Abstract
Purpose of the review
To review current controversies in the transcatheter device closure of ostium secundum atrial septal defects (ASD).
Recent findings
Trans-catheter device closure of ASD (TC-ASD) has well-established efficacy and safety. For most individual patients with suitable anatomy, TC-ASD is the preferred method for treating ASD. The availability of large multicenter datasets has made it possible to study practice patterns at a range of hospitals across the United States. These studies have revealed differences in practice that were not previously appreciated. 1) Interpretation of the indications for TC-ASD, specifically the definition of right ventricular volume overload varies between hospitals. 2) In response to concern about device erosion, an increasing proportion of patients are being referred for operative ASD closure. 3) Over the last decade, the average age at which ASD closure occurs has decreased. These trends demonstrate previously under-appreciated differences in opinion between cardiologists across the country, and suggest that further research is necessary to address knowledge gaps limiting consistency of practice.
Summary
As TC-ASD and congenital interventional cardiology mature as a field, studies of real-world practice provide increasingly valuable information about aspects of care where there are disagreements about best practices and where further research is necessary.
Keywords: erosion, pediatric cardiology, transcatheter intervention, outcomes
Introduction
Ostium secundum atrial septal defects (ASD) are one of the most common forms of congenital heart disease with an incidence of 6 to 10 per 10,000 live births[1]. Transcatheter device closure of ASD (TC-ASD) was first reported by King and Mills in 1976[2]. TC-ASD with the current generation of devices has favorable rates of technical success and risk of adverse events when compared to operative closure of ASD (O-ASD)[3–8]. TC-ASD is the dominant technique for closing ASD; >80% of isolated ASD treated at primary pediatric hospitals in the United States are closed in the catheterization laboratory[9]. Though new devices continue to be developed, the technique for TC-ASD has remained essentially unchanged since the introduction of the Amplatzer Septal Occluder (ASO) in the early 2000’s. Recent studies have taken advantage of large multicenter datasets, providing an opportunity to study real-world practice in an innovative fashion. These studies have revealed significant variability in hospital practice in closure of ASD and thus an opportunity to improve the quality of care for patients with ASD.
Current options for device closure of ASD
The history of device development has been summarized elsewhere[10]. Two devices are currently widely available in the United States (Figure 1): the ASO (St. Jude Medical, St. Paul MN) (Figure 1A) and the Gore Cardioform device (W.L. Gore and Associates, Flagstaff, AZ) (Figure 1B). In multicenter series of US centers, the ASO is used in between 70-86% of cases, with the HSO being used in 5-21% of cases[11,12]. The ASO was the first device approved by the FDA for TC-ASD demonstrating safety and efficacy in a non-randomized IDE trial[3], which was reinforced in a subsequent multicenter registry study[13]. It has been in use long enough for several large single-center case series to report excellent medium and long-term outcomes[14–16]. The Gore Helex Septal Occluder (W.L. Gore and Associates, Flagstaff, AZ) also demonstrated excellent safety and efficacy in both short[17–19] and middle term outcomes[20], but is no longer sold in the US. It has been replaced by the Gore Cardioform device, which has a Nitinol wire frame covered with an expanded tetrafluoroethylene membrane. Like the Helex before it, this device was not self-centering and limited to relatively small defects. The initial device trial and continuing access series are complete with manuscripts pending at this time. Gore has produced a second Cardioform device – the Cardioform Atrial Septal Defect Occluder (C-ASDO)-with a larger diameter central waist and an expanded range of diameters, both designed to facilitate closure of larger diameter ASD’s (Figure 1C). The newer C-ASDO device is currently undergoing an FDA Pivotal trial(Gore ASSURED Trial; Clinical Trials.gov Identifier NCT02985684) in the United States. In a multicenter series from Canada, both Gore Cardioform devices have demonstrated similar safety and efficacy to previous devices[21]. The Amplatzer multi-fenestrated septal occluder (“Cribriform” device) (St. Jude Medical, St. Paul, MN) resembles the ASO device but has symmetric discs and a narrow waist, designed to allow it to cover the septum when there are multiple ASD.
Figure 1. Current TC-ASD Devices Available in the United States.
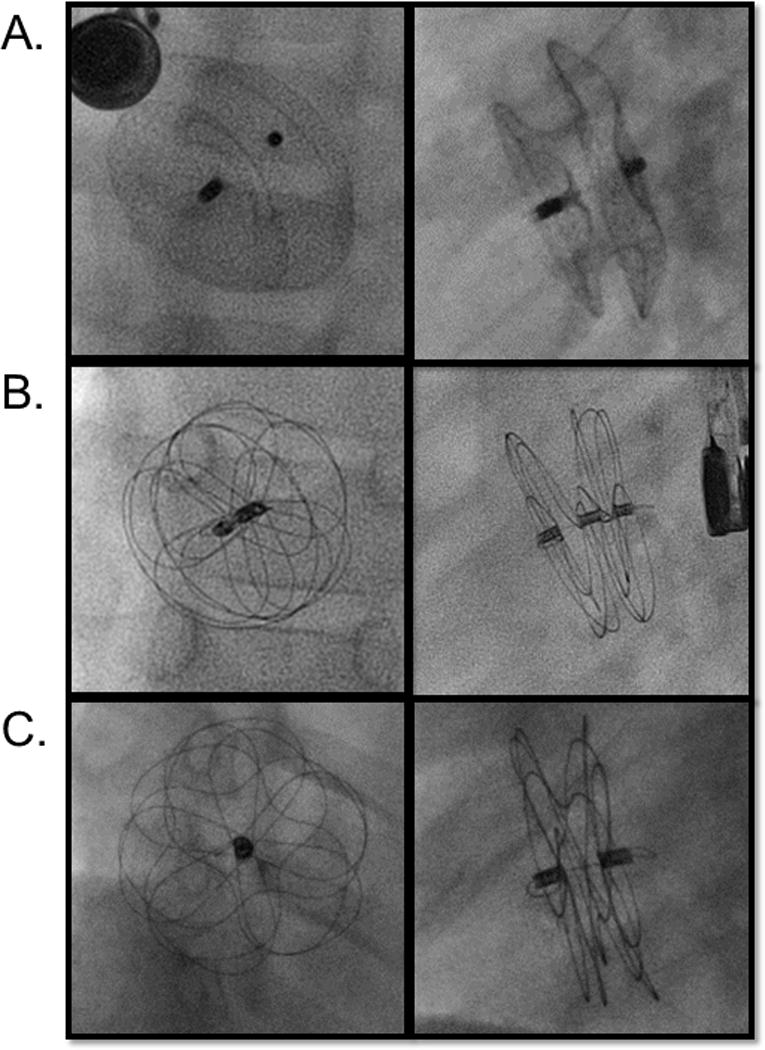
Digital acquisition images of A) Amplatzer septal occlude (anterior posterior and lateral projections), B) Gore Cardioform device (en face and orthogonal views), C) Cardioform Atrial Septal Defect Occluder (en face and orthogonal views).
