Abstract
The halophyte Salicornia europaea L. is a widely distributed salt-tolerant plant species that produces numerous dimorphic seeds. We studied germination and recovery in dimorphic seeds of Central Asian S. europaea under various salinity conditions. We also tested the effects of various salts on Na+ and K+ accumulation during plant development from germination to anthesis under greenhouse conditions. We found good germination (close to control) of large seeds under NaCl between 0.5 and 2%, Na2SO4 and 2NaCl + KCl + CaCl between 0.5 and 3%, and 2Na2SO4 + K2SO4 + MgSO4 between 0.5 and 5%. For the small seeds, we found stimulating effects of chloride salts (both pure and mixed) under 0.5–1% concentrations, and sulfate salts under 0.5–3%. Both types of seeds showed high germination recovery potential. Salt tolerance limits of the two seed types during germination and at the later stages of development were very similar (4–5%). During plant growth the optimal concentrations of mixed chloride and sulfate salts ranged from 0.5 to 2%. The mechanisms of salt tolerance in the two seed types of S. europaea appear to differ, but complement each other, improving overall adaptation of this species to high salinity.
Keywords: Dimorphic seeds, Germination, Salinity, Recovery, Ion content
1. Introduction
The annual halophyte Salicornia europaea L. almost ubiquitously occupies inland salt marshes of the globe. This species has long attracted interest due to its high salt tolerance, reclamation potential, and high pharmaceutical and culinary value (Ungar, 1962, Waisel, 1972, Keiffer and Ungar, 2001).
S. europaea reproduces sexually and produces a large number of dimorphic seeds that differ in size and plant position. A median, larger seed (seed type 1) is located in the central part of the nodal segment, while smaller seeds (seed type 2) are located on both sides of the nodal segment just beneath the large seed. Ungar (1979) demonstrated that large central seeds are more salt tolerant. These two seed types demonstrate differential germination which has been hypothesized to have a positive impact on species survival in unpredictable arid conditions (Philipupillai and Ungar, 1984). Philipupillai and Ungar (1984) have also suggested that more dormant lateral seeds of S. europaea are important for maintaining a long term viable soil seed bank. This suggestion is supported by experiments in which lateral and median seeds of S. europaea showed germination percentage of 68.6% and 0%, respectively, after 9 years of storage in paper bags under room temperature (Orlovsky, unpublished).
Seed heteromorphism in halophytes is considered an adaptation to harsh and unpredictable desert environments, whereby two seed morphs germinate under different environmental conditions (Song et al., 2008, Gul et al., 2003, Wang et al., 2015, Ameixa et al., 2016).
Previous research has found that S. europaea seeds germinate at low levels when treated with NaCl solutions between 1 and 5%, while germination levels are close to the controls (distilled water) when treated at or below 1% NaCl (Ungar, 1977). However, it is not known whether the two seed types in S. europaea have different salt tolerance limits.
Many halophytes show differential ecotypic response to salinity during germination (e.g. Francois, 1976, Semushina and Morozova, 1979, Pujol et al., 2000, Qu et al., 2007). Differential salt compositions in the soil can lead to the formation of halophyte ecotypes. For example, morphologically distinct ecotypes have been detected among three populations of S. europaea in Ohio (USA) inhabiting soils with different salinity types (Ungar, 1987).
Often, soil salinity types are not pure, but mixed. Central Asian salinized soils are often mixed, commonly including chlorides (chlorides of Na+, Ca2+ and Mg2+ prevail), sulfates, chloride-sulfates, as well as others (Lobova, 1967). Mixed salts inhibit seed germination less severely than pure salts (Tobe et al., 2004, Orlovsky et al., 2011). Dimorphic seeds respond differently to salt types and salt concentrations at various stages of plant development (Ungar, 1978). Therefore, studying germination and growth of S. europaea exposed to different salt types and concentrations may provide insights into the adaptive importance of seed dimorphism in this species.
S. europaea is an obligate halophyte with very high recovery potential (Waisel, 1972). Keiffer and Ungar (1997) showed that hypersaline conditions can stimulate seed germination in distilled water. Unfortunately, previous studies have not examined differential responses of the two seed types. Despite their lower salt tolerance, we hypothesize that lateral seeds of S. europaea have the same recovery capacity as median seeds.
Our experiments differ from those of Ungar (1979) and Philipupillai and Ungar (1984) in several aspects. The above authors analyzed a response of two seed morphs of S. europaea only to NaCl and only at the seed stage. The focus of this study was to understand how variations in salinity affect dimorphic seed germination and plant growth. We treated S. europaea seeds with four different salts at a range of concentrations (1) to examine germination and recovery in dimorphic seeds of S. europaea under various salinity conditions, and (2) to test the effects of various salts on Na+ and K+ accumulation during seedling development.
