Abstract
We conducted a 26-month follow-up of a previously reported 12-month study that compared mindfulness-based cognitive therapy (MBCT) to a rigorous active control condition (ACC) for depressive relapse/recurrence prevention and improvements in depressive symptoms and life satisfaction. Participants in remission from major depression were randomized to an 8-week MBCT group (n=46) or ACC (n=46). Outcomes were assessed at baseline, post-intervention, and 6, 12, and 26 months. Intention-to-treat analyses indicated no differences between groups for any outcome over the 26 month follow-up. Time to relapse results (MBCT vs. ACC) indicated a hazard ratio (HR) = .82, 95% CI [.34, 1.99]. Relapse rates were 47.8% for MBCT and 50.0% for ACC. Piecewise analyses indicated that steeper declines in depressive symptoms in the MBCT vs. the ACC group from post-intervention to 12 months were not maintained after 12 months. Both groups experienced a marginally significant rebound of depressive symptoms after 12 months but were still improved at 26 months compared to baseline (b=-4.12, p<=.008). Results for life satisfaction were similar. In sum, over a 26-month follow-up, MBCT was no more effective for preventing depression relapse/recurrence, reducing depressive symptoms, or improving life satisfaction than a rigorous ACC. Based on epidemiological data and evidence from prior depression prevention trials, we discuss the possibility that both MBCT and ACC confer equal therapeutic benefit. Future studies that include treatment as usual (TAU) control conditions are needed to confirm this possibility and to rule out the potential role of time-related effects. Overall findings underscore the importance of comparing MBCT to TAU as well as to ACCs.
Keywords: mindfulness-based cognitive therapy, depression relapse/recurrence prevention, depressive symptoms, active control condition, long-term follow-up
Major depressive disorder (MDD) is a leading cause of disability worldwide (Ferrari et al., 2013). The 50–80% rate of depression relapse (Judd, 1997) has prompted a focus on treatments aimed at preventing relapse/recurrence. Mindfulness-Based Cognitive Therapy (MBCT) has been shown to reduce depression relapse/recurrence compared with usual care (Ma & Teasdale, 2004; Teasdale, Williams, Ridgeway, Soulsby, & Lau, 2000) in individuals with three or more episodes of MDD. Further, three randomized controlled trials (RCTs) have shown MBCT combined with support to taper or discontinue antidepressant medications (ADM) is as effective as maintenance ADM (mADM) for reducing risk of relapse/recurrence (Kuyken et al., 2008, 2015, 2016; Segal, Martin, & Joseph, 2010). One recent inferiority trial, however, demonstrated that MBCT following discontinuation of ADM was not superior to mADM (Huijbers et al., 2016). While it is unclear whether MBCT is a viable alternative to mADM, meta-analyses support that it is an efficacious intervention for depressive relapse/recurrence prevention, relative to treatment as usual (TAU) and mADM (Kuyken et al., 2016; Piet & Hougaard, 2011).
Despite this research supporting the benefits of MBCT, three gaps in the evidence for MBCT warrant further attention. First, little is known about the effects of MBCT compared to psycho-educational active control conditions (ACCs). Comparing MBCT to rigorous ACCs is important because MBCT is not yet widely available and other psycho-educational interventions may produce similar benefits with cost, acceptability, accessibility, and dissemination advantages (Parikh et al., 2012). Second, to understand the active ingredients of MBCT, it is necessary to compare it to structurally equivalent and therapeutically credible ACCs that are matched to MBCT on non-specific therapeutic factors but that lack mindfulness and cognitive therapy components. This is important because although mindfulness and cognitive therapy are widely assumed to be the components of MBCT that are responsible for its effects (Williams et al., 2014), non-specific factors such as amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance and alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes have each been shown to contribute to psychological treatment outcomes (Huibers & Cuijpers, 2014; Wampold, 2015). Further, some of these variables may account for more of the variance in outcomes than specific psycho-educational treatment approaches (Chatoor & Kurpnick, 2001). Third, the longer-term effects (i.e., greater than 12 months) of MBCT vs. ACCs are poorly understood. Longer-term follow-up of depression outcomes is critical because depression follows a chronic and recurrent course (Bockting, Hollon, Jarrett, Kuyken, & Dobson, 2015). Extended follow-up studies of psychosocial treatments, in particular, are needed to understand the trajectory and duration of effects and which treatments are most appropriate for the prevention of depressive relapse/recurrence over the longer-term.
The broader literature on mindfulness-based interventions (MBIs) includes a range studies that have compared MBIs to ACCs. However, the MBIs, ACCs and the samples in these studies don’t sufficiently overlap with our investigation to be included in our focused literature review. Thus, we focus on MBCT versus ACC studies in participants with a history of recurrent major depressive disorder.
Only a handful of studies have compared MBCT to ACCs. Evidence for the efficacy of MBCT compared to an ACC is supported by at least one RCT that found MBCT to be superior to a psycho-educational control condition for reducing depressive symptoms over a 26-week follow-up (Chiesa et al., 2015). We are aware of only two RCTs that have compared MBCT to an ACC for the prevention of depression relapse/recurrence (Meadows et al., 2014; Williams et al., 2014). Neither study found MBCT to be superior to an ACC for extending time to relapse/recurrence except in sub-groups of participants. For example, Meadows et al. (2014) found that MBCT was associated with an extended time to relapse/recurrence in participants who attended 4 or more sessions and who received specialized care or pharmacological treatment. In the Williams et al. (2014) study, time to relapse/recurrence was extended in individuals who had a history of childhood trauma. Despite evidence supporting that certain types of individuals may benefit more from MBCT versus a psycho-educational ACC, the specificity, active ingredients, and longer-term effects of MBCT remain unclear for two reasons.
First, the respective ACCs in each of these studies did not control for the range of nonspecific psychological components that may impart therapeutic benefit. For example, the ACC in the Meadows trial was developed primarily to reduce the discrepancy between groups in treatment expectation. Thus, their ‘Depression Relapse Active Monitoring’ (DRAM) control condition, which included a brief training in self-monitoring during the intake assessment interview, was not matched to MBCT on amount of group contact, treatment-related activity outside of class (i.e., home-based practice), interaction with a facilitator, therapeutic allegiance and alliance, and perceived social support. Although considerably more comprehensive in terms of matching on the range of plausible non-specific therapeutic ingredients, the ACC in the Williams study, a ‘Cognitive Psychological Education’ control group, did not include equivalent home-based practice. Thus, the active ingredients of MBCT cannot be clearly determined from these investigations because neither of them included control conditions that were matched to MBCT on the most comprehensive range of therapeutically probable non-specific components—namely, amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance and alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes.
Second, of these two studies, only the Meadows trial examined effects beyond 12 months. Although this investigation found no group differences for extending time to relapse/recurrence, results indicated that fewer MBCT vs. ACC participants experienced a relapse over a 24-month follow-up. These findings support the possibility that effects of MBCT vs. an ACC may emerge after a longer follow-up period (e.g., beyond 12 months) and substantiate the need for longer-term investigations of MBCT vs. ACCs for depression relapse prevention (Bockting et al., 2015).
