantations in the country, established late 1940s) and pines (especially Pinus caribaea Morolet). The planting of exotic species has become increasingly emphasised in forestry over the years, for instance through an adjustment to forest policy in 1971–1973 (Lockwood Consultants Ltd., 1973), in a new forest policy in 2001 and a concentration on exotics in most carbon capture projects. It is hoped that these plantings will help meet projected shortfalls in the supply of wood and take pressure off indigenous forests, taking advantage of potentially rapid growth rates (Jacovelli et al., 2009). However, whether pressure is actually reduced on indigenous forest will depend on several factors, such as whether the types of people who currently support their livelihoods by harvesting products from indigenous forest to sell will also benefit financially from the planting of exotics.
Perhaps diverted by this concentration on exotics, less effective attention has been given to the management of natural forests and the planting of indigenous trees. Roadside nurseries often offer large numbers of seedlings of pines and eucalyptus for sale, but it can be hard to find seedlings of indigenous trees (Galabuzi et al., 2014). Ecosystem services have also become institutionally neglected, as epitomised in this quotation from the forest policy adjustment of 1971–1973: “there are secondary objectives such as the protection of water catchments, soils, wildlife and amenity of land. These however cannot be measured and are dependent on responsible behaviour by (Forest) Department officials in their provision” (Lockwood Consultants Ltd., 1973). The rate of deforestation, at 2.72% per year, has now become one of the highest in the world (FAO, 2010a, FAO, 2010b). According to the first chairperson of the board of the National Forest Authority (NFA) (see below) (Kabogozza, 2011): “there is no sustainable management of planted or natural forests in Uganda. … The government has not supported the forest sector with enough resources. … Also, given the fact that 64% of the current forests are on private land, and nothing is done in terms of forestry extension, it is not surprising that the highest degradation rate is found in these areas”.
There have been two studies of forest loss and degradation at the national level. The first, based on analysis of satellite imagery and examination of Forest Department records covers the period to 1982 (Hamilton, 1984). It found that the annual reports of the Forest Department recorded only minor infringements of forestry regulations in the years preceding 1970, but that there was evidence of alarming levels of forest loss and degradation thereafter. The second, a National Biomass Survey, based on analysis of satellite imagery backed up by sample plots, found that 9% of CFRs and 43% of LFRs had become completely deforested by 2002 (Drichi, 2002). (No differentiation is made in these percentages between forest reserves covered by rainforest and those covered by other woody vegetation types.) Appendix 2 provides examples of forest loss and degradation since 1970.
The areas of rainforest under different forms of ownership in 2011 were reported as: National Forest Authority (NFA, 2836 km2), Uganda Wildlife Authority (UWA, 2510 km2), private (2344 km2), joint NFA/UWA (2346 km2) and LFRs (2.43 km2) (Kabogozza, 2011). The figure for LFRs is startlingly low, considering that the total area of LFRs was reported to be 3028 km2 in 1965, of which 1036 km2 was rainforest (Webster and Osmaston, 2003). Confirmation that there has been a drastic reduction in the total area of LFRs is provided by another figure (50 km2), published in 2010 (LTS, 2010). The high figure for private forests also merits comment. According to interviews with senior foresters (see Acknowledgements), very little of this is on the mailo land that was allocated to ‘chiefs and private landowners’ under the provisions of the Uganda Agreement in 1900, because most of the considerable area of rainforest that once stood on such land has by now been destroyed. Rather, most of this forest is either on land in Buganda that was assigned to the Protectorate Government under the Uganda Agreement and/or are former Forest Reserves that have become private property (or, at any rate, have become so regarded).
Forestry has been institutionally unstable over recent decades (Table 5). There were two episodes of complete decentralisation of the forest estate during the 1990s, followed by total or partial recentralisation. It has been implied that this was related to competing arguments about the best administrative structure to manage natural resources (Banana et al., 2007), but actually, according to interviews, the decentralisations (to district level) were nearer to accidental by-products of major political events (general decentralisation of government 1993; new constitution 1997) with forestry given no particular consideration. In any case, the decentralisations were not made with reference to the ethnic geography of the country (Nsita, 2005). The number of districts, the pivotal unit of local government, has been multiplying, from 56 in 2002 to 111 in 2015.
Table 5.
Institutional history of forestry in Uganda.
| 1898 | First director of a new Scientific and Forestry Department appointed. |
| 1917 | Forest Department created. |
| 1929 | First formulation of forest policy, concentrating on forest reservation for environmental protection and timber production. |
| 1929–1951 | Large forests made Central Forest Reserves (CFRs) under the central government and small forests made Local Forest Reserves (LFRs) under local governments. (However, a survey in 1956–1960 found that a considerable area of forest in Buganda had come to fall under private hands.) |
| 1967 | CFRs and LFRs merged into the unitary category of Forest Reserves under the central government. |
| 1971–1973 | Forest policy adjusted favouring enlargement of conifer plantations for volume wood production. Little emphasis given to natural forest, either for its protective functions or for productive purposes. |
| 1993 | All forest reserves decentralised to local government, except five of the larger forests (Bwindi-Impenetrable, Elgon, Kibale, Mgahinga, Rwenzori) which were made national parks and transferred to the Uganda Wildlife Authority. |
| 1995 | All forest reserves recentralised. |
| 1997 | All forest reserves decentralised. |
| 1998 | Forest reserves over 100 ha recentralised (and labelled CFRs); forest reserves under 100 ha remaining with local authorities (and labelled LFRs). |
| 2001 | New forest policy agreed, emphasising a greater role for the private sector in forestry operations. |
| 2003 | Forest Department replaced by: (1) National Forest Authority (NFA), responsible for CFRs; (2) District Forest Services (DFS), responsible for LFRs and advice to private forest owners; (3) Forest Sector Support Department (FSSD), responsible for coordination and regulation. |
| 2006 | Board members of NFA resign or summarily dismissed, relating to conflict with the government over allocating parts of Mabira CFR and forests on the Ssese Islands to investors. New board members appointed. |
The Forest Department itself was abolished in 2003 and replaced by three new bodies, National Forest Authority (NFA), District Forest Services (DFS) and Forest Sector Support Department (FSSD). The idea was that NFA would manage CFRs, DFS would manage LFRs plus provide advice to private forest owners, and FSSD would be a coordinator and regulator, without line responsibilities. NFA was created as a semi-autonomous entity, making it free to keep its own revenues, so potentially less dependent on the vagaries of government funding. Government support for forestry had declined greatly during the previous decade, one study in Mpigi District finding that funding had been cut by 89% and staffing by 68% over 1993–1995 (Banana et al., 2007).
A subsequent review of the forestry sector found that support for DFS and FSSD never materialised and that these institutions have been ineffective (LTS, 2010). In contrast, NFA was initially put into operation effectively, though soon constrained by a presidential ban (2005) prohibiting the eviction of encroachers from CFRs. The number of such encroachers stood at 180,000 in 2005 (officially registered), mushrooming to an estimated 270,000 by 2010. The original Board of NFA resigned or was replaced in 2006, reportedly due to its opposition to government plans to hand over certain areas of forest reserves for oil palm or sugarcane planting (BirdLife International, 2008, Nakkazi, 2011, Tenywa, 2005, Tenywa, 2013, van Schaik and Tickell, 2015, Veit, 2010) (Appendix 2). The review found that NFA had declined greatly in effectiveness by 2010.
One reason for the failure to launch DFS and FSSD properly is reported to be the withdrawal or reduction in levels of support given by the Norwegian Aid for Development Cooperation (NORAD) and the UK's Department for International Development (DFID) to the Uganda Forest Sector Umbrella Programme (UFSUP, 1999–2003), charged with the restructuring of forestry. In DFID's case, internal changes may have contributed, since, at the time, DFID was moving towards a single-minded focus on ‘poverty reduction’ and a shift from ‘projects to programmes’ (Killick, 2005). DFID's support for UFSUP was reportedly classified as a project. The standard of forestry advice available to DFID may anyway have declined, given that the prestigious Oxford Forestry Institute was closed in the 1980s, with DFID's own tropical forestry team later disbanded (Mills, 2006). NORAD has been the most consistent ‘donor’ to forestry in Uganda, including through supporting the founding of a Forestry Department at Makerere University (late 1960s), a National Tree Seed Centre (NTSC, in 1993) and a National Biomass Study (1989–2002). The original intention was that NTSC would make systematic collections of the germplasm of indigenous trees (leaving the actual supply of seedlings to the private sector), but today it concentrates mostly on exotic species.
Concerns about climate change have led to a market in carbon credits developing in Uganda, mainly REDD (or REDD+) schemes (REDD = Reducing Emissions from Deforestation and Forest Degradation), with 97% of carbon credits sold to voluntary purchasers in the European Union (Bulafu et al., 2013). REDD has been severely criticised in general (Brown, 2013) and in relation to Uganda in particular, where controversies over land ownership and tenure and levels of corruption are regarded as unacceptably high (Twongyirwe et al., 2015). A similar conclusion has been reached for Kenya (Entenmann et al., 2014). Third party certification, as would be necessary to establish credibility for REDD schemes, is difficult to establish successfully in Uganda, as research into Fairtrade tea and coffee has shown (Cramer et al., 2014).
REDD projects are considered too expensive to mount for owners of smaller forest patches in Uganda (Bulafu et al., 2013), but larger-scale projects have been launched by companies registered in the Netherlands, Norway and the UK. The project of the FACE Foundation (Forests Absorbing Carbon dioxide Emission) on Mt Elgon differs from the others in its concentration on planting indigenous rather than exotic trees. Launched in 1994 and based on an agreement between the Dutch Electricity Board and UWA, the objective is to plant indigenous trees on 250 km2 of land that earlier had been illegally deforested (Appendix 2) (White and Wanyama, 2006). Provisions include a ban on the logging of the trees for a period of 99 years and for all carbon credits to accrue to The Netherlands.
Several organisms introduced by people into Uganda pose problems for forest management. They include the rampant shrub Lantana camara L., which can suppress the regeneration of indigenous species, and the fast-growing paper mulberry tree (Broussonetia papyrifera (L.) L. Vent.), which has spread rapidly in Budongo and Mabira Forests, becoming dominant in parts of the latter (Kisekka, 2012). The introduction of the Nile Perch (Lates niloticus Linnaeus, 1758) into Lake Victoria in the 1950s has resulted in added pressure on lakeside forests via several intermediate steps. This fish has driven to extinction or near extinction hundreds of species of small indigenous cichlid fish (enkejje) that used to form the basis of a sizable artisanal fishing industry, the fishes being sun-dried and then carried inland on bicycles to supply villagers with an affordable source of protein. Nile Perch is too oily to dry properly in the sun, so wood fuel is being used instead, hence the added pressure on the forests. Cypress has suffered from damaging attacks of cancer since the 1960s and eucalyptus and pines would seem vulnerable to similar epidemics of pests or diseases, given that they have been planted in extensive monospecific stands.
