Abstract
Background
Practice variation is a potentially important measure of healthcare quality. The IMPACT® registry provides a representative national sample with which to study practice variation in trans-catheter interventions for congenital heart disease.
Methods
We studied cases for closure of atrial septal defect (ASD) and patent ductus arteriosus (PDA) in IMPACT® between 1/1/2011 and 9/30/2015, using hierarchical multivariate models studying 1) the distribution of indications for closure and 2) in patients whose indication for closure was left (LVVO) or right ventricular volume overload(RVVO), the factors influencing probability of closure of a small defect (either in size or in terms of the magnitude of shunt).
Results
Over the study period, 5,233 PDA and 4,459 ASD cases were performed at 77 hospitals. The indications for ASD closure were RVVO in 84% and stroke prevention in 13%. Indications for PDA closure were LVVO in 57%, endocarditis prevention in 36%, and pulmonary hypertension in 7%. There was statistically significant variability in indications between hospitals for PDA and ASD procedures (median rate ratio (MRR): 1.3 and 1.1; both p<0.001). The proportion of cases for volume overload with a Qp:Qs<1.5:1 decreased with increasing PDA and ASD procedural volume (p=0.04 and 0.05). For ASD, the proportion was higher at hospitals with a larger proportion of adult cases (p=0.0007). There was significant variation in practice in the risk of closing PDA<2mm for LVVO (MRR: 1.4, p<0.001).
Conclusion
There is measurable variation in transcatheter closure of PDA and ASD. Further research is necessary to study whether this affects outcomes or resource utilization.
Keywords: Outcomes research, Heart catheterization, Health services research, Pediatric cardiology, Congenital heart disease
INTRODUCTION
Practice variation, defined as observed differences in health care service delivery that are not otherwise explained by patient illness, medical need, or evidence-based recommendations, is a novel outcome measure in the study of interventional cardiology for congenital heart disease. It has become an important measure of quality across many fields of medicine1, and reducing variability in practice variation has been shown to improve both resource utilization and traditional patient outcomes for adult cardiac patients in both inpatient2,3 and outpatient4–11 settings, as well as adult12 and pediatric13–17 patients with congenital heart disease. Measuring variation in real-world practice identifies aspects of care of potential interest for future research and means of improving quality of care.
In the field of pediatric or congenital interventional cardiology, practice guidelines have been published18. However, there may continue to be large center- and practitioner-level variability in the conduct of transcatheter interventions for congenital heart disease. To our knowledge, no studies have attempted to measure this practice variation. Previously, the dearth of standardized data about individual procedures from a large, diverse pool of centers was an obstacle to this type of research. The Improving Adult and Congenital Treatment® (IMPACT®) Registry, a multi-center clinical registry of catheterization procedures performed at hospitals in the United States provides an opportunity to address these questions. We performed a retrospective multicenter observational study to measure practice variation in device closure of ostium secundum atrial septal defect (ASD) and patent ductus arteriosus (PDA).
We sought to measure practice variation in two ways. First, we studied the distribution of indications for ASD and PDA closure. Second, in cases with ventricular volume overload as the indication, we measured the proportion in which there was a relatively small defect (either anatomically or in terms of the ratio of systemic to pulmonary blood flow). Variation was measured in two ways: 1) whether hospital characteristics appeared to influence outcomes of interest and 2) whether there was otherwise unaccounted for inter-hospital variation. Presence of otherwise unexplained variation in practice patterns not explained by patient factors identifies aspects of care (whether at the level of referral patterns or at the individual patient level) where there is uncertainty and where there is potential to improve healthcare quality.
METHODS
Data source
IMPACT® is a clinical registry funded by the American College of Cardiology and managed by the National Cardiovascular Data Registry with data from 86 pediatric and general hospitals performing cardiac catheterizations in children and adults with congenital heart disease at the time of this analysis19–21 (Figure 1). Participating centers collect information on all patients undergoing cardiac catheterization, including patient demographics, medical/surgical history, procedural information and adverse events through hospital discharge. Data are recorded using standardized data elements and definitions. The database is subject to rigorous quality assurance standards22. Auditing procedures are still being developed23. The current study used data from IMPACT v1.0.1. The project uses de-identified data analyzed and does not represent human subjects research in accordance with the Common Rule (45 CFR 46.102(f)).
Figure 1. IMproving Pediatric and Adult Congenital Treatment (IMPACT®) Registry.
This map depicts the distribution of hospitals in the IMPACT® Registry during the study period. At that time, there were 86 pediatric and general hospitals contributing data distributed across the United States. Individual hospitals are marked with red circles. For clarity, census regions are also identified: Midwest (blue), Northeast (yellow), South (white), and West (green).
Funding sources
Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). The analysis in this manuscript was funded by the American College of Cardiology and National Cardiovascular Data Registry. The proposed project and manuscript were reviewed by IMPACT® Research and Publications Committee. The funding agencies had no role in the drafting of the manuscript or influencing its content. This manuscript represents the opinion of the authors alone.
Study population and study procedures
The study population was identified by direct query of IMPACT® by analysts at Mid America Heart Institute. Subjects of all ages who underwent transcatheter closure of an isolated ASD or PDA recorded in IMPACT® between January 1, 2011 and September 30, 2015 were included in the study. Cases from hospitals with >15% incomplete data, contributing data for less than 6 months or at which less than 10 total ASD or PDA procedures were performed over the study period were excluded from analysis as described previously21,27. Individual cases were excluded if the ASD or PDA was not treated. They were also excluded if the patient had previous cardiac surgery, additional interventions during the same catheterization, a diagnosis of single ventricle heart disease, or other surgical or transcatheter procedures during the same admission. Analysis was restricted to isolated ASD and PDA to establish a homogenous study population in which differences in the practice of treatment of these lesions without confounding from a small number of more complex patients. Including these patients, might be expected to erroneously inflate estimates of practice variation. Because the indication was vital for analysis, cases with the indication missing were also excluded.
Data collected included demographic data, pre-catheterization clinical history, indications for closure, hemodynamics, and details about the conduct of the case. Characteristics about hospitals collected included center volume, percentage of procedures performed in patients older than 18 years, geographic region (Northeast, Midwest, South, or West, Figure 1), hospital type (government, private/community, or university) and hospital setting (rural, suburban, or urban). Center volumes are an annualized volume over the period that each hospital has contributed data to IMPACT®. Major adverse events were defined as previously described24,25.
Data analysis
Analyses of ASD and PDA cases were performed separately. Descriptive statistics were calculated with categorical variables expressed as counts and percentages with 95% confidence intervals (CI). Continues variables were expressed as mean ± standard deviation or median (range, interquartile range) as appropriate.
