Abstract
‘Cassette’-ISES (In Situ Enzymatic Screening) Identifies Complementary Chiral Scaffolds for Hydrolytic Kinetic Resolution Across a Range of Epoxides
A new ‘Cassette’-In Situ Enzymatic Screen (ISES) for combinatorial catalysis is introduced. This allows the experimentalist to obtain an information-rich readout, in real time, providing an estimate of the sense and magnitude of enantioselectivity across more than one substrate. In its first iteration, the screen identified CoIII-salen catalysts with β-pinene- and α-naphthylalanine-derived chiral scaffolds with broad, yet complementary, substrate specificities.
Keywords: asymmetric catalysis, combinatorial chemistry, cobalt, salen, UV/vis spectroscopy
For combinatorial catalysis,[1,2] rapid and information-rich screening methods are very useful. Toward, this end, we describe herein a new ‘cassette’-ISES approach which allows the experimentalist to obtain a parallel readout on substrate specificity, as well as sense and magnitude of enantioselectivity.[3] In its first iteration, ‘cassette’-ISES is used to ‘cherry pick’ only those catalysts in the array that show high ISES-ee readouts across both test substrates. Two such catalysts are then investigated further, yielding promising results.
In our ISES approach, typically the reaction product[4] or byproduct[5] diffuses from an organic layer into an aqueous layer containing the ‘reporting enzyme.’ [6] There, an enzyme-catalyzed reaction leads to a spectroscopic signal that is monitored in real time. The approach complements other emerging screens using chiroptical techniques,[7] liquid crystalline arrays,[8] IR thermography,[9] mass,[10] NMR,[11] IR[12] and fluorescence spectroscopy.[13] The technique is sensitive (i.e. 10 nmol of product gives rise to Abs340 ~ 0.12 for a dehydrogenase reporting enzyme in a 500 μL aq. volume), allowing one to get information on catalyst performance at relatively early conversions/short reaction times. Catalysts may be screened in parallel in a standard spectrophotometer with a multicell changer, without the need to draw aliquots or work-up the reaction. Moreover, the need to install a chromophore (adding steps and potentially altering substrate reactivity) is obviated.
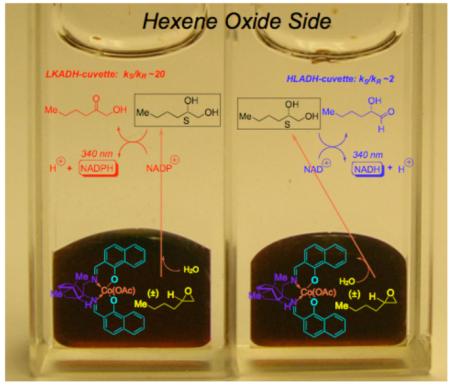
In this Communication (Scheme 1), we describe a new pair of reporting enzymes, capable of differentiating the 1,2-hexanediol antipodes (LKADH – highly S-selective kS/kR ~20 and HLADH – modestly S-selective kS/kR ~2.2). This allows one to obtain simultaneous enantioselectivity readouts on two distinct substrates for the CoIII-salen-mediated HKR (hydrolytic kinetic resolution) of epoxides,[14] presenting both ‘short’ (propylene oxide; R = Me)[4] and long (hexene oxide: R = Bu) R groups. In this way, one can begin to address the question of substrate generality so important in asymmetric catalysis today.[15,16]
Scheme 1.
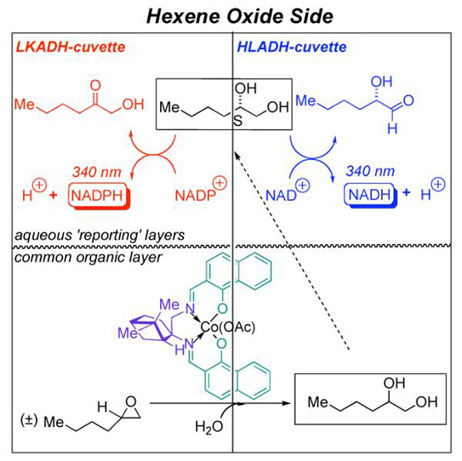
A suitable pair of ‘reporting enzymes’ for 1,2-hexanediol permits for a ‘cassette’-ISES evaluation of HKR catalyst candidates. Each ’side’ of the cassette screens for a particular substrate, and comprises a pair of cuvettes, with identical (lower – CH2Cl2) organic layers, but distinct reporting DH’s in the (upper) aqueous layer.
