Abstract
Objective
Acute kidney injury (AKI) occurs early in pediatric intensive care unit (PICU) admission and increases risks for poor outcomes. We evaluated the feasibility of a multi-centre AKI biomarker urine collection protocol and measured diagnostic characteristics of urine neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18) and liver fatty acid binding protein (LFABP) to predict AKI and prolonged AKI.
Design:
Prospective observational pilot cohort study.
Setting:
Four Canadian tertiary healthcare PICUs.
Patients:
Eighty-one children 1 month-18 years old. Exclusions: cardiac surgery; baseline severe kidney disease; inadequate urine or serum for PICU days 1 to 3.
Interventions:
PICUs performed standardized urine collection protocol to obtain early PICU admission urine samples, with deferred consent.
Measurements and main results:
Study barriers and facilitators were recorded. AKI was defined based on Kidney Disease: Improving Global Outcomes (KDIGO) serum creatinine (SCr) criteria (AKISCr) and by SCr and urine output criteria (AKISCr+UO) Prolonged AKI was defined as AKI duration ≥48 hours. PICU days 1 to 3 NGAL, IL-18 and LFABP were evaluated for AKI prediction (area under the curve [AUC]). Biomarkers on the first day of AKI attainment (day 1 AKI) were evaluated for predicting prolonged AKI. Eighty-two to 95% of subjects had urine collected from PICU days 1 to 3. Sixteen subjects (20%) developed AKISCr; 38 (47%) developed AKISCr+UO. On PICU day 1, IL-18 predicted AKISCr with AUC=0.82, but NGAL and LFABP predicted AKISCr with AUC’s ≤0.69; on PICU day 2, AUC’s higher (not shown). IL-18 and LFABP on day 1 AKI predicted prolonged AKISCr (AUC’s 0.74 and 0.83, respectively). When AKISCr+UO was used to define AKI, biomarker AUC’s were globally lower.
Conclusions:
Protocol urine collection to procure early admission samples is feasible. Individual biomarker AKI prediction performance is highly variable and modest. Larger studies should evaluate utility and cost effectiveness of using early AKI biomarkers.
Keywords: feasibility, critical care unit, renal, child, diagnostic testing
INTRODUCTION
Acute kidney injury (AKI) occurs in 10–20% of patients in the pediatric intensive care unit (PICU)(1,2). AKI often develops early in PICU admission and is consistently reported to be a risk factor for prolonged ventilation, longer hospital stay and increased PICU mortality(1–4).
AKI definition utilizes serum creatinine (SCr) rise from baseline or urine output (UO) decrease, per the international Kidney Disease: Improving Global Outcomes (KDIGO) definition(5). However, SCr rise with AKI is delayed, resulting in late diagnosis, treatment and prevention of AKI complications(6). Several treatments may attenuate renal tissue injury (7–10) but have been understudied or unsuccessful in humans, at least partially due to the shortcoming of SCr to promptly detect AKI(6,11).
Several early AKI biomarkers have been studied in cardiac surgery patients(12–14) and less so in non-cardiac surgery patients(15–17). Most are urine proteins upregulated in renal tubule cells with cell (or tissue) injury, as opposed to SCr which rises due to renal function change. Thus, biomarkers have been referred to as “structural” AKI biomarkers(14,18). New AKI biomarkers may rise before functional impairment becomes evident or serve as measures of AKI severity. Neutrophil gelatinase-associated lipocalin (NGAL) is extruded into urine with AKI, involved in injury and repair of renal tubule cells(19). Other recently studied AKI biomarkers include interleukin-18 (IL-18) which plays a role in activating macrophages, T cell-immunity and mediating ischemic tubule injury(20) and liver-type fatty acid binding protein (LFABP), a proximal tubule cytoplasmic fatty acid carrier that binds intermediates of peroxidation(21). These biomarkers have shown promise for early AKI diagnosis in children undergoing cardiac surgery or single-centre studies of non-cardiac populations(12,13,15–17,22–23). Few AKI biomarker studies have been published in the non-cardiac surgery PICU population and no multi-centre PICU biomarker studies have been published. This research gap may be contributed to by the fact that recruiting such patients into early AKI diagnostic biomarker studies is difficult. Because AKI occurs early in PICU admission(1,2), recruiting patients early enough in PICU admission to collect urine specimens for biomarker measurement is challenging.
