Abstract
Background: The issue of whether radiation-induced thyroid cancer is pathologically different from sporadic remains not fully answered. This study compared structural characteristics and invasive features of papillary thyroid carcinoma (PTC) in two age-matched groups: patients who were children (≤4 years old) at the time of the Chernobyl accident and who lived in three regions of Ukraine most contaminated by radioactive iodine 131I (“radiogenic” cancer), and those who lived in the same regions but who were born after 1987 and were not exposed to 131I (“sporadic” cancer). Further, the histopathologic features of PTC were analyzed in relation to age and individual 131I thyroid dose.
Methods: The study included 301 radiogenic and 194 sporadic PTCs. According to age at surgery, patients were subdivided into children (≤14 years old), adolescents (15–18 years old), and adults (19–28 years old). Statistical analyses included univariate tests and multivariable logistic regression within and across the age subgroups. Analyses of morphological features related to 131I doses were conducted among exposed patients on categorical and continuous scales controlling for sex and age.
Results: Among children, radiogenic PTC displayed a significantly higher frequency of tumors with a dominant solid growth pattern, intrathyroidal spread, extrathyroidal extension, lymphatic/vascular invasion, and distant metastases. Exposed adolescents more frequently displayed extrathyroidal extension, lymphatic/vascular invasion, and distant metastases. Exposed adults more frequently had intrathyroidal spread and extrathyroidal extension. The frequency of PTC with dominant papillary pattern and oxyphilic cell metaplasia was significantly lower in radiogenic compared to sporadic tumors for all age groups. Manifestations of tumor aggressiveness were most frequent in children compared to adolescents and adults regardless of etiology.
Conclusions: Radiogenic PTC is less likely to demonstrate a dominant papillary growth pattern and more likely to display more aggressive tumor behavior than sporadic PTC. Histopathologic tumor aggressiveness declines with patient age in both radiogenic and sporadic cases.
Keywords: : papillary thyroid carcinoma, Chernobyl, radiation, pathology, age-matched groups, 131I thyroid dose
Introduction
Amajor health consequence of the Chernobyl nuclear power plant (CNPP) accident for the population was a sharp increase in the incidence of thyroid cancer among subjects aged ≤18 years living in Ukraine, Belarus, and parts of the Russian Federation in 1986 (1–7). The incidence of thyroid cancer in Ukraine has been shown to be substantially higher in six northern regions closest to the CNPP site, and particularly in three of those—Kiev, Zhytomyr, and Chernihiv—which were the regions most contaminated by radioactive iodine (131I). The highest 131I dose-dependent excess in incidence was observed in children whose age in 1986 was four years or younger (6–11).
Many studies consistently reported that papillary thyroid carcinoma (PTC) is the main histological type of post-Chernobyl thyroid cancer, but a unique “structural portrait” of “radiogenic” PTC has not been established (12–18). Further, most histopathologic characteristics of Ukrainian PTCs were shown to change with patient age at surgery and/or time since the Chernobyl accident (i.e., with latency) (15). Specifically, the frequency of architecturally less differentiated PTC with the solid or solid-follicular growth pattern and of more aggressive tumors featuring intrathyroidal spread, extrathyroidal extension, lymphatic/vascular invasion, and regional and distant metastases was found to decrease with older age at surgery and/or longer time since the accident. The frequency of more differentiated PTCs with the papillary or papillary–follicular growth pattern, ≤10 mm in size, and fully encapsulated tumors was found to increase with age/time. The latter characteristics may be considered favorable, since small and encapsulated PTCs are, in general, clinically more indolent than large and non-encapsulated tumors. Thus, based on morphological characteristics of post-Chernobyl PTCs, it appears that these become less aggressive with age/time since the accident. In support, PTCs detected after a longer period of latency in patients exposed to Chernobyl radiation have been shown to display fewer of the ultrasound features characteristic of a malignant process (19). Interestingly, the structural characteristics of “sporadic” PTCs diagnosed in unexposed to 131I individuals are also known to change with increasing age at surgery (20,21).
The primary objectives of the current study were (i) to compare structural characteristics and invasive features of PTCs in three age-matched groups of patients (≤14, 15–18, and 19–28 years of age) exposed to 131I in early childhood as a result of the Chernobyl accident (i.e., radiogenic PTC) to those born after the Chernobyl accident in 1987 or later and therefore unexposed to 131I (i.e., sporadic PTC), and (ii) to evaluate how structural and invasive characteristics of PTCs change with age. As histopathologic features of PTC invasiveness were associated with 131I thyroid dose in the Belarusian (22) and Ukrainian screening cohorts (16), the study also evaluated if these were related to individual thyroid doses among exposed patients.
Methods
Patients
The study protocol was approved by the ethics committee of the Institute of Endocrinology and Metabolism (IEM) of NAMS of Ukraine (Kiev, Ukraine).
The study included 495 Ukrainian PTC cases selected among 3991 PTCs operated at IEM between 1990 and 2015, when a significant rise in thyroid cancer incidence was registered in Ukraine. Considering that the highest risk of thyroid cancer following the Chernobyl accident was observed in the youngest children residing in the most contaminated regions (6–11), the radiogenic cases were defined as those who were four years old or younger in 1986 and who lived in the Kiev, Chernihiv, or Zhytomyr regions. The comparison group included sporadic PTC cases selected among those who were born after the accident (January 1, 1987, or later and not exposed to 131I) and lived in the same regions as the radiogenic cases or Kiev city. Sporadic cases were closely matched to radiogenic cases on age at surgery (Table 1). Because, as of 2015, sporadic cases could not be >28 years of age, and in order to ensure comparability on age, no radiogenic cases >28 years of age were included in the study. Active screening for the detection of sporadic thyroid carcinomas cases was not performed in Ukraine. In total, there were 301 radiogenic PTCs (121 children, 66 adolescents, and 114 adults) selected from 3460 cases born before the Chernobyl accident on age at surgery, age at exposure, and residence. There were also 194 sporadic PTCs (60 children, 54 adolescents, and 80 adults) selected from 531 cases born after the Chernobyl accident on age at surgery and residence.
Table 1.
