Abstract
Aim
To compare implant survival rate and marginal bone loss (MBL) of immediately loaded single implants inserted by using ultrasonic implant site preparation (UISP) (test) and conventional rotary instrumentation (control).
Methods
Two single implants were inserted for each patient: after randomization, test site was prepared by using an ultrasonic device (Piezosurgery Touch, Mectron, Italy) and control site was prepared by using the drills of the selected implant system (Premium AZT, Sweden & Martina, Italy), until reaching a final diameter of 3 mm in both groups. Identical implants (3.8x11.5 mm) were inserted in all sites at crestal level. Impressions were taken and screwed resin single crowns with platform-switched provisional abutments were delivered with 48 hours. Periapical radiographs were taken at provisional crown insertion (T0), 6 months (T1) and one year (T2) after prosthetic loading to measure MBL. All data were tested for normality and subsequently analyzed by paired samples t-test and forward multiple linear regression.
Results
Forty-eight patients were treated in six centers with the insertion of ninety-six implants (48 test; 48 control). Four implants in four patients failed within the first six months of healing (two in test group; two in control group; no difference between groups). Forty patients (age 60.1±10.7 years; 22 female, 18 male) were included in the final analysis. Mean MBL after six months of loading was 1.39±1.03 mm in the test group and 1.42±1.16 mm in the control group (p>0.05) and after one year was 1.92±1.14 mm and 2.14±1.55 mm in test and control, respectively (p>0.05).
Conclusions
No differences in survival rate and MBL were demonstrated between UISP and conventional site preparation with rotary instruments in immediately loaded dental implants: UISP, with its characteristics of enhanced surgical control and safety in proximity of delicate structures, may be used as a reliable alternative to the traditional drilling systems.
1. Introduction
Implant-supported restoration is currently considered as a predictable treatment option for single tooth loss, showing high success rate after 5 years [1]. The original two-stage procedure with delayed implant loading [2, 3] has been modified over the years with the introduction of early and immediate loading protocols, in the attempt to reduce treatment time and patient discomfort [4, 5]. According to recent studies, immediately and conventionally loaded implant-supported single crowns showed equally successful clinical outcomes regarding implant survival rate and marginal bone loss [6, 7]; moreover, patient's oral health-related quality of life was demonstrated to improve significantly after the application of immediately loaded implant-supported fixed prosthesis [8, 9]. However, an accurate presurgical planning and strict adherence to validated protocols are necessary to obtain optimal functional and aesthetic results when approaching these advanced techniques [10–12].
An adequate primary stability is the main prerequisite to apply an immediate loading protocol; a secure mechanical retention of the implant into the host bone is necessary to prevent detrimental micromovements which could lead to a failure of the osseointegration process [13–15]. There is no universal consensus about the minimum primary stability threshold to reach for a safe application of immediate loading protocols; however implant stability quotients (ISQ) >60-65 or peak insertion torques >35 Ncm are mostly accepted as minimum values [6, 16]. Moreover, it should be considered that primary stability decreases during the first month after implant insertion due to peri-implant bone remodeling following surgical trauma [17]: modifications of implant microgeometry have been introduced to enhance and accelerate bone healing response and limit this problem [18, 19].
Piezoelectric bone surgery has been introduced into clinical practice as an alternative possibility of performing osteotomies by using ultrasonic surgical systems [20]. In the last twenty years, various clinical applications of ultrasonic bone cutting in oral and maxillofacial surgery were widely investigated, obtaining promising results in terms of surgical control, precision, and safety [21–29].
Ultrasonic implant site preparation (UISP) has been analyzed in biomolecular and histologic animal studies, showing evidence of favourable bone healing response in the early period after implant insertion [30, 31]. Clinical studies showed that, if compared to the traditional drilling technique, UISP resulted in limited decrease of implant primary stability and in an earlier transition towards an increasing stability pattern, representing a potential additional benefit in immediate loading protocols [32–34].
The objective of this parallel-group, superiority randomized clinical trial (RCT) was to compare the clinical outcomes of immediately loaded single implants inserted by using two different techniques: UISP as test and rotary instruments as control.
2. Materials and Methods
2.1. Study Design
The present study was designed as a multicenter randomized controlled clinical trial, following CONSORT guidelines, and was conducted in six clinical centers in accordance with the Good Clinical Practice Guidelines (GCPs) and with the recommendations of the Declaration of Helsinki as revised in Fortaleza (2013) for investigations with human subjects. The study protocol had been authorized by Azienda Ospedaliero Universitaria “Città della Salute e della Scienza”, Torino, Italy, and recorded in a public register of clinical studies (www.clinicaltrials.gov, n° NCT03357406). A meeting had been held among all the clinical centers before the beginning of the study, in order to illustrate surgical and prosthetic protocols and ensure that clinical operators applied a standardized approach. One clinical operator for each center received written instructions regarding the assessment of experimental parameters in order to obtain acceptable interexaminer consistency in data collection.
Prior to enrollment, all patients were asked to sign an informed consent form to document that they understood the aims of the study (including procedures, follow-up evaluations, and any potential risk involved). Patients were allowed to ask questions pertaining to this study and were thoroughly informed of alternative treatments.