There is also a knowledge gap regarding the impact of UO criteria of AKI definition, on AKI biomarker performance. Almost all AKI biomarker studies have only applied the KDIGO SCr criteria to define AKI, ignoring UO criteria. AKI ascertainment may differ substantially when SCr criteria alone are used vs. applying both SCr and UO criteria to define AKI(3,24). The extent to which applying UO criteria in AKI definition affects AKI biomarker diagnostic performance is unknown.
We hypothesized that urine NGAL, IL-18 and LFABP are early diagnostic markers of AKI and predict prolonged AKI duration (as a marker of AKI severity), in non-cardiac surgery children admitted to the PICU and that inclusion of UO criteria to define AKI modifies biomarker diagnostic performance. We also hypothesized that instituting a PICU clinical urine collection protocol would reduce the challenge of studying of early AKI biomarkers. We performed a pilot multi-centre study to evaluate feasibility of a urine collection protocol on all children admitted to PICU for procurement of early AKI biomarkers. We used this opportunity to evaluate and compare urine NGAL, IL-18, LFABP for early diagnosis of AKI defined using SCr criteria and AKI defined using both SCr and UO criteria.
MATERIALS AND METHODS
Design and setting
This was a prospective, observational cohort study, performed at four Canadian PICUs: the Montreal Children’s Hospital, Montreal (July-October 2013), Children’s Hospital of Winnipeg, Winnipeg (November 2013-January 2014), British Columbia’s Children’s Hospital, Vancouver (January-April 2014), and Stollery Children’s Hospital, Edmonton (June-September 2014). Inclusion criteria were age 1 month-18 years old. Clinical exclusion criteria were: known baseline estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2; immediately post-renal transplantation or cardiac surgery; admitted with a primary renal disease (e.g., nephritis); admitted to PICU for <2 calendar days. Enrollement occurred at the latest by PICU day 3. Research ethics board approval was obtained to perform research activities at all institutions.
Recruitment
The subject eligibility evaluation procedure is shown in Supp. online Figure 1. To maximize multi-centre staff participation and encourage recruitment, we performed a 2–4 week nurse and physician pre-study education phase (e-mails, in-services, study posters), informing on the study rationale and protocol. Following this, a PICU urine collection protocol was instituted. This protocol included urine collection daily (from existing urinary catheters, urine collection bag or cotton balls in children wearing diapers) for the first 3 PICU days on all PICU patients. Urine samples were not used for study purposes until the patient was recruited (deferred consent)(25), but were placed in a 4°C fridge within the PICU until the patient was screened for eligibility. Every 1–3 days, the research team performed eligibility screening (inclusion, exclusion criteria) for all PICU patients. Eligible patients were screened for urine specimen number adequacy and measures of SCr. Of eligible patients (as per criteria described above), only those with a) ≥1 urine specimen if admitted for 2 days or ≥2 urine specimens if admitted for 3 days and b) ≥1 SCr measurement if admitted for 2 days or ≥2 SCr measurements if admitted ≥3 calendar days, were considered for recruitment. Patients fulfilling clinical and specimen eligibility criteria were approached for informed consent and/or patient assent (if appropriate). Urine from patients not eligible or refusing participation was immediately discarded.
Study procedure and clinical data collection
The study occurred over 6 to 10 weeks at each site. Once a patient was recruited (at the latest PICU day 3), daily urine collection continued for up to 5 days from PICU admission. Urine specimens were centrifuged (1000g, 15 minutes, 21°C), aliquoted and stored at −80°C until biomarker measurement. Urine specimens remained in the 4°C fridge ≤72 hours. Prior studies suggest biomarkers are stable under similar conditions(26).
