Abstract
Many behaviors posing significant risks to public health are characterized by repeated decisions to forego better long-term outcomes in the face of immediate temptations. Steeply discounting the value of delayed outcomes often underlies a pattern of impulsive choice. Steep delay discounting is correlated with addictions (e.g., substance abuse, obesity) and behaviors such as seatbelt use and risky sexual activity. As evidence accumulates suggesting steep delay discounting plays a causal role in these maladaptive behaviors, researchers have begun testing methods for reducing discounting. In this first systematic and comprehensive review of this literature, the findings of 92 articles employing different methodologies to reduce discounting are evaluated narratively and meta-analytically. While most of the methods reviewed produced significant reductions in discounting, they varied in effect sizes. Most methods were ideal for influencing one-off choices (e.g., framing and priming manipulations) although other successful manipulations, such as episodic future thinking, could be incorporated into existing therapies designed to produce longer-lasting changes in decision-making. The largest and longest-lasting effects were produced by learning-based manipulations; although, translational research is needed to determine the generality and clinical utility of these methods. Methodological shortcomings in the existing literature and suggestions for ameliorating these issues are discussed. This review reveals a variety of methods with translational potential, which, through continued refinement, may prove effective in reducing impulsive choice and its associated maladaptive decisions that negatively impact quality of life
Keywords: delay discounting, impulsive choice, meta analysis, manipulations, systematic review
In our daily lives, we encounter intertemporal choice opportunities that tempt us toward the “dark side.” Do you stay up longer binge-watching Game of Thrones or do you go to sleep so you can be rested, focused, and productive at work tomorrow? Do you enjoy another cocktail now or do you opt for the benefits of a sober drive home at the close of the evening? Do you choose fried instead of baked chicken, preferring the immediate crunch over the desire to lose weight? These encounters with immediate temptation that are at odds with our long-term interests are commonplace. Examined in isolation, the outcomes of these choices may be trivial; but when combined into a temporally extended pattern of behavior, they can influence wealth, health, and psychological well-being (Rachlin, 1995; Schroeder, 2007).
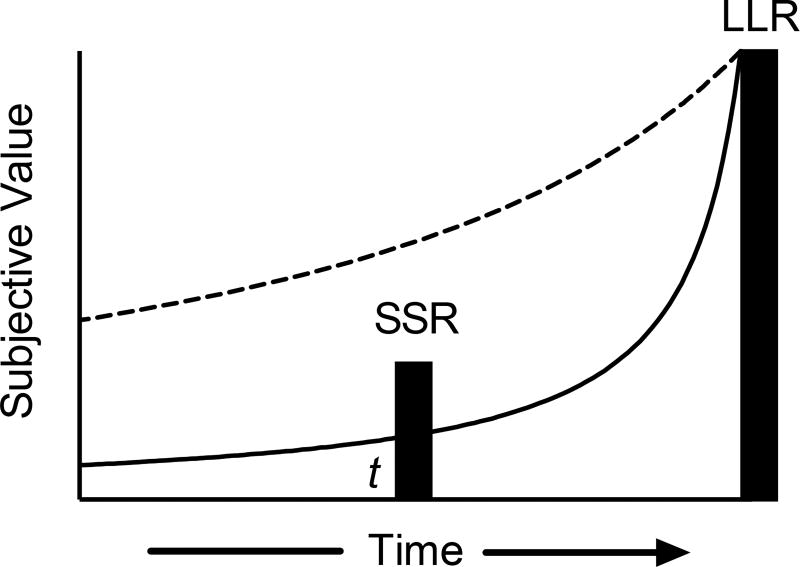
Delay discounting describes the devaluation of an outcome because it is delayed (Madden & Johnson, 2010). To illustrate, Figure 1 depicts how two individuals, represented by the dashed and solid curves, discount the value of a larger-later reward (LLR). Across human and nonhuman species, discounting functions are hyperbolic (or approximately so), which is revealed by a steep decline in reward value at short delays, and a more shallow decline at longer delays (Green & Myerson, 2004; Kirby & Herrnstein, 1995; Madden, Bickel, & Jacobs, 1999; Mazur, 1987). This form holds regardless of reward type (real, hypothetical, drug, food, etc.; Friedel, DeHart, Madden, & Odum, 2014, Johnson & Bickel, 2002; Jiruma, Myerson, Hilgard, Braver, & Green, 2009) or delay type (e.g., Jimura et al., 2009; Johnson, Herrman, & Johnson, 2015). At time t in Figure 1, a smaller-sooner reward (SSR) is available immediately and its undiscounted value is given by the height of the bar. The subjective value of the LLR is given by the height of the discounting function at t. All else being equal, the steep discounter will choose the subjectively more valuable SSR – the impulsive choice. By contrast, for the individual whose choices are described by the dashed curve, the subjective value of the LLR exceeds that of the SSR at t, and hence the LLR (the self-control choice) is selected.1
Figure 1.
Discounted value of a larger-later reward (LLR) plotted as a function of time to reward delivery. At time t the smaller-sooner reward (SSR) is available immediately while the LLR reward is delayed. Solid and dashed curves show high- and low-rate hyperbolic delay discounting.
Among humans, steeply discounting the future is correlated with maladaptive preferences for SSRs that pose significant public health concerns. For example, steep delay discounting is associated with substance use and dependence, including cigarette smoking (Baker, Johnson, & Bickel, 2003; Bickel, Odum, & Madden, 1999; Mitchell, 1999), problematic alcohol use (MacKillop et al., 2010; Vuchinich & Simpson, 1998) and alcohol dependence (Mitchell, Fields, D’Esposito, & Boettiger, 2005; Petry, 2001), heroin use (Kirby & Petry, 2004; Kirby, Petry, & Bickel, 1999), and illicit stimulant use (Heil, Johnson, Higgins, & Bickel, 2006; Kirby & Petry, 2004; Monterosso et al., 2007). In addition, steep delay discounting is related to obesity (Fields, Sabet, Peal, & Reynolds, 2011; Jarmolowicz et al., 2014; see Amlung et al., 2016 for review); pathological gambling (Alessi & Petry, 2003; Dixon, Marley, & Jacobs, 2003); and sub-clinical yet impactful health-related behaviors such as wearing sunscreen, using seatbelts, visiting the dentist, early sexual activity, and relationship infidelity (Daugherty & Brase, 2010; Reimers, Maylor, Stewart, & Chater, 2009).
Accumulating evidence of the predictive validity of steepness of discounting suggests an etiological role in the development of addictions. Longitudinal studies illustrate that steep discounting is predictive of the initiation of cigarette smoking in adolescents (Audrain-McGovern et al., 2009), future alcohol use in adolescents (Fernie et al., 2013; Khurana et al., 2014), and increases in drug use in young adulthood (cigarette, marijuana, and alcohol use; Brody et al., 2014); for an exception to these findings see Isen, Sparks, and Iacono (2014). Further, discounting does not increase after initiation of cigarette smoking in adolescence (Audrain-McGovern et al., 2009), counter to expectation if steeper discounting was due to nicotine exposure. Also consistent with an etiological role, steep delay discounting is often predictive of poor outcomes during (Stanger et al., 2012; Washio et al., 2011) and after substance-abuse treatment (MacKillop & Kahler, 2009; Sheffer et al., 2014), relapse after spontaneously quitting (Yoon et al., 2007), as well as relapse in analogue laboratory treatment settings (Mueller et al., 2009).
Non-human animal research provides some support for the hypothesis that steep delay discounting precedes and predicts drug taking. High-impulsive rats more often initiate (Perry, Larson, German, Madden, & Carroll, 2005) and escalate (Anker, Perry, Gliddon, & Carroll, 2009) cocaine self-administration, and show more persistent demand for nicotine and cocaine when the price of the drug increases (i.e., responses per dose; Diergaarde, Van Mourik, Pattij, Schoffelmeer, & De Vries, 2012; Koffarnus & Woods, 2013). However, the relation between impulsive-choice and other drugs is inconsistent (see Stein & Madden, 2013 for review).
Extensive research has evaluated the alternative possibility that steep delay discounting is a result of problem drug use. The findings are discrepant: human and nonhuman discounting can be increased, decreased, or unaffected by a wide variety of drugs and doses, with little consistency between published studies (see de Wit & Mitchell, 2010; Stein & Madden, 2013; Weafer, Mitchell, & De Wit, 2014, for discussion and reviews). If acute or chronic drug use influences delay discounting, the effects are complicated by poorly understood genetic factors, dose of drug, drug type, baseline levels of discounting, and the discounting task itself (Stein & Madden, 2013; Weafer et al., 2014).
Thus, the weight of the current evidence favors (but does not establish) an etiological role of delay discounting in addictions and health-impacting behaviors. Bickel and colleagues (2012) suggested steep delay discounting is a trans-disease process underlying these maladaptive behaviors. This proposal and its implications have been echoed by researchers calling for interventions to reduce steepness of delay discounting as a preventive measure for those at risk of addictions (e.g., Gray & MacKillop, 2015; Volkow & Baler, 2015) or as a component of a comprehensive treatment for those already afflicted with health deficits caused by persistent patterns of impulsive choice (Schroeder, 2007).
If the above hypotheses are supported empirically, then it will be important to identify effective methods for experimentally reducing delay discounting and impulsive choice. These experimental manipulations are also important in evaluating the causal role, if any, of delay discounting on the maladaptive behaviors with which it correlates. The present review and meta-analysis identified and evaluated the efficacy of methods used to reduce delay discounting or impulsive choice. The few existing reviews of this literature have either not been systematic (i.e., explicitly defined and replicable search and inclusionary procedures; e.g., Gray & MacKillop, 2015; Lempert & Phelps, 2016) or were not comprehensive (Koffarnus, Jarmolowicz, Mueller, & Bickel, 2013). The present comprehensive review focuses on environmental manipulations (i.e., non-pharmacological or neurological) designed to reduce delay discounting or impulsive choice.
Method
Identification of Studies
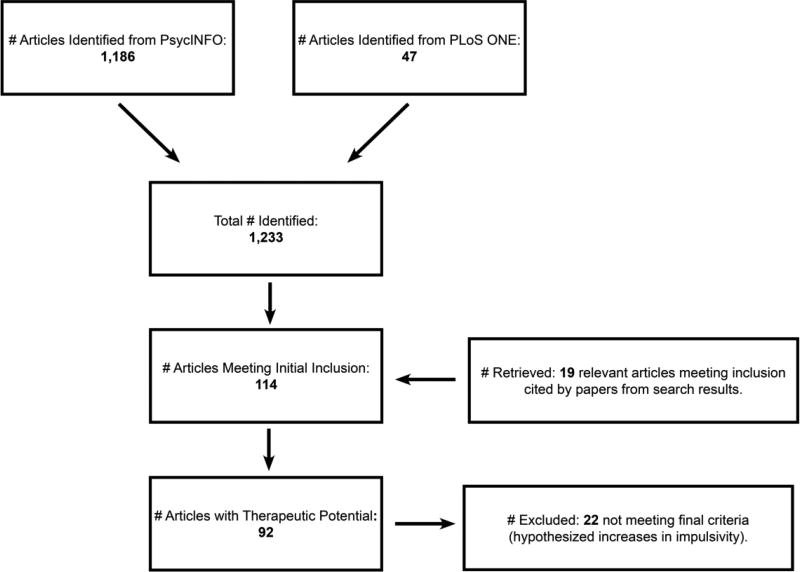
Studies employing an experimental manipulation designed to reduce the steepness of delay discounting or prevalence of impulsive choice were identified via one of two methods. First, the PsycINFO and PLoS One2 databases were searched on March 1, 2016, limiting results to peer-reviewed papers written in English. Articles were included if the abstracts contained at least one term in each of two groups of terms: 1 [intertemporal choice OR delay discounting OR delayed gratification OR “impulsive choice” OR “impulsive decision making” OR “intertemporal decision making”], 2 [expectancy OR certainty OR manipulat* OR train* OR improv* OR intervention OR chang* OR alter* OR effect* OR affect* OR reduc* OR increas*]. The PsycINFO and PLoS One searches yielded 1,186 and 47 records, respectively. Second, references of articles identified and included in the review were searched, with 19 additional articles identified.
Exclusion Criteria
The 1,233 articles were screened according to six exclusion criteria (see Figure 2). First, articles needed to include at least one experimental manipulation that was neither pharmacological nor neural (e.g., lesion). The rationale for this exclusion was, in part, practical. Including the large number of studies attempting to influence impulsive choice through pharmacological or neural manipulations would make the review unwieldy, and reviews on these topics already exist (e.g., Stein & Madden, 2013; Weafer et al., 2014; Winstanley, 2010). Second, articles were excluded if they did not include a control or comparison procedure (e.g., a control group or pre-intervention baseline) or if they were case studies. Third, if delay discounting or impulsive choice was not unambiguously measured (e.g., studies in which the effects of delay and effort were confounded) the article was excluded. Fourth, articles were excluded if their therapeutic potential was limited to select contexts; e.g., experimental manipulations of reward magnitude or sign (gains vs. losses), participant income, and commodity type (monetary vs. food rewards) are impractical in clinical and certain field settings. Fifth, articles were excluded if there were confirmed violations of the assumptions of inferential statistical tests (e.g., violations of normality) and the authors did not respond to requests to provide individual-participant data for the purpose of non-parametric re-analyses.
Figure 2.
Diagram depicting the number of articles retrieved, included, and excluded following the criteria developed for the present review.
Articles that passed the first five criteria (n = 113) were categorized based on the type of experimental manipulation (e.g., framing, cueing, priming) and the direction of the hypothesized effect (increase/decrease). A sixth criterion excluded categories of manipulations that were uniformly hypothesized to increase delay discounting/impulsive choice (n = 22; e.g., sexual cues hypothesized to increase delay discounting in men; Van den Bergh, Dewitte, & Warlop, 2008).
Computation of Effect Size
Effect sizes were calculated when the necessary data were provided in-text, could be obtained from the published graphs using GraphClick, or were provided by the corresponding author. Authors were contacted if the article was published within the last 10 years, reasoning that older data were unlikely to be retained. If data were not provided after two requests, the article was retained for narrative purposes but not included in meta-analysis or graphical displays of effect sizes.
Effect size was first calculated using Cohen’s d, but depending on the study design, the effect size was calculated differently. For between-subjects designs, when the means and standard deviations (or standard errors of the mean) were available, Cohen’s d was calculated as follows:
| Eq. (1) |
where x̅1 and x̅2 are the means for each of two groups of interest, n1 and n2 are the sample sizes for those groups, and SD1 and SD2 are the standard deviations of the mean for each of those groups, respectively (Lakens, 2013). If the results of a t-test were reported for a between-subjects comparison, then the following formula was used:
| Eq. (2) |
where t is the test statistic, and n1 and n2 are as described above (Lakens, 2013). When the number of participants/subjects assigned to each group was not specified, the sample size per group was approximated as the total number of subjects in the analytic sample divided by the number of groups being compared.
For within-subjects designs, the standardizer of the mean difference was the average of the standard deviations across measurements, which was calculated as follows:
| Eq. (3) |
where x̅diff is the difference in means across two assessments, and SD1 and SD2 are as described previously. Equation 3 was chosen so effect sizes across between- and within-subjects designs would be more comparable (see Lakens, 2013 for discussion).
For studies using impulsive choice assessments with dichotomous outcomes (e.g., single-item discounting questions) the proportions of individuals choosing the SSR or LLR were used in lieu of averages for calculating effect size. Specifically, the proportions were treated as means (of a distribution of 0s and 1s), with their difference divided by the pooled standard deviation of these means; this version of Cohen’s dbtw was calculated as outlined in DeCoster (2009):
| Eq. (4) |
In Equation 4, the subscripts 1 and 2 represent the two groups being compared; n is as in previous calculations, p is the percentage of participants selecting the target response (e.g., LLR), and q is 1 – p. For within-subjects designs with a proportional dependent measure, Cohen’s dwin was calculated similarly to dbtw-prop (but with subscripts referring to measurements across baseline and intervention assessments):
| Eq. (5) |
The formula for dwin-prop was extrapolated from the calculation for dbtw-prop; although note that both formulas result in the same values. These particular formulas for proportion data were chosen over others (e.g., Cohen’s h; Cohen, 1988) because, based on simulations (not reported here), they yielded effect sizes of a more comparable range to that of Cohen’s dbtw and dwin.
After calculating the appropriate version of Cohen’s d, effect sizes were corrected for small sample sizes because Cohen’s d tends to overestimate effect sizes when groups are small (Cumming, 2011). To correct for small sample size bias, Hedge’s g was calculated by multiplying d by one of the following correction factors (Cumming, 2011; Lakens, 2013). For between-subjects designs, the correction (j) was:
| Eq. (6) |
and for within-subjects designs, the correction was:
| Eq. (7) |
For consistency across studies, the correction was applied regardless of sample size. Thus, the final reported effect sizes, and those used in meta-analyses, are all Hedge’s g.
Next, the sampling variance of the effect sizes was calculated using the following formula (Morris & DeShon, 2002):
| Eq. (8) |
where ñ is equal to (n1 * n2)/ (n1 + n2) for between-subjects designs and N for within-subjects designs. All others terms in equation 8 are as previously described and are the same across study types with the exception that the second term (N – 2/ N – 4) is (N – 1/ N – 3) for within-subjects designs. While variance calculations for within-subjects designs more typically incorporate the correlation between repeated measurements, the majority of studies employing within-subjects designs did not report this information. Application of Equation 8 to within-subject data is identical to the within-subject calculation of variance suggested by Morris and DeShon (2002) while assuming a correlation of 0.5 between measurements.
Finally, effect sizes were subjected to meta-analysis to broadly examine which categories and subcategories of manipulations were successful for reducing steepness of discounting. Meta-analysis was conducted using the metafor package (Viechtbauer, 2010) in R (R Core Team, 2013). The efficacy of the different categories of manipulations was examined using a mixed-effects model, including category as a moderator and no intercept. Next, similar models were conducted for each category but with subcategory included as a moderator (unless there were no subcategories, which reduced to a simple random-effects model). All effect sizes were included except where noted in the footnote below.3 A measure of effect size heterogeneity (I2) indicates the percentage of study variability, or the amount of variability in effect sizes not accounted for by chance (J. P. T. Higgins, Thompson, Deeks, & Altman, 2003).