This superiority trial tested the null hypothesis of no differences in survival rate and marginal bone loss between UISP (test group) and conventional site preparation with rotary instruments (control group) in immediately loaded dental implants.
2.2. Study Population
Eligible participants were all adult patients (age ≥18 years), needing two implant-supported single crowns with immediate loading in the upper or lower arch (in incisor, canine, or premolar area), based on accurate diagnosis and treatment planning. Patients underwent clinical examination to evaluate periodontal and occlusal conditions, and bone volume in the areas of interest was analyzed basing on cone-beam computed tomography.
Inclusion criteria were the following:
healed bone crest (at least six months elapsed after tooth loss)
residual bone crest with minimum width of 6 mm and minimum height of 13 mm
both implant sites inserted in similar bone quality (i.e., adjacent or contralateral teeth)
peak insertion torque comprised between 35 and 60 Ncm
patient willing to and fully capable of complying with the study protocol
written informed consent given
Exclusion criteria were the following:
acute myocardial infarction within the past 2 months
uncontrolled coagulation disorders
poorly controlled diabetes (HBA1c > 7.5%)
radiotherapy to the head/neck district within the past 24 months
immunocompromised patient (HIV infection or chemotherapy within the past 5 years)
present or past treatment with intravenous bisphosphonates
psychological or psychiatric problems
alcohol or drugs abuse
full mouth plaque score and/or full mouth bleeding score >20%
2.3. Surgical Procedures
After performing anaesthesia (articaine 4% with epinephrine 1:100.000, Artin, Omnia, Italy) and raising a minimally invasive flap, the randomization sealed opaque envelope was opened by a clinical assistant and the surgeon was advised on the location of test and control sites. The preparation of the test site was performed by using an ultrasonic device (Piezosurgery Touch, Mectron, Italy) and the control site was prepared by using the drills of the selected implant system, following in both cases the sequence recommended by the manufacturer until a final diameter of 3 mm was reached in both groups (Figures 1 and 2). Internal hex implants with a sandblasted/etched surface (Premium AZT, Sweden & Martina, Italy), measuring 3.8x11.5 mm, were inserted in all sites at crestal level with healing abutment of reduced diameter (3.3 mm) (Figure 3). In case of adjacent implants, a minimal distance of 3 mm was respected between the two fixtures.
Figure 1.
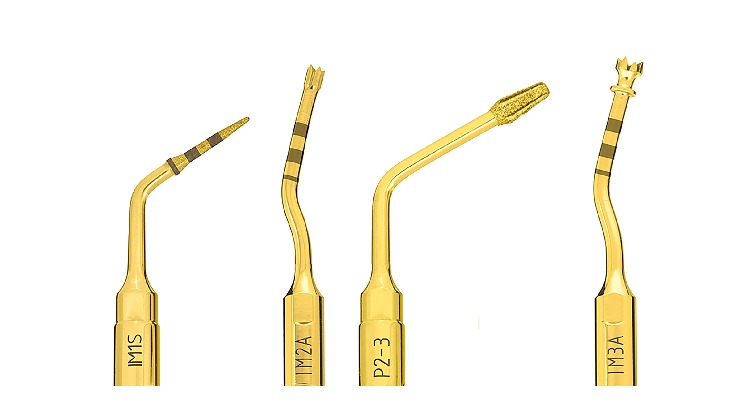
Sequence of ultrasonic inserts used for implant site preparation in the test group (final diameter 3.0 mm).
Figure 2.

Sequence of rotary instruments used for implant site preparation in control group (final diameter 3.0 mm).
Figure 3.

Internal hex implants with a sandblasted/etched surface (Premium AZT, Sweden & Martina, Italy), measuring 3.8x11.5 mm, were inserted in all sites at crestal level with healing abutment of reduced diameter (3.3 mm).
A clinical assistant recorded the peak insertion torque for both implants and the duration of the implant insertion procedure (time elapsed from the first cortical perforation to the complete insertion of the implant in the final position) for both techniques.
After suturing, a polyether impression (Impregum, 3 M Espe, USA) was performed by using an open tray and pick-up copings. Provisional restorations (screwed resin single crowns with platform-switched provisional abutments) were delivered with 48 hours, applying a nonfunctional loading.
Patients were prescribed with antibiotics for 6 days (amoxicillin 1 g twice a day or, in allergic patients, clarithromycin 250 mg twice a day) and nonsteroidal anti-inflammatory drug (ibuprofen 600 mg), when needed. Sutures were removed after 7 days. Definitive screwed metal ceramic crowns were delivered after 6 months of healing.
Periapical radiographs were performed with long cone paralleling technique using a film holding device, customized for each patient with a polyvinylsiloxane jig. Marginal bone level was assessed using a measuring software (Image J, National Institutes of Health, USA) by a single blinded and calibrated examiner (AR). All measurements were repeated three times at three different time points as suggested by Gomez-Roman and Launer [35] and each radiograph was calibrated using the known thread pitch of the implant as a reference. Examiner calibration was performed by assessing 20 radiographs, with another author (CS) who served as “reference examiner”. Intraexaminer and interexaminer concordances were 92.4% and 88.5%, respectively, for linear measurements within ±0.1 mm. The linear distance from the abutment/implant junction to the first bone contact was measured on mesial and distal aspect of the implant at provisional crown insertion (T0), 6 months (T1), one year (T2), and two years (T3) of prosthetic loading. Marginal bone loss (MBL) was defined as the difference among T0 and follow-up measurements (mean value between mesial and distal measurements was considered for each implant).