We conducted a 12-month study to compare MBCT to a structurally equivalent and validated ACC that was matched to MBCT on a comprehensive range of non-specific factors (amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance and alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes) that have been shown in prior studies to contribute to depression outcomes (Huibers & Cuijpers, 2014; Parsons, Crane, Parsons, Fjorback, & Kuyken, 2017; Wampold, 2015) but have not been included in prior efficacy trials of MBCT. Results from this study indicated no differences between groups for depressive relapse/recurrence rates, time to relapse/recurrence, or levels of depressive symptoms and life satisfaction (Shallcross et al., 2015).
Although relapse outcomes and levels of depressive symptoms were not different between groups, the trajectory of depressive symptom improvement did vary by group. As reported in (Shallcross et al., 2015), analyses indicated a significant Group X Quadratic Time interaction such that the ACC experienced immediate symptom reduction, which was followed by a gradual increase in symptoms over the 12-month follow-up. In contrast, the MBCT group experienced continued reductions in depressive symptoms (with no increase) over the 12-month follow-up. This finding could be explained by differential rates of therapeutic benefit associated with intervention-specific skills acquisition.
For example, the knowledge and skill focus of the ACC, which included physical activity, functional movement, nutrition, and music therapy, may lead to more immediate reductions in depressive symptoms through distraction, improved self-efficacy, or neurohormonal mechanisms (e.g., increases in serotonin, dopamine) (Craft & Perna, 2004; Maratos, Crawford, & Procter, 2011; Opie, O’Neil, Itsiopoulos, & Jacka, 2014). These effects, however, might not be sustained over the longer-term. On the other hand, the neurocognitive improvements in attention regulation and self-referential processing that are associated with mindfulness meditation (Chiesa & Serretti, 2011; Goldin, Ziv, Jazaieri, & Gross, 2012) might lead to more gradual (Grossman, Niemann, Schmidt, & Walach, 2004) but cumulative and sustained reductions in depressive symptoms.
Theory and evidence supporting the benefits of MBCT for reducing key cognitive vulnerability factors associated with depressive relapse (e.g., rumination and cognitive reactivity) (Michalak, Holz, & Teismann, 2011) suggest that differences between groups may emerge after 12-months. This idea is consistent with a study showing long-term and significant reductions in depressive symptoms in MBCT vs. TAU over a 56- week follow-up (Godfrin & van Heeringen, 2010). Additionally, two single-arm trials have shown sustained reductions in depressive symptoms after MBCT training. One study demonstrated reductions in symptoms for 34 months (Mathew, Whitford, Kenny, & Denson, 2010) and another showed sustained reductions for 58.9 months (Munshi, Eisendrath, & Delucchi, 2013).
This evidence supports the possibility that the downward trajectory of depressive symptoms from post-intervention to 12-months may continue in the MBCT group, and these reductions could translate to an emergent effect of MBCT vs. ACC for depression relapse/recurrence rates over an extended follow-up period. An equally likely possibility, however, is that in the absence of supportive or supplementary treatment beyond the 8-week intervention, depressive symptoms may level off or increase in the MBCT group after 12 months, thus mimicking the trajectory of increasing symptoms in the ACC group. In sum, these considerations raise the question of whether MBCT would show significant advantage over the ACC at follow-up assessments past 12 months.
The present study examined this question by extending our earlier investigation and comparing MBCT to an ACC over total follow-up period of 26 months. Outcomes include depression relapse/recurrence rates, time to relapse/recurrence, depressive symptoms, and life satisfaction. To examine whether subgroups of participants may benefit differentially from MBCT vs. ACC, we also conducted exploratory analyses of several theoretically and empirically supported moderators that include: age of onset of depression, baseline levels of depressive symptoms and life satisfaction, antidepressant medication use, sex, intervention dose, and self-reported practice. Each one of these moderators was tested in our original 12-month follow-up trial (Shallcross et al., 2015) and was selected because it had been shown in prior studies to be associated with depression outcomes or to moderate effects of MBCT (Braun, Gregor, & Tran, 2013; Kuyken et al., 2016; Parsons et al., 2017).
Method
Study Design and Participants
Complete details of the method are described in our original investigation (Shallcross et al., 2015). The study design was a randomized, single blind, 8-week parallel comparison of MBCT (n=46) and a validated ACC (n=46). Consent and IRB approval were obtained. Eligibility was assessed via a combination of an online screening assessment and an in-person assessment using the Structured Clinical Interview for the DSM-IV (SCID-IV) (First, Spitzer, Gibbon, & Williams, 2002).
Interventions
Mindfulness-based cognitive therapy (MBCT)
MBCT is an 8-week group intervention that combines cognitive therapy with mindfulness training and was delivered in accordance with the published MBCT protocol (Segal, Williams, & Teasdale, 2002). Each MBCT group was led by one of two PhD-level clinical psychologists and co-facilitated with one of two fourth-year doctoral trainees in clinical psychology. Both study therapists attended a 7-day residential training with one of the developers of the MBCT protocol and each of them had a personal mindfulness practice spanning five or more years. The two co-facilitators received organized training in MBCT facilitated by one of the study therapists before the start of the study. All MBCT and ACC group sessions were audiotaped. Therapists’ degree of adherence to the MBCT protocol was monitored using the 17-item MBCT Adherence Scale (Segal, Teasdale, Williams, & Gemar, 2002). Adherence results obtained from an independent, master’s-level psychologist with familiarity with MBCT indicated quality adherence to the MBCT protocol. Complete details of MBCT therapist fidelity are reported in the original investigation (Shallcross et al., 2015).
Active control condition
The ACC is based on the validated and manualized Health Enhancement Program (HEP) (MacCoon et al., 2012), which was designed as an active control for mindfulness-based interventions. It includes classes in physical activity, functional movement, music therapy, and nutrition. The ACC is structurally equivalent to MBCT and controls for nonspecific effects including: amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance and alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes. Facilitators included a board-certified music therapist, a certified personal trainer and group fitness instructor, and a masters-level nutrition therapist. A bachelor’s-level trainee with understanding of and familiarity with the HEP manual assessed HEP treatment fidelity using the 17-item HEP Adherence Scale (HEP-AS) (Eisendrath et al., 2014), a newly developed but not yet validated measure modeled after the MBCT Adherence Scale. Ratings indicated that all facilitators demonstrated quality adherence to the HEP intervention manual. Complete details of HEP facilitator fidelity are reported in the original investigation (Shallcross et al., 2015).