Because of the heterogeneity in experimental procedures both between- (e.g., momentary framing vs. months-long training regimens) and within-categories (e.g., methods of measuring and quantifying impulsive choice), as well as the settings (e.g., controlled laboratory vs. outpatient clinic) and participant populations (e.g., economics students vs. laboratory rats), moderators of effect size other than category and subcategory were not examined. For these reasons, we also did not conduct comparisons of effect sizes across categories, nor did we provide metrics of publication bias. We chose not to provide the latter because in many instances there were varying numbers of papers for which effect sizes could not be calculated, which would ultimately bias the resulting measure. Thus, the meta-analytic technique primarily served to provide an objective method of determining manipulation efficacy, which was contextualized within narrative review.
Results
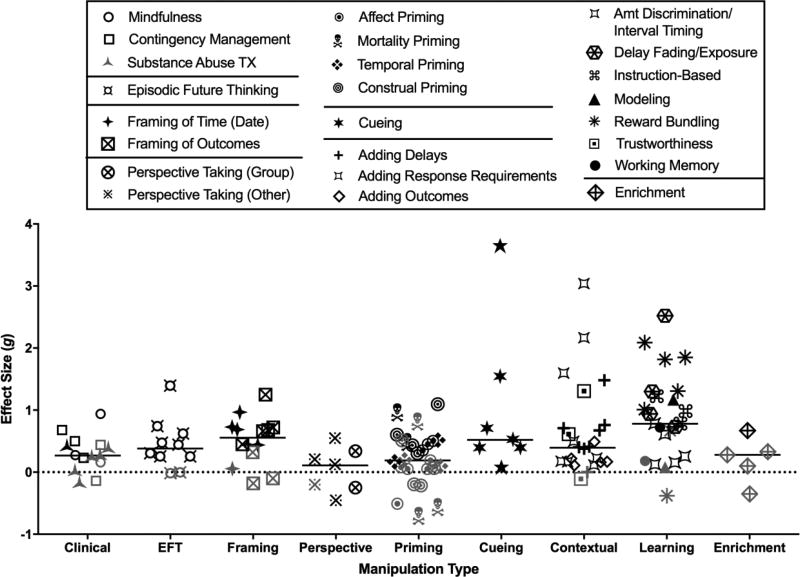
After applying the exclusion criteria, 92 papers qualified for review. These articles fell into the nine categories of experimental manipulations shown in Figure 3 (symbols represent effect sizes from each paper). Some categories were divided into the sub-categories outlined in the legend. The primary meta-analysis revealed that overall, the interventions were successful in reducing discounting, Q(9) = 224.68, p < .0001. Moderate heterogeneity in effect sizes reveal differences across studies (I2 = 64%). In the sections below, we discuss the significance of, and theory behind each of the categories and their subcategories as relevant. The categories are organized sequentially from applied to translational to basic research. As such, the first six sections summarize research conducted exclusively with human participants, while the remaining sections include human and nonhuman research subjects.
Figure 3.
Effect sizes (Hedge’s gbtw or gwin) by manipulation type. The effect sizes are either averages by publication (when a single study had more than one experiment or condition examining the same manipulation) or individual effect sizes (when a publication reported the result of one study or found a significant moderator of the effect). Larger effect sizes reflect greater preference for larger, delayed outcomes. Horizontal lines reflect the median effect size for that category; symbols for effect sizes are jittered to reduce overlap. Gray symbols indicate that the effect was not statistically significant.
Clinical Interventions
Twelve studies meeting the inclusion criteria examined the effects of clinical interventions; see Table 1 and Figure 4. For most of these studies, delay discounting was not a primary target of the intervention, but changes in discounting were examined because of their relevance to the problem behavior(s) (e.g., addictions). Clinical interventions overall produced significant reductions in discounting (B = 0.23, SE = 0.08; z = 2.78, p = .005), with magnitude and significance varying by subcategory. After accounting for subcategories, study heterogeneity was small to moderate (I2 = 38%) suggesting that a relatively larger percentage of variability in effect sizes was due to manipulation type, rather than study heterogeneity.
Table 1.
Effect sizes for Clinical Manipulations: Estimated subcategory averages (and SEM) from the Clinical manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Mindfulness-based approaches | 0.32 (0.17) | .06 | |||||
| Hendrickson & Rasmussen (2013) | College students | Mindful eating (vs. nutritional info control) | %LLR | ↓ | 0.27 (food) | ||
| ↓ | n.s. | 0.16 (money) | |||||
| Morrisson et al. (2014) | College students | ACT (vs. waitlist control) | AUC | ↓ | 0.94‡ | ||
| Contingency Management | 0.36 (0.15) | .02 | |||||
| Weidberg et al. (2015) | Treatment-seeking cigarette smokers (≥ 10 cigs/day) | CM + Cognitive Behavioral Therapy (CBT) (vs. CBT alone) | AUC | ↓ | 0.50* (women) | ||
| ↑ | 0.23* (men) | ||||||
| Yi et al. (2008) | Non-treatment-seeking cigarette smokers (≥ 20 cigs/day) | CM (vs. no monetary incentive control) | k | ↓ | 0.50 (money) | ||
| ↓ | 0.23 (cigarettes) | ||||||
| Yoon et al. (2009) | Non-treatment-seeking cigarette smokers (≥ 10 cigs/day) | CM (vs. non-contingent rewards) | k | ↓ | n.s. | 0.44 | |
| Other Substance-Use Treatments | 0.16 (0.08) | .03 | |||||
| Aklin et al. (2009) | Substance users | Residential substance use treatment (within-subjects) | k | ↓ | n.s. | 0.26 | |
| Black & Rosen (2011) | Cocaine, or cocaine+ alcohol abusing individuals | Money-management based substance use treatment (vs. minimal attention control) | k | ↓ | n.s. | 0.37 | |
| De Wilde et al. (2013) | Polysubstance dependent individuals | Residential substance use treatment (within-subjects) | k | – | n.s. | <0.01 | |
| Dennhardt et al. (2015) | Heavy-drinking college students | Motivational intervention (MI) and substance-free activity sessions (vs. MI with educational component) | k | ↓ | n.s. | 0.23 | |
| Landes et al. (2012) | Treatment-seeking opioid dependent individuals | Combinations of CM, buprenorphine counseling, etc. (within-subjects) | AUC | ↓ | 0.40* | ||
| Lee et al. (2015) | Adolescent and adult cannabis use/dependence | Outpatient treatment (combinations of CBT, movational enhancement, CM, etc. [within-subjects]) | k | ↓ | CNC | ||
| Littlefield et al. (2015) | Individuals with various SUDs | Residential substance use treatment (12-step, counseling, etc. [within-subjects]) | k | ↑ | 0.19 | ||
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Trend level significance for this between-subjects comparison (p < .06); the within-subjects comparison was statistically significant.
Effect size was calculated based on change scores at post-treatment for each group by sex.
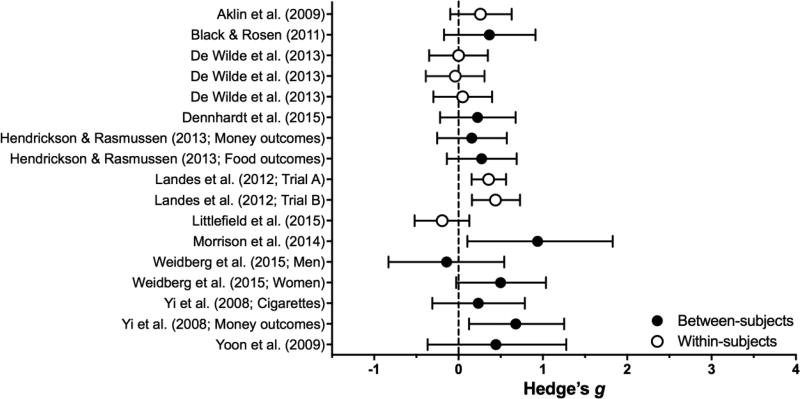
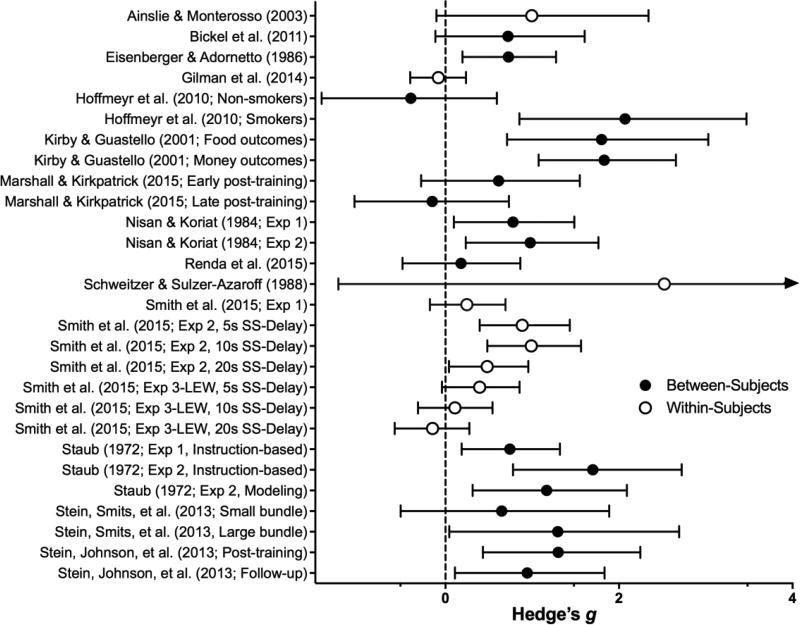
Figure 4.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals for manipulations in the Clinical category. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes.
Mindfulness-Based Approaches
Mindfulness is nonjudgmental awareness of present-moment events (e.g., thoughts, sensations), which can be employed as a stand-alone intervention or within mindfulness-based therapies such as Acceptance and Commitment Therapy (ACT; Hayes, Strosahl, & Wilson, 1999). Overall, the reduction in discounting produced by mindfulness interventions only approached significance (z = 1.89, p = .059). Mindful-eating produced a small decrease in discounting of hypothetical food, but not money (Hendrickson & Rasmussen, 2013), whereas a brief course of ACT produced significant within-subjects reductions in discounting (Morrison, Madden, Odum, Friedel, & Twohig, 2014). In the latter, however, the between-group difference (ACT vs. wait-list control) did not achieve statistical significance. Continued research to clarify outcome-specificity of effects while employing better controls (e.g., sham-therapy in lieu of wait-list controls) is warranted.
Contingency Management
In contingency management (CM) of substance abuse, rewards (e.g., money, vouchers) are provided contingent on biologically confirmed drug abstinence (Dallery, Glenn, & Raiff, 2007; S. T. Higgins & Petry, 1999; Silverman et al., 1996). If steep delay discounting is a consequence of frequent drug use, then abstinence-producing interventions like CM should decrease delay discounting (unless such changes are permanent). Three studies examining the effect of CM for reducing cigarette smoking revealed inconsistent effects, although when combined they produced significant decreases in discounting (z = 2.39, p = .02). In one study, a five-day CM intervention reduced cigarette smoking and discounting of delayed monetary- and cigarette-rewards (Yi et al., 2008). However, Weidberg et al. (2015) found that a longer course of CM decreased discounting in women only, and regression to the mean likely accounted for the effect. Yoon, Higgins, Bradstreet, Badger, and Thomas (2009) reported no effect of CM on delay discounting.
Other Substance Use Treatments
The effects of multi-component substance use treatments on delay discounting have been examined in five studies. Three of these found no significant reductions in steepness of discounting (Aklin, Tull, Kahler, & Lejuez, 2009; De Wilde, Bechara, Sabbe, Hulstijn, & Dom, 2013; Littlefield et al., 2015), but when combined they produced small, significant effects (z = 2.16, p = .03). In the two studies in which significant reductions were observed, CM was a component of the intervention (Landes, Christensen, & Bickel, 2012; Lee, Stanger, & Budney, 2015).
Two additional studies evaluated the effects of more specific treatment components on discounting. A financial-planning-based treatment for cocaine use (e.g., clients were encouraged to restrict current spending and to plan for future expenses) nominally reduced delay discounting (p = .052; Black & Rosen, 2011). Likewise, counseling clients to increase engagement in non-substance related activities (e.g., those related to educational and career goals) reduced drug value and use, but not discounting (Dennhardt, Yurasek, & Murphy, 2015).
Overall, substance-use treatments do not consistently reduce discounting, and their overall utility is modest (model-estimated d = .16). Heterogeneity in procedures and treatments makes it difficult to rectify these inconsistencies. When considered in light of the hypothesis that regular drug use produces neuroadaptations that increase delay discounting (e.g., Mendez et al., 2010; Yi, Mitchell, & Bickel, 2010), these findings offer no simple support for the position that drug abstinence would reverse these effects. Perhaps the neuroadaptations are longer lasting than the treatments in these studies (2 to 36 weeks) or that treatment-produced abstinence (not simply being in treatment) coincides with reductions in delay discounting. The latter analysis was conducted in only one study in this review (Weidberg et al., 2015) and they found no relation between smoking abstinence and reductions in discounting. Future substance-use treatment studies which include delay discounting or impulsive choice as a dependent measure should conduct much needed mediation analyses to evaluate the causal pathway that delay discounting might hold.
Episodic Future Thinking
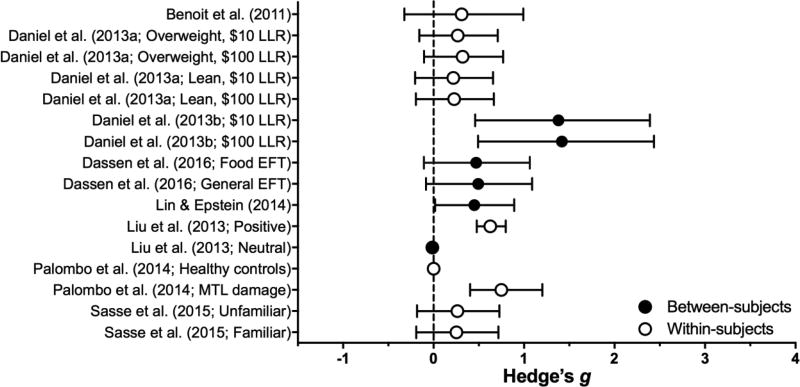
Episodic future thinking (EFT) is the act of vividly imagining one’s future, which involves episodic simulation (pre-experiencing an event in its entirety: associated feelings, sensations, emotions, etc.) as opposed to generating semantic details (facts, general knowledge; Atance & O’Neill, 2001). When applied to delay discounting, participants are first asked to identify and vividly imagine positive future events (e.g., Daniel, Stanton, & Epstein, 2013b; Peters & Büchel, 2010) and then are cued to imagine these events while completing a delay discounting task. EFT putatively reduces discounting by increasing the salience of future events or response-outcomes that would otherwise not be considered (Dassen, Jansen, Nederkoorn, & Houben, 2016; Lin & Epstein, 2014), and/or that it inhibits hyper-valuation of immediate rewards (Snider, LaConte, & Bickel, 2016). Based on the ten studies in this review, EFT produces sizeable (B = 0.38, SE = .09), significant reductions in discounting (z = 4.02, p < .0001) with little study variability (I2 = 3%); see Table 2 and Figure 5.
Table 2.
Effect sizes for Episodic Future Thinking Manipulations: Individual-publication effect sizes.
| Study | Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | |
|---|---|---|---|---|---|---|
| Benoit et al. (2011) | Adults | Imagine spending LLR (vs. estimate what LLR can buy after delay) | %LLR | ↓ | 0.31 | |
| Daniel et al. (2013a) | Overweight (BMI ≥ 25) & lean (BMI ≤ 25) women | Imagine positive future events (vs. vivid scenes from Pinocchio) | AUC | ↓ | 0.26* | |
| Daniel et al. (2013b) | Overweight women (BMI ≥ 25) | Imagine positive future events (vs. vivid events from travel blog) | AUC | ↓ | 1.40* | |
| Dassen et al. (2016) | Adult women | Imagine future food- or unconstrained events (vs. recent food or unconstrained events) | k | ↓ | 0.48* | |
| Kwan et al. (2015) | Healthy and amnesiac adults | Imagine future events (vs. no cues) | AUC | ↓ | CNC | |
| Lin & Epstein (2014) | Adults | Imagine positive or neutral future events (vs. very near-future [present] events) | k | ↓ | 0.45 | |
| Liu et al. (2014) | College students | Imagine experimenter-provided positive future events (vs. no imagined events) | %SSR | ↓ | 0.63 | |
| Same, but neutral future events | – | n.s. | 0.01 | |||
| Palombo et al. (2014) | Healthy adults | Imagine spending reward in the future (vs. no explicitly imagined events) | LLR Accrual** | ↓ | 0.75 | |
| Amnesiacs | – | n.s. | 0.00 | |||
| Peters & Büchel (2010) | Adults | Episodic cues present (vs. absent) | k | ↓ | CNC | |
| Sasse et al. (2015) | Adults | Imagine meeting un/familiar person in the future (vs. no imagined event) | k | ↓ | 0.26* | |
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Effect sizes averaged across experiments or comparable conditions.
Across-trial “earnings” calculated as the total amount awarded above and beyond that which would have been obtained by exclusively choosing the immediate option.
Figure 5.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals for Episodic Future Thinking manipulations. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes
EFT procedures have been shaped by empirical findings regarding moderators of its efficacy. First, the episodic thinking must be future-oriented: EFT reduces delay discounting relative to present (Lin & Epstein, 2014), past (Dassen et al., 2016), and temporally-neutral thinking (Daniel, Stanton, & Epstein, 2013a; Daniel et al., 2013b). Second, EFT produces larger effects when future events are more vividly imagined (Palombo, Keane, & Verfaellie, 2015; Peters & Büchel, 2010) and personally/emotionally relevant (Benoit, Gilbert, & Burgess, 2011). Thus, when engaging in EFT participants are often encouraged to imagine many details about the future events (e.g., where will this happen, what will you see/smell/hear; Dassen et al., 2016; Kwan et al., 2015a; Palombo, Keane, & Verfaellie, 2015b) and are explicitly instructed to imagine the cued events while completing the discounting task (cf. Peters & Büchel, 2010). The presented cues are often temporally-matched with the LLR (e.g., “Graduation in 1 year” is shown when the LLR is “$100 in 1 year”).