Descriptive Characteristics of “Radiogenic” and “Sporadic” PTC
| Children (4 to <14 years at surgery) | Adolescents (15 to 18 years at surgery) | Adults (≥19 to 28 years at surgery) | All cases (4–28 years at surgery) | |||||
|---|---|---|---|---|---|---|---|---|
| Radiogenic (n = 121) | Sporadic (n = 60) | Radiogenic (n = 66) | Sporadic (n = 54) | Radiogenic (n = 114) | Sporadic (n = 80) | Radiogenic (n = 301) | Sporadic (n = 194) | |
| Characteristics | n (%) or mean (SD)a | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) |
| Female | 72 (59.5) | 49 (81.7) | 44 (66.7) | 39 (72.2) | 86 (75.4) | 67 (83.8) | 202 (67.1) | 155 (79.9) |
| Male | 49 (40.5) | 11 (18.3) | 22 (33.3) | 15 (27.8) | 28 (24.6) | 13 (16.2) | 99 (32.9) | 39 (20.1) |
| Age at exposure, years | 1.9 (1.2) | — | 1.6 (1.1) | — | 2.0 (1.3) | — | 1.9 (1.2) | — |
| Age at surgery, years | 11.5 (2.4) | 12.6 (2.1) | 16.7 (1.1) | 17.0 (1.0) | 23.9 (3.1) | 23.0 (2.5) | 16.9 (6.0) | 17.6 (4.9) |
| Oblast | ||||||||
| Kievb | 53 (43.8) | 41 (68.3) | 19 (28.8) | 42 (77.8) | 54 (47.4) | 66 (82.4) | 126 (41.9) | 149 (76.8 |
| Chernihiv | 35 (28.9) | 9 (15.0) | 23 (34.8) | 7 (13.0) | 44 (38.6) | 7 (8.8) | 102 (33.9) | 23 (11.9) |
| Zhytomyr | 33 (27.3) | 10 (16.7) | 24 (36.4) | 5 (9.2) | 16 (14.0) | 7 (8.8) | 73 (24.2) | 22 (11.3) |
| 131I thyroid dose, mGy | 984.0 (2113.3) | — | 1333.3 (3438.4) | — | 667.5 (1409.2) | — | 940.7 (2271.3) | — |
| CTB dose group | ||||||||
| 1 | 9 (7.4) | — | 12 (18.2) | — | 26 (22.8) | — | 47 (15.6) | — |
| 2 | 1 (0.8) | — | 2 (3.0) | — | 2 (1.8) | — | 5 (1.7) | — |
| 3-1 | 66 (54.6) | — | 19 (28.8) | — | 40 (35.1) | — | 125 (41.5) | — |
| 3-2 | 15 (12.4) | — | 8 (12.1) | — | 9 (7.9) | — | 32 (10.6) | — |
| 3-3 | 30 (24.8) | — | 25 (37.9) | — | 37 (32.4) | — | 92 (30.6) | — |
% for count data, SD for thyroid dose.
Including Kiev city for sporadic cases.
PTC, papillary thyroid carcinoma; SD, standard deviation; 131I, radioactive iodine; CTB, Chernobyl Tissue Bank.
Histopathology
Histopathologic examination of hematoxylin and eosin stained paraffin sections was initially performed at the Laboratory of Morphology of Endocrine System (IEM, Kiev, Ukraine) by two experienced pathologists (T.B. and L.Z.). The pathological classification was based on World Health Organization (WHO) definitions (23). All diagnoses were reviewed and confirmed by international experts in the course of joint research projects and/or at the Chernobyl Tissue Bank (CTB) Pathology Panel sessions (24). Tumors were further classified according to the dominant histological growth pattern into three categories: papillary, follicular, or solid-trabecular (when corresponding structure represented >50% of a tumor). In addition, PTCs were assigned main histological variants according to the WHO classification (23) as follows: classic papillary, follicular, solid-trabecular, diffuse-sclerosing, or Warthin-like (when >80% of the tumor section had corresponding structure).
Previous studies showed that invasive properties of PTC significantly differed between fully encapsulated and non-encapsulated or partially encapsulated tumors (15). Further, in the new fourth edition of the WHO histological classification, encapsulated PTCs are considered as a separate PTC variant (25). Therefore, an analysis of morphological parameters was performed for all tumors and, separately, for fully encapsulated and non-encapsulated tumors.
The study also evaluated oxyphilic (oncocytic/Hürthle) cell metaplasia (focal and severe, including oxyphilic cell PTC). Tumor stage was defined according to the seventh edition of the TNM classification system (26), which assigns minimal extrathyroidal extension pT3 category. Only marked intrathyroidal spread of the tumor to the lobe(s) was considered positive, including the diffuse-sclerosing variant (DSV)-like spread. Distant metastases to the lung were determined by radioactive iodine scans performed following thyroidectomy.
Dosimetry
Estimated thyroid doses due to 131I intake were based on the thyroid dose system used for CTB cases (“TD-CTB”) (10,11). All patients were subdivided into dose groups and subgroups, depending on the availability of radioactivity measurements performed in May–June 1986 either directly on the subject or upon other persons in the same settlement.
Group 1 included subjects who had a direct thyroid measurement performed in May–June 1986 and who were administered a special interview concerning their consumption of contaminated foods (essentially cow's milk and leafy vegetables), as well as possible changes of residence in May–June 1986. The interviews of these subjects were performed in the framework of the Ukraine–USA cohort study (27).
Group 2 included subjects who had individual direct thyroid measurements carried out in May–June 1986 but no personal interviews.
Group 3 included subjects with neither individual direct thyroid measurements in May–June 1986 nor personal interviews. These subjects were divided into three subgroups depending on their residence or non-residence in the settlements of different regions where direct measurements were performed among the local population:
Subgroup 3-1 included subjects who resided in May–June 1986 in settlements where direct thyroid measurements were performed on some inhabitants.
Subgroup 3-2 included subjects who resided in settlements where no direct thyroid measurements were performed, but such measurements were performed on some inhabitants of neighboring settlements of the region.
Subgroup 3-3 included subjects who were resident of settlements where direct thyroid measurements were not carried out at all.
Thyroid doses were estimated for every patient using the ecological and dosimetric model that includes iodine ecological transport and iodine bio-kinetic models. The model was adapted to available information about each patient's residence, his/her diet, and individual direct thyroid measurements in May–June 1986. Dose uncertainties were calculated by Monte Carlo simulation procedure, with 1000 trials for every subject (10,11).
Statistical analysis
Univariate Fisher's exact test or chi-square test for categorical data and Mann–Whitney or Kruskal–Wallis tests for continuous quantitative data were used to compare characteristics of radiogenic and sporadic PTC cases. Multivariable logistic regression models were adjusted for age at surgery and sex. Analyses were performed using IBM SPSS Statistics for Windows v21 (IBM Corp., Armonk, NY). Analyses with very small numbers of outcomes (<5 per cell) were conducted using exact logistic regression using the SAS v9.3 (SAS Institute, Inc., Cary, NC). The p-values were two-sided and were considered significant if p < 0.05.
Changes in histopathologic characteristics of PTCs were assessed across age subgroups using the chi-square test for trend (Cochran–Armitage test) for categorical and Jonckheere–Terpstra test for quantitative data. Heterogeneity of age trends was evaluated using a one degree of freedom likelihood ratio test for nested logistic regression models with and without an interaction term between exposure indicator and age. This analysis was performed using Epicure software (Risk Sciences International, Ottawa, Canada). Note that age at surgery in exposed individuals is a linear combination of age at exposure and time since exposure. Hence, trends in histopathologic characteristics of radiogenic PTCs also reflect time elapsed since the date of the Chernobyl accident or latency.