Measures
The primary outcomes were depressive relapse/recurrence rates and time to relapse/recurrence, defined as meeting relevant SCID criteria for MDD for at least 2 weeks. Relapse/recurrence was assessed at 6, 12, and 26 months post-intervention and covered the full duration of time since the prior interview. Clinical interviews at 6 and 12 months were conducted by two fifth-year doctoral trainees in psychology. Reliability between interviewers for the diagnosis of a depressive episode yielded an average Kappa coefficient of .84 based on the 6- and 12- month interviews. Only one of the two interviewers was available to conduct the 26-month assessment. Thus, assessment of reliability at this time point was not possible. In support of the diagnostic validity of the 26-month assessment, the remaining interviewer was an advanced PhD-level clinical psychology student (who received her PhD during the study period) and who had extensive training in clinical psychological assessment, including seven years of experience conducting SCID interviews for this and other clinical trials. She additionally achieved high reliability with the interviewer at the 6- and 12-month clinical assessments (Kappa = .84).
Blinding of the interviewers over the entire study period with regard to study hypotheses and participant group assignment was maintained via several methods. First, neither interviewer was part of the core research team. Thus, interviewers were not involved with study-related discussions and did not have access to data or results from the initial trial, which were published after the final 26-month interview. Second, interviewers followed a standardized script, which included an explicit reminder to participants at the beginning of each interview not to reveal information about their group assignment. Finally, interviewers were supervised by an experienced research clinician who was also blind to group assignment. As a part of this supervision, the importance of remaining blind was emphasized. One incident of unintentional unblinding at the 12-month follow-up was reported. The audiotape for this interview was edited to remove the segment where group assignment was inadvertently revealed. The edited audio was evaluated by the second interviewer who made the final diagnosis.
Additional outcomes included depressive symptoms and life satisfaction assessed at baseline, post-intervention, and at 6, 12, and 26 months measured with self-report questionnaires (i.e., the BDI-II (Beck, Steer, & Brown, 1996) and the Satisfaction with Life Scale (SWL) (Diener, Emmons, Larsen, & Griffen, 1985), respectively. Internal consistency for all BDI-II and SWL assessments was α >.85.
Putative moderator variables included age of onset of depression (assessed via SCID-IV), baseline depressive symptoms, life satisfaction, and ADM use (assessed via self-report); sex; per-protocol completion (based on attendance of ≥ 4 classes); self-report of total practice time outside of class during intervention (sum of minutes of practice logged during 8-week intervention); and self-report of total practice time since the intervention (sum of retrospective estimation of number of minutes of practice each week assessed at 6, 12, and 24 months).
Data Analytic Strategy
Depressive relapse/recurrence and time to relapse/recurrence
Fisher’s exact test was used to compare the proportions of depressive relapse/recurrence between groups. Cox proportional hazards were used to estimate survival curves. Participants with missing data and those who did not experience a relapse/recurrence were treated as censored observations. Primary outcomes were analyzed separately for intention-to-treat (ITT) and per-protocol (PP) samples. The PP sample included participants who received the minimum effective dose of MBCT (≥ 4 sessions (Teasdale et al., 2000)) or an equivalent dose of ACC.
Depressive symptoms and satisfaction with life
Full information maximum likelihood (FIML) mixed effects regression models were used to analyze depressive symptoms and life satisfaction across time between groups. Models were based on a piecewise analysis of time. Piecewise analyses allow for the representation of multiple discrete time periods by modeling separate variables (and therefore separate coefficients and slopes) for these periods. Using this approach, the original follow-up period (reported in our original investigation) and the extended follow-up period can be conceptualized as discrete and yet represented within the same model. This allowed us to test for emergent benefits during the extended follow-up period, while controlling for effects during the original follow-up period. Piece 1 was the period from pretreatment to post-treatment, Piece 2 was the original follow-up period from post-treatment to 12 months. Piece 3 was the extended follow-up from 12 months to 26 months. Time was mean-centered in all analyses.
We followed an iterative process to build the mixed effects models predicting change in depressive symptoms and satisfaction with life over time. First, the random effects of each Piece (i.e., time period) were added sequentially, and statistically significant (p<.05) effects were retained. Next, fixed effects of Group, Piece, and all Group X Piece interactions were added to the model. Interaction terms were retained when statistically significant.
Missing data
Attrition rates and PP completion did not differ between groups at any time point. Comparisons of outcomes between PP completers vs. non-completers and comparisons between participants with present vs. missing data in the PP and ITT samples were conducted to assess if data were missing at random. For PP completers vs. non-completers, no differences emerged in any outcome variable (p>.15). For PP completers with present vs. missing data, participants with missing data had lower incidence of relapse/recurrence at 26 months (p=.023). In the ITT sample, participants with missing data had lower baseline depressive symptoms (p=.013) and lower incidence of relapse/recurrence at 26 months (p=.012). Because data were not missing completely at random, we used pattern-mixture models (Tasca & Gallop, 2009) to test whether effects on time to relapse/recurrence and depressive symptoms were independent of missing data patterns.
Cox proportional hazards were used to estimate survival curves for time to relapse/recurrence as a function of missing data patterns. Participants with missing data were compared to participants with no missing data during the 26-month follow-up period (Pattern 1). There were no group differences in missing data patterns for the PP or ITT samples (ps>.41). There was a marginally significant main effect of Pattern 1 on time to relapse/recurrence for the ITT sample only (p=.08). Thus, Pattern 1 was entered as a covariate into Cox proportional hazards models to control for the effect of missing data patterns in the ITT sample.
For pattern effects on depressive symptoms, participants with missing data during Pieces 1–3 (Patterns 1–3, respectively) were compared to participants with no missing data during the 26-month follow-up period. Nonsignificant group differences for missing data patterns and for random, fixed, and interaction effects (Pattern X Piece X Group) indicate that missingness did not meaningfully affect the rate of change in depressive symptoms by intervention group. For this reason and because primary missing data comparisons did not differ for SWL, full information maximum likelihood estimation was used to model depressive symptoms and SWL.
Sample size
We had .80 power to detect a Cohen’s h value of .58 for relapse/recurrence (medium effect size) and a hazard ratio of .36 for time to relapse/recurrence (large effect size). These effect sizes are similar to those observed in previous studies that compared MBCT to TAU. For the mixed effects model predicting depressive symptoms, we calculated sample size based on the number of observations in the group with the smallest number of observations. Using this conservative approach to calculating sample size, we had .80 power to detect a beta of .16 (small effect size).
Results
Preliminary Analyses
Figure 1 shows previously reported information on screening, randomization, and attrition. Table 1 shows clinical characteristics of the sample.
Figure 1.