Some findings have called into question the necessity of episodic prospection for EFT benefits. Kwan et al. (2015) reported that EFT reduced delay discounting in amnesiacs with serious deficits in episodic prospective ability, and that changes in delay discounting were unrelated to the extent of these deficits. Notably, participants in Kwan et al. identified personally-relevant future events. By contrast,Palombo et al. (2015), who supplied future events to participants, reported no beneficial effect of EFT in a similar sample. Because vividness and personal/emotional relevance are related to EFT’s efficacy (Benoit, Gilbert, & Burgess, 2011b; Palombo et al., 2015; Peters & Büchel, 2010), this procedural difference may account for the discrepant results. Kwan et al. (2015) also suggested that personal cues may enable other types of future prospection (e.g., semantic), which may similarly enhance future perspective.
The importance of other aspects of typical EFT procedures are less well researched. Where one study suggested benefits of EFT were dependent upon imagining positive-valence future events (Liu, Feng, Chen, & Li, 2013), another found that imagining neutral-valence events reduced steepness of delay discounting (Lin & Epstein, 2014). These studies used different discounting tasks and dependent measures, so additional research is needed to resolve the issue of valence.
Some research has evaluated individual-differences that moderate the effects of EFT. EFT is less effective in those with low working memory capacity (Lin & Epstein, 2014), low goal persistence (Daniel et al., 2013a), and high consideration of the future (Benoit et al., 2011a); i.e., those who are already future-oriented do not benefit as much from EFT (but see Daniel et al., 2013a for a failure to replicate with a different measure of time perspective). Given working memory deficits among substance-dependent individuals (e.g., Bechara & Martin, 2004), EFT interventions may not be as successful for such populations.
The modally discussed psychological mechanism by which EFT reduces delay discounting is increasing future orientation or broadening temporal horizon (e.g., Lin & Epstein, 2014; Snider et al., 2016). The one study meeting inclusion criteria which has evaluated this hypothesis indicated EFT did not increase future orientation (Dassen et al., 2016).4 The authors speculated that the Consideration of Future Consequences scale (Strathman, Gleicher, Boninger, & Edwards, 1994), a measure of future orientation, is not sensitive to state changes.
An alternative account of the effect of EFT on delay discounting is that it may be a byproduct of demand characteristics (Rung & Madden, 2018). That is, if the experimental hypothesis is deduced by the participant, then he/she may behave in accord with it (Orne, 1962; see Nichols & Maner, 2008 for a demonstration of such bias)–i.e., choose the LLR. Presenting future cues (e.g., “vacation in 2 years”) delay-matched to the LLR (e.g., $10 now vs. $100 in 2 years) increases concerns for a demand-characteristic effect. Indeed, the majority of participants who read a description of typical EFT procedures deduced that the experimenter expected the participant to choose the LLR (Rung & Madden, 2018). Evaluating the contribution (if any) of demand characteristics to the effect of EFT should be a priority for future research.
Framing
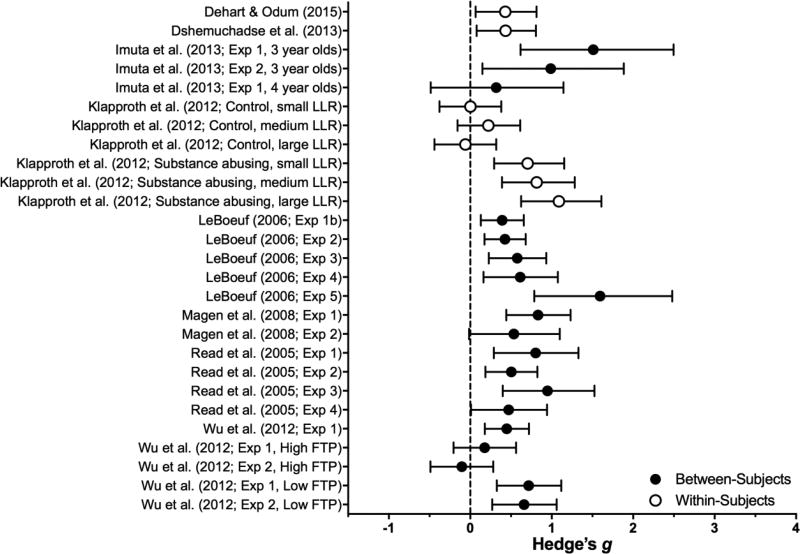
Framing manipulations vary the description of an intertemporal choice while holding functionally equivalent the outcomes across different descriptions/frames (Kühberger, 1998). For instance, in a classic example of framing (the Asian disease problem; Tversky & Kahneman, 1981), participants choose how to address the outbreak of a disease. One frame indicates the number who can be saved, and the other the number who will die. Across frames, the outcomes are the same, but choice is influenced by the gain/loss framing. Ten studies meeting the inclusion criteria examined the effects of different choice frames (see Table 3 and Figure 6), which produced medium-to-large (B = 0.47, SE = .06), significant reductions in discounting (z = 7.48, p < .0001). Across framing studies, there was a moderate degree of study heterogeneity (I2 = 68%).
Table 3.
Effect sizes for Framing Manipulations: Estimated subcategory averages (and SEM) from the Framing manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Framing of Time | 0.45 (0.08) | <.001 | |||||
| DeHart & Odum (2015) | College students | Date framing (vs. delay) | AUC | ↓ | CNC | ||
| Dshemuchadse et al. (2013) | College students | Date framing (vs. delay) | AUC | ↓ | 0.34 | ||
| Klapproth (2012) | Substance Users | Date framing (vs. days framing) | k | ↓ | 0.96* | ||
| Healthy Controls | ↓ | 0.05*◊ | |||||
| LeBoeuf (2006) | College students | Date framing (vs. delay) | LLR amount/delay | ↓ | 0.72* | ||
| Read et al. (2011) | Adults | Date framing (vs. delay) | % Choosing LLR, indifference pt | ↓ | 0.68* | ||
| Framing of Outcomes | 0.54 (0.12) | < .001 | |||||
| Reward segregation | |||||||
| Grace & McLean (2005) | College students | LLR separated into SSR amount plus bonus | k | ↓ | CNC | ||
| Imuta et al. (2013) | 3-year olds | LLR displayed as SSR amount plus bonus | LLR Choices | ↓ | 1.25* | ||
| 4-year olds | ↓ | n.s. | 0.32 | ||||
| Explicit Zero | |||||||
| Magen et al. (2008) | Online volunteers | Explicit zero (vs. usual task) | SSR Choices | ↓ | 0.68* | ||
| Radu et al. (2015) | College students | Explicit zero (vs. usual task) | SSR Choices | ↓ | CNC | ||
| Wu & He (2012) | College students | Explicit zero (vs. usual task) | LLR Choices | ↓ | 0.45 | ||
| ↓ | 0.72 (low future orientation) | ||||||
| ↓ | n.s. | 0.18 (high future orientation) | |||||
| % Choosing LLR | ↓ | 0.66‡ (low future orientation) | |||||
| ↑ | 0.10‡ (high future orientation) | ||||||
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Effect sizes averaged across experiments or comparable conditions.
Average is a combination of significant and non-significant effects.
Statistical analyses comparing the proportions choosing LLR were not conducted for these particular group comparisons in the original paper.
Figure 6.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals for Framing manipulations. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes.
Framing of Time
Describing LLRs as an outcome to be delivered on a specific date (e.g., $100 obtained on _____ [insert date 1 year from today]) instead of delayed by the same interval of time (e.g., $100 in 1 year) consistently and significantly reduces delay discounting (z = 5.39, p < .0001) (Read, Frederick, Orsel, & Rahman, 2005; LeBoeuf, 2006). This finding has been replicated (DeHart & Odum, 2015; Dshemuchadse, Scherbaum, & Goschke, 2013; Klapproth, 2012), with effects of medium to large magnitude (B = 0.45).
Four theoretical accounts of the date-delay framing effect are noteworthy. First, date framing may shift attention from the delay and increase sensitivity to the difference in the SSR and LLR monetary amounts (LeBoeuf, 2006; Read et al., 2005). Second, presenting dates may interfere with computational strategies or heuristics typically used to judge the subjective values of delayed outcomes (Read et al., 2005). Third, the effect could be due to subadditivity: specifying the delay as a date prevents participants from considering, for example, six separate 1-week delays when the delay is specified as “6 weeks” (LeBoeuf, 2006; Read, 2001). While not included in tables or graphs herein (exclusion criteria 6), support for the subadditivity hypothesis is mixed: one study provides support (days vs. date conditions in DeHart & Odum, 2015) and two provide evidence against (Experiment 5 in LeBoeuf, 2006; Experiment 1 in Read et al., 2005). Finally, date-delay framing may reduce subjective estimates of time duration (see Experiment 6 in LeBoeuf, 2006). For example, drug dependent populations overestimate time durations (e.g., Wittmann, Leland, Churan, & Paulus, 2007), and Klapproth (2012) reported that date framing reduced delay discounting so much in this population (Mdn g = 0.96) that their discounting rates were not significantly different from those of a non-drug-using comparison group.
Framing of Outcomes
The remaining framing studies manipulated the presentation of the SSR and LLR outcomes themselves, which produced significant reductions in discounting (z = 4.32, p < .0001). In the most common outcome frame, the explicit zero manipulation, the mutual exclusivity of SSR and LLR alternatives is highlighted by noting that selecting one alternative means nothing will be received at the time the foregone option would have been obtained (Magen, Dweck, & Gross, 2008). For example, instead of choosing between $50 now vs. $100 in 1 year, the zero outcomes are made explicit by reframing the choice as $50 now and $0 in 1 year vs. $0 now and $100 in 1 year. Explicit-zero framing significantly reduces discounting with medium to large effects (Magen et al., 2008; Radu, Yi, Bickel, Gross, & McClure, 2011; Wu & He, 2012). While the zero is typically made explicit in both the SSR and LLR alternatives, Wu and He (2012) found that the delayed zero ($50 now and $0 in 1 year) is largely responsible for the effect. Presenting the immediate zero alone produced no significant reduction.
Magen et al. (2008) proposed that a preference for improving sequences can explain the explicit zero effect (e.g., Loewenstein & Prelec, 1993). That is, choosing the LLR arranges an improving sequence from $0 now to money in the future, whereas the SSR yields a decreasing sequence from something now to nothing later. This account was challenged by Radu et al. (2011): explicit zeros reduce past discounting, in which preference for the larger more-distal reward produces a decreasing sequence (e.g., $100 26-days ago and $0 one hour ago). Combined with the null effect of the present zero (Wu & He, 2012), the improving sequence hypothesis appears refuted. Radu and colleagues (2011) suggest instead that the explicit-zero increases temporal attention to more distal outcomes, thereby broadening the temporal window across which choice outcomes are integrated. That the explicit-zero effect is muted among those high in future time perspective (average g = 0.04; Wu & He, 2012) is consistent with this account.
Other outcome framing manipulations were infrequent (n = 2). In Grace and McLean (2005), the LLR was presented as two amounts: the amount of the SSR plus the difference between the SSR and LLR amounts. For example, a choice between $150 now vs. $200 in 1 year was reframed as $150 now vs. $150 plus a $50 bonus, both delivered in 1 year. Segregating the LLR significantly reduced discounting. Imuta, Hayne, and Scarf (2014) found similar reductions in impulsive choice when children were shown the SSR (stickers), and then additional stickers were added to comprise the LLR. Grace and McLean (2005) explained the effect as the result of diminishing marginal utility of rewards; i.e., the subjective value of a reward increases as a concave function of objective amount (Galanter, 1962). Therefore, when the LLR is separated into two outcomes, the value of each is calculated separately and the SS amount + bonus is subjectively more valuable than the single-quantity LLR. Given the initial successes of these manipulations, additional empirical attention appears warranted.
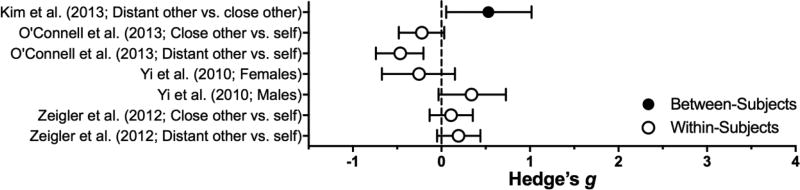
Perspective Taking
Making decisions on behalf of a group or another person does not, overall, significantly affect delay discounting (z = 0.09, p = .93; I2 = 84%; see Table 4 and Figure 7). Instructional differences may account for some discrepant between-study effects (see Ziegler & Tunney, 2012), but at this time there are too few studies representing the different instruction types (and potentially important participant characteristics) to objectively support this via evaluation of moderator(s).
Table 4.
Effect sizes for Perspective Manipulations: Estimated subcategory averages (and SEM) from the Perspective manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Group Perspective | 0.05 (0.28) | .86 | |||||
| Charlton et al. (2013) | College students | Group (vs. self) | k | ↓ | CNC | ||
| Yi et al. (2010) | Adults | Group (vs. self) | k | ↑ | 0.34 (females) | ||
| ↓ | 0.25 (males) | ||||||
| Another’s Perspective | 0.01 (0.16) | .98 | |||||
| Kim et al. (2013) Experiment 3 | Adults | Choosing on stranger’s behalf (vs. close other) | Indifference point | ↓ | 0.53 | ||
| O’Connell et al. (2013) | College students | Choosing from perspective of distant or close other (vs. self) | AUC | ↑ | 0.47 | ||
| ↑ | n.s. | 0.22 | |||||
| Weatherly & Ruthig (2013) | College students | Choosing on other’s behalf | AUC | – | n.s. | CNC^ | |
| Ziegler & Tunney (2012) | College students | Choosing on other’s behalf (of varying closeness, vs. self) | k | ↓ | 0.19 (distant) | ||
| ↓ | 0.11 (close) | ||||||
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Data were obtained from the authors but could not be transformed to normality for effect size calculation.
Figure 7.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals manipulations in the Perspective Taking category. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes.
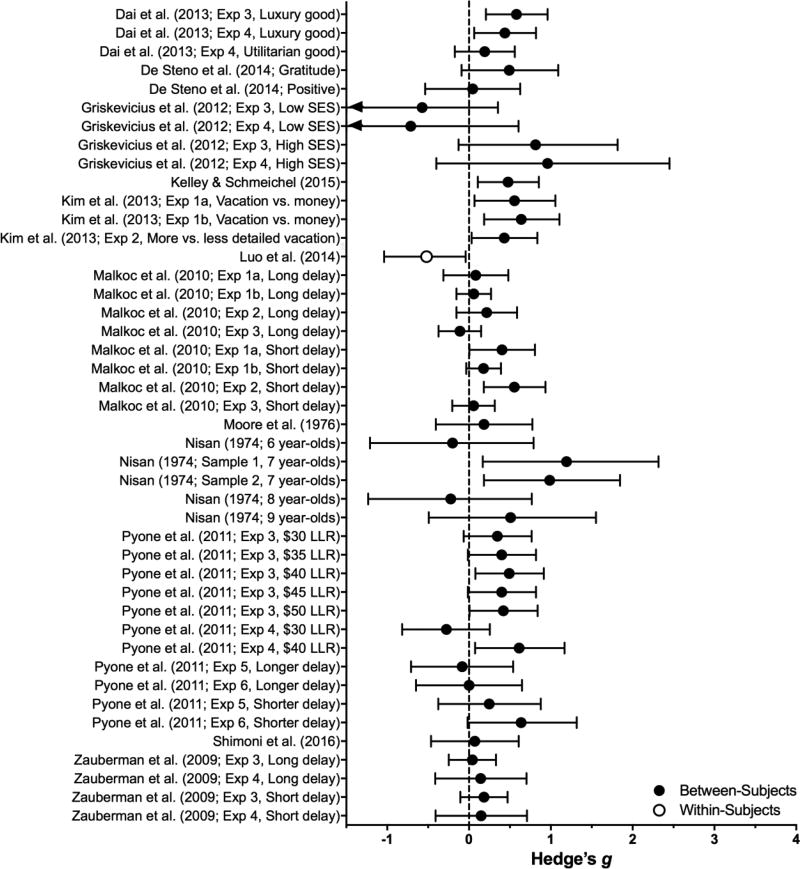
Priming
Priming involves experimental manipulations of participants’ affect or cognitive content, typically arranged through a preliminary task and often framed as part of a different experiment than the discounting task. While priming manipulations produced modest (B = 0.24, SE = 0.06), significant reductions in discounting (z = 4.18, p < .0001) their effects are often context-specific (see Table 5 and Figure 8). The latter is both empirically (see below) and statistically supported by moderate study heterogeneity (I2 = 42%).
Table 5.
