Abstract
Alginate has long been the material of choice for immunoprotection of islets due to its low cost and ability to easily form microspheres. Unfortunately, this seaweed-derived material is notoriously prone to fibrotic overgrowth in vivo, resulting in premature graft failure. The purpose of this study was to test an alternative, hyaluronic acid (HA-COL), for in vitro function, viability, and allogeneic islet transplant outcomes in diabetic rats. In vitro studies indicated that the HA-COL gel had diffusion characteristics that would allow small molecules such as glucose and insulin to enter and exit the gel, whereas larger molecules (70 and 500 kDa dextrans) were impeded from diffusing past the gel edge in 24 h. Islets encapsulated in HA-COL hydrogel showed significantly improved in vitro viability over unencapsulated islets and retained their morphology and glucose sensitivity for 28 days. When unencapsulated allogeneic islet transplants were administered to the omentum of outbred rats, they initially were normoglycemic, but by 11 days returned to hyperglycemia. Immunohistological examination of the grafts and surrounding tissue indicated strong graft rejection. By comparison, when using the same outbred strain of rats, allogeneic transplantation of islets within the HA-COL gel reversed long-term diabetes and prevented graft rejection in all animals. Animals were sacrificed at 40, 52, 64, and 80 weeks for evaluation, and all were non-diabetic at sacrifice. Explanted grafts revealed viable islets in the transplant site as well as intact hydrogel, with little or no evidence of fibrotic overgrowth or cellular rejection. The results of these studies demonstrate great potential for HA-COL hydrogel as an alternative to sodium alginate for long-term immunoprotected islet transplantation.
Keywords: : islet transplantation, encapsulation, alginate, hyaluronic acid, hydrogel, immunoprotection
Introduction
Islet transplantation will most likely remain an experimental treatment, whereas immunosuppressive therapy is required. The reality is that, for the majority of type 1 diabetics (T1D), the health risks of chronic immunosuppression may outweigh the potential enhanced quality of life granted by an islet transplant. Indeed, between 1999 and 2007, less than 400 islet transplantations were performed in the United States, whereas prevalence of this disease stands at ∼1.25 million cases of T1D nationwide.1,2
Extensive effort has been put forth to address this striking treatment gap. Numerous strategies have been employed toward eliminating the requirement of chronic immunosuppression, including immunomodulation via antigen-specific regulatory T cells, patient-specific stem cell-derived autologous beta cells, and a trove of immunoprotective devices, capsules, and coatings.3–5 Of these, immunoprotection by hydrogel encapsulation has received the greatest consideration as one of the most readily translatable approaches for the clinic.
Immunoprotection by gel encapsulation, in general, provides two key advantages. First, the extensive body of research surrounding this concept spans more than three decades, providing a wealth of fundamental knowledge and robust evidence of safety and efficacy to better facilitate translation.6–8 Second, immunoprotection enables transplantation of allo and xenogeneic (e.g., porcine) islets, the latter being a promising approach to the critical barrier of donor scarcity.9,10 To this point, immunoprotective hydrogel systems could have tremendous future utility in the safe delivery of stem cell-derived islet surrogates.11 Scharp and Marchetti recently published an extensive review summarizing numerous immunoprotection strategies along with several attempts to commercialize them.5 Of the various strategies, hydrogel encapsulation remains a leading approach to achieving widespread clinical success of islet transplantation.
Inexpensive alginate hydrogel microcapsules have dominated the islet encapsulation realm since their introduction in 1980.8 The nearly instantaneous gelation mechanism of these seaweed-derived alginate polymers enables simple fabrication of microcapsules that can be easily injected into a patient. Furthermore, these capsules have been established as both durable and non-toxic to host organisms.6,12,13
However, due to the foreign nature of the material, they are notoriously prone to fibrotic overgrowth, ultimately leading to necrosis of encapsulated cells and premature graft failure.12,14,15 Some progress has been made with the development of ultra-purification processes, surface treatments, co-encapsulated materials, and more stringent control of capsule microstructure, but despite these advances, the performance of alginate microcapsules still does not meet the clinical needs for islet transplants.13,14,16–18
Recently, native, “raw,” and biomimetic materials have been gaining attention as improved cellular scaffolds and delivery systems.19,20 Such materials are intrinsically biocompatible with a lower probability of inducing fibrosis, and they may better sustain or direct cellular function of encapsulated tissues. Lim et al. demonstrated enhanced in vitro function and survival of rat islets within a self-assembling biomimetic peptide gel.21 Liao et al. achieved similar results by using an injectable saccharide-peptide gel.22 In yet another study, a “biosynthetic” hydrogel loaded with vascular endothelial growth factor (VEGF) reversed diabetes in syngeneic mice at a 40% reduction in islet dose.23 However, these advanced, bioinspired hydrogels typically degrade too quickly to be effective for long-term immunoprotection of islet transplants.
Another material of particular interest for this application is hyaluronic acid (HA). HA-based hydrogels have been steadily gaining recognition as an interesting class of biomaterial for tissue engineering and cell therapy applications due to their unique mechanical and biological properties.24–26 In 2009, Vanderhooft et al. described the versatile rheological characteristics and tunable durability of a hydrogel system comprising thiolated HA and denatured collagen (COL) and a polyethylene glycol diacrylate (PEGDA) cross-linker.27 These HA-COL-derived hydrogels are easily prepared under physiological conditions, with shear moduli ranging from 11 to 3500 Pa. A commercially available version of this gel, sold under the brand HyStem-C, has recently been used in vivo for myocardial infarct repair in SCID mice and for osteochondral defect repair in rabbits.28,29
In light of the properties of this HA-COL-derived hydrogel, the present study sought to evaluate this biomaterial as a replacement for alginate hydrogels in encapsulated islet transplantation, particularly with regard to graft failure related to fibrosis. Unlike previous work, the animal studies were designed to test duration of the islet transplant.
Materials and Methods
Islet isolation
Canine islet isolation
Pancreata were obtained from canine donors at local veterinary clinics from animals scheduled for euthanasia for other purposes and with no known pancreatic disorders. Euthanasia was performed by the licensed veterinarian overseeing the care of each animal, and the clinical veterinarian confirmed death with loss of heart function. Collection of the donor pancreata after death from animals euthanized for reasons other than tissue procurement was determined to be exempt from review by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Canine islets were isolated from donors by using a method adapted from Vrabelova et al.30 Pancreata were removed after euthanasia at the clinic and transported to the laboratory on ice for islet isolation. Pancreata were digested with Liberase T-Flex (Roche Custom Biotech) and purified by discontinuous density gradient centrifugation with an iodixanol/HTK-based density gradient medium (OptiPrep; Cosmo Bio USA, Inc.), as is reported elsewhere.31
The isolated canine islets were cultured in Connaught Medical Research Laboratories (CMRL) 1066 media supplemented with 10% fetal bovine serum, 2 mM glutamine, and an antibiotic-antimycotic at 37°C and 5% CO2. Islets were quantified by conversion to islet equivalents (IEQs) by using standard methods.32 Islet purity was evaluated via dithizone staining (0.2 mg/mL dithizone in phosphate-buffered saline [PBS]), and islets had to have a purity 60% or higher to be included in the studies.
Rat islet isolation
The use of rats for islet isolation and transplantation was approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. Islets were isolated from pancreata and were procured from male and female Sprague–Dawley rats by collagenase digestion followed by discontinuous density gradient centrifugation with a Ficoll-based density gradient medium (Histopaque 1119 and 1077; Sigma Aldrich) as previously described.33–35 Islets were quantified and cultured as described earlier.33
Preparation of hydrogels and encapsulation of islets
Molecular diffusion in HA-COL gels
For diffusion studies, HA-COL hydrogels (HyStem-C; ESI Bio) were prepared according to the manufacturer's instructions with PEGDA as the cross-linker and poured into standard 24-well plates by using custom silicone dividers to create gels with a single exposed vertical edge to facilitate uniform lateral diffusion. Gels were stored in PBS overnight before testing to allow hydrogel swelling. Gels were ∼5 mm in height at equilibrium swelling.