To study the distribution of indications for the procedure, we reported the distribution of patient- and hospital-level characteristics by indication for each procedure. Differences in the distributions of these characteristics were assessed using analysis of variance, Kruskal-Wallis, or Chi-square tests. To assess for significant practice variation, we calculated a series of hierarchical multivariate models adjusting for patient-level characteristics. Patient-level covariates included as fixed effects were age, sex, prior catheterization, genetic condition, chronic lung disease, coagulopathy, diabetes mellitus, hepatic disease, renal insufficiency, sickle cell disease, prior stroke, and seizure disorder. The number of covariates was limited to 1 covariate for every 10 events to prevent over-fitting the model26. A random intercept for each hospital was included in the model to account for covariance. To avoid bias, no model refinement was performed. A series of models was created to assess for the effects of hospital-characteristics (region, setting, procedural volume, proportion of adult cases, and teaching status) on outcome and to assess for additional inter-hospital variation. To accomplish this last goal, a median rate ratio (MRR) was calculated. MRR has been used in previous studies to measure otherwise unaccounted for inter-hospital variation9. In a sample of multiple hospitals, MRR reflects the probability that two identical patients treated at two of the sample hospitals selected at random would receive different care7. The MRR value is always ≥1. As an example of how this value is interpreted, a MRR = 1.2 means that two identical patients would have a 20% probability of being treated differently at two randomly selected hospitals. An MRR ≥1.2 has been used previously as a threshold for large magnitude variation. Statistical significance is expressed with conventional p values and confidence intervals9. A secondary analysis was performed adding ASD static diameter to the multivariate model, to assess the degree to which it influenced variation.
For ASD and PDA interventions, the majority of cases listed suspected ventricular volume overload (of the right ventricle in ASD and the left ventricle in PDA) as the indication. We investigated whether there was variation in the likelihood of attributing volume overload to a “small defect.” A “small defect” was defined first by the diameter of the defect (ASD static dimension <5mm or PDA minimal luminal diameter <2mm as conservative estimates) and in a second analysis by the magnitude of the shunt, calculated using the Fick principle to calculate the ratio of pulmonary to systemic blood flow (Qp:Qs) with 1.5:1 chosen as the threshold. The rate at which small defects are closed reflects how aggressive an individual practitioner or hospital system is in pursuing transcatheter closure of defects based on the outpatient assessment of the patient. Recommendations regarding closure are not specific about how one should define ventricular volume overload, so this decision is a potential aspect of care in which significant variation might be expected. We acknowledge that the thresholds are somewhat arbitrary. However, the distribution of both defect size and Qp:Qs are such that changing the threshold should not bias the estimates of inter-hospital variability or those of systematic differences in practice associated with hospital characteristics. We chose to analyze size of the defect using both anatomic size and magnitude of the shunt, because both measures are imperfect measures. However, they provide complementary analyses, and if results were consistent, inclusion of both would support the validity of the analyses in spite of the acknowledged limitations of each individually. The proportions of cases with small shunts at each center were calculated and reported. Next, a series of hierarchical multivariate models were calculated, adjusted for patient characteristics, to assess whether hospital characteristics, such as census region, hospital setting, teaching status, procedural volume, ASD/PDA closure volume, and for ASD the proportion of hospital catheterizations performed in patients older than 18 years, affected risk and whether there was significant inter-hospital variation (measured by calculating the MRR as above).
To provide an estimate of the risk associated with closing smaller defects, an additional secondary analyses was performed. Specifically, the risk of major adverse events for ASD and PDA cases with small defects (defined either by anatomic size or Qp:Qs) was measured and compared to the risk in subjects with larger defects, using a chi-squared test.
A threshold for statistical significance was set at p<0.05. All analyses were performed using SAS 9.4 (Cary, NC, USA)
RESULTS
Study population
Between 1/1/2011 and 9/30/2015, 5,233 PDA closure procedures and 4,549 ASD cases meeting inclusion criteria were performed at 77 hospitals (Figure 2). These accounted for 13% (9782/77116) of all cases in the IMPACT® registry over this period. PDA patients were 64% female, 67% white, with a median age of 2 years (IQR: 0–4 years) (Table 1a). ASD patients were 63% female, 75% white, with a median age of 8 years (IQR: 4–16 years) (Table 1b).
Figure 2.

Study population
Table 1a.
Characteristics of patent ductus arteriosus subjects by indication
| Total (n=5233) | Left ventricular volume overload (n=2980) | Prevention of endocarditis (n=1861) | Pulmonary hypertension (n=392) | p | |