2.4. Outcomes
This study evaluated the following outcome measures:
MBL: marginal bone loss at T1 and T2, using T0 as a reference
Implant failure: implant mobility and/or any situation suggesting implant removal
Biological and mechanical complications: any complication defined as an unexpected deviation from the normal treatment outcome, both biological (e.g., mucositis, peri-implantitis) and mechanical (e.g., implant fracture, prosthesis fracture, fixation screw loosening, etc.)
2.5. Sample Size and Statistical Power
The calculation was performed to detect a significant difference between the groups in marginal bone loss at 12 months of at least 0.2 mm with an expected standard deviation of 0.4 mm. Based on these data, a sample of 34 patients (68 implants; 34 test and 34 control cases) was needed to reach 80% of statistical power with α set at 0.05. Each clinical center treated 8 patients for a total of 48 (96 implants; 48 test, 48 control) to compensate eventual drop-outs occurring during the follow-up period.
2.6. Randomization
A table was prepared by using a web-based software (www.randomization.com) with a balanced, randomly permuted block approach, distributing first and second site of each patient into two groups (test=UISP; control=drills). The randomization codes were enclosed in numbered, sealed, opaque envelopes which were opened by a clinical assistant after flap elevation in the first site. Treatment allocation was then concealed to the surgeon in charge of recruiting and treating the patients included in this clinical trial.
2.7. Statistical Analysis
Statistical analysis was performed by using a statistical software package (SPSS 22.0, SPSS Inc., Germany). Parametric methods were used for all the datasets. Data normality (with the exception of age) was assured by root square transformation, as assessed through the Kolmogorov-Smirnov test. However, mean and standard deviations of nontransformed data were used for descriptive purposes. The significance of the difference in surgical time between the two groups was assessed through a paired sample t-test [36]. The significance of the difference in marginal bone loss between the groups within each time point and between the time points within each group was assessed by a paired sample t-test.
Finally, for each group, a forward multiple linear regression was used to evaluate the association between marginal bone loss at 12 months (dependent variable) and other independent variables (age, gender, smoking habits, history of periodontal disease, and implant insertion area). The cut-off levels of significance used were 0.05 and 0.10 for entry and removal, respectively.
A p value <0.05 was considered statistically significant.
3. Results
Forty-eight patients were enrolled, randomized, and treated with the insertion of two implants: each clinical center contributed with 8 patients. Eight patients dropped out from the study: eight implants in four patients did not reach a sufficient primary stability to be immediately loaded (35 Ncm) and were submerged under the soft tissues (four in test group; four in control group; no difference between groups); four implants in four patients failed within the first six months of healing (two in test group; two in control group; no difference between groups; 4.5% cumulative failure rate). No additional implants were lost and no other biological or mechanical complications were recorded during the first two years after implant positioning.
Forty patients (age 60.1±10.7 years; range 39–79 years; 22 female, 18 male) with eighty implants (40 test; 40 control) were included in this study. Twenty-six patients referred to be no smokers, eleven light smokers, and three heavy smokers. Main demographic characteristics are summarized in Table 1. Mean MBL of the entire sample after six months of loading was 1.41±1.09 mm, after one year was 2.03±1.36 mm, and after two years was 2.11±1.07 mm. Mean MBL after six months of loading in the test group was 1.39±1.03mm, after one year was 1.92±1.14 mm, and after two years was 1.95±0.99 mm. Mean MBL after six months of loading in the control group was 1.42±1.16mm, after one year was 2.14±1.55 mm, and after two years was 2.22±1.04 mm. Differences in marginal bone loss within test and control groups at 6 months and one year was statistically significant (p<0.0001). Differences between test and control group at six months and one year were not statistically significant (p>0.05).
Table 1.
| Demographic characteristics | |
|---|---|
| Male | 18 (45%) |
| Female | 22 (55%) |
| Mean age (range) | 60.1 (39-79) |
| No smoker | 26 (65%) |
| Light smoker (<10) | 11 (27.5%) |
| Heavy smoker (≥10) | 3 (7.5%) |
Mean surgical time was 395.2±171.3 sec (range 120-810 sec) in the test group and 304.6±148.0 sec (range 120-600 sec) in the control group: difference between the two groups was statistically significant (p=0.001).
Multiple linear regression analysis did not demonstrate a significant association between marginal bone loss and any of the explanatory variables (age, gender, smoking habits, history of periodontal disease, and implant insertion area; data not shown).
4. Discussion
UISP was previously clinically tested on a large number of patients showing that this novel approach could represent a reliable alternative to traditional drilling protocols [37, 38]. Recent studies showed that UISP leads to a limited decrease of primary stability during the early phases of bone healing [32–34], likely due to a slightly different biochemical response in the osteotomy area. In particular, researchers focused on the receptor activator of nuclear factor kappa-B-ligand (RANKL) and osteoprotegerin molecular system, which control the cellular cascade regulating bone resorption process [39]. A recent human study demonstrated lower RANKL levels in implant sites prepared by ultrasonic devices compared to sites prepared by using traditional drilling systems, suggesting decreased osteoclastic activity [40].