TC-ASD has demonstrated equal efficacy at closure with excellent safety in comparisons to O-ASD[3,6,8]. In addition, as a relatively non-invasive technology TC-ASD results in less discomfort, superior cosmesis, and shorter hospital length of stay. All of these are potentially preferable to patients, and when accompanied by improved economic cost represent superior value from the perspective of the entire health system. Comparing current outcomes following TC-ASD and O-ASD is challenging. There are relatively few contemporary series comparing the results of TC-ASD and O-ASD. Large multicenter series are necessary for comparisons because of systematic differences between patients undergoing O-ASD and TC-ASD and the need for statistical adjustment to account for confounding by indication. Representative multi-center series have demonstrated that in-hospital mortality after TC-ASD is between 0-0.015%[11,12,22]. Using data from the Society for Thoracic Surgeons Congenital Heart Surgeons database, the estimated risk of in-hospital mortality for O-ASD is between 0.3-0.9% even after adjusting for pre-operative risk factors[23]. In terms of peri-procedural morbidity, analysis of data from the Pediatric Health Information Systems Database (PHIS) by Ooi and colleagues demonstrated that the adjusted risk of complications and infection were higher following O-ASD than TC-ASD[24]. Morbidity is also reflected in a longer length of stay following O-ASD[5]. Measuring economic cost provides a way to compare the impact of O-ASD and TC-ASD on patients, integrating resource utilization and risk of adverse events in a single measure. Early attempts to compare cost of TC-ASD and O-ASD had equivocal results[25–28], but more recent studies with sufficient sample sizes for case-mix adjustment have demonstrated that the total hospital costs of TC-ASD (including cost of device) are less than those associated with O-ASD[5,24]. Cost savings arise not only from a shorter length of stay but also from reduced pharmacy, radiology, and laboratory costs[5].
The introduction of minimally invasive cardiac surgery (MICS), through “mini-sternotomy” or video assisted thoracoscopic surgery (VATS) (with or without robotic assistance) has the potential to provide a method of closing ASD with reduced morbidity, shorter length of stay, and improved cosmesis compared to O-ASD[29]. In the past year, three case series of MICS for O-ASD have been published[30–32]. The largest series demonstrates that MICS patients were exposed to increased cardiopulmonary bypass times but spent less time on the ventilator [30]. In each of the three series, the median lengths of stay remain between 5-7 days[30–32], which remains significantly longer than LOS for TC-ASD (usually less than a day). Cost comparison of MICS to traditional O-ASD, or TC-ASD for that matter, were not attempted. Given longer length of stay, one would expect that MICS will be more expensive than either O-ASD or TC-ASD. At this time, enthusiasm for MICS is limited to a few centers, and its future as an alternative to conventional O-ASD and TC-ASD remains uncertain.
Practice variation in ASD closure
Traditional studies of ASD closure, either as part of a device trial or through retrospective review of cases, have been effective at demonstrating the safety and efficacy of various ASD closure strategies. Recent access to multicenter databases has allowed research about variability in real-world practice between different hospitals. The presence of inter-center variability points toward a lack of consensus in the delivery of care and an opportunity to improve quality of care. Reducing practice variation through standardization in practice has demonstrably improved resource utilization and traditional patient outcomes for adult cardiac patients in both inpatient[33,34] and outpatient[35–42] settings, as well as adult[43] and pediatric[44–47] patients with congenital heart disease. In the case of closure of ASD, studies have demonstrated significant practice variation in 1) the distribution and interpretation of indications for TC-ASD, 2) choice between TC-ASD and O-ASD to close ASD, and 3) the age at which ASD closure is pursued (regardless of method). These findings are the results of the first studies of their kind. They highlight the importance of this type of research and suggest avenues for further research with the potential to continue to improve care of patients with ASD.
Indications for ASD closure
Indications for TC-ASD were published by the American Heart Association in 2011 (Table 1)[48]. The IMproving Pediatric and Adult Congenital Treatment (IMPACT®) Registry provides the first opportunity to study how the practices in transcatheter procedures vary between hospitals. IMPACT® is an American College of Cardiology Foundation and National Cardiovascular Data Registry funded clinical registry of hospitals performing transcatheter procedures in children and adults with congenital and acquired cardiac disease and contains demographic, clinical, and procedural information about patients and their procedures[22,49,50]. Special attention is given to six procedures including TC-ASD[50], about which more detailed procedural information is collected. TC-ASD account for 6% of all cases recorded in IMPACT®[11,22,51]. Indications for TC-ASD in IMPACT® are right ventricular volume overload (RVVO), failure to thrive, recurrent respiratory infections/chronic lung disease, cyanosis and stroke prevention, with the majority (83%) of cases having RVVO as an indication.
Table 1.