|---|---|---|---|---|---|
| Age (years) | 2.0 (IQR: 0–4) | 1.0 (IQR: 0.0–3.0) | 3.0 (IQR: 1–7) | 0.0 (IQR: 0–1.0) | <0.001 |
| Age >18 years | 2.5% (132) | 1.9% (44) | 3.6% (66) | 2.8% (11) | 0.001 |
| Female sex | 64% (3370) | 66% (1957) | 64% (1188) | 57% (225) | 0.004 |
| Weight (kg) | 11.7 (IQR: 8.2–18.0) | 10.5 (7.5–15.5) | 15.4 (10.8–26.2) | 6.6 (4.6–9.3) | <0.001 |
| Race | <0.001 | ||||
| White | 67% (3518) | 67% (2002) | 70% (1298) | 56% (218) | |
| Black | 18% (958) | 17% (495) | 19% (353) | 28% (110) | |
| Other | 14% (757) | 16% (483) | 11% (210) | 16% (64) | |
| Trisomy 21 | 8% (399) | 7%(198) | 6% (118) | 21% (83) | <0.001 |
| Other genetic condition | 1% (66) | 1% (31) | 1% (22) | 3.3% (13) | <0.001 |
| Chronic lung disease | 8% (409) | 6% (186) | 3% (57) | 42% (166) | <0.001 |
| Renal insufficiency | 0.4% (22) | 0.4% (12) | 0.3% (5) | 1.3% (5) | 0.02 |
| History of stroke | 0.3% (15) | 0.3% (8) | 0.1% (2) | 1.3% (5) | <0.001 |
| Hospital status | <0.001 | ||||
| Outpatient | 52% (2720) | 51% (1506) | 60% (1116) | 25% (98) | |
| 23 hour observation | 34% (1777) | 35% (1039) | 34% (630) | 28% (108) | |
| Admit to inpatient floor | 4% (192) | 4% (127) | 3% (48) | 4% (17) | |
| Admit to ICU | 3% (176) | 4% (113) | 2% (38) | 6% (25) | |
| Return to inpatient floor | 2% (87) | 2% (59) | 0.4% (7) | 5% (21) | |
| Return to ICU | 5% (266) | 4% (127) | 1% (17) | 31% (122) | |
| Missing | 15 | 9 | 5 | 1 | |
| Anesthesia/sedation | 0.04 | ||||
| General anesthesia | 87% (4563) | 87% (2605) | 86% (1605) | 90% (353) | |
| IV sedation | 12% (629) | 12% (345) | 13% (246) | 10% (38) | |
| Other/Missing | 1% (41) | 1% (30) | 1% (10) | 0% (1) | |
| Trainee present | 55% (2873) | 57% (1683) | 53% (981) | 53% (209) | 0.03 |
| Access | <0.001 | ||||
| Arterial only | 6% (318) | 3% (98) | 11% (211) | 2% (9) | |
| Venous only | 2% (82) | 2% (47) | 1% (23) | 3% (12) | |
| Both | 92% (4830) | 95% (2834) | 87% (1625) | 94% (371) | |
| Systemic heparinization | 83% (4362) | 85% (2533) | 80% (1485) | 88% (344) | <0.001 |
| PDA minimal lumenal diameter (mm) | 2.5±1.6 | 2.7±1.5 | 1.8±1.3 | 3.2±1.9 | <0.001 |
| Anatomic type | <0.001 | ||||
| Conical | 62% (3173) | 66% (1927) | 61% (1103) | 38% (143) | |
| Window | 2% (80) | 2% (46) | 1% (27) | 2% (7) | |
| Tubular | 19% (964) | 18% (520) | 16% (296) | 39% (148) | |
| Complex | 5% (242) | 5% (138) | 5% (91) | 6% (23) | |
| Elongated | 13% (660) | 10% (308) | 16% (296) | 15% (56) | |
| Missing | 104 | 41 | 48 | 15 | |
| Ratio of pulmonary to systemic blood flow (Qp:Qs) | 1.6±1.1 (n=4225) | 1.8±1.3 (n=2406) | 1.3±0.6 (n=1309) | 1.9±1.1 (n=332) | <0.001 |
| Qp:Qs<1.5:1 | 58% (2463) | 47% (1169) | 83% (1165) | 39% (129) | <0.001 |
| Indexed pulmonary vascular resistance (WU*m2) | 1.6±1.4 | 1.4±1.1 | 1.4±1.0 | 3.6±2.6 | <0.001 |
| Hospital region | <0.001 | ||||
| Northeast | 11% (567) | 12% (352) | 9% (168) | 12% (47) | |
| Midwest | 27% (1368) | 25% (735) | 29% (528) | 27% (105) | |
| South | 44% (2239) | 42% (1228) | 47% (846) | 43% (165) | |
| West | 18% (946) | 21% (600) | 15% (275) | 18% (71) | |
| Missing | 113 | 65 | 44 | 4 | |
| Hospital setting | <0.001 | ||||
| Rural | 4% (209) | 3% (89) | 5% (97) | 6% (23) | |
| Suburban | 10% (509) | 8% (252) | 12% (219) | 10% (38) | |
| Urban | 86% (4515) | 89% (2639) | 83% (1545) | 84%(331) | |
| Hospital type | 0.50 | ||||
| Government | 2% (113) | 2% (65) | 2% (44) | 1% (4) | |
| Private/community | 48% (2493) | 48% (1425) | 47% (874) | 49% (194) | |
| University | 50% (2627) | 50% (1490) | 51% (943) | 49% (194) |
Table 1b.
Characteristics of atrial septal defect subjects by indication
| Total (n=4549) | Right ventricular volume overload (n=3793) | Chronic lung disease or recurrent respiratory infection (n=75) | Failure to thrive (n=38) | Cyanosis (n=50) | Stroke prevention (n=593) | P | |
|---|---|---|---|---|---|---|---|
| Age (years) | 8 (IQR: 4–16) | 6 (IQR: 4–14) | 3 (IQR: 1–9) | 2 (IQR: 1–3) | 17 (IQR: 4–46) | 38 (IQR: 16–54) | <0.001 |
| Age >18 years | 22% (1007) | 14% (545) | 16% (12) | 3% (1) | 50% (25) | 72% (424) | <0.001 |
| Female sex | 63% (2887) | 64% (2439) | 60% (45) | 74% (28) | 68% (34) | 58% (341) | 0.01 |
| Weight (kg) | 27 (IQR: 17–60) | 23 (IQR: 17–53) | 14 (IQR: 9–38) | 11 (IQR: 8–13) | 56 (21–78) | 72 (56–86) | <0.001 |
| Race | <0.001 | ||||||
| White | 75% (3432) | 74% (2798) | 73% (55) | 82% (31) | 96% (48) | 84% (500) | |
| Black | 11% (500) | 11% (434) | 15% (110 | 3% (1) | 4% (2) | 9% (52) | |
| Other | 14% (617) | 15% (561) | 12% (9) | 16% (6) | 0% (0) | 7% (41) | |
| Trisomy 21 | 3% (143) | 3% (131) | 11% (8) | 5% (2) | 0% (0) | 0.3% (2) | <0.001 |
| Other genetic condition | 1% (58) | 1% (55) | 1% (1) | 5% (2) | 0% (0) | 0.3% (2) | 0.18 |
| Chronic lung disease | 3% (155) | 3% (96) | 53% (40) | 5% (2) | 8% (4) | 2% (13) | <0.001 |
| Renal insufficiency | 0.8% (34) | 0.6% (21) | 3% (2) | 0% (0) | 0% (0) | 2% (11) | 0.002 |
| History of stroke | 7.6% (343) | 0.8% (30) | 0% (0) | 0% (0) | 4% (2) | 53% (311) | <0.001 |
| Hospital status | <0.001 | ||||||
| Outpatient | 20% (899) | 18% (694) | 16% (12) | 18% (7) | 14% (7) | 30% (179) | |
| 23 hour observation | 64% (2923) | 66% (2511) | 55% (41) | 55% (21) | 64% (32) | 54% (318) | |
| Admit to inpatient floor | 10% (465) | 10% (390) | 9% (7) | 13% (5) | 2% (1) | 10% (62) | |
| Admit to ICU | 4% (174) | 4% (151) | 8% (6) | 11% (4) | 8% (4) | 2% (9) | |