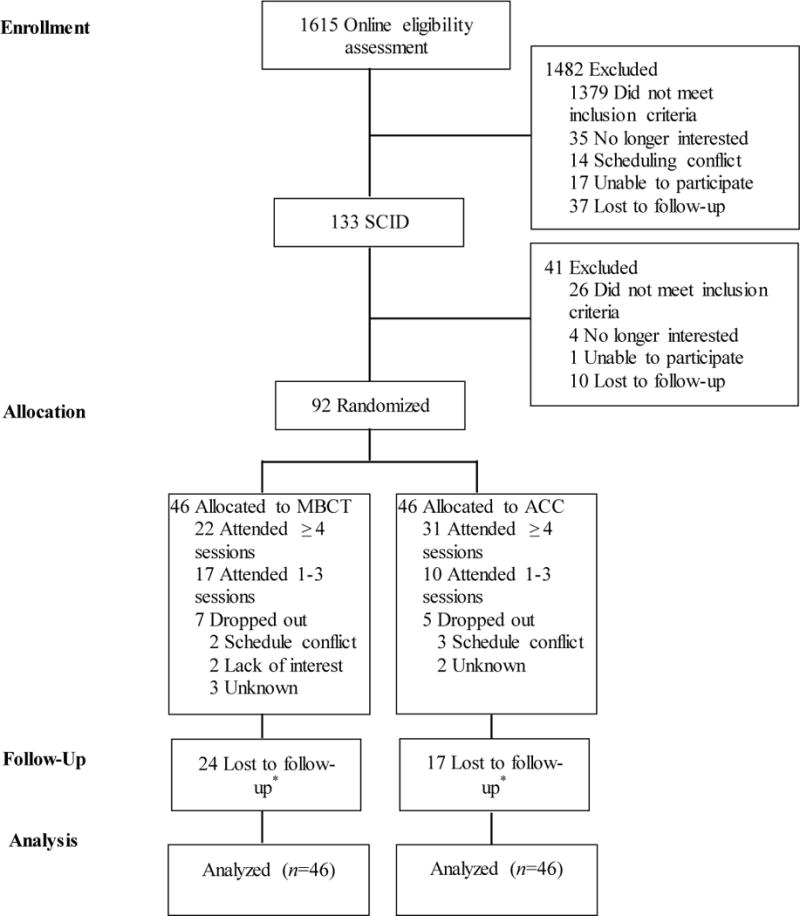
CONSORT flow diagram. MBCT=mindfulness based cognitive therapy; ACC=active control condition; SCID=Structured Clinical Interview for DSM-IV. * Depressive symptoms and life satisfaction were assessed immediately following intervention and at 6, 12, and 24 months. Depressive relapse was assessed at 6, 12, and 24 months. Lost to follow-up values represent the number of people who failed to complete at least one follow-up assessment. Attrition varied across follow-up assessments due to attempts made to collect follow-up data even if participants missed previous assessments.
Table 1.
Clinical characteristics
| Group | |||
|---|---|---|---|
| Variable | MBCT (n = 46) |
ACC (n = 46) |
|
| Clinical characteristics (baseline) | |||
|
| |||
| BDI-II M (SD) | 12.1 (7.5) | 11.9 (6.6) | |
| SWL M (SD) | 3.3 (1.5) | 3.3 (1.4) | |
| Age (in years) of first onset of depression M (SD) | 15.7 (8.2) | 16.5 (5.7) | |
| Number of previous episodes of depression (≥3) | 44 (95.7) | 43 (93.5) | |
| Current antidepressant medication use | 14 (30.4) | 12 (26.1) | |
|
| |||
| Clinical Characteristics (over 26-month follow-up) | MBCT | ACC | P valuea |
| BDI-II (post-intervention) M (SD) | 11.9 (7.2) | 7.1 (6.49) | <.01 |
| BDI-II (6 months) M (SD) | 8.2 (6.9) | 6.2 (5.7) | .295 |
| BDI-II (12 months) M (SD) | 7.0 (6.1) | 7.2 (6.0) | .914 |
| BDI-II (26 months) M (SD) | 10.8 (11.1) | 8.01 (6.9) | .582 |
| SWL (post-intervention) M (SD) | 3.5 (1.5) | 4.0 (1.8) | .198 |
| SWL (6 months) M (SD) | 3.9 (1.7) | 4.3 (1.7) | .451 |
| SWL (12 months) M (SD) | 4.3 (1.4) | 4.1 (1.6) | .603 |
| SWL (26 months) M (SD) | 4.0 (1.6) | 4.1 (1.5) | .879 |
| Treatment outside of interventions | |||
| Antidepressant use | 14 (77.8) | 10 (47.6) | .098 |
| One or more depression-related visits to GP | 16 (88.9) | 16 (76.2) | .418 |
| Counseling or psychotherapy | 11 (61.1) | 16 (76.2) | .488 |
| Psychiatric treatment | |||
| Outpatient | 3 (16.7) | 5 (23.8) | .702 |
| Day patient | 0 | 0 | |
| Inpatient | 0 | 0 | |
| Per-protocol completion (attended ≥4 sessions) | 22 (47.8) | 31 (67.4) | .091 |
| Number of intervention sessions completed M (SD) | 3.87 (2.93) | 4.72 (2.60) | .213 |
Note. Data are presented as number (percentage) unless otherwise indicated. BDI–II = Beck Depression Inventory II; SWL=Satisfaction with Life; GP=General Practitioner.
Fisher’s exact test for proportions and Mann–Whitney U-test for continuous and ordinal variables.
Depressive Relapse/Recurrence and Time to Relapse/Recurrence
For ITT relapse/recurrence rates, sensitivity analyses were conducted whereby missing values in the MBCT and ACC conditions were imputed based on the observed relapse/recurrence rates in the opposite arm (Proschan et al., 2001). Over the 26-month study period1, relapse/recurrence rates in the ITT sample were: 47.8% (22/46) for MBCT and 50.0% (23/46) for ACC (OR=1; 95% CI [0.443, 2.24]; p= 99). The difference between 47.8% relapse and 50.0% relapse yields an h value of .043, indicating a small effect size. Relapse/recurrence rates in the PP sample were: 47.4% (9/19) for MBCT and 51.9% (14/27) for ACC (OR=.84; 95% CI [0.258, 2.71]; p=.76).2 A Cox regression indicated no difference in time to relapse/recurrence between the two groups: ITT: Wald (3, n=92)=3.42; hazard ratio (HR)=.76, CI[.30, 1.89], p=. 55; and PP: Wald (1, n=46)=0.32, HR=.78, CI[.33, 1.86], p=.57 (see Figure 2). The pattern and significance of results remain unchanged when excluding individuals with ≤ 2 prior episodes (n=5), who have not benefited from MBCT in prior studies (Teasdale et al., 2000).3 Effect sizes for PP and ITT analyses from OR and HR models were small.
Figure 2.
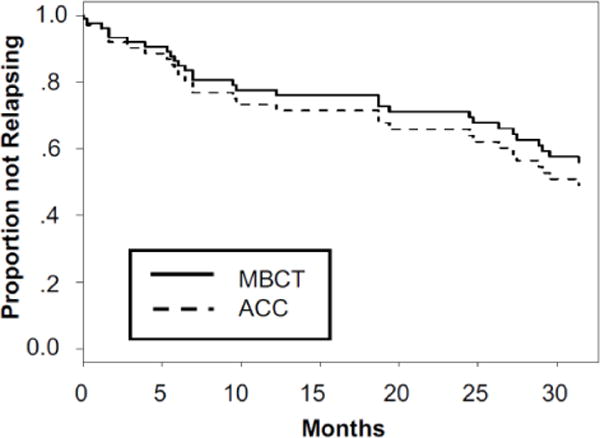
Survival curves showing proportion not relapsing for participants in the MBCT and ACC groups. 0 = baseline (pre-intervention). The final censoring time of 31.4 months is due to attempts to acquire follow-up data from a maximum number of participants (see Footnote 1).