Clinical data was collected for up to 14 days of PICU admission on case report forms with a detailed instructions manual to ensure cross-site data consistency. The central site coordinator performed training for each site coordinator at study initiation, followed by regular phone and email contact throughout study. Clinical variables included: age, gender, weight, height, past medical history, primary PICU diagnosis, fluid balance, sepsis or infection (defined by a positive body fluid culture or infection diagnosis [sepsis, pneumonia, meningitis] in admission or discharge summaries), death, mechanical ventilation, extra-corporeal membrane oxygenation, dialysis, available variables for the Pediatric Risk of Mortality II (PRISM) score (which does not include a renal component)(27), and medications including vasopressors, selected nephrotoxins (including non-steroidal anti-inflammatory agents, vancomycin, aminoglycosides, acyclovir/ganciclovir derivatives and amphotericin) and diuretics. Urine output at every 8 hour interval was recorded (to apply the AKI definition described below) for up to 7 PICU days. Our previously performed single-centre study(28) informed us that UO is very poorly recorded in most patients admitted for over 7 days. Routinely collected SCr values were recorded. When SCr was not measured by the clinical team, if leftover serum from other tests performed that day was available in the hospital laboratory, we obtained this serum to measure SCr when feasible. Data were entered into a secure REDCap database, cleaned every 1–2 months, with 10% data re-entry to capture errors and sites were queried for missing or unclear data.
Baseline SCr was defined as the lowest SCr within 3 months before PICU admission(5). When baseline SCr was not available, baseline estimated glomerular filtration rate (eGFR) of 120 ml/min/1.73m2 was assumed and baseline SCr was estimated by back-calculating from the Chronic Kidney Disease in Children eGFR formula and height(29). When no height or baseline SCr were available, eGFR was estimated using a height-independent eGFR equation derived in Europe and validated in Canada (30,31), with back-calculation of SCr to estimate baseline SCr.
Throughout the study, sites systematically recorded barriers and facilitators to recruitment, specimen and data collection feasibility.
Laboratory measurements
SCr measurements were performed at each site’s central laboratory, to maintain within-subject assay consistency for evaluating SCr change from baseline. All sites report IDMS-traceable SCr. Frozen urine was shipped to the MCH at the end of the study period and stored at −80°C. Biomarkers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory, Ohio, USA. NGAL was measured using a commercial ELISA kit (Bioporto, Gentogte, Denmark). LFABP was measured using a microbead-based assay (MesoScale Discovery [MSD] Gaithersburg, MD) on a luminex system detected by a Sector Imager 2400 (MSD). IL-18 was measured using an IL-18 ELISA kit (Medical & Biological Laboratories, Nagoya, Japan). Verified inter and intra assay biomarker assay coefficient of variations were ≤10%. Biomarkers were measured in duplicate and corrected for urine creatinine. Personnel measuring biomarkers were blinded to clinical outcomes.
Outcomes
The primary study feasibility outcome was achieving urine collection on Days 1, 2 and 3 of PICU admission. The primary outcome for biomarker analyses was AKI based on the KDIGO definition(5). Because few AKI biomarker studies have used the UO criteria to define AKI, and few pediatric studies have evaluated the UO criteria for associations with clinical outcomes(24), we defined AKI in two ways. The first was to only apply KDIGO SCr criteria (hereafter, AKISCr). Peak SCr during PICU admission was divided by baseline SCr to calculate percentage SCr rise. Subjects with SCr rise ≥50% or ≥26.5 umol/L from baseline were classified as AKISCr stage 1; subjects with SCr doubling were classified as stage 2; those with SCr tripling, requiring dialysis or eGFR <35 ml/min/1.73m2 were classified as stage 3. We also defined AKI using both SCr and UO criteria for AKI definition, whereby a patient may be classified as having AKI if they either fulfill SCr criteria (described above) or UO criteria. The UO criteria were stage 1 AKI, if UO was <0.5 ml/kg/hour for 8 hours, stage 2 if UO was <0.5 ml/kg/hour for 16 hours, or stage 3 if the patient was anuric for 12 hours or had UO < 0.3 ml/kg/hour for 24 hours. When evaluating consecutive 8-hour intervals, we performed this in a contiguous manner for different PICU days (e.g., evaluating the last 8 hours of the prior day and the first 8 hours of the current day to determine that 16 hour UO severity stratum). Of note, the KDIGO guideline defines UO AKI in adults using 6-hour, rather than 8-hour intervals. However, almost all published pediatric AKI studies have defined AKI using the 8-hour intervals used in this study, from the original published pediatric AKI modified definition (3, 24). For consistency with past research, we utilized these pediatric-specific UO criteria, referring to the combined SCr or UO-based definition as AKISCr+UO, (or “complete” KDIGO AKI definition).