Effect sizes for Priming Manipulations: Estimated subcategory averages (and SEM) from the Priming manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Affect priming | 0.17 (0.09) | .06 | |||||
| DeSteno et al. (2014) | College students | Positive (vs. neutral) | δt | – | n.s. | 0.04 | |
| Gratitude (vs. neutral) | ↓ | 0.49 | |||||
| Hirsh et al. (2010) | College students | Positive (vs. neutral) | k | ↑ | n.s. | CNC | |
| Luo et al. (2014) | Adult humans | Positive (vs. neutral) | %LLR | ↑ | n.s. | 0.52 | |
| Moore et al. (1976) | 3–5 year olds | Positive (vs. neutral) | %LLR | ↓ | n.s. | .18 | |
| Pyone & Isen (2011) § | College students | Positive (vs. neutral) | %LLR | ↓ | 0.34*◊ | ||
| ↓ | 0.44* (short delay) | ||||||
| ↓ | n.s. | 0.04* (long delay) | |||||
| Shimoni et al. (2016) | 3rd graders | Positive (vs. neutral) | AUC | – | n.s. | 0.07 | |
| Mortality priming | 0.30 (0.19) | .12 | |||||
| Griskevicius et al. (2011) | College students | High violent crime stats (vs. no prime) | %LLR | ↓ | 0.89*◊ (high SES) | ||
| ↑ | n.s. | 0.64* (low SES) | |||||
| Kelley & Schmeichel (2015) | College students | Think about your own death (vs. dental procedure) | k | ↓ | 0.48 | ||
| Kelley et al. (2015) | College students | Think about your own death (vs. uncertainty prime) | Indifference point | – | n.s. | CNC (overall) | |
| ↓ | CNC (disgust-sensitive) | ||||||
| Temporal priming | 0.25 (0.10) | .02 | |||||
| Dai & Fishbach (2013) | College students | Consider time since last reward (vs. no prime) | %LLR | ↓ | 0.51* (chocolate) | ||
| ↓ | n.s. | 0.19 (flashdrive) | |||||
| Zauberman et al. (2009) | College students | Estimate time durations (vs. estimate calories) | Discount rate | ↓ | 0.16*◊ (short delay) | ||
| – | 0.09* (long delay) | ||||||
| Construal priming | 0.26 (0.07) | <.001 | |||||
| Kim et al. (2013) Experiments 1–2 | College students | Concrete construal of SSR and LLR (vs. usual monetary discounting task) | Indifference point | ↓ | 0.60* | ||
| Online volunteers | More concrete (vs. less concrete vacation discounting task) | ↓ | 0.43 | ||||
| Nisan (1974) | 6 to 9 year-old children | SSR and LLR visually available (vs. not shown) | %LLR | ↑ | n.s. | 0.20 (6 year olds) | |
| ↓ | 1.09* (7 year olds) | ||||||
| ↑ | n.s. | 0.22 (8 year olds) | |||||
| ↓ | n.s. | 0.51 (9 year olds) | |||||
| Malkoc et al. (2010) | College students | Abstract (vs. concrete) thinking | Delay premium | ↓ | 0.30*# (short delay) | ||
| ↑ | – | 0.06*# (long delay) | |||||
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Effect sizes from Exp 3 ($30, $45, and $40 LLR magnitudes) were omitted from the meta-analysis but are included in the averages in the table.
Average is a combination of significant and non-significant effects.
Effect sizes averaged across experiments or comparable conditions.
There was a significant interaction between the prime and the reward delay; post-hoc tests were not conducted, thus the significance of the by-delay effects are not indicated.
Figure 8.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals manipulations in the Priming category. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes, and arrows on the end of a confidence interval indicate that the limits extended beyond the axes.
Affect Priming
Affect priming typically involves the presentation of emotion-inducing stimuli (e.g., pictures, words) or directed remembering (e.g., think of a positive event in your past). Across six papers, positive-affect priming had small (B = 0.17, SE = .09), inconsistent, and overall non-significant effects on discounting (z = 1.87, p = .06). Only one of these papers reported significant reductions in discounting: Pyone and Isen (2011) found that positive-affect primes reduced impulsive choice in three of four experiments, and that the effects were dependent on the magnitude or delay of the larger-later rewards5. The remaining five papers either reported no significant effect of positive affect (DeSteno, Li, Dickens, & Lerner, 2014; Luo, Ainslie, & Monterosso, 2014; Moore, Clyburn, & Underwood, 1976; Shimoni, Asbe, Eyal, & Berger, 2016) or the opposite among extraverted participants (Hirsh, Guindon, Morisano, & Peterson, 2010). DeSteno et al. (2014), however, argued that the specific feeling of gratitude should increase altruism (which is generally motivated by long-term interests) and thereby reduce delay discounting. Consistent with this hypothesis, priming gratefulness produced moderate reductions in discounting.
Mortality Priming
Studies inducing thoughts of one’s mortality, either now or in the future, have a small (B = 0.30, SE = .19) nonsignificant impact on delay discounting (z = 1.56, p = .12; see Table 5 and Figure 5). Any significant effects of mortality priming on discounting are complicated by the specific primes used and participant characteristics (e.g., high vs. low SES; high vs. low disgust-sensitivity; Griskevicius, Tybur, Delton, & Robertson, 2011; Kelley, Crowell, Tang, Harmon-Jones, & Schmeichel, 2015)
Temporal Priming
The few studies examining effects of temporal primes on steepness of discounting have produced modest (B = 0.25, SE = .10) but significant reductions in discounting (z = 2.41, p = .02; see Table 5 and Figure 5). For example, Zauberman, Kim, Malkoc, & Bettman (2009) demonstrated that nonlinear perception of time could partially account for the hyperbolic shape of the delay discounting function (see also McKerchar et al., 2009). From this, they hypothesized that priming attention to time would shift discounting from hyperbolic (steep declines at short delays that give way to shallow declines at long delays) to exponential (constant rate of discounting at all delays). Temporal priming was achieved by having participants estimate a variety of time durations (i.e., how long does it take to…). Consistent with a shift from hyperbolic to exponential discounting, time-primed participants discounted modestly less at a one-month, but not a three-month delay to the LLR. This effect was not, however, replicated in a follow-up experiment in the same report.
Construal Primes
Construal-level theory (Trope & Liberman, 2003) posits that information processing occurs on a continuum from concrete (detailed, context-dependent, focused on the present situation) to abstract (broad, decontextualized, focused beyond the present situation). Applied to intertemporal choice, the SSR is imminent so it should be construed at a concrete level, whereas the LLR should be construed relatively abstractly (general thoughts about the nonspecific life-context in which the LLR would be received). While construal primes vary in their implementations, they yield the most consistent and significant effects of all primes reviewed herein (B = .26, SE = .07; z = 3.56, p = .0004).
Malkoc, Zauberman, & Bettman (2010) hypothesized that abstract construal of the SSR (vs. the concrete default) should render the discounting function more exponential. In so doing, discounting should decrease in the shorter range of LLR delays, similar to the effects of temporal priming in Zauberman et al. (2009). In support of their hypothesis, priming abstract thinking prior to an intertemporal choice task produced a small reduction in discounting at brief, but not long delays relative to concrete and control primes (i.e., a significant interaction with delay).
In contrast,Kim et al. (2013) suggested the mismatch in construal across the SSR (concrete) and LLR (abstract) impedes comparison of these outcomes and increases impulsive choice. Across three experiments, providing concrete visual and/or verbal details about immediate and delayed Paris vacations reduced discounting. Nisan (1974) conducted a similar concrete-construal manipulation by visually (vs. verbally) presenting the SSR and LLR prior to the choice.6 Visual presentation decreased impulsive choice in 7-year olds, but had no effect in younger or older children who were putatively too impulsive or self-controlled, respectively, to benefit from the manipulation.
Viewed from the perspective of construal-level theory, EFT might be conceptualized as an all-concrete manipulation. That is, if the default construal of the SSR is concrete, then thinking vividly about the LLR may render its construal more concrete (e.g., I will be getting married in two years when I receive the $1,000). If this analysis is correct, then EFT represents a subset of construal-based manipulations, in which its mechanism is construal-level parity.
Cueing
Cueing involves the presentation of a functional stimulus prior to decision-making. The function of the stimulus may be acquired through ontogenetic learning or phylogenetic evolution. While few studies have evaluated the ability of cues to reduce discounting, those that have show promise. Combined, the five studies examining cueing effects produce significant (z = 4.75, p < .0001) and moderate reductions in discounting (B = 0.63, SE = 0.13). The large degree of study heterogeneity in this category (I2 = 88%) is likely attributed to studies examining effects of learned cues, which had sparse representation (see discussion below).
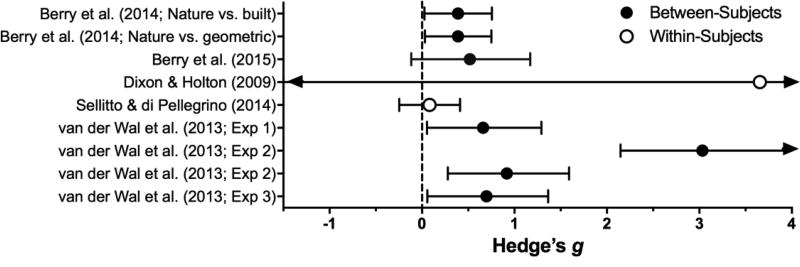
Humans show an affinity for looking at natural landscapes depicting resource abundance (Purcell, Peron, & Berto, 2001), which has beneficial effects on affect and attention (Bowler, Buyung-Ali, Knight, & Pullin, 2010) and slows the perception of how quickly time passes (Rudd, Vohs, & Aaker, 2012). Three studies report medium to large reductions in delay discounting following presentation of nature cues (see Table 6 and Figure 9; z = 2.81, p = .005). Nature cueing produces reductions in discounting both in-lab (i.e., nature photos, Berry et al., 2015; Berry, Sweeney, Morath, Odum, & Jordan, 2014; van der Wal et al., 2013) and in-vivo (i.e., spending time outdoors, van der Wal et al., 2013). Some mechanisms for the effects of nature cues have been evaluated: the studies above found no differences in session-time estimation across nature- and urban-cue conditions (Berry et al., 2014), and discounting rate was not significantly correlated with time estimation (Berry et al., 2015) nor with changes in affect (van der Wal et al., 2013). Thus, the prevailing hypothesis is that nature cues signal a safe, rich environment in which waiting is evolutionarily adaptive. Given that exposure to nature cues consistently reduces discounting with medium-to-large significant effects, further research evaluating dose- and the duration of its effects is warranted.
Table 6.
Effect sizes for Cueing Manipulations: Estimated subcategory averages (and SEM) from the Cueing manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Nature | 0.89 (0.31) | .005 | |||||
| Berry et al. (2014) | College students | Nature cues (vs. built environment) | AUC | ↓ | 0.39 | ||
| (vs. geometric shapes) | ↓ | 0.39 | |||||
| Berry et al. (2015) | College students | Nature cues (vs. built environment) | AUC | ↓ | 0.52 | ||
| Van der Wal et al. (2013) | College students | Nature cues (vs. built environment, or vs. no images) | Indifference point | ↓ | 1.54* | ||
| Community sample | Walk in nature (vs. built environment) | ↓ | 0.70 | ||||
| Learned Cues | 0.29 (0.77) | .71 | |||||
| Dixon & Holton (2009) | Pathological gamblers | Relational training (vs. pre-training baseline) | AUC | ↓ | 3.65 | ||
| Sellito & di Pellegrino (2014) | Female college students | High error rate cue (vs. low error rate) | k | ↓ | 0.08 (within-Ss) | ||
AUC, area under the discounting curve
Effect sizes averaged across experiments or comparable conditions.
Figure 9.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals manipulations in the Cueing category. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes, and arrows on the end of a confidence interval indicate that the limits extended beyond the axes.
Cues acquiring meaning as a function of learning history can also affect delay discounting; however, the small number of studies in this subcategory (n = 2) combined with the large differences in the magnitude of their effects, and sample sizes used (which produced an extremely wide confidence interval in one case) rendered their combined efficacy non-significant (z = 0.38, p = .71). Given the substantial differences in the theory driving these studies, this non-significant result should be interpreted with caution. In brief, Sellitto and di Pellegrino (2014) demonstrated small, significant reductions in discounting by presenting cues established to recruit enhanced top-down cognitive control. By contrast, cues trained with “better than” and “worse than” functions substantially decreased delay discounting in pathological gamblers when the “better than” cue was paired with the LLR (Dixon and Holton, 2009). As discussed in the context of EFT, in the latter study participants may have deduced the experimenter’s intent during the discounting task, so demand characteristics are a concern.
Context
Thirteen studies falling into the broad category of contextual manipulations produced significant reductions in impulsive choice (B = 0.37, SE = 0.05; z = 6.90, p < .0001; see Table 7 and Figure 10). Given the breadth of this category and theories behind the approaches, the moderate to large degree of study variability is unsurprising (I2 = 68%). Context manipulations involve changing features of the choice scenario that do not fall within the scope of framing manipulations; i.e., these manipulations do not produce economically equivalent outcomes across conditions. Where possible, similar manipulations are grouped together.
Table 7.
Effect sizes for Contextual Manipulations: Estimated subcategory averages (and SEM) from the Contextual manipulation-only meta-analytic mode and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Adding Delays | 0.49 (.08) | <.001 | |||||
| Ainslie & Herrnstein (1981) | Pigeons | Added 4-s delay (vs. no added delay) | %LLR | ↓ | ‡ | ||
| Added 12-s delay (vs. no added delay) | ↓ | ‡ | |||||
| Dai & Fishbach (2013) Experiments 1–2b | College students | Added delay (vs. small added delay) | Proportion choosing LLR | ↓ | 0.40*◊ | ||
| Same, but choice made when delay to SSR 90% complete (vs. small added delay) | ↓ | 0.70* | |||||
| Green et al. (2005)§ Experiments 1–2 | College students | Added 2-year delay (vs. no added delay) | AUC | ↓ | −0.01*# | ||
| Added 5-year delay (vs. no added delay) | ↓ | 0.39*# | |||||
| Added 10-year delay (vs. no added delay) | ↓ | 0.66*# | |||||
| Mitchell & Wilson (2012) Experiments 1–2 | Nonsmokers | Added 12-week delay (vs. no added delay) | k | ↓ | n.s. | 0.37* | |
| Smokers | ↓ | 0.75* | |||||
| Rachlin & Green (1972) | Pigeons | Added 4-s delay (vs. no added delay) | Relative response rate | ↓ | ‡ | ||
| Added 12-s delay (vs. no added delay) | ↓ | ‡ | |||||
| Seigel & Rachlin (1995) | Pigeons | Added delay (vs. no added delay) | %SSR | ↓ | 1.47 | ||
| Adding Response Requirements | 2.14 (1.17) | .07 | |||||
| Fortes et al. (2015) | Pigeons | High response rate during LLR delay (vs. no responding during delay) | Indifference delay | ↓ | ‡ | ||
| Low response rate during LLR delay (vs. no responding during delay) | ↑ | ‡ | |||||
| Huskinson & Anderson (2013) | Rats | High pre-choice response requirement (vs. single response) | AUCb | ↓ | 3.03 | ||
| Half of the pre-choice response requirement (vs. single-response) | ↓ | 2.16 | |||||
| Mazur (2012) | Rats | Pre-choice response requirement (vs. single response) | %LLR | ↓ | ‡ | ||
| Pigeons | — | ‡ | |||||
| Seigel & Rachlin (1995) | Pigeons | Pre-choice response requirement (vs. single response) | %LLR | ↓ | 1.59* | ||
| Adding Outcomes (vs. no added outcome) | 0.24 (0.06) | < .001 | |||||
| Kowal & Faulkner (2015) | College students | Added decoy LLR alternative | k | ↓ | 0.16◊* | ||
| Added decoy SSR/LLR alternative | k | ↓ | 0.11 | ||||
| Scholten & Read (2014) | Online volunteers | Improving sequence (LLR) | Proportion choosing LLR | ↓ | 0.17* | ||
| Deteriorating sequence (SSR) | ↓ | 0.49* | |||||
| Urminsky & Kivetz (2011)˚ | Adults | Add small, near-immediate reward to SSR and LLR | Proportion choosing & preference for | ↓ | 0.22◊* | ||
| Trustworthiness | 0.76 (0.17) | < .001 | |||||
| Mahrer (1956) | Stimulant addicts | Working memory training (vs. sham) | Proportion choosing LLR | ↓ | 0.60 | ||
| Michaelson et al. (2013) | Online volunteers | Trustworthy LLR provider (vs. neutral provider) | Probability of choosing LLR | ↓ | 1.29 (within-Ss) | ||
| ↑ | n.s. | 0.12 (between-Ss) | |||||
n.s., No statistically significant effect; AUC, area under the discounting curve
The effect of the added delay was examined using an ANOVA, with the no-added delay as the reference condition; there was a main effect of added-delay, but post-hoc tests were not reported (and thus, statistical significance for each added delay in the table is unknown).
The effect sizes for the $10k and $100k LLRs were omitted from the meta-analysis (>3 effect sizes in the same subjects) but are included in the averages presented in the table.
Effect sizes from Exp 1b ($50 and $100 token amounts), Exp 3, and Exp 5a ($100, $300, $700, and $900) were omitted from the meta-analysis (>8 effect sizes from the same publication) but are included in the averages presented in the table.
Effect sizes were not calculated due to small sample size.
Effect sizes averaged across experiments or comparable conditions.
Average is a combination of significant and non-significant effects.
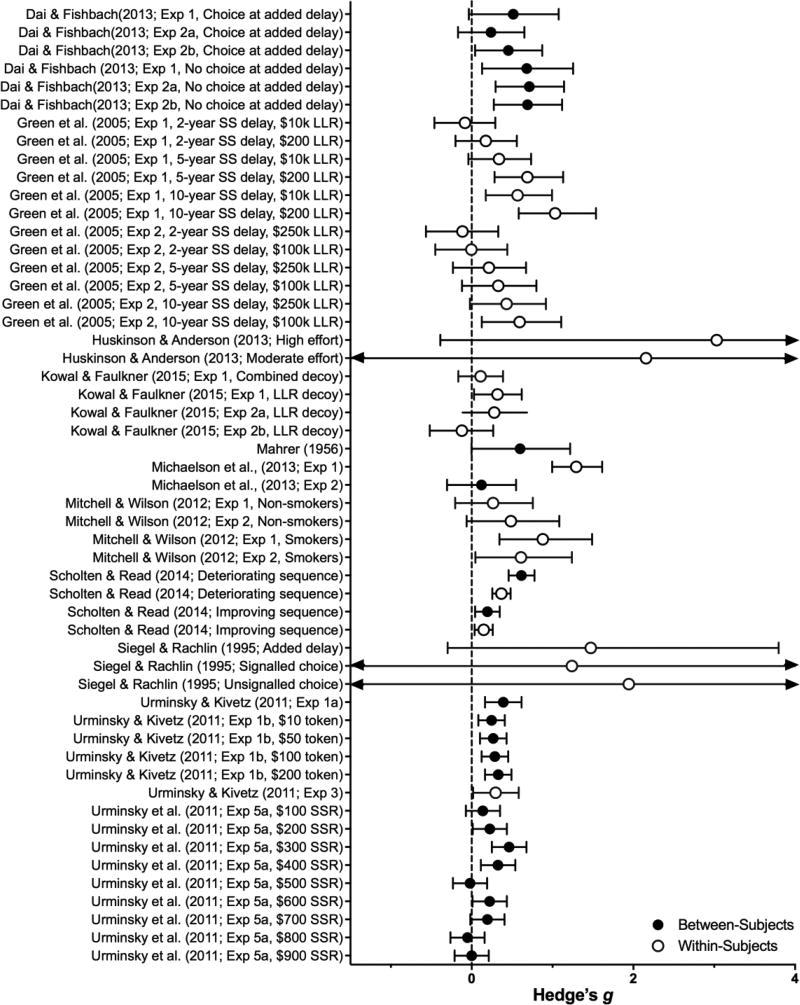
Figure 10.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals manipulations in the Context category. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes, and arrows on the end of a confidence interval indicate that the limits extend beyond the axes.