4.3. Planting and conservation of individual forest species
Small-holding farmers commonly plant a few species of indigenous forest trees, while many householders grow a few species of medicinal plants (mostly herbs or shrubs) and herbal doctors can have extensive collections. Several non-governmental organisations (NGOs) have promoted the planting of indigenous species by communities, sometimes developing nurseries to supply seedlings, including Rukararwe Development Centre (Bushenyi), Joint Ethnobotanical Research and Advocacy (JERA), Uganda Group of the African Network of Ethnobotanists and Ethnoecologists (UGANEB) and Promotion de la Médicine Traditionelle (PROMETRA-Uganda). Tooro Botanical Garden has developed the concept of a ‘first aid herbal toolkit’, a collection of about 20 species of medicinal plants (some indigenous) intended for planting in home gardens (Hamilton, 2008) and is also involved in forest restoration (BGCI, 2013). Especially valuable for supporting general biodiversity conservation is the growing of plants in the buffer zones of protected areas to provide alternatives to species being overharvested within them, as at Bwindi Impenetrable National Park (Cunningham, 1996, Wild and Mutebi, 1997). A manual on how to raise and plant 80 species of indigenous trees has been produced (Meunier et al., 2010). The Forest Department started to develop techniques for the enrichment planting of natural forests in the 1930s, involving mahoganies (Khaya anthotheca C. DC. and Entandrophragma spp.) in Budongo Forest and the general purpose timber species musizi (Maesopsis eminii Engl.) in logged-over forests near Kampala, but such attention to detail has become largely superseded by events.
At one time the Forest Department established nature reserves within its larger forests, but these have little practical recognition today (Howard, 1991). Only forests lying within national parks and one forest reserve (Mpanga) receive much management attention aimed specifically at biological conservation. A substantial field survey of 12 principal forests in 1985–1988, involving inventories of tree species, primates, birds and butterflies, resulted in calculation of a biodiversity importance value for each forest (Howard, 1991, Howard et al., 2000). This was then weighed up against the interests of commercial forestry and the harvesting of minor forest products, to provide optimal management objectives for each forest, treating the whole forest estate as a single planning unit. The Forest Department was thwarted from further development of this approach when it lost 5 of its larger forests to UWA in 1993.
The conservation status of trees in Uganda (829 species, of which 455 live in ‘moist forest’) has been evaluated, finding that only 1% are endangered (Kalema and Beentje, 2012). This evaluation refers to the conservation status of species over their entire ranges (few of the species are totally confined to Uganda) and the authors concede: “There are, in fact, many species under threat in Uganda through habitat loss or habitat erosion, through over-harvesting, and through other reasons”. Over-reliance on conservation in neighbouring countries could be risky in the case of Uganda, given that several of its neighbours have suffered from severe political challenges (DR Congo, Rwanda, South Sudan). A follow-up national-level Red List assessment would be useful, as well as initiatives to conserve species identified as endangered. There is a Uganda National Gene Bank in Entebbe Botanical Gardens, housing mainly varieties of crops.
4.4. Banana diversity and its conservation
The British Empire, as with other European empires, was strongly founded on trade in plants, so, following the common practice, a botanical garden (Entebbe Botanic Gardens) was established (1898) to serve as a testing station to identify species and varieties of economic worth (Biggs, 1940, Tothill, 1940). Cotton and coffee were soon recognised as prime candidates for general promotion, their planting then being encouraged by imposing a hut tax to encourage farmers to enter the monetary economy. Trials on crops were shifted to agricultural stations in 1910 (Table 6), by which date many varieties of bananas had been imported, including from Ceylon (Sri Lanka), Dominica, India, Jamaica and the UK (Kew Botanical Gardens) (Thomas, 1940). Varieties of bananas have continued to be introduced since 1910, in recent times principally to Kawanda Agricultural Research Institute (KARI) and to its field collection of bananas at Mbarara.
Table 6.
Field collections of bananas in Uganda. Sources: (Biggs, 1940, Blomme et al., 2012, Karamura and Mgenzi, 2004, Kikulwe et al., 2007, Thomas, 1940, Tothill, 1940).
| Institute | History |
|---|---|
| Entebbe Botanic Gardensa | Founded 1898, especially to test the local suitability of potential economic crops. Banana varieties were introduced from many countries. Work on bananas was transferred to agricultural stations from 1910. |
| Kampala Plantation | Banana varieties accumulated from Buganda and Ankole (1919–1925) and trials of cooking bananas started (1927). The collection no long exists, related to expansion of Kampala city. |
| Bukalasa Agricultural College (formerly Bukalasa Substation)b | Trials on banana varieties started 1927 using materials obtained from Kampala Plantation. The work was largely transferred to Kawanda in about 1940. A banana collection was re-established in the 1960s, eventually containing 600 accessions, but lost by 1985. |
| Kawanda Agricultural Research Institute (KARI)a | Banana collection started 1940, but later lost. It was restarted in 1989, triggered by concern over discovery of Black Sikokota in Uganda (1988). When Uganda started a banana breeding (2003), the Kawanda collection was transferred to MZARDI. Kawanda then became developed as a collection of breeding lines. |
| Makerere University Agricultural Research Institute – Kabanyolo | Banana collection established 1989, but lost by 2004. A few representative samples exist in tissue culture. |
| Namulonge Agricultural Research Institutea | Collection established in 2006–8 as part of a plan to move all research on crops from Kawanda to Namulonge. It contains about 100 accessions. A banana collection belonging to the International Institute of Tropical Agriculture (IITA) was started at Sendusu, near Namulonge, in the 1990s. |
| Mbarara Zonal Agricultural Research and Development Institute (MZARDI)a | Banana collection started 1998 as a reference collection and to duplicate the collection at Kawanda. The collection became a regional collection in 2008 (for DR Congo, Kenya, Tanzania, Uganda) within the framework of the Banana Research Network for Eastern and Southern Africa (BARNESA). It currently has 450 accessions. It is run by the National Banana Programme (based in KARI) of NARO, backstopped by Bioversity International. The altitude is 1410 m. |
Today under National Agricultural Research Organisation (NARO).
Today under Ministry of Agriculture, Animal Industry and Fisheries.
A decline in the productivity of bananas in the central area of Buganda was noted in the 1950s, thought to be related to exhausted soils (Wrigley, 1989), and then again from the 1970s, with banana diversity also diminishing, the causes reported as ravages by pests and diseases, decreased soil fertility and socio-economic trends (Karamura and Mgenzi, 2004, Mulumba et al., 2004). Some serious pests and diseases that were seemingly absent before 1900 are thought to have been introduced inadvertently along with banana materials imported for planting (Blomme et al., 2012). They include the banana weevil (Cosmopolites sordidus (Germar), which was present by 1908), some damaging nematodes (notably Radopholus similis (Cobb, 1893) Thorne, 1949), the pathogens responsible for Sigatoka leaf spot (first seen 1938), Black Sigatoka (first recorded 1988), banana Xanthomonas wilt (BXW, first reported 2001) and Fusarium wilt (first reported 1953) (Blomme et al., 2012). All banana varieties in Uganda succumb to BXW, while AAA-EA bananas are especially susceptible to weevils, R. similis and Black Sigatoka, particularly at altitudes below 1400 m. The heart of commercial banana growing has shifted from the Central to slightly higher parts of the Western Region since 1980, partly because of reduced problems with pests and diseases.
The maintenance of banana productivity and diversity in Uganda has become a major concern for agricultural scientists, given the importance of the crop in the diet (especially AAA-EA varieties) and because the country is a major global centre of genetic diversity for the crop. In situ conservation approaches have mainly been limited to studying how farmers maintain banana diversity in their gardens. Ex situ techniques suitable for conservation of banana germplasm include field gene banks, in vitro culture and cryopreservation, the first being the most useful for plant breeders (Pillay et al., 2004). Various field gene banks have been started over the years, the principal current ones being at Sendusu, associated with Namulonge Agricultural Research Institute, and at Mbarara Zonal Agricultural and Development Institute (MZARDI). The former belongs to the International Institute of Tropical Agriculture (IITA), while the latter falls under the National Agricultural Research Organisation (NARO) (Table 6). Several field collections of bananas in Uganda have been lost over time, the causes including inadequate funding and civil strife. MZARDI is currently subject to a land claim by an ex-army officer (Mukombozi, 2013).
Conventional banana breeding is one method that has been tried in Uganda to make AAA-EA bananas less susceptible to pests and diseases. AAA-EA bananas are triploid (3×) and therefore sterile, so do not normally produce viable seed. However, it has been found that hand pollination with pollen from wild bananas, especially the strain Musa acuminata spp. burmannicoides ‘Calcutta 4’, sometimes results in some bananas in a bunch having viable seeds and, further, that some of the banana plants grown from these seeds are resistant to pests and disease (Pillay et al., 2004). M. acuminata is diploid (2×) and the seeds produced from this crossing are tetraploid (4×) and so potentially fertile and may have viable seeds (few to many). Consumers dislike bananas containing seeds, so plant breeders have then back-crossed the tetraploids with improved diploid varieties (2×) to produce secondary triploids (3×) that are seedless.
The discovery in 1988 of Black Sigatoka, a particularly devastating disease, caused the banana to rapidly become the priority crop for urgent attention by plant breeders in Uganda. It triggered the re-establishment of a banana collection at KARI (1989), the development of a collaborative programme between NARO and IITA and the launch of a formal banana breeding programme (1994) (Kikulwe et al., 2007). Useful levels of resistance to pests and diseases have not been found in Uganda, so germplasm of cultivars for the breeding programme have been imported from elsewhere (in the form of in vitro plantlets) (Kikulwe et al., 2007, Smale and Tushemereirwe, 2007). Tissue culture techniques have been developed for the multiplication of planting materials for delivery to farmers, with commercial laboratories involved. Height, suckering ability, time to flower, time to fill, bunch traits, yield, and organoleptic quality have received attention in the breeding programme.
A genetic engineering laboratory was established at KARI in 2003. Support for capacity-building has been received from Biodiversity International, a conservation group that incorporates the former International Network for the Improvement of Banana and Plantain (INIBAP). A second potential avenue has thus been opened up to develop strains based on AAA-EA bananas that have resistance to pests and diseases or have other useful traits. Introduced genes have come from varieties of bananas and a range of other organisms (plants, animals and bacteria). Legislation does not currently allow the release of genetically modified (GM) crops to farmers.
5. Discussion: the future and some conservation suggestions
The present is distinctive in its rapid rates of change and high interconnectivity. Uganda as a political entity was created recently by historical standards, bringing together people with diverse cultures. Some incidents of loss of forests or collections of banana germplasm have occurred at times of disrupted government related to tensions between the (centralised) state and traditional political allegiances. Increased connectivity has led to the entry of invasive species and new pests and diseases, heightening threats to indigenous plant diversity. It is not surprising that, under these circumstances, it has been hard to find effective ways to achieve conservation of plants. Two predicted trends will make this task even harder in the future – population growth (magnifying pressures on natural resources) and climate change (causing shifts in zones of natural vegetation and agriculture). The population was ca. 1.8 million in 1906 (HMSO, 1906) and will be over 100 million by 2050 if present trends continue (World Bank, 2015). Average annual temperatures could rise by 1.5 °C by 2030 (USAID, 2013).
Conservation of plant diversity will be advanced by people aware of the issues and prepared to make efforts for improvements. The suggestions for conservation that follow deal with general matters of orientation and coordination, rather than with particular areas of specialisation in conservation, since those already involved will be aware of the immediate challenges they face. We assume that the present unfavourable enabling environment will continue, while hoping for policy change.