A secondary biomarker outcome was prolonged AKI duration, defined as AKI criteria fulfillment for ≥48 hours from the day of first evidence of AKI (e.g., the first day that SCr rises by at least 50%)(3).
Statistical Analysis
Continuous variables were compared between groups using t-tests, one-way analysis of variance, Mann-Whitney-U or Kruskal-Wallis tests. Categorical variables were compared using Fisher’s exact or Chi-square tests. The multi-centre recruitment goal was 15–20 patients/site within 4–6 weeks. Analyses were performed using STATA® version 10, College Station, Texas, USA. Analyses were performed separately using AKISCr and AKISCr+UO definitions.
Biomarkers for early AKI diagnosis
Biomarker concentrations on PICU days 1 to 3 were plotted and compared between AKI and non-AKI groups, in subjects with biomarkers available from all three days.
Calculation of area under the receiver operating characteristic curve (AUC, 95% confidence interval [CI]) was used to evaluate biomarkers from PICU days 1 to 3 (in patients with biomarkers available on those individual days), to predict AKI development. All non-AKI subjects with biomarkers available were included in this AUC calculation; only AKI subjects who had not yet developed AKI by the day of assessment were included in these analyses.
Biomarker associations with prolonged AKI
In AKI subjects, biomarkers measured on the first day of AKI attainment were evaluated for predicting prolonged AKI duration using AUC analysis.
RESULTS
Study population
Our final cohort included 81 children (Figure 1, study flow). Sixteen subjects (20%) developed AKISCr (stage 1, n=11; stage 2 or worse, n=5); 38 (47%) developed AKISCr+UO (stage 1, n=25; stage 2 or worse, n=13). Baseline SCr was available in 37 (46%) patients. About 95% of all AKI developed by PICU day 4. There was 73% agreement between AKISCr and AKISCr+UO designations (Kappa statistic = 0.44; Supp. Table 1 shows AKI staging severity by each definition). Primary reasons for PICU admission were respiratory (n=21, 26%), neurosurgical (n=15, 19%), orthopedic or trauma (n=14, 17%), hematologic-oncologic (n=6, 7%), or other (n=25, 31%), with no significant difference between AKI and non-AKI groups (not shown). In general, differences in patient characteristics between AKI vs. non-AKI groups were more striking, with increased illness severity markers (i.e., higher PRISM, more vasopressor use, longer stay, Table 1) when AKI was defined by SCr criteria, compared to defining AKI by SCr or UO criteria (Table 1).
Figure 1. Study flow to analysis population and urine collection protocol performance.
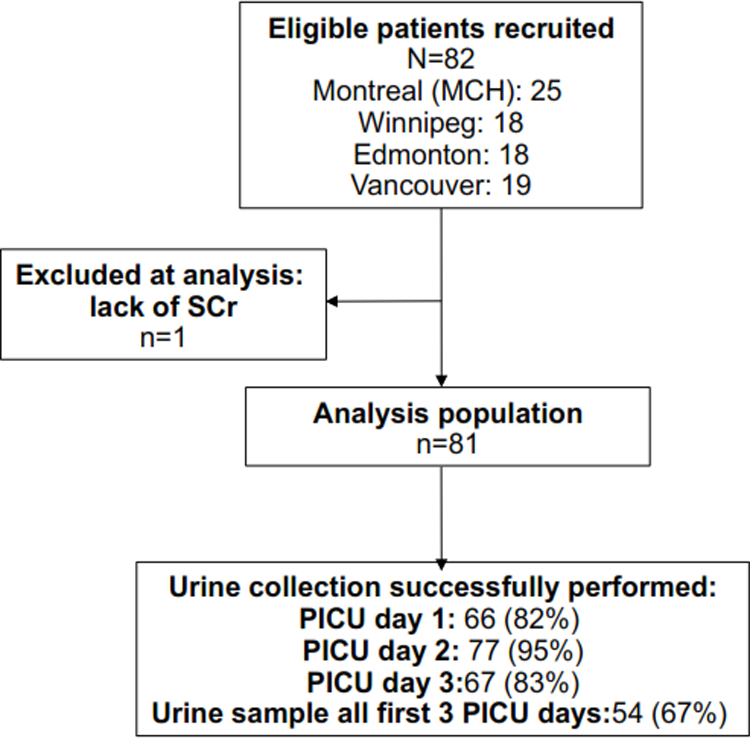
Describes the performance of urine collection protocol, as measured by proportions of patients with a urine specimen collected on each of the first 3 PICU admission days. Abbreviations: SCr: serum creatinine; PICU: pediatric intensive care unit.