Indications for transcatheter closure of atrial septal defects
| Indication | Class | LOE |
|---|---|---|
| Closure is indicated | ||
| Hemodynamically significant ASD with suitable anatomic features | I | B |
| Transient right to left shunt with history of sequelae of paradoxical emboli | IIA | B |
| Right to left shunt with symptomatic cyanosis who do not require the communication to maintain cardiac output | IIA | B |
| Small ASD who are believed to be at high risk of thromboembolic events | IIB | C |
| Closure is not indicated | ||
| Small secundum ASD without hemodynamically significant shunt with other risk factors | III | B |
| ASD other than secundum ASD | III | C |
| Patients with advanced pulmonary vascular obstructive disease | III | C |
Abbreviations: ASD atrial septal defect, LOE level of evidence
Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, et al.: Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 2011, 123:2607–2652.
Analysis of this dataset demonstrated significant differences in the distribution of these indications by both census region and hospital setting (urban vs. suburban or rural), which could not be explained by measurable confounders[51]. Further analysis studied the subset of cases with RVVO as an indication, measuring the proportion of these cases at each hospital where there was a small shunt (as defined by either the ratio of pulmonary to systemic blood flow (Qp:Qs) or the size of the defect). In this study, 33% of patients whose ASD were closed with RVVO as the listed indication had a Qp:Qs <1.5:1. Importantly, the proportion of these cases at individual hospitals varied systematically with hospital characteristics. Specifically, hospitals with a smaller TC-ASD volume and those with a larger adult catheterization volume had a higher proportion of TC-ASD for the stated indication of RVVO with a small magnitude shunt[51]. It is not possible in this analysis to differentiate between variation in referral patterns and variation in interventional cardiologist practice. However, variation of this kind potentially exposes patients to greater risk of adverse outcome and increases resource utilization unnecessarily. At a minimum, it asserts that current practice guidelines have not resulted in consensus regarding the indications for TC-ASD.
Practice variation in TC-ASD vs. O-ASD
In analysis of data from the PHIS database, TC-ASD accounted for >80% of ASD closure procedures at US pediatric hospitals[9]. However, there was significant variation in how individual centers chose to pursue ASD closure with the range of ASD closure performed in the catheterization laboratory between 30 and nearly 100%[9]. Even after adjusting for differences in case-mix, there remained significant inter-hospital variability in the choice between O-ASD and TC-ASD[9]. To our knowledge, no other studies have assessed practice variation in ASD closure, but their presence reflects lack of consensus in deciding the strategy to close ASD.
Device erosion and its potential impact on practice patterns
Clinical decision-making is multi-factorial, and it is not possible to assert a causal relationship in an observational study. However, device erosion remains a singular concern for physicians caring for patients with ASD. Erosions of ASO were first reported in a series of case reports in 2003 and 2004[52–54], and a board of physicians was convened to review known cases[55]. Deficient anterior-superior or retro-aortic rim (along with device over-sizing) was identified as a risk factor present in all of their cases. Concern regarding erosions precipitated a United States Food and Drug Administration Panel Review in May of 2012 followed by revision of the manufacturers Indication for Use, labeling a retro-aortic rim <5mm in diameter as a relative contraindication to TC-ASD with an ASO device[56–58]. Subsequent research has demonstrated that the prevalence of deficient retro-aortic rim is between 40-60% of children referred for TC-ASD[11,16,59,60] and slightly lower in adult patients[61].
Concern for device erosion has persisted [62–66], but limitations in longitudinal follow-up of implanted devices has made it impossible to accurately measure the number of devices implanted, the total number of erosions, and the risk of erosion. Best estimates of risk are between 0.04 and 0.3% of device implants[55,57,62,63,67]. The majority of cases present early after device implantation, but remote erosions as late as 8 years after device implantation have been reported[68,69]. The low overall rates of erosion and limited experience at individual centers has complicated identification of patient- and procedure-level risk factors for device erosion. Neither the first version of the IMPACT® [11] nor C3PO [12] contained data about post-discharge adverse events. Since that time, a prospective post-market surveillance study of the ASO was initiated. However, enrollment was stopped in 12/2016, and the results have not been published. The second version of the IMPACT® registry includes the capacity to include follow-up data for pre-specified interventions including TC-ASD. Access to other large observational data-sets (e.g. insurance claims data) may improve our estimates of post-procedural risk.
Moreover, our understanding of the mechanism underlying erosions and identification of the patients at highest risk of erosion has not progressed. Specifically, it is unclear whether 1) anatomic variations (bare vs. small retro-aortic rim or concomitant deficient superior tissue rim) or 2) a combination of anatomy and choice of device (a patient with deficient retro-aortic or superior rim and a large or over-sized device) can provide predict superior risk stratification[55,67,70]. A recent case-control study by McElhinney and colleagues using data from the Erosion Board’s collection of cases and controls derived from the ASO Post-Approval Study reiterated that deficient retro-aortic and superior vena cava rims were present in a much higher proportion of cases than controls. In cases with erosion, ASD were larger in diameter and in proportion to patient weight than in controls. Finally, several factors consistent with device over-sizing (balloon size much larger than static defect size or device much larger than static defect size) were more common in cases and controls[67]. Although this study confirms risk factors for erosion, it was unable to identify a subgroup of patients in which the risk of erosion exceeds the risk of O-ASD.
Moreover, the effect of the erosion debate on actual practice has until recently been challenging to study. Single center studies are subject to small sample sizes and limited generalizability to the general population[71]. Analysis of clinical registry data demonstrated that patients with deficient retro-aortic rim were no less likely to receive ASO devices than those with larger retro-aortic rims[11]. More recently, analysis of administrative data allowed, for the first time, measurement of temporal trends in practice, specifically the propensity to pursue O-ASD and TC-ASD[9]. It demonstrated that prior to 2013, the proportion of TC-ASD was increasing, but that between 2013 and 2015 this trend reversed and the proportion of O-ASD increased slightly relative to TC-ASD (Figure 2).
Figure 2. Estimated probability of TC-ASD vs. O-ASD 2007-2015.