2. Materials and methods
The mature fruits of S. europaea were collected from a natural population located in the north-eastern part of Turkmenistan within the Amudarya river basin (Dashoguz oasis) in November 2002. The interannual average temperature in January and July is −4 and 27 °C, respectively. Summer is long and hot; winter is mild with moderate frosts lasting more than three months. The maximum air temperature can reach +46 °C. Annual rainfall is 100–115 mm with major rainy events from November to May. During June–October precipitation represents only about 7–9% of the annual rainfall (Orlovsky, 1994).
The fruits were stored in paper bags under +6 °C. Germination experiments started in June 2004, after 1.5 years of storage. The fruits were classified as suggested by Ungar (1979) into two size categories: large (>1.5 mm) and small (<1.4 mm). Seeds were germinated on light in distilled water (control), chloride and sulfate solutions in Petri dishes. There were 50 seeds per Petri dish and three replicates per treatment. To each Petri dish 7 ml of either 0.5, 1, 2, 3 or 5% solution was added. Temperature was kept +6 °C during the first 20 days and +22 °C till the end of experiment. In order to separate ionic and osmotic salinity effects (Kokina, 1938) we applied pure and mixed salts, viz. i) NaCl vs. mixture of NaCl, KCl and CaCl2, and ii) Na2SO4 vs. mixture of Na2SO4, K2SO4 and MgSO4. In the mixed salts the components were in a proportion 2:1:1.
Soil salinity affects not only germination but also survival and growth of seedlings. Seedling response to different salinity was studied in a greenhouse. Greenhouse experiments were conducted from February to April 2004. Seeds were sown in pots filled with peat and seedling were transferred to 4-L plastic boxes with half strength Hoagland media when seedlings reached 2 cm in height. Eight seedlings were raised in each box. Salinity treatments included addition of either NaCl, Na2SO4, NaCl + KCl + CaCl2 (ratio 2:1:1) or Na2SO4 + K2SO4 + MgSO4 (ratio 2:1:1) to the half strength Hoagland solution at concentrations of 0, 0.5, 1, 2, 3, 4 or 5%, respectively. In the non-control salinity treatments, salt concentration was gradually increased to the final concentration. The solutions were refreshed every 7 days. The height of plants was measured every week. Seedlings were harvested after two months of growth and measured for height and fresh weight of shoots and roots. Then the measured shoots and roots were rinsed 3 times in deionized water and oven dried for 3 days at 55 °C. The dried plant material was ground into powder and the samples of 0.25 g were digested in 5 ml of acid mixture of HClO4 and HNO3 (15:85% v/v) in ‘digestion glass tubes’. It