To examine whether intervention effects for relapse/recurrence rates and time to relapse/recurrence differed for subgroups of participants, we tested each putative moderator in separate ITT regression analyses.4 No moderation term was statistically significant (ps>.05). The interaction between baseline depressive symptoms and intervention group was marginal (p=.07). For participants with lower baseline depressive symptoms, MBCT (vs. ACC) was associated with a marginally longer time to relapse/recurrence. For participants with higher baseline symptoms, MBCT (vs. ACC) was associated with a marginally shorter time to relapse/recurrence. Effect sizes from moderation analyses were small.
Depressive Symptoms and Life Satisfaction
The final model for depressive symptoms, summarized in Table 2, included random effects of Piece 1 and Piece 3, fixed effects of Group, Pieces 1–3, Piece 1 X Group, and Piece 2 X Group. Analyses indicated a significant overall linear decrease in symptoms during Piece 1 (from pre-intervention to post-intervention). This was qualified by a significant Piece 1 X Group interaction, such that the ACC group, compared to MBCT, experienced steeper declines in depressive symptoms. The Piece 2 (from post-intervention to 12 months) X Group interaction was also statistically significant, such that the MBCT group, but not the ACC group, experienced a linear decrease in depressive symptoms. The fixed effect of Piece 3 (from 12 months to 26 months) was not statistically significant, although there was an upward trend (p=.07) in depressive symptoms for both groups (see Figure 3).
Table 2.
Summary of Fixed Effects of Intervention Group and Piecewise Time on Depressive Symptoms (BDI-II) and Satisfaction with Life (SWL)
| Effect | ß | B | SEB | T | p | 95% CI |
|---|---|---|---|---|---|---|
| Depressive Symptoms (BDI) | ||||||
| Intercept | 8.1360 | 0.8249 | 9.86 | .000 | [6.4910, 9.7809] | |
| Group | .10 | 1.4636 | 1.1816 | 1.24 | .219 | [−0.8913, 3.8184] |
| Piece 1 | −.31 | −1.5087 | 0.0822 | −4.22 | .000 | [−0.5101, −0.1839] |
| Piece 2 | −.02 | −0.0281 | 0.0169 | −0.38 | .703 | [0.0399, 0.02702] |
| Piece 3 | .12 | 0.2154 | 0.0273 | 1.8 | .074 | [−0.0049, 0.1040] |
| Piece 1 X Group | .17 | 1.1661 | 0.1157 | 2.12 | .022 | [0.0389, 0.4975] |
| Piece 2 X Group | −.14 | −0.2869 | 0.0246 | −2.68 | .008 | [−0.1147, −0.0173] |
| Satisfaction with Life | ||||||
| Intercept | 3.8944 | 0.1990 | 19.57 | .000 | [3.4994, 4.2894] | |
| Group | −.01 | −0.0305 | 0.2682 | −0.11 | .910 | [−0.5631, 0.5022] |
| Piece 1 | .14 | 0.1466 | 0.0098 | 3.41 | .001 | [0.0140, 0.0535] |
| Piece 2 | .13 | 0.0370 | 0.0034 | 2.49 | .014 | [0.0018, 0.0153] |
| Piece 3 | −.07 | −0.0284 | 0.0035 | −1.88 | .063 | [−0.01342, 0.0004] |
Figure 3.
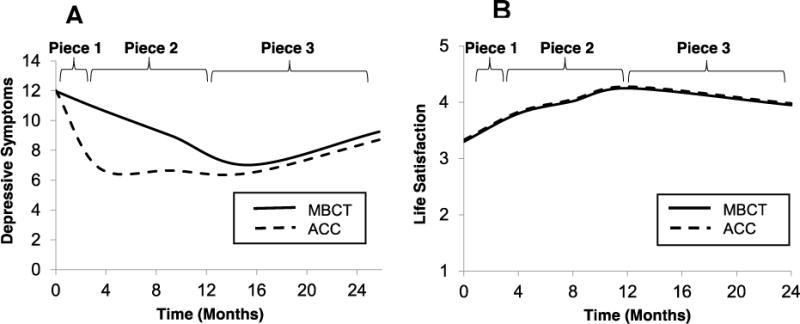
Depressive symptoms (Beck Depression Inventory—II [BDI]; Panel A) and life satisfaction (Satisfaction with Life Scale [SWL]; Panel B) in the MBCT and ACC groups. 0 = baseline (pre-intervention). Piece 1=baseline to post-intervention; Piece 2 = post-intervention to 12 months; Piece 3 = 12 months to 26 months. BDI–II scale range: 0 – 63; SWL scale range: 1–7.
The final model for life satisfaction, summarized in Table 2, included random effects of Piece 1 and Piece 2, fixed effects of Group, and Pieces 1–3. Analyses indicated no significant effects of intervention group or Piece X Group interactions. Fixed effects of Piece 1 and Piece 2 were statistically significant, such that there was a linear increase in life satisfaction from pre-intervention to post-intervention and from post-intervention to 12 months for both intervention groups. The fixed effect of Piece 3 was not significant, though there was a downward trend (p=.06) in life satisfaction for both groups (see Figure 3). Effect sizes for the trajectory of depressive symptoms and life satisfaction over the 26-month follow-up were small.
Mixed effect models comparing depressive symptom and life satisfaction scores at baseline to scores at 26 months indicated that depressive symptoms at 26 months were significantly lower than baseline symptoms, b=−4.12, p=.008 (ß=−.55, large effect size), and there was no interaction with Group, p=.30. Similarly, life satisfaction at 26 months was significantly higher than baseline satisfaction for both groups, b=0.77, p=.001 (ß=.47, medium effect size), and there was no interaction with Group, p=.84.
Discussion
To our knowledge this is the longest follow-up study comparing the effects of MBCT to a structurally equivalent and validated ACC, which was matched to MBCT on non-specific components including: amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance and alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes. Results indicated no differences between MBCT and ACC for relapse/recurrence rates, time to relapse/recurrence, depressive symptoms, or life satisfaction over the 26-month follow-up. Piecewise analyses, which modeled both the original 12-month follow-up period as well as the extended 12–26-month follow-up, corroborated the same pattern of results that were reported in the original investigation from baseline to 12 months. Specifically, both groups experienced a significant reduction in depressive symptoms over the 12-month follow-up. The ACC group experienced initial declines in symptoms, which were followed by a gradual increase in symptoms up to the 12-month follow-up, while the MBCT group experienced steady declines in symptoms from post-intervention to the 12-month follow-up. Results for the extended follow-up period (12–26 months) indicated that the potentially favorable trajectory of symptomatic improvement in the MBCT versus the ACC group from the end of intervention to the 12-month follow-up was not sustained beyond 12 months. Instead, both the MBCT and ACC group experienced a marginally significant increase in depressive symptoms from the 12-month to the 26-month follow-up. However, symptoms in both groups remained significantly lower than baseline at the 26-month assessment. The overall pattern of results for life satisfaction was similar, although slopes of improvement over the 12-month follow-up did not differ between groups. Effect sizes of between-group differences for relapse/recurrence, depressive symptoms, and life satisfaction were all small. Effect sizes for improvements in depressive symptoms and life satisfaction from baseline to the 26-month follow-up were large and medium, respectively.