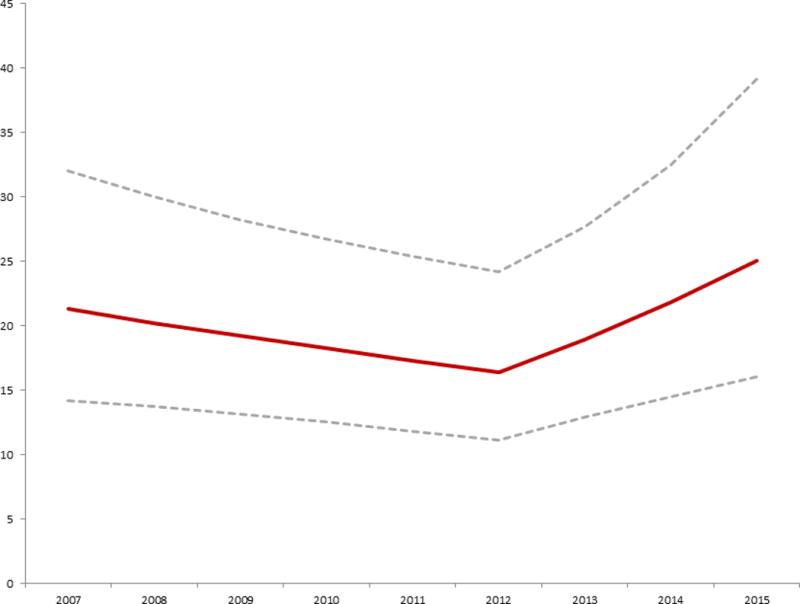
Probability of operative ASD closure versus transcatheter ASD closure
Conditional standardization was used to calculate an adjusted probability of operative ASD closure vs. transcatheter device closure, for a hypothetical white male six year old boy with no co-morbid conditions (maroon line with 95% CI represented by the dashed grey lines). This was based on the mixed effects multivariate generalized linear model summarized in Table 2. The probability of operative ASD closure decreased significantly from 2007 until 2012 (OR: 0.95 per year, p=0.02). In 2013, there was a significant shift in probability favoring ASD (OR: 1.21 per year, p=0.006).
Taken from O’Byrne et al 2017 Increasing Propensity to Pursue Operative Closure of Atrial Septal Defects Following Changes in the Instructions for Use of the Amplatzer Septal Occluder Device An Observational Study Using Data from the Pediatric Health Information Systems Database. Am Heart J (in press)
Though referring patients for O-ASD will inevitably reduce the risk for erosion, this practice only results in a net reduction in harm to patients if the benefit exceeds the inherently higher risks of O-ASD. This can only be accomplished by identifying subgroup(s) of patients with a significantly higher risk of erosion than the overall population risk. The heterogeneity of practice and anatomy combined with the low event rate are real challenges to solving this problem. Post-market surveillance studies have the potential to address these concerns but have not to date made progress. Alternative research strategies to address these concerns may be necessary.
Timing of ASD closure
Another important decision in the care of patients with ASD is the age at which to intervene. From the outset of TC-ASD, there has been interest in treating young (and small) patients with ASD. The original trial for the ASO device did not restrict age or size for the TC-ASD arm. The median age was 9.8 years but with a range from 0.6 to 82 years[3]. The first multicenter study reporting real-world use of the ASO device was a case series from the Mid-Atlantic Group of Interventional Cardiology (MAGIC), which reported the results of 478 cases from 13 centers performed between 2004 and 2007[13]. The median age of patients in this series was 6 years, but again with a broad range (spanning from infancy until 80’s). At that time, 33% of reported cases were performed in small patients (defined as <16 kg). Multiple case series demonstrate that TC-ASD can be performed even in patients <10 kg[59,72–76]. At the same time, the majority of cases continue to be performed in school-age patients. Multicenter series from IMPACT® report that the median age of closure remains between 5-7 years)[11,22], and in a study from C3PO, 85% of subjects were older than 3 years[12].
Natural history studies of large ASD demonstrated a decrease in life expectancy, but in almost all patients childhood symptoms due to congestive heart failure are rare, as is the development of pulmonary vascular disease [77–79]. The majority of small defects found in infants close spontaneously[80–82], but the natural history of larger defects has not been well defined. In cross-sectional analyses, an association has been demonstrated between older patient age at diagnosis and larger defect size[81,82]. This may be due to spontaneous closure of smaller defects, but some have hypothesized that some ASD increase in size over time [83]. This has been used as a justification for early intervention. No studies have followed ASD longitudinally to confirm if some ASD do grow over time. Even if some ASD did grow, it is unclear whether how often that growth would complicate TC-ASD.
In the face of this uncertainty, a recent study of trends in ASD closure using PHIS data demonstrated that patients undergoing ASD closure at primary pediatric hospitals in the US were progressively younger between 2007 and 2015[9]. This was independent of measurable patient-level confounders. It is impossible to determine in this study design the reasons behind this trend. Sensitivity analyses demonstrated that this was not driven by the aforementioned trend to increasing use of O-ASD and was present regardless of closure method. There is also no evidence that the trend is the result of increasing prevalence of pulmonary disease or prematurity that might aggravate the physiological effects of an atrial level shunt.
This trend has the potential to have real ramifications on patient safety. The effect of small size on the risk of adverse events or technical failure has been equivocal in multiple studies [11,12,16]. However, McElhinney and colleagues demonstrated that larger defect size to patient size was a risk factor for device erosion[67]. Therefore, for TC-ASD, the optimal age for closure is not clear. We recommend discussing ASD cases with an interventional cardiologist to determine the right timing for each individual patient.
CONCLUSION
Transcatheter device closure of ostium secundum atrial septal defects has been performed for over forty years. Over that time it has become the gold-standard therapy for closure of most ASD, comparing favorably to operative ASD closure in efficacy, safety, and cost. Large multicenter data-sets have allowed researchers to study practice patterns in the community and identify areas where uncertainty persists. It is our hope that identification of these knowledge gaps will motivate future research that will improve outcomes of patients with ASD.
KEY POINTS.
Transcatheter device closure of ostium secundum atrial defects (ASD) has a lower risk of mortality, lower risk of morbidity, shorter length of stay, and lower cost than operative closure of the same defect.
Minimally invasive surgical approaches to operative closure of ASD are being used. In skilled hands and appropriately selected patients, they have similar risk of adverse events and length of stay (but improved cosmesis) to conventional surgical approaches, but remain inferior to transcatheter device closure.