was mixed and predigested at least 4 h in a fume-hood. Digestion was processed under gradual increase of temperature from room temperature to 195 °C. After cooling, 2.5 ml of HCl (10%) or HNO3 were added to digests and whirling mixed and warmed to 80 °C for 30 min. The final volume was brought to 10 ml with double distilled water and re-warmed to 80 °C for another 30 min. Samples were diluted to a ratio of 1:500 and 1:1000. Measurements of sodium and potassium were made using Atomic Absorption Spectrophotometer (AAS) (Perkin Elmer 1100B).
Results were analyzed by ANOVA using SPSS version 13.0 (SPSS, Inc., Chicago IL).
3. Results
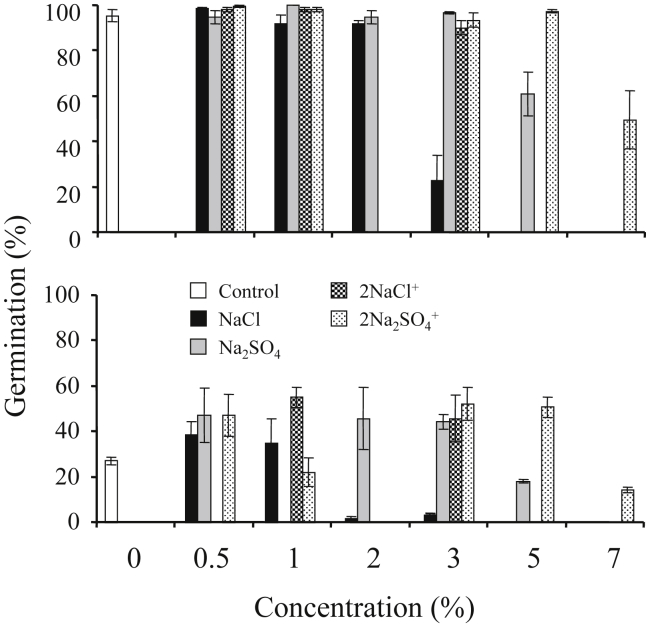
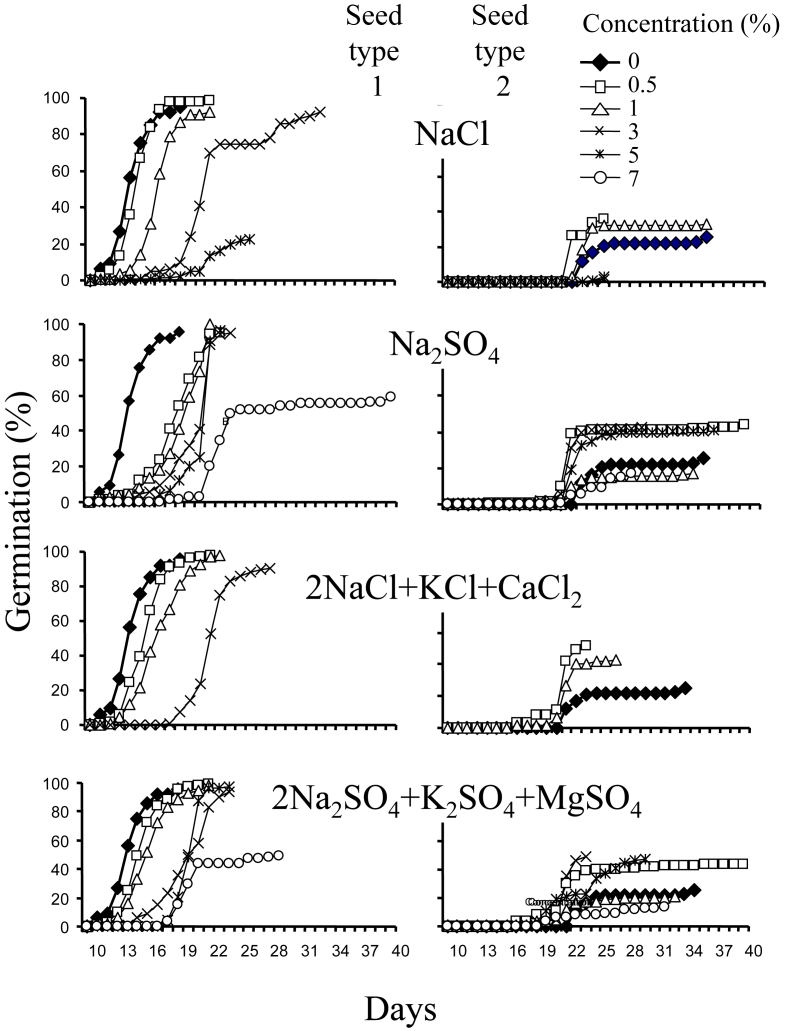
Two-way ANOVAs for each salt type tested showed that germination and rate of germination were significantly affected by seed type, salt concentration and their interaction (P < 0.0001) (Table 1, Table 2). Final germination percentage and rate of germination were higher in large seeds than in small seeds (Fig. 2 and Table 3). The first seedlings from large seeds appeared on day 11 and from small seeds on day 23. Both seed types demonstrated a gradual pattern of germination that halted after 20 days (large seeds) and 34 days (small seeds) (Fig. 1). For large seeds, germination was stimulated by only one salt treatment, 0.5% NaCl. However, germination was generally high, and close to control levels under the following treatments: 1–2% NaCl, 0.5–3% Na2SO4, 0.5–3% 2NaCl + KCl + CaCl2 and 0.5–5% 2Na2SO4 + K2SO4 + MgSO4. Small seed germination was stimulated by chlorides (pure and mixed) at 0.5–1%, and sulfates 0.5–3%. The stimulating effect of salts on small seeds was not as pronounced as on large seeds, although under some concentrations it was twice as large as the control (Fig. 1, Fig. 2). For both seed types, germination percentage and germination rate decreased as salinity increased (P < 0.0001). Salt tolerance limits for large seeds were 4% for NaCl and 8% for Na2SO4; whereas, for small seeds they were 3% and 5%, respectively.
Table 1.
Results of two-way ANOVA on effect of salinity type (S) and concentration (C) on cumulative percentage and rate of seed germination, and on height and weight of S. europaea plants.
| Source | Large (type 1) seeds |
Small (type 2) seeds |
Plant height | Plant weight | ||
|---|---|---|---|---|---|---|
| Seed germination | Rate germination | Seed germination | Rate germination | |||
| Salinity type | 39.7*** | 22.3*** | 37.3*** | 4.3** | 32.8*** | 4.8** |
| Concentration | 124.9*** | 125.4*** | 8.6*** | 7.2*** | 18.9*** | 12.4*** |
| C*S | 48.5*** | 30.2*** | 5.7*** | 10.2*** | 7.2*** | 1.4 NS |
***P < 0.001, **P < 0.01, NS not significant.
Table 2.