We suggest an opportunistic approach to engagement at field level, taking advantage of favourable local circumstances as they arise. Given the present high levels of forest loss and degradation, the retention or restoration of any area of rainforest can be considered of value for the conservation of forest species. Efforts at conservation across the landscape will anyway be needed to facilitate the survival of species under predicted climate change.
The local is the key level towards which conservation efforts should be orientated, that is, where people are in direct physical contact with plants. Progress at any other level (for instance, identifying priority areas for conservation action or selling carbon credits abroad) must feed into improved management on the ground to be counted a success. Ex situ conservation should be closely supportive of in situ conservation in the Ugandan context to attract necessary support.
Species of plants have typically taken a very long time to evolve, therefore the assessment that 20% of them are in danger of imminent global extinction represents a calamitous loss of natural capital. However, people who are alive today, even if intellectually aware of the problem, can have overriding concerns about their own immediate livelihoods and the future of their children. Conservationists interested in the survival of plant diversity need social allies. Those concerned with plant genetic resources in Uganda engage with much the same systems of plant resource use and management at field level as do those concerned with wild plant species. We suggest closer coordination. In any case, wild plants do not form a well-bounded category. There are many species of ‘wild’ plants that are semi-domesticated, the planting of ‘wild’ plants by households is common, there is probably no forest in Uganda free of human influence, and planting trees for forest restoration or enrichment are recognised management practices.
Plant conservation is distinctive, in comparison to conservation applied to other taxonomic categories, in that plants almost everywhere provide products that contribute to human economies and because of the major roles that they play in delivering many ecosystem services. With few exceptions, it is unrealistic in Uganda to devise systems of management to conserve species of wild plants, unless attention is given to extractive use (of the same or other species). Awareness of the roles that plants play in delivering ecosystem services may reveal new possibilities for partnerships. For instance, conservationists concerned about forest species may be able to build alliances with elements of society concerned about the availability of future water supplies, given the common perception that a cover of indigenous forest helps to maintain flows in streams. Those concerned with the survival of banana diversity may be able to find allies among people anxious about cultural survival, given the cultural significance of the banana garden in parts of Uganda.
Assessing the conservation approaches and methodologies that work best is a challenge, given the many potentially influencing variables. We suggest an evidence-based approach to establishing best practice, similar to that which has been so successful in advancing medicine (Hamilton, 2011, Sackett et al., 1996, Sutherland et al., 2004). This involves assessing where and why more desirable (system) states exist and then using this knowledge to promote improvements elsewhere. For example, it would be interesting to know why some farmers maintain a greater diversity of indigenous varieties of bananas than others, and the processes followed in Mpigi District that have led to the survival of some patches of forest, while others have been lost (Appendix 2).
One reason why we have laid stress on institutional history in relation to forestry and collections of banana germplasm is because of the potential of institutions for directional effort over time, gaining greater proficiency through experience and institutional memory. A programme designed to answer the question ‘How can communities best conserve their medicinal plants?’, involving three case studies in Uganda (among others), has identified the central roles that NGOs can play in conservation initiatives (Hamilton, 2008, Pei et al., 2010). Local, national and international NGOs can take complementary roles. National NGOs are pivotal, on the one hand connecting with community groups seeking local development supportive of conservation goals, and, on the other hand, with international NGOs able to facilitate exchanges of experiences between countries and sometimes having easier access to funds.
Building capacity for the approach to plant conservation suggested here is a challenge. Ethnobotany is a key discipline because it covers both the social and botanical aspects of plant conservation and draws on both indigenous and scientific knowledge (Cunningham, 2001, Martin, 1995, Tuxill and Nabhan, 2001). Ethnobotany has been taught at Makerere University since 1998.
6. Conclusions
In many countries, plants hold a central position in the economy, but suffer from low levels of support for plant conservation, ever rising demands on natural resources, a tendency to place short-term economic development above environmental concerns, and vulnerability to climate change. It is suggested that they too could benefit from an ecosystem-based approach to plant conservation, covering sustainable use and delivery of ecosystem services, additional to conservation of species and genetic diversity. Few of them, it is further contended, will be able to achieve sustainable economic development, unless due attention is given to management of the natural environment, in which plants form such a prominent part.
We suggest that international organisations concerned with the two schools of plant conservation that have developed historically (dealing with conservation of plant genetic resources and wild plant species respectively) collaborate to advance the discipline of plant conservation conceptually, making it more useful for countries like Uganda. Realistic protocols for field implementation will be required.
Acknowledgements
Many thanks to Dr David Balikowa (Director of Research) for welcoming a visit to Mbarara Zonal Agricultural Research and Development Institute (MZARDI) and to Mr. Sedrach Muhangi (Research Assistant) for a tour of the field collection of bananas. Professor Maud Kamatenesi Mugisha (Vice Chancellor, Bishop Stuart University) provided hospitality in Mbarara and transport to the institute. We are grateful to those who contributed information during interview for the ‘forests and forestry’ section of the paper. Comments labelled ‘reported’ are mostly from these sources. They are Professor John Kaboggoza (former Professor of Forestry, Makerere University, and first Chairperson of the Board of Uganda Forestry Authority (UFA) 2003–2006), John Kamugisha (Director of Field Operations for UFA 2004–2006 and earlier Acting Executive Director), Rachel Musoke (Acting Commissioner, Forest Department 1998–1999; Commissioner, Forestry Sector Support Programme 2008–2013) and Bill Farmer (Technical Team Leader for DFID on Uganda Forest Sector Umbrella Programme 1999–2003; currently Chairman, Uganda Carbon Bureau). Kirsty Shaw of Botanic Gardens Conservation International (BGCI) provided information on botanic gardens. Dr Patrick Hamilton assisted with access to the literature and Mike Lagan helped with computing.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Appendix 1.
Detection of human influences on forests in pollen diagrams from Uganda (See Table 3).
-
1.
High altitude sites
These sites (>2900 m altitude) are on the Virunga Volcanoes and Rwenzori (Livingstone, 1967, McGlynn et al., 2013). Changes in the abundance of pollen believed to have come from plants growing at lower altitudes reveal a major reduction in forest at ∼1000 CE. A principal signal of this decline is a fall in Celtis, a pollen type produced by several species of lowland forest trees. There is no indication in the pollen diagrams of any major climatic change at ∼1000 CE, while contemporaneous peaks in charcoal in the Virunga sediments are consistent with the theory that forest reduction was caused by clearance for agriculture. Differences between pollen diagrams from west- and east-facing valleys on Rwenzori suggest that reduction in forest was greater to the east of the range (Hamilton, 1972).
A changed floristic composition of montane forest and woodland on the Virunga Volcanoes at ∼1000 CE is indicated by increases in the pollen of Hagenia abyssinica Willd. and Myrica (most likely from Morella salicifolia (Engl.) Verdc. and Polhill in this context). Both are small trees of secondary montane woodland, Hagenia being additionally common in higher altitude montane forest. This is evidence that people influenced the floristic composition of forests at some distance from where they were practicing agriculture. The Virunga Volcanoes are unsuitable for agriculture or permanent settlement above 2100 m, having very steep slopes, rocky soils and little surface water.
The dating of forest clearance around Rwenzori relies on more extensive extrapolation from available radiocarbon dates than is the case with the Virunga Volcanoes. Nevertheless, there is no confusion between changes in pollen abundance in the diagrams indicating forest clearance and changes believed consequent to the mid-Holocene drying in climate at ∼2050–1850 BCE, an event well marked (at lower levels in the Rwenzori diagrams) by notable increases in Acalypha and Podocarpus.
Pollen diagrams are also available for high altitude sites on Mt Elgon, actually in Kenya but close to the Ugandan border (Hamilton, 1982, Hamilton, 1987). No episode of deforestation (at any time) is apparent, though this does not necessarily mean that early forest clearance in the surrounding lowlands did not occur. Mt Elgon differs from the Virunga Volcanoes and Rwenzori, which have precipitous slopes, in having gentle slopes and much more extensive montane forest. Several species of plants known to be high pollen producers are abundant in the montane forest, most notably Afrocarpus gracilior (Pilg.) C.N. Page and Podocarpus milanjianus Rendle (both producing the pollen type known as Podocarpus). Palynological signs of past human influence at lower altitudes could thus be masked.
-
2.
Sites in the Rukiga Highlands
These pollen diagrams are for sediments under mires and a lake (Bunyonyi) at altitudes of 1830–2260 m (Hamilton et al., 1986, Hamilton et al., 1989, Marchant et al., 1997, Morrison, 1968, Morrison and Hamilton, 1974, Taylor, 1990). The sites are only 17–34 km from Mt Muhavura, the nearest of the Virunga Volcanoes. All lie today within intensively cultivated farmland, except for those above 2000 m, but the whole area is climatically suitable for moist montane forest. The slopes around one of the higher altitude sites (Muchoya Swamp, 2260 m) are covered largely by mountain bamboo (Yushania alpina (K. Schum.) W.C. Lin), a species that typically forms a zone on wetter mountains in East Africa at 2450–3050 m and which is here dominant at an exceptionally low altitude (2260–2450 m). It is possible that bamboo has replaced broad-leafed montane forest following human disturbance (Hamilton, 1982, Jolly et al., 1997). The other higher altitude site is Mubwindi Swamp (2100 m), which lies within the moist broad-leaved montane forest of Bwindi-Impenetrable National Park.
Two of the pollen diagrams (Muchoya, Ahakagyezi) are well dated for the period of interest here, but no radiocarbon dates are available for Katenga and Bunyonyi, and Mubwindi shows a hiatus in sedimentation stretching back several thousand prior to 1 CE. Rates of sediment accumulation (as measured by sediment thickness) can be highly variable with time in East Africa (Hamilton and Taylor, 1986, Thompson and Hamilton, 1983), so extrapolation of intermediate ages from available radiocarbon dates needs to be approached with caution. A low rate of sediment accumulation during the earlier (wetter) part of the Holocene is a common feature.
Pollen diagrams from the Rukiga Highlands provide a more intimate record of human influence on vegetation than is the case with the Virunga Volcanoes and Rwenzori. Considered together, they provide clear evidence of major forest reduction in the past, believed to be related to agriculture. Higher altitude forest (≥2100 m) was less affected than that at lower altitudes and there may never have been substantial agriculture in the Mubwindi catchment.
There are signs that soil erosion and degradation accompanied the agriculture. They include a rise in the pollen of Dodonaea viscosa Jacq., seen at all sites, but especially prominent at Bunyonyi. Dodonaea is a shrub typical of degraded soils. Another is an increase in Typha seen in several of the pollen diagrams. Typha is a wetland genus known to be responsive to heavy siltation (Lind, 1956). The volume of soil eroded was large, judging by the great thickness of sediment (5 m) that accumulated under Lake Bunyonyi since the time when forest clearance began. This thickness is for the site used for sediment coring, 0.5 km from the nearest shore and where the water depth is 40 m. Human influence is likely to have been responsible for a major change in the vegetation of Muchoya Swamp. It is dominated today by a sedge (Pycreus nigricans (Steud.) C.B. Clarke), accompanied by scattered bushes of tree heather (Erica kingaensis Engl.). A dense layer of fossil Erica wood in the peat, shows that Pycreus has largely replaced dense Erica scrub, 14C dates showing that this happened during the 1st millennium CE, a timing suggestive of a human cause.