Molecular diffusion into HA-COL hydrogels was examined via fluorescein isothiocyanate (FITC)-labeled dextrans of an increasing molecular weight (dextran-fluorescein 3, 70, and 500 kDa; Molecular Probes). Wells were loaded with FITC-dextran solution (0.1 mg/mL in PBS), and fluorescence at the gel/liquid interface was monitored for 24 h in a Cytation 5 Cell Imaging Multi-Mode Reader (Biotek Instruments, Inc.). Care was taken that the FITC-dextran solution did not cover the top of the gels to prevent diffusion from the z-direction.
Diffusion was further analyzed by generating plot profiles of the fluorescent micrographs by using NIH ImageJ (black = 0 intensity) to quantify fluorescence intensity at given x coordinates within the gels. Specifically, the x coordinates were chosen to represent 200, 500, and 1000 μm from the edge of the gel, with y values representing the average (line analysis) across the gel. Data are the average gray value of all pixels in the y-direction at a given x coordinate along the micrographs. Data were normalized to the average gray value of the surrounding medium outside the gel.
HA-COL gel encapsulation
HA-COL hydrogels were prepared according to the manufacturer's instructions for encapsulating cells (HyStem-C; ESI Bio). Canine islets were suspended in HA-COL gel precursor at ∼5000 IEQ/mL. IEQ is the standard measure of islet volume, with 1 IEQ equating to a spherical islet that is 150 μm in diameter.32 The cross-linker solution (PEGDA, MW 3400 in PBS) was added, and the suspension was thoroughly mixed. Finally, the gel precursor containing islets was distributed in 5 μL aliquots into 24-well non-tissue-treated plates. Each well contained one 5 μL gel/islet construct with ∼25 IEQ per gel. Gels were allowed to cross-link for 60 min. Subsequently, 500 μL of CMRL culture media (described earlier) was added to each well. Final constructs contained 1% gel polymer by weight.
Alginate encapsulation
Ultrapure, sterile “RGD” conjugated sodium alginate (Novatech MVG GRGDSP peptide-coupled alginate; FMC Biopolymer) was reconstituted in sterile deionized water containing 300 mM mannitol (to maintain isotonicity) at a concentration of 1% by weight to match that of the HA-COL gels. Islets were suspended in the alginate solution at ∼5000 IEQ/mL, and 5 μL droplets were added to individual wells of 24-well non-tissue-treated plates containing 2.0 mL of cross-linking solution (100 mM calcium chloride, 5 mM barium chloride, and 5 mM HEPES at pH 7.4).
Spherical gels formed on contact with the cross-linking solution, and they were allowed to cross-link for 10 min under mild orbital agitation. Gels underwent a 10-min hardening phase in HBSS with 10 mM calcium chloride. Finally, the hardening solution was removed from each well and replaced with 500 μL of CMRL islet culture medium. All islets were encapsulated between 2 and 4 days after isolation and incubated at 37°C and 5% CO2 in the aforementioned CMRL-based islet culture medium. Media were exchanged 50% by volume three times/week in all groups, including non-encapsulated controls.
In vitro assessment of encapsulated islets
Islet morphology, viability, and survival
Color micrographs of dithizone-stained islets were taken to evaluate islet morphology over long-term culture (Axio Vert.A1 Inverted Microscope; Zeiss International).
Encapsulated islet viability and long-term survival were assessed in all groups at 3, 7, 14, and 21 days after encapsulation via propidium iodide staining and fluorescence microscopy (Cytation 5 Cell Imaging Multi-Mode Reader; Biotek Instruments, Inc.).36 Percent viability, or viable cell fraction, was calculated by using procedures reported elsewhere.37 Results shown are the average viable cell fraction of at least 25 individually analyzed islets pooled from two separate islet donors for each group at every time point.
Glucose-stimulated insulin secretion
Glucose-stimulated insulin secretion (GSIS) was tested on days 3, 7, 14, 21, and 28 by using a method adapted from the standard Integrated Islet Distribution Program protocol.38 Unencapsulated (control) islets were tested in Transwell inserts (8.0 μm pore size). The islets within the inserts were moved between glucose solutions by using sterile forceps after each incubation step. Conversely, islet gels remained in their original wells and glucose solutions were exchanged with a micropipette.
Glucose solutions were made in an Earl's balanced salt solution buffer with 0.1% bovine serum albumin and sodium bicarbonate added, pH 7.4 at 37°C. Incubation in different glucose concentrations was done at 37°C and 5% CO2.
Islets were first equilibrated to the basal medium with 2.8 mM glucose for 1 h and then exposed to fresh 2.8 mM (low) glucose and 22.4 mM (high) glucose for 90 min each. Supernatant media were collected after each incubation and stored at −80°C until insulin quantification was performed. Tests were performed in triplicate on islets from three different canine donors for all experimental groups. Insulin concentration was quantified via Perkin Elmer alphaLISA insulin assays in conjunction with a Perkin Elmer EnSpire® plate reader. All assays were performed in triplicate according to the manufacturer's instructions by using a 12-point standard curve fit to a five-parameter logistic curve. Insulin concentration data were normalized to IEQ and reported as microIU/mL/IEQ.
Encapsulated islet transplantation
Diabetic rat models
Outbred streptozotocin-induced diabetic (immune-competent) Sprague–Dawley rats were generated as allogeneic islet transplant recipients as previously described.36 Diabetes was defined as an average non-fasting blood glucose (NFBG) above 300 mg/dL over three consecutive daily readings. Four diabetic rats received immunoprotected (encapsulated) islet transplants within the HA-COL gel, and two diabetic rats received islets without gel as controls to validate allo-rejection in the present model.
Three diabetic rats were given sham surgeries (i.e., without islets) as diabetic controls. Three non-diabetic rats were also included to monitor long-term blood glucose levels in aging rats.
Islet transplantation in HA-COL hydrogel and non-encapsulated controls
Rat surgeries followed our previously published protocols.33,34 Briefly, animals were anesthetized by ketamine/xylazine and isoflurane (as needed) and positioned in dorsal recumbency; abdominal fur was shaved, and surrounding skin was sterilized with iodine. A vertical midline incision was made through the abdominal muscle, and the stomach and greater omentum were gently exteriorized and placed on a small sterile pad.
A thin layer of HA-COL gel precursor solution containing the PEGDA cross-linker was applied to the entire omentum and was allowed to cross-link until the gel solution was no longer fluid. Islets, suspended in ∼200 μL of gel precursor, were applied to the omentum on top of the base gel layer. Care was taken that the islets were located near large blood vessels to maximize nutrient exchange. The islet/gel layer was given sufficient time to cross-link, and the omentum was folded into a pouch-like structure and sutured onto the stomach wall. Finally, the stomach/gel/omentum construct was gently returned into the peritoneal cavity, and the abdominal incision was closed.
Islets were transplanted at a minimum dose of 10,000 IEQ/kg into the omentum for all recipients. For encapsulated transplants, a thin layer of blank HA-COL gel was applied to the omental surface, followed by deposition of the islets suspended in HA-COL gel precursor. After gelation was complete, the omentum was sutured to the stomach wall to secure the transplant. For animals receiving transplants without hydrogel, islets were mixed with a sterile microfribrillar collagen hemostat (INSTAT MCH; Ethicon, Inc.) to form a paste-like substance to hold the islets in place.
Animal monitoring
The NFBG measurements were taken daily for the first 14 days post-transplant or until graft failure was observed, in which case the animals were sacrificed. Over time, the frequency of glucose readings was reduced to a minimum of once per week. Graft failure was defined as a return to a diabetic state as described earlier.