Logistic regression models were used to evaluate associations between individual histopathologic characteristics and 131I thyroid dose in exposed individuals. Odds ratio (OR) was determined for each characteristic with several dose categories chosen to assure reasonable distribution of cases. Continuous dose trends were also evaluated based on linear and linear-quadratic (on a log scale) dose–response models. All dose–response models were adjusted for sex and age at surgery unless otherwise specified. Dose–response analyses were performed using SAS software. All tests were two-sided and based on maximum likelihood ratios. A p-value of <0.05 was considered statistically significant.
Results
Descriptive characteristics of radiogenic and sporadic PTC in age-matched groups
The age at surgery in radiogenic and sporadic cases was comparable due to study design. In all age groups, female patients were more common (Table 1), although their proportion tended to be lower in radiogenic compared to sporadic PTCs, particularly in children (odds ratio [OR] = 0.33; p = 0.003). In both series, radiogenic and sporadic, the frequency of PTC from the Kiev region was generally higher than from the Chernihiv or Zhytomyr regions, especially in the sporadic series due to the inclusion in the analysis the cases from Kiev city (Table 1).
The average thyroid dose in the radiogenic series was 940.7 mGy (Table 1). The doses in childhood and adolescent groups did not differ significantly, but in adults they were lower, reaching significance compared to the childhood group (p = 0.027).
Only 52 (17.3%) patients with radiogenic PTC had direct radioactivity measurements (dose groups 1 and 2). Among the remaining 249 (82.7%) patients without direct measurements (dose group 3), 157 were in dose subgroups 3-1 and 3-2, and 92 were in in dose subgroup 3-3 (Table 1).
Comparative histopathologic characteristics of radiogenic and sporadic PTC in age-matched groups
In all age groups, non-encapsulated PTCs were most frequent, accounting for 70–95% of tumors. The proportion of non-encapsulated tumors was generally higher in radiogenic compared to sporadic PTCs, but the difference was significant only in children (p = 0.003; Fig. 1 and Supplementary Table S1 for statistical details; Supplementary Data are available online at www.liebertpub.com/thy).
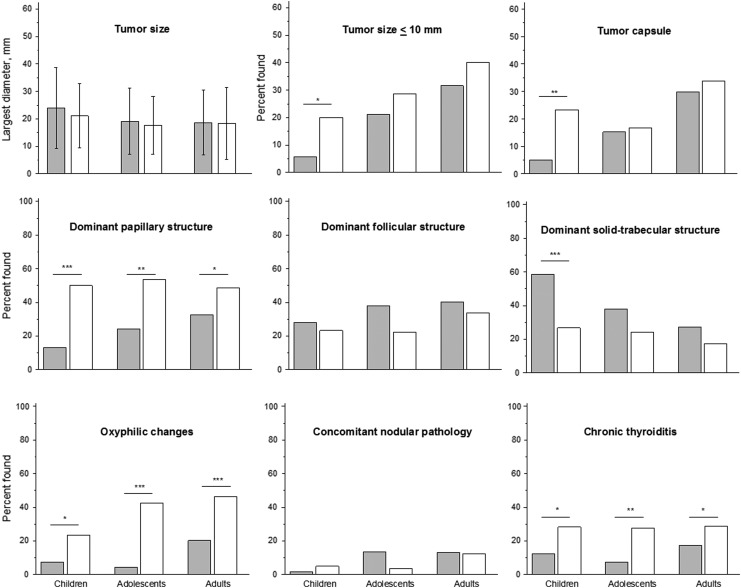
FIG. 1.
Histopathologic characteristics of all cases of “radiogenic” (filled bars) and “sporadic” (open bars) papillary thyroid carcinoma (PTC) in different age subgroups of patients. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
There were no significant differences between radiogenic and sporadic PTCs in mean size of tumors in age-matched groups. Within each age group, the ORs for microcarcinoma in radiogenic relative to sporadic PTC were <1, reaching significance in children (p = 0.020; Fig. 1 and Supplementary Table S1).
Analysis of the dominant growth pattern showed that in all age groups, the frequency of tumors with papillary structure was significantly lower in radiogenic than sporadic PTC. This was mainly due to non-encapsulated tumors (p < 0.05 for each group; Fig. 1 and Supplementary Table S1). A dominant follicular growth pattern tended to be more frequent in radiogenic PTC, particularly in non-encapsulated tumors, reaching significance in adolescents (p = 0.024). Similarly, dominant solid-trabecular pattern was more frequent in radiogenic PTC. The difference, in comparison to sporadic tumors, was significant for all tumors and non-encapsulated PTCs in children (p < 0.0001) and fully encapsulated PTCs in adults (p = 0.034; Supplementary Table S1).
It should be noted that the dominant growth pattern largely reflects histological subtype/variant of PTC or the prevalent structural component of tumors with mixed structure. Rarer PTC subtypes such as DSV and Warthin-like were identified in a small number of cases. DSV was observed in all age groups of radiogenic PTC: in 8/121 (6.6%) cases in children, 1/66 (1.5%) in adolescents, and 1/114 (0.9%) in adults. In all radiogenic cases, DSV tumors had solid foci spreading to both thyroid lobes. In sporadic PTC, DSV was detected only in children (2/60; 3.3%). Overall, DSV was 3.3 times more frequent in radiogenic (10/301; 3.3%) than sporadic PTC (2/194; 1.0%), but the difference was not significant (p = 0.138).
The Warthin-like variant was absent in children but was found in 1/66 (1.5%) of radiogenic and 1/54 (1.9%) of sporadic adolescent PTC, and in 4/80 (5.0%) of sporadic adult PTC. All Warthin-like PTCs had a dominant papillary pattern. Overall, the Warthin-like variant was 8.7 times less frequent in radiogenic (1/301; 0.3%) than sporadic PTCs (5/194; 2.6%); the difference was statistically significant (p = 0.036). No tall-cell or columnar-cell variants of PTC were found in the entire case series.
In all age groups, oxyphilic cell metaplasia was significantly more common in sporadic cases (p < 0.05 for any comparison except for children and adolescents with fully encapsulated tumors; Supplementary Table S2). Radiogenic PTC in all age groups had a significantly lower frequency of concomitant diffuse thyroid pathology, specifically chronic thyroiditis, than sporadic PTC for all and non-encapsulated tumors (p < 0.05 for any comparison except for fully encapsulated tumors; Supplementary Table S2).