Table 1.
Patient characteristics by acute kidney injury (AKI) status, as defined by serum creatinine (SCr) criteria and by SCr or urine output (UO) criteria.
| Variable | No AKI SCr (n=65) |
AKI SCr (n=16) |
No AKI SCr+UO (n=43) |
AKI SCr+UO (n=38) |
|
|---|---|---|---|---|---|
| Age (years) | 7.7 (6.2) | 10.7 (5.9) | 6.5 (6.3) | 10.3 (5.5)* | |
| Male gender | 35 (53.9%) | 8 (50.0%) | 22 (51.2%) | 21 (55.3%) | |
| PRISM score | 6.4 (5.7) | 12.1 (6.1)* | 6.6 (5.9) | 8.6 (6.4) | |
| Baseline eGFR(ml/min/1.73m2) | 104.4 (65.4) | 138.8 (26.3)* | 92.3 (48.4) | 131.4 (65.3)* | |
|
Bladder
catheter |
PICU day 1 | 43 (67.2%) | 13 (81.2%) | 27 (64.3%) | 29 (76.3%) |
| PICU day 2 | 48 (73.9%) | 12 (75.0%) | 30 (69.8%) | 30 (79.0%) | |
| PICU day 3 | 27 (69.2%) | 8 (72.7%) | 19 (79.1%) | 16 (61.5%) | |
| Infection |
Primary diagnosis |
2 (3.1%) | 2 (12.5%) | 2 (4.7%) | 2 (5.3%) |
| During PICU | 11 (16.9%) | 8 (50.0%)* | 10 (23.3%) | 9 (23.7%) | |
|
Number of nephrotoxins in
PICU |
0.7 (0.8) | 0.9 (1.2) | 0.8 (0.8) | 0.7 (0.9) | |
| Diuretics |
Diuretics first
2 PICU days |
15 (23.1%) | 7 (43.8%) | 13 (30.2%) | 9 (23.7%) |
|
# PICU Days
with diuretics |
0.9 (2.4) | 2.8 (3.9)* | 1.1 (2.5) | 1.5 (3.1) | |
|
Mechanical
ventilation |
Ventilated | 30 (46.2%) | 11 (68.8%) | 19 (44.2%) | 22 (57.9%) |
|
# days of
mechanical ventilation |
2.3 (3.6) | 4.1 (4.5) | 2.7 (4.1) | 2.6 (3.5) | |
| Vasopressors used | 6 (9.2%) | 5 (31.3%)* | 6 (14.0%) | 5 (13.2%) | |
| Death | 1 (1.5%) | 0 (0%) | 1 (2.3%) | 0 (0%) | |
| Dialysis | 0 (0%) | 1 (6.3%)* | 0 (0%) | 1 (2.6%) | |
| PICU length of stay (days) | 7.9 (16.7) | 10.1 (10.5) | 10.0 (20.2) | 6.5 (7.5) | |
| Hospital length of stay (days) | 17.2 (26.3) | 37.3 (67.2) | 19.5 (30.4) | 22.9 (45.6) | |
Abbreviations: PRISM: pediatric risk of mortality score; eGFR: estimated glomerular filtration rate; PICU: pediatric intensive care unit;
Continuous variables are expressed as Mean (Standard deviation); categorical values are expressed as number and column percent.