Results of two-way ANOVA on effect of salt concentration (C) and type of seed (T) of S. europaea on final germination percentage and rate of germination in different salts.
| Salts | Source | df | F |
|
|---|---|---|---|---|
| Final germination | Rate germination | |||
| NaCl | Concentration | 5 | 51.8*** | 43.8*** |
| Type of seeds | 1 | 245.2*** | 241.2*** | |
| C*T | 5 | 18.4*** | 17.3*** | |
| Na2SO4 | Concentration | 5 | 5.7*** | 11.7*** |
| Type of seeds | 1 | 219.8*** | 406.5*** | |
| C*T | 5 | 2.2 NS | 8.0*** | |
| 2NaCl + KCl + CaCl2 | Concentration | 5 | 657.7*** | 98.0*** |
| Type of seeds | 1 | 4684.1*** | 307.9*** | |
| C*T | 5 | 490.4*** | 44.2*** | |
| 2Na2SO4 + K2SO4 + MgSO4 | Concentration | 5 | 64.1*** | 55.9*** |
| Type of seeds | 1 | 1145.3*** | 476.7*** | |
| C*T | 5 | 49.9*** | 21.8*** | |
P < 0.001 NS not significant.
Fig. 2.
Percentage of total germination of S. europaea seeds of type 1 (top) and type 2 (bottom) under different salt types and concentration (means ± SE).
Table 3.
Germination rate (mean ± SE) of two types of Salicornia europaea seeds.
| Concentration (%) | Salt type |
|||
|---|---|---|---|---|
| NaCl | Na2SO4 | 2NaCl + KCl + CaCl2 | 2Na2SO4 + K2SO4 + MgSO4 | |
| Large seeds | ||||
| 0 | 4.64 ± 0.5 | 4.64 ± 0.5 | 4.64 ± 0.5 | 4.64 ± 0.5 |
| 0,5 | 4.79 ± 0.4 | 4.06 ± 0.3 | 4.46 ± 0.2 | 4.54 ± 0.4 |
| 1 | 4.23 ± 0.5 | 4.35 ± 0 | 4.2 ± 0.1 | 4.26 ± 0.1 |
| 2 | 3.45 ± 0.9 | 4.0 ± 0.3 | ||
| 3 | 0.97 ± 0.9 | 4.0 ± 0.05 | 3.43 ± 0.6 | 4.0 ± 0.4 |
| 5 | 0 | 1.87 ± 0.7 | 0 | 4.12 ± 0.2 |
| 7 | 1.82 ± 0.8 | |||
| Small seeds | ||||
| 0 | 0.72 ± 0.1 | 0.72 ± 0.1 | 0.72 ± 0.1 | 0.72 ± 0.1 |
| 0.5 | 1.44 ± 0.5 | 1.3 ± 0.4 | 1.6 ± 0.7 | |
| 1 | 1.12 ± 0.6 | 0.6 ± 0.6 | 2.14 ± 0.35 | 0.7 ± 0.3 |
| 2 | 0.05 ± 0.1 | 1.6 ± 0.9 | ||
| 3 | 0.08 ± 0.1 | 1.3 ± 0.2 | 1.67 ± 0.81 | 0.7 ± 0.5 |