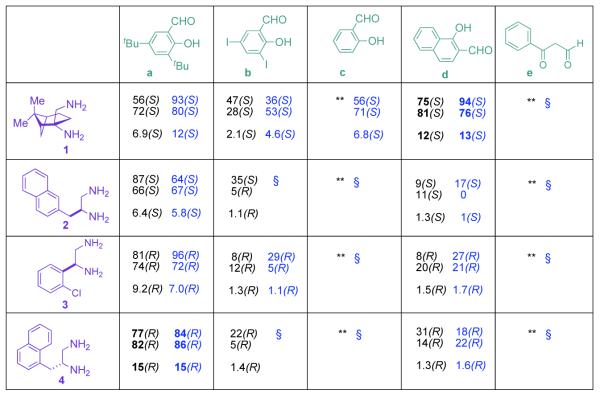
To demonstrate proof of principle for ‘cassette’-ISES, we employed a focused chiral salen array (Figure 1) that crosses chiral space variation in the constituent 1,2-diamines with considerable steric[17], and electronic variation in the ‘salicylaldehyde partners’ (including benzoylacetaldehyde = baen[18] precursor). Figure 2 illustrates the ‘four coordinate’ SER (structure-enantioselectivity relationship) data that one obtains from such a ‘cassette’-ISES protocol. The x and y axes represent the two structural variables in the salen array. The directionality and length of the z-vector provide the sense and magnitude of enantioselection, respectively. The fourth dimension is substrate variation, and this is represented by color-coded bars.
Figure 1.
Structure vs. enantioselectivity profile for the CoIII-acetate catalysts derived from this 5 × 4 salen/baen ligand library. Within a box, the entries (top to bottom) represent (a) ISES-estimated ee; (b) observed ee (flask conditions: neat, rt) and (c) calc’d E value. Black (left) and blue (right) columns designate results with propylene oxide, and hexene oxide, respectively. ** and § denote slow catalysts showing enzymatic reporting rates ≤ 15 mAbs min−1, for propylene oxide, and hexene oxide, respectively.
Figure 2.
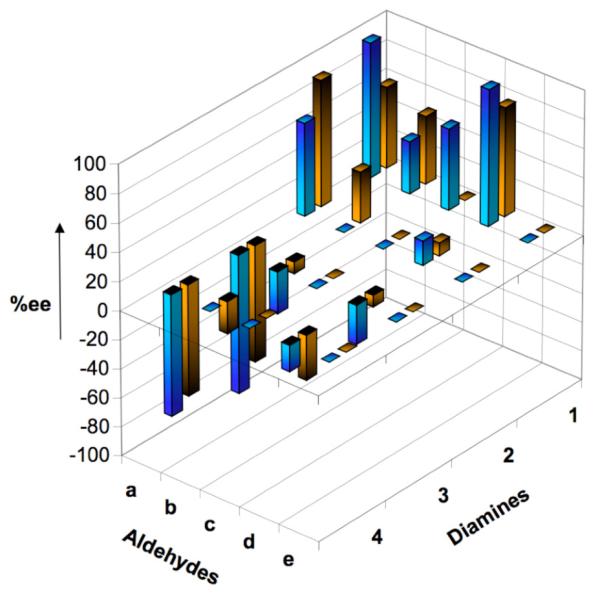
Three dimensional view of the enantioselectivity output from cassette-ISES for the 5 × 4 ligand array. For a given catalyst, brown and blue bars represent predicted ee’s for propylene oxide and hexene oxide, respectively. A positive deflection indicates S-selectivity, whereas a negative deflection indicates R-selectivity (convention follows the 1,2-propanediol optical rotation sign).
Several trends are apparent from this ‘cassette’-ISES readout. Unfortunately, the baen ligands (e) do not appear to produce effective CoIII-based HKR catalysts. Conversely, the most versatile diamine partner in the array is 1, successfully conferring S-selectivity, particularly for the hexene oxide test substrate, upon all of its derivative salens. This chiral element was first constructed through a clever MnIII-mediated diazidation of β-pinene by Snider,[19] and appears from these findings to have significant (as yet untapped) potential in asymmetric catalysis.
In seeking to ‘cherry pick’ the catalysts with the most promise for both high enantioselectivity and generality,[15] one quickly gravitates to ligands 1d, 3a and 4a. We chose 1d and 4a, as representative S- and R-selective cassette-ISES hits, for further development. These were next exposed to a more extensive battery of epoxides, differing in functionality, sterics, polarity and position and electronic nature of π-surfaces presented.