Studying practice patterns in ASD closure has identified that smaller hospitals and hospitals with predominantly adult patients are more likely to close a small defect with the indication of right ventricular volume overload. The effect of this on clinical outcomes is not clear but it highlights that there are persistent differences in the interpretation of the indications for transcatheter intervention.
Erosion of devices after transcatheter closure of ASD remains a concern. The overall risk is between 0.04-0.3% of device implants. The most recent data found that cases were more likely than controls to have smaller superior rims, larger defects relative to the septum and patient size, and were more likely to have an oversized device. Further research is necessary to determine whether a specific subset of patients can be identified whose risk of device erosion exceeds the risks of operative ASD closure.
Studying trends in practice have found that transcatheter ASD closure is the predominant method of ASD closure in children and young adults, but that in recent years there is a trend towards increasing use of operative ASD closure. This is coincident with concern for the risk of device erosions. The effect of this trend on outcomes is not clear at this time, but deserves additional attention.
Acknowledgments
None
Funding Sources: Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). Dr. Glatz receives research support from the Children’s Heart Foundation. The funding agencies had no role in the drafting of the manuscript or influencing its content.
Footnotes
Conflicts of Interest: No other relevant disclosures.
This manuscript represents the opinion of the authors alone. There are no other relevant financial disclosures.
References
- 1.Hoffman JIE, Kaplan S. The Incidence of Congenital Heart Disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976;235:2506–2509. [PubMed] [Google Scholar]
- 3.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer Investigators Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosas M, Zabal C, Garcia-Montes J, Buendia A, Webb G, Attie F. Transcatheter versus surgical closure of secundum atrial septal defect in adults: impact of age at intervention. A concurrent matched comparative study. Cong heart dis. 2007;2:148–155. doi: 10.1111/j.1747-0803.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J. 2015;169:727–735.e2. doi: 10.1016/j.ahj.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutty S, Abu Hazeem A, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA. Long-Term (5- to 20-Year) Outcomes After Transcatheter or Surgical Treatment of Hemodynamically Significant Isolated Secundum Atrial Septal Defect. Am J Cardiol. 2012;109:1348–1352. doi: 10.1016/j.amjcard.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Butera G, Biondi-Zoccai G, Sangiorgi G, Abella R, Giamberti A, Bussadori C, Sheiban I, Saliba Z, Santoro T, Pelissero G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention. 2011;7:377–385. doi: 10.4244/EIJV7I3A63. [DOI] [PubMed] [Google Scholar]
- 8.Kotowycz MA, Therrien J, Ionescu-Ittu R, Owens CG, Pilote L, Martucci G, Tchervenkov C, Marelli AJ. Long-Term Outcomes After Surgical Versus Transcatheter Closure of Atrial Septal Defects in Adults. JACC Cardiovasc Interv. 2013;6:497–503. doi: 10.1016/j.jcin.2012.12.126. [DOI] [PubMed] [Google Scholar]
- 9**.O’Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing Propensity to Pursue Operative Closure of Atrial Septal Defects Following Changes in the Instructions For Use of the Amplatzer Septal Occluder Device: An Observational Study Using Data from the Pediatric Health Information Systems Database. Am Heart J. 2017 doi: 10.1016/j.ahj.2017.07.012. In Press. This retrospective multicenter observational study using data from the Pediatric Health Information Systems Database is the first to assess trends in practice across primary pediatric hospitals in the United States, demonstrating 1) significant inter-hospital variation in the decision to pursue operative or transcatheter ASD closure, 2) that in the period following Food and Drug Administration Review of device erosion risk that the propensity to pursue operative ASD closure increased modestly, and 3) that over the study period ASD closure is being pursued in progressively younger patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TD, Mills NL. Historical Perspective on ASD Device Closure. In: Hijazib ZM, Feldman T, Abdullah Al-Qbandi MH, Sievert H, editors. Transcatheter Closure of ASDs and PFOs. Cardiotext; 2010. pp. 37–64. [Google Scholar]
- 11*.O’Byrne ML, Gillespie MJ, Kennedy KF, Dori Y, Rome JJ, Glatz AC. The influence of deficient retro-aortic rim on technical success and early adverse events following device closure of secundum atrial septal defects: An Analysis of the IMPACT Registry(®) Catheter Cardiovasc Interv. 2017;89:102–111. doi: 10.1002/ccd.26585. Retrospective multicenter cohort study using data from the IMPACT® Registry which is the first report of short-term outcomes of ASD closure in real-world practice, demonstrating that deficient retro-aortic rim was not associated with increased risk of adverse event or technical failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Said H, Hegde S, Foerster S, Hellenbrand W, Kreutzer J, Trucco SM, Holzer R, Burch G, Mirani A, Nicolas R, et al. Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv. 2015;85:227–233. doi: 10.1002/ccd.25684. [DOI] [PubMed] [Google Scholar]