On these premises, the present trial was specifically designed to assess the clinical impact of different implant site preparation techniques on the outcomes of immediately loaded single implants.
Eight implants in four patients did not reach an insertion torque ≥35 Ncm and were not immediately loaded, dropping out from the study: these implants were all inserted in low quality maxillary bone where both test and control site preparation technique failed in reaching a sufficient primary stability. This is in accordance with the studies by Baker et al. [41] and Gandhi et al. [42], demonstrating that UISP affords similar primary implant stability in comparison to conventional rotary instrumentation.
Four implants in four patients failed during the first six months of healing and no other implants were lost at two-year follow-up: cumulative survival rate was 95.5%, which is an acceptable result considering that a recent meta-analysis by Sanz et al. (2015) stated that immediately loaded single implants are at greater risk of failure, when compared to immediately loaded bridges or full arch restorations [43]. However, no difference was found between the two arms of the present study (2 failed implants in both groups).
The traditional drilling protocol required a lower operative time than UISP: difference in surgical time between the two groups reached statistical significance in the present trial (p=0.001). This finding is in accordance with all previously published studies comparing the two techniques in terms of duration of the intervention [32, 40, 44]; however, even if the difference was statistically significant, it could be considered clinically irrelevant if balanced by surgical or biological advantages.
Mean interproximal MBL was 1.41 mm after 6 months and 2.03 mm after one year: even if implants inserted with UISP technique resulted in a slightly lower MBL than the control group (1.39 and 1.42 mm at six months; 1.92 and 2.14 mm at one year, respectively), no statistically significant differences were demonstrated between the two groups. Between one-year and two-year follow-up, no statistically significant difference in MBL was demonstrated in both groups, suggesting a stabilization of marginal bone levels. The multivariate analysis did not show a significant influence of patient-related variables (age, gender, smoke, history of periodontitis, and implant insertion site) on MBL.
However, in the present study both test and control groups resulted in a greater mean MBL if compared to data present in literature: seven RCT included in a recent systematic review on immediately loaded single implants reported MBL ranging from 0.24 to 0.91 mm at one-year follow-up [6].
Numerous variables, including patient habits [45], surgical technique [46], soft tissue and alveolar bone thickness [47–49], implant and abutment design (e.g., macro- and microgeometry, connection characteristics, implant crest module, and abutment height) [50–53], number of abutment disconnections [54], and prosthetic features (e.g., screwed versus cemented retention, inadequate occlusion) [55, 56] have been identified as influencing factors in the genesis of peri-implant bone resorption. However, due to their simultaneous action, the exact role and importance of each factor, together with their complex interactions, are not completely clarified yet [57]. In the present study, most of these confounding factors have been controlled by inclusion and exclusion criteria, in order to evaluate the effect of implant site preparation technique. Therefore, the analysis of the factors causing MBL in this trial should be focused on two main factors: implant crest module and characteristics of provisional abutment. Some authors demonstrated that a parallel-sided implant crest module with smooth surface results in a greater shear stress in the crestal region than an angled crest module with rough surface, increasing the risk of marginal bone resorption [50, 51, 58]. Moreover, other studies showed that the presence of microthreads in the implant neck could provide a positive contribution to bone implant contact and to the preservation of the marginal bone [59–61]. The implant used in this study had a parallel-sided crest module with a polished collar without microthreads: these features could have favoured the transmission to the crestal bone of a greater amount of shear forces in comparison to compressive and tensile components. The detrimental role of this force distribution could have a particularly negative influence on marginal bone stability in immediate loading conditions: in fact, a previous study using the same implants but applying a delayed loading (3 months after insertion) reported a mean MBL of 0.8 mm at three-year follow-up [62].
The second factor to be evaluated for its contribution to MBL is the height of the provisional abutment. Numerous authors demonstrated a strict relationship between prosthetic abutment height and peri-implant bone loss, possibly due to a reestablishment of the biological width [63–66]: in particular, Galindo-Moreno and coworkers suggested that a prosthetic abutment height <2 mm is significantly related to higher MBL rates than longer abutments, irrespective of the presence of a platform-switched connection [53]. The height of the provisional abutment used in this study was 1.5 mm (Figure 4): this factor could also have contributed to promote marginal bone resorption during the healing period.
Figure 4.

The provisional abutment used in this study presented 1.5 mm height from the implant platform.
The possible negative impact of the selected implant-abutment system on the maintenance of marginal bone level represented the major limitation of this study. Other possible limitations were represented by the relatively low number of patients and by the partial standardization of bone quality within the single patient. The number of patients included in the final analysis was sufficient to satisfy the minimum sample size requirements of the study, but trials on a broader population are recommended. Moreover, a split-mouth design should be considered, in order to minimize variability between test and control site in terms of bone quality.