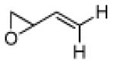
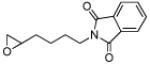
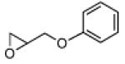
As can be seen in Table 1, catalyst CoIII-1d-OAc does indeed appear to retain ‘S’-antipodal selectivity across the epoxide library. Several substrates presenting π-surfaces at intermediate chain lengths from the epoxide are well matched with this catalyst. Particularly striking are the HKR’s with O-phenylglycidol (E ~20) and 4-benzyloxybut-1-ene oxide (E ~65!). Moreover, the selectivity associated with the HKR of the 2’-acetyl-4’-nitro-O-phenylglycidol [final entry, remaining epoxide (96% ee) has the correct handedness[20] for the drug], though not quite as spectacular, provides a formal new route[21] into the β-blocker celiprolol.
Table 1.
Kinetic Resolutions with the CoIII-1d-OAc Catalyst
| Epoxide | Loading (mol%) |
Cond.[a] | Isolated epoxide (% yield, % ee, E-value) |
Isolated diol (% yield, % ee, E-value) |
|---|---|---|---|---|
|
5 | A | 51, 45 R, 4 | 37, 52 S,[b] 4 |
|
5 | B | 46, 70 (R), 8 | 52, 67 (S),[c] 11 |
|
|
2 | B | 71, 27 (R), 6 | 29, 71 (S),[c] 8 |
|
|
2 | B | 70, 33 (R), 11 | 29, 79 (S),[c] 12 |
|
2 | B | 50, 80 S,[d] 22 | 45, 80 R,[d],[e] 18 |
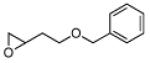 |
3 | B | 48, 96 R, 65 | 50, 91 S, [e] 67 |
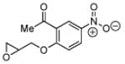 |
10 | C | 37, 96 S,[d],[f] 13 | 63, 55 R,[d],[g] 11 |
Cond. A: 12 h, 0°C, neat; Cond. B: 12 h, 0°C, THF; Cond. C: 24 h, rt, THF;
Stereochemistry assigned by comparison of HPLC ret’n time with that of authentic standards (ref 17) (see SI for details);
In these cases absolute stereochemistry was assigned by analogy to catalyst selectivity with structurally related substrates;
Note: The sense of enantioselection is the same for these examples, but substituent priorities lead to the opposite configurational assignment;
Absolute stereochemistry assigned by comparison of the observed optical rotation with literature value (ref 17);
The R-epoxide was independently synthesized from S-glycidol, and matches the minor peak here (Chiralcel OD);
Stereochemistry assigned by relative HPLC retention time (Chiralcel OD -ref 23).
In a complementary fashion, the CoIII-4a-OAc catalyst apparently displays rather general ‘R’-bias for epoxide ring-opening (Table 2). Here, epoxides bearing long chain alkyl groups such as 1,7-octadien-1-oxide,[22] and 6-t-butyldiphenylsilyloxy-hex-1-ene oxide appear to be especially well-resolved. And while O-phenylglycidol shows almost perfectly mirrored enantioselectivity here, the celiprolol substrate is processed much less selectively.
Table 2.
Kinetic Resolutions with the CoIII-4a-OAc Catalyst
| Epoxide | Loading (mol%) |
Cond.[c] | Isolated epoxide (% yield, % ee, E-value) |
Isolated diol (% yield, % ee, E-value) |
|---|---|---|---|---|
|
1 | A | 70, 33 S, 11 | 26, 72 R,[c] 8 |
|
|
1 | A | 44, 92 S, 20 | 56, 72 R,e19 |
|
|
4 | B | 59, 55 (S), 16 | 39, 80 (R),[d] 15 |
|
2 | B | 59, 53 (S), 13 | 37, 75 (R),[d] 11 |
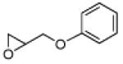
|
2 | B | 45, 93 R,[e] 25 | 55, 72 S,[e],[f] 17 |
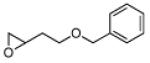
|
1 | B | 82, 17 S, 9 | 18, 80 R,[f] 11 |
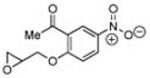
|
10 | B | 62, 28 R,[g] 3.5 | 37, 46 S,[e] 3.5 |
as for Table 1;
Assigned by comparison of optical rot'n with that of the known R-diol (ref 25);
– see notes
, Table 1;
The sign of the optical rotation was found to differ with that reported [the S-epoxide is said to levorotatory (ref 24) but the R-epoxide was found to be levorotatory here]. To confirm the absolute stereochemistry, the R-epoxide was independently from S-glycidol.
note
, Table 1.