Comparative morphological features of tumor invasiveness of radiogenic and sporadic PTC in age-matched groups
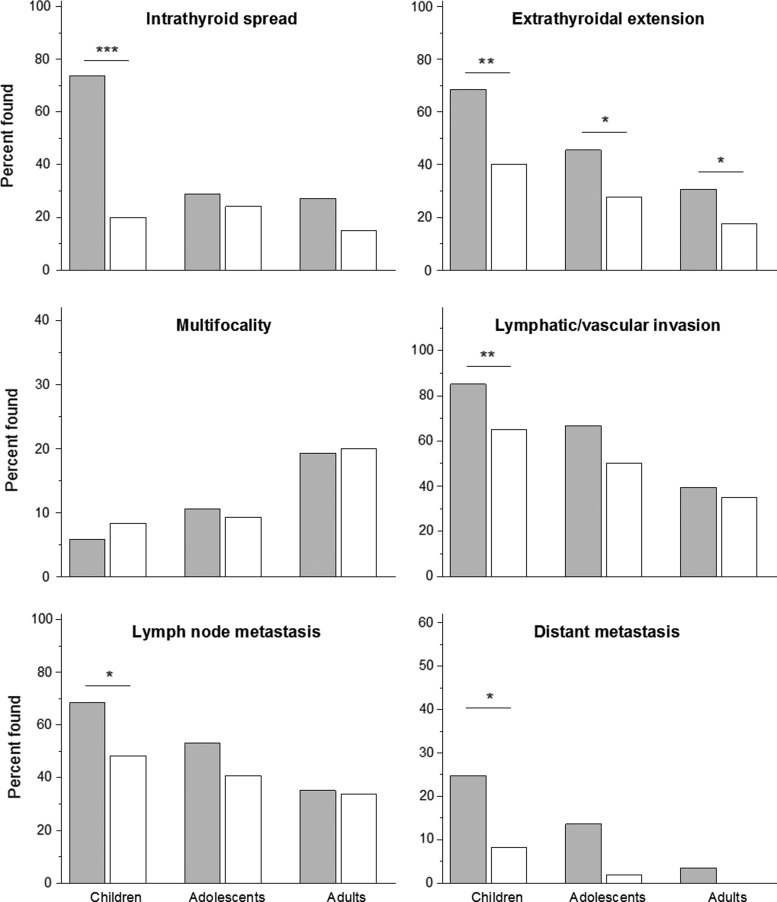
In children, considering all cases, a higher frequency of the following aggressive features was associated with exposure status: intrathyroidal spread (p < 0.0001), extrathyroidal extension (p = 0.002), lymphatic/vascular invasion (p = 0.003), regional lymph node (p = 0.041), and distant metastases (p = 0.026; Fig. 2 and Supplementary Table S3). In exposed adolescents, the frequencies of extrathyroidal extension (p = 0.050), lymphatic/vascular invasion (p = 0.052), and distant metastases (p = 0.054), were marginally significant. In exposed adults, only extrathyroidal extension was marginally more frequent (p = 0.046).
FIG. 2.
Invasive features of all cases of radiogenic (filled bars) and sporadic (open bars) PTC in different age subgroups of patients. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
In analysis of non-encapsulated tumors in children, the significant association for intrathyroidal spread persisted (p < 0.0001), while extrathyroidal extension became marginally significant (p = 0.050; Supplementary Table S3). In adolescents, the associations for extrathyroidal extension (p = 0.050), lymphatic/vascular invasion (p = 0.033), and distant metastases (p = 0.041) were statistically significant. In exposed adults, one significant association was found for intrathyroidal spread (p = 0.041).
Considering fully encapsulated tumors, a significantly higher OR was found for lymphatic/vascular invasion (p = 0.033) in exposed children, and for tumor capsule invasion (p = 0.034) in adults (Supplementary Table S3). The overall frequency of encapsulated PTC with dominant follicular growth pattern was 42% (21/50: 4 in children, 5 in adolescents, and 12 in adults) in the radiogenic series and 54% (27/50: 8 in children, 5 in adolescents, and 14 in adults) in the sporadic series (p = 0.736). Invasive properties of these tumors were not statistically different in any age subgroup between the radiogenic and sporadic series. At the same time, all ORs for invasive features in the combined radiogenic versus combined sporadic group analysis were >1, from 1.76 for tumor spread beyond own capsule to 3.30 for lymphatic/vascular invasion to the tumor capsule vessels (data not shown). In addition, only in the radiogenic series, encapsulated PTC with dominant follicular architecture displayed extrathyroidal extension to the adjacent fat and connective tissues in one childhood patient and in one adult patient, and lymph node metastasis in one childhood patient. These observations may suggest a higher morphological aggressiveness of follicular-patterned encapsulated radiogenic tumors, but larger number of relevant cases would be necessary for statistical assessment.
Age-related trends of histopathologic characteristics of radiogenic and sporadic PTC
Several morphologic tumor characteristics in radiogenic PTC varied with age (Table 2). The proportion of fully encapsulated tumors (ptrend < 0.0001) as well as of micro-PTC (ptrend < 0.0001) for both all and non-encapsulated tumors, but not for encapsulated PTC (ptrend = 0.839; Supplementary Table S4), increased with patient age. A similar trend for small-sized tumors was found in sporadic PTC (ptrend = 0.010 and ptrend = 0.005 for all and non-encapsulated tumors, respectively), but not for encapsulated PTC (ptrend = 0.631; Supplementary Table S4).
Table 2.
Age-Related Trends for Different Characteristics of Radiogenic and Sporadic PTC in Age-Matched Groups
| Characteristics | Radiogenic ptrend | Sporadic ptrend | phet |
|---|---|---|---|
| Histopathology | |||
| Capsule | <0.0001↑ | 0.127 | 0.014 |
| Size (mean, mm) | |||
| All cases | 0.002↓ | 0.030↓ | 0.001 |
| Non-encapsulated | <0.0001↓ | <0.0001↓ | <0.0001 |
| Size ≤10 mm | |||
| All cases | <0.0001↑ | 0.010↑ | 0.094 |
| Non-encapsulated | <0.0001↑ | 0.005↑ | 0.289 |
| Dominant pattern | |||
| Papillary | |||
| All cases | <0.001↑ | 0.851 | 0.014 |
| Non-encapsulated | <0.0001↑ | 0.671 | 0.006 |
| Follicular | |||
| All cases | 0.048↑ | 0.150 | 0.956 |
| Non-encapsulated | 0.016↑ | 0.133 | 0.943 |
| Solid-trabecular | |||
| All cases | <0.0001↓ | 0.187 | 0.098 |
| Non-encapsulated | <0.0001↓ | 0.387 | 0.021 |
| Oxyphilic changes | 0.003↑ | 0.007↑ | 0.627 |
| Concomitant nodular pathology | 0.001↑ | 0.082 | 0.680 |
| Concomitant diffuse pathology | 0.110 | 0.733 | 0.198 |
| Chronic thyroiditis | 0.254 | 0.950 | 0.492 |
| Invasive features | |||
| Intrathyroid spread | |||
| All cases | <0.0001↓ | 0.408 | 0.002 |
| Non-encapsulated | <0.0001↓ | 0.526 | 0.015 |
| Extrathyroidal extension (T3) | |||
| All cases | <0.0001↓ | 0.003↓ | 0.357 |
| Non-encapsulated | <0.0001↓ | 0.009↓ | 0.781 |
| Multifocality (Tm) | |||
| All cases | 0.001↑ | 0.038↑ | 0.728 |
| Non-encapsulated | 0.003↑ | 0.046↑ | 0.866 |
| Lymphatic/vascular invasion | |||
| All cases | <0.0001↓ | <0.001↓ | 0.047 |
| Non-encapsulated | <0.0001↓ | 0.001↓ | 0.413 |
| Lymph node metastases (N1) | |||
| All cases | <0.0001↓ | 0.081 | 0.089 |
| Non-encapsulated | 0.001↓ | 0.242 | 0.354 |
| Distant metastases (M1) | |||
| All cases | <0.0001↓ | 0.006↓ | 0.256 |
| Non-encapsulated | <0.0001↓ | 0.008↓ | 0.183 |
phet, p-value for heterogeneity; ↑, an uptrend; ↓, a downtrend.