Therefore, the aforementioned findings allowed us to accept the null hypothesis of the study of no differences in survival rate and marginal bone loss between UISP (test group) and conventional site preparation with rotary instruments (control group) in immediately loaded dental implants: UISP might be used as a reliable alternative to the traditional drilling systems, coupling similar clinical outcomes with the characteristics of enhanced surgical control and safety in proximity of delicate structures.
Future research should focus on long-term follow-up to determine both implant- and patient-based outcomes of UISP. Comparative clinical trials with split-mouth design in different clinical situations (delayed loading, immediate loading, single implant, and multiple implants) should be designed and conducted.
5. Conclusions
Within the limitations of this study, UISP for immediately loaded single implants resulted in similar clinical outcomes (implant survival rate and marginal bone loss), when compared to conventional rotary instrumentation. Further clinical trials on greater samples and additional long-term studies are necessary to confirm these findings and completely understand the possible clinical advantages of bone healing process after ultrasonic surgery.
Acknowledgments
The authors wish to thank Dr. Francesco Oreglia and Dr. Giuseppe Sepe for their valuable help in conducting this study.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Sweden & Martina, the manufacturer of the implants used in this investigation, partially supported this trial; however, data belonged to the authors and the sponsor did not interfere in the study conduction or in the publication of the results.
References
- 1.Weber H.-P., Sukotjo C. Does the type of implant prosthesis affect outcomes in the partially edentulous patient? The International Journal of Oral & Maxillofacial Implants. 2007;22:140–172. [PubMed] [Google Scholar]
- 2.Lewis S. G., Beumer J., Perri G. R., Hornburg W. P. Single tooth implant supported restorations. The International Journal of Oral & Maxillofacial Implants. 1988;3(1):25–30. [PubMed] [Google Scholar]
- 3.Jemt T., Lekholm U., Gröndahl K. 3-year followup study of early single implant restorations ad modum Brånemark. The International Journal of Periodontics and Restorative Dentistry. 1990;10(5):340–349. [PubMed] [Google Scholar]
- 4.Ericsson I., Nilson H., Lindh T., Nilner K., Randow K. Immediate functional loading of Brånemark single tooth implants: An 18 months' clinical pilot follow-up study. Clinical Oral Implants Research. 2000;11(1):26–33. doi: 10.1034/j.1600-0501.2000.011001026.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen E., Haanaes H. R., Knutsen B. M. Immediate loading of single-tooth ITI implants in the anterior maxilla: a prospective 5-year pilot study. Clinical Oral Implants Research. 2002;13(3):281–287. doi: 10.1034/j.1600-0501.2002.130307.x. [DOI] [PubMed] [Google Scholar]
- 6.Benic G. I., Mir-Mari J., Hämmerle C. H. F. Loading protocols for single-implant crowns: a systematic review and meta-analysis. The International Journal of Oral & Maxillofacial Implants. 2014;29(supplement):222–238. doi: 10.11607/jomi.2014suppl.g4.1. [DOI] [PubMed] [Google Scholar]
- 7.Gjelvold B., Kisch J., Chrcanovic B. R., Albrektsson T., Wennerberg A. Clinical and radiographic outcome following immediate loading and delayed loading of single-tooth implants: Randomized clinical trial. Clinical Implant Dentistry and Related Research. 2017;19(3):549–558. doi: 10.1111/cid.12479. [DOI] [PubMed] [Google Scholar]
- 8.Raes S., Raes F., Cooper L., et al. Oral health-related quality of life changes after placement of immediately loaded single implants in healed alveolar ridges or extraction sockets: a 5-year prospective follow-up study. Clinical Oral Implants Research. 2017;28(6):662–667. doi: 10.1111/clr.12858. [DOI] [PubMed] [Google Scholar]
- 9.Berton F., Stacchi C., Lombardi T., Rapani A., Rizzo R., Di Lenarda R. How does immediately loaded implant-supported fixed rehabilitation influence the oral health-related quality of life? Global Journal of Oral Science. 2018;4(1):1–7. doi: 10.30576/2414-2050.2018.04.1. [DOI] [Google Scholar]
- 10.Wang H.-L., Ormianer Z., Palti A., Perel M. L., Trisi P., Sammartino G. Consensus conference on immediate loading: the single tooth and partial edentulous areas. Implant Dentistry. 2006;15(4):324–333. doi: 10.1097/01.id.0000246248.55038.3a. [DOI] [PubMed] [Google Scholar]