The date of forest reduction varied between the sites according to the available radiocarbon dates. The first signs of disturbance at Muchoya are at ∼225 BCE, intensifying from ∼1250 CE, but disturbance is only detectable from ∼1650 CE at Mubwindi and may reflect activities at a considerable distance from the sample site. Ahakagyezi is the most intriguing, with two phases of disturbance identified, one at ∼1700–750 BCE, and another beginning at ∼700 CE and continuing to the present, the final clearance of ridge top forest being at ∼1150 CE.
The evidence for the earlier phase of forest disturbance at Ahakagyezi (∼1700–750 BCE) includes small peaks in the pollen of Bidens sim., Dodonaea viscosa and Rumex (all herbs or shrubs of open land), as well as Myrica (possibly M. salicifolia) and Nuxia congesta Comm. ex Lam. (a tree of early forest succession). An initial interpretation of the pollen diagram (for the upper 10 m of sediment only, collected in 1982) found evidence of human disturbance at several levels, including right at the base (dated ∼3550 BCE) (Hamilton et al., 1986, Hamilton et al., 1989). This interpretation became disputed once a longer core (22.84 m) was collected (in 1984) from a site very close to that used for the first coring (Taylor, 1990). A longer temporal context for interpreting the properties of the sediments then became available. As the person responsible for collecting and analysing the first core, I (AH) now agree with most of the criticisms, but contend that the above-mentioned evidence of very early forest disturbance at ∼1700–550 BCE still stands.
Interpreting the Ahakagyezi diagram is complicated because possible early human influences must be disentangled from climatic effects associated with the transition to a drier mid-Holocene climate at 2050–1850 BCE. One of the characteristic indicators of this transition in pollen diagrams from Uganda is a rise in Podocarpus pollen, which, in the case of the Ahakagyezi diagram, started distinctly earlier (∼1950 BCE) than the first phase of human disturbance suggested above (beginning 1700 BCE). Dating control for the upper sediment at Ahakakyezi is excellent because the radiocarbon dates show a very even rate of accumulation of peat with time and the rate of peat accumulation is the highest known for Africa (1 m per 500 years). Ahakagyezi is noteworthy hydrologically for being a source of the River Nile in two directions, water flowing out of the swamp at both ends, subsequently passing through Lakes Victoria and Edward, respectively, and finally becoming reunited in Lake Albert after travelling their separate ways for several hundred kilometres.
A decline in the pollen of Alchornea hirtella Benth. was formerly held to be another indication of very early human disturbance, but this is an understory tree found today in valleys in lower montane forest in nearby Bwindi-Impenetrable Forest and it could have been adversely influenced by the drying in climate. Particular weight was given in the initial interpretation to high values of inorganic matter, grass pollen and charcoal at places in the 10 m core, including at its base. These were interpreted as being related to slash and burn agriculture, but, with the longer temporal context now available, it is now accepted that changes in these parameters have been strongly influenced by changes in the sedimentary environment, that could have been largely unrelated to agriculture.
-
3.
Crater sediments, Kasenda Volcanic Field
These pollen diagrams are for sediments beneath two small lakes and a swamp (Kabata) occupying extinct volcanic craters (Ryves et al., 2011, Ssemmanda et al., 2005, Taylor et al., 1999). The sites are at altitudes of 1170–1370 m and about 40 km east of those used for pollen analysis on Rwenzori. All the diagrams show forest clearance, which is especially obvious and well dated at Kasenda and Wandakara (occurring at ∼900–1000 CE). The same two diagrams also show signs of forest regrowth from ∼1700 to 1750 CE (especially from the late 1800s), marked by an initial increase in Securinega pollen followed by increases in Celtis, Phoenix and other trees. Securinega pollen may have come from Flueggea virosa (Roxb. ex Willd.) Voigt, a bush or small tree with edible fruits found on forest margins. Phoenix pollen is from the wild date palm, a plant having many uses, and it is possible that these plants were wild-cultivated. Papyrus (Cyperus papyrus L.) overgrew a lake at Kabata sometime during the period 400–1400 CE, a date suggestive of a human cause.
The pollen diagram from Kabata is less precisely dated than those from Kasenda and Wandakara, and there is an apparent hiatus in sedimentation during the early Holocene. The uppermost sediments were not analysed. Forest disturbance from ∼1600 CE is indicated by a fall in Celtis and a rise in Cyathea. Cyathea is a genus of tree ferns found in open areas of moist forest. A possible earlier phase of forest disturbance (poorly dated at sometime between 1350 BCE and 400 CE) is suggested by higher values of Dodonaea and Pteridium. Pteridium spores come from Pteridium aquilinum (L.) Kuhn, a fern of open ground, found especially on impoverished soils. A claim has been made for very early forest disturbance (predating ∼1350 BCE) at Kabata, based largely on high values of Acalypha (Taylor et al., 1999). Acalypha is a very small and well-dispersed pollen type, which in pollen diagrams from Rwenzori, Lake Victoria and Mt Elgon shows increased values associated with the mid-Holocene transition to a drier climate (∼2050–1850 BCE) (Hamilton, 1987, Kendall, 1969, Livingstone, 1967). Its increase at Kabata may have had a similar cause.
The cores from Kasenda and Wandakara date back only to 750 CE and 1300 CE respectively. Ricinus pollen, which is present throughout the pollen diagrams, comes from R. communis (castor bean plant), a shrub to small tree of disturbed ground and forest gaps, habitats that could have been encouraged by people. Ricinus may have been wild-growing or cultivated. Kasenda contains a greater quantity of tree pollen (Celtis, Ficus, Phoenix, Vepris) than Wandakara. Forest at Kasenda has received greater protection than Wandakara over recent decades. It lies within a private estate that has aimed to retain forest, while the steep slopes around Wandakara are covered by small-scale agricultural plots.
-
4.
Swamp associated with a Later Iron Age settlement at Munsa
Munsa is a Later Iron Age site with large-scale earthworks constructed at some time between 1400 and 1650 CE, and abandoned by 1700 CE (Robertshaw et al., 1997). The site used for sediment analysis is a small papyrus swamp lying within the earthworks (Lejja et al., 2005, Lejju et al., 2006). Analyses of the well-dated sediments at the sample site (pollen, fungal spores, phytoliths, charcoal), plus archaeological evidence, show forest clearance at ∼1100 CE and the establishment of an economy based on cereal cultivation, large-scale cattle keeping and iron-smelting. Analysis of glass beads from archaeological contexts show that the inhabitants were in contact with exchange networks extending to South Asia. A papyrus swamp expanded over the sediment site at about the time when forest was cleared, spreading inwards from the margins, suggesting that its development was related to human occupancy. There was some forest recovery from ∼1780 CE.
-
5.
Large lowland lakes
A well-dated pollen diagram is available for a core of sediment from Pilkington Bay, a virtually enclosed bay off Buvuma Island close to the northern shore of Lake Victoria (Kendall, 1969). Buvuma was largely forested at the time of collection of the core, contrasting with the nearby mainland which was then covered by a mosaic of secondary woodland, grassland and agriculture. The quantity of tree pollen in the sediments starts to decline relative to grass pollen from ∼1 CE and, in absolute terms (number of grains becoming incorporated per unit time), between 1750 and 1250 BCE. Celtis is one of the types of tree pollen to decline, just as it does in pollen diagrams from the Virunga Volcanoes and Rwenzori, but here the decline begins much earlier.
Wetland vegetation changed at ∼1 CE, as shown by increased amounts of Nymphaea and Typha in the pollen diagram. It has been suggested that this may be related to a shallowing of the lake (Kendall, 1969), but an increase in Typha pollen is associated with forest clearance in the Rukiga Highlands and both of these changes could have had anthropogenic causes.
A few pollen counts have been made on another core from Lake Victoria, collected from Kome Channel about 100 km west of Pilkington Bay (Kendall, 1969). The pollen diagram broadly confirms the picture of vegetation change reconstructed from Pilkington Bay. A core of sediment has been collected from Lake Albert (altitude 619 m) and its upper sediments (those of the age of interest here) subject to outline pollen analysis (Beuning et al., 1997). A decline in forest pollen and a rise in grass pollen is evident from 1725 BCE, considered by the researchers to be due to a drier climate and perhaps human influence.
Appendix 2.
Examples of tropical forest loss and degradation in Uganda since 1970.
-
1.
Budongo Forest
A study of households adjacent to Budongo Central Forest Reserve (CFR) found that average household income rose significantly following transfer of authority over the forest in 2003 from the Forest Department to the National Forest Authority (NFA) (Jagger, 2008). The gains were mainly made from the sale of illegally harvested timber and accrued only to richer households.
-
2.
Bugala and Buvuma Islands, Lake Victoria
BIDCO, an oil palm company, was granted permission to plant oil palms in Mugoye, Banya, Nkoma and Towa CFRs on Bugala Island in Lake Victoria in 2005 (Tenywa, 2005). Altogether, BIDCO has been allocated 65 km2 of the island's total area of 290 km2, with a further 35 km2 being made available for an out-grower scheme (Tenywa, 2013). The planting of oil palms was expanded onto Buvuma Island in 2012 through Oil Palm Uganda Limited (OPUL, 90% of which is owned by BIDCO), its operations resulting in the destruction of 36 km2 of rainforest by 2015 (van Schaik and Tickell, 2015).
-
3.
Bwindi Impenetrable Forest Reserve (National Park from 1993)
A biological and forest utilization survey was carried out in the forest in 1983–1984, finding large-scale illegal pit-sawing for timber (an estimated 140–200 illegal pit-sawers operating), widespread illegal collection of fuelwood, poles and bamboo, rampant small-scale gold mining and poaching (Butynski, 1984). Later, further research revealed that 9–12 mountain gorillas had been killed in the forest over an 18 month period in 1986–1987, a serious loss considering that this is one of only two sites globally for mountain gorilla and there were only 115 gorillas in the forest at the time (Butynski, 1990).
The forest was transferred from the Forest Department to Uganda National Parks (later Uganda Wildlife Authority – UWA) in 1993 and much tighter controls over activities in the forest instigated. An establishment plan prepared for this transfer received evidence from the Conservation Education Assistants (CEAs) of a Conservation through Education Project (drawn from local villages) that there had been three incidents of agricultural encroachment into the forest on its northern side (Hamilton et al., 1990). They believed that local forestry officials had authorised forest destruction for the planting of crops, the idea supposedly being that they would inter-plant them with valuable trees. Corruption was suspected. Senior forestry officials from Kampala intervened and were able to stop one of the encroachments.
Relationships between the new park and the local people were poor at first, with fires set or not extinguished in the forest and threats made to kill the gorillas (Hamilton et al., 2000). Three schemes were then instituted to provide benefits to adjacent communities and involve them in park management, these being agreements on the controlled harvesting of certain resources in the park (Cunningham, 1996, Wild and Mutebi, 1996, Wild and Mutebi, 1997), the receipt of some revenues from tourism and establishment of a trust fund for community development. The situation eventually stabilised. Based on analysis of satellite imagery, the National Biomass Survey reported in 2002 that there was no evidence of forest depletion in the 31,046 ha of forest then present in the national park (total area 32,019 ha) (Drichi, 2002).
-
4.