The mean size of all and non-encapsulated tumors tended to decrease with increasing age, regardless of exposure status (ptrend < 0.05 for any comparison).
Clear age trends were found for the dominant growth pattern in radiogenic PTC (Table 2). Frequencies of tumors with the papillary (ptrend < 0.001 and ptrend < 0.0001 for all and non-encapsulated PTC, respectively) or follicular (ptrend = 0.048 and ptrend = 0.016 for all and non-encapsulated PTC, respectively) growth patterns increased with patient age. By contrast, the frequency of tumors with solid-trabecular structure decreased with age (ptrend < 0.001 and ptrend < 0.001 for all and non-encapsulated PTC, respectively). No age-related trends were found for the dominant growth pattern in sporadic PTC.
Significant positive age trends were also observed for oxyphilic cell metaplasia in both radiogenic and sporadic PTC (ptrend = 0.003 and ptrend = 0.007, respectively; Table 2). For concomitant nodular pathology, an increasing with age trend was found in radiogenic (ptrend = 0.001) but not in sporadic PTC.
The detected age trends were significantly more pronounced in radiogenic than in sporadic PTC for the increasing frequency of encapsulated tumors (phet = 0.014; Table 2) and tumors with the papillary dominant growth pattern (phet = 0.014 and phet = 0.006 for all and non-encapsulated PTCs, respectively), and for the decreasing frequency of non-encapsulated tumors with the solid-trabecular structure (phet = 0.021).
Age-related trends of morphological invasiveness of radiogenic and sporadic PTC
The frequency of most invasive tumor features significantly decreased with patient age in both radiogenic and sporadic PTC overall and in non-encapsulated tumors (Table 2).
In radiogenic PTC, an age-related decrease was observed in all tumors combined and in non-encapsulated tumors for intrathyroidal spread and extrathyroidal extension (ptrend < 0.0001 for any comparison), lymphatic/vascular invasion (ptrend < 0.0001 for any comparison), lymph node metastases (ptrend < 0.0001 and ptrend = 0.001, respectively), and distant metastases (ptrend < 0.0001 for any comparison). An age-related decrease was also significant for lymphatic/vascular invasion and lymph node metastases (ptrend < 0.001 and ptrend = 0.036, respectively) in fully encapsulated tumors (Supplementary Table S4).
In sporadic PTC, an age-related decrease in tumor invasiveness was found in all tumors combined and non-encapsulated tumors for extrathyroidal extension (ptrend = 0.003 and ptrend = 0.009, respectively), lymphatic/vascular invasion (ptrend < 0.001 and ptrend = 0.001, respectively), and distant metastases (ptrend = 0.006 and ptrend = 0.008, respectively). In contrast with other characteristics of tumor invasiveness, multifocality showed significant uptrend with age for all and non-encapsulated tumors in both radiogenic (ptrend = 0.001 and ptrend = 0.003, respectively) and sporadic PTC (ptrend = 0.038 and ptrend = 0.046, respectively; Table 2).
Age-related trends for most histopathologic characteristics of tumor invasiveness did not show heterogeneity between radiogenic and sporadic tumors (Table 2). The decrease in frequency of intrathyroidal spread for all and non-encapsulated PTCs (phet = 0.002 and phet = 0.015, respectively) and of lymphatic/vascular invasion for all cases (phet = 0.047) were the only trends that were more pronounced in radiogenic that in sporadic PTC.
Associations between histopathologic characteristics of radiogenic PTCs and 131I dose
The mean 131I thyroid dose in radiogenic PTC cases was 984.0 mGy (SD = 2113.3 mGy) in children, 1333.3 mGy (SD = 3438.4 mGy) in adolescents, and 667.5 mGy (SD = 1409.2 mGy) in adults (Table 1). In categorical and continuous analyses of 131I dose, combining all age groups, little evidence was found of an independent dose–response with 131I dose for most morphological or invasive PTC characteristics (Supplementary Table S5). The only associations detected were for tumor size for which the overall OR per Gy for ≤10 mm versus >10 mm PTCs was significantly elevated (OR = 1.20 [confidence interval (CI) 1.05–1.38], p = 0.004). However, this increase was solely attributed to individuals exposed to ≥2 Gy). The other associations were before adjustment for tumor size, concomitant nodular thyroid pathology (a positive correlation: 1.24 [CI 1.08–1.45]; p = 0.003), and extrathyroidal extension (an inverse association with the increasing dose: 0.87 [CI 0.74–0.98]; p = 0.031).
Discussion
This study is the first detailed comparative histopathologic analysis of radiogenic PTC associated with the Chernobyl fallout and sporadic tumors (detected in patients born after the Chernobyl accident) in groups of children, adolescents, and adults matched on age at surgery. The few previous studies addressing this issue did not find significant differences between potentially radiogenic (born before Chernobyl) and sporadic (born after Chernobyl) PTC in children and adolescents from Ukraine and Belarus (14,17,28), or used a different study design (18), making direct comparison with the current results difficult.
By contrast to a previous study (29), here, cases of sporadic PTC were selected from the same regions as cases of radiogenic PTC to control for potential confounding by geographical differences (e.g., by variable degree of iodine deficiency). Histopathologic features were also analyzed in fully encapsulated and non-encapsulated (including partially encapsulated) PTC separately because fully encapsulated PTC is overall morphologically less aggressive (15). Inclusion of childhood, adolescent, and adult cases of PTC allowed us to assess age-dependent (and/or latency-related, applicably to radiogenic PTC) changes in histopathologic characteristics of radiogenic and sporadic tumors.
