Abstract
Objective
To assess the relationships among obesity, insulin sensitivity, and testosterone in pubertal boys.
Participants
This study included 20 lean, obese, and type 2 diabetic (T2DM) males, the majority of whom underwent a hyperinsulinemic-euglycemic clamp (n=16).
Methods
Glucose disposal (M value), serum testosterone, and body mass index (BMI) z-score were measured. Differences in testosterone were evaluated by group (lean vs. obese vs. T2DM), while regression was performed to evaluate the relationships among testosterone, obesity and insulin sensitivity.
Results
Controlling for Tanner stage, testosterone concentration was significantly lower in obese (p=0.02) and T2DM males (p=0.001) compared to lean males. Furthermore, M value was significantly associated with serum testosterone, even after controlling for BMI and Tanner stage.
Conclusions
These data suggest that obese adolescent boys have lower serum testosterone than controls of the same Tanner stage, and echo the data in adult males associating obesity and insulin resistance with hypogonadism.
Keywords: insulin resistance, insulin sensitivity, obesity, hypogonadism, puberty, type 2 diabetes mellitus
INTRODUCTION
The observation that pubertal onset is occurring at younger ages in females in the past several decades was reported as early as 19971, and has received considerable attention in both the scientific literature and the lay press. A recent panel concluded that there is sufficient evidence to suggest that breast development is starting at an earlier age when data from recent cohort studies, such as Pediatric Research in Office Settings Network (PROS), Blood Institute’s National Growth and Health Survey, the National Health and Nutrition Examination Surveys (NHANES) and the Bogalusa Heart Study, are compared with older studies of pubertal development2. Furthermore, the earlier age of pubertal onset appears to coincide with the increasing prevalence of obesity3. Girls from the NHANES cohort who matured earlier had a greater body mass index (BMI) than late-onset girls4. The PROS study, which was specifically designed to assess pubertal timing in females, similarly found a correlation between breast Tanner stage and BMI z-score in a given age group1.
In contrast, one small Italian study reported delayed genital development in obese males5, a pattern opposite to that reported in females. Similarly, analysis of the NHANES cohort suggests that timing of pubertal onset in males is positively correlated with BMI, i.e. leaner males start puberty earlier4. A German study found that lean males reached Tanner stage 5 pubic hair at a younger age than obese males, and had higher testosterone concentrations, although no differences in testicular volume were detected6. Results of a recent longitudinal study in the US of 401 boys also suggest that obese males start puberty later than lean males7. However, other results are conflicting8, and mechanistic studies are lacking. Therefore, effects of obesity on gonadal development in males remain unclear.
Studies in adult males support a relationship between obesity and decreased total testosterone, sex hormone binding globulin (SHBG), and free testosterone, potentially due to gonadotropin secretory defects9–11. The relationship between obesity and hypogonadism appears to be stronger in adult males with insulin resistance, T2DM, and other components of the metabolic syndrome, rather than isolated obesity12,13. To our knowledge, the relationship between insulin resistance and gonadal function has not been assessed in obese adolescent males.
Thus, while multiple longitudinal and cross-sectional studies have confirmed the relationship between BMI and pubertal onset in girls14–17, data regarding the effect of obesity on pubertal timing in males, and the relationship between testosterone and insulin resistance in pubertal males, are limited. The objective of this study was to compare gonadal function during pubertal development, as assessed by serum testosterone concentration, in lean males and in obese males with and without T2DM. Furthermore, we aimed to assess the relationship between testosterone and insulin sensitivity in this cohort.
METHODS
Subjects
A total of 20 pubertal males between the ages of 12 and 19 years were recruited for a study of obesity and insulin resistance in youth. Height and weight were measured for determination of BMI. BMI z-score was calculated using BMI and age. Subjects were classified by BMI percentile as lean (≤85th percentile) or obese (>95th percentile). This group has been previously described in detail18. This cohort includes lean, obese, and T2DM adolescents. Obese subjects were matched for BMI with T2DM subjects. Mean age was 15 ± 2 years and Tanner stage was 4.1 ± 0.8 (range 3–5). Pubertal development was assessed by a single pediatric endocrinologist using the criteria established by Tanner and Marshall for pubic hair and genital development. In addition, testicular volume was measured using an orchidometer by a single pediatric endocrinologist and volumes were assigned an equivalent Tanner stage as follows: Tanner 2 = 3–5 ml vol., Tanner 3 =6–8 ml , Tanner 4 =10–12 ml , Tanner 5 =>12 ml. There was no significant difference in Tanner stage across the 3 groups as assessed by pubic hair and Tanner stage equivalent of testicular volume. A subset of these subjects (n=16) also had insulin sensitivity assessed by hyperinsulinemic euglycemic clamp.