- 11.Colombo M., Mangano C., Mijiritsky E., Krebs M., Hauschild U., Fortin T. Clinical applications and effectiveness of guided implant surgery: A critical review based on randomized controlled trials. BMC Oral Health. 2017;17, article 150 doi: 10.1186/s12903-017-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs R., Salmon B., Codari M., Hassan B., Bornstein M. M. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018;18(1) doi: 10.1186/s12903-018-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szmukler-Moncler S., Salama H., Reingewirtz Y., Dubruille J. H. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 1998;43(2):192–203. doi: 10.1002/(sici)1097-4636(199822)43:2lt;192::aid-jbm1462;3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Hsu J.-T., Wu A. Y.-J., Fuh L.-J., Huang H.-L. Effects of implant length and 3D bone-to-implant contact on initial stabilities of dental implant: A microcomputed tomography study. BMC Oral Health. 2017;17(1) doi: 10.1186/s12903-017-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldi D., Lombardi T., Colombo J., et al. Correlation between insertion torque and implant stability quotient in tapered implants with knife-edge thread design. BioMed Research International. 2018;2018:7. doi: 10.1155/2018/7201093.7201093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maló P., Rangert B., Dvärsäter L. Immediate function of Brånemark implants in the esthetic zone: A retrospective clinical study with 6 months to 4 years of follow-up. Clinical Implant Dentistry and Related Research. 2000;2(3):138–146. doi: 10.1111/j.1708-8208.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 17.Raghavendra S., Wood M. C., Taylor T. D. Early wound healing around endosseous implants: a review of the literature. The International Journal of Oral & Maxillofacial Implants. 2005;20(3):425–431. [PubMed] [Google Scholar]
- 18.Oates T. W., Valderrama P., Bischof M., et al. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. The International Journal of Oral & Maxillofacial Implants. 2007;22(5):755–760. [PubMed] [Google Scholar]
- 19.Vlacic-Zischke J., Hamlet S. M., Friis T., Tonetti M. S., Ivanovski S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFβ/BMP signalling in osteoblasts. Biomaterials. 2011;32(3):665–671. doi: 10.1016/j.biomaterials.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Vercellotti T. Piezoelectric surgery in implantology: a case report—a new piezoelectric ridge expansion technique. The International Journal of Periodontics and Restorative Dentistry. 2000;20(4):359–365. [PubMed] [Google Scholar]
- 21.Nordera P., Spanio Di Spilimbergo S., Stenico A., Fornezza U., Volpin L., Padula E. The cutting-edge technique for safe osteotomies in craniofacial surgery: The piezosurgery bone scalpel. Plastic and Reconstructive Surgery. 2007;120(7):1989–1995. doi: 10.1097/01.prs.0000287328.56050.4e. [DOI] [PubMed] [Google Scholar]
- 22.Vercellotti T., Podesta A. Orthodontic microsurgery: A new surgically guided technique for dental movement. International Journal of Periodontics and Restorative Dentistry. 2007;27(4):325–331. [PubMed] [Google Scholar]
- 23.Schaeren S., Jaquiéry C., Heberer M., Tolnay M., Vercellotti T., Martin I. Assessment of nerve damage using a novel ultrasonic device for bone cutting. Journal of Oral and Maxillofacial Surgery. 2008;66(3):593–596. doi: 10.1016/j.joms.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Stacchi C., Costantinides F., Biasotto M., Di Lenarda R. Relocation of a malpositioned maxillary implant with piezoelectric osteotomies: A case report. International Journal of Periodontics and Restorative Dentistry. 2008;28(5):489–495. [PubMed] [Google Scholar]
- 25.Sammartino G., Riccitiello F., Trosino O., Marenzi G., Cioffi A., Mortellaro C. Use of piezosurgery device in management of oral surgery complications: clincal case and clinical experience report. Minerva Stomatologica. 2012;61(5):225–231. [PubMed] [Google Scholar]
- 26.Robiony M., Costa F., Politi M. Ultrasound endoscopic bone cutting for rapid maxillary expansion. Journal of Oral and Maxillofacial Surgery. 2014;72(5):980–990. doi: 10.1016/j.joms.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Stacchi C., Vercellotti T., Toschetti A., Speroni S., Salgarello S., Di Lenarda R. Intraoperative complications during sinus floor elevation using two different ultrasonic approaches: A two-center, randomized, controlled clinical trial. Clinical Implant Dentistry and Related Research. 2015;17(supplement 1):e117–e125. doi: 10.1111/cid.12136. [DOI] [PubMed] [Google Scholar]
- 28.Al-Moraissi E. A., Elmansi Y. A., Al-Sharaee Y. A., Alrmali A. E., Alkhutari A. S. Does the piezoelectric surgical technique produce fewer postoperative sequelae after lower third molar surgery than conventional rotary instruments? A systematic review and meta analysis. International Journal of Oral and Maxillofacial Surgery. 2016;45(3):383–391. doi: 10.1016/j.ijom.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Stacchi C., Lombardi T., Cusimano P., et al. Bone scrapers versus piezoelectric surgery in the lateral antrostomy for sinus floor elevation. The Journal of Craniofacial Surgery. 2017;28(5):1191–1196. doi: 10.1097/SCS.0000000000003636. [DOI] [PubMed] [Google Scholar]
- 30.Preti G., Martinasso G., Peirone B., et al. Cytokines and growth factors involved in the osseointegration of oral titanium implants positioned using piezoelectric bone surgery versus a drill technique: a pilot study in minipigs. Journal of Periodontology. 2007;78(4):716–722. doi: 10.1902/jop.2007.060285. [DOI] [PubMed] [Google Scholar]