| 5 | 0 | 0.6 ± 0 | 1.6 ± 0.3 | |
| 7 | 0 | 0.5 ± 0.4 | ||
Fig. 1.
Effect of different salts and their concentrations on seed germination in two types of S. europaea seeds.
One-way ANOVA revealed that recovery germination for both seed types was significantly and positively affected by 5% NaCl compared with controls (P < 0.001). Also, we found a significant difference in recovery germination rate between the two seed morphotypes (P < 0.0001), with final germination percentages of 98% for the large seeds and 50% for small seeds.
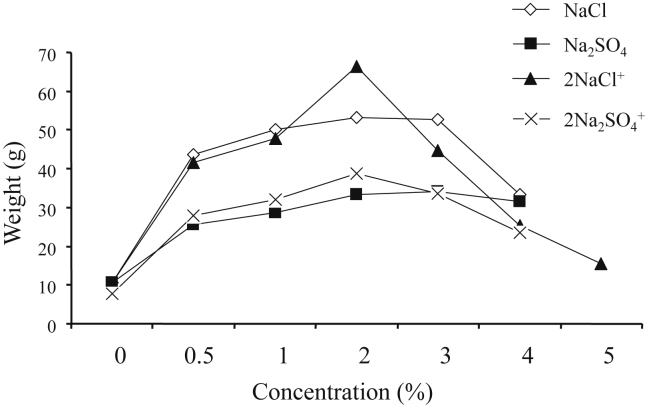
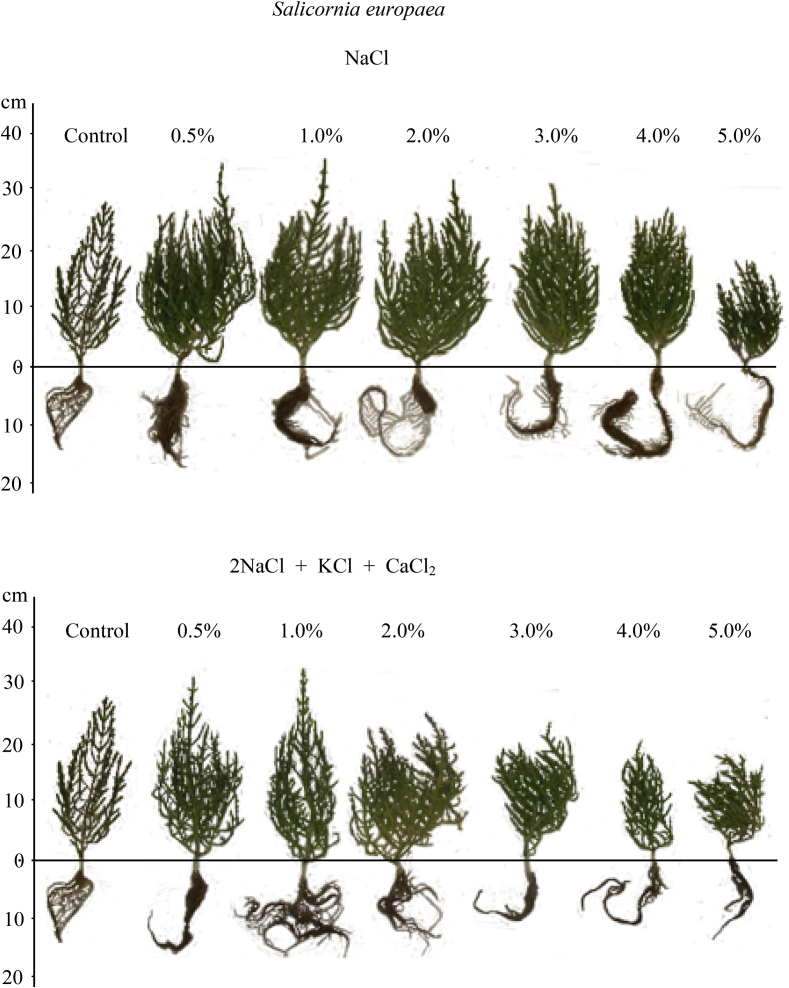
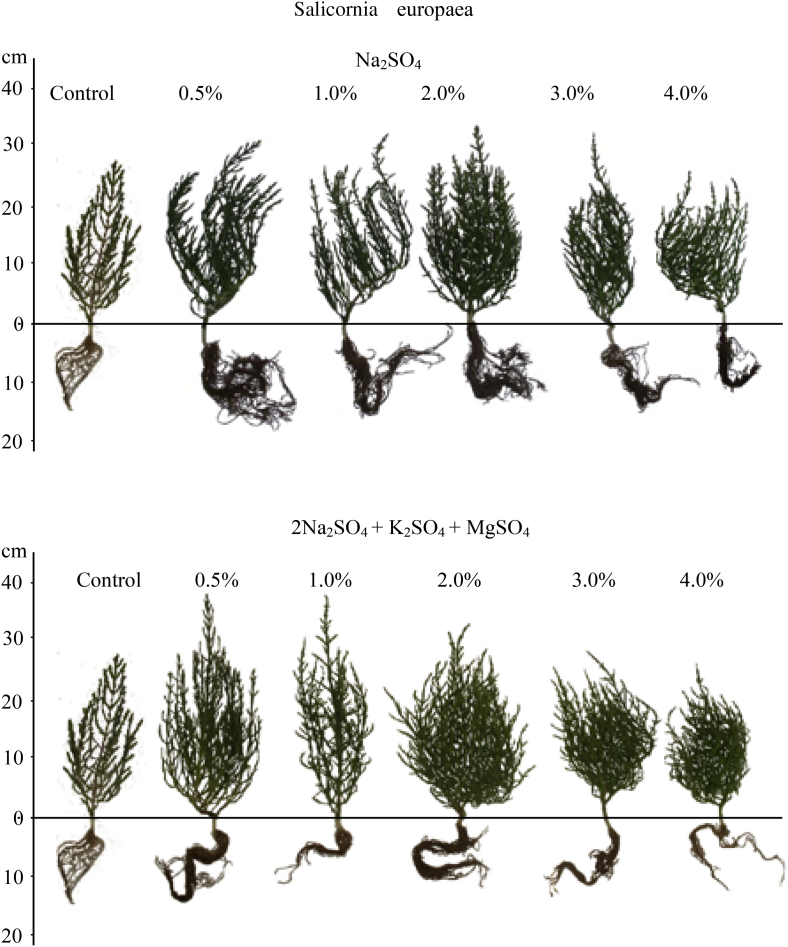
Plant growth was significantly affected by salinity type, salt concentration, and their interaction (Table 1). However, only salt type (P < 0.0001) and concentration (P < 0.05), but not their interaction, had an effect on plant biomass. We observed that, compared with controls, plant growth improves when exposed to chlorides (both pure and mixed; concentration range 0.5–2%) or sulfates (concentration range 0.5–3%). We also found that salt tolerance limits from germination to flowering are 5% for NaCl and slightly higher for sulfates. Height increment was much greater in sulfate salts (especially mixed) than in chloride salts. In contrast, fresh biomass increase was higher in plants treated with chloride salts compared with sulfate salts (Fig. 3). Maximum fresh biomass was observed at 2% NaCl and gradually decreased at higher NaCl concentrations. Furthermore, 0.5–2% chloride salt solutions produced more side branches. As salt concentrations increased, branches became greenish and distinctly thicker (Fig. 5). In contrast, the branches of S. europaea treated with sulfates were thin, almost awl-shaped and dark green (Fig. 6).