Graft explantation and evaluation
On sacrifice, the omenta of transplant recipients were removed and preserved for histological evaluation. The preserved tissues were embedded in paraffin and sectioned at 7 μm thicknesses. A total of 40 sections were collected and stained with either hematoxylin and eosin (H&E) or a triple fluorescent stain for insulin, glucagon, and somatostatin as previously described.39 Micrographs were examined to assess islet health, morphology, and cell composition at the transplant site, and to evaluate biocompatibility and durability of the HA-COL gel. For cell composition analysis, every fifth section was evaluated to ensure that cells were not counted multiple times, and a total of 70 unique islet sections were counted. When a potential overlapping islet was identified, its cells were not analyzed.
Data analysis
In vitro data were evaluated for significant differences by one-way repeated-measures ANOVA. Pairwise comparisons were evaluated via the Holm–Sidak test for significant differences between individual group means (p < 0.05).
Results
Diffusion properties of HA-COL hydrogels
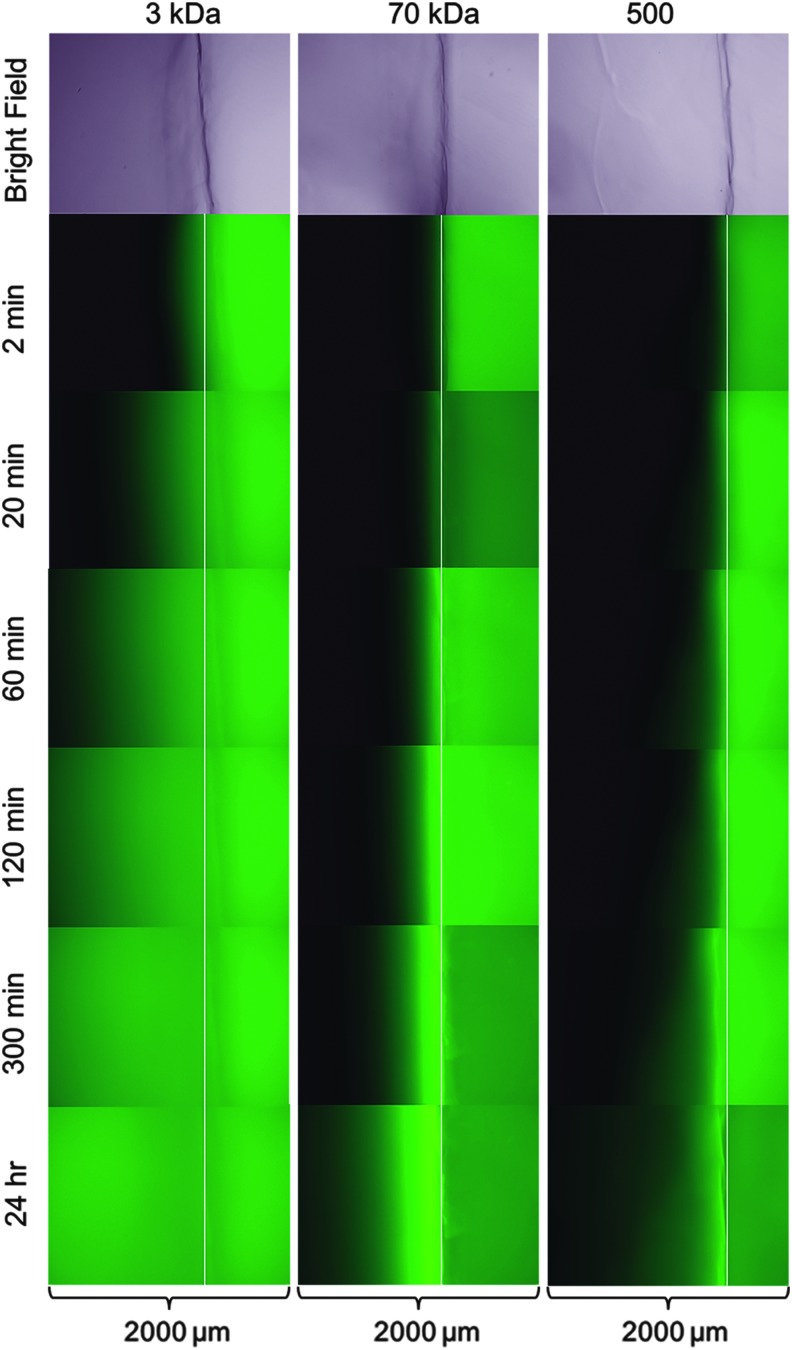
Micrographs of diffusion using fluorescent dextrans were acquired over a 24-h period (Fig. 1). Diffusion of the 3 kDa probe was very rapid, with significant penetration into the gel after just 2 min. Little diffusion of both 70 and 500 kDa probes was observed at the 2-min time point. Diffusion of the 500 kDa probe appeared minimal even after 24 h of incubation. Interestingly, accumulation of the 70 kDa probe near the gel edge was observed and appeared to increase with time, as indicated by more intense fluorescence relative to the surrounding medium. Some accumulation of the 500 kDa probe near the gel edge also appeared to be present at later time points, but it was minor. Edge accumulation was not observed with the 3 kDa probe.
FIG. 1.
Diffusion into HA-COL hydrogels. Fluorescence micrographs of 3 kDa (left), 70 kDa (center), and 500 kDa (right) FITC-dextran diffusion into HA-COL hydrogels were captured over 24 h. Bright-field images (40 × ) of the hydrogels are included at the top of each column for reference. Overlaid white lines represent the gel edge for each group. Dimensions of each individual micrograph were 1.4 × 2.0 mm. FITC, fluorescein isothiocyanate; HA-COL, hyaluronic acid-collagen.
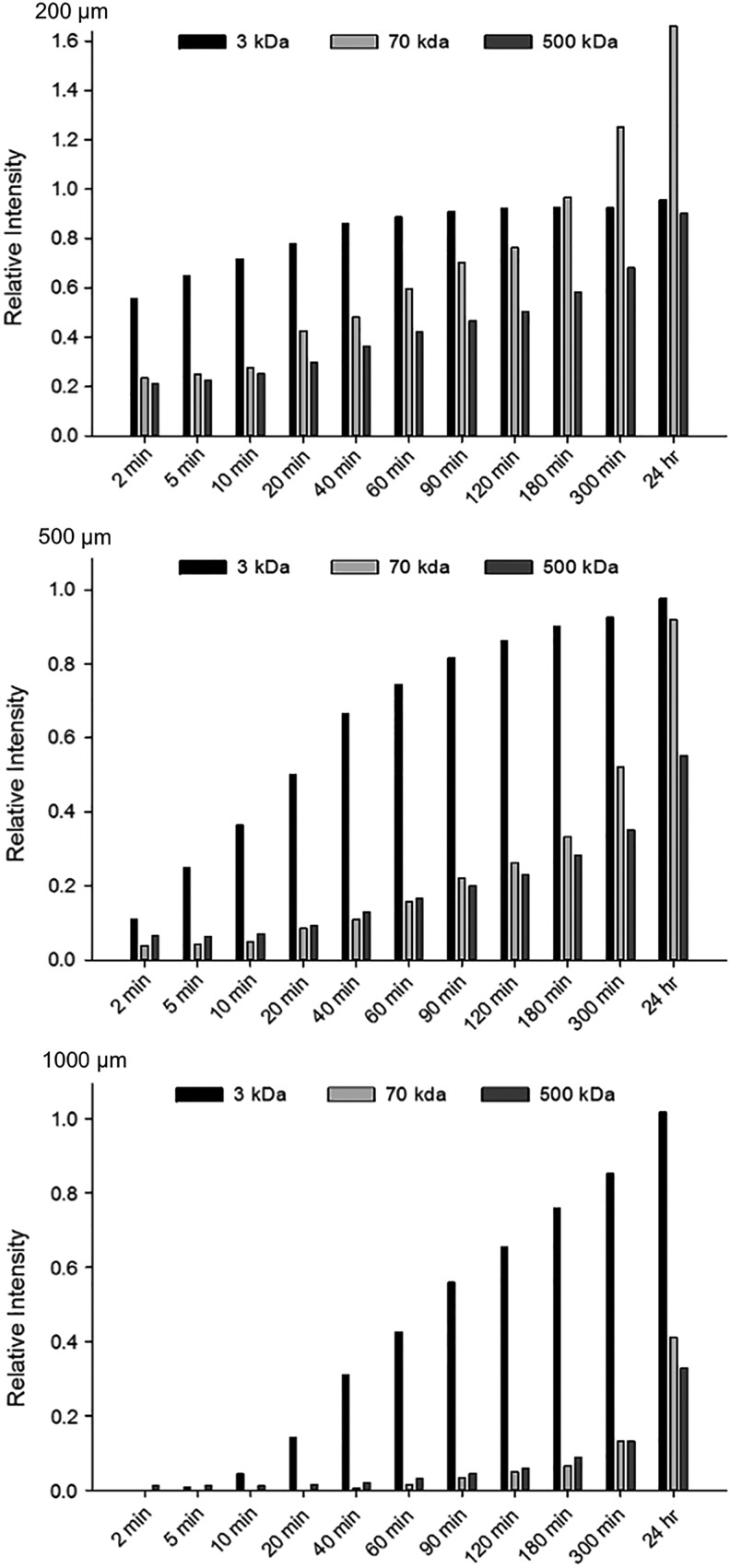
Images were further analyzed by quantifying the relative fluorescence intensity (pixel intensity) at various distances from the gel edge over time, normalized to the fluorescence of the liquid medium. Figure 2 provides histograms of the fluorescence intensities of the probes over time at 200, 500, and 1000 μm from the gel edge. All probes eventually showed substantial diffusion to 200 μm after 24 h, with the 3 kDa probe reaching equilibrium with the medium by the 90-min time point. At 5 and 24 h, the 70 kDa dextran intensities were 125% and 166% of the medium at 200 μm, respectively, corroborating the fluorescence accumulation observed near the interface. By comparison, the 500 kDa probe showed 68% and 90% relative fluorescence at the same time points.