- 31.Sirolli M., Mafra C. E. S., dos Santos R. A. B., Saraiva L., Holzhausen M., César Neto J. B. Influence of piezosurgery on bone healing around titanium implants: A histological study in rats. Brazilian Dental Journal. 2016;27(3):278–283. doi: 10.1590/0103-6440201600161. [DOI] [PubMed] [Google Scholar]
- 32.Stacchi C., Vercellotti T., Torelli L., Furlan F., Di Lenarda R. Changes in implant stability using different site preparation techniques: twist drills versus piezosurgery. A single-blinded, randomized, controlled clinical trial. Clinical Implant Dentistry and Related Research. 2013;15(2):188–197. doi: 10.1111/j.1708-8208.2011.00341.x. [DOI] [PubMed] [Google Scholar]
- 33.Da Silva Neto U. T., Joly J. C., Gehrke S. A. Clinical analysis of the stability of dental implants after preparation of the site by conventional drilling or piezosurgery. British Journal of Oral and Maxillofacial Surgery. 2014;52(2):149–153. doi: 10.1016/j.bjoms.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Canullo L., Peñarrocha D., Peñarrocha M., Rocio A.-G., Penarrocha-Diago M. Piezoelectric vs. conventional drilling in implant site preparation: Pilot controlled randomized clinical trial with crossover design. Clinical Oral Implants Research. 2014;25(12):1336–1343. doi: 10.1111/clr.12278. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Roman G., Launer S. Peri-implant bone changes in immediate and non-immediate root-analog stepped implants—a matched comparative prospective study up to 10 years. International Journal of Implant Dentistry. 2016;2(1) doi: 10.1186/s40729-016-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perinetti G. StaTips Part I: Choosing statistical test when dealing with differences. South European Journal of Orthodontics and Dentofacial Research. 2017;3(1) doi: 10.5937/sejodr3-15216. [DOI] [Google Scholar]
- 37.Vercellotti T., Stacchi C., Russo C., et al. Ultrasonic implant site preparation using piezosurgery: a multicenter case series study analyzing 3,579 implants with a 1- to 3-year follow-up. International Journal of Periodontics and Restorative Dentistry. 2014;34(1):11–18. doi: 10.11607/prd.1860. [DOI] [PubMed] [Google Scholar]
- 38.Makary C., Rebaudi A., Demircioglu A., Lahoud P., Naaman N. Standard drilling versus ultrasonic implant site preparation: a clinical study at 4 weeks after insertion of conical implants. Implant Dentistry. 2017;26(4):547–552. doi: 10.1097/ID.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 39.Belibasakis G. N., Bostanci N. The RANKL-OPG system in clinical periodontology. Journal of Clinical Periodontology. 2012;39(3):239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 40.Peker Tekdal G., Bostanci N., Belibasakis G. N., Gürkan A. The effect of piezoelectric surgery implant osteotomy on radiological and molecular parameters of peri-implant crestal bone loss: A randomized, controlled, split-mouth trial. Clinical Oral Implants Research. 2016;27(5):535–544. doi: 10.1111/clr.12620. [DOI] [PubMed] [Google Scholar]
- 41.Baker J. A., Vora S., Bairam L., Kim H.-I., Davis E. L., Andreana S. Piezoelectric vs. conventional implant site preparation: Ex vivo implant primary stability. Clinical Oral Implants Research. 2012;23(4):433–437. doi: 10.1111/j.1600-0501.2011.02286.x. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi S. A., Baker J. A., Bairam L., Kim H.-I., Davis E. L., Andreana S. Primary stability comparison using piezoelectric or conventional implant site preparation systems in cancellous bone: a pilot study. Implant Dentistry. 2014;23(1):79–84. doi: 10.1097/id.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 43.Sanz-Sánchez I., Sanz-Martín I., Figuero E., Sanz M. Clinical efficacy of immediate implant loading protocols compared to conventional loading depending on the type of the restoration: A systematic review. Clinical Oral Implants Research. 2015;26(8):964–982. doi: 10.1111/clr.12428. [DOI] [PubMed] [Google Scholar]
- 44.Atieh M. A., Alsabeeha N. H. M., Tawse-Smith A., Duncan W. J. Piezoelectric versus conventional implant site preparation: a systematic review and meta-analysis. Clinical Implant Dentistry and Related Research. 2018;20(2):261–270. doi: 10.1111/cid.12555. [DOI] [PubMed] [Google Scholar]
- 45.Galindo-Moreno P., Fauri M., Ávila-Ortiz G., Fernández-Barbero J. E., Cabrera-León A., Sánchez-Fernández E. Influence of alcohol and tobacco habits on peri-implant marginal bone loss: A prospective study. Clinical Oral Implants Research. 2005;16(5):579–586. doi: 10.1111/j.1600-0501.2005.01148.x. [DOI] [PubMed] [Google Scholar]
- 46.Marconcini S., Giammarinaro E., Toti P., Alfonsi F., Covani U., Barone A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clinical Implant Dentistry and Related Research. 2018;20(3):322–332. doi: 10.1111/cid.12586. [DOI] [PubMed] [Google Scholar]
- 47.Spray J. R., Black C. G., Morris H. F., Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Annals of Periodontology. 2000;5(1):119–128. doi: 10.1902/annals.2000.5.1.119. [DOI] [PubMed] [Google Scholar]