Forests in Mpigi District and other forests near Kampala
A sample of 9 forests lying in Mpigi District (20–70 km west of Kampala) showed a 62% decline in biomass and 74% decline in tree density between 1994/1995 and 1999/2000, attributed to tree harvesting for firewood and timber, and clearance for agriculture (Banana et al., 2007). Not all forests suffered equally. Mpanga CFR, classified as a nature reserve, was little affected, benefitting from relatively high level of staffing through funding from the European Union. One other government-owned forest that did well was Kizzikibbi, where negotiations between government officials and the local community resulted in the development and enforcement of strict rules over harvesting. One of the two private forests included in the survey (Namungo) was also well managed. The forest owner is said to have worked closely with the village council and neighbouring community to develop agreements that effectively regulated harvesting. The National Biomass Study reported in 2002 that, of the total area of Mpanga CFR (1012 ha), only 43 ha had been deforested and 14 ha degraded (Drichi, 2002), confirming that it was substantially intact. However, a permanent plot monitored over 40 years in the forest revealed several cases of illegal tree felling, including of Antiaris toxicaria Lesch. and Funtumia africana (Benth.) Stapf for carving into drums (Taylor et al., 2008).
A return survey revealed that 11 out of 22 forest patches lying within a distance of 35 km of Kampala disappeared between 1990 and 2010 (Bulafu et al., 2013). Three of the surviving forests were government-owned (one belonging to the NFA and the other two to research institutes), four belonged to churches, three to individuals and one was communal (a sacred forest). However, all the surviving forests, except those belonging to the government, had become severely degraded, the causative factors considered to be selective logging and fragmentation effects (increased mortality of exposed large trees near forest edges).
-
5.
Forest Reserves in Busoga
Half of Bukaleba CFR was transferred to the Ministry of Animal Resources in 1975, the remaining part becoming heavily encroached (Hamilton, 1984). It was reported that South Busoga CFR became heavily encroached after 1975, about half of its 163 km2 being lost by 1982 (Hamilton, 1984). The National Biomass Study reported in 2002 that, of the total area of Bukaleba CFR as then constituted (9536 ha), 1293 ha had become deforested and 231 degraded (Drichi, 2002). The equivalent figures for South Busoga CFR (total area 16,107 ha) were 493 ha (deforested) and 12,539 ha (degraded).
-
6.
Kibale and Kisangi Forest Reserves (Kibale a National Park from 1993)
Schemes for the resettlement of people from densely populated Kigezi in southwest Uganda have existed since the 1930s. One area chosen for resettlement has been land close to these two forest reserves, within one of which (Kibale) encroachment by the resettled people is reported to have started in 1971–1972 and in Kisangi a year or two later (Hamilton, 1984). All of Kisangi was reported to have been felled by 1982. A ground survey of southern Kibale in 1980 found that about 7000 people had settled in the forest reserve, 97 km2 of which had become disturbed by 1982 (Van Orsdol, 1983). The National Biomass Study reported in 2002 that, of the total area of rainforest (49,447 ha) in Kibale National Park (total area 74,396 ha), 5351 ha had become degraded. Of that part of Kisangi that has remained as a CFR after creation of the national park (481 ha), 344 ha were classified as deforested and 98 ha as degraded.
-
7.
Mabira Forest Reserve
Encroachment was not regarded as a serious problem before the 1970s (Earl, 1971). Forest protection started to collapse in the mid-1970s, when a government minister is alleged to have encouraged settlement in the forest reportedly in response to the government's call to ‘double agricultural production’ following expulsion of ‘Asians’ from the country in 1972. There were many reports of agricultural encroachment and uncontrolled charcoal burning in the forest by 1982, the forest being described as looking like a hollow shell from the air (Hamilton, 1984). The National Biomass Study reported in 2002 that, of the total area of Mabira CFR (29,566 ha), 1215 had become deforested and 7099 degraded (Drichi, 2002).
The government sought to degazette 7100 ha of the forest in 2007 to clear for the growing of sugarcane by the Sugar Corporation of Uganda (Veit, 2010). This became a major political issue in Uganda with demonstrations held in Kampala (resulting in some fatalities) (Nakkazi, 2011). Concern for conservation was expressed internationally (BirdLife International, 2008). The King of Buganda (Kabaka) declared that he would find an equivalent area of non-forested land elsewhere for the sugar estate to prevent the forest's loss. Theats to Mabira continue.
-
8.
Mt Elgon Forest Reserve (National Park from 1993)
Encroachment started at several places during the 1970s, most seriously on the western side where 82 km2 of forest was reported to have been lost by 1982, involving about 5000–7000 farmers (Hamilton, 1984). Cultivation was then extending in places up to the bamboo zone (lower altitudinal limit 2450 m), which itself was being heavily grazed by cattle. At Bumbo in the southwest, an old man was given a temporary permit in the 1960s to reside and cultivate in the forest, together with his small immediate family. By 1980 the ‘family’ had expanded to 230 people and the encroachment was out of control. Satellite images showed that a slice of forest running along part of the northern boundary had become lost by 1982 (Carvalho, 1982). This is an area that had been affected by ethnic disturbances in the preceding years, resulting in difficulties in administration for the Forest Department.
A study of changes in forest extent on Mt Elgon between 1973 and 2009, based on comparison of satellite images and ground survey, found that 25% of the forest had been destroyed illegally between 1973 and 1988, fuelled by land grabs for agriculture (Sassen, 2014, Sassen et al., 2013). Greater discipline was restored when the reserve was made a national park in 1993, resulting in some vegetation regrowth, though hampered by continuing illegal collection of firewood (up to >1 km inside the park boundary). The National Biomass Study reported in 2002 that, of the total area of forest on Mt Elgon (55,826 ha), 29,606 had become degraded (Drichi, 2002). This figure for the area of degraded forest is comparable with that of 25,000 ha, the size of the area allocated for a tree planting scheme to gain carbon credits under an agreement reached between the FACE Foundation in the Netherlands and UWA in 1994 (White and Wanyama, 2006). Exceptionally, deforestation has continued in the southeastern sector, especially since 2005, when multi-party politics resumed in Uganda and rival electoral candidates are reported to have competed with ‘giving away’ land to farmers (Sassen, 2014).
References
- Adams J.S., McShane T.O. W.W. Norton and Company; New York and London: 1992. The Myth of Wild Africa. [Google Scholar]
- Adia M.M., Anywar G., Byamukama R., Kamatenesi-Mugisha M., Sekagya Y., Kakudidi E.K., Kiremire B.T. Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. J. Ethnopharmacol. 2014;155:580–588. doi: 10.1016/j.jep.2014.05.060. [DOI] [PubMed] [Google Scholar]
- Banana A.Y., Vogt N.D., Bahati J., Gombya-Ssembajjwe W. Decentralized governance and ecological health: why local institutions fail to moderate deforestation in Mpigi district of Uganda. Sci. Res. Essays. 2007;2:434–445. [Google Scholar]
- Beuning K.R.M., Talbot M.R., Kelts K. A revised 30,000-year paleoclimatic and paleohydrologic history of Lake Albert, East Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997;136:239–279. [Google Scholar]
- BGCI . Enhancing Tree Conservation and Forest Restoration in Africa: Report of the Regional Workshop Held in Entebbe, Uganda, 30th July-1st August 2013. Botanic Gardens Conservation International; Kew: 2013. [Google Scholar]
- Biggs C.E.G. Government experiment stations and farms. In: Tothill J.D., editor. Agriculture in Uganda. Oxford University Press; London: 1940. pp. 101–111. [Google Scholar]
- BirdLife International . 2008. Campaign to Save Mabira Forest in Uganda from Sugarcane Plantation for Biofuels.http://www.birdlife.org/datazone/sowb/casestudy/231 (accessed 28.08.15.) [Google Scholar]
- Blench R. Bananas and plantains in Africa: re-interpreting the linguistic evidence. Ethnobot. Res. Appl. 2009;7:363–380. [Google Scholar]
- Blomme G. A historical overview of the appearance and spread of Musa pests and pathogens on the African continent: highlighting the importance of clean Musa planting materials and quarantine measures. Ann. Appl. Biol. 2012;162:314–326. [Google Scholar]
- Brown M.I. Earthscan; London: 2013. Redeeming REDD. [Google Scholar]
- Bulafu C., Baranga D., Eycott A.E., Mucunguzi P., Telford R.J., Vandvik V. Structural changes are more important than compositional changes in driving biomass loss in Ugandan forest fragments. J. Trop. For. Environ. 2013;3:23–38. [Google Scholar]
- Butynski T.M. Ministry of Tourism and Wildlife; Kampala: 1984. Ecological Survey of the Impenetrable (Bwindi) Forest, Uganda, and Recommendations for its Conservation and Management. [Google Scholar]
- Butynski T.M. Ministry of Tourism and Wildlife; Kampala: 1990. Technical Report on Gorilla Tourism. [Google Scholar]
- Byarugaba D. Integrating indigenous knowledge, active conservation and wild food plant use with reference to Dioscorea species in Bwindi forest, southwestern Uganda. Afr. J. Ecol. 2008;48:539–540. [Google Scholar]
- Carvalho J. Forest Research Centre, Forest Department; Kampala: 1982. A Preliminary Report on Preparation of an Inventory of Forestry Resources Using Data Available at the Nairobi Remote Sensing Facility with Reference to Uganda. [Google Scholar]
- Colonial Office . Her Majesty's Stationery Office; London: 1961. Buganda Agreement. [Google Scholar]
- Cramer C., Johnston D., Oya C., Sender J. Fairtrade cooperatives in Ethiopia and Uganda uncensored. Rev. Afr. Polit. Econ. 2014;41:115–127. doi: 10.1080/03056244.2014.976192. doi. [DOI] [Google Scholar]