- 13.Everett AD, Jennings J, Sibinga E, Owada C, Lim DS, Cheatham J, Holzer R, Ringewald J, Bandisode R, Ringel R. Community Use of the Amplatzer Atrial Septal Defect Occluder: Results of the Multicenter MAGIC Atrial Septal Defect Study. Pediatr Cardiol. 2008;30:240–247. doi: 10.1007/s00246-008-9325-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang J-K, Tsai S-K, Wu M-H, Lin M-T, Lue H-C. Short- and intermediate-term results of transcatheter closure of atrial septal defect with the Amplatzer Septal Occluder. Am Heart J. 2004;148:511–517. doi: 10.1016/j.ahj.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM. Long-Term Follow Up of Secundum Atrial Septal Defect Closure with the Amplatzer Septal Occluder. Cong heart dis. 2010;5:32–37. doi: 10.1111/j.1747-0803.2009.00358.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Byrne ML, Glatz AC, Sunderji S, Mathew AE, Goldberg DJ, Dori Y, Rome JJ, Gillespie MJ. Prevalence of Deficient Retro-Aortic Rim and Its Effects on Outcomes in Device Closure of Atrial Septal Defects [Internet] Pediatr Cardiol. 2014;35:1181–1190. doi: 10.1007/s00246-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent RN, Raviele AA, Diehl HJ. Single-center experience with the HELEX septal occluder for closure of atrial septal defects in children. J Interv Cardiol. 2003;16:79–82. doi: 10.1046/j.1540-8183.2003.08005.x. [DOI] [PubMed] [Google Scholar]
- 18.Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R, Kanter K. Results of the U.S. Multicenter Pivotal Study of the HELEX Septal Occluder for Percutaneous Closure of Secundum Atrial Septal Defects. J Am Coll Cardiol. 2007;49:2215–2221. doi: 10.1016/j.jacc.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Latson LA, Jones TK, Jacobson J, Zahn E, Rhodes JF. Analysis of factors related to successful transcatheter closure of secundum atrial septal defects using the HELEX septal occluder. Am Heart J. 2006;151:1129.e7–1129.e11. doi: 10.1016/j.ahj.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Correa R, Zahn E, Khan D. Mid-term Outcomes of the Helex Septal Occluder for Percutaneous Closure of Secundum Atrial Septal Defects. Cong heart dis. 2013;8:428–433. doi: 10.1111/chd.12043. [DOI] [PubMed] [Google Scholar]
- 21*.de Hemptinne Q, Horlick EM, Osten MD, Millán X, Tadros V-X, Pighi M, Gonzalez Barlatey F, Alnasser SM, Miró J, Asgar AW, et al. Initial clinical experience with the GORE(®) CARDIOFORM ASD occluder for transcatheter atrial septal defect closure. Catheter Cardiovasc Interv. 2017 doi: 10.1002/ccd.26907. This case series is the first published series describing use of both Gore Cardioform devices in 24 patients in three centers in Canada, demonstrating good safety and efficacy over a range of patient and defect sizes. At this time it is the only published series describing the use of these devices. [DOI] [PubMed] [Google Scholar]
- 22.Moore JW, Vincent RN, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Li Y, Ringel R, et al. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the national cardiovascular data registry. J Am Coll Cardiol. 2014;64:2439–2451. doi: 10.1016/j.jacc.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 24*.Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc Interv. 2016;9:79–86. doi: 10.1016/j.jcin.2015.09.028. Multicenter retrospective cohort study using data from the Pediatric Health Information Systems Database (PHIS) to compare the relative cost of operative and transcatheter closure of ASD, demonstrating convincingly the moderate magnitude but consistent cost savings associated with transcatheter closure. [DOI] [PubMed] [Google Scholar]
- 25.Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol. 2004;94:690–692. doi: 10.1016/j.amjcard.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Hughes ML, Maskell G, Goh TH, Wilkinson JL. Prospective comparison of costs and short term health outcomes of surgical versus device closure of atrial septal defect in children. Heart. 2002;88:67–70. doi: 10.1136/heart.88.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker SS, O’Laughlin MP, Jollis JG, Harrison JK, Sanders SP, Li JS. Cost implications of closure of atrial septal defect. Catheter Cardiovasc Interv. 2001;55:83–87. doi: 10.1002/ccd.10079. [DOI] [PubMed] [Google Scholar]
- 28.Thomson JDR. Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and cost. Heart. 2002;87:466–469. doi: 10.1136/heart.87.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacha E, Kalfa D. Minimally invasive paediatric cardiac surgery. Nat Rev Cardiol. 2014;11:24–34. doi: 10.1038/nrcardio.2013.168. [DOI] [PubMed] [Google Scholar]
- 30.Burkhart HM, Suri RM. Minimally invasive video assisted surgical closure of secundum atrial septal defect. Ann Cardiothorac Surg. 2017;6:60–63. doi: 10.21037/acs.2017.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Kodaira M, Kawamura A, Okamoto K, Kanazawa H, Minakata Y, Murata M, Shimizu H, Fukuda K. Comparison of Clinical Outcomes After Transcatheter vs. Minimally Invasive Cardiac Surgery Closure for Atrial Septal Defect. Circ J. 2017;81:543–551. doi: 10.1253/circj.CJ-16-0904. [DOI] [PubMed] [Google Scholar]
- 32**.Schneeberger Y, Schaefer A, Conradi L, Brickwedel J, Reichenspurner H, Kozlik-Feldmann R, Detter C. Minimally invasive endoscopic surgery versus catheter-based device occlusion for atrial septal defects in adults: reconsideration of the standard of care. Interactive CardioVascular and Thoracic Surgery. 2016 doi: 10.1093/icvts/ivw366. The two largest series of minimally invasive cardiac surgery (MICS) for operative ASD compared with transcatheter closure, demonstrating feasibility and safety similar to the conventional operative approach. In these small series in which risk adjustment is not possible there were no significant differences in the risk of mortality between MICS and TC-ASD closure. Length of stay following MICS continued to exceed that following TC-ASD. [DOI] [PubMed] [Google Scholar]