- 48.Linkevicius T., Apse P., Grybauskas S., Puisys A. Influence of thin mucosal tissues on crestal bone stability around implants with platform switching: A 1-year pilot study. Journal of Oral and Maxillofacial Surgery. 2010;68(9):2272–2277. doi: 10.1016/j.joms.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Vervaeke S., Dierens M., Besseler J., De Bruyn H. The influence of initial soft tissue thickness on peri-implant bone remodeling. Clinical Implant Dentistry and Related Research. 2014;16(2):238–247. doi: 10.1111/j.1708-8208.2012.00474.x. [DOI] [PubMed] [Google Scholar]
- 50.Misch C. E., Bidez A. A scientific rationale for dental implant. In: Misch C. E., editor. Contemporary Implant Dentistry. 2nd. St. Louis: Mosby: 1999. pp. 329–343. [Google Scholar]
- 51.Oh T., Yoon J., Misch C. E., Wang H. The causes of early implant bone loss: myth or science? Journal of Periodontology. 2002;73(3):322–333. doi: 10.1902/jop.2002.73.3.322. [DOI] [PubMed] [Google Scholar]
- 52.Cochran D. L., Bosshardt D. D., Grize L., et al. Bone respones to loaded implants with non-matching implant-abutment diameters in the canine mandible. Journal of Periodontology. 2009;80(4):609–617. doi: 10.1902/jop.2009.080323. [DOI] [PubMed] [Google Scholar]
- 53.Galindo-Moreno P., León-Cano A., Ortega-Oller I., et al. Prosthetic abutment height is a key factor in peri-implant marginal bone loss. Journal of Dental Research. 2014;93(7):80–85. doi: 10.1177/0022034513519800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atieh M. A., Tawse-Smith A., Alsabeeha N. H. M., Ma S., Duncan W. J. The one abutment-one time protocol: A systematic review & meta-analysis. Journal of Periodontology. 2017;88(11):1173–1185. doi: 10.1902/jop.2017.170238. [DOI] [PubMed] [Google Scholar]
- 55.Misch C. E., Dietsh-Misch F., Hoar J., Beck G., Hazen R., Misch C. M. A bone quality-based implant system: first year of prosthetic loading. Journal of Oral Implantology. 1999;25(3):185–197. doi: 10.1563/1548-1336(1999)025<0185:ABQISF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 56.Linkevicius T., Puisys A., Vindasiute E., Linkeviciene L., Apse P. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clinical Oral Implants Research. 2013;24(11):1179–1184. doi: 10.1111/j.1600-0501.2012.02570.x. [DOI] [PubMed] [Google Scholar]
- 57.Traini T., Berardini M., Congedi F., Sinjari B., Trisi P., Caputi S. Impact of second stage surgery on bone remodeling around new hybrid titanium implants: a prospective clinical study in humans. Implant Dentistry. 2017;26(1):121–128. doi: 10.1097/ID.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 58.Khanna V., Khanna G., Bansal A., Malik R. Predictable biomechanics and implications of implant crest module: a clinical note. Annals of Medical Health Sciences Research. 2014;4(S1):S71–S72. doi: 10.4103/2141-9248.131735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin S.-Y., Han D.-H. Influence of a microgrooved collar design on soft and hard tissue healing of immediate implantation in fresh extraction sites in dogs. Clinical Oral Implants Research. 2010;21(8):804–814. doi: 10.1111/j.1600-0501.2010.01917.x. [DOI] [PubMed] [Google Scholar]
- 60.Aparna I. N., Dhanasekar B., Lingeshwar D., Gupta L. Implant crest module: A review of biomechanical considerations. Indian Journal of Dental Research. 2012;23(2):257–263. doi: 10.4103/0970-9290.100437. [DOI] [PubMed] [Google Scholar]
- 61.Niu W., Wang P., Zhu S., Liu Z., Ji P. Marginal bone loss around dental implants with and without microthreads in the neck: A systematic review and meta-analysis. Journal of Prosthetic Dentistry. 2017;117(1):34–40. doi: 10.1016/j.prosdent.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Negri M., Galli C., Smerieri A., et al. The effect of age, gender, and insertion site on marginal bone loss around endosseous implants: results from a 3-year trial with premium implant system. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/369051.369051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collaert B., De Bruyn H. Early loading of four or five Astra Tech fixtures with a fixed cross-arch restoration in the mandible. Clinical Implant Dentistry and Related Research. 2002;4(3):133–135. doi: 10.1111/j.1708-8208.2002.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 64.Galindo-Moreno P., León-Cano A., Monje A., Ortega-Oller I., O'valle F., Catena A. Abutment height influences the effect of platform switching on peri-implant marginal bone loss. Clinical Oral Implants Research. 2016;27(2):167–173. doi: 10.1111/clr.12554. [DOI] [PubMed] [Google Scholar]
- 65.Spinato S., Galindo-Moreno P., Bernardello F., Zaffe D. Minimum abutment height to eliminate bone loss: Influence of implant neck design and platform switching. The International Journal of Oral & Maxillofacial Implants. 2018;33(2):405–411. doi: 10.11607/jomi.5604. [DOI] [PubMed] [Google Scholar]
- 66.Blanco J., Pico A., Caneiro L., Nóvoa L., Batalla P., Martín-Lancharro P. Effect of abutment height on interproximal implant bone level in the early healing: A randomized clinical trial. Clinical Oral Implants Research. 2018;29(1):108–117. doi: 10.1111/clr.13108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.