- Cunningham A.B. Division of Ecological Sciences, United Nations Educational, Scientific and Cultural Organization; Paris: 1993. African Medicinal Plants: Setting Priorities at the Interface between Conservation and Primary Healthcare. People and Plants Working Paper no. 1. [Google Scholar]
- Cunningham A.B. Division of Ecological Sciences, United Nations Educational, Scientific and Cultural Organization; Paris: 1996. People, Park and Plant Use. People and Plants. Working Paper no. 4. [Google Scholar]
- Cunningham A.B. Earthscan; London: 2001. Applied Ethnobotany: People, Wild Plant Use and Conservation. [Google Scholar]
- Daniells J., Karamura D. Banana brew and stew on Uganda's menu. Aust. Banan. Mag. 2013-2014:36–37. [Google Scholar]
- Davey M.W., Gudimella R., Harikrishna J.A., Sin Lee Wan, Khalid N., Keulemans J. A draft Musa balbisiana genome sequence for molecular genetics in polyploid, inter- and intra-specific Musa hybrids. BMC Genom. 2013;14 doi: 10.1186/1471-2164-14-683. http://www.biomedcentral.com/1471-2164/14/683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.D. International Union for Conservation of Nature and Natural Resources; Gland: 1986. Plants in Danger: What Do We Know? [Google Scholar]
- De Langhe E. The establishment of traditional plantain cultivation in the African rain forest: a working hypothesis. In: Denham T., Iriate J., Vrydaghs L., editors. Rethinking Agriculture. Left Coast Press, Inc; Walnut Creek: 2007. pp. 361–370. [Google Scholar]
- De Langhe E., Swennen R., Vuylsteke D. Plantain in the early Bantu world. Azania. 1994-5;29–30:147–160. [Google Scholar]
- deMenocal P.B. Climate shocks. Sci. Am. 2014;311:32–37. doi: 10.1038/scientificamerican0914-48. [DOI] [PubMed] [Google Scholar]
- Denham T.P., Haberle S.G., Lentfer C., Fullagar R., Field J., Therin M., Porch N., Winsborough B. Origins of agriculture in the Kuk Swamp in the highlands of New Guinea. Science. 2003;301:189–193. doi: 10.1126/science.1085255. [DOI] [PubMed] [Google Scholar]
- Derek N., Philippson G. Towards a historical classification of the Bantu languages. In: Derek N., Philippson G., editors. The Bantu Languages. Routledge; London: 2003. pp. 164–181. [Google Scholar]
- Diamond A.S., Hamilton A.C. The distribution of forest passerine birds and Quaternary climatic change in tropical Africa. J. Zool. 1980;191:379–402. [Google Scholar]
- Drichi P. Forest Department; Kampala: 2002. National Biomass Study 1996–2002, Technical Report. [Google Scholar]
- Duminil J., Mona S., Mardulyn P., Doumenge C., Walmacq F., Doucet J.-L., Hardy O.J. Late Pleistocene molecular dating of past population fragmentation and demographic changes in African rain forest tree species supports the forest refuge hypothesis. J. Biogeogr. 2015;42:1–12. [Google Scholar]
- Earl D.E. The Mabira forest. Uganda J. 1971;60:229–241. [Google Scholar]
- Edel M.M. Oxford University Press; New York and Toronto: 1957. The Chiga of Western Uganda. [Google Scholar]
- Edmeades S., Karamura D. Banana taxonomy for Uganda. In: Smale M., Tushemereirwe W.K., editors. An Economic Assessment of Banana Genetic Improvement and Innovation in the Lake Victoria Region of Uganda and Tanzania. International Food Policy Research Institute; Washington: 2007. pp. 165–169. [Google Scholar]
- Edmeades S., Smale M., Kikulwe E.M., Nkuba J.M., Byabachwezi M.S.R. Characteristics of banana-growing households and banana cultivars in Uganda and Tanzania. In: Smale M., Tushemereirwe W.K., editors. An Economic Assessment of Banana Genetic Improvement and Innovation in the Lake Victoria Region of Uganda and Tanzania. International Food Policy Research Institute; Washington: 2007. pp. 49–71. [Google Scholar]
- Eggeling W.J. Government Printer; Entebbe: 1952. The Indigenous Trees of the Uganda Protectorate. [Google Scholar]
- Ehret C. Linguistic inferences about Early Bantu history. In: Ehret C., Posnansky M., editors. The Archaeological and Linguistic Reconstruction of African History. University of California Press; Berkeley: 1982. pp. 57–65. [Google Scholar]
- Ehret C. James Currey; Oxford: 1998. An African Classical Age: Eastern and Southern Africa in World History: 1000 BC to 400 AD. [Google Scholar]
- Ehret C. University of California Press; Berkeley: 2011. History and the Testimony of Language. [Google Scholar]
- Entenmann S.K., Schmitt C.B., Konold W. REDD+-related activities in Kenya: actors' views on biodiversity and monitoring in a broader policy context. Biodivers. Conserv. 2014;23:2561–2586. [Google Scholar]
- FAO . Food and Agricultural Organization of the United Nations; Rome: 1992. International Code of Conduct for Plant Germplasm Collecting and Transfer. [Google Scholar]
- FAO . Food and Agricultural Organization of the United Nations; Rome: 1998. The State of the World's Plant Genetic Resources for Food and Agriculture. [Google Scholar]
- FAO . Food and Agricultural Organization of the United Nations; Rome: 2010. Global Forest Resources Assessment 2010. [Google Scholar]
- FAO . Forestry Department, Food and Agriculture Organization of the United Nations; Rome: 2010. Global Forest Resources Assessment: Country Report for Uganda. [Google Scholar]
- FOE . Friends of the Earth International; Amsterdam: 2012. Land, Life and Justice: How Land Grabbing in Uganda Is Affecting the Environment, Livelihoods and Food Sovereignty of Communities. [Google Scholar]
- Galabuzi C., Eilu G., Mulugo L., Kakudidi E.K., Tabuti J., Sibelet N. Strategies for empowering the local people to participate in forest restoration. Agroforest Syst. 2014;88:719–734. [Google Scholar]
- Galabuzi C., Nabanoga G.N., Ssegawa P., Obua J., Eilu G. Double jeopardy: bark harvest for malaria treatment and poor regeneration threaten tree population in a tropical forest of Uganda. Afr. J. Ecol. 2015;53:214–222. [Google Scholar]
- Given D.R. Timber Press; Portland: 1994. Principles and Practice of Plant Conservation. [Google Scholar]
- Gold C.S., Kiggundu A., Abera A.M.K., Karamura D. Diversity, distribution and farmer preference of Musa cultivars in Uganda. Exp. Agric. 2002;38:39–50. [Google Scholar]
- Hamilton A.,C., editor. Medicinal Plants in Conservation and Development: Case Studies and Lessons Learnt. Plantlife International; Salisbury: 2008. [Google Scholar]
- Hamilton A.C. The interpretation of pollen diagrams from highland Uganda. Palaeoecol. Afr. 1972;7:45–149. [Google Scholar]
- Hamilton A.C. The dispersal of forest tree species in Uganda during the Upper Pleistocene. Boissiera. 1975;24:29–32. [Google Scholar]
- Hamilton A.C. The significance of patterns of distribution shown by forest plants and animals in tropical Africa for the reconstruction of upper Pleistocene palaeo-environments: a review. Palaeoecol. Afr. 1976;9:63–97. [Google Scholar]
- Hamilton A.C. Academic Press; London: 1982. Environmental History of East Africa: a Study of the Quaternary. [Google Scholar]
- Hamilton A.C. Oxford University Press; Nairobi: 1984. Deforestation in Uganda. [Google Scholar]
- Hamilton A.C. Vegetation and climate of Mt Elgon during the late Pleistocene and Holocene. Palaeoecol. Afr. 1987;18:283–304. [Google Scholar]
- Hamilton A.C. Guenon Evolution and forest history. In: Gautier-Hion A., Bourlière F., Gautier J.-P., Kingdon J., editors. A Primate Radiation: Evolutionary Biology of the African Guenons. Cambridge University Press; Cambridge: 1988. pp. 13–34. [Google Scholar]
- Hamilton A.C. Makerere University Printery; Kampala: 1991. A Field Guide to Uganda Forest Trees. [Google Scholar]
- Hamilton A.C. History of forests and climate. In: Sayer J.A., Harcourt C.S., Collins N.M., editors. The Conservation Atlas of Tropical Forests: Africa. Macmillan Publishers Ltd; Basingstoke: 1992. [Google Scholar]
- Hamilton A.C. An evidence-based approach to conservation through medicinal plants. Med. Plant Conserv. 2011;14:2–7. [Google Scholar]
- Hamilton A.C., Aumeeruddy-Thomas Y. Maintaining resources for traditional medicine: a global overview and a case study from Buganda (Uganda) Plant Divers Resour. 2013;35:407–423. [Google Scholar]
- Hamilton A.C., Baranga J., Tindigarukayo J. Uganda National Parks; Kampala: 1990. Proposed Bwindi (Impenetrable) National Park: Results of a Public Inquiry and Recommendations for its Establishment. [Google Scholar]
- Hamilton A.C., Bensted Smith R. International Union for Conservation of Nature and Natural Resources; Gland and Cambridge: 1989. Forest Conservation in the East Usambara Mountains, Tanzania. [Google Scholar]
- Hamilton A.C., Cunningham A., Byarugaba D., Kayanja F. Conservation in a region of political instability. Conserv. Biol. 2000;14:1722–1725. doi: 10.1111/j.1523-1739.2000.99452.x. [DOI] [PubMed] [Google Scholar]
- Hamilton A.C., Hamilton P.B. Earthscan; London: 2006. Plant Conservation: an Ecosystem Approach. [Google Scholar]
- Hamilton A.C., Perrott R.A. Modern pollen deposition on a tropical African mountain. Pollen Spores. 1980;22:437–468. [Google Scholar]
- Hamilton A.C., Perrott R.A. A study of altitudinal zonation in the Montane Forest Belt of Mt. Elgon, East Africa. Vegetatio. 1981;45:107–125. [Google Scholar]
- Hamilton A.C., Taylor D. Mire sediments in East Africa. In: Frostick L.E., Renaut R.W., Reid I., Tiercelin J.J., editors. Sedimentation in the African Rifts. Geological Society Special Publication; London: 1986. pp. 211–217. [Google Scholar]
- Hamilton A.C., Taylor D. History of climate and forests in tropical Africa during the last 8 million years. Clim. Change. 1991;19:65–78. [Google Scholar]
- Hamilton A.C., Taylor D., Vogel J.C. Early forest clearance and environmental degradation in south-west Uganda. Nature. 1986;320:164–167. [Google Scholar]
- Hamilton A.C., Taylor D., Vogel J.C. Neolithic forest clearance at Ahakagyezi, western Uganda. In: Mahaney W.C., editor. Quaternary and Environmental Research on East African Mountains. Balkema; Rotterdam: 1989. pp. 435–463. [Google Scholar]
- Harris D.J., Poulsen A.D., Frimodt-Möller C., Preston J., Cronk Q. Rapid radiation in Aframomum (Zingiberaceae): evidence from nuclear ribosomal DNA internal transcribed (ITS) sequences. Edinb. J. Bot. 2000;57:377–395. [Google Scholar]
- Hart T.B., Hart J.A., Dechamps R., Fournier M., Ataholo M. Changes in forest composition over the last 4000 years in the Ituri basin, Zaire. In: van der Maesen L.J.G., van der Burgt X.M., van Medanback de Rooy J.M., editors. The Biodiversity of African Plants. Kluwer Academic Publishers; Dordrecht: 1996. pp. 541–563. [Google Scholar]
- Hawkes J.G., Maxted N., Ford-Lloyd B.V. Kluwer Academic Publishers; London: 2001. The ex situ Conservation of Plant Genetic Resources. [Google Scholar]