The Context category is the first in which nonhuman animal research is presented. It is appropriate to review animal studies with those on humans because experimental reductions of nonhuman impulsive choice have often proven effective in reducing human impulsive choice (e.g., Mazur & Logue, 1978; Schweitzer & Sulzer-Azaroff, 1988). For the sake of stimulating future research, we note where manipulations have proven effective in animals but have not yet been evaluated in humans. Effect sizes for several animal studies are not reported because the number of subjects was too small to evaluate assumptions of normality and thus appropriately calculate effect sizes.
Adding Delays
The hyperbolic shape of the delay-discounting function predicts that impulsive choice will be reduced if a common delay is added to the delivery of both the SSR and LLR (e.g., $5 now vs. $10 in 2 weeks becomes $5 in 1 week vs. $10 in 3 weeks). Overall, this technique is successful for reducing impulsive choice (z = 6.34, p < .0001). It is robust in pigeons, whether implemented by adding a common delay (Ainslie & Herrnstein, 1981) or fixed-interval schedule (Siegel & Rachlin, 1995) prior to the delivery of both rewards. Similarly, if at the beginning of a trial (when SSR and LLR are both delayed) pigeons are allowed to pre-commit to the LLR, they will do so (Rachlin & Green, 1972). Green, Myerson, and Macaux (2005) replicated this effect in humans when adding a common delay to the SSR and LLR alternatives in a discounting task. They found the effect held with various added delay durations (e.g., 5 years and 10 years), LLR reward magnitudes (e.g., $200 to $250,000); and others have found it generalizes to cigarette smokers and different reward types (hypothetical or potentially real money; S. H. Mitchell & Wilson, 2012). Dai and Fishbach (2013) replicated this general effect in one of three experiments; the lack of significance in the other two cases was attributed to the small difference between the SSR and LLR rewards (approx. $5 USD).
In a variant of the adding-delays procedure in humans, Dai and Fishbach (2013) produced consistently large reductions in impulsive choice by simply informing participants of the choice alternatives before the choice-point. The authors hypothesized that this pre-choice waiting produced a sense of investment, which increased the subjective value of the LLR. Changes in the perceived value of the LLR completely mediated the manipulation effect, providing strong evidence for this account. This finding deserves continued exploration.
Adding Response Requirements
Four animal studies have examined the effects of adding a response requirement prior to the selection of SSR and LLR outcomes. Due to the small number of studies contributing effect sizes in this category (n = 2) and the small-samples in each, the effect was large (B = 2.14) but non-significant (z = 1.83, p = .07). Siegel and Rachlin (1995) reported that adding a pre-choice response requirement that could be completed across either choice-key significantly reduced pigeons’ impulsive choice, and similar reductions have been observed in rats whether the pre-choice response requirement is arranged on a non-choice lever (Mazur, 2012), independent requirements are arranged on each choice-lever (Huskinson & Anderson, 2013), or only on the SSR alternative (Fortes, Vasconcelos, & Machado, 2015). To date, this manipulation has not been investigated in humans.
From a theory-evaluation perspective, a shortcoming of existing studies that add common pre-choice response requirements is the confounding of added delay with added responses. Siegel and Rachlin (1995) reported reductions in impulsive choice whether the addition was response- or time-based; but, added delays were not yoked to time spent completing the response requirement. Theory aside, adding pre-choice response requirements has produced greater reductions in impulsive choice than adding delays alone (see individual effect sizes in Table 7), which underscores the need to investigate the therapeutic potential of this effect in humans.
Adding Outcomes
Decreases in discounting have been found by adding outcomes to the choice scenario (z = 4.22, p = .0001), although with smaller effects than the above contextual manipulations (B = 0.24). Kowal and Faulker (2016) offered mixed evidence for the discounting-reducing effects of adding a third alternative (a decoy) to the usual two-choice task. Adding a decoy that was the same size as the LLR but delivered after a longer delay; or one that was smaller than the SSR and more delayed than the LLR reduced delay discounting. However, the effects were not robust across task sequences and/or were dependent on data exclusions. Urminsky and Kivetz (2011) found reductions in impulsive choice by adding a small, near-immediate reward (referred to as a token) to both the SSR and LLR alternatives; but this effect was often confined to conditions in which SSR and LLR magnitudes were similar. Scholten and Read (2014) reduced discounting to a modest, although significant degree by using a token immediate payment to arrange an improving sequence of events on the LLR (pay now, big reward later). Relatively larger, significant reductions were obtained when a token payment was used to create a deteriorating sequence on the SSR (small reward now, pay token amount later).
Trustworthiness
Increasing trustworthiness of the LLR source significantly reduces discounting (z = 4.46, p <. 0001), although not consistently so. Mahrer (1956) increased trustworthiness of an experimenter by initially having him/her deliver all promised rewards to children. Subsequently, children in this high-trust group made less impulsive choices, but the effect did not generalize to a novel experimenter. Michaelson, De la Vega, Chatham, and Munakata (2013) reduced discounting by simply describing hypothetical LLR-providers as trustworthy, but the effect was only significant in a within-subjects manipulation (not between-).
Michaelson et al. (2013) and Mahrer (1956) also decreased the trustworthiness of the source of the LLR, and this produced consistent increases in impulsive choice. The aforementioned findings parallel reductions in impulsive choice when the probability of LLR receipt is reduced in human (Vanderveldt, Green, & Myerson, 2015) and nonhuman research (Mazur, 1985). As these manipulations were hypothesized to increase impulsive choice, these studies did not meet the inclusion criteria of this review and are not presented in tables or figures.
Learning-Based Approaches
A variety of learning-based approaches to reducing delay discounting have produced some of the most reliable and large reductions in delay discounting (B = 0.62, SE = 0.08; z = 7.43, p < .0001; see Table 8 and Figure 11). The moderate to large degree of study variability (I2 = 60%) reflects the variety of learning-based approaches employed.
Table 8.
Effect sizes for Learning-Based Manipulations: Estimated subcategory averages (and SEM) from the Learning manipulation-only meta-analytic model and individual-publication effect sizes.
| Subcategory or Study |
Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | p | |
|---|---|---|---|---|---|---|---|
| Reward Bundling (vs. no bundling) | 1.16 (0.25) | <.001 | |||||
| Ainslie & Monterosso (2003) | Rats | 3-reward bundling | k | 0.99 | |||
| Hoffmeyr et al. (2010) | College students | 3-reward bundling | %LLR | ↑, | n.s. | 0.40 | |
| Student smokers | 3-reward bundling | ↓ | 2.07 | ||||
| Kirby & Guastello (2001) | College students | 5-reward bundling | %LLR | ↓ | 1.83 ($) | ||
| ↓ | 1.80 (pizza) | ||||||
| Stein et al. (2013a) | Rats | 3-reward bundling history | Change in AUC | ↓, | n.s. | 0.65 | |
| 9-reward bundling history | ↓ | 1.29 | |||||
| Delay Fading/Exposure | 0.98 (0.31) | .002 | |||||
| Eisenberger & Adornetto (1986) | 2nd & 3rd graders | Delayed reward exposure (vs. immediate reward) | %LLR | ↓ | 0.73 | ||
| (vs. no-exposure) | ?^ | CNC | |||||
| Mazur & Logue (1978) | Pigeons | Delay fading (vs. no SSR delay) | %LLR | ↓ | ‡ | ||
| Schweitzer & Sulzer-Azaroff (1988) | Impulsive preschoolers | Delay fading (vs. baseline) | %LLR | ↓ | 2.52 | ||
| Stein et al. (2013b) | Rats | Delayed reward exposure (vs. immediate reward) | %LLR | ↓ | 1.30 | ||
| ↓ | 0.94 (9-wks post) | ||||||
| Interval-Timing and Reward-Magnitude Training | 0.41 (0.18) | .02 | |||||
| 0.22 (0.42) | .59 | ||||||
| Marshall & Kirkpatrick (2015) | Rats | Reward-magnitude training (vs. none) | %LLR | ↓ | 0.61 (session 1) | ||
| ↓ | 0.15 (session 6) | ||||||
| Smith et al. (2015) | Rats (WKY) | DRL (pre vs. post) | Log-odds LLR | ↓ | 0.25 | ||
| FI or VI schedule (pre vs. post) | ↓ | 0.78† | |||||
| Rats (LEW) | FI or VI schedule (pre vs. post) | ↓ | 0.12† | ||||
| Working Memory Training | 0.42 (0.39) | .28 | |||||
| Bickel et al. (2011) | Stimulant addicts | Working memory training (vs. sham) | k | ↓ | 0.72 | ||
| Renda et al. (2015) | Rats | Delayed match-to-position training (vs. sham) | MAD | ↓, | n.s. | 0.18 | |
| Modeling | 0.35 (0.35) | .31 | |||||
| Atwood et al. (1978) | Impulsive 4th & 5th grade children | Watch video of child model choose LLR (vs. no model) | %LLR | ↓ | CNC | ||
| Bandura & Mischel (1965) | Impulsive 4th & 5th graders | Watch adult model choose LLR (vs. no model) | %LLR | ↓ | CNC | ||
| Text description of adult model choosing LLR (vs. no model) | %LLR | ↓ | CNC | ||||
| Gilman et al. (2014) | Adults | Virtual peer chooses LLR (vs. no model) | k | n.s. | 0.08 | ||
| Staub (1972) | 7th grade children | Watch adult model choose LLR (vs. no model) | %LLR | ↓ | 1.17 | ||
| Stumphauzer (1972) | Prison inmates | Admirable inmate chooses LLR (vs. no model) | %LLR | ↓ | CNC | ||
| Instruction-Based | 0.99 (.27) | <.001 | |||||
| Nisan & Koriat (1984) | Kindergarteners | Explain why a child would choose LLR (vs. no reason-giving) | % choosing LLR | ↓ | 0.78 | ||
| ↓ | 0.98 | ||||||
| Provide objective reason why child chose LLR (vs. no reason-giving) | |||||||
| Staub (1972) | 7th grade children | Explain positive consequences of choosing LLR (vs. neutral information) | %LLR | ↓ | 1.22* | ||
n.s., No statistically significant effect; AUC, area under the discounting curve; CNC, could not calculate effect size
Effect sizes were not calculated due to low sample size and/or inferential statistics not conducted in the original paper.
Effect size averaged across different delays to the SSR. We excluded the 20-s delay because of a ceiling effect.
Effect sizes averaged across experiments or comparable conditions.
No statistics were conducted to evaluate this difference, nor was a measure of variability provided with central tendency. As a result, the direction for this effect was not coded.
Figure 11.
Effect sizes (gbtw or gwin; filled and open circles, respectively) and 95% confidence intervals for Learning manipulations. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes, and arrows on the end of a confidence interval indicate that the limits extended beyond the axes.
Reward bundling
In accord with Figure 1, if the discounted value of the LLR is less than the undiscounted value of the SSR, the impulsive choice will be made. However, if a single choice determines not just the next outcome, but a bundle of the same outcomes (e.g., choosing the SSR locks the decision-maker into three SSRs delivered over the next three trials) this should reduce impulsive choice (for quantitative details of this prediction see Stein, Smits, Johnson, Liston, & Madden, 2013).
Several studies have evaluated this bundling prediction, typically finding successful reductions in discounting (z = 4.57, p < .0001). Kirby and Guastello (2001) found that bundling five rewards together (monetary or food), caused a large percentage of participants to reverse an initial preference for an SSR to LLR. This effect has been replicated in rats (Ainslie & Monterosso, 2003) and human cigarette smokers (Hofmeyr, Ainslie, Charlton, & Ross, 2011), although the latter failed to find an effect of reward bundling in nonsmokers. Stein, Smits, et al. (2013) evaluated if experience with bundled rewards would reduce impulsive choice when outcomes were subsequently unbundled. Relative to a no-bundle control group, experience with 9-reward bundles (but not 3-reward bundles) decreased impulsive choice for unbundled SS and LL rewards. There was no significant relation between impulsive choice in the bundling (training) phase and impulsive choice during testing (unbundled rewards), suggesting that greater exposure to delays during bundling exposure (i.e., the 9-reward bundle group) appeared responsible for the reduction in unbundled impulsive choice.
Delay fading/exposure
Exposure to delayed rewards via fading techniques or prolonged experience has produced large (B = 0.98) and significant effects (z = 3.13, p = .002) on impulsive choice. Taking a systematic approach to exposing animals to delayed reinforcers, Mazur and Logue (1978) first established pigeons’ preference for large food rewards vs. small food rewards that were both delayed by 6 s. Then, the delay to the smaller reward was gradually reduced (faded out) while maintaining preference for the LLR until the SSR was available immediately. Compared to a no-fading control group, delay-fading produced far fewer impulsive choices. This effect was maintained when the position of the SSR and LLR keys (i.e., left/right) was reversed and when the pigeons were retested 11 months later (Logue & Mazur, 1981). The short-term impulsive-choice reducing effects of delay-fading have been replicated in a laboratory setting in children identified as impulsive or hyperactive (Schweitzer & Sulzer-Azaroff, 1988).
Stein et al. (2013) evaluated the effects of delay exposure without choice opportunities during training. In their study, delay-exposed rats completed 4 months of daily sessions in which pressing a single lever delivered two food pellets after a 17.5 s delay. In both a post-training impulsive-choice assessment and a 9-week follow-up, delay-exposed rats chose a three-pellet LLR delayed by 15 s (vs. 1 pellet now) near-exclusively, relative to a group of immediacy-exposed rats. A weakness of the study is the lack of a no-training control group, which leaves the possibility that immediate reward exposure increased impulsive choice during training. The single experiment evaluating the effects of delay exposure in human children produced positive effects in some conditions, but it is unclear if the increase in self-control was present when compared to a no-training control group (Eisenberger & Adornetto, 1986).
Delay timing and amount discrimination
Inaccurate interval timing occurs when the duration of a delay is over- or under-estimated; if an interval’s duration is overestimated, time is perceived as passing slowly, which should increase impulsive choice. However, few studies support this hypothesis (see Baumann & Odum, 2012 for an exception). Instead, the amount of unsystematic variability in timing from trial to trial (timing precision) correlates with impulsive choice in rats (Marshall, Smith, & Kirkpatrick, 2014; McClure, Podos, & Richardson, 2014). Timing imprecision may undermine the ability to discriminate when (and perhaps whether; e.g., McGuire & Kable, 2012) rewards will be delivered. Smith et al. (2015) evaluated the effects of interventions to improve timing precision in rats (differential reinforcement of low rate [DRL], fixed-interval [FI], or variable-interval [VI] schedules). These interventions improved timing precision and reduced impulsive choice (z = 2.30, p = .02).
By contrast, an extended history of choosing between small and large rewards (to train amount discrimination) produced a transitory, but not lasting reduction in impulsive choice (z = 0.53, p = .59; Marshall & Kirkpatrick, 2016). This long-term null effect is consistent with the finding that reward magnitude sensitivity is unrelated to impulsive choice in rats (Marshall et al., 2014).
Working-memory training
Observing that individuals who steeply discount delayed rewards also tend to score poorly on tests of working memory, and reasoning that working memory is important in imagining one’s future experiences, Bickel, Yi, Landes, Hill and Baxter (2011) provided working-memory training to treatment-seeking stimulant-addicts, while a control group received sham training. While Bickel et al. found this training reduced rates of delay discounting, Renda, Stein, and Madden (2015) failed to replicate this finding in rats, even though working-memory training improved working memory (g = 2.21). As it stands, working memory training has no significant overall effect on discounting (z = 1.09, p = .28), but given the small number of studies on the topic and potential cross-species differences, the effects of working-memory training are in need of further investigation. Particularly useful would be investigating if improvements in working memory mediate changes in delay discounting. That 8 of 13 sham-trained participants in Bickel et al. (2011) had higher discounting rates at the post-training evaluation, and working-memory training had no effect on transfer tests of working memory (i.e., working memory assessments not used during training) raises the possibility of a Type I error.
Modeling
Six studies examined the effects of social learning – that is, how observing a model choose a larger-later consequence influences observer decision-making. Unfortunately, effect sizes could only be calculated for two of these studies, with one being the sole study finding a null effect (Gilman, Curran, Calderon, Stoeckel, & Eden Evins, 2014); thus, modeling yielded a non-significant effect on discounting in the meta-analysis (z = 1.01, p = .31) despite most reporting positive effects on impulsive choice. For example, Bandura and Mischel (1965) evaluated how viewing (or reading about) adult models choosing and subsequently explaining their reasons for selecting LLRs, affected decisions of 4th and 5th grade children. They found that 10% fewer children selected the SSR after observing or reading about a model, relative to a no-model control group; the effect persisted 4–5 weeks later at a reassessment of choice. While this effect has been replicated with children (Atwood, Ruebush, & Everett, 1978; Staub, 1972) and prison inmates (Stumphauzer, 1972) a recent study revealed that adults seeing LLR choices made by virtual peers on a computer screen did not reduce delay discounting (Gilman et al., 2014).
Instruction-based procedures
In two studies, asking participants to consider the consequences of (and reasons for) their choices reduced impulsive choice (z = 3.64, p = .0003). For instance, in Nisan and Koriat (1984), kindergarteners who were instructed to generate reasons why another child might choose the LLR (after the participant just chose the SSR) subsequently increased their selection of the LLR. Likewise, experimenter-provided reasons why another child chose the LLR (e.g., “because he wanted lots, and two tomorrow is more than one today”) also shifted preference toward the LLR. Similarly, Staub (1972) found that instructing children about the positive consequences of choosing the LLR increased the number of LLR choices.