Laboratory measures
Insulin sensitivity was calculated from a 3-hour hyperinsulinemic euglycemic clamp (80 mU*m−2*min−1 insulin) following 3 days of limited physical activity and a 3-day fixed-macro-nutrient, weight-maintenance diet, using the glucose disposal rate (M)19 as previously described18.
Testosterone was measured on a morning fasting sample, drawn prior to the insulin clamp, by chemiluminescence using the Beckman-Coulter Immunoassay Access System (intra- and inter-assay coefficients of variation (CV) are 2.1% and 5.1%, respectively). Serum glucose was measured using a Cobas Mira Plus Chemistry Analyzer with intra- and inter-assay CV of 0.66 – 1.24% and 3.9 – 4.6%, respectively. Serum insulin was measured by competitive radio-immunoassay (Diagnostic Systems Laboratories, Inc; DSL-1600) with intra- and inter-assay CV of 5.2 and 9.8%, respectively.
Statistical analysis
All analyses assume a two-sided test of hypothesis with an overall significance level of 0.05 (unless otherwise noted) and were performed in SAS v9.2 (SAS Institute Inc., Cary, NC). Kruskal-Wallis and Fisher’s exact tests were used to compare characteristics across groups. Pubic hair Tanner stage was used for all subjects in these analyses. ANCOVA regression was used to model testosterone as a function of the following set of variables: group (lean, obese and T2DM) and Tanner stage. Linear regression was used to model testosterone as a function of the following set of variables: Tanner stage, BMI z-score, and M value.
The study was approved by the Colorado Multiple Institution review board, and appropriate consent and assent were obtained.
RESULTS
Subject characteristics
From the total sample of 20 subjects, 7 (35%) were classified as lean, 6 (30%) as obese without diabetes and 7 (35%) as obese with T2DM (Table 1). As expected, BMI z-score was significantly lower in lean subjects (−0.03 ± 0.7, BMI=19.8 ± 0.8 mg/m2) compared to obese (2.0± 0.43, BMI=30.4 ± 2.3 mg/m2) or T2DM (2.3 ± 0.41, BMI=32.9 ± 2.7 mg/m2) subjects, but obese and T2DM subjects had a similar BMI z-score by design.
TABLE 1.
Subject Characteristics
| Characteristic | All (n=20) | Lean (n=7) | Obese (n=6) | T2DM (n=7) | p-value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (sd) | Median (range) | Mean (sd) | Median (range) | Mean (sd) | Median (range) | Mean (sd) | Median (range) | ||
| Age | 14.9 (2.0) | 14.5 (12, 19) | 15.7 (1.7) | 15.0 (14, 18) | 14.5 (2.6) | 13.5 (12, 19) | 14.3 (1.8) | 14.0 (12, 17) | 0.31 |
| BMI z-score | 0.69 (1.3) | 0.81 (−2.6, 2.8) | −0.03 (0.70) | −0.11 (−0.79, 0.96) | 2.0 (0.43) | 1.9 (1.5, 2.7) | 2.3 (0.41) | 2.4 (1.7, 2.8) | < 0.0001 |
| Race/Ethnicity** | |||||||||
| White | 8 (40%) | 5 (71%) | 2 (33%) | 1 (14%) | 0.15† | ||||
| Hispanic | 8 (40%) | 1 (14%) | 2 (33%) | 5 (71%) | |||||
| Other | 4 (20%) | 1 (14%) | 2 (33%) | 1 (14%) | |||||
| Tanner Stage** | |||||||||
| 3 | 3 (15%) | 1 (14%) | 1 (17%) | 1 (14%) | 1.0† | ||||
| 4 | 9 (45%) | 3 (43%) | 3 (50%) | 3 (43%) | |||||
| 5 | 8 (40%) | 3 (43%) | 2 (33%) | 3 (43%) | |||||
Kruskal-Wallis Test
N (%)
Fisher’s Exact Test
Obesity and serum testosterone concentration
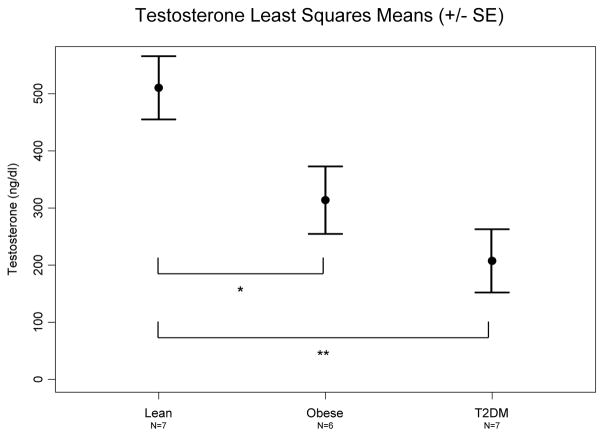
Serum testosterone concentration was significantly different in the overall comparison across groups (p=0.004) and post-hoc tests revealed significant differences between both the lean and obese (p=0.02) and the lean and T2DM (p=0.001) subjects when controlling for Tanner stage (Figure 1).