PICU urine collection protocol/study feasibility
Sites easily attained recruitment goals (Figure 1). Study barriers and facilitators were identified from discussions with PICU staff, coordinators and investigators (Table 2). In general, barriers were related to inter-site differences in data availability, care processes, impeding on protocol adherence. These were mostly resolvable by inter-site regular communication, clarifying instructions. Study facilitators were mainly related to open, consistent communication between study and clinical staff and considering site-specific processes during protocol development. Figure 1 displays that the urine collection protocol resulted in a urine specimen available 82%, 95% and 83% of subjects on PICU days 1 to 3, respectively. Sixty-seven percent of subjects had urine specimens available all 3 days. There was no significant difference in protocol urine collection between AKISCr and non-AKISCr subjects (2.3±0.7 vs. 2.6±0.5 urines within the first 3 PICU days, respectively) nor between AKISCr+UO and AKISCr+UO subjects (2.5 vs. 2.7 urine samples, respectively). There were no significant differences in patient characteristics between subjects who did vs. did not have a PICU day 1 urine specimen available (not shown). On different PICU days, up to 14% of SCr measurements were performed using leftover samples from other blood tests performed by the clinical team.
Table 2.
Representative examples of barriers and facilitators to performing the multi-centre prospective biomarker study and PICU institutional urine collection protocol.
| Barriers | ||
| Barrier | Explanation and Implication | Rectification/Improvements |
| Data issues | ||
| PICU admission time discrepancy between sources |
Discrepant in electronic records (EMR) and paper chart. Affects eligibility and Pediatric Risk of Mortality (PRISM) score window |
PICU clerk keeps time log. Use if available. If not, chart, then EMR |
| Glascow coma score (GCS): sedated/non-sedated assessment |
Sedation status not always collected, thus unable to be certain recorded GCS was definitely non- sedated |
Queries sent; sedatives list added to case report form (CRF) instructions |
| Daily drugs, events data Incomplete |
Clarity issues on which study day corresponded to a PICU day |
CRF table titles and instructions modified, simpler. Queries sent |
| CRF instructions lacking | Assuming research assistant (RA) knowledge for some variables causes data variability |
Every data point described in detail in final CRF instructions |
| Data entry errors | Certain variables more error prone | Monthly data clean; 10% reentry |
| Height not always available | Crucial to estimate baseline glomerular filtration rate (GFR); often not recorded in chart |
Systematically measure height at recruitment or shortly after |
| Clinical staff issues | ||
| Day 1 –3 urine not always collected per protocol |
PICU nurses did not always collect daily urine during the clinical protocol (in most cases, stated to have forgotten) |
Order form to collect daily urine, added to admission package |
| Urine not collected on PICU discharge day |
Ward nurses not all informed of study. PICU nurse sometimes did not collect urine on day of PICU to ward transfer |
RA sees patient every study day; Involve parents; reminders; Fridays, speak to nurse and clerk |
| Sites differ in nurse “shift intervals” |
Affects study day start time. Affects time needed for data collection |
A uniform CRF to account for inter-site variations in shift times |
| PICU staff schedule changes | Nurse and resident rotations, “new faces” not always familiar with the study |