Environmental Enrichment/Deprivation
Childhood trauma is predictive of substance use in humans (Chassin, Ritter, Trim, & King, 2003) and is correlated with steep delay discounting in men (van den Berk-Clark, Myerson, Green, & Grucza, 2018). This relation, and the finding that early isolation impairs response-inhibition in rats (Hall, 1998), motivated research examining the effects of rats’ rearing environments on impulsive choice. Despite good external validity, rearing manipulations overall had no significant impact on impulsive choice (B = .27, SE = 0.19; z = 1.44, p = .15; see Table 9 and Figure 12). Similarity in animal laboratory procedures may explain the low study variability (I2 = 0%; i.e., the observed variability is due to chance). The exception is a study by Perry, Stairs, and Bardo (2008), who reported lower discounting among socially-enriched rats, relative to rats raised in isolation. However, the effect was only evaluated for 5 days and it appeared to lessen over time. Hellemans et al. (2005) reported temporary benefits of enrichment that did not continue through steady-state assessment. Thus, there is little evidence that environmental enrichment decreases delay discounting.
Table 9.
Effect sizes for Environmental Enrichment/Deprivation: Individual-publication effect sizes.
| Study | Population | Manipulation | DV | Effect on Impulsivity |
Effect Size | |
|---|---|---|---|---|---|---|
| Baarendse et al. (2013) | Rats | 3-wk enrichment | %LLR | n.s. | 0.10 | |
| Botanas et al. (2016) § | Rats – SHR | 4-wk enrichment | %LLR | ↓, | n.s. | 0.28* |
| Rats – WKY | ↓, | n.s. | 0.35* | |||
| Hellemans et al. (2005) | Rats | 12-wk isolation | %LLR | ↓, | n.s. | CNC |
| 12-wk enrichment | ↓, | n.s. | ||||
| Kirkpatrick et al. (2013) | Rats | 5-wk enrichment | %LLR | ↓, | n.s. | 0.33 |
| Perry et al. (2008) | Rats | 5-wk enrichment | MAD | ↓ | 0.67 | |
n.s., No statistically significant effect; CNC, could not calculate effect size
Effect sizes averaged across experiments or comparable conditions.
The effect sizes for the intervention at the 10-s LLR delay are included in the averages presented in the table, but omitted from the meta-analysis (>3 effect sizes in the same subjects).
Figure 12.
Effect sizes (gbtw) and 95% confidence intervals for Environmental Enrichment/Deprivation manipulations. Only studies for which effect sizes could be calculated are included. Larger effect sizes reflect greater preference for larger, delayed outcomes
Discussion
Working under the assumption that steeply discounting future outcomes plays a role in the development and/or continuation of maladaptive behaviors (Bickel et al., 2012; Bickel, Mackillop, Madden, Odum, & Yi, 2015), a large number of studies have investigated environmental manipulations designed to reduce delay discounting or impulsive choice. Given the infancy of this effort, the majority of the research reviewed above was conducted in laboratory settings. Where clinical work has been conducted, delay discounting is often an incidental dependent measure and the effects of the intervention have been inconsistent or not compared to adequate control groups. It is appropriate, therefore, that laboratory research with human and nonhuman subjects continues to explore and refine methods to more consistently reduce delay discounting.
The effect sizes in Figure 3, and the forest plots in Figures 4–12, allow for informal cross-category and -experiment comparisons of effect sizes. Although efforts were made to select an effect size measure that was comparable across different types of experiments and different sample sizes, there are several reasons to interpret these data with caution. First, a wide variety of tasks, reward amounts/types, delay durations, and associated dependent measures were used in the reviewed experiments. If these task/reward/delay/measure combinations are differentially sensitive to experimental manipulations, comparison of effect sizes must be done judiciously. Second, effect sizes could not be calculated for some studies reviewed. Thus, some manipulations that produce potentially useful reductions in delay discounting are under-represented (e.g., modeling) in the figures. Third, comparing effect sizes across laboratory and clinical settings must consider that greater control of extraneous variables will increase effect sizes; this may be particularly true in nonhuman animal research, although a preliminary analysis of differences in effect sizes across humans and non- revealed no significant differences.7 We believe the latter supports the applicability of research on discounting with non-human subjects for efforts to improve the human condition. Finally, the effect sizes entail some degree of error. In many instances we estimated information (e.g., means and variability via GraphClick software; assuming a moderate correlation between repeated measures) as opposed to directly retrieving it. The effect sizes and results of the meta-analysis, therefore, should be considered a means of providing relative, as opposed to absolute information of size and precision.
With these cautions in mind, we note that several brief experimental manipulations have produced across-laboratory reductions in delay discounting. For example, a short period of time spent with nature (cueing) or engaged in episodic future thinking (EFT) consistently reduces delay discounting unless the imagined future event has a negative valence (Liu et al., 2014). Likewise, arranging the environment so decisions are made when neither the SSR nor LLR are immediately available (adding delays, adding response requirements) produces large reductions in impulsive choice. Perhaps the shortest duration manipulation producing reliable reductions in impulsive choice (barring ceiling effects) are the date framing and explicit-zero framing interventions. As currently implemented, these manipulations do not produce, nor are they designed to produce, long-term changes in impulsive choice. That is, making explicit that selecting the SSR entails nothing will be received at a future date (explicit zero framing) can reduce a one-off impulsive choice, but this reframing is unlikely to influence a later decision to binge watch Netflix instead of getting the sleep needed to perform better the next day.
Such acute manipulations need not have limited utility. Many important decisions are one-offs and, in these cases, acute manipulations are all that is needed. Consider the decision to save money for retirement or to start a college savings account for a child. Once a savings plan is initiated, it is rarely reversed (Samuelson & Zeckhauser, 1988) so influencing choice just once can profoundly affect one’s life. Using a version of the Adding Delays manipulation, Thaler and Benartzi’s (2004) Save More Tomorrow™ program strategically delays contributions to a retirement savings plan until a later date - when the employee receives their first pay raise. Similar programs might use the effective discounting manipulations reviewed in this paper to influence other important one-off choices. For example, sales of energy efficient appliances might be increased by simply specifying the amount and date by which energy-bill savings will be realized (the date framing effect; DeHart & Odum, 2015; Dshemuchadse et al., 2013; Klapproth, 2012; LeBoeuf, 2006; Read et al., 2005). Additional increases might be produced by explicitly noting the lack of savings if the cheaper appliance were purchased (the explicit zero effect; Magen et al., 2008; Radu et al., 2011; Wu & He, 2012). These effective means of acutely reducing delay discounting should empower researchers to creatively arrange choice contexts that nudge behavior in advantageous directions.
Unexplored to date is the development of interventions designed to teach decision-makers to consistently reframe for themselves intertemporal choice alternatives so as to minimize impulsive choices. Training might begin by teaching participants to recognize intertemporal choice contexts, to identify the SSR and LLR, and then to apply one or more of the strategies summarized in this review. Beyond learning to reframe the choice alternatives, participants might learn to bring to mind social experiences for which the individual is grateful (DeSteno et al., 2014) or engage in EFT activities (e.g., Lin & Epstein, 2014).
Indeed, evidence supporting the development of such therapeutic interventions comes from the EFT literature. Sze, Daniel, Kilanowski, Collins, and Epstein (2015) trained overweight parent-child dyads in EFT and audio-recorded their future-thinking cues (including how losing weight would enhance the future event) so they could be later accessed to prompt EFT activities prior to meals. Following a 4-week trial, parents assigned to EFT reduced their BMI and percent overweight more than those assigned to a nutrition-education group that also received daily prompting (p = .01). This is an encouraging finding serving as proof of concept that individuals prone to making impulsive choices can learn to self-initiate therapeutic behaviors prior to making a decision with health implications.
Learning-based approaches that produce large and long-lasting reductions in delay discounting have a long history, mostly in the animal laboratory where arranging extended training programs is more feasible than with free-ranging humans. One study that met the current inclusion criteria demonstrated the efficacy of a variant of delay-fading training in children with impulse control issues (Schweitzer & Sulzer-Azaroff, 1988), and several studies that did not meet our inclusion criteria have replicated this effect (e.g., Dixon et al., 1998; Fisher, Thompson, Hagopian, Bowman, & Krug, 2000). Similarly, bundling greatly reduces delay discounting and impulsive choice in rats (Ainslie & Monterosso, 2003; Stein, Smits, et al., 2013) and humans (Hofmeyr et al., 2011; Kirby & Guastello, 2001). Nonetheless, most of this translational research remains laboratory based, with few human studies evaluating duration of efficacy or generalization to novel settings, commodities, etc. Where duration and/or efficacy have been evaluated in small-N studies, the findings with fading-related manipulations are encouraging (Dunkel-Jackson, Dixon, & Szekely, 2016; Neef, Bicard, & Endo, 2001).
Given these encouraging findings, future research should explore the efficacy of procedures such as delay fading (Mazur & Logue, 1987) or delay-exposure training (Stein et al., 2013) in preschool settings. As above, children might first be taught to discriminate situations in which SSRs and LLRs are available, and then given supported opportunities to select the LLR, experience the delay, and obtain the better of the two outcomes. It may be particularly important to ensure the LLR is always received, given that impulsive choice is a maximization strategy when the source of the delayed reward is untrustworthy (Mahrer, 1956; Michaelson et al., 2013; Mazur, 1985). Embedding these didactic and experiential-learning techniques into, for example, the Head Start curriculum in the U.S. could prove an effective preventive measure in children at risk of substance use and abuse.
As these translational efforts begin, it is important to be cognizant of the distinction between “statistically significant” and “clinically significant,” and to consider the different interpretations of effect sizes. For example, as the definition of the standardized mean difference entails–an effect size of, for example, 0.5, would mean that those in the intervention group on average improved by half a standard deviation. However, such an interpretation is best reserved for within-subjects designs; more appropriate to a between-subjects design, an effect size of 0.5 means that 70% of the participants in the intervention group scored better (e.g., fewer impulsive choices) than the control group and thus not all participants may benefit from such an intervention. Many of the effect sizes in Figure 3–12 are smaller than this; it is important to consider all of these implications when evaluating effect sizes. In addition, to further assist researchers in evaluating the practical significance of an interventions’ efficacy, we urge researchers to graphically represent individual participant data (see e.g., Kwan et al., 2015).
Theoretical Issues
Within most of the categories of intervention strategies reviewed above, there is no clear theoretical understanding of how the manipulation influences delay discounting. Inclusion of formal mediation analyses is rare, although discussion of potential mediators is not. Similarly, in the absence of a straightforward way to measure some hypothesized mediators, few studies have designed procedures to disambiguate between similar processes of change.
There are at least two benefits to uncovering the processes by which delay discounting and impulsive choice are reduced. First, understanding how a manipulation affects discounting will facilitate more efficient translation to clinical interventions. In the absence of this knowledge, ineffective components may be carried forward, effective components omitted, and interventions may be applied in contexts or with populations who would not benefit. Second, further identifying the processes by which delay discounting is momentarily or permanently changed may guide the exploration of the neurological bases of discounting and aid in the development of better quantitative models of the discounting process.
Identifying the processes underlying changes in delay discounting requires an empirical base of studies with strong internal validity. At present, some of the promising interventions reviewed above have threats to internal validity and these must be addressed before translational research is undertaken. As noted above, a prominent threat to internal validity is demand characteristics. These are of particular concern when the experimental manipulation overlaps in content with the discounting task (e.g., EFT cues whose future time corresponds with the delay to the LLR) or when control procedures do not equate for expectancy of change (e.g., wait-list control groups). Several tactics are available for addressing these issues of internal validity (Boot, Simons, Stothart, & Stutts, 2013). For example, control tasks could equate demand characteristics across groups (e.g., semantic future thinking vs. episodic future thinking; Chiou & Wu, 2017) or less transparent/less easily faked delay-discounting tasks could be used.
Methodological Recommendations
As research in delay discounting and impulsive choice continues to explore methods for reducing these important choices, it will be important for the purpose of between-experiment comparison to standardize the assessment and quantification of these behaviors. This is particularly important in human laboratory research, as these findings are most likely to influence which manipulations will be translated to clinical trials. Because i) several different tasks are available for quickly obtaining delay discounting rates (e.g., Du, Green, & Myerson, 2002; Kirby & Maraković, 1995; Rachlin, Raineri, & Cross, 1991), ii) these rates tend to significantly correlate across tasks (Epstein et al., 2003; Hardisty, Thompson, Krantz, & Weber, 2013; Holt, Green, & Myerson, 2012; Koffarnus & Bickel, 2014; Kowal, Yi, Erisman, & Bickel, 2007), iii) preferences between SSRs and LLRs may be derived from these discounting rates, and iv) discounting rates do not appear to be systematically different whether the rewards are real or hypothetical (Johnson & Bickel, 2002; Lagorio & Madden, 2005) we recommend that future studies evaluating therapeutic manipulations use a delay-discounting task, rather than an impulsive-choice task (see Madden & Johnson, 2010 for a primer on these tasks and quantitative methods). Such standardization of the dependent measure will facilitate evaluation of the relative merits of different approaches to reducing delay discounting.
With the exception of the Multiple-Choice Questionnaire (Kirby et al., 1999), delay discounting tasks yield indifference points that may be plotted and fit with a discounting equation. The variety of these equations and the theoretical underpinnings of each are beyond the scope of this review. Suffice it to say, comparison across published articles and scholarly disciplines (e.g., economics and psychology) would be facilitated if a common metric of delay discounting were used. The area under the curve (AUC) formed by indifference points (Myerson, Green, & Warusawitharana, 2001) is a candidate metric, because it is already frequently used and is usually normally distributed, facilitating the use of parametric statistical analyses. AUC is theoretically neutral and may be applied to any data set.
Last, we provide several suggestions, based on our observations in conducting this review, that would improve the meta-analyzability of this literature for future reviews. First, and most simply, researchers should take care to publish data needed in the calculation of effect sizes. This includes clear and explicitly identified descriptive statistics (i.e., measures of central tendency and variability), specific identification of group sizes, and correlations between repeated measurements. Where effect sizes are reported, the authors must also identify the method of calculation because there are several calculations for Cohen’s d for within-subjects designs, and they can yield very different effect size estimates.
Our second suggestion is to incorporate more systematic approaches into the study of manipulations of discounting. The heterogeneity in the execution of experimental manipulations, the measures used, the settings, and participants/species precluded identification of meaningful moderators of effect sizes between-categories (e.g., only two categories used both humans and non-; clinical populations were seldom recruited outside of clinical manipulations). In many instances there were similar issues within-category (e.g., most human studies used hypothetical monetary outcomes, with very few using non-monetary or [potentially-] real outcomes). In other words, most potential moderators were confounded with categories themselves, or too infrequently represented to yield meaningful analysis. These statements are supported by the generally moderate degree of effect-size variability at the category and subcategory level. A more systematic approach in planning future studies will facilitate our understanding of the efficacy of the manipulations, their limitations, and relative utility. To this end, researchers will need to work collectively to build a cohesive body of work, rather than a collection of studies.
Summary & Conclusions
Several methods for reducing delay discounting and impulsive choice were reviewed. Although some promising manipulations have been identified, very little translational research has adapted these techniques in clinical or practical settings. Some manipulations, like EFT, may be easily integrated into talk-based therapies (e.g., Acceptance & Commitment Therapy; Hayes et al., 1999), and substance-abuse treatment trials that include a behavior-therapy component should consider this integration. Because framing manipulations so consistently shift choice toward the LLR, future research should evaluate the efficacy of these manipulations in influencing one-off choices that affect the health and well-being of decision-makers, and the world in which they live. Finally, learning-based manipulations enjoy a robust empirical base and have shown successful initial translation to humans (e.g., delay fading; Schweitzer & Sulzer-Azaroff, 1988). These encouraging findings should be further developed into a practical curriculum for broad-scale dissemination and evaluation of long-term outcomes. Such resiliency-building programs hold promise for improving the pattern of decision-making that underlies physical and ecological health (Volkow & Baler, 2015).
Acknowledgments
This research was supported in part by a grant from the National Institute on Drug Abuse awarded to the second author (R21 DA042174).
Footnotes
It should be noted that delay discounting is a description of a pattern of choices between SSRs and LLRs; it is not an explanation. There are several factors that can explain why delayed outcomes are discounted in value. For example, delays typically come with opportunity costs (Paglieri, 2013; Stevens & Stephens, 2010) and often either signal a local collection risk (the LLR is promised, but not delivered; e.g., Mahrer, 1956) or a stable collection risk in the phylogenetic history of the species (Stevens & Stephens, 2010). It is also important to note that sensitivity to delay is not the only factor that can influence impulsive decision-making. For example, if sensitivity to differences in reward magnitude declines, preference for a LLR will shift toward the SSR. Likewise, failure to couple the LLR with the response that produced it will render the SSR as the only functional response-outcome contingency (Killeen, 2011). These complexities open considerably the range of experimental variables that can influence the SSR- and LLR-choices from which a delay-discounting function is derived. Several of these variables appear in the review that follows.
A separate search was conducted in PLoS One because some eligible papers published in that journal did not appear in the PsycINFO data base. The functionality of the PLoS One search engine was more limited so the search procedures were slightly modified from that used in PsycINFO.
If a study yielded > 3 effect sizes with the same participants/subjects, or if a study contributed > 8 effect sizes in total, individual effect sizes were omitted from the meta-analysis such that the total number of effect sizes for those participants/studies did not exceed the previously stated criteria. Sometimes, the effects of a manipulation were examined over a wide range of parameters (e.g., larger-later reward amounts/delays), which inflated the number of effect sizes for a given publication. To control for inflation of effect sizes due to unknown correlations within participants/studies, individual effect sizes from qualifying studies (n = 4) were omitted such that the mean of the effect sizes for that study remained similar to that with all effect sizes, and that the effect sizes across levels of the moderating variable (e.g, larger-later reward delays) remained represented. These cases are noted in the corresponding effect size tables.
The only other known EFT study that measured temporal horizon did not meet the inclusion criteria. Cheng, Shein, and Chiou (2012) found that engaging in an EFT-like exercise produced greater future orientation as measured with the Zimbardo Time Perspective Inventory (ZTPI; Zimbardo & Boyd, 1999), and that ZTPI scores mediated the effect of EFT in reducing steepness of discounting. The paper was excluded from this review because the authors confirmed that assumptions of their statistical analyses were violated but failed to make available individual participant data for supplemental analyses.
Nonsignificant effects in Pyone and Isen (2011) were often observed when strong preferences were produced by choice parameters. For example, positive affect primes did not decrease impulsive choice when the LLR amount was only nominally larger than the SSR (e.g., $25 now vs. $30 in 4 weeks), or when the LLR was strongly preferred without the prime because the difference in reward amounts was large (e.g., $25 now vs. $50 in 4 weeks). In Figure 9, these differences are indicated as “Easier” vs. “Harder” magnitude pairs.