| Return to inpatient floor | 1% (48) | 1% (20) | 1% (1) | 3% (1) | 6% (3) | 4% (23) | |
| Return to ICU | 0.6% (29) | 0.5% (17) | 11% (8) | 0% (0) | 6% (3) | 0.2% (1) | |
| Missing | 11 | 10 | 0 | 0 | 0 | 1 | |
| Anesthesia/sedation | <0.001 | ||||||
| General anesthesia | 84.0% (3823) | 90% (3423) | 79% (59) | 95% (36) | 72% (36) | 45% (269) | |
| IV sedation | 15.5 % (705) | 9% (356) | 20% (15) | 3% (1) | 28% (14) | 54% (319) | |
| Other/Missing | 0.5% (21) | 0.4% (14) | 1% (1) | 3% (1) | 0% (0) | 1% (5) | |
| Trainee present | 56% (2525) | 59% (2227) | 47% (35) | 42% (16) | 47% (23) | 38% (224) | <0.001 |
| Access | <0.001 | ||||||
| Venous only | 60% (2719) | 58% (2190) | 61% (46) | 55% (22) | 50% (25) | 74% (437) | |
| Both | 40% (1818) | 42% (1592) | 37% (28) | 45% (17) | 50% (25) | 26% (156) | |
| Systemic heparinization | 99% (4479) | 99% (3663) | 97% (73) | 97% (45) | 100% (50) | 98% (580) | 0.44 |
| Balloon sizing performed | 83% (3752) | 85% (3209) | 57% (43) | 70% (26) | 75% (36) | 74% (438) | <0.001 |
| ASD static size | 12 (IQR: 8–16) | 13 (IQR: 9–17) | 10 (IQR: 7–15) | 10 (IQR: 7–14) | 8 (IQR: 6–10) | 8 (IQR: 5–10) | <0.001 |
| Total septal length | 34.1±10.1 | 34.1±9.5 | 26.2±9.5 | 25.7±8.2 | 33.6±11.6 | 36.4±12.8 | <0.001 |
| Ratio of pulmonary to systemic blood flow (Qp:Qs) | 1.8±0.7 | 1.8±0.7 | 1.7±0.6 | 1.9±0.6 | 1.1±0.6 | 1.3±0.6 | <0.001 |
| Qp:Qs<1.5:1 | 36% (1344) | 33% (1064) | 43% (29) | 46% (18) | 90% (11) | 74% (208) | <0.001 |
| Indexed pulmonary vascular resistance (WU*m2) | 1.1 (IQR: 0.8–1.6) | 1.1 (IQR: 0.8–1.5) | 2.0 (IQR: 1.2–3.1) | 1.5 (IQR: 1.0–1.7) | 1.9 (IQR: 1.3–2.6) | 1.4 (IQR: 1.0–2.2) | <0.001 |
| Hospital region | <0.001 | ||||||
| Northeast | 11% (499) | 13% (468) | 4% (3) | 8% (3) | 10% (5) | 3% (20) | |
| Midwest | 27% (1201) | 28% (1025) | 36% (27) | 26% (10) | 24% (12) | 21% (127) | |
| South | 42% (1840) | 39% (1437) | 27% (20) | 21% (8) | 48% (24) | 59% (351) | |
| West | 20% (881) | 20% (736) | 32% (24) | 45% (17) | 18% (9) | 16% (95) | |
| Missing | 128 | 127 | 1 | 0 | 0 | 0 | |
| Hospital setting | <0.001 | ||||||
| Rural | 4% (171) | 4% (143) | 7% (5) | 5% (2) | 4% (2) | 3% (19) | |
| Suburban | 15% (668) | 10% (385) | 15% (11) | 21% (8) | 16% (8) | 54% (318) | |
| Urban | 82% (3710) | 86% (3265) | 79% (59) | 74% (28) | 80% (40) | 43% (256) | |
| Hospital type | <0.001 | ||||||
| Government | 3% (128) | 3% (127) | 1% (1) | 0% (0) | 0% (0) | 0% (0) | |
| Private/community | 46% (2094) | 48% (1818) | 51% (38) | 55% (21) | 38% (19) | 33% (198) | |
| University | 51% (2327) | 49% (1848) | 48% (36) | 45% (17) | 62% (31) | 66% (395) |
Indications for closure
In the PDA cohort, the indication was LVVO in 57% (n=2980), prevention of spontaneous bacterial endocarditis (SBE) in 36%% (n=1861), and pulmonary hypertension (PH) in 7% (n=392). Clustering cases by their indications demonstrates significant differences in both subject characteristics and the characteristics of hospitals (Table 1a). Relative to other indications, patients undergoing PDA closure for PH were more likely to be younger and lower in weight (p<0.001 for both). They were more likely to have trisomy 21, other genetic syndromes, chronic lung disease, and a tubular (type C) PDA (for all p<0.001). They were also more likely to return to an ICU after catheterization. For cases where the PDA was closed to prevent SBE, the patients were older, had smaller PDA minimal luminal diameters, and a smaller Qp:Qs (p<0.001 for all).
PDA closure with SBE prevention as the indication were less common than expected in the Northeast and more common in the Midwest and South (p<0.001). Similarly, a larger proportion of SBE and PH cases were performed in suburban hospitals with a larger proportion of LVVO cases performed in urban hospitals than expected (p<0.001).
After adjusting for patient level characteristics, significant differences were noted in the distribution of case indication by hospital setting with LVVO significantly more likely to be the indication for PDA closure at urban hospitals compared to those in suburban and rural locations (p<0.001) (Table 2a), while census region, teaching status, and proportion of overall cases in adults did not affect the distribution of indications. There was significant between-center variation (MRR: 1.3 (95% CI 1.2–1.4), p<0.001).
Table 2a.
Distribution of indications for device closure of PDA
| Variable | Comparison | OR (95% CI) | p-value | Global p-value |
|---|---|---|---|---|
| Site | Median rate ratio | 1.3 (1.2–1.4) | --- | <0.001 |
| Census Region | Northeast vs. West | 1.0 (0.8–1.3) | 0.87 | 0.39 |
| Midwest vs. West | 0.9 (0.7–1.1) | 0.22 | ||
| South vs. West | 0.9 (0.7–1.1) | 0.22 | ||
| Northeast vs. South | 1.2 (0.9–1.5) | 0.21 | ||
| Midwest vs. South | 1.0 (0.8–1.2) | 0.91 | ||
| Northeast vs. Midwest | 1.2 (0.9–1.5) | 0.21 | ||
| Hospital setting | Rural vs. Suburbs | 0.8 (0.7–1.1) | 0.15 | 0.001 |
| Urban vs. Suburbs | 1.2 (1.0–1.3) | 0.03 | ||
| Rural vs. Urban | 0.7 (0.6–0.9) | 0.02 | ||
| Teaching status | Yes vs. No | 1.1 (0.9–1.4) | --- | 0.53 |
| Proportion of cases in adults | Low vs. High | 1.0 (0.7–1.4) | 0.98 | 0.33 |
| Moderate vs. High | 1.1 (0.7–1.5) | 0.72 |
Each row represents a separate model adjusted for subject age, previous catheterization, genetic condition, chronic lung disease, coagulation disorder, diabetes mellitus, hepatic disease, renal insufficiency, seizures, sickle cell disease, prior stroke.