- 33.Lewis WR, Peterson ED, Cannon CP, Super DM, LaBresh KA, Quealy K, Liang L, Fonarow GC. An organized approach to improvement in guideline adherence for acute myocardial infarction: results with the Get With The Guidelines quality improvement program. Arch Intern Med. 2008;168:1813–1819. doi: 10.1001/archinte.168.16.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonarow GC, Yancy CW, Heywood JT, ADHERE Scientific Advisory Committee, Study Group, and Investigators Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 35.Arnold SV, Spertus JA, Masoudi FA, Daugherty SL, Maddox TM, Li Y, Dodson JA, Chan PS. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–1801. doi: 10.1016/j.jacc.2013.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63:2876–2877. doi: 10.1016/j.jacc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Hira RS, Kennedy K, Nambi V, Jneid H, Alam M, Basra SS, Ho PM, Deswal A, Ballantyne CM, Petersen LA, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65:111–121. doi: 10.1016/j.jacc.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice-level variation in use of recommended medications among outpatients with heart failure: Insights from the NCDR PINNACLE program. Circulation: Heart Failure. 2013;6:1132–1138. doi: 10.1161/CIRCHEARTFAILURE.113.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2014;63:539–546. doi: 10.1016/j.jacc.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program) Am J Cardiol. 2011;108:1136–1140. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole-Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- 43.Engelfriet P, Tijssen J, Kaemmerer H, Gatzoulis MA, Boersma E, Oechslin E, Thaulow E, Popelová J, Moons P, Meijboom F, et al. Adherence to guidelines in the clinical care for adults with congenital heart disease: the Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2006;27:737–745. doi: 10.1093/eurheartj/ehi718. [DOI] [PubMed] [Google Scholar]
- 44.Angoff GH, Kane DA, Giddins N, Paris YM. Regional implementation of a pediatric cardiology chest pain guideline using SCAMPs methodology. Pediatrics. 2013 doi: 10.1542/peds.2013-0086/-/DCSupplemental. [DOI] [PubMed] [Google Scholar]
- 45.Harahsheh AS, O’Byrne ML, Pastor B. Pediatric Chest Pain—Low-Probability Referral. Clin Pediatr. 2017 doi: 10.1177/0009922816684605. e-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farias M, Friedman KG, Powell AJ, de Ferranti SD, Marshall AC, Brown DW, Kulik TJ. Dynamic evolution of practice guidelines: analysis of deviations from assessment and management plans. Pediatrics. 2012;130:93–98. doi: 10.1542/peds.2011-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman KG, Rathod RH, Farias M, Graham D, Powell AJ, Fulton DR, Newburger JW, Colan SD, Jenkins KJ, Lock JE. Resource utilization after introduction of a standardized clinical assessment and management plan. Cong heart dis. 2010;5:374–381. doi: 10.1111/j.1747-0803.2010.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 49.Vincent RN, Moore J, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Ringel R, Rome J, et al. Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiology in the Young. 2016;26:70–78. doi: 10.1017/S1047951114002637. [DOI] [PubMed] [Google Scholar]
- 50.Martin GR, Beekman RH, Ing FF, Jenkins KJ, McKay CR, Moore JW, Ringel RE, Rome JJ, Ruiz CE, Vincent RN. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Sem Thorac and Cardiovasc Surg: Ped Card Surg Annual. 2010;13:20–25. doi: 10.1053/j.pcsu.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 51**.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variability of Practice Patterns in Device Closure of Atrial Septal Defects and Patent Ductus Arteriosus: An Analysis of Data From the Impact® Registry. Circulation. 2016;134(Suppl 1):A15978–A15978. doi: 10.1016/j.ahj.2017.10.018. Observational study using data from the IMPACT® registry, studying for the first time practice variation in the pediatric and congenital catheterization laboratory, demonstrating 1) significant inter-hospital variation in the distribution of indications for ASD closure, and 2) that the proportion of cases whose indication was right ventricular volume overload with a small shunt differed significantly based on hospital characteristics. Specifically this proportion was higher in hospitals with lower ASD closure volume and in hospitals with a larger proportion of adult patients in their catheterization laboratory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preventza O, Sampath-Kumar S, Wasnick J, Gold JP. Late cardiac perforation following transcatheter atrial septal defect closure. Ann Thoracic Surg. 2004;77:1435–1437. doi: 10.1016/S0003-4975(03)00901-9. [DOI] [PubMed] [Google Scholar]
- 53.Chun DS, Turrentine MW, Moustapha A, Hoyer MH. Development of aorta-to-right atrial fistula following closure of secundum atrial septal defect using the Amplatzer septal occluder. Cathet Cardiovasc Intervent. 2003;58:246–251. doi: 10.1002/ccd.10434. [DOI] [PubMed] [Google Scholar]
- 54.Trepels T, Zeplin H, Sievert H, Billinger K, Krumsdorf U, Zadan E, Horvath K. Cardiac perforation following transcatheter PFO closure. Cathet Cardiovasc Intervent. 2003;58:111–113. doi: 10.1002/ccd.10371. [DOI] [PubMed] [Google Scholar]
- 55.Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Cathet Cardiovasc Intervent. 2004;63:496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 56.Amplatzer Septal Occluder Delivery System: Instructions for Use. professional.sjm.com. 2012 [no volume] [Google Scholar]
- 57.Rare Serious Erosion Events Associated with St. Jude Amplatzer Atrial Septal Occluder (ASO) [Internet] United States Food and Drug Administration; 2013. [Google Scholar]
- 58.Mallula K, Amin Z. Recent Changes in Instructions for Use for the Amplatzer Atrial Septal Defect Occluder: How to Incorporate These Changes While Using Transesophageal Echocardiography or Intracardiac Echocardiography? Pediatr Cardiol. 2012;33:995–1000. doi: 10.1007/s00246-012-0323-7. [DOI] [PubMed] [Google Scholar]
- 59.Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF. Percutaneous Atrial Septal Defect Closure in Infants and Toddlers: Predictors of Success. Pediatr Cardiol. 2012;34:220–225. doi: 10.1007/s00246-012-0413-6. [DOI] [PubMed] [Google Scholar]