Fig. 1.
Comparison of estimated testosterone between lean, obese and T2DM males. Testosterone concentrations are adjusted for Tanner stage. *Lean vs. Obese p=0.02, **Lean vs. T2DM p=0.001.
Testosterone and insulin sensitivity
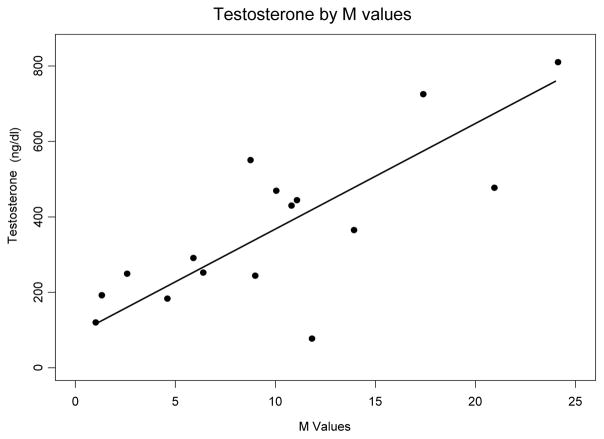
In order to further assess whether insulin sensitivity is an independent predictor of serum testosterone concentration, a regression analysis was performed modeling testosterone as a function of M value, BMI z-score and Tanner stage. In these subjects, M value was significantly associated with serum testosterone after controlling for BMI z-score and Tanner stage (p=0.006). For each unit (mmol* kg−1 *min−1) decrease in M value, testosterone decreased by 176 nmol/l [28 ng/dL decrease in testosterone for each unit (mg*kg−1 *min−1) decrease in M value (95% CI (9.7, 46.3 ng/dl)] (Figure 2).
Fig. 2.
Regression analysis of testosterone by M value, controlling for BMI and Tanner stage. p=0.006 (to convert values for testosterone to SI units (nmol/l), multiply by 0.037)
DISCUSSION
Our results suggest that serum testosterone, after adjusting for Tanner stage, is lower in obese boys with and without T2DM than in lean boys. These data are consistent with reports of lower testosterone concentrations in obese adults.
Furthermore, while BMI contributes to lower serum testosterone concentration in pubertal males, these data suggest that the relative hypogonadism in obese males is related to the degree of insulin resistance. To our knowledge, we are the first to report these relationships among insulin resistance, obesity, and serum testosterone in pubertal adolescent males.
The paucity of recent data regarding pubertal development in males in general and, more specifically, about the effects of obesity on male pubertal development is surprising, especially given the considerable recent attention that these topics have received in females. Two small studies, performed in European populations, suggest that obese boys may have delayed pubertal development. One of these studies also reported lower testosterone concentrations in obese boys5,6. Data from NHANES suggest that pubertal development in obese males is not advanced, and may even be delayed4.
Two more recent studies specifically address effects of obesity on age at onset of pubertal development, and have conflicting results. A large Danish study compared a cross-sectional cohort of boys from 2006 with one from 19918. They found that age of onset of Tanner 2 pubic hair and genital development was negatively correlated with BMI. They also report that age-adjusted testosterone is positively correlated with BMI in the 2006 cohort. Our study does not include males in Tanner 2 puberty. However, it is possible that puberty starts on time in obese males, but that pubertal progression is slower. This possibility is not addressed in the Danish paper. Furthermore, our study includes a more diverse, insulin resistant population. Both our study and the Danish study are cross-sectional, making it difficult to truly assess onset of pubertal development. However, a recent longitudinal study done in the US and specifically designed to assess the relationship between BMI and the onset of pubertal development7, found that boys in the highest BMI trajectory were more likely to be prepubertal at a given age than their lean counterparts. Our results support the argument that BMI influences testosterone and pubertal development in males. Further longitudinal studies in a larger cohort of young males are needed to explore these relationships.
The association of decreasing serum testosterone concentration with decreasing insulin sensitivity, as measured in these subjects by the gold standard hyperinsulinemic euglycemic clamp, suggests that the relationship between poor gonadal function and insulin resistance described in older adult males is present as early as adolescence. The possibility that insulin resistance may be a significant contributor to hypogonadism in young obese males is a novel finding in this study.