The odds ratio depicts the relative likelihood of having the indication of LVVO for closure (vs. all others).
In the ASD cohort, the indication was RVVO in 83% (n=3793), stroke prevention in 13% (n=593), chronic lung disease or recurrent respiratory infections in 2% (n=75), cyanosis in 1% (n=50), and failure to thrive in 1% (n=38). Similar to the PDA cohort, separation of the cohort by indication revealed significant differences in both subject and hospital characteristics (Table 1b). Relative to RVVO cases, cases for cyanosis and stroke prevention were performed in older, heavier patients, with smaller ASD size, and lower Qp:Qs (p<0.001 for all). Chronic lung disease cases had a higher proportion of trisomy 21 with higher indexed pulmonary vascular resistance (PVRI) (both p<0.001). A disproportionate percentage of failure to thrive, cyanosis, and stroke prevention cases were performed in white patients (p<0.001). In terms of the conduct of the procedure, balloon sizing was performed at a much lower rate in chronic lung disease patients (p<0.001), who were also much more likely to return to an ICU (p<0.001).
In terms of hospital characteristics, failure to thrive and chronic lung disease cases were over-represented in the West census region, while stroke prevention and cyanosis cases were more prevalent in the South census region. Stroke prevention cases were also more prevalent in suburban hospitals (vs. rural or urban hospitals p<0.001) and university hospitals (as opposed to government or private hospitals, p<0.001).
After adjusting for patient characteristics, hospital setting was associated with differences in indication (p=0.01), with RVVO being significantly more likely to be the indication for ASD closure in an urban hospital compared to a suburban hospital (Table 2b). In addition, though census region was not significantly influential overall, there was a relatively large magnitude difference between Northeast and South regions (OR: 1.2, 95% CI: 1.0–1.3, p=0.02), with RVVO being the more likely indication provided in the Northeast. Between-hospital variability was statistically significant but relatively small in magnitude (MRR: 1.1, p<0.001). Other hospital characteristics were not associated with significant differences in the distribution of indications. With additional adjustment for static ASD diameter, census region (p=0.04) and hospital setting (p=0.03) were both associated with significant variability in distribution of indications. Though the magnitude of the MRR was similar (1.04) the measurement of inter-center variability was no longer significant (p=0.07) (Supplementary Table 1).
Table 2b.
Distribution of indications for device closure of ASD
| Variable | Comparison | OR (95% CI) | p-value | Global p-value |
|---|---|---|---|---|
| Site | Median rate ratio | 1.1 (1.01–1.1) | --- | <0.001 |
| Census Region | Northeast vs. West | 1.1 (1.0–1.3) | 0.08 | 0.13 |
| Midwest vs. West | 1.0 (0.9–1.2) | 0.49 | ||
| South vs. West | 1.0 (0.9–1.1) | 0.68 | ||
| Northeast vs. South | 1.2 (1.0–1.3) | 0.02 | ||
| Midwest vs. South | 1.1 (1.0–1.2) | 0.21 | ||
| Northeast vs. Midwest | 1.1 (1.0–1.2) | 0.20 | ||
| Hospital setting | Rural vs. Suburbs | 1.2 (1.0–1.4) | 0.10 | 0.01 |
| Urban vs. Suburbs | 1.2 (1.1–1.3) | 0.003 | ||
| Rural vs. Urban | 1.0 (0.8–1.2) | 0.95 | ||
| Teaching status | Yes vs. No | 1.0 (0.9–1.2) | --- | 0.81 |
| Proportion of ASD cases in adults | Low vs. High | 1.1 (1.0–1.2) | 0.13 | 0.32 |
| Moderate vs. High | 1.1 (1.0–1.2) | 0.21 | ||
| Proportion of all cases in adults | Low vs. High | 1.2 (0.9–1.4) | 0.23 | 0.46 |
| Moderate vs. High | 1.1 (0.9–1.3) | 0.32 |
Each row represents a separate model adjusted for subject age, previous catheterization, genetic condition, chronic lung disease, coagulation disorder, diabetes mellitus, hepatic disease, renal insufficiency, seizures, sickle cell disease, prior stroke.
The odds ratio depicts the relative likelihood of having the indication of RVVO for closure (vs. all others).
Volume overload in patients with small defects and low magnitude Qp:Qs
In cases with the indication of volume overload, we measured the proportion of cases that had either a relatively small magnitude shunt (Qp:Qs<1.5:1) or a small defect (PDA minimal luminal diameter<2mm or ASD static dimension <5mm). In PDA cases for LVVO, 47% were performed in subjects with a Qp:Qs <1.5, compared to 33% in ASD cases whose indication was RVVO (p<0.001). In multivariable models, after adjusting for subject sex, age, and insurance type, the likelihood of closure of a PDA with a Qp:Qs<1.5 decreased with increasing PDA closure procedural volume (OR: 0.87 for each additional 10 procedures, p=0.04) (Table 3a). Similarly, the likelihood of closure of an ASD in patient with Qp:Qs<1.5 was decreased with increasing ASD procedural volume (OR: 0.91 per 10 procedures, p=0.05), while the risk increased in centers with increasing annual proportional volume of adults (OR: 1.16, p=0.0007) (Table 3b).
Table 3a.
Multivariate model for predicting a Qp:Qs <1.5:1 in PDA closed with LVVO as the indication
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Male sex | 1.37 | 1.11–1.69 | 0.003 |
| Age (per 10 years) | 2.74 | 2.08–3.61 | <0.001 |
| Private insurance vs. public insurance | 1.09 | 0.89–1.33 | 0.40 |
| PDA procedural volume (per 10 procedures) | 0.87 | 0.77–1.00 | 0.04 |
| Proportion of procedures performed in adults (per 10% adult patients) | 0.92 | 0.76–1.12 | 0.43 |
Table 3b.