Similar to previous reports, the present study suggests that a single structural “portrait” of radiogenic PTC, which could be used to discriminate this etiological form of PTC, is unlikely to exist in any age subgroup. At the same time, by reducing the number of parameters characterizing tumor architecture from nine histological subtypes/variants (29) to three dominant growth patterns, independent associations were found for the dominant solid-trabecular growth pattern with younger age at surgery and radiation exposure (see Fig. 1 and Supplementary Table S1). Interestingly, a significant association was observed for the dominant solid-trabecular pattern with radiogenic PTC in two age groups—children and adults—but the invasive features of tumors were radically different in these groups. In children, the solid-trabecular dominant pattern was most frequently observed in non-encapsulated (more aggressive) tumors (see Supplementary Table S1), while in adults, on the contrary, these were found in fully encapsulated (least aggressive) PTCs. This finding was consistent with the opposite age-related trends for the dominant solid-trabecular pattern in non-encapsulated (a downtrend) and fully encapsulated radiogenic PTC (an uptrend). Note also that a solid growth pattern is frequently associated with RET/PTC3 rearrangements in childhood PTC, while in PTC from adult patients, this genetic abnormality is rare (21,30,31).
The dominant papillary pattern was common in sporadic PTC in all age groups in non-encapsulated tumors. The frequency of tumors with dominant papillary growth pattern in sporadic cases did not change significantly with increasing patient age at surgery, but increased with age in radiogenic PTC. This difference was statistically significant (see Table 2). The dominant follicular pattern was more common in radiogenic than sporadic PTC, but the difference was significant only for non-encapsulated tumors in adolescents. A significant increasing trend with age was also found for this structure in radiogenic PTC, not detectable in sporadic tumors, although there was no statistically significant heterogeneity in age trends between the two etiological forms of PTC (see Table 2).
Oxyphilic cell metaplasia was more common in sporadic tumors, although its frequency significantly increased with age in both radiogenic and sporadic forms of PTC (see Fig. 1 and Supplementary Table S2). The observed age trends might be due partly to the increasing frequency of tumors composed of oxyphilic cells such as Warthin-like variant of PTC with age. Potential somatic mutations underlying these trends remain to be clarified, in particular a possible relationship between oxyphilic changes and the BRAF mutation.
The inverse age trends in mean size of non-encapsulated tumors and proportion of small-size tumors, as well as the increasing frequency of tumors with the dominant papillary pattern (see Table 2), could also be partly explained by the higher frequency of BRAF mutations in older patients. The slower growth of tumors driven by a BRAF mutation compared to gene rearrangement(s) was suggested in the authors' earlier work (32). The BRAFV600E mutation is known to occur with higher frequency in PTCs with the papillary growth pattern, and its rate increases with patient age (21,25). Another explanation for the higher frequency of smaller tumors in older patients could be the increased use of ultrasound equipment and/or improved medical surveillance in recent years.
Two observations were made with regard to concomitant thyroid pathology. First, despite the fact that frequencies of coexisting nodular lesions did not differ between radiogenic and sporadic tumors in any age group, the age-related trend was significant in radiogenic PTC, while no statistically significant trend was found in sporadic PTC. Concomitant chronic thyroiditis was more common in sporadic tumors, which is consistent with a report from Belarus (18), but there was no age trend. It is possible that different types of concomitant thyroid pathology may be associated with exposure to different environmental factors. For example, some nodular lesions in radiogenic PTC might have been related to radiation exposure, as was reported in the Ukrainian and Belarusian screening cohorts for follicular adenoma (22,33), while a chronic inflammatory process in the thyroid might have been triggered by another environmental factor, so far unidentified, other than radiation.
For analysis of invasiveness, tumors were divided into fully encapsulated and non-encapsulated. The study showed that this was reasonable, especially for children, where the proportion of encapsulated tumors was significantly different between radiogenic and sporadic PTC (see Supplementary Table S1). While a significant difference was found for intrathyroidal spread, extrathyroidal extension, lymphatic/vascular invasion, lymph node, and distant metastases in the analysis of all tumors in children (see Fig. 2), the three latter features were no longer significant when analysis was limited to non-encapsulated tumors (see Supplementary Table S3). This indicates that such important indicators of tumor aggressiveness as lymphatic/vascular invasion and regional and distant metastases are rather associated with childhood age of patients, regardless of the presence or absence of a radiation history. By contrast, the difference for intrathyroidal spread and extrathyroidal extension remained significant in the analysis of non-encapsulated tumors. Thus, tumor stratification into fully encapsulated and non-encapsulated provides a more correct distinction of patients' age influence.
More pronounced histopathologic characteristics of tumor invasiveness were observed in non-encapsulated radiogenic PTC, not only in children but also in older age groups (see Supplementary Table S3). These included extrathyroidal extension, lymphatic/vascular invasion, and distant metastases in adolescents, and intrathyroidal spread in young adults. At the same time, the decreasing trends with age for the majority of invasive characteristics seen both in radiogenic and sporadic PTC, which did not show marked heterogeneity between radiogenic and sporadic tumors for most characteristics, may indicate the presence of common mechanisms underlying lesser tumor aggressiveness in older patients which, for example, could be due to a different spectrum of somatic mutations driving carcinogenesis compared to younger patients.
It should also be noted that both radiogenic and sporadic fully encapsulated tumors were not completely devoid of certain invasive features such as invasion into the tumor capsule and its vessels, focal tumor spread beyond the tumor capsule, or even extrathyroidal extension or nodal disease in isolated cases (see Supplementary Table S3). The encapsulated follicular variant of PTC also exhibited signs of invasiveness in both radiogenic and sporadic series. In cases with features of focal invasiveness, tumors had a mixed architecture with prominent (>20%) areas of papillary or solid structures or both. This generally indicates that these were bona fide malignant tumors and not lesions, such as well-differentiated tumor of uncertain malignant potential or non-invasive follicular thyroid neoplasm with papillary-like nuclear features that occupy an intermediate position between follicular adenoma and fully encapsulated PTC (20,25). Further, fully encapsulated PTC showed an independent association with radiation exposure: more frequent lymphatic/vascular invasion in children and more frequent tumor capsule invasion in adults (see Supplementary Table S3).
This study has several strengths and limitations. Radiogenic and sporadic cases were selected from the same source (i.e., the IEM database) and were well matched by age at surgery and place of residence. While these were not matched by sex, all models were appropriately adjusted, and therefore confounding by sex is unlikely. Also, since sporadic cases in the study were not detected by active screening, their overall less pronounced aggressiveness could not be attributed to early cancer detection. To assure that sporadic cases were unexposed to 131I, these were selected among individuals born in 1987 or later. Therefore, the possibility cannot be excluded that some observed differences between radiogenic and sporadic cases may be due to birth cohort or period effects. In addition, because the focus was on PTC cases that occurred in individuals exposed to 131I when aged four years or younger, the most sensitive period to radiation, this may have reduced variability in thyroid doses (as 131I doses are inversely related to age at exposure) and thus chances to detect the dose–response. Finally, some statistically significant associations detected in this work need to be considered with caution in view of a large number of statistical tests performed.