The etiology of this relative hypogonadism in obese pubertal males remains unclear. Leptin concentrations increase early in puberty in both males and females. However, in males, unlike in females, leptin normally declines as puberty progresses20,21. It has been postulated that the paradoxically persistently elevated leptin reported in obese males may be responsible for suppression of gonadal function22. Alternatively, increased aromatase activity in excessive adipose tissue may cause increased conversion of testosterone to estrogen, increased ratio of estrogens to androgens, and resulting suppression of gonadotropins. However, neither of these explanations takes into account our demonstration of the apparent relationship between insulin resistance and serum testosterone concentration. It is certainly plausible that, as in females, insulin resistance and resulting hyperinsulinemia result in direct gonadal dysfunction in males. However, while insulin resistance-associated gonadal dysfunction in females leads to excess androgen production, in males it may lead to decreased testosterone production.
There are several limitations to our study design. First, this is a small study which lacks longitudinal follow-up of individual subjects. Future studies following obese males during pubertal development are planned to provide such longitudinal information. An additional limitation is that only total testosterone was measured. Reductions in SHBG, previously reported in obese boys6, could at least partially explain the reductions in total testosterone. Despite a similar relationship between insulin resistance/obesity and SHBG, adult male studies importantly still show a reduction in free testosterone, arguing that decreased total testosterone levels in obese males are not solely an artifact of decreased SHBG. Furthermore, a recent study suggests that lower SHBG is an independent risk factor for progression to T2DM23.
Despite these limitations, our finding that BMI contributes to hypogonadism in obese males supports and extends the limited data that have been previously published. More importantly, our finding that insulin resistance may be an important contributor to this relationship is unique, and warrants further investigation. These findings are very different from those reported in obese females, who appear to be precocious in sexual development. However, similar to obese females with polycystic ovarian syndrome, the obese boys in the cohort have evidence of dysfunction of gonadal steroid synthesis associated with insulin resistance. These results address a poorly studied area and clearly demonstrate that a more rigorous longitudinal assessment of pubertal progression, gonadal function and insulin resistance in males is necessary.
References
- 1.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 2.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TIA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 3.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–10. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 5.Vignolo M, Naselli A, Di BE, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr. 1988;147:242–4. doi: 10.1007/BF00442687. [DOI] [PubMed] [Google Scholar]
- 6.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes (Lond) 2007;31:1509–19. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body Mass Index and Timing of Pubertal Initiation in Boys. Arch Pediatr Adolesc Med. 2010 Feb 1;164(2):139–44. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen K, Aksglaede L, Petersen JH, Juul A. Recent Changes in Pubertal Timing in Healthy Danish Boys: Associations with Body Mass Index. J Clin Endocrinol Metab. 2009 Nov 19; doi: 10.1210/jc.2009-1478. jc. [DOI] [PubMed] [Google Scholar]
- 9.Allan CA, Strauss BJ, McLachlan RI. Body composition, metabolic syndrome and testosterone in ageing men. Int J Impot Res. 2007;19:448–57. doi: 10.1038/sj.ijir.3901552. [DOI] [PubMed] [Google Scholar]
- 10.az-Arjonilla M, Schwarcz M, Swerdloff RS, Wang C. Obesity, low testosterone levels and erectile dysfunction. Int J Impot Res. 2008;21:89–98. doi: 10.1038/ijir.2008.42. [DOI] [PubMed] [Google Scholar]
- 11.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–8. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 12.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively High Incidence of erectile dysfunction observed in these men? Journal Urol. 2006;176:1524–8. doi: 10.1016/j.juro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US adolescent girls. Am J Public Health. 2001;91:642–4. doi: 10.2105/ajph.91.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 16.Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, McClanahan BS. Early sexual maturation, body composition, and obesity in African-American girls. Obes Res. 2004;(Suppl):64S–72S. doi: 10.1038/oby.2004.270. [DOI] [PubMed] [Google Scholar]
- 17.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–53. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 18.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed ML, Ong KKL, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84:899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82:2849–55. doi: 10.1210/jcem.82.9.4235. [DOI] [PubMed] [Google Scholar]
- 22.Kaplowitz P. Delayed puberty in obese boys: comparison with constitutional delayed puberty and response to testosterone therapy. J Pediatr. 1998;133:745–9. doi: 10.1016/s0022-3476(98)70144-1. [DOI] [PubMed] [Google Scholar]
- 23.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009 Sep 17;361(12):1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]