- HMSO . His Majesty's Stationery Office; London: 1906. Census of the British Empire 1901. [Google Scholar]
- Howard P.C. International Union for Conservation of Nature and Natural Resources; Gland: 1991. Nature Conservation in Uganda's Tropical Forest Reserves. [Google Scholar]
- Howard P.C. Protected area planning in the tropics: Uganda's national system of forest nature reserves. Conserv. Biol. 2000;14:1–19. [Google Scholar]
- Iles L. Impressions of banana pseudostem in iron slag from eastern Africa. Ethnobot. Res. Appl. 2009;7:283–291. [Google Scholar]
- Jacobs B.F. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Philos. Trans. Roy. Soc. B. 2004;359:1573–1583. doi: 10.1098/rstb.2004.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacovelli P. Sawlog Production Grant Scheme; Kampala: 2009. Tree Planting Guidelines for Uganda. revised edn. [Google Scholar]
- Jagger P. Consultative Group on International Agricultural Research; Washington: 2008. Forest Incomes after Uganda's Forest Sector Reform. [Google Scholar]
- Jolly D., Taylor D., Marchant R., Hamilton A., Bonnefille R., Buchet G., Riollet G. Vegetation dynamics in central Africa since 18,000 BP: records from the interlacustrine highlands of Burundi, Rwanda and western Uganda. J. Biogeogr. 1997;24:495–512. [Google Scholar]
- Kabogozza J. African Forest Forum; Nairobi: 2011. Forest Plantations and Woodlots in Uganda. Working Paper. [Google Scholar]
- Kalema J., Beentje H. Royal Botanic Gardens; Kew: 2012. Conservation Checklist of the Trees of Uganda. [Google Scholar]
- Karamura D., Karamura E., Tushemereirwe W.K., Rubaihayo P.R., Markham R. Somatic mutations and their implications to the conservation strategies of the East African highland bananas (Musa spp.) Acta Hortic. 2010;879:615–622. [Google Scholar]
- Karamura D., Kiggundu A., Karamura E. The complementarity of farmers' and botanical descriptors of the East African Highland banana cultivars (Musa, AAA) Acta Hortic. 2011;897:107–112. [Google Scholar]
- Karamura D., Mgenzi B. On farm conservation of Musa diversity in the great lakes region of East Africa. Afr. Crop Sci. J. 2004;12:75–83. [Google Scholar]
- Karamura D., Mgenzi B., Karamura E., Sharrock S. Exploiting indigenous knowledge for the management and maintenance of Musa biodiversity on farm. Afr. Crop Sci. J. 2004;12:67–74. [Google Scholar]
- Karamura D., Pickersgill B. A classification of the clones of East African Highland bananas (Musa) found in Uganda. Plant Genet. Resour. Newsl. 1999;119:1–6. [Google Scholar]
- Katende A.B., Birnie A., Tengnäs B. Regional Soil Conservation Unit, Swedish International Development Cooperation Agency; Nairobi: 1995. Useful Trees and Shrubs for Uganda. [Google Scholar]
- Katuura E., Waako P., Ogwal-Okeng J., Bukenya-Ziraba R. Traditional treatment of malaria in Mbarara district, western Uganda. Afr. J. Ecol. 2007;45:48–51. [Google Scholar]
- Kendall R.L. An ecological history of the Lake Victoria basin. Ecol. Monogr. 1969;39:121–176. [Google Scholar]
- Kew . Royal Botanic Gardens; Kew: 2012. Plants under Pressure – a Global Context. [Google Scholar]
- Kiage L.M., Liu Kam-biu. Late Quaternary paleoenvironmental changes in East Africa: a review of multiproxy evidence from palynology, lake sediments, and associated records. Prog. Phys. Geogr. 2006;30:633–658. [Google Scholar]
- Kikulwe E.M., Nowakunda K., Byabachwezi M.S.R., Talengera D., Katungi E., Tushemereirwe W.K. Development and dissemination of improved banana cultivars and management practices in Uganda and Tanzania. In: Smale M., Tushemereirwe W.K., editors. An Economic Assessment of Banana Genetic Improvement and Innovation in the Lake Victoria Region of Uganda and Tanzania. International Food Policy Research Institute; Washington: 2007. pp. 37–48. [Google Scholar]
- Killick T. Understanding British aid to Africa: a historical perspective. Dev. Policy Rev. 2005;23:665–681. [Google Scholar]
- Kisekka J.W. Uganda Coalition for Sustainable Development; 2012. Paper Mulberry: Good or Bad for Uganda?http://www.ugandacoalition.or.ug/content/paper-mulberry-good-or-bad-uganda (accessed 23.06.15.) [Google Scholar]
- Langdale-Brown I., Osmaston H.A., Wilson J.G. Government Printer; Entebbe: 1964. The Vegetation of Uganda (Excluding Karamoja) and its Bearing on Land Use. [Google Scholar]
- Lange D. Trade in plant material for medicinal and other purposes. Traffic Bull. 1997;17:21–32. [Google Scholar]
- Lejja B.J., Taylor D., Robertshaw P. Late-Holocene environmental variability at Munsa archaeological site, Uganda: a multicore, multiproxy approach. Holocene. 2005;15:1044–1061. [Google Scholar]
- Lejju J.B., Taylor D., Robertshaw P. Africa's earliest bananas? J. Archaeol. Sci. 2006;33:102–113. [Google Scholar]
- Lind E.M. Studies in Uganda swamps. Uganda J. 1956;20:166–176. [Google Scholar]
- Livingstone D.A. Postglacial vegetation of the Ruwenzori Mountains in Equatorial Africa. Ecol. Monogr. 1967;37:25–52. [Google Scholar]
- Lockwood Consultants Ltd . Canadian International Development Agency; Republic of Uganda. Quebec: 1973. Forest Resource Development Study. [Google Scholar]
- LTS . LTS International; Penicuik: 2010. Review of the Forestry Sector in Uganda: Proposals for Improving Governance and Effective Management in the Forestry Sector. [Google Scholar]
- Lunyiigo S.L. Wavah Books Ltd; Kampala: 2011. The Struggle for Land in Buganda 1888–2005. [Google Scholar]
- Lwanga J. 1992. Traditional Healers Baseline Survey in Gomba County (Uganda)http://www.popline.org/node/328013 (accessed 29.08.15.) [Google Scholar]
- Lye K.A., Bukenya-Ziraba R., Tabuti J.R.S., Waako P.J. Makerere University Herbarium; Kampala: 2008. Plant-medicinal Dictionary for East Africa. [Google Scholar]
- Lyons K., Richards C., Westoby P. The Oakland Institute; Oakland: 2014. The Darker Side of Green: Plantation Forestry and Carbon Violence in Uganda. [Google Scholar]
- Marchant R., Taylor D., Hamilton A.C. Late Pleistocene and Holocene history at Mubwindi swamp, southwest Uganda. Quat. Res. 1997;47:316–328. [Google Scholar]
- Martin G. Chapman and Hall; London: 1995. Ethnobotany: a Methods Manual. [Google Scholar]
- Mbida C., Doutrelepont H., Vrydaghs L., Swennen R.J., Beeckman H., de Langhe E., de Maret P. First archaeological evidence of banana cultivation in central Africa during the third millennium before present. Veg. Hist. Archaeobotany. 2001;10:1–6. [Google Scholar]
- McGlynn G., Mooney S., Taylor D. Palaeoecological evidence for Holocene environmental change from the Virunga volcanoes in the Albertine Rift, central Africa. Quat. Sci. Rev. 2013;61:32–46. [Google Scholar]
- Meunier Q., Lemmens R., Morin A. GraphiConsult (U) Ltd; Kampala: 2010. Alternatives to Exotic Species in Uganda: Growth and Cultivation of 85 Indigenous Trees. [Google Scholar]
- Mills R. 2006. Preserving the Past for the Future: the Importance of Archival Information in Forestry.http://www.istl.org/46-supp/article9.html (accessed 29.08.15.) [Google Scholar]
- Morrison M.E.S. Vegetation and climate in the uplands of south-western Uganda during the Later Pleistocene Period, 1. Muchoya Swamp, Kigezi District. J. Ecol. 1968;56:363–384. [Google Scholar]
- Morrison M.E.S., Hamilton A.C. Vegetation and climate in the uplands of south-western Uganda during the later Pleistocene period, 2: forest clearance and other vegetational changes in the Rukiga Highlands during the past 8000 years. J. Ecol. 1974;62:1–31. [Google Scholar]
- Mukombozi R. 2013. NARO Workers Evict Armed Herdsmen.http://www.monitor.co.ug/News/National/NARO-workers-evict-armed-herdsmen/-/688334/1670994/-/pqnv7k/-/index.html (accessed 29.08.15.) [Google Scholar]
- Mulumba J.W., Nkwiine C., Male-Kayiwa B., Kalanzi A., Karamura D. Evaluation of farmers' best practices for on-farm conservation of rare banana cultivars in the semi-arid region of Lwengo sub-county, Uganda. Uganda J. Agric. Sci. 2004;9:281–288. [Google Scholar]
- Mutengesa S. The Independent; 2012. Mailo Akenda Is Mengo's Emblem of Self-deception.http://www.independent.co.ug/column/opinion/5131-mailo-akenda-is-mengos-emblem-of-self-deception (accessed 28.08.15.) [Google Scholar]
- Nakkazi E. 2011. Ugandans Mobilize to Save Mabira Forest from Sugarcane Plantation.http://www.theecologist.org/campaigning/wildlife/1057616/ugandans_mobilise_to_save_mabira_forest_from_sugarcane_plantation.html (accessed 29.08.15.) [Google Scholar]
- Nantale G., Kakudidi E.K., Karamura D.A., Karamura E., Soka G. Scientific basis for banana cultivar proportions on-farm in East Africa. Afr. Crop Sci. J. 2008;16:41–49. [Google Scholar]
- Newmann K., Hildebrand E. Early bananas in Africa: the state of the art. Ethnobot. Res. Appl. 2009;7:353–362. [Google Scholar]
- Nicholson J.W. Government Printer; Entebbe: 1929. The Future of Forestry in Uganda. [Google Scholar]
- Nsita S.A. Decentralisation and forest management in Uganda. In: Colfer C.J.P., Capistrano D., editors. The Politics of Decentralization: Forests, Power and People. Earthscan; London: 2005. [Google Scholar]
- Nuwagaba T.F. Taga Nuwagaba and Nathan Kiwere; Kampala: 2014. Totems of Uganda: Buganda Edition. [Google Scholar]
- Onyango M., Karamura D., Keely S., Manshardt R., Haymar D. Morphological characterisation of East African AAB and AA dessert bananas (Musa spp.) Acta Hortic. 2011;897:95–105. [Google Scholar]
- Oslisly R., White L. Human impact and environmental exploitation in Gabon during the Holocene. In: Denham T.P., Iriate J., Vrydaghs L., editors. Rethinking Agriculture: Archaeological and Ethnoarchaeological Perspectives. Left Coast Press; Walnut Creek: 2007. pp. 347–360. [Google Scholar]
- Osmaston H.A. Almqvist and Wiksells Boktryckeri AB; Uppsala: 1968. Uganda. Hedberg I, Hedberg O. Conservation of Vegetation in Africa South of the Sahara. [Google Scholar]
- Pei Shengji. The road to the future? The biocultural values of the Holy Hill Forests of Yunnan Province, China. In: Verschuuren B., Wild R., McNeely J.A., Oviedo G., editors. Sacred Natural Sites. Earthscan; London: 2010. pp. 98–106. [Google Scholar]
- Pei Shengji, Hamilton A.C., Yang Lixin, Huai Huyin, Yang Zhiwei, Gao Fu, Zhang Quangxin. Conservation and development through medicinal plants: a case study from Ludian (Northwest Yunnan, China) and presentation of a general model. Biodivers. Conserv. 2010;19:2619–2636. [Google Scholar]
- Pei Shengji, Huai Huyin, Hamilton A.C., Hamilton P.B. China Environmental Science and Technology Press; Beijing: 2009. Plant Resource Conservation. [Google Scholar]