FIG. 2.
Fluorescence intensity of FITC-dextran diffusion into HA-COL gels over time. Fluorescence intensities of 3, 70, and 500 kDa FITC-dextran probes were quantified at the x coordinates, representing 200 μm (top), 500 μm (middle), or 1000 μm (bottom) from the gel edge and normalized to the surrounding liquid medium at several time points over 24 h. Fluorescence intensity values were obtained by analyzing the micrographs such as those shown in Figure 1 by using NIH ImageJ. The data shown represent the average gray value of all pixels in the y-direction of the micrograph at a specified x coordinate (i.e., distance from gel edge).
Fluorescence of the 3 kDa probe at 1000 μm was detected quickly at only 10 min, surpassed 50% relative intensity by 90 min, and was equal to that of the liquid medium at the 24-h time point. There was negligible fluorescence measured from the 70 and 500 kDa probes at 1000 μm until the 60-min time point, and relative fluorescence intensity reached 41% and 33% at 24 h, respectively.
In vitro encapsulated canine islet assessment
Viability and survival
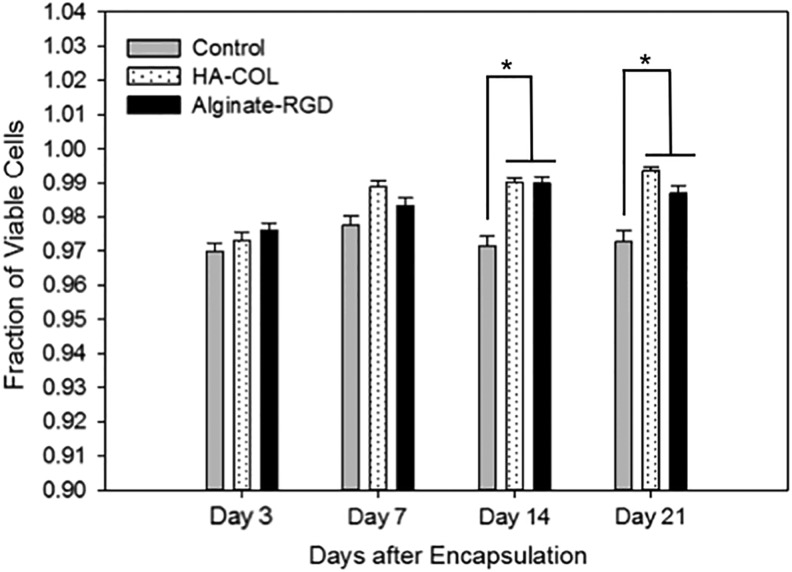
The percentage of viable canine islet cells on day 3 was consistent across all groups, as no significant differences were detected and all were between 97% and 98%. Differences in cell survival were non-significant through day 7 of the study period, though the HA-COL gel groups showed a slight increase in the viable cell fraction over day 3 values (Fig. 3). On days 14 and 21, cell viability was statistically higher in both gel groups compared with the controls, though viability was still more than 97% for each group.
FIG. 3.
Encapsulated canine islet viability over 3 weeks. Viability of canine islets was measured at 3, 7, 14, and 21 days after encapsulation in gel with comparison to unencapsulated controls. Values shown are the mean viable fraction of individual islets for each group and time point from two combined donors. Significant differences are denoted with an asterisk (n = 25 islets or more group and time point; p < 0.05).
Islet morphology
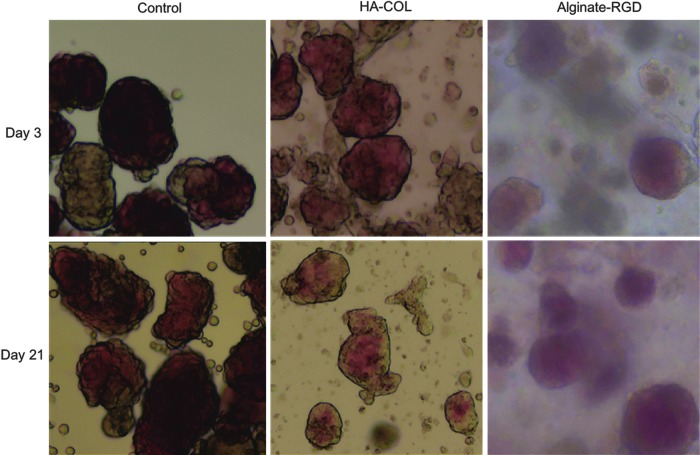
Figure 4 displays representative micrographs of encapsulated and non-encapsulated control islets that were stained with dithizone at 3 and 21 days in culture. Images of alginate-encapsulated islets were cloudy due to the optical properties of the alginate material. Islets in all groups appeared healthy and showed robust dithizone staining over the duration of the study. In the gel groups, islets generally remained separated from one another as well as from exocrine and ductal tissue carried over through the islet isolation process. However, in the control unencapsulated group, islets (dark red stained) were more likely to fuse to remnant ductal and/or exocrine tissue (brown, unstained) present in the culture dish, as seen in the left column of Figure 4.
FIG. 4.
Encapsulated canine islet morphology. Representative micrographs (40 × ) were taken of encapsulated and control canine islets that were stained with dithizone at 3 and 21 days for morphological evaluation. Dithizone, red color, indicates the presence of insulin, and, thus, identifies healthy beta cells. Islets are characterized by their expected appearance with smooth, rounded edges. In the control group at day 21, however, single cells were seen sloughing off from the islets, which was not observed in the encapsulated islet groups. The cloudy appearance of the islets in alginate is a property of the gel.
By day 21, cells appeared to be sloughing off from unencapsulated (control) islets but not the islets in gel (Fig. 4, bottom row), which is consistent with the increased cell death observed in the control group at later time points. Although single cells were also seen in the HA-COL images, they were, most likely, small tissue debris/fragments generated during the islet isolation process that, subsequently, became trapped in the gel. In the control group, this small debris was removed during media exchanges. Thus, on day 3, the control islets appeared clean compared with the gel groups. At day 21, however, cells in the control group appeared to originate directly from the islets, where in the HA-COL group, single cells were isolated and appeared similar to those in the day 3 images.