Confidence in these findings is strengthened by several considerations. First, the two interventions did not differ across four outcomes, thus corroborating the robustness of the present results. Further, all between-group effect sizes were small, suggesting that lack of statistical significance was not due to lack of power. Second, two other studies that have compared MBCT and MBSR to the same ACC used in the present study (Health Enhancement Program) have similarly found a lack of group differences on depression outcomes (Eisendrath et al., 2016; MacCoon et al., 2012). This precedence helps to support the veracity of the present study’s results, which generalize only to MBCT and not a broader range of MBIs in the literature. Third, the strong design sensitivity of this trial (e.g., validity/reliability of measures, stratified randomization, and stringent statistical analyses) minimizes the likelihood of Type II error. Finally, support for internal validity of the intervention comes from high therapist adherence and treatment expectancy and credibility ratings (see original investigation for complete details) that were comparable to other MBCT studies (Kuyken et al., 2008; Meadows et al., 2014; Segal et al., 2010). The quality of the delivery of MBCT is additionally supported by the 48% relapse/recurrence rate observed in the MBCT group, which is comparable to at least three other rigorous MBCT trials (Kuyken et al., 2008, 2015; Williams et al., 2014) and supports that MBCT was delivered as intended. In sum, the lack of differences between MBCT and ACC are substantiated by the consistency of small effect sizes across three indicators of depression (relapse rates, time to relapse, depressive symptoms) as well as life satisfaction, the corroboration with other study findings that have used the same ACC, the strong design sensitivity, and evidence supporting high internal validity.
Moderation analyses for relapse/recurrence outcomes indicated no significant effect modification by age of onset of depression, baseline depressive symptoms, life satisfaction, or ADM use, sex, per-protocol completion, or self-reported practice (ps>.07, all small effect sizes). A marginal interaction between severity of baseline depressive symptoms and intervention group (p=.07) indicated that in individuals with lower baseline depressive symptoms, MBCT vs. ACC was associated with a marginally longer time to relapse/recurrence over 26 months, while in individuals with higher baseline symptoms, MBCT vs. ACC was associated with a marginally shorter time to relapse/recurrence. This result must be interpreted with caution, however, given that our sample size was minimally powered to detect interaction effects and we did not correct for multiple comparisons. Also, this finding is inconsistent with evidence supporting that greater severity of depressive symptoms at baseline is associated with a more beneficial effect of MBCT compared to other active treatments (including rigorous psycho-educational controls) for relapse/recurrence prevention (Kuyken et al., 2016) and depressive symptom reduction (Chiesa et al., 2015). Replication in future, well-powered trials is needed to follow up on this marginal finding. Collectively, however, results from our moderation analyses add to the developing knowledge about for whom MBCT (vs. other psycho-educational interventions) may be effective.
One important consideration is that although depressive relapse/recurrence rates in this study were comparable to those of other MBCT trials, room for improvement remains given that 50% of participants still experienced a depressive relapse/recurrence. Evidence from this 26-month follow-up study supports the possible utility of continued support or booster sessions beyond the 8-week treatment and indicates the need for such support before 12 months post-intervention, when depressive symptoms begin to increase.
Overall, the present results indicate that benefits of MBCT vs. ACC for depression outcomes did not emerge beyond a 12-month follow-up and that MBCT is not more effective than an ACC for preventing depressive relapse/recurrence or reducing depressive symptoms and improving life satisfaction over a 26-month follow-up. Epidemiological data and benchmarks from prior depression prevention trials support that both treatments may have been associated with enduring positive outcomes in terms of relapse/recurrence, depressive symptoms, and life satisfaction. For example, relapse/recurrence rates in people with three or more previous episodes are as high as 80% over two years in placebo-control groups (Kupfer et al., 1992). Further, the 47.8% (MBCT) and the 50% (ACC) relapse/recurrence rates observed in this study are comparable to longer-term relapse rates (e.g., 15 months and 24 months) observed in individuals assigned to mADM conditions in prior studies (Kuyken, 2008; Kuyken et al., 2015). Meta-analyses consistently support that mADM treatment reduces the odds of relapse by two-thirds compared with usual care or placebo (Geddes et al., 2017). Additionally, relapse rates in the current investigation were lower than the 60–80% relapse rates observed in TAU groups in the two seminal MBCT trials (Ma & Teasdale, 2004; Teasdale et al., 2000) and one other investigation of relapse prevention with a 2-year follow-up (Bockting et al., 2005). Although more recent MBCT investigations have found lower relapse/recurrence rates in TAU groups (ranging from 33%–53%) (Bondolfi et al., 2010; Williams et al., 2014) than those observed in earlier trials, these lower rates are hypothesized to be driven by the high caliber of mental health care received in the TAU groups in these studies. For example, improvements in depression management since the seminal MBCT studies (due to amended NICE guidelines in the U.K.) may have yielded lower TAU relapse rates than those found in earlier studies (Williams et al., 2014). Similarly, the high accessibility to mental healthcare in the Swiss healthcare system, where psychiatrists rather than general practitioners are the first line of care, may have resulted in a more rigorous TAU group than would be expected in studies conducted in countries with inferior healthcare systems (Bondolfi et al., 2010). One final consideration in support of the potential efficacy of both interventions tested here is that despite being at high risk for experiencing symptomatic deterioration, depressive symptoms remained lower than baseline levels and did not increase over the 26-month follow-up. Collectively, these considerations support the possibility that both interventions may lead to enduring positive outcomes. However, three-arm studies are needed that compare MBCT vs. ACCs vs. TAU to rule out time-related effects and to confirm the therapeutic benefit of both interventions.
The potential value of both interventions for long-term well-being is significant because mADM is the most common approach to relapse/recurrence prevention and viable options for non-pharmacological management are needed. Also, there are limited options for group-based preventative treatments for depression, which may have cost, and hence scalability, advantages over individual-based approaches for depression prevention. Finally, results highlight that psychosocial treatments that incorporate some of the specific components of the HEP (e.g., physical activity) may be viable treatment options for individuals who cannot access MBCT, which is not yet widely available.
Limitations
The present study had three notable limitations. First, our assessments of relapse/recurrence did not allow us to differentiate between these two outcomes. Distinguishing between depressive relapse (the reemergence of depressive symptoms following remission but preceding recovery) and recurrence (the onset of a new episode following recovery) is important for understanding the efficacy and consistency of effects of preventative interventions for depression and for informing trial design and methods for future investigations (Bockting et al., 2015).