Other studies have manipulated the presence vs. absence of rewards, but used appetitive rewards designed to increase impulsive choice. These studies did not meet the inclusion criteria of the present review.
To conduct a comparison of effect sizes using humans (n = 6) and non-humans (n = 5) as research subjects, the Delay-Fading/Exposure and Bundling subcategories (Learning category) were collapsed. Then, “Population” (human vs. non-) was examined as the sole moderator of effect sizes. Studies using non-humans (B = −0.13, SE = 0.48) did not yield significantly different effect sizes from those with humans (z = −0.27, p = 0.79). We call this comparison preliminary because Population is necessarily confounded with an array of procedural differences. A systematic line of research on the subject would need to be developed to rigorously test this difference.
None of the authors have any real or potential conflict(s) of interest, including financial, personal, or other relationships with organizations or pharmaceutical/biomedical companies that may inappropriately influence the research and interpretation of the findings. All authors have contributed substantively to this review and have read and approved this final manuscript.
Portions of this research were presented at the 2016 annual convention for the Association for Behavior Analysis International in Chicago, IL, USA.
References
- Ainslie G, Herrnstein RJ. Preference reversal and delayed reinforcement. Animal Learning & Behavior. 1981 doi: 10.3758/BF03209777. [DOI]
- Ainslie G, Monterosso JR. Building blocks of self-control: increased tolerance for delay with bundled rewards. Journal of the Experimental Analysis of Behavior. 2003;79(1):37–48. doi: 10.1901/jeab.2003.79-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklin WM, Tull MT, Kahler CW, Lejuez CW. Risk-taking propensity changes throughout the course of residential substance abuse treatment. Personality and Individual Differences. 2009;46(4):454–459. doi: 10.1016/j.paid.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64(3):345–354. doi: 10.1016/S0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychological Medicine. 2016;46(11):2423–34. doi: 10.1017/S0033291716000866. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology Biochemistry and Behavior. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. Episodic future thinking. Trends in Cognitive Sciences. 2001 doi: 10.1016/S1364-6613(00)01804-0. [DOI] [PubMed]
- Atwood MD, Ruebush BK, Everett FL. The effects of modeling and role playing on children’s delay of gratification behavior. Child Study Journal. 1978;8(3):149–163. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Counotte DS, O’Donnell P, Vanderschuren LJMJ. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38(8):1485–1994. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: Similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112(3):382–392. doi: 10.1037/0021-843X.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bandura A, Mischel W. Modification of self-imposed delay of reward through exposure to live and symbolic models. Journal of Personality and Social Psychology. 1965;2(5):698–705. doi: 10.1037/h0022655. [DOI] [PubMed] [Google Scholar]
- Baumann AA, Odum AL. Impulsivity, risk taking, and timing. Behavioural Processes. 2012;90(3):408–414. doi: 10.1016/j.beproc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–62. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. The Journal of Neuroscience. 2011a;31(18):6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. The Journal of Neuroscience. 2011b;31(18):6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MS, Repke MA, Nickerson NP, Conway LG, Odum AL, Jordan KE. Making time for nature: Visual exposure to natural environments lengthens subjective time perception and reduces impulsivity. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MS, Sweeney MM, Morath J, Odum AL, Jordan KE. The nature of impulsivity: Visual exposure to natural environments decreases impulsive decision-making in a delay discounting task. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0097915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology and Therapeutics. 2012 doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed]
- Bickel WK, Mackillop J, Madden GJ, Odum AL, Yi R. Experimental manipulations of delay discounting & related processes: An introduction to the special issue. Journal of the Experimental Analysis of Behavior. 2015;103(1):1–9. doi: 10.1002/jeab.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/PL00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AC, Rosen MI. A money management-based substance use treatment increases valuation of future rewards. Addictive Behaviors. 2011;36(1-2):125–128. doi: 10.1016/j.addbeh.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn M, El-Deredy W. The future is risky: Discounting of delayed and uncertain outcomes. Behavioural Processes. 2013;94:9–18. doi: 10.1016/j.beproc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Boot WR, Simons DJ, Stothart C, Stutts C. The Pervasive Problem With Placebos in Psychology: Why Active Control Groups Are Not Sufficient to Rule Out Placebo Effects. Perspectives on Psychological Science. 2013;8(4):445–454. doi: 10.1177/1745691613491271. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, Lee H, de la Peña JB, dela Peña IJ, Woo T, Kim HJ, Cheong JH. Rearing in an enriched environment attenuated hyperactivity and inattention in the Spontaneously Hypertensive Rats, an animal model of Attention-Deficit Hyperactivity Disorder. Physiology and Behavior. 2016;155:30–37. doi: 10.1016/j.physbeh.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Bowler DE, Buyung-Ali LM, Knight TM, Pullin AS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health. 2010;10(1):456. doi: 10.1186/1471-2458-10-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Mackillop J, Miller GE, Chen E, Obasi EM, Beach SRH. Catecholamine levels and delay discounting forecast drug use among African American youths. Addiction. 2014;109(7):1112–1118. doi: 10.1111/add.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton SR, Yi R, Porter C, Carter AE, Bickel W, Rachlin H. Now for me, later for us? Effects of group context on temporal discounting. Journal of Behavioral Decision Making. 2013;26(2):118–127. doi: 10.1002/bdm.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Ritter J, Trim RS, King KM. Adolescent substance use disorders. Child Psychopathology ( (2) 2003 Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=psyc4&NEWS=N&AN=2003-04378-004.
- Cheng YY, Shein PP, Chiou WBin. Escaping the impulse to immediate gratification: The prospect concept promotes a future-oriented mindset, prompting an inclination towards delayed gratification. British Journal of Psychology. 2012;103(1):129–141. doi: 10.1111/j.2044-8295.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- Chiou W-B, Wu W-H. Episodic Future Thinking Involving the Nonsmoking Self Can Induce Lower Discounting and Cigarette Consumption. Journal of Studies on Alcohol and Drugs. 2017;78(1):106–112. doi: 10.15288/jsad.2017.78.106. https://doi.org/10.15288/jsad.2017.78.106. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Statistical Power Analysis for the Behavioral Sciences. 1988 doi: 10.1234/12345678. [DOI]
- Cumming G. Understanding The New Statistics : Effect Sizes, Confidence Intervals, and Meta-Analysis. New York: Routledge; 2011. [Google Scholar]
- Dai X, Fishbach A. When waiting to choose increases patience. Organizational Behavior and Human Decision Processes. 2013;121(2):256–266. doi: 10.1016/j.obhdp.2013.01.007. [DOI] [Google Scholar]
- Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence. 2007;86(2-3):230–238. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: Comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite. 2013a;71:120–125. doi: 10.1016/j.appet.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science. 2013b;24(11):2339–2342. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassen FCM, Jansen A, Nederkoorn C, Houben K. Focus on the future: Episodic future thinking reduces discount rate and snacking. Appetite. 2016;96:327–332. doi: 10.1016/j.appet.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Daugherty JR, Brase GL. Taking time to be healthy: Predicting health behaviors with delay discounting and time perspective. Personality and Individual Differences. 2010;48(2):202–207. doi: 10.1016/j.paid.2009.10.007. [DOI] [Google Scholar]
- De Wilde B, Bechara A, Sabbe B, Hulstijn W, Dom G. Risky decision-making but not delay discounting improves during inpatient treatment of polysubstance dependent alcoholics. Frontiers in Psychiatry. 2013 Sep;4:1–7. doi: 10.3389/fpsyt.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Mitchell SH. Drug Effects of Delay Discounting. Impulsivity: The Behavioral and Neurological Science of Discounting. 2010:213–241. [Google Scholar]
- DeCoster J. Meta-Analysis Notes. 2009 Retrieved April 19, 2016, from http://www.stat-help.com/notes.html.
- DeHart WB, Odum AL. The effects of the framing of time on delay discounting. Journal of the Experimental Analysis of Behavior. 2015;103(1):10–21. doi: 10.1002/jeab.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennhardt AA, Yurasek AM, Murphy JG. Change in delay discounting and substance reward value following a brief alcohol and drug use intervention. Journal of the Experimental Analysis of Behavior. 2015;103(1):125–140. doi: 10.1002/jeab.121. [DOI] [PubMed] [Google Scholar]
- DeSteno D, Li Y, Dickens L, Lerner JS. Gratitude: A tool for reducing economic impatience. Psychological Science. 2014;25(6):1262–1267. doi: 10.1177/0956797614529979. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addiction Biology. 2012;17(3):576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Hayes LJ, Binder LM, Manthey S, Sigman C, Zdanowski DM. Using a self-control training procedure to increase appropriate behavior. Journal of Applied Behavior Analysis. 1998;31(2):203–210. doi: 10.1901/jaba.1998.31-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Holton B. Altering the magnitude of delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2009;42(2):269–275. doi: 10.1901/jaba.2009.42-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36(4):449–r58. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dshemuchadse M, Scherbaum S, Goschke T. How decisions emerge: Action dynamics in intertemporal decision making. Journal of Experimental Psychology: General. 2013;142(1):93–100. doi: 10.1037/a0028499. [DOI] [PubMed] [Google Scholar]
- Du W, Green L, Myerson J. Cross-Cultural Comparisons of Discounting Delayed and Probabilistic Rewards. Psychological Record. 2002;52(4):479–492. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=7730084&lang=ja&site=ehost-live. [Google Scholar]
- Dunkel-Jackson SM, Dixon MR, Szekely S. Self-control as generalized operant behavior by adults with autism spectrum disorder. Journal of Applied Behavior Analysis. 2016;49(3):705–710. doi: 10.1002/jaba.315. [DOI] [PubMed] [Google Scholar]
- Eisenberger R, Adornetto M. Generalized self-control of delay and effort. Journal of Personality and Social Psychology. 1986;51(5):1020–1031. doi: 10.1037/0022-3514.51.5.1020. [DOI] [Google Scholar]
- Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Experimental and Clinical Psychopharmacology. 2003;11(2):131–138. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108(11):1916–1923. doi: 10.1111/add.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields SA, Sabet M, Peal A, Reynolds B. Relationship between weight status and delay discounting in a sample of adolescent cigarette smokers. Behavioural Pharmacology. 2011;22(3):266–8. doi: 10.1097/FBP.0b013e328345c855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher WW, Thompson RH, Hagopian LP, Bowman LG, Krug A. Facilitating tolerance of delayed reinforcement during functional communication training. Behavior Modification. 2000;24(1):3–29. doi: 10.1177/0145445500241001. [DOI] [PubMed] [Google Scholar]
- Fortes I, Vasconcelos M, Machado A. The effect of response rate on reward value in a self-control task. Journal of the Experimental Analysis of Behavior. 2015;103(1):141–152. doi: 10.1002/jeab.123. [DOI] [PubMed] [Google Scholar]
- Galanter E. The direct measurement of utility and subjective probability. The American Journal of Psychology. 1962;75:208–220. [PubMed] [Google Scholar]
- Gilman JM, Curran MT, Calderon V, Stoeckel LE, Eden Evins A. Impulsive social influence increases impulsive choices on a temporal discounting task in young adults. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace RC, McLean AP. Integrated versus segregated accounting and the magnitude effect in temporal discounting. Psychonomic Bulletin & Review. 2005;12(4):732–739. doi: 10.3758/BF03196765. [DOI] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Impulsive delayed reward discounting as a genetically-influenced target for drug abuse prevention: a critical evaluation. Frontiers in Psychology. 2015;6:1104. doi: 10.3389/fpsyg.2015.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Macaux EW. Temporal Discounting When the Choice Is Between Two Delayed Rewards. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(5):1121–1133. doi: 10.1037/0278-7393.31.5.1121. [DOI] [PubMed] [Google Scholar]
- Griskevicius V, Tybur JM, Delton AW, Robertson TE. The influence of mortality and socioeconomic status on risk and delayed rewards: A life history theory approach. Journal of Personality and Social Psychology. 2011;100(6):1015–1026. doi: 10.1037/a0022403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews in Neurobiology. 1998;12(1-2):129–62. doi: 10.1615/CritRevNeurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hardisty DJ, Thompson KF, Krantz DH, Weber EU. How to measure time preferences: An experimental comparison of three methods. Judgment and Decision Making. 2013;8(3):236–249. doi: 10.1007/s10826-012-9600-6. [DOI] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. 1999 Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=psyc3&NEWS=N&AN=1999-04037-000.
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors. 2006;31(7):1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: Relationship to forebrain 5-HT1A receptor binding. Behavioural Brain Research. 2005;159(2):207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Hendrickson KL, Rasmussen EB. Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behaviour Research and Therapy. 2013;51(7):399–409. doi: 10.1016/j.brat.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ : British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Petry NM. Contingency Management Incentives for Sobriety. Alcohol Research and Health. 1999;23(2):122–127. Retrieved from http://pubs.niaaa.nih.gov/publications/arh23-2/122-127.pdf. [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, Guindon A, Morisano D, Peterson JB. Positive mood effects on delay discounting. Emotion. 2010;10(5):717–721. doi: 10.1037/a0019466. [DOI] [PubMed] [Google Scholar]
- Hofmeyr A, Ainslie G, Charlton R, Ross D. The relationship between addiction and reward bundling: An experiment comparing smokers and non-smokers. Addiction. 2011;106(2):402–409. doi: 10.1111/j.1360-0443.2010.03166.x. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Estimating the subjective value of future rewards: Comparison of adjusting-amount and adjusting-delay procedures. Behavioural Processes. 2012;90(3):302–310. doi: 10.1016/j.beproc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of different fixed-ratio requirements on delay discounting in rats. Behavioural Processes. 2013;100:18–22. doi: 10.1016/j.beproc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Imuta K, Hayne H, Scarf D. I want it all and I want it now: Delay of gratification in preschool children. Developmental Psychobiology. 2014;56(7):1541–1552. doi: 10.1002/dev.21249. [DOI] [PubMed] [Google Scholar]
- Isen JD, Sparks JC, Iacono WG. Predictive validity of delay discounting behavior in adolescence: a longitudinal twin study. Experimental and Clinical Psychopharmacology. 2014;22(5):434–443. doi: 10.1037/a0037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowicz DP, Cherry JBC, Reed DD, Bruce JM, Crespi JM, Lusk JL, Bruce AS. Robust relation between temporal discounting rates and body mass. Appetite. 2014;78:63–67. doi: 10.1016/j.appet.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77(2):129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley NJ, Crowell AL, Tang D, Harmon-Jones E, Schmeichel BJ. Disgust sensitivity predicts defensive responding to mortality salience. Emotion. 2015;15(5):590–602. doi: 10.1037/a0038915. [DOI] [PubMed] [Google Scholar]
- Kelley NJ, Schmeichel BJ. Thinking about Death Reduces Delay Discounting. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0144228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working Memory Ability Predicts Trajectories of Early Alcohol Use in Adolescents: The Mediational Role of Impulsivity. 2014;108(3):506–515. doi: 10.1111/add.12001.Working. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR. Models of trace decay, eligibility for reinforcement, and delay of reinforcement gradients, from exponential to hyperboloid. Behavioural Processes. 2011;87(1):57–63. doi: 10.1016/j.beproc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kim H, Schnall S, White MP. Similar psychological distance reduces temporal discounting. Personality and Social Psychology Bulletin. 2013;39(8):1005–16. doi: 10.1177/0146167213488214. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Guastello B. Making choices in anticipation of similar future choices can increase self-control. Journal of Experimental Psychology. Applied. 2001;7(2):154–164. doi: 10.1037/1076-898X.7.2.154. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Herrnstein RJ. Preference reversals due to myopic discounting of delayed reward. Psychological Science. 1995;6(2):83–89. doi: 10.1111/j.1467-9280.1995.tb00311.x. [DOI] [Google Scholar]
- Kirby KN, Maraković NN. Modeling myopic decisions: Evidence for hyperbolic delay-discounting within subjects and amounts. Organizational Behavior and Human Decision Processes. 1995 doi: 10.1006/obhd.1995.1086. [DOI]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology. General. 1999;128(1):78–87. doi: 10.1037/0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Clarke J, Cain ME. Environmental rearing effects on impulsivity and reward sensitivity. Behavioral Neuroscience. 2013;127(5):712–724. doi: 10.1037/a0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapproth F. The date-delay framing effect in temporal discounting depends on substance abuse. Behavioural Processes. 2012;90(3):420–423. doi: 10.1016/j.beproc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Experimental and Clinical Psychopharmacology. 2014;22(3):222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing Delay Discounting in the Light of the Competing Neurobehavioral Decision Systems Theory: A Review. Journal of Experimental Analysis of Behavior. 2013;99(1):32–57. doi: 10.1002/jeab.2.Changing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addiction Biology. 2013;18(1):8–18. doi: 10.1111/j.1369-1600.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal BP, Faulkner JL. Delay discounting of hypothetical monetary rewards with decoys. Behavioural Processes. 2016;122:26–35. doi: 10.1016/j.beproc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Kowal BP, Yi R, Erisman AC, Bickel WK. A comparison of two algorithms in computerized temporal discounting procedures. Behavioural Processes. 2007;75(2 SPEC. ISS.):231–236. doi: 10.1016/j.beproc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kühberger A. The Influence of Framing on Risky Decisions: A Meta-Analysis. Organizational Behavior and Human Decision Processes. 1998;75(1):23–55. doi: 10.1006/obhd.1998.2781. [DOI] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Gao F, Black SE, Rosenbaum RS. Cueing the personal future to reduce discounting in intertemporal choice: Is episodic prospection necessary? Hippocampus. 2015;25(4):432–443. doi: 10.1002/hipo.22431. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes. 2005;69:173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013 Nov;4 doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes RD, Christensen DR, Bickel WK. Delay discounting decreases in those completing treatment for opioid dependence. Experimental and Clinical Psychopharmacology. 2012;20(4):302–309. doi: 10.1037/a0027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf RA. Discount rates for time versus dates: The sensitivity of discounting to time-interval description. Journal of Marketing Research. 2006;43(1):59–72. doi: 10.1509/jmkr.43.1.59. [DOI] [Google Scholar]