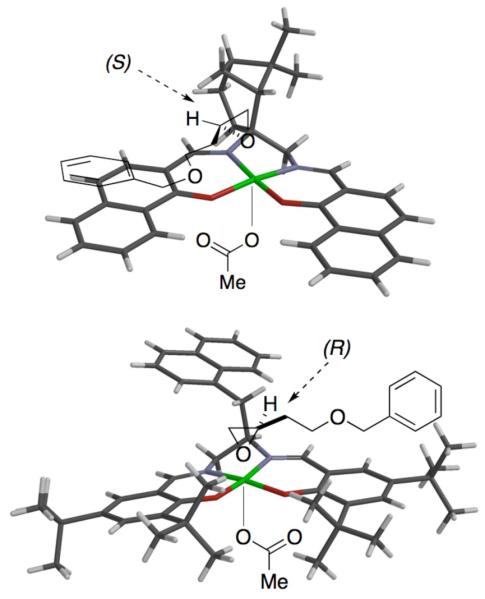
Three dimensional X-ray structures of both CoII-1d and CoII-4a have been obtained. If one assumes that upon oxidation to CoIII, the acetate ligand coordinates axially to the least hindered face,[23] then one can be begin to examine the available chiral epoxide-binding surface at the opposite face.
A schematic model[24] is presented in Figure 3, wherein preferred approach of the S-antipode from the ‘front left quadrant,’ as drawn, is proposed for CoIII-1d-OAc system. This raises the intriguing possibility of favorable π-π interactions between the α-hydroxy-β-naphthaldehyde platform and aryl substituents on the best resolved substrates. On the other hand, for CoIII-4a-OAc, ‘front right quadrant’ approach of the opposite enantiomer is suggested. No such π-π-interactions would be accessible to intermediately spaced aryl substituents here. Rather, these might have to ‘thread the needle’ in avoiding the bulky t-butyl groups, upon coordinating to the CoIII center.
Figure 3.
Three dimensional tube representation (Spartan 1.0.3) of X-ray crystallographic structures determined for the Co(II)-1d and Co(II)-4a complexes. Supermimposed upon the structures is presented a model consistent with the observed Co(III)-salen-enantiopreferences.
In conclusion, the ‘cassette’-procedure described herein, with readout on both enantio- and substrate selectivity, makes for an especially information rich in situ enzyme-based parallel screen. An interesting subtlety is that fact that the two 1,2-hexanediol reporting enzymes introduced here (LKADH and HLADH), are both S-selective, but differ greatly in the magnitude of that selectivity. That difference in selectivity allows us to pick up both S- and R-selective catalysts for hexene oxide ring opening. On the discovery side, this combinatorial approach has uncovered some rather unconventional scaffolds for asymmetric catalysis. For example, one of our best HKR ligands, 1d, is assembled from a non-C2-symmetric, terpene-derived chiral diamine and a sterically unencumbered α-hydroxy-β-naphthaldehyde partner, yet shows remarkable enantiodiscrimination (E ~ 65 for 4-benzyloxybutene oxide!). Given the rapidly expanding menu of metal-salen mediated C-X bond-forming reactions, this ligand scaffold likely will find application well beyond the HKR chemistry reported here.
Supplementary Material
Footnotes
This work was carried out with support from the NSF (CHE-0616840), ONR (05PR07809-00) and Nebraska Research Initiative. We acknowledge the NSF (CHE-0091975, MRI-0079750) and NIH (SIG-1-510-RR-06307) for NMR instrumentation, and the NIH (RR016544) for facilities renovation. For questions about the X-ray crystallography, contact D.R.P. or C.H. ADH = alcohol dehydrogenase; HL = horse liver; TB = Thermoanaerobium brockii; LK = Lactobacillus kefir.
Contributor Information
Sangeeta Dey, Department of Chemistry University of Nebraska Lincoln, NE 68588 (USA).
Douglas R. Powell, Department of Chemistry and Biochemistry University of Oklahoma Norman, OK 73019 (USA)
Chunhua Hu, Department of Chemistry University of Nebraska Lincoln, NE 68588 (USA).
David B. Berkowitz, Department of Chemistry University of Nebraska Lincoln, NE 68588 (USA).