Second, attrition rates over the follow-up assessment period were higher than other MBCT trials. Attrition did not vary between groups and data were missing completely at random for all models with the exception of the hazard model for the ITT sample whereby Pattern 1 was entered as a covariate. Thus, analyses were conducted with appropriate rigor, and statistical model assumptions were met. Higher attrition in the present study may be explained by unique demographic characteristics of the study sample that have been associated with lower retention in other clinical trials (Alexander et al., 2017; Ejiogu et al., 2011). For example, the present sample had greater racial/ethnic diversity, lower average socio-economic status, and lower average age compared to other MBCT studies (Segal et al., 2010; Williams et al., 2014). Thus, greater attrition may be expected due at least in part to potential social and financial disadvantages among these groups (Mein et al., 2012). Relatedly, Caucasian compared with non-Caucasian participants in this trial attended a greater number of sessions (r=.242, p=.02), which may reflect the need to culturally tailor the MBCT and HEP protocols. Overall, lower adherence in this and at least one other community-based trial of MBCT (Meadows et al., 2014) points to potential challenges with the feasibility and acceptability of the delivery of MBCT for relapse prevention in community-based settings with socioeconomically disadvantaged and racially/ethnically diverse participants and in sites away from where the majority of MBCT trials have been conducted (e.g., Western European and Canadian academic medical centers and clinics).
Finally, the sample size for the current investigation was based on available effect sizes in the literature at the start of this trial, which did not include those based on studies that compared MBCT to ACCs. Given effect sizes comparing MBCT to an ACC are likely smaller than those comparing MBCT to TAU, sample sizes needed for studies comparing MBCT to TAU are likely smaller than those needed for studies comparing MBCT to an ACC. This points to a potential issue with power in this study. For example, for our primary outcomes, based on our observed hazards ratio for time to relapse/recurrence (HR = .76), 425 participants per group would be needed to have 80% power to detect group differences at alpha =.05. Based on the difference between relapse/recurrence rates in MBCT vs. ACC (Cohen’s h = −.043), a sample size of >6000 participants per group would be needed to have 80% power to detect group differences at alpha =.05. Future studies should balance the practicality of conducting studies that would require these large sample sizes with considerations for the sample size needed to detect the smallest clinically meaningful difference. In sum, our primary null results are unlikely due to power. Further, these results provide estimates of effect sizes for replication studies and for future Stage III-V effectiveness trials MBCT for depression (Dimidjian & Segal, 2015).
Conclusion
We did not find any evidence that the therapeutic components specific to MBCT (e.g., mindfulness and cognitive therapy) are more effective than the therapeutic components specific to the ACC (e.g., nutrition, physical activity/functional movement, and music therapy) over a 26- month follow-up. Nonetheless, across 26 months, individuals in both treatment conditions experienced lower depressive relapse/recurrence than what has been shown in TAU control groups in prior investigations as well as sustained reductions in depressive symptoms and improvements in life satisfaction from baseline. Future studies that include passive control conditions (e.g., treatment as usual) are needed to examine whether both MBCT and HEP, compared to TAU, may provide benefit for individuals with recurrent major depressive disorder.
Highlights.
Study tested effects MBCT vs. active control condition (ACC) beyond 12-month trial
No emergent effects of MBCT vs. ACC were found over 26-month follow-up
Symptom reduction that initially favored MBCT was not sustained past 12 months
MBCT is not more effective than ACC for depression outcomes over 26-month follow-up
No evidence for effect moderation was found for any outcome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical standards and conflict of interest: Informed consent was obtained from all participants in this study. The authors declare no conflict of interest.
A final censoring time of 31.4 months was used for survival analyses in order to accommodate data collection from a maximum number of participants. Although follow-up times were slightly longer for MBCT vs. ACC (t(55)=−2.40, p=.02), results for relapse rates and time to relapse were unchanged when using the same follow-up period (25.7 months) that was used for depressive symptoms. Final follow-up time for depressive symptoms was based on targeted assessment time (8 weeks of training + 24 months) for both groups.
Sample size for per-protocol analysis was restricted to the 46 participants who completed ≥4 sessions and who provided follow-up data on relapse/recurrence.
Results when including only individuals with ≥3 prior episodes of depression (n=87): Over the 24-month study period, in the ITT sample, 50.0% (22/44) of the MBCT participants relapsed and 51.2% (22/43) of the ACC participants relapsed. In the PP sample, 50.0% (9/16) of the MBCT participants relapsed, compared with 50.0% (13/25) of the ACC participants. Cox regression indicated no difference in incidence and time to relapse between the two treatment groups; ITT: Wald (3, n=87)=3.61; hazard ratio (HR)=.82, CI[0.34, 1.99], p=.67; and PP: Wald (1, n=50)=0.23, HR=.84, CI[0.41, 1.72], p=.63.
Only ITT analyses were used to examine moderators because of limited power in PP sample.
Contributor Information
Amanda J. Shallcross, NYU, School of Medicine
Emily C. Willroth, University of California, Berkeley
Aaron Fisher, University of California, Berkeley.
Sona Dimidjian, University of Colorado, Boulder.
James J. Gross, Stanford University
Pallavi D. Visvanathan, Manhattan Mindfulness-Based Cognitive Behavioral Therapy
Iris B. Mauss, University of California, Berkeley
References
- Alexander GL, Dixit-Joshi S, Kushi LH, Coleman LA, Sundaram ME, Clancy HA, Thompson FE. Comparison of recruitment and retention among demographic subgroups in a large diverse population study of diet. Contemporary Clinical Trials Communications. 2017;6:140–146. doi: 10.1016/j.conctc.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory-II San Antonio. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bockting CLH, Schene AH, Spinhoven P, Koeter MWJ, Wouters LF, Huyser J, Kamphuis JH. Preventing relapse/recurrence in recurrent depression with cognitive therapy: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2005;73(4):647–657. doi: 10.1037/0022-006X.73.4.647. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review. 2015;41:16–26. doi: 10.1016/j.cpr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, Van der Linden M, Gex-Fabry M, Bizzini L, Rouget BW, Bertschy G. Depression relapse prophylaxis with mindfulness-based cognitive therapy: replication and extension in the Swiss health care system. Journal of Affective Disorders. 2010;122(3):224–231. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun SR, Gregor B, Tran US. Comparing bona fide psychotherapies of depression in adults with two meta-analytical approaches. PLoS ONE. 2013;8(6):e68135. doi: 10.1371/journal.pone.0068135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatoor I, Kurpnick J. The role of non-specific factors in treatment outcome of psychotherapy studies. European Child & Adolescent Psychiatry. 2001;10(1):S19. doi: 10.1007/s007870170004. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Castagner V, Andrisano C, Serretti A, Mandelli L, Porcelli S, Giommi F. Mindfulness-based cognitive therapy vs. psycho-education for patients with major depression who did not achieve remission following antidepressant treatment. Psychiatry Research. 2015;226(2–3):474–483. doi: 10.1016/j.psychres.