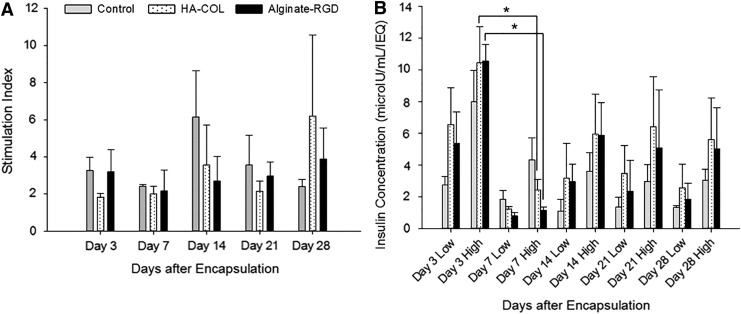
Glucose-stimulated insulin secretion
A common measure of islet function is the “stimulation index” (SI): the ratio of secreted insulin at high (22.4 mM) to low (2.8 mM) glucose. The SI values were between 2 and 6 throughout the study, indicating proper secretory function (Fig. 5A). Due to substantial variation in the SI values within and between groups, no statistically significant differences in SIs were detected at any time point. Further analysis of the insulin secretion data revealed that there was a drop-off in insulin secretion in high glucose for all three groups between days 3 and 7 (Fig. 5B), which then resolved by day 14. This decrease reached statistical significance in the gel groups, but not for the control. Importantly, insulin secretion decreased between days, whereas there was no statistical difference in the high GSIS between the three groups at any time point.
FIG. 5.
Glucose-stimulated insulin secretion of encapsulated islets. (A) Stimulation indices (ratio of high glucose insulin secretion to low glucose secretion) were measured at days 3, 7, 14, 21, and 28 in vitro for encapsulated islets in HA-COL and alginate-RGD hydrogels and for unencapsulated controls. The stimulation index for control cells in media did not change significantly over time, which was also true for islets encapsulated in HA-COL and alginate (n = 3 donor animals). (B) Actual secreted insulin concentrations in response to low and high glucose incubations normalized to IEQ are summarized. Data shown are the mean values from three separate donors measured in triplicate for each group at 3, 7, 14, 21, and 28 days. Significant differences were denoted with an asterisk (p < 0.05). Incubation periods were 90 min for both low and high glucose conditions. IEQ, islet equivalents.
Islet transplantation in HA-COL hydrogel and non-encapsulated controls
Novel method of bulk encapsulation
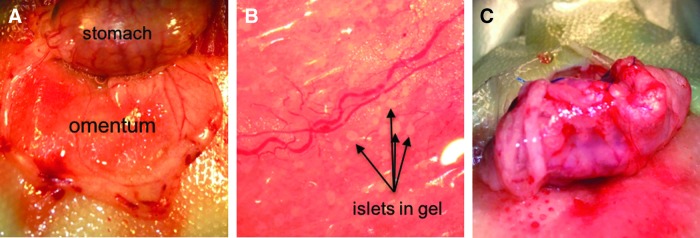
In vitro work with the hydrogel was conducted by using canine islets, whereas transplantation studies into diabetic rats were designed to determine the degree of graft rejection within animal species, because xenotransplantation adds additional confounding complications40 even when encapsulated.41,42 Thus, rat islets were encapsulated to be transplanted into diabetic rats. Due to the slow-hardening properties of the HA-COL gel, a new method of bulk encapsulation had to be developed. As described in more detail in the Materials and Methods section, a thin layer of HA-COL gel precursor solution containing the cross-linker (PEGDA) was applied to the entire omentum and given time to harden (Fig. 6A).
FIG. 6.
Photographs of the surgical procedure for transplantation of islets encapsulated in HA-COL hydrogel. Islets were encapsulated and transplanted into the rat omentum by following a novel three-step protocol. (A) The omentum was first exteriorized, and a base layer of gel was applied and allowed to cross-link. (B) Islets, suspended in an HA-COL precursor solution, were applied along large blood vessels for optimum nutrient exchange and the gel solution was allowed to solidify. (C) The omentum was then rolled up and sutured to the stomach wall to minimize disturbance of the islet graft.
Islets suspended in the precursor gel were applied to the top of the base layer (Fig. 6B), locating the islets near clearly visible blood vessels to maximize nutrient exchange. The islet gel layer was again given time to cross-link so that islets were later attached to the base of the gel. Next, the omentum was folded and sutured to the stomach wall, creating a pouch (Fig. 6C), which was then returned to the peritoneal cavity.
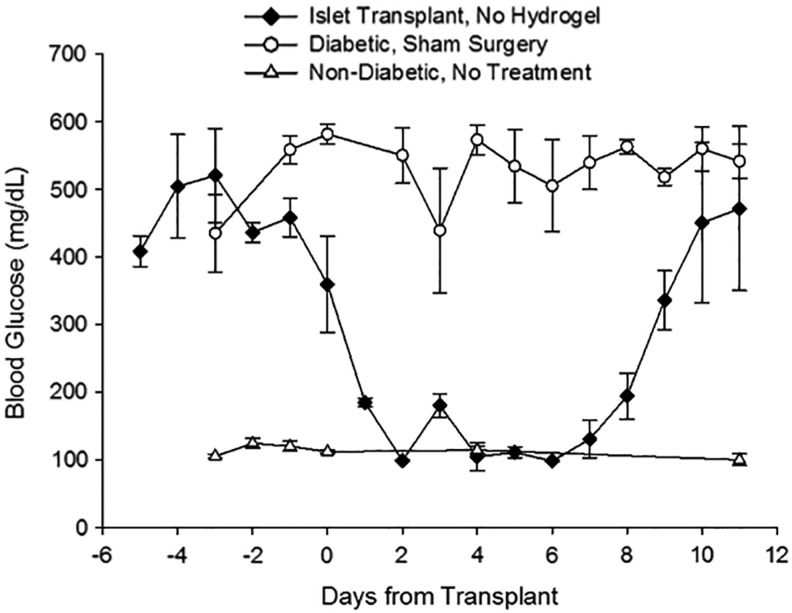
Allogeneic rejection model validation (non-encapsulated islet transplants)
To validate the presence of graft rejection in the outbred rat model, diabetic rats received allogeneic islet transplants without hydrogel. Recipients were non-diabetic within 2 days after transplantation and displayed normal NFBG, as shown in Figure 7. On day 8, NFBG levels began to sharply increase in the control animals; by day 11, the rats were overtly diabetic. By comparison, diabetic sham rats failed to have a single blood glucose reading within the normal range (Fig. 7).
FIG. 7.
Blood glucose levels of control animals. Non-encapsulated rat islet recipients (black diamonds, n = 2) achieved normoglycemia initially, but by 11 days returned to a diabetic state after apparent allo-rejection of the transplanted tissue. Diabetic animals receiving sham surgery (open circles, n = 3) without islets were hyperglycemic throughout the entire study period. For comparison, blood glucose levels of healthy, non-diabetic rats (open triangles, n = 3) are also shown.
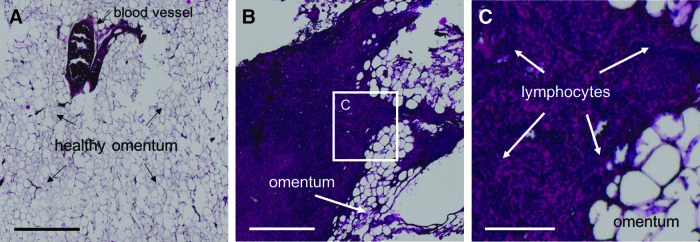
Non-encapsulated omental transplants were recovered after graft failure was confirmed. Tissue from a healthy, non-diabetic rat omentum, stained with H&E, is also shown for comparison, predominantly composed of acellular fat deposits and blood vessels (Fig. 8A). A representative image of the explanted tissue is displayed at low and high magnification (Fig. 8B, C, respectively). Explanted grafts contained areas with large populations of densely packed nuclei with very little cytoplasm, indicative of a massive infiltration of lymphocytes and associated graft rejection. Furthermore, no islets could be identified in any of the tissue sections analyzed from each animal (six sections per rat).