Multivariate model for predicting a Qp:Qs <1.5:1 in ASD closed with RVVO as the indication
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Male sex | 1.18 | 1.00–1.39 | 0.05 |
| Age (per 10 years) | 0.97 | 0.91–1.04 | 0.38 |
| Private insurance vs. public insurance | 1.22 | 0.82–1.14 | 0.65 |
| ASD procedural volume (per 10 procedures) | 0.91 | 0.83–1.00 | 0.05 |
| Proportion of procedures performed in adults (per 10% adult patients) | 1.16 | 1.06–1.26 | 0.0007 |
This can also be represented by the percentage of cases performed in patients with anatomically small lesions (Figure 3). For PDA cases, 30% of hospitals performed device closure of small defects (for the indication of volume overload) in >30% of cases. For ASD cases, only one of the 77 hospitals in the study sample had a rate of small defects closed >10%. This was reflected in multivariable models (Supplementary Table 2a and 2b). After adjusting for age and sex, there was significant variability by centers for PDA (MRR 1.4 p<0.001) but not for ASD (p=0.17). In the same analysis, increasing hospital catheterization volume decreased the probability of closing a small PDA for volume overload (OR: 0.97 for each increment of 50 cases, 95% CI: 0.95–0.99, p=0.02). Other center factors were not associated with the risk of device closing small PDA or ASD.
Figure 3. Percentage of cases for volume overload with small defects at each center.
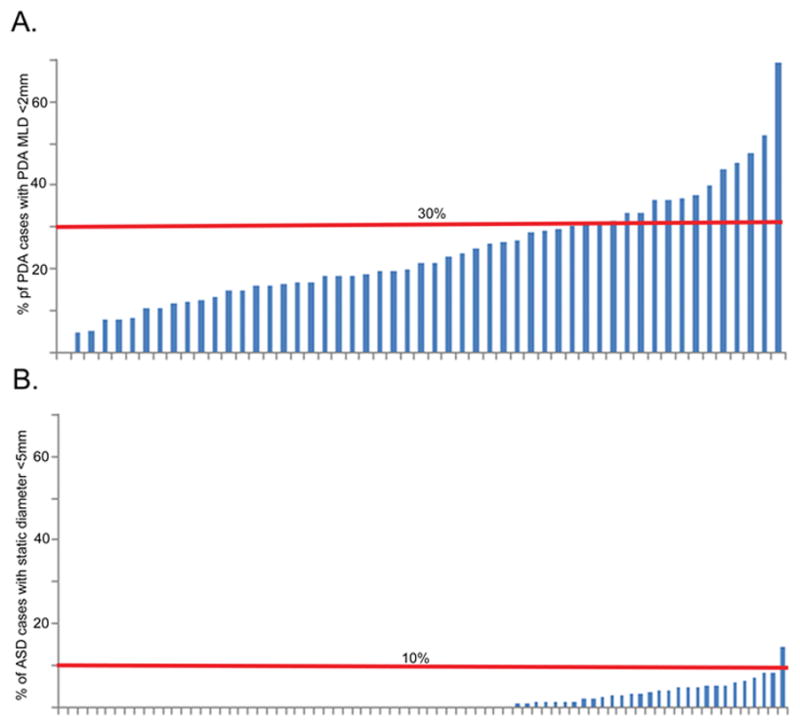
A bar graph of the percentage of A) PDA and B) ASD cases performed at a hospital for defects that are small (<2mm in PDA, <5mm in ASD). Centers are sorted from lowest percentage of small defect closures to largest. Horizontal reference lines are plotted in red.
The risk of major adverse events was compared between the population with relatively small shunts and those with larger shunts. For PDA cases, the risk of major adverse events was lower in subjects with anatomically small defects (0.2%) compared to those with larger defects (1.3% p=0.04 95% CI: −2% to −0.1%), as well as those with a Qp:Qs<1.5:1 (0.2%) when compared to those with a Qp:Qs≥1.5:1 (1.5%, p=0.002, 95% CI: −2.4% to −0.5%). For ASD cases, the risk of major adverse events was lower in subjects with a Qp:Qs<1.5:1 (1.1%) compared to those with a larger Qp:Qs (2.6%, p=0.01, 95% CI: −2.5% to −0.1%), but there was not a significant difference with anatomically small defects (0%) vs. those with larger defects (1.5%, p=0.40, 95% CI: −2.0% to 5.9%).
DISCUSSION
In this multicenter retrospective study, we were able identify significant practice variation in transcatheter procedures to device-close ASD and PDA that could not be explained by measured patient-level characteristics. The distribution of indications for closure varied by hospital setting, and in addition to this there was significant inter-hospital variation in the distribution of indications. For cases with volume overload as the indication, several significant patterns in the probability that cases had small shunts were observed. Higher hospital procedural volumes were associated with a reduced risk, meaning that the proportion of cases with small shunts was lower at higher volume hospitals. For ASD cases, hospitals with a higher proportion of catheterizations per year in adults had a higher proportion of cases in which the reported indication for closure was volume overload and a small magnitude shunt. For PDA closure, there was additional inter-hospital variation in the risk that was not explained by either patient or hospital factors. These are indicators of practice variation.
ASD and PDA closure are two of the most common procedures performed in congenital cardiac catheterization, representing a sizable minority of total cases in the IMPACT registry. The current generation of closure devices have been available in the United States for over a decade. US Food and Drug Administration approval for the Amplatzer Septal Occluder (St. Jude Medical, St. Paul, MN) was granted in 2000, with approval for the Amplatzer Duct Occluder (St. Jude Medical, St. Paul, MN) following in 2003. Though there have been innovations in the range of devices available and shifts in practice, ASD and PDA closure are mature procedures for which one might expect little controversy in their conduct. Yet, there remains practice variation that is not explicable in terms of patient characteristics.
There was significant variation in the distribution of RVVO and LVVO relative to other indications for both procedures. In unadjusted analyses, hospital location (by census region) and setting (urban vs. suburban vs. rural) appeared to be associated with the distribution of indications. In analyses adjusting for patient factors, the association between hospital location and the distribution of indications was no longer seen. After adjustment, the proportion of cases for volume overload was still not distributed evenly between urban, suburban, and rural centers. For PDA cases, urban centers were more likely than rural or suburban centers to have cases with LVVO as an indication. For ASD cases, rural and urban hospitals had more cases for RVVO than suburban hospitals. However, these observations may not represent true practice variation. They may instead represent the referral patterns of community cardiologists of patients with volume load due to ASD or PDA to urban hospitals. This is in contrast to the observed differences in the distribution of indications for ASD closure between Northeastern and Southern hospitals. The distribution of patients is unlikely to vary between regions of the country, and one would not expect unbalanced referral patterns at the regional level when there are high-volume urban centers available in all geographic regions. This makes the difference in the adjusted distribution of indications for ASD closure between the Northeast and South regions harder to explain, and is more likely to represent true practice variation. Additional evidence of practice variation is the significant inter-hospital variation in the distribution of indications for both PDA and ASD cases (represented by statistically significant MRR). This represents independent variability in practice between hospitals, and true practice variation. The implications of this variation are unclear. The study is not designed to measure the effect of this practice variation on clinical outcomes or resource utilization.