- 60.O’Byrne ML, Glatz AC, Goldberg DJ, Shinohara R, Dori Y, Rome JJ, Gillespie MJ. Accuracy of Transthoracic Echocardiography in Assessing Retro-aortic Rim prior to Device Closure of Atrial Septal Defects. Cong heart dis. 2014 doi: 10.1111/chd.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butera G, Romagnoli E, Carminati M, Chessa M, Piazza L, Negura D, Giamberti A, Abella R, Pomè G, Condoluci C, et al. Treatment of isolated secundum atrial septal defects: Impact of age and defect morphology in 1,013 consecutive patients. Am Heart J. 2008;156:706–712. doi: 10.1016/j.ahj.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 62.DiBardino DJ, McElhinney DB, Kaza AK, Mayer JE., Jr Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg. 2009;137:1334–1341. doi: 10.1016/j.jtcvs.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delaney JW, Li JS, Rhodes JF. Major Complications Associated with Transcatheter Atrial Septal Occluder Implantation: A Review of the Medical Literature and the Manufacturer and User Facility Device Experience (MAUDE) Database. Cong heart dis. 2007;2:1–9. doi: 10.1111/j.1747-0803.2007.00107.x. [DOI] [PubMed] [Google Scholar]
- 64.DiBardino DJ, Mayer JE., Jr Continued controversy regarding adverse events after Amplatzer septal device closure: Mass hysteria or tip of the iceberg? J Thorac Cardiovasc Surg. 2011;142:222–223. doi: 10.1016/j.jtcvs.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Diab K, Kenny D, Hijazi ZM. Erosions, erosions, and erosions! Device closure of atrial septal defects: How safe is safe? Cathet Cardiovasc Intervent. 2012;80:168–174. doi: 10.1002/ccd.24517. [DOI] [PubMed] [Google Scholar]
- 66.Moore J, Hegde S, El-Said H, Beekman R, Benson L, Bergersen L, Holzer R, Jenkins K, Ringel R, Rome J, et al. Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv. 2013;6:433–442. doi: 10.1016/j.jcin.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 67**.McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation. 2016;133:1738–1746. doi: 10.1161/CIRCULATIONAHA.115.019987. Case control study using data from Erosion Board and Amplatzer Septal Occluder Post-Approval Study, which is the largest series of erosion cases in the literature with which to assess modifiable and non-modifiable risk factors for ASO device erosion. It reiterated findings from previous studies that cases with erosion were more likely to have had deficient retro-aortic and superior vena cava rims, larger defects (relative to septal length or patient size), and to have a relatively over-sized device. [DOI] [PubMed] [Google Scholar]
- 68.Taggart NW, Dearani JA, Hagler DJ. Late erosion of an Amplatzer septal occluder device 6 years after placement. J Thorac Cardiovasc Surg. 2011;142:221–222. doi: 10.1016/j.jtcvs.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Roberts WT, Parmar J, Rajathurai T. Very late erosion of Amplatzer septal occluder device presenting as pericardial pain and effusion 8 years after placement. Catheter Cardiovasc Interv. 2013;82:E592–4. doi: 10.1002/ccd.24755. [DOI] [PubMed] [Google Scholar]
- 70.Amin Z. Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. Catheter Cardiovasc Interv. 2014;83:84–92. doi: 10.1002/ccd.25175. [DOI] [PubMed] [Google Scholar]
- 71.Mitchelson B, O’Donnell C, Ruygrok P, Wright J, Stirling J, Wilson N. Transcatheter closure of secundum atrial septal defects: has fear of device erosion altered outcomes? Cardiology in the Young. 2017 doi: 10.1017/S1047951116002663. [DOI] [PubMed] [Google Scholar]
- 72.Wyss Y, Quandt D, Weber R, Stiasny B, Weber B, Knirsch W, Kretschmar O. Interventional Closure of Secundum Type Atrial Septal Defects in Infants Less Than 10 Kilograms: Indications and Procedural Outcome. J Interv Cardiol. 2016;29:646–653. doi: 10.1111/joic.12328. [DOI] [PubMed] [Google Scholar]
- 73.Abu-Tair T, Wiethoff CM, Kehr J, Kuroczynski W, Kampmann C. Transcatheter Closure of Atrial Septal Defects using the GORE® Septal Occluder in Children Less Than 10 kg of Body Weight. Pediatr Cardiol. 2016;37:778–783. doi: 10.1007/s00246-016-1350-6. [DOI] [PubMed] [Google Scholar]
- 74.Tanghöj G, Odermarsky M, Naumburg E, Liuba P. Early Complications After Percutaneous Closure of Atrial Septal Defect in Infants with Procedural Weight Less than 15 kg. Pediatr Cardiol. 2016;38:255–263. doi: 10.1007/s00246-016-1507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer G, Smevik B, Kramer HH, Bjørnstad PG. Catheter-based closure of atrial septal defects in the oval fossa with the Amplatzer® device in patients in their first or second year of life. Cathet Cardiovasc Intervent. 2009;73:949–955. doi: 10.1002/ccd.21866. [DOI] [PubMed] [Google Scholar]
- 76.Fraisse A, Losay J, Bourlon F, Agnoletti G, Lusson JR, Godart F, De Geeter B, Petit J, Piechaud JF. Efficiency of transcatheter closure of atrial septal defects in small and symptomatic children. Cardiology in the Young. 2008;18:343–347. doi: 10.1017/S1047951108002291. [DOI] [PubMed] [Google Scholar]
- 77.Campbel M. Natural history of atrial septal defect. Br Heart J. 1970;32:820–826. doi: 10.1136/hrt.32.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craig RJ, Selzer A. Natural History and Prognosis of Atrial Septal Defect. Circulation. 1968;37:805–815. doi: 10.1161/01.cir.37.5.805. [DOI] [PubMed] [Google Scholar]
- 79.Andersen M, Lyngborg K, Moller I, Wennevold A. The natural history of small atrial septal defects: Long-term follow-up with serial heart catheterizations. Am Heart J. 1976;92:302–307. doi: 10.1016/s0002-8703(76)80111-1. [DOI] [PubMed] [Google Scholar]
- 80.Radzik D, Davignon A, van Doesburg N, Fournier A, Marchand T, Ducharme G. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol. 1993;22:851–853. doi: 10.1016/0735-1097(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 81.Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C. Predictors of Spontaneous Closure of Isolated Secundum Atrial Septal Defect in Children: A Longitudinal Study. Pediatrics. 2006;118:1560–1565. doi: 10.1542/peds.2005-3037. [DOI] [PubMed] [Google Scholar]
- 82.Helgason H, Jonsdottir G. Spontaneous closure of atrial septal defects. Pediatr Cardiol. 1999;20:195–1999. doi: 10.1007/s002469900439. [DOI] [PubMed] [Google Scholar]
- 83.McMahon CJ, Feltes TF, Fraley JK, Bricker JT, Grifka RG, Tortoriello TA, Blake R, Bezold LI. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256–259. doi: 10.1136/heart.87.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]