- Lee DC, Stanger C, Budney AJ. A comparison of delay discounting in adolescents and adults in treatment for cannabis use disorders. Experimental and Clinical Psychopharmacology. 2015;23(2):130–137. doi: 10.1037/a0038792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Phelps EA. The Malleability of Intertemporal Choice. Trends in Cognitive Sciences. 2016;20(1):64–74. doi: 10.1016/j.tics.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Epstein LH. Living in the moment: Effects of time perspective and emotional valence of episodic thinking on delay discounting. Behavioral Neuroscience. 2014;128(1):12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Stevens AK, Cunningham S, Jones RE, King KM, Schumacher JA, Coffey SF. Stability and change in multi-method measures of impulsivity across residential addictions treatment. Addictive Behaviors. 2015;42:126–129. doi: 10.1016/j.addbeh.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Liu L, Feng T, Chen J, Li H. The value of emotion: How does episodic prospection modulate delay discounting? PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0081717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein GE, Prelec D. Preferences for Sequences of Outcomes. Psychological Review. 1993;100(1):91–108. doi: 10.1037/0033-295X.100.1.91. [DOI] [Google Scholar]
- Logue AW, Mazur J. Maintenance of self-control acquired through a fading procedure: follow-up on Mazur and Logue (1978) BEHAVIOR ANALYSIS LETTERS. 1981;1:131–137. [Google Scholar]
- Luo S, Ainslie G, Monterosso J. The behavioral and neural effect of emotional primes on intertemporal decisions. Social Cognitive and Affective Neuroscience. 2014;9(3):283–291. doi: 10.1093/scan/nss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug and Alcohol Dependence. 2009;104(3):197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. Journal of Abnormal Psychology. 2010;119(1):106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs Ea. Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions? Experimental and Clinical Psychopharmacology. 1999;7(3):284–293. doi: 10.1037/1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A Delay-Discounting Primer. Impulsivity: The Behavioral and Neurological Science of Discounting. 2010:11–37. doi: 10.1037/12069-001. [DOI]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Experimental and Clinical Psychopharmacology. 2009;17(5):283–90. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen E, Dweck CS, Gross JJ. The hidden-zero effect: Representing a single choice as an extended sequence reduces impulsive choice. Psychological Science. 2008;19(7):648–649. doi: 10.1111/j.1467-9280.2008.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrer AR. The role of expectancy in delayed reinforcement. Journal of Experimental Psychology. 1956;52(2):101–106. doi: 10.1037/h0040837. [DOI] [PubMed] [Google Scholar]
- Malkoc SA, Zauberman G, Bettman JR. Unstuck from the concrete: Carryover effects of abstract mindsets in intertemporal preferences. Organizational Behavior and Human Decision Processes. 2010;113(2):112–126. doi: 10.1016/j.obhdp.2010.07.003. [DOI] [Google Scholar]
- Marshall AT, Kirkpatrick K. Mechanisms of impulsive choice: III. The role of reward processes. Behavioural Processes. 2016;123:134–148. doi: 10.1016/j.beproc.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, Smith AP, Kirkpatrick K. Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing. Journal of the Experimental Analysis of Behavior. 2014;102(1):86–101. doi: 10.1002/jeab.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. Probability and delay of reinforcement as factors in discrete-trial choice. Journal of the Experimental Analysis of Behavior. 1985;43(3):341–351. doi: 10.1901/jeab.1985.43-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An Adjusting Procedure for Studying Delayed Reinforcement. In: Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior: V. The Effect of Delay and of Intervening Events on Reinforcement Value. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Effects of pre-trial response requirements on self-control choices by rats and pigeons. Journal of the Experimental Analysis of Behavior. 2012;97(2):215–230. doi: 10.1901/jeab.2012.97-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Logue A. Choice in a “self-control” paradigm: effects of a fading procedure. Journal of the Experimental Analysis of Behavior. 1978;30(1):11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure J, Podos J, Richardson HN. Isolating the delay component of impulsive choice in adolescent rats. Frontiers in Integrative. 2014 Jan;8:1–9. doi: 10.3389/fnint.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Kable JW. Decision makers calibrate behavioral persistence on the basis of time-interval experience. Cognition. 2012;124(2):216–226. doi: 10.1016/j.cognition.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral Neuroscience. 2010;124(4):470–7. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson L, de la Vega A, Chatham CH, Munakata Y. Delaying gratification depends on social trust. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00355. Retrieved from http://dist.lib.usu.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2013-26776-001&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29(12):2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. https://doi.org/00000374-200512000-00010 [pii] [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146(4):455–464. doi: 10.1007/PL00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Wilson VB. Differences in delay discounting between smokers and nonsmokers remain when both rewards are delayed. Psychopharmacology. 2012;219(2):549–562. doi: 10.1007/s00213-011-2521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslle G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BS, Clyburn A, Underwood B. The role of affect in delay of gratification. Child Development. 1976;47(1):273–276. doi: 10.2307/1128312. [DOI] [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7(1):105–125. doi: 10.1037//1082-989X.7.1.105. [DOI] [PubMed] [Google Scholar]
- Morrison KL, Madden GJ, Odum AL, Friedel JE, Twohig MP. Altering impulsive decision making with an acceptance-based procedure. Behavior Therapy. 2014;45(5):630–639. doi: 10.1016/j.beth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller ET, Landes RD, Kowal BP, Yi R, Stitzer ML, Burnett CA, Bickel WK. Delay of smoking gratification as a laboratory model of relapse: effects of incentives for not smoking, and relationship with measures of executive function. Behavioural Pharmacology. 2009;20(5-6):461–73. doi: 10.1097/FBP.0b013e3283305ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef Na, Bicard DF, Endo S. Assessment of impulsivity and the development of self-control in students with attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis. 2001;34(4):397–408. doi: 10.1901/jaba.2001.34-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AL, Maner JK. The good-subject effect: investigating participant demand characteristics. The Journal of General Psychology. 2008;135(2):151–165. doi: 10.3200/GENP.135.2.151-166. [DOI] [PubMed] [Google Scholar]
- Nisan M. Exposure to rewards and the delay of gratification. Developmental Psychology. 1974;10(3):376–380. doi: 10.1037/h0036431. [DOI] [Google Scholar]
- Nisan M, Koriat A. The effect of cognitive restructuring on delay of gratification. Child Development. 1984;55(2):492–503. doi: 10.2307/1129960. [DOI] [Google Scholar]
- O’Connell G, Christakou A, Haffey AT, Chakrabarti B. The role of empathy in choosing rewards from another’s perspective. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orne MT. On the social psychology of the psychological experiment: with particular reference to demand characteristics and their implication. American Psychologist. 1962;17(11):776–783. doi: 10.1037/h0043424. [DOI] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus. 2015;25(3):345–353. doi: 10.1002/hipo.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178(2-3):193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behavioural Brain Research. 2008;193(1):48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Mediotemporal Interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Purcell T, Peron E, Berto R. Why Do Preferences Differ Between Scene Types? Environment and Behavior. 2001;33(1):93–106. doi: 10.1177/00139160121972882. [DOI] [Google Scholar]
- Pyone JS, Isen AM. Positive affect, intertemporal choice, and levels of thinking: Increasing consumers’ willingness to wait. Journal of Marketing Research. 2011;48(3):532–543. doi: 10.1509/jmkr.48.3.532. [DOI] [Google Scholar]
- Rachlin H. Self-control: Beyond commitment. Behavioral and Brain Sciences. 1995;18(1):109–121. doi: 10.1017/S0140525X00037602. [DOI] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17(1):15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55(2):233–44. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R Software. R: A Language and Environment for Statistical Computing 2013 [Google Scholar]
- Radu PT, Yi R, Bickel WK, Gross JJ, McClure SM. A mechanism for reducing delay discounting by altering temporal attention. Journal of the Experimental Analysis of Behavior. 2011;96(3):363–385. doi: 10.1901/jeab.2011.96-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D. Is Time-Discounting Hyperbolic or Subadditive? Journal of Risk and Uncertainty. 2001;23(1):5–32. doi: 10.1023/A:1011198414683. [DOI] [Google Scholar]
- Read D, Frederick S, Orsel B, Rahman J. Four Score and Seven Years from Now: The Date/Delay Effect in Temporal Discounting. Management Science. 2005;51(9):1326–1335. doi: 10.1287/mnsc.1050.0412. [DOI] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personality and Individual Differences. 2009;47(8):973–978. doi: 10.1016/j.paid.2009.07.026. [DOI] [Google Scholar]
- Renda CR, Stein JS, Madden GJ. Working-memory training: Effects on delay discounting in male long Evans rats. Journal of the Experimental Analysis of Behavior. 2015;103(1):50–61. doi: 10.1002/jeab.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd M, Vohs KD, Aaker J. Awe expands people’s perception of time, alters decision making, and enhances well-being. Psychological Science. 2012;23(10):1130–6. doi: 10.1177/0956797612438731. [DOI] [PubMed] [Google Scholar]
- Rung JM, Madden GJ. Demand Characteristics in Episodic Future Thinking: Delay Discounting and Healthy Eating. Experimental and Clinical Psychopharmacology. 2018 doi: 10.1037/pha0000171. [DOI] [PubMed]
- Samuelson W, Zeckhauser R. Status quo bias in decision making. Journal of Risk and Uncertainty. 1988;1(1):7–59. doi: 10.1007/BF00055564. [DOI] [Google Scholar]
- Sasse LK, Peters J, Büchel C, Brassen S. Effects of prospective thinking on intertemporal choice: The role of familiarity. Human Brain Mapping. 2015;36(10):4210–4221. doi: 10.1002/hbm.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten M, Read D. Better is worse, worse is better: Violations of dominance in intertemporal choice. Decision. 2014;1(3):215–222. doi: 10.1037/dec0000014. [DOI] [Google Scholar]
- Schroeder SA. We Can Do Better — Improving the Health of the American People. New England Journal of Medicine. 2007;357(12):1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- Schweitzer J, Sulzer-Azaroff B. Self-Control: Teaching Tolerance for Delay in Implulsive Children. Journal of the Experimental Analysis of Behavior. 1988;50(2):173–186. doi: 10.1901/jeab.1988.50-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M, Di Pellegrino G. Errors affect hypothetical intertemporal food choice in women. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: A strong prognostic indicator of smoking relapse. Addictive Behaviors. 2014;39(11):1682–1689. doi: 10.1016/j.addbeh.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni E, Asbe M, Eyal T, Berger A. Too proud to regulate: The differential effect of pride versus joy on children’s ability to delay gratification. Journal of Experimental Child Psychology. 2016;141:275–282. doi: 10.1016/j.jecp.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Siegel E, Rachlin H. Soft commitment: self-control achieved by response persistence. 1995;2(2):117–128. doi: 10.1901/jeab.1995.64-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug and Alcohol Dependence. 1996;41(2):157–165. doi: 10.1016/0376-8716(96)01246-X. [DOI] [PubMed] [Google Scholar]
- Smith AP, Marshall AT, Kirkpatrick K. Mechanisms of impulsive choice: II. Time-based interventions to improve self-control. Behavioural Processes. 2015;112:29–42. doi: 10.1016/j.beproc.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, LaConte SM, Bickel WK. Episodic Future Thinking: Expansion of the Temporal Window in Individuals with Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2016:1–9. doi: 10.1111/acer.13112. [DOI] [PMC free article] [PubMed]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2012;20(3):205–12. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub E. Effects of persuasion and modeling on delay of gratification. Developmental Psychology. 1972;6(1):166–177. doi: 10.1037/h0032212. [DOI] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ. Early and prolonged exposure to reward delay: effects on impulsive choice and alcohol self-administration in male rats. Experimental and Clinical Psychopharmacology. 2013;21(2):172–180. doi: 10.1037/a0031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Madden GJ. Delay discounting and drug abuse: Empirical, conceptual, and methodological considerations. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. 2013:165–208. doi: 10.1111/b.9781119978268.2013.00010.x. [DOI]
- Stein JS, Smits RR, Johnson PS, Liston KJ, Madden GJ. Effects of reward bundling on male rats’ preference for larger–later food rewards. Journal of the Experimental Analysis of Behavior. 2013;99(2):150–158. doi: 10.1002/jeab.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathman A, Gleicher F, Boninger DS, Edwards CS. The consideration of future consequences: Weighing immediate and distant outcomes of behavior. Journal of Personality and Social Psychology. 1994;66(4):742–752. doi: 10.1037/0022-3514.66.4.742. [DOI] [Google Scholar]
- Stumphauzer JS. Increased delay of gratification in young prison inmates through imitation of high-delay peer models. Journal of Personality and Social Psychology. 1972;21(1):10–17. doi: 10.1037/h0032064. [DOI] [PubMed] [Google Scholar]
- Sze YY, Daniel TO, Kilanowski CK, Collins RL, Epstein LH. Web-Based and Mobile Delivery of an Episodic Future Thinking Intervention for Overweight and Obese Families: A Feasibility Study. JMIR mHealth and uHealth. 2015;3(4):e97. doi: 10.2196/mhealth.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler RH, Benartzi S. Save More Tomorrow ™ : Using Behavioral Economics to Increase Employee Saving Save More Tomorrow : Using Behavioral Economics to Increase Employee Saving Shlomo Benartzi. Journal of Political Economy. 2004;112:S164–S187. February 2004. [Google Scholar]
- Trope Y, Liberman N. Temporal Construal. Psychological Review. 2003;110(3):403–421. doi: 10.1037/0033-295X.110.3.403. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Urminsky O, Kivetz R. Scope insensitivity and the “mere token” effect. Journal of Marketing Research. 2011;48(2):282–295. doi: 10.1509/jmkr.48.2.282. [DOI] [Google Scholar]
- Van den Bergh B, Dewitte S, Warlop L. Bikinis instigate generalized impatience in intertemporal choice. Journal of Consumer Research. 2008;35(1):85–97. doi: 10.1086/525505. [DOI] [Google Scholar]
- van den Berk-Clark C, Myerson J, Green L, Grucza RA. Past trauma and future choices: differences in discounting in low-income, urban African Americans. Psychological Medicine. 2018:1–8. doi: 10.1017/S0033291718000326. [DOI] [PubMed] [Google Scholar]
- van der Wal AJ, Schade HM, Krabbendam L, van Vugt M, Wal AJ, van der Schade HM, Vugt M van. Do natural landscapes reduce future discounting in humans? Proceedings. Biological Sciences / The Royal Society. 2013;280(1773):20132295. doi: 10.1098/rspb.2013.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderveldt A, Green L, Myerson J. Discounting of monetary rewards that are both delayed and probabilistic: Delay and probability combine multiplicatively, not additively. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2015;41(1):148–162. doi: 10.1037/xlm0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software. 2010;36(3):1–48. doi: 10.1103/PhysRevB.91.121108. [DOI] [Google Scholar]
- Volkow ND, Baler RD. NOW vs LATER brain circuits: Implications for obesity and addiction. Trends in Neurosciences. 2015;38(6):345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6(3):292–305. doi: 10.1037/1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Washio Y, Higgins ST, Heil SH, McKerchar TL, Badger GJ, Skelly JM, Dantona RL. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Experimental and Clinical Psychopharmacology. 2011;19(3):243–8. doi: 10.1037/a0023617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Mitchell SH, De Wit H. Recent Translational Findings on Impulsivity in Relation to Drug Abuse. Current Addiction Reports. 2014;1(4):289–300. doi: 10.1007/s40429-014-0035-6.Recent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly JN, Ruthig JC. Degree of Delay Discounting as a Function of Who Receives the Outcome and the Discounter’s Perceived Level of Social Support. Current Psychology. 2013;32(1):1–17. doi: 10.1007/s12144-012-9160-3. [DOI] [Google Scholar]
- Weidberg S, Landes RD, García-Rodríguez O, Yoon JH, Secades-Villa R. Interaction Effect of Contingency Management and Sex on Delay-Discounting Changes Among Treatment-Seeking Smokers. Experimental and Clinical Psychopharmacology. 2015;23(5):1–9. doi: 10.1037/pha0000043. [DOI] [PubMed] [Google Scholar]
- Weidberg S, Landes RD, López-Núñez C, Pericot-Valverde I, González-Roz A, Yoon JH, Secades-Villa R. Contingency management effects on delay discounting among patients receiving smoking cessation treatment. Psicothema. 2015;27(4):309–316. doi: 10.7334/psicothema2015.184. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The neural and neurochemical basis of delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. American Psychological Association; 2010. pp. 95–121. [Google Scholar]
- Wittmann M, Leland DS, Churan J, Paulus MP. Impaired time perception and motor timing in stimulant-dependent subjects. Drug and Alcohol Dependence. 2007;90(2–3):183–192. doi: 10.1016/j.drugalcdep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-Y, He G-B. The effects of time perspective and salience of possible monetary losses on intertemporal choice. Social Behavior and Personality. 2012;40(10):1645–1654. doi: 10.2224/sbp.2012.40.10.1645. [DOI] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, Bickel WK. The Effects of Reduced Cigarette Smoking on Discounting Future Rewards: an Initial Evaluation. The Psychological Record. 2008;58(2):163–174. doi: 10.1007/bf03395609. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3697871&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, King LF, Carter AE, Landes RD, Bickel WK. Intertemporal decision-making for a group. The Psychological Record. 2010;60(4):577–586. doi: 10.1007/bf03395733. Retrieved from http://dist.lib.usu.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2010-23608-003&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Mitchell SH, Bickel WK. Delay discounting and substance abuse-dependence. Impulsivity: The Behavioral and Neurological Science of Discounting. 2010:191–211. doi: 10.1037/12069-007. [DOI]
- Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology. 2009;205(2):305–318. doi: 10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15(2):176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- Zauberman G, Kim BK, Malkoc SA, Bettman JR. Discounting Time and Time Discounting: Subjective Time Perception and Intertemporal Preferences. Journal of Marketing Research. 2009;46(4):543–556. doi: 10.1509/jmkr.46.4.543. [DOI] [Google Scholar]
- Ziegler FV, Tunney RJ. Decisions for Others Become Less Impulsive the Further Away They Are on the Family Tree. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbardo PG, Boyd JN. Putting Time in perspective: a valid, reliable individual differences metric. Journal of Personality and Social Psychology. 1999;6(77):1271–1288. doi: 10.1037//0022-3514.77.6.1271. [DOI] [Google Scholar]