In conclusion, this study demonstrates that, overall, radiogenic PTC in subjects aged four years or younger at the time of exposure differed from sporadic PTC in age-matched groups for a number of histopathologic parameters. The analyses revealed significant differences in both histological architecture and invasive features of radiogenic PTC, demonstrating that a more aggressive behavior occurs in children and adolescents. Changes in histopathologic characteristics with age suggest that the phenotype of both radiogenic and sporadic PTCs becomes less invasive at older ages. It would be necessary to reevaluate PTC in adult patients in the future as new cases occur to determine whether the observed differences will remain or be lost over time. Analyses of correlations between morphological features of tumor aggressiveness and clinical aggressiveness to ascertain differences in tumor recurrence and/or cause-specific death rates in patients with radiogenic and sporadic PTC, as well as advanced molecular studies in relatively large age-matched groups of PTC of different etiology, will also be of importance.
Supplementary Material
Acknowledgements
We thank the staff of the Laboratory of Morphology of Endocrine System who prepared all pathological material, and the staff of the Department of Surgery of Endocrine System of IEM who operated these patients. The authors gratefully acknowledge the confirmation of diagnoses provided by the International Pathology Panel of the Chernobyl Tissue Bank: Professors A. Abrosimov, T. Bogdanova, G. Fadda, J. Hunt, M. Ito, V. Livolsi, J. Rosai, and E.D. Williams, and Dr. N. Dvinskyh. This work was supported in part by KAKENHI (grant number 16H02774) from the Japan Society for the Promotion of Science.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kazakov VS, Demidchik EP, Astakhova LN. 1992. Thyroid cancer after Chernobyl. Nature 359:21. [DOI] [PubMed] [Google Scholar]
- 2.Likhtarev IA, Sobolev BG, Kairo IA, Tronko ND, Bogdanova TI, Oleinic VA, Epshtein EV, Beral V. 1995. Thyroid cancer in the Ukraine. Nature 375:365. [DOI] [PubMed] [Google Scholar]
- 3.Jacob P, Bogdanova TI, Buglova E, Chepurniy M, Demidchik Y, Gavrilin Y, Kenigsberg J, Kruk J, Schotola C, Shinkarev S, Tronko MD, Vavilov S. 2006. Thyroid cancer among Ukrainians and Belarusians who were children or adolescents at the time of the Chernobyl accident. J Radiol Prot 26:51–67 [DOI] [PubMed] [Google Scholar]
- 4.Cardis E, Hatch M. 2011. The Chernobyl accident—an epidemiological perspective. Clin Oncol (R Coll Radiol) 23:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saenko V, Ivanov V, Tsyb A, Bogdanova T, Tronko M, Demidchik Y, Yamashita S. 2011. The Chernobyl accident and its consequences. Clin Oncol (R Coll Radiol) 23:234–243 [DOI] [PubMed] [Google Scholar]
- 6.Tronko M BT, Saenko V, Thomas GA, Likhtaterv , Yamashita S. 2014. Thyroid Cancer in Ukraine after Chernobyl: Dosimetry, Epidemiology, Pathology, Molecular Biology. IN-TEX, Nagasaki, Japan [Google Scholar]
- 7.Tronko M, Bogdanova T, Shpak V, Gulak L. 2016. Thyroid cancer in Ukraine during 1986–2014. In: Bazyka D, Sushko V, Chumak A, Chumak V, Yanovich L. (eds) Health Effects of Chornobyl Accident Thirty Years Aftermath. DIA, Kiev, Ukraine, pp 85–103 [Google Scholar]
- 8.Jacob P, Goulko G, Heidenreich WF, Likhtarev I, Kairo I, Tronko ND, Bogdanova TI, Kenigsberg J, Buglova E, Drozdovitch V, Golovneva A, Demidchik EP, Balonov M, Zvonova I, Beral V. 1998. Thyroid cancer risk to children calculated. Nature 392:31–32 [DOI] [PubMed] [Google Scholar]
- 9.Tronko M, Bogdanova T, Voskoboynyk L, Zurnadzhy L, Shpak V, Gulak L. 2010. Radiation induced thyroid cancer: fundamental and applied aspects. Exp Oncol 32:200–204 [PubMed] [Google Scholar]
- 10.Likhtarov I, Thomas G, Kovgan L, Masiuk S, Chepurny M, Ivanova O, Gerasymenko V, Tronko M, Bogdanova T, Bouville A. 2013. Reconstruction of individual thyroid doses to the Ukrainian subjects enrolled in the Chernobyl Tissue Bank. Radiat Prot Dosimetry 156:407–423 [DOI] [PubMed] [Google Scholar]
- 11.Likhtarov I, Kovgan L, Masiuk S, Talerko M, Chepurny M, Ivanova O, Gerasymenko V, Boyko Z, Voilleque P, Drozdovitch V, Bouville A. 2014. Thyroid cancer study among Ukrainian children exposed to radiation after the Chornobyl accident: improved estimates of the thyroid doses to the cohort members. Health Phys 106:370–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronko MD, Bogdanova TI, Komissarenko IV, Epstein OV, Oliynyk V, Kovalenko A, Likhtarev IA, Kairo I, Peters SB, LiVolsi VA. 1999. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl nuclear accident: statistical data and clinicomorphologic characteristics. Cancer 86:149–156 [DOI] [PubMed] [Google Scholar]
- 13.Williams ED, Abrosimov A, Bogdanova T, Demidchik EP, Ito M, LiVolsi V, Lushnikov E, Rosai J, Sidorov Y, Tronko MD, Tsyb AF, Vowler SL, Thomas GA. 2004. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. Br J Cancer 90:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LiVolsi VA, Abrosimov AA, Bogdanova T, Fadda G, Hunt JL, Ito M, Rosai J, Thomas GA, Williams ED. 2011. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol) 23:261–267 [DOI] [PubMed] [Google Scholar]
- 15.Bogdanova T, Zurnadzhy L, LiVolsi VA, Williams ED, Ito M, Nakashima M, Thomas GA. 2014. Thyroid cancer pathology in Ukraine after Chernobyl. In: Tronko M, Bogdanova T, Saenko V, Thomas GA, Likhtarov I, Yamashita S. (eds) Thyroid Cancer in Ukraine after Chernobyl: Dosimetry, Epidemiology, Pathology, Molecular Biology. IN-TEX, Nagasaki, Japan, pp 109–135 [Google Scholar]