FIG. 8.
H&E staining of the omenta of unencapsulated islet transplant controls. (A) H&E stained section of a typical healthy, untreated rat omentum, shown for comparison (scale = 0.5 mm; magnification = 40 × ). (B) Omentum of rats transplanted with unencapsulated islets, explanted 11 days post-transplant after blood glucose levels, indicated that graft rejection had occurred (scale = 0.5 mm; magnification = 40 × ). (C) Higher magnification of the center image (white square frame) showing significant lymphocyte infiltration in the region (scale = 150 μm; magnification = 200 × ). H&E, hematoxylin and eosin.
Encapsulated islet transplantation outcomes
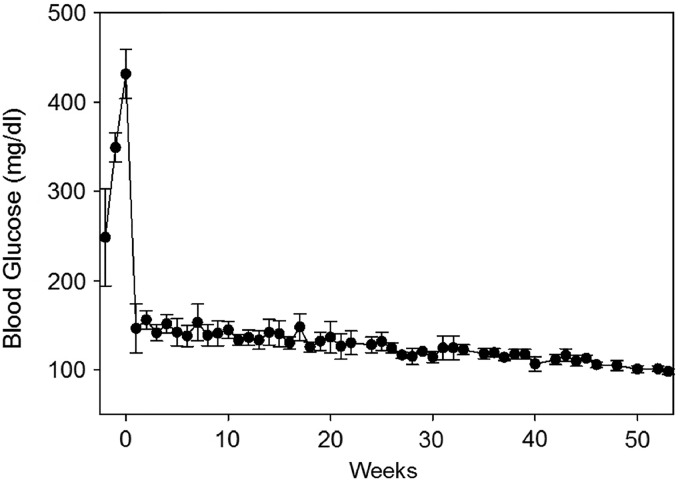
When diabetic rats were transplanted with HA-COL encapsulated islets, all were insulin independent and considered non-diabetic immediately after transplantation. Average NFBG levels of the encapsulated transplant recipients over 1 year are shown in Figure 9. Data beyond 40 weeks include only three animals, as one was sacrificed at that time. Three of these rats were well controlled, with no measured excursions falling within the diabetic range. The fourth rat had occasional values in the 200–250 range in the first 50 days, but those normalized from that time point forward to termination at 52 weeks.
FIG. 9.
Transplantation of islets in hydrogel. Average non-fasting blood glucose levels of four diabetic rats transplanted with islets encapsulated in HA-COL hydrogel over 1 year. “0” marks the day of the islet transplant surgery. Values to the left of “0” were taken before transplant to confirm a diabetic state. All animals showed normal blood glucose levels at sacrifice. Data points beyond 40 weeks are from only three animals, as one animal was sacrificed at this time point.
The HA-COL gel-transplanted animals were sacrificed for ethical reasons when indications of poor health, not related to diabetes, were observed. Axillary and mammary tumors were the primary justification for early termination in these studies among other neoplastic lesions. Such tumors are common in this strain when the animals reach advanced ages.43 When possible, the islet grafts were recovered at the time of sacrifice and preserved for evaluation. No gel was noted in any portion of the peritoneal cavity outside of the omentum pouch.
Histological evaluation of explanted encapsulated islet grafts
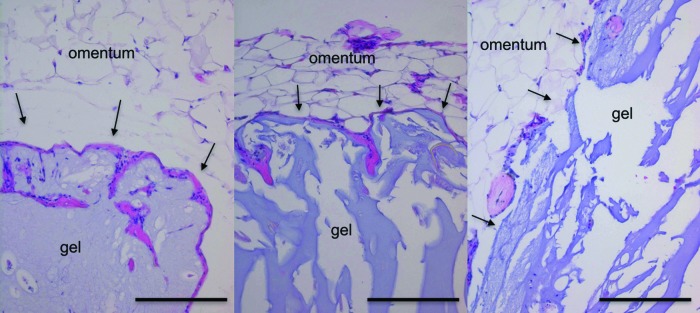
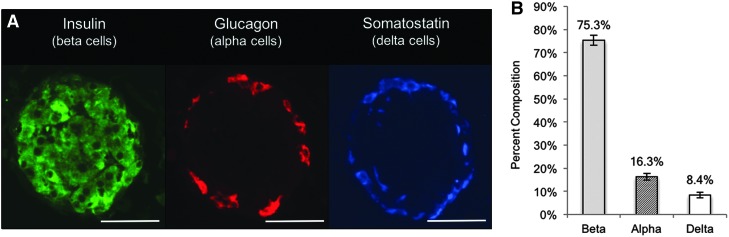
Explanted islet graft sections stained with H&E revealed intact hydrogel, characterized by light blue/purple acellular regions adjacent to the typical adipose tissue of the omentum (Fig. 10). Minimal eosin positive staining around the perimeter of the gel indicated a lack of fibrosis. Figure 11A contains representative immunofluorescence images of an encapsulated islet from the explants, containing brightly stained islets comprising all three major islet cell types. The average percent composition of the beta/alpha/delta cells from 70 islet sections from the explant is shown (Fig. 11B).
FIG. 10.
H&E staining of encapsulated islet transplants. Micrographs (200 × ) of H&E stained sections from the explanted graft showed intact hydrogel and very little or no fibrosis at the gel/tissue interface, indicated with black arrows. Tissues were explanted from rats that received encapsulated islet transplants at 40 (left), 52 (middle), and 64 (right) weeks post-transplant. The large open spaces around the explanted hydrogel material were due to changes in the gel's physical properties during paraffin embedding because of its high water content, which leaves the gel brittle and difficult to section. Scale bars = 200 μm.
FIG. 11.
Immunohistochemistry of explanted islet graft. (A) Islets within the recovered graft displayed strong staining for the three major islet cell types—beta, alpha, and delta. Insulin is shown in green, glucagon in red, and somatostatin in blue. (B) Histogram of cell composition of islets found in explanted tissue (n = 70 islet sections) showing normal distribution of rat islet cell types. Scale bars = 50 μm, magnification = 400 × .
Discussion
This study evaluated the potential of an HA-COL hydrogel as an encapsulant to improve long-term islet transplantation without immunosuppression. Although HA hydrogels are becoming increasingly common in tissue engineering research, they have, to our knowledge, never been used for encapsulation and immunoprotection of pancreatic islets.
Immunoprotective materials must facilitate diffusion of nutrients and signaling molecules (e.g., glucose and insulin) while also preventing direct contact between immune cells and the encapsulated cells. Thus, we evaluated the diffusional properties of the HA-COL gel using fluorescent dextrans. A small 3 kDa dextran diffused rapidly into the hydrogel, suggesting that insulin would encounter little diffusional resistance (MW ∼5.8 kDa).
Interestingly, we observed marked accumulation of the 70 kDa probe at the gel/liquid interface. Thus, the probe was able to enter the gel fairly readily, but there was likely some form of thermodynamically favorable interaction between the large dextran and the hydrogel (e.g., conformational effects or van der Waals forces) that did not exist in sufficient magnitude with the smaller 3 kDa probe.44 This may suggest that moderately sized proteins, such as albumin (∼66.5 kDa), could diffuse into the gel in vivo, but with a marked attenuation at greater diffusion lengths.