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Research. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Primary Care Companion to the Journal of Clinical Psychiatry. 2004;6(3):104–111. doi: 10.1093/heapro/dar059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffen S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Eisendrath SJ, Gillung E, Delucchi KL, Segal ZV, Nelson JC, McInnes LA, Feldman MD. A randomized controlled trial of mindfulness-based cognitive therapy for treatment-resistant depression. Psychotherapy and Psychosomatics. 2016;85(2):99–110. doi: 10.1159/000442260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendrath SJ, Gillung EP, Delucchi KL, Chartier M, Mathalon DH, Sullivan JC, Feldman MD. Mindfulness-based cognitive therapy (MBCT) versus the health-enhancement program (HEP) for adults with treatment-resistant depression: a randomized control trial study protocol. BMC Complementary and Alternative Medicine. 2014;14:95. doi: 10.1186/1472-6882-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the healthy aging in neighborhoods of diversity across the life span study. Gerontologist. 2011;51(SUPPL. 1) doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine. 2013;10(11) doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders. New York State Psychiatric Institute; 2002. Retrieved from http://www.scid4.org/revisions/november_2001_02.htm. [Google Scholar]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. The Lancet. 2017;361(9358):653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behavior Research and Therapy. 2010;48(8):738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Goldin P, Ziv M, Jazaieri H, Gross JJ. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: effects on the self-referential brain network in social anxiety disorder. Frontiers in Human Neuroscience. 2012 Nov;6:295. doi: 10.3389/fnhum.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Huibers MJH, Cuijpers P. The encyclopedia of clinical psychology. John Wiley & Sons, Inc; 2014. Common (nonspecific) factors in psychotherapy. [DOI] [Google Scholar]
- Huijbers MJ, Spinhoven P, Spijker J, Ruhé HG, van Schaik DJF, van Oppen P, Speckens AEM. Discontinuation of antidepressant medication after mindfulness-based cognitive therapy for recurrent depression: Randomised controlled non-inferiority trial. The British Journal of Psychiatry : The Journal of Mental Science. 2016;208(4):366–73. doi: 10.1192/bjp.bp.115.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL. The clinical course of unipolar major depressive disorders. Archives of General Psychiatry. 1997;54(11):989–991. doi: 10.1001/archpsyc.1997.01830230015002. [DOI] [PubMed] [Google Scholar]
- Kupfer D, Frank E, Perel J, Cornes C, Mallinger A, Thase M, Grochocinski V. Five-year outcome for maintenance therapies in recurrent depression. Archives of General Psychiatry. 1992;49(10):769–73. doi: 10.1001/archpsyc.1992.01820100013002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1417428. [DOI] [PubMed] [Google Scholar]
- Kuyken W. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):13. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): A randomised controlled trial. The Lancet. 2015;386(9988):63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, Dalgleish T. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse. JAMA Psychiatry. 2016;73(6):565. doi: 10.1001/jamapsychiatry.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Lutz A. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behaviour Research and Therapy. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos A, Crawford MJ, Procter S. Music therapy for depression: It seems to work, but how? British Journal of Psychiatry. 2011;199(2):92–93. doi: 10.1192/bjp.bp.110.087494. [DOI] [PubMed] [Google Scholar]
- Mathew KL, Whitford HS, Kenny MA, Denson LA. The long-term effects of mindfulness-based cognitive therapy as a relapse prevention treatment for major depressive disorder. Behavioural and Cognitive Psychotherapy. 2010 Apr;38:1–16. doi: 10.1017/S135246581000010X. [DOI] [PubMed] [Google Scholar]
- Meadows GN, Shawyer F, Enticott JC, Graham AL, Judd F, Martin PR, Segal Z. Mindfulness-based cognitive therapy for recurrent depression: A translational research study with 2-year follow-up. Australian and New Zealand Journal of Psychiatry. 2014;48(8):743–755. doi: 10.1177/0004867414525841. [DOI] [PubMed] [Google Scholar]
- Mein G, Johal S, Grant RL, Seale C, Ashcroft R, Tinker A. Predictors of two forms of attrition in a longitudinal health study involving ageing participants: An analysis based on the Whitehall II study. BMC Medical Research Methodology. 2012;12(1):164. doi: 10.1186/1471-2288-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak J, Holz A, Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychol Psychother. 2011;84(2):230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- Munshi K, Eisendrath S, Delucchi K. Preliminary long-term follow-up of mindfulness-based cognitive therapy-induced remission of depression. Mindfulness (N Y) 2013;4(4):354–361. doi: 10.1007/s12671-012-0135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie RS, O’Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public Health Nutr. 2014:1–20. doi: 10.1017/S1368980014002614. [DOI] [PMC free article] [PubMed]
- Parikh SV, Zaretsky A, Beaulieu S, Yatham LN, Young LT, Patelis-Siotis I, Streiner DL. A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: A Canadian Network for Mood and Anxiety Treatments (CANMAT) study. Journal of Clinical Psychiatry. 2012;73(6):803–810. doi: 10.4088/JCP.11m07343. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Crane C, Parsons LJ, Fjorback LO, Kuyken W. Home practice in mindfulness-based cognitive therapy and mindfulness-based stress reduction: A systematic review and meta-analysis of participants’ mindfulness practice and its association with outcomes. Behaviour Research and Therapy. 2017;95(Supplement C):29–41. doi: 10.1016/j.brat.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clinical Psychology Review. 2011;31(6):1032–1040. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Proschan MA, McMahon RP, Shih JH, Hunsberger SA, Geller NL, Knatterud G, Wittes J. Sensitivity analysis using an imputation method for missing binary data in clinical trials. Journal of Statistical Planning and Inference. 2001;96(1):155–165. doi: 10.1016/S0378-3758(00)00332-3. [DOI] [Google Scholar]
- Segal ZV, Teasdale JD, Williams JM, Gemar MC. The mindfulness-based cognitive therapy adherence scale: Inter-rater reliability, adherence to protocol and treatment distinctiveness. Clinical Psychology and Psychotherapy. 2002;9(2):131–138. doi: 10.1002/cpp.320. [DOI] [Google Scholar]
- Segal ZV, Martin L, Joseph S. Antidepressant monotherapy versus sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168.Antidepressant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Williams M, Teasdale JD. Mindfulness based cognitive therapy for depression: A new approach to preventing relapse. New York, NY: The Guilford Press; 2002. [Google Scholar]
- Shallcross AJ, Gross JJ, Visvanathan PD, Kumar N, Palfrey A, Ford BQ, Mauss IB. Relapse prevention in major depressive disorder: Mindfulness-based cognitive therapy versus an active control condition. Journal of Consulting and Clinical Psychology. 2015;83(5):964–975. doi: 10.1037/ccp0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca GA, Gallop R. Multilevel modeling of longitudinal data for psychotherapy researchers: I. The basics. Psychotherapy Research. 2009 Mar;19:429–437. doi: 10.1080/10503300802641444. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68(4):615–623. doi: 10.1037//0022-006X.68.4.615. [DOI] [PubMed] [Google Scholar]
- Wampold BE. How important are the common factors in psychotherapy? An update. World Psychiatry. 2015;14(3):270–277. doi: 10.1002/wps.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Russell IT. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: A randomized dismantling trial. Journal of Consulting and Clinical Psychology. 2014;82(2):275–286. doi: 10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]