- 16.Bogdanova TI, Zurnadzhy LY, Nikiforov YE, Leeman-Neill RJ, Tronko MD, Chanock S, Mabuchi K, Likhtarov IA, Kovgan LM, Drozdovitch V, Little MP, Hatch M, Zablotska LB, Shpak VM, McConnell RJ, Brenner AV. 2015. Histopathological features of papillary thyroid carcinomas detected during four screening examinations of a Ukrainian–American cohort. Br J Cancer 113:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demidchik Y, Fridman M, Schmid KW, Reiners C, Biko J, Mankovskaya S. 2012. Papillary thyroid cancer in childhood and adolescence with specific consideration of patients after radiation exposure. In: Fahey TJ. (ed) Updates in the Understanding and Management of Thyroid Cancer. InTech, Rijeka, Croatia, pp 163–188 [Google Scholar]
- 18.Fridman M, Lam AK, Krasko O, Schmid KW, Branovan DI, Demidchik Y. 2015. Morphological and clinical presentation of papillary thyroid carcinoma in children and adolescents of Belarus: the influence of radiation exposure and the source of irradiation. Exp Mol Pathol 98:527–531 [DOI] [PubMed] [Google Scholar]
- 19.Drozd VM, Lushchik ML, Polyanskaya ON, Fridman MV, Demidchik YE, Lyshchik AP, Biko J, Reiners C, Shibata Y, Saenko VA, Yamashita S. 2009. The usual ultrasonographic features of thyroid cancer are less frequent in small tumors that develop after a long latent period after the Chernobyl radiation release accident. Thyroid 19:725–734 [DOI] [PubMed] [Google Scholar]
- 20.Rosai J. 2011. Thyroid Gland. Tenth edition. Mosby, Edinburgh, United Kingdom [Google Scholar]
- 21.Nikiforov Y, Biddinger PW, Nikiforova MN, Thompson LDR. 2012. Diagnostic Pathology and Molecular Genetics of the Thyroid. Second edition. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 22.Zablotska LB, Nadyrov EA, Rozhko AV, Gong Z, Polyanskaya ON, McConnell RJ, O'Kane P, Brenner AV, Little MP, Ostroumova E, Bouville A, Drozdovitch V, Minenko V, Demidchik Y, Nerovnya A, Yauseyenka V, Savasteeva I, Nikonovich S, Mabuchi K, Hatch M. 2015. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997–2008) of a cohort of children and adolescents from belarus exposed to radioiodines after the Chernobyl accident. Cancer 121:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLellis RA, R.Lloyd, Ph.Heitz, Ch.Eng 2004. WHO Classification of Tumors. Vol.8 Pathology and Genetics of Tumours of Endocrine Prgans. Third edition. IARC Press, Lyon, France [Google Scholar]
- 24.Thomas GA, Williams ED, Becker DV, Bogdanova TI, Demidchik EP, Lushnikov E, Nagataki S, Ostapenko V, Pinchera A, Souchkevitch G, Tronko MD, Tsyb AF, Tuttle M, Yamashita S. 2000. Chernobyl tumor bank. Thyroid 10:1126–1127 [DOI] [PubMed] [Google Scholar]
- 25.Lloyd RV, Osamura RY, Kloppel G, Rosai J. 2017. The WHO Classification of Tumours of Endocrine Organs. Fourth edition. IARC Press, Lyon, France [Google Scholar]
- 26.Sobin LH, Gospodarowicz MK, Wittekind Ch. (eds) 2010. TNM Classification of Malignant Tumours. Seventh edition. Wiley-Blackwell, Chichester, United Kingdom [Google Scholar]
- 27.Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, Minenko VF, Ostapenko VA, Petrenko SV, Polyanskaya ON, Rzheutski VA, Tronko MD, Bobylyova OO, Bogdanova TI, Ephstein OV, Kairo IA, Kostin OV, Likhtarev IA, Markov VV, Oliynik VA, Shpak VM, Tereshchenko VP, Zamotayeva GA, Beebe GW, Bouville AC, Brill AB, Burch JD, Fink DJ, Greenebaum E, Howe GR, Luckyanov NK, Masnyk IJ, McConnell RJ, Robbins J, Thomas TL, Voilleque PG, Zablotska LB; Chornobyl Thyroid Diseases Study Group of Belarus, Chornobyl Thyroid Diseases Study Group of Ukraine, Chornobyl Thyroid Diseases Study Group of the USA 2004. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res 161:481–492 [DOI] [PubMed] [Google Scholar]
- 28.Williams ED, Abrosimov A, Bogdanova T, Demidchik EP, Ito M, LiVolsi V, Lushnikov E, Rosai J, Tronko MD, Tsyb AF, Vowler SL, Thomas GA. 2008. Morphologic characteristics of Chernobyl-related childhood papillary thyroid carcinomas are independent of radiation exposure but vary with iodine intake. Thyroid 18:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdanova T, Saenko V, Zurnadzhy L, Likhtarov I, Kovgan L, Masiuk S, Kashcheev V, LiVolsi VA, Williams ED, Ito M, Mine M, Thomas GA, Tronko M, Yamashita S. 2014. Comparative pathological analysis of papillary thyroid carcinoma in age-matched groups of patients born before and after Chernobyl. In: Tronko M, Bogdanova T, Saenko V, Thomas GA, Likhtarov I, Yamashita S. (eds) Thyroid Cancer in Ukraine After Chernobyl: Dosimetry, Epidemiology, Pathology, Molecular Biology. IN-TEX, Nagasaki, Japan, pp 65–108 [Google Scholar]
- 30.Thomas G. 2017. Somatic genomics of childhood thyroid cancer. In: Yamashita S, Thomas G. (eds) Thyroid Cancer and Nuclear Accidents—Long Term After Effects of Chernobyl and Fukushima. Elsevier, Amsterdam, The Netherlands, pp 121–132 [Google Scholar]
- 31.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova T, Thomas GA, Mason CE, Fagin JA. 2013. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest 123:4935–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsutake N, Fukushima T, Matsuse M, Rogounovitch T, Saenko V, Uchino S, Ito M, Suzuki K, Suzuki S, Yamashita S. 2015. BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci Rep 5:16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zablotska LB, Bogdanova TI, Ron E, Epstein OV, Robbins J, Likhtarev IA, Hatch M, Markov VV, Bouville AC, Olijnyk VA, McConnell RJ, Shpak VM, Brenner A, Terekhova GN, Greenebaum E, Tereshchenko VP, Fink DJ, Brill AB, Zamotayeva GA, Masnyk IJ, Howe GR, Tronko MD. 2008. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: dose-response analysis of thyroid follicular adenomas detected during first screening in Ukraine (1998–2000). Am J Epidemiol 167:305–312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.