The 500 kDa probe exhibited the least extensive diffusion, but it was not blocked from the gel matrix entirely. However, it is important to note in considering a “molecular weight cut-off” that dextrans are flexible and mostly linear polymers, whereas immunoglobulins and antibodies are rigid, globular structures. Pluen et al. demonstrated that diffusion of linear polymers into hydrogels was substantially greater than globular proteins of a similar molecular mass, noting that the latter became quickly entrapped within gel pores.45 Thus, diffusion of high-molecular-weight globular proteins through this gel would likely be more limited compared with the FITC-dextran probes.
Although we did not characterize the average pore size of the hydrogels explicitly, infiltration of the 500 kDa dextran probe suggests that the average pore size of the HA-COL gel is large compared with other common hydrogels such as those made from low-molecular-weight PEGDA. For example, Durst et al. described a 15% PEGDA (MW 3.4 kDa) gel with an average pore size of 5.94 nm.46 By comparison, the reported hydrodynamic radius of a 500 kDa dextran is 15.9 nm,47 Furthermore, because porosity and pore size are strongly correlated to cross-link density and polymer mass fraction of the hydrogel, these parameters could be easily controlled, if desired, by simply increasing the concentration of HA/COL, PEGDA (the cross-linker), or both.
When comparing the long-term islet viability, the alginate-RGD gel performed as well as the natural HA-COL gel over 3 weeks. The results were somewhat surprising given the more biocompatible characteristics of HA-COL. This result may be attributable to the RGD motif present in both gels, which has a well-documented benefit to islet viability in vitro.21,48,49 Cheng et al. evaluated cardiosphere-derived cells in the same HA-COL gel with and without the RGD-containing (COL) component, and they found that the HA-COL gel led to higher cell survival than the gel with HA alone.28 Although our data did not show a significant difference from the alginate-RGD material in terms of islet viability in vitro, the HA-COL gel facilitated a significant reduction in cell death over unencapsulated islets during long-term culture.
Islets in all groups, including controls, remained responsive to glucose stimulation throughout the entire 28-day study. Other publications have shown that cultured islets generally have near or complete loss of GSIS after 14 days,23,24 but we did not see this in our studies. One difference in our procedure was the presence of acinar or ductal cells in our cultures, which have been shown to possess pro-islet properties, compared with typical in vitro studies done with highly pure or handpicked islets.50 In fact, Murray et al. reported that pancreatic duct-derived epithelial cells improved glucose sensitivity in vitro compared with control islets, which lost nearly all responses after 10 days.51
Interestingly, we observed a significant decrease in insulin secretion in the encapsulated groups on day 7 that recovered by day 14. Lim et al. reported a similar pattern in a previous islet encapsulation study, but they did not comment on whether the temporary reduction in insulin secretion was statistically significant.21 Regardless, these observations may imply that the introduction of a solid, three-dimensional environment in vitro leads to a temporary alteration in the cellular machinery of the islets and, thus, their ability to produce, sense, and/or secrete insulin. However, the underlying molecular mechanisms behind this phenomenon are still highly unclear, and additional studies would be required to draw conclusions as to the consistency and origin of these observations.
Allogeneic transplants of islets in HA-COL hydrogels into diabetic rats reversed diabetes and showed no evidence of graft rejection or fibrosis in the recipients for as many as 18 months. Immunofluorescence staining of explanted HA-COL grafts revealed healthy islets containing all three major islet cell types in a ratio and spatial distribution that is characteristic of healthy rat islets.36,52
These results corroborate the excellent glycemic control observed in vivo, and they demonstrate the robust long-term biocompatibility and immunoprotective capacity of the HA-COL gel. This is encouraging given the long history of biocompatibility issues surrounding alginate-based islet transplants, which are prone to early graft failure due to fibrosis,7,12,15,53–55 although some progress has been made in this regard through advanced alginate purification and capsule optimization protocols.14,16,17,56–58 However, the processes for improving alginate biocompatibility are complex and expensive; thus, an alternative encapsulant for islet transplantation is appealing.
There are clearly differences between the bulk-gel transplantation method described here and alginate microsphere-based transplants. Advantageously, we found that the targeted deposition of islets to the highly vascularized regions of the omentum produced consistent, high-quality transplant outcomes. However, this method is, of course, more invasive and less convenient than a simple injection of alginate beads, and, therefore, not ideal for clinical use. Considering this, our lab is currently developing a novel method for producing uniform islet microspheres by using this HA-COL gel, enabling a more clinically relevant delivery of the material.
The bio-stability of encapsulating materials is a critical factor for maintaining long-term immunoprotection of an islet graft. Several extracellular matrix- or bio-inspired hydrogels have been evaluated for islet transplantation, including self-assembling peptide gels and a VEGF-loaded PEG maleimide gel.21–23
Although these gels improved engraftment, they degraded much too quickly to be appropriate for long-term function in vivo. Conversely, the present HA-COL gel forms more durable covalent cross-links, and it can be easily tuned by modifying the component concentrations to improve gel strength further. For example, Vanderhooft et al. showed that when concentrations of HA and PEGDA were increased from 0.4% and 0.2% (formulation used in this study) to 1.6% and 1.6%, respectively, the shear modulus increased from 37 to 3500 Pa.27 Even at the relatively low gel concentration used in this study, the HA-COL gel persisted for as many as 18 months in the rat omentum.
In conclusion, this study demonstrates the potential of an HA-COL-derived hydrogel as an effective alternative to alginate for long-term immunoprotected islet transplantation at a time when clinical outcomes of alginate encapsulated islet transplants have been disappointing. These promising results support further investigation of this HA-COL hydrogel for encapsulated islet transplantation.
Acknowledgments
The authors would like to thank Jing Huang and Sarah Tague for their assistance with tissue processing, immunohistochemistry, and confocal microscopy. They also wish to extend their gratitude to Dr. Travis Hagedorn, DVM, for his assistance in interpreting histological images. This work was supported by the Smith Intellectual and Developmental Disabilities Research Center (NIH HHS NICHD P30 HD002528) at the University of Kansas Medical Center and by Likarda, LLC, Kansas City, KS.
Disclosure Statement
L.S.B. and K.R. and the University of Kansas have financial interests in Likarda.