Reference
- [1] a).Tan KL, Jacobsen EN, Angew. Chem, 2007, 119, 1337; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 2007, 46, 1315; [Google Scholar]; b) Zhao Y, Rodrigo J, Hoveyda AH, Snapper ML, Nature, 2006, 443, 67; [DOI] [PubMed] [Google Scholar]; c) Reyes SJ, Burgess K, Chem. Soc. Rev, 2006, 35, 416; [DOI] [PubMed] [Google Scholar]; d) Akullian LC, Snapper ML, Hoveyda AH, J. Am. Chem. Soc, 2006, 128, 6532; [DOI] [PubMed] [Google Scholar]; e) Reetz MT, Li X, Angew. Chem, 2005, 117, 3022; [Google Scholar]; Angew. Chem, 2005, 44, 2962;15827970 [Google Scholar]; f) Lee J-Y, Miller JJ, Hamilton SS, Sigman MS, Org. Lett, 2005, 7, 1837; [DOI] [PubMed] [Google Scholar]
- [2] a).Reetz MT, Compr. Coord. Chem, 2004, 9, 509; [Google Scholar]; b) Lavastre O, Bonnette F, Gallard L, Curr. Opin. Chem. Biol, 2004, 8, 311; [DOI] [PubMed] [Google Scholar]; c) Hoveyda AH, Murphy KE, Compr. Asymm. Catalysis, Supplement, 2004, 1, 171; [Google Scholar]; d) Stambuli JP, Hartwig JF 2003 Curr. Opin. Chem. Biol 7, 420; [DOI] [PubMed] [Google Scholar]; Reviews on Combi-catalysis:
- [3] a).Traverse JF, Snapper ML, Drug Discovery Today, 2002, 7, 1002; [DOI] [PubMed] [Google Scholar]; b) Reetz MT, Angew. Chem, 2002, 114, 1391; [Google Scholar]; Angew. Chem., Int. Ed, 2002, 41, 1335; [Google Scholar]; c) Finn MG, Chirality, 2002, 14, 534; [DOI] [PubMed] [Google Scholar]
- [4].Dey S, Karukurichi KR, Shen W, Berkowitz DB, J. Am. Chem. Soc, 2005, 127, 8610; [DOI] [PubMed] [Google Scholar]
- [5] a).Berkowitz DB, Shen W, Maiti G, Tetrahedron: Asymmetry, 2004, 15, 2845; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Berkowitz DB, Maiti G, Org. Lett, 2004, 6, 2661; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Berkowitz DB, Bose M, Choi S, Angew. Chem Angew. Chem., Int. Ed, 20022002, 11441, 1673, 1603; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6] a).Sprout CM, Seto CT, Org. Lett, 2005, 7, 5099; [DOI] [PubMed] [Google Scholar]; b) Onaran MB, Seto CT, J. Org. Chem, 2003, 68, 8136; [DOI] [PubMed] [Google Scholar]; c) Abato P, Seto CT, J. Am. Chem. Soc, 2001, 123, 9206; [DOI] [PubMed] [Google Scholar]; d) Janes LE, Lowendahl AC, Kazlauskas RJ, Chem. Eur. J, 1998, 4, 2324; [Google Scholar]; e) Hamberg A, Lundgren S, Penhoat M, Moberg C, Hult K, J. Am. Chem. Soc, 2006, 128, 2234; [DOI] [PubMed] [Google Scholar]; f) Z Li, L Buetikofer, B Witholt, Angew. Chem Angew. Chem., Int. Ed, 20042004, 11643, 1730, 1698; [Google Scholar]; g) Badalassi F, Klein G, Crotti P, Reymond J-L, Eur. J. Org. Chem, 20042557; [Google Scholar]; h) Klein G, Reymond J-L, Helv. Chim. Acta, 1999, 82, 400; [Google Scholar]
- [7] a).Matsushita H, Yamamoto N, Meijler MM, Wirsching P, Lerner RA, Matsushita M, Janda KD, Molecular BioSystems, 2005, 1, 303; [DOI] [PubMed] [Google Scholar]; b) Matsushita M, Yoshida K, Yamamoto N, Wirsching P, Lerner RA, Janda KD, Angew. Chem, 2003, 115, 6166; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 2003, 42, 5984; [Google Scholar]; c) Taran F, Gauchet C, Mohar B, Meunier S, Valleix A, Renard PY, Creminon C, Grassi J, Wagner A, Mioskowski C, Angew. Chem, 2002, 114, 132; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 2002, 41, 124; [Google Scholar]
- [8].Michaud M, Jourdan E, Villet A, Ravel A, Grosset C, Peyrin E, J. Am. Chem. Soc, 2003, 125, 8672; [DOI] [PubMed] [Google Scholar]