Fig. 3.
Effect of various salts on wet biomass of S. europaea.
Fig. 5.
The effect of various chloride and chloride-sulfate salts and their concentration on the development of the sprouts in S. europaea.
Fig. 6.
The effect of various sulfate and sulfate-chloride salts and their concentration on the development of the sprouts of S. europaea.
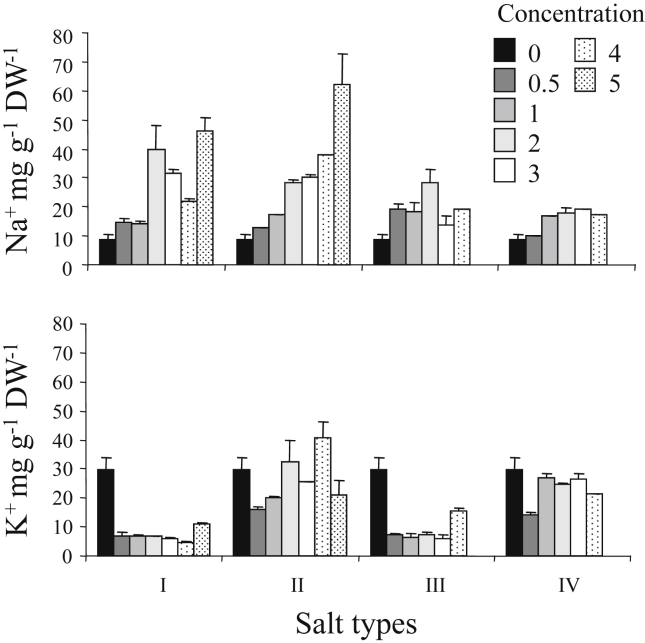
The effects of various salts on accumulation of Na+ and K+ are summarized in Fig. 4. When NaCl + KCl + CaCl2 (ratio 2:1:1) was added to growth media, the highest Na+ concentration was at 5% salt concentration, similar to the pure NaCl treatment. However, when Na2SO4 was added to growth media, a 3% salt solution produced the highest Na+ concentration. Adding Na2SO4 + K2SO4 + MgSO4 (ratio 2:1:1) to growth media had no significant effect on accumulation of Na+. For growth media treated with NaCl, Na2SO4 and Na2SO4 + K2SO4 + MgSO4 (ratio 2:1:1), the highest K+ concentration in the shoots was in the control, with salt concentration in the growth medium having no effect on K+ accumulation. For growth media treated with NaCl + KCl + CaCl2 (ratio 2:1:1), a 4% salt concentration produced the highest K+ concentration (Fig. 4).
Fig. 4.
Effect of various salts concentrations on the accumulation of Na+ and K+ in S. europaea (means ± SE). I- NaCl; II- NaCl + KCl + CaCl2; III- Na2SO4: IV- Na2SO4 + K2SO4 + MgSO4.
4. Discussion
Salt tolerance during germination varies among species of Salicornia from 850 to 1700 mM NaCl (Khan and Gul, 2006). Seeds of S. europaea from salt marshes of Kansas (USA) germinate in up to 5% NaCl (Ungar, 1962). For Central Asian S. europaea seeds this limit ranges from 4 to 7% depending on temperature (Orlovsky, unpublished). Dimorphic seeds of S. europaea germinate in different seasons: large seeds, in early spring; small seeds, from late spring to mid-summer (Philipupillai and Ungar, 1984). In this study, germination of large seeds was 3–4 times higher than of small seeds in the controls and under 0.5–2% of all salts tested. Small seeds are innately dormant, and this dormancy can be partly broken by applying chloride or sulfate salts in concentrations ranging from 0.5 to 2%. This treatment doubles germination percentage compared to the control (Fig. 1). Keeping seeds in 5% NaCl for one month followed by a transfer to distilled water also doubled the germination recovery of small seeds. We suggest that the best means of achieving high germination in S. europaea involves optimizing the combination of salts, their concentration and duration of seed exposure to salinity. The observed differences between the two S. europaea seed types in response to different salt concentrations demonstrate that there are distinct mechanisms of salt tolerance for large and small seeds. Differential salt tolerance of the two seed morphs apparently results from the difference in their plant position and order of development. Because heterocarpy is thought to be an adaptation to unpredictable environmental conditions (Harper et al., 1970), it is possible that large seeds originated later than small ones, and their higher salt tolerance evolved as an adaptive response to increasing aridization and soil salinization.