- Perrier X., De Langhe E., Donohue M., Lentfer C., Vrydaghs L., Bakry F., Carreel F. Multidisciplinary perspectives on banana (Musa spp.) domestication. PNAS U. S. A. 2011;108:11311–11318. doi: 10.1073/pnas.1102001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay M., Dimkpa C., Ude G., Makumbi D., Tushemereirwe W.K. Genetic variation in banana cultivar ‘sukali ndiizi’ grown in different regions of Uganda. Afr. Crop Sci. J. 2003;11:87–95. [Google Scholar]
- Pillay M., Ssebuliba R., Hartman J., Vuylsteke D., Talengera D., Tushemereirwe W.K. Conventional breeding strategies to enhance the sustainability of Musa biodiversity conservation for endemic cultivars. Afr. Crop Sci. J. 2004;12:59–65. [Google Scholar]
- Plana V. Mechanisms and tempo of evolution in the African Guineo-Congolian rainforest. Philos. Trans. R. Soc. B. 2004;349:1585–1594. doi: 10.1098/rstb.2004.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott-Allen R., Prescott-Allen C. Earthscan; London: 1988. Genes from the Wild: Using Wild Genetic Resources for Food and Raw Materials. [Google Scholar]
- Ray B.C. Oxford University Press; Oxford: 1991. Myth, Ritual, and Kingship in Buganda. [Google Scholar]
- Reimer P.J. IntCal09 and Marine09 radiocarbon age calibration curves, 0-50,000 years cal BP. Radiocarbon. 2009;51:1111–1150. [Google Scholar]
- Richardson J.E., Chatrou L.W., Mols J.B., Erkens R.H.J., Pirie M.D. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos. Trans. R. Soc. B. 2004;359:1495–1508. doi: 10.1098/rstb.2004.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertshaw P., Kamuhangire E.R., Reid A., Young R., Childs S.T., Pearson N. Archaeological research in Bunyoro-Kitara: preliminary results. Nyame Akuma. 1997;48:70–77. [Google Scholar]
- Rossignol-Strick M. African monsoons, an immediate climate response to orbital insolation. Nature. 1983;304:46–49. [Google Scholar]
- Rossignol-Strick M., Nesteroff W., Olive P., Vergnaud-Grazzini C. After the deluge: Mediterranean stagnation and sapropel formation. Nature. 1982;295:105–110. [Google Scholar]
- Rugadya M. 1999. Land Reform: the Ugandan Experience.http://www.mokoro.co.uk/files/13/file/lria/land_reform_ugandan_experience.pdf (accessed 29.08.15.) [Google Scholar]
- Ryves D.,B. Environmental change over the last millennium recorded in two contrasting crater lakes in western Uganda, eastern Africa (Lakes Kasenda and Wandakara) Quat. Sci. Rev. 2011;30:555–569. [Google Scholar]
- Sackett D.L., Rosenberg W.M.C., Gray J.A.M., Haynes R.B., Richardson W.S. Evidence-based medicine: what it is and what it isn't. Br. Med. J. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassen M. Wageningen University; Wageningen: 2014. Conservation in a Crowded Place: Forest and People on Mount Elgon, Uganda. [Google Scholar]
- Sassen M., Sheil D., Giller K.E., Ter Braak C.J.F. Complex contexts and dynamic drivers: understanding four decades of forest loss and recovery in an East African protected area. Biol. Conserv. 2013;159:257–268. [Google Scholar]
- Schippmann U., Leaman D.J., Cunningham A.B. Cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In: Bogers R.J., Craker L.E., Lange D., editors. Medicinal and Aromatic Plants. 17th ed. Springer; Dordrecht: 2006. (Wageningen UR Frontis Series). [Google Scholar]
- Schoenbrun D.L. Cattle herds and banana gardens: the historical geography of the western Great Lakes region, ca AD 800-1500. Afr. Archaeol. Rev. 1993;11:39–72. [Google Scholar]
- Schoenbrun D.L. We are what we eat: ancient agriculture between the great lakes. J. Afr. Hist. 1993;34:1–31. [Google Scholar]
- Schoenbrun D.L. The contours of vegetation change and human agency in eastern Africa's Great Lakes region. Hist. Afr. 1994;21:269–302. [Google Scholar]
- Schoenbrun D.L. Heinemann; Portsmouth: 1998. A Green Place, a Good Place: Agrarian Change, Gender and Social Identity in the Great Lakes Region to the 15th Century. [Google Scholar]
- Sembajjwe W.S.G. Sacred forests in Ganda society. Uganda J. 1995;42:32–44. [Google Scholar]
- Smale M., Tushemereirwe W.K., editors. An Economic Assessment of Banana Genetic Improvement and Innovation in the Lake Victoria Region of Uganda and Tanzania. International Food Policy Research Institute; Washington: 2007. [Google Scholar]
- Sosef M.S.M. Refuge begonias: taxonomy, phylogeny and historical biogeography of Begonia sect. Loasibeongia and sect. Scutobegonia in relation to glacial rainforest refuges. In: van der Maesen L.J.G., van der Burgt X.M., Medenbach de Rooy J.M., editors. The Biodiversity of African Plants. Kluwer; Dordrecht: 1994. pp. 602–611. [Google Scholar]
- Ssemmanda I., Ryves D.,B., Bennike O., Appleby P.G. Vegetation history in western Uganda during the last 1200 years: a sediment-based reconstruction from two crater lakes. Holocene. 2005;15:119–132. [Google Scholar]
- Sutherland W.J., Pullin A.S., Dolman P.M., Knight T.M. The need for evidence-based conservation. Trends Ecol. Evol. 2004;19:305–308. doi: 10.1016/j.tree.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Tabuti J.R.S. Herbal medicines used in the treatment of malaria in Budiope, Uganda: plants, use and administration. J. Ethnopharmacol. 2008;88:19–44. doi: 10.1016/j.jep.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Tabuti J.R.S., Dhillion S.S., Lye K.A. Traditional medicine in Bulamogi country, Uganda: its practitioners, users and viability. J. Ethnopharmacol. 2003;85:119–129. doi: 10.1016/s0378-8741(02)00378-1. [DOI] [PubMed] [Google Scholar]
- Tattersall I. If I had a hammer. Sci. Am. 2014;311:39–43. doi: 10.1038/scientificamerican0914-54. [DOI] [PubMed] [Google Scholar]
- Taylor D., Hamilton A.C., Lewis S.L., Nantale G. Thirty-eight years of change in a tropical forest: plot data from Mpanga Forest Reserve, Uganda. Afr. J. Ecol. 2008;46:655–667. [Google Scholar]
- Taylor D., Marchant R.A., Robertshaw P. A sediment-based history of medium altitude forest in central Africa: a record from Kabata Swamp, Ndale volcanic field, Uganda. J. Ecol. 1999;87:303–315. [Google Scholar]
- Taylor D.M. Late Quaternary pollen records from two Ugandan mires: evidence for environmental change in the Rukiga Highlands of southwest Uganda. Palaeogeogr. Palaeoecol. 1990;80:283–300. [Google Scholar]
- Tenywa G. New Vision; Kampala, Uganda: 2005. BIDCO Gets Bugala Island Forest Land. 14 September 2005. [Google Scholar]
- Tenywa G. New Vision; Kampala, Uganda: 2013. Oil Palm Growing Threatens Buggala Island Forest Cover. 17 April 2013. [Google Scholar]
- Thomas A.S. Uganda banana varieties and their uses. In: Tothill J.D., editor. Agriculture in Uganda. Oxford University Press; Oxford: 1940. pp. 116–120. [Google Scholar]
- Thompson K., Hamilton A.C. Elsevier; Amsterdam: 1983. Peatland and Swamps of the African Continent. Gore AJP. Mires: Swamp, Bog, Fen and Moor, B: Regional Studies; pp. 331–373. [Google Scholar]
- Tierney J.A., Lewis S.C., Cook B.I., LeGrande A.N., Schmidt G.A. Model, proxy and isotopic perspectives on the East African humid period. Earth Planet Lett. 2011;307:103–112. [Google Scholar]
- Tothill J.D., editor. Agriculture in Uganda. Oxford University Press; Oxford: 1940. [Google Scholar]
- Tugume A.K., Lubega G.W., Rubaihayo P.R. Genetic diversity of East African Highland bananas using AFLP. Infomusa. 2002;11:28–32. [Google Scholar]
- Tuxill J., Nabhan G.P. Earthscan; London: 2001. People, Plants and Protected Areas. [Google Scholar]
- Twongyirwe R., Sheil D., Sandbrook C.G., Sandbrook L.C. REDD at the crossroads? The opportunities and challenges of REDD for conservation and human welfare in South West Uganda. Int. J. Environ. Sust. Dev. 2015;14:273–298. [Google Scholar]
- USAID . United States Agency for International Development; Arlington: 2013. Uganda Climate Change Vulnerability Assessment Report. [Google Scholar]
- USAID . 2015. Uganda: Property Rights and Resource Governance.http://usaidlandtenure.net/sites/default/files/country-profiles/full-reports/USAID_Land_Tenure_Uganda_Profile.pdf (accessed 09.06.15.) [Google Scholar]
- Van Grunderbeek M.-C., Roche E. Multidisciplinary evidence of mixed farming during the Early Iron Age in Rwanda and Burundi. In: Denham T., Iriate J., Vrydaghs L., editors. Rethinking Agriculture. Left Coast Press, Inc; Walnut Creek: 2007. pp. 299–319. [Google Scholar]
- Van Orsdol K.G. Ministry of Agriculture and Forestry; Kampala: 1983. The Status of Kibale Forest Reserve in Western Uganda and Recommendations for its Conservation and Management. [Google Scholar]
- van Schaik A., Tickell O. 2015. UN, Banks and Oil Palm Giants Feast on the Stolen Land of Uganda's Dispossessed.http://www.theecologist.org/News/news_analysis/2759987/un_banks_and_oil_palm_giants_feast_on_the_stolen_land_of_ugandas_dispossessed.html (accessed 29.08.15.) [Google Scholar]
- Veit P. World Resources Institute; Washington: 2010. Land for Private Investors and Economic Development: Uganda. [Google Scholar]
- Vleminckx J. Soil charcoal to assess the impacts of past human disturbances on tropical forests. PLOS One. 2014;9:1–9. doi: 10.1371/journal.pone.0108121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G., Osmaston H.A. Commonwealth Secretariat; London: 2003. A History of the Uganda Forest Department 1951–1965. [Google Scholar]
- West H.W. Uganda Government; Entebbe: 1965. The Mailo System in Buganda. [Google Scholar]
- White S., Wanyama F. Forestry Department, Food and Agriculture Organization of the United Nations; Rome: 2006. Forest Management Working Paper: the Case of Mt Elgon UWA/FACE Carbon Sequestration Project in Uganda. [Google Scholar]
- Wild R.G., Mutebi J. Division of Ecological Sciences, United Nations Educational, Scientific and Cultural Organization; Paris: 1996. Conservation through Community Use of Plant Resources. People and Plants. Working Paper no. 5. [Google Scholar]
- Wild R.G., Mutebi J. Bwindi impenetrable forest, Uganda: conservation through collaborative management. Nat. Resour. 1997;33:33–51. [Google Scholar]
- World Bank . 2015. Population Estimates and Projections.http://datatopics.worldbank.org/hnp/popestimates (accessed 09.08.15.) [Google Scholar]
- Wrigley C.C. Bananas in Buganda. Azania. 1989;24:64–70. [Google Scholar]
- Zawedde B.M., Harris C., Alajo A., Hancock J., Grumet R. Factors influencing diversity of farmer's varieties of sweet potato in Uganda: implications for conservation. Econ. Bot. 2014;68:337–349. [Google Scholar]