There also is significant variation in the distribution of the size of the shunt (either anatomically or physiologically) between hospitals. Specifically, as center PDA and ASD procedural volume increased, defects with a small shunt constituted a smaller proportion of cases in which the indication for closure was volume overload, and for ASD cases, the proportion of cases with small shunts was larger at hospitals serving predominantly adult patients. Whether this pattern is the result of the pattern of referrals to these hospitals and/or the practice of the interventional cardiologists cannot be discerned from the current data. The diagnosis of volume overload in an individual patient may be based on the interpretation of an echocardiogram by the referring cardiologist, an echocardiographer, or the interventionalist performing the case rather than on procedural imaging and oximetry. Intrinsic differences in the two diagnostic tests, as well as differences in physiologic state (sedation or anesthesia versus an awake patient) could result in discrepancies between the diagnosis of volume overload and the size of shunt. However, persistent differences in the proportion of patients with small shunts indicates differences in the practices of different hospitals and the population undergoing these procedures with predictable ramifications. A hospital where a larger number of defects with smaller shunts (either by anatomic size or magnitude of shunt) are treated, will expose a larger number of patients overall to the risks of anesthesia and the intervention itself. We acknowledge 1) that transcatheter closure of ASD and PDA both have well-established records for safety and 2) that in this study the risk of major adverse events was lower in subjects with smaller shunts than those with larger shunts. However, a more aggressive practice pattern inevitably increases resource utilization and total healthcare spending. Potentially unnecessary procedures expose patients to risk. The balance of risk and benefit should be considered carefully at the level of the individual patient. From the perspective of a health system, it is important to be circumspect about the incentives for these procedures at each level and to consider if these incentives are supportive of the best care for patients. The current study has an observational design that does not allow for an analysis of these structures, so we it is not possible to elaborate further on this. Even accepting that the relative merits of different hospitals’ strategies is not known at this time, the variation observed reflects uncertainty and lack of consensus regarding how volume overload is defined as an indication for transcatheter device closure of ASD and PDA. This identifies a need for further research into what represents best practices regarding these procedures.
A third observation is that there appears to be greater otherwise unaccounted for variability in closure of PDA than ASD. Specifically, in terms of the distribution of indications, the MRR for PDA closure is 1.3, and the MRR for ASD is 1.1. This means that between two otherwise identical patients with PDA there is a 30% chance that at two randomly selected hospitals in the sample the indication for their case would be different, whereas it is only 10% in an ASD patient. The greater variation in practice in PDAs is also demonstrated in greater variation in defining a hemodynamically significant PDA. This is seen in the large number of hospitals classifying small PDA as hemodynamically significant and reinforced by the large magnitude MRR (1.4) in the multivariate model for closure of a PDA <2mm. We acknowledge that this may be due to difficulty in interpreting pre-procedural echocardiographic imaging of PDA. However, it is more likely that this degree of practice variation reflects a more fundamental uncertainty in selecting and referring patients for transcatheter PDA closure that deserves attention.
There are several limitations to this study. First, though widely used, the Fick principle has some limitations. Patients undergoing catheterization are almost universally under anesthesia or are receiving sedation, both of which potentially alter the hemodynamic conditions significantly from the patient’s baseline while awake. In a PDA, there are additional limitations to the use of Fick. Preferential streaming of oxygenated blood to the left pulmonary artery can result in different distal pulmonary artery saturations and some uncertainty regarding the magnitude of the shunt. Depending how this is addressed, this has the potential to introduce variation between hospitals. For ASD, at times, there may be a discrepancy between the shunt measured by Fick and the qualitative appraisal of RV size on echocardiography. Echocardiographic assessments of left or right ventricular size are not included in IMPACT®. Ultimately, however, neither of these limitations would explain the systematic associations between hospital characteristics and the proportion of patients with relatively small shunts. Indeed reliance on qualitative estimates of left ventricular and right ventricular dilation may be part of the etiology of the observed inter-hospital variation.
An important limitation to the study is that both PDA and ASD closure can not only performed in the catheterization laboratory but also the operating room. Data about surgical cases, which would provide a more comprehensive view of practice in closing ASD and PDA, are not included in IMPACT®. A previous study has demonstrated that in a contemporary cohort of ASD’s treated at primary pediatric hospitals there is significant inter-hospital variability in the propensity to pursue operative or transcatheter closure of ASD after adjusting for measurable covariates27. Though it is conjecture, we would propose that this is also likely true for PDA. This is another aspect of practice variability that deserves attention. In the future, linkage of multiple registries or use of novel sources of data (administrative or claims databases) will allow for this type of analysis. When unforeseen adverse events (such as device erosion associated with the Amplatzer Septal Occluder) come to light and as novel devices are introduced for transcatheter interventions, studying the effects of these experiences on the decision-making and practice patterns of interventional cardiologists becomes increasingly important.
This study uses data from the IMPACT® registry, for which data are collected and recorded by staff at each hospital. Definitions of each data point are standardized and available to each center, but local differences in the interpretation and coding of data could increase variability between hospitals. An important limitation is only a single indication can be marked for each procedure, and there some plausible cases in which more than one indication can exist. Though this might be expected to introduce uncertainty in individual case reporting that might inflate inter-hospital variation. As noted previously, variability in practice patterns includes the behavior of both referring physicians and the interventional cardiologist. Specifically, IMPACT® does not measure the numbers and characteristics of patients with ASD and PDA who are not referred for catheterization. These data would help to study differences in referral patterns. For this reason, we focused in the current study on the operator’s classification of small anatomic shunts, which is solely the province of the interventional cardiologist. Moreover, one might argue that current guideline definitions of RV and LV volume overload 18 are ambiguous, and left to interpretation. As a result, we were forced to define thresholds for the size of defects and ratio of Qp:Qs for a hemodynamically significant lesion. These were chosen based on our clinical experience. However, it is important to recognize that practice variation identified is not dependent on the value of the threshold, and that the same variation would be seen regardless of where the threshold was defined. Additionally, IMPACT® does not contain data to compare ASD/PDA cases where the indication was not volume overload between centers, including potentially important covariates (e.g. history of endocarditis as part of the indication for PDA closure for SBE prophylaxis). Finally, to date, guidelines and practice recommendations have not identified process measures or institutional environmental factors that strongly influence outcome and which can be used as additional quality metrics. Thus, the scope of any investigation of practice variation is limited to the recommendations of published guidelines. We hope that subsequent research will help to identify practices or institutional factors that are influential on outcome and indicate centers of excellence.