We found that sulfates do no affect S. europaea dimorphic seeds as strongly as chloride salts. Higher germination and tolerance limits in mixed chloride and sulfate salts is consistent with previous research and is probably due to the alleviating effects of Ca2+ and Mg2+ (Tobe et al., 2004, Gul and Khan, 2006). Salinization damages ion balance in the cells and application of salts containing Ca2+ and Mg2+ ions corrects this imbalance and reduces negative impacts of Na+ and Cl−.
At later stages of ontogenesis salt tolerance limits may be lower, higher or the same as during germination (Ungar, 1978). Our results show that salt tolerance limits in S. europaea are slightly higher during growth than during germination. Plant growth responses varied following treatment with different salts at low concentrations. Mixed chlorides, and especially sulfate salts, stimulated height increases, but the plant weight was higher in chloride salts. More side branches were produced in 0.5–2% chloride salt solutions. In addition, branches became greenish and distinctly thicker at increased salt concentrations (Fig. 5). In contrast, the branches of S. europaea treated with sulfates were thin, almost awl-shaped and dark green (Fig. 6). At high concentrations no differences were detected between the effects of various pure salts or salt mixtures on plant growth. Height and weight increments were substantially reduced under chloride and sulfate salinity above 2% and 3%, respectively (Fig. 5, Fig. 6). These findings are consistent with previous research showing that salinity induces branching and an increase in biomass in S. europaea, while non-saline conditions inhibit branching and total biomass production (Keiffer et al., 1994).
Seeds of S. europaea from Central Asia were found to be highly tolerant to chloride and sulfate salts, applied either separately or as a mix. Salt tolerance limits were similar (4–5%) during germination and later stages of development. There were minor differences in these limits between seeds of different origin (two populations in USA; Philipupillai and Ungar, 1984 vs. a population in Turkmenistan; this study). Mixed chloride and sulfate salts stimulated both seed germination and plant growth to some degree at later stages (optimal concentrations 0.5–2% for all tested salts). The mechanisms of salt tolerance of the two dimorphic seed types of S. europaea differ, appear to complement one another, and by that may reinforce adaptations to high salinity environments.
We found that raising salt concentrations increases sodium, but decreases potassium concentration in the shoots of Saliconia europaea. This relationship between sodium and potassium concentration has been reported for other salt-tolerant species such as Kochia prostrate (Karimi et al., 2005), Haloxylon recurvum (Khan et al., 2000) and Atriplex prostrate (Karimi and Ungar, 1984). The accumulation of sodium and the concomitant decrease of potassium levels in the shoot appears to be one of the general characteristics of halophytes. This result agrees with the notion that sodium competes with potassium for the same binding sites, and therefore interferes with potassium transport into the cell by using its physiological transport systems (Flowers and Lauchli, 1983).
5. Conclusions
Our study provides evidence of high salt tolerance limits of dimorphic seeds of S. europaea from Central Asia. Germination of large seeds was 3–4 times higher than of small seeds under control and 0.5–2% of all salts tested. Germination and plant growth of S. europaea in mixed sulfate-chloride salts was distinctly higher than in the pure chloride salts, suggesting a mitigating effect of Ca2+ and Mg2+ ions on the cell ion balance reducing negative impact of ions of Na+ and Cl−.
Small seeds exhibited deep innate dormancy. However, application of 0.5–2% of chloride and sulfate salts stimulated germination in these seeds suggesting that, in comparison with large seeds, there is a different mechanism of salt tolerance. Small seeds develop earlier, are more dormant and less salt-tolerant than large seeds. It is possible, that small seeds are evolutionarily older, and large seeds in S. europaea evolved later, when this species habitat had become more saline as a result of species range expansion or increased salinity of already occupied environment. As a result, seed dimorphism made the species more flexible in its response to varying salinity and more adapted to salt and temperature stresses.