References
- 1.Alejandro R., Barton F.B., Hering B.J., and Wease S. 2008 update from the collaborative islet transplant registry. Transplantation 86, 1783, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Division of Diabetes Translation. National Diabetes Statistics Report, 2014. National Center for Chronic Disease Prevention and Health Promotion: Centers for Disease Control and Prevention; 2014 [Google Scholar]
- 3.Graham J.G., Zhang X., Goodman A., Pothoven K., Houlihan J., Wang S., et al. . PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A 19, 1465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., et al. . Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 106, 15768, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharp D.W., and Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 67, 35, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Calafiore R., and Basta G. Clinical application of microencapsulated islets: actual prospectives on progress and challenges. Adv Drug Deliv Rev 67, 84, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Buder B., Alexander M., Krishnan R., Chapman D.W., and Lakey J.R. Encapsulated islet transplantation: strategies and clinical trials. Immune Netw 13, 235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim F., and Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Dufrane D., and Gianello P. Macro- or microencapsulation of pig islets to cure type 1 diabetes. World J Gastroenterol 18, 6885, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott R.B., Escobar L., Tan P.L., Muzina M., Zwain S., and Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14, 157, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kirk K., Hao E., Lahmy R., and Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res 12, 807, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Tuch B.E., Keogh G.W., Williams L.J., Wu W., Foster J.L., Vaithilingam V., et al. . Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32, 1887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T., Adcock J., Kühtreiber W., Qiang D., Salleng K.J., Trenary I., et al. . Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 85, 331, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Dufrane D., Goebbels R.-M.M., Saliez A., Guiot Y., and Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81, 1345, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gotfredsen C.F., Stewart M.G., O'Shea G.M., Vose J.R., Horn T., and Moody A.J. The fate of transplanted encapsulated islets in spontaneously diabetic BB/Wor rats. Diabetes Res 15, 157, 1990 [PubMed] [Google Scholar]
- 16.Langlois G., Dusseault J., Bilodeau S., Tam S.K., Magassouba D., and Hallé J.-P.P. Direct effect of alginate purification on the survival of islets immobilized in alginate-based microcapsules. Acta Biomater 5, 3433, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hillberg A.L., Kathirgamanathan K., Lam J.B.B., Law L.Y., Garkavenko O., and Elliott R.B. Improving alginate-poly-L-ornithine-alginate capsule biocompatibility through genipin crosslinking. J Biomed Mater Res Part B Appl Biomater 101, 258, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Campanha-Rodrigues A.L., Grazioli G., Oliveira T.C., Campos-Lisbôa A.C., Mares-Guia T.R., and Sogayar M.C. Therapeutic potential of laminin-biodritin microcapsules for type 1 diabetes mellitus. Cell Transplant 24, 247, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Mohan N., Gupta V., Sridharan B., Sutherland A., and Detamore M.S. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnol Bioeng 111, 829, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murali R., Ponrasu T., Cheirmadurai K., and Thanikaivelan P. Biomimetic hybrid porous scaffolds immobilized with platelet derived growth factor-BB promote cellularization and vascularization in tissue engineering. J Biomed Mater Res A 104, 388, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Lim D.-J.J., Antipenko S.V., Anderson J.M., Jaimes K.F., Viera L., Stephen B.R., et al. . Enhanced rat islet function and survival in vitro using a biomimetic self-assembled nanomatrix gel. Tissue Eng Part A 17, 399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao S.W., Rawson J., Omori K., Ishiyama K., Mozhdehi D., Oancea A.R., et al. . Maintaining functional islets through encapsulation in an injectable saccharide-peptide hydrogel. Biomaterials 34, 3984, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phelps E.A., Headen D.M., Taylor W.R., Thulé P.M., and García A.J.J. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 34, 4602, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prestwich G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release 155, 193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burdick J.A., and Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater Weinheim 23, H41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prestwich G.D. Engineering a clinically-useful matrix for cell therapy. Organogenesis 4, 42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderhooft J.L., Alcoutlabi M., Magda J.J., and Prestwich G.D. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol Biosci 9, 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng K., Blusztajn A., Shen D., Li T.-S.S., Sun B., Galang G., et al. . Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel. Biomaterials 33, 5317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Shu X.Z., and Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng 12, 3405, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Vrabelova D., Adin C.A., Kenzig A., Gilor C., Xu F., Buss J.L., et al. . Evaluation of a high-yield technique for pancreatic islet isolation from deceased canine donors. Domest Anim Endocrinol 47, 119, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Jin S.-M.M., Lee H.-S.S., Oh S.-H.H., Park H.J., Park J.B., Kim J.H., et al. . Adult porcine islet isolation using a ductal preservation method and purification with a density gradient composed of histidine-tryptophan-ketoglutarate solution and iodixanol. Transplant Proc 46, 1628, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Ricordi C., Gray D.W., Hering B.J., Kaufman D.B., Warnock G.L., Kneteman N.M., et al. . Islet isolation assessment in man and large animals. Acta Diabetol Lat 27, 185, 1990 [DOI] [PubMed] [Google Scholar]
- 33.MacGregor R.R., Williams S.J., Tong P.Y., Kover K., Moore W.V., and Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab 290, E771, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Williams S.J., Huang H.-H.H., Kover K., Moore W., Berkland C., Singh M., et al. . Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis 6, 115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams S.J., Wang Q., Macgregor R.R., Siahaan T.J., Stehno-Bittel L., and Berkland C. Adhesion of pancreatic beta cells to biopolymer films. Biopolymers. 91, 676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran K., Williams S.J., Huang H.-H.H., Novikova L., and Stehno-Bittel L. Engineering islets for improved performance by optimized reaggregation in a micromold. Tissue Eng Part A 19, 604, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Williams S.J., Schwasinger-Schmidt T., Zamierowski D., and Stehno-Bittel L. Diffusion into human islets is limited to molecules below 10 kDa. Tissue Cell 44, 332, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Integrated Islet Distribution Program. Standard Operating Procedure for Potency Test: Glucose Stimulated Insulin Release Assay. National Institute of Diabetes and Digestive and Kidney Diseases; 2012 [Google Scholar]
- 39.Novikova L., Smirnova I.V., Rawal S., Dotson A.L., Benedict S.H., and Stehno-Bittel L. Variations in rodent models of type 1 diabetes: islet morphology. J Diabetes Res 2013, 965832, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham M.L., Bellin M.D., Papas K.K., Hering B.J., and Schuurman H.-J.J. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation 18, 328, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T., Harb G., Rajotte R.V., Korbutt G.S., Mallett A.G., Arefanian H., et al. . Immune mechanisms associated with the rejection of encapsulated neonatal porcine islet xenografts. Xenotransplantation 13, 547, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Safley S.A., Cui H., Cauffiel S.M., Xu B.-Y.Y., Wright J.R., and Weber C.J. Encapsulated piscine (tilapia) islets for diabetes therapy: studies in diabetic NOD and NOD-SCID mice. Xenotransplantation 21, 127, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Nakazawa M., Tawaratani T., Uchimoto H., Kawaminami A., Ueda M., Ueda A., et al. . Spontaneous neoplastic lesions in aged Sprague-Dawley rats. Exp. Anim 50, 99, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Gehrke S.H., Fisher J.P., Palasis M., and Lund M.E. Factors determining hydrogel permeability. Ann N Y Acad Sci 831, 179, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Pluen A., Netti P.A., Jain R.K., and Berk D.A. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys J 77, 542, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durst C.A., Cuchiara M.P., Mansfield E.G., and West J.L. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater 7, 2467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong J.K., Wenby R.B., Meiselman H.J., and Fisher T.C. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J 87, 4259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinkse G.G., Bouwman W.P., Jiawan-Lalai R., Terpstra O.T., Bruijn J.A., and de Heer E. Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes 55, 312, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Weber L.M., Hayda K.N., Haskins K., and Anseth K.S. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28, 3004, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Bertelli E., and Bendayan M. Association between endocrine pancreas and ductal system. More than an epiphenomenon of endocrine differentiation and development? J Histochem Cytochem 53, 1071, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Murray H.E., Paget M.B., Bailey C.J., and Downing R. Sustained insulin secretory response in human islets co-cultured with pancreatic duct-derived epithelial cells within a rotational cell culture system. Diabetologia 52, 477, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Sujatha S.R., Pulimood A., and Gunasekaran S. Comparative immunocytochemistry of isolated rat & monkey pancreatic islet cell types. Indian J Med Res 119, 38, 2004 [PubMed] [Google Scholar]
- 53.Duvivier-Kali V.F., Omer A., Lopez-Avalos M.D., O'Neil J.J., and Weir G.C. Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am J Transplant 4, 1991, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K., Bonner-Weir S., Trivedi N., Yoon K.H., Hollister-Lock J., Colton C.K., et al. . Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation 66, 21, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Juste S., Lessard M., Henley N., Ménard M., and Hallé J. Effect of poly‐L‐lysine coating on macrophage activation by alginate‐based microcapsules: assessment using a new in vitro method. J Biomed Mater Res A 72A, 389, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Zhang W.J., Laue C., Hyder A., and Schrezenmeir J. Purity of alginate affects the viability and fibrotic overgrowth of encapsulated porcine islet xenografts. Transplant Proc 33, 3517, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Park H.-S.S., Kim J.-W.W., Lee S.-H.H., Yang H.K., Ham D.-S.S., Sun C.-L.L., et al. . Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation. J Tissue Eng Regen Med 2015. [Epub ahead of print]; DOI: 10.1002/term.2029 [DOI] [PubMed] [Google Scholar]
- 58.Veiseh O., Doloff J.C., Ma M., Vegas A.J., Tam H.H., Bader A.R., et al. . Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 14, 643, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]