- [9] a).Zhu L, Shabbir SH, Anslyn EV, Chem. Eur. J, 2006, 13, 99; [DOI] [PubMed] [Google Scholar]; b) Li Z-B, Lin J, Pu L, Angew. Chem, 2005, 117, 1718; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 2005, 44, 1690; [Google Scholar]; c) Lee SJ, Lin W, J. Am. Chem. Soc, 2002, 124, 4554; [DOI] [PubMed] [Google Scholar]
- [10] a).Schoenfeld DL, Bornscheuer UT, Anal. Chem, 2004, 76, 1184; [DOI] [PubMed] [Google Scholar]; b) Mikami K, Angelaud R, Ding K, Ishii A, Tanaka A, Sawada N, Kudo K, Senda M, Chem. Eur. J, 2001, 7, 730; [DOI] [PubMed] [Google Scholar]
- [11].Eelkema R, van Delden RA, Feringa BL, Angew. Chem, 2004, 116, 5123; [Google Scholar]; Angew. Chem., Int. Ed, 2004, 43, 5013; [Google Scholar]
- [12].Reetz MT, Becker MH, Kuhling KM, Holzwarth A, Angew. Chem, 1998, 110, 2792; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 1998, 37, 2647; [Google Scholar]
- [13] a).Markert C, Pfaltz A, Angew. Chem, 2004, 116, 2552; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 2004, 43, 2498; [Google Scholar]; b) Reetz MT, Becker MH, Klein H-W, Stockigt D, Angew. Chem, 1999, 111, 1872; [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed, 1999, 38, 1758; [Google Scholar]
- [14] a).Reetz MT, Tielmann P, Eipper A, Ross A, Schlotterbeck G, Chem. Commun, 20041366; [DOI] [PubMed] [Google Scholar]; b) Evans MA, Morken JP, J. Am. Chem. Soc, 2002, 124, 9020; [DOI] [PubMed] [Google Scholar]
- [15].Tielmann P, Boese M, Luft M, Reetz MT, Chem. Eur. J, 2003, 9, 3882; [DOI] [PubMed] [Google Scholar]
- [16] a).Korbel GA, Lalic G, Shair MD, J. Am. Chem. Soc, 2001, 123, 361; [DOI] [PubMed] [Google Scholar]; b) Jarvo ER, Evans CA, Copeland GT, Miller SJ, J. Org. Chem, 2001, 66, 5522; [DOI] [PubMed] [Google Scholar]
- [17].S E. Schaus, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN, J. Am. Chem. Soc, 2002, 124, 1307; [DOI] [PubMed] [Google Scholar]
- [18].Jacobsen EN, 'Understanding selective yet general chiral catalysts,' Abstracts of Papers, 233rd ACS National Meeting, Chicago, IL, United States, March 25-29 2007 2007, ORGN228. [Google Scholar]
- [19].Satyanarayana T, Kagan HB, Adv. Synth. Catal, 2005, 347, 737; [Google Scholar]
- [20].Berkessel A, Ertuerk E, Adv. Synth. Catal, 2006, 348, 2619; [Google Scholar]
- [21].Averill DF, Broman RF, Inorg. Chem, 1978, 17, 3389; [Google Scholar]
- [22].Snider BB, Lin H, Syn. Comm, 1998, 28, 1913; [Google Scholar]
- [23].Jolly SR, Van Inwegen R, Schwab A, Pruss TP, Smith RD, Wolf PS, Drug Development Research, 1988, 14, 45; [Google Scholar]
- [24].Sayyed IA, Thakur VV, Nikalje MD, Dewkar GK, Kotkar SP, Sudalai A, Tetrahedron, 2005, 61, 2831; [Google Scholar]
- [25].Botes AL, Weijers CAGM, Botes PJ, Van Dyk MS, Tetrahedron: Asymmetry, 1999, 10, 3327; [Google Scholar]
- [26].Nielsen LPC, Stevenson CP, Blackmond DG, Jacobsen EN, J. Am. Chem. Soc, 2004, 126, 1360; [DOI] [PubMed] [Google Scholar]
- [27].We put forth this model simply for consideration here, recalling, at the same time, the complex mechanistic situation reported for Jacobsen’s catalyst that may have bearing on the catalysts under study here. These include the observed bimolecular dependence on metal-salen catalyst, and the suggestion that the antipodal epoxides may have similar binding energies to the catalyst, yet very different HKR rates (ref 26).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.