Acknowledgments
This study was supported by a grant from the United States Agency for International Development, Bureau for Economic Growth, Agriculture, and Trade, project number TA-MOU-03-CA23-032.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
References
- Ameixa O., Marques B., Fernandes V.S., Soares Amadeu M., Calado R., Lilleb A.I. Dimorphic seeds of Salicornia ramosissima display contrasting germination responses under different salinities. Ecol. Eng. 2016;87:120–123. [Google Scholar]
- Flowers T.J., Lauchli A. Inorganic plant nutrition. V 3. Sodium versus potassium: substitution and compartmentation. Encycl. Plant Physiol. 1983;15B:651–681. [Google Scholar]
- Francois L.E. Salt tolerance of prostrate summer cypress Kochia prostrata. Agron. J. 1976;68:455–456. [Google Scholar]
- Gul B., Ansari R., Flowers T., Khan M. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2003;92:4–18. [Google Scholar]
- Gul B., Khan M.A. Role of calcium in alleviating salinity effects in coastal halophytes. In: Khan M.A., Weber D.J., editors. Ecophysiology of High Salinity Tolerant Plants. Springer; The Netherlands: 2006. pp. 107–114. [Google Scholar]
- Harper S.L., Lovell P.H., Moore K.G. The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1970;1:327–356. [Google Scholar]
- Karimi G., Ghorbanli M., Heidari H., Khavari R., Assareh M. The effects of NaCl on growth, water relations, osmolytes and ion content in Kochia prostrata. Biol. Plant. 2005;49:301–304. [Google Scholar]
- Karimi S.H., Ungar I.A. The effect of salinity on the ion content and water relations of Atriplex triangularis. In: Tiedemann A.R., McArthur E.D., Stutz H.C., Stevens R., Johnson K.L., editors. Proceeding of the Symposium on the Biology of Atriplex and Related Chenopods. 1984. pp. 124–130. [Google Scholar]
- Keiffer C.H., McCarthy B.C., Ungar I.A. Effect of salinity and waterlogging on growth and survival of Salicornia europaea L., an inland halophyte. Ohio J. Sci. 1994;94:70–73. [Google Scholar]
- Keiffer C.H., Ungar I.A. The effect of extended exposure to hypersaline conditions of the germination of five inland halophyte species. Am. J. Bot. 1997;84:104–111. [Google Scholar]
- Keiffer C.H., Ungar I.A. The effect of competition and edaphic conditions on the establishment of halophytes on brine effected soils. Wetl. Ecol. Manag. 2001;9:469–481. [Google Scholar]
- Khan M.A., Gul B. Halophyte seed germination. In: Khan M.A., Weber D.J., editors. Ecophysiology of High Salinity Tolerant Plants. Springer; The Netherlands: 2006. pp. 11–30. [Google Scholar]
- Khan M.A., Ungar I.A., Showalter A.M. Effects of sodium chloride treatments on growth and ion accumulation of the halophyte Haloxylon recurvum. Commun. Soil Sci. Plant Anal. 2000;31:2763–2774. [Google Scholar]
- Kokina S.I. The effect of various salts and their concentrations on the germination of the seeds and the development of the sprouts of Haloxylon species. Bot. J. USSR. 1938;23:287–303. [Google Scholar]
- Lobova E.V. Academy of Sciences of USSR; Moscow: 1967. Soils of the Desert Zone of the U.S.S.R. [Google Scholar]
- Orlovsky N.S. Climate of Turkmenistan. In: Fet V., Atamuradov K.I., editors. Biogeography and Ecology of Turkmenistan. Kluwer Academic Publishers; Netherlands: 1994. pp. 23–48. [Google Scholar]
- Orlovsky N.S., Japakova U.N., Shulgina I., Volis S. Comparative study of seed germination and growth of Kochia prostrata and Kochia scoparia (Chenopodiaceae) under salinity. J. Arid Environ. 2011;75:532–537. [Google Scholar]
- Philipupillai J., Ungar I.A. The effect of seed dimorphism on the germination and survival of Salicornia europaea L. populations. Am. J. Bot. 1984;71:542–549. [Google Scholar]
- Pujol J.A., Calvo J.F., Ramirez-Diaz L. Recovery of germination from different osmotic conditions by four halophytes from Southeastern Spain. Ann. Bot. 2000;85:279–286. [Google Scholar]
- Qu X.X., Huang Z.Y., Baskin J.M., Baskin C.C. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Ann. Bot. 2007;101:293–299. doi: 10.1093/aob/mcm047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semushina L.A., Morozova A.G. Diagnosis of Kochia prostrata (L.) Shrad. salt resistance by seed germination method. Bot. Zhurnal. 1979;64:254–258. [Google Scholar]
- Song J., Fan H., Zhao Yu., Du X., Wang B. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquat. Bot. 2008;88:331–337. [Google Scholar]
- SPSS Inc. 2005. SPSS 13.0 for Windows. [Computer software]. Chicago. [Google Scholar]
- Tobe K., Li X., Omasa K. Effects of five different salts on seed germination and seedling growth of Haloxylon ammodendron (Chenopodiaceae) Seed Sci. Res. 2004;14:345–353. [Google Scholar]
- Ungar I.A. Influence of salinity on seed germination in succulent halophytes. Ecology. 1962;4:763–764. [Google Scholar]
- Ungar I.A. Salinity, temperature, and growth regulator effects on seed germination of Salicornia europaea L. Aquat. Bot. 1977;3:329–335. [Google Scholar]
- Ungar I.A. Halophyte seed germination. Bot. Rev. 1978;44:233–263. [Google Scholar]
- Ungar I.A. Seed dimorphism in Salicornia europaea L. Bot. Gaz. 1979;140:102–108. [Google Scholar]
- Ungar I.A. The distribution and growth of Salicornia europaea L. on an inland salt pan. Ecology. 1987;60:329–336. [Google Scholar]
- Waisel Y. Academic Press; New York, NY: 1972. Biology of Halophytes. [Google Scholar]
- Wang F., Xu Y., Wang Sh., Shi W., Liu R., Feng G., Song J. Salinity effects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol. Biochem. 2015;95:41–48. doi: 10.1016/j.plaphy.2015.07.005. [DOI] [PubMed] [Google Scholar]