Despite these limitations, the durable presence of variability between centers forces us to acknowledge that even in the most common and “simplest” transcatheter interventions for congenital heart disease there remains broad variation in practice. This reflects a significant knowledge gap about what constitutes best practice for these procedures, and represents an opportunity for further research to improve outcomes and optimize healthcare utilization.
Supplementary Material
Footnotes
Disclosures: No conflicts of interest to disclose.
References
- 1.Bonow RO, Masoudi FA, Rumsfeld JS, Delong E, Estes NAM, Goff DC, Grady K, Green LA, Loth AR, Peterson ED, Piña IL, Radford MJ, Shahian DM American College of Cardiology, American Heart Association Task Force on Performance Measures. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2008;52:2113–2117. doi: 10.1016/j.jacc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Lewis WR, Peterson ED, Cannon CP, Super DM, LaBresh KA, Quealy K, Liang L, Fonarow GC. An organized approach to improvement in guideline adherence for acute myocardial infarction: results with the Get With The Guidelines quality improvement program. Arch Intern Med. 2008;168:1813–1819. doi: 10.1001/archinte.168.16.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonarow GC, Yancy CW, Heywood JT ADHERE Scientific Advisory Committee, Study Group, and Investigators. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SV, Spertus JA, Masoudi FA, Daugherty SL, Maddox TM, Li Y, Dodson JA, Chan PS. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–1801. doi: 10.1016/j.jacc.2013.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63:2876–2877. doi: 10.1016/j.jacc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice-level variation in use of recommended medications among outpatients with heart failure: Insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6:1132–1138. doi: 10.1161/CIRCHEARTFAILURE.113.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2014;63:539–546. doi: 10.1016/j.jacc.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program) Am J Cardiol. 2011;108:1136–1140. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole-Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- 11.Hira RS, Kennedy K, Nambi V, Jneid H, Alam M, Basra SS, Ho PM, Deswal A, Ballantyne CM, Petersen LA, Virani SS. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65:111–121. doi: 10.1016/j.jacc.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Engelfriet P, Tijssen J, Kaemmerer H, Gatzoulis MA, Boersma E, Oechslin E, Thaulow E, Popelová J, Moons P, Meijboom F, Daliento L, Hirsch R, Laforest V, Thilén U, Mulder B. Adherence to guidelines in the clinical care for adults with congenital heart disease: the Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2006;27:737–745. doi: 10.1093/eurheartj/ehi718. [DOI] [PubMed] [Google Scholar]
- 13.Farias M, Friedman KG, Powell AJ, de Ferranti SD, Marshall AC, Brown DW, Kulik TJ. Dynamic evolution of practice guidelines: analysis of deviations from assessment and management plans. Pediatr. 2012;130:93–98. doi: 10.1542/peds.2011-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman KG, Rathod RH, Farias M, Graham D, Powell AJ, Fulton DR, Newburger JW, Colan SD, Jenkins KJ, Lock JE. Resource utilization after introduction of a standardized clinical assessment and management plan. Cong Heart Dis. 2010;5:374–381. doi: 10.1111/j.1747-0803.2010.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen NL, Drezner JA, Salerno JC. Sudden cardiac death screening in adolescent athletes: an evaluation of compliance with national guidelines. Br J Sports Med. 2013;47:172–177. doi: 10.1136/bjsports-2012-091670. [DOI] [PubMed] [Google Scholar]
- 16.Angoff GH, Kane DA, Giddins N, Paris YM, Moran AM, Tantengco V, Rotondo KM, Arnold L, Toro-Salazar OH, Gauthier NS, Kanevsky E, Renaud A, Geggel RL, Brown DW, Fulton DR. Regional implementation of a pediatric cardiology chest pain guideline using SCAMPs methodology. Pediatr [Internet] 2013;132:e1010–e1017. doi: 10.1542/peds.2013-0086. Available from: http://pediatrics.aappublications.org/content/early/2013/09/04/peds.2013-0086.abstract. [DOI] [PubMed] [Google Scholar]
- 17.Harahsheh AS, O’Byrne ML, Pastor B, Graham DA, Fulton DR. Pediatric Chest Pain—Low-Probability Referral. Clin Pediatr. 2017:1–8. doi: 10.1177/0009922816684605. e-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, Mullins CE, Taubert KA, Zahn EM American Heart Association Congenital Cardiac Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Radiology and Intervention, American Heart Association. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circ. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 19.Vincent RN, Moore J, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Ringel R, Rome J, Martin GR. Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiology in the Young. 2016;26:70–78. doi: 10.1017/S1047951114002637. [DOI] [PubMed] [Google Scholar]
- 20.Martin GR, Beekman RH, Ing FF, Jenkins KJ, McKay CR, Moore JW, Ringel RE, Rome JJ, Ruiz CE, Vincent RN. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Sem Thorac and Cardiovasc Surg: Ped Card Surg Annual. 2010;13:20–25. doi: 10.1053/j.pcsu.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 21.O’Byrne ML, Gillespie MJ, Kennedy KF, Dori Y, Rome JJ, Glatz AC. The influence of deficient retro-aortic rim on technical success and early adverse events following device closure of secundum atrial septal defects: An Analysis of the IMPACT Registry(®) Catheter Cardiovasc Interv. 2017;89:102–111. doi: 10.1002/ccd.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messenger JC, Ho KKL, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Moore JW, Vincent RN, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Li Y, Ringel R, Rome J, Martin GR NCDR IMPACT Steering Committee. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the national cardiovascular data registry. J Am Coll Cardiol. 2014;64:2439–2451. doi: 10.1016/j.jacc.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Jayaram N, Spertus JA, O’Byrne ML, Chan PS, Kennedy KF, Bergersen L, Glatz AC. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: A report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J. 2017;183:118–128. doi: 10.1016/j.ahj.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaram N, Beekman RH, Benson L, Holzer R, Jenkins K, Kennedy KF, Martin GR, Moore JW, Ringel R, Rome J, Spertus JA, Vincent R, Bergersen L. Adjusting for Risk Associated With Pediatric and Congenital Cardiac Catheterization: A Report From the NCDR IMPACT Registry. Circ. 2015;132:1863–1870. doi: 10.1161/CIRCULATIONAHA.114.014694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 27.O’Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing Propensity to Pursue Operative Closure of Atrial Septal Defects Following Changes in the Instructions for Use of the Amplatzer Septal Occluder Device. Am Heart J. 2017:1–10. doi: 10.1016/j.ahj.2017.07.012. e-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