Study reminders; urine collection order sheet; new staff tracking |
| Lab issues | ||
| Site lab results reporting (units) differ |
E.g., bilirubin, FiO2, Mostly affected PRISM calculation |
Conversion tables added to CRF instructions |
| Leftover serum use, when no routine SCr measured |
Leftover serum from central lab used to measure SCr when not available. Some sites not always able to easily obtain |
Some sites need specific process to obtain serum from central lab. Site- specific process set up |
| Other | ||
| Missing weekend patients potential |
Mostly Friday admission with Monday discharge to home and not ward. Reviewed: small number |
Protocol dictates urine collection 7 days/week. Monday AM priority to recruit such patients first |
| No routine PICU blood draws | Results in no SCr. Reviewed: small number, low- risk, short stay patients |
Minimum SCr criteria included in inclusion criteria; draw study blood if not routinely drawn |
| Parent accessibility, significant “back and forth to PICU” |
Parents leave PICU often, challenge for consent; patient tests (e.g. imaging); time and cost concern |
Provide RA/site investigator pager to nurses; involve site investigator on non- RA hours. |
| Facilitators | ||
| Facilitator | Explanation | Product |
| Involving Head Nurse in planning |
Re: study initiation and maintenance | Nurses more aware, reminded of urine protocol. Head nurses is research team member or involved in designing site- specific study feasibility methods |
| RA/site investigator PICU presence |
Having one study RA puts a face to the study; helped remind staff of study |
Prefer consistent RA over “hospital research service”, unless research service has constant presence. |
| Deferred consent, during/after PICU-wide urine protocol collection |
At recruitment, urine often available from the first 2–3 PICU days; i.e. protocol successful to obtain early PICU urines; accepted by nurse staff |
The PICU-wide urine collection protocol will be used for future studies |
| Simple PICU-wide urine collection protocol for nurses |
Focus on urine collection without nurse screening work; all patients; PICU staff not confused by the protocol |
The PICU-wide urine collection protocol will be used for future studies. |
Biomarkers excretion from PICU days 1 to 3 by AKI status
Figure 2A shows that patients who developed AKISCr during PICU admission had higher PICU days 1 to 3 biomarker concentrations than non-AKISCr patients (statistically significant [p<0.05] for PICU days 2 and 3 NGAL, day 1 IL-18, day 2 LFABP, Figure 2A). Figure 2B shows that there was no significant difference in biomarker concentrations between subjects with vs. without AKISCr+UO on PICU days 1 to 3.
Figure 2. Biomarker excretion in the first 3 days of pediatric intensive care unit (PICU) admission by acute kidney injury status.
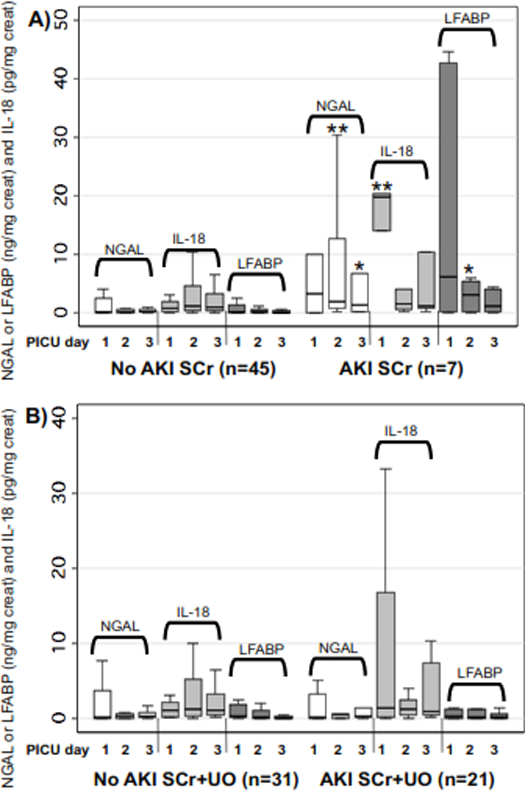
Boxplots of urinary NGAL, IL-18 and LFABP on days 1, 2 and 3 of PICU admission, only including subjects with biomarkers available on all three PICU days. Left side of both graphs are biomarkers in subjects without AKI; on the right side of graphs are subjects who developed AKI. A) AKI defined using the serum creatinine criteria. B) AKI defined using both serum creatinine or urine output criteria. * indicates statistically significant difference in biomarker concentration between AKI vs. non-AKI on that PICU day (* = p<0.05; ** = p<0.005). Abbreviations: NGAL: neutrophil gelatinase associated lipocalin; LFABP: liver fatty acid binding protein’ creat: urine creatinine; IL-18: interleukin-18; PICU: pediatric intensive care unit; AKI SCr: acute kidney injury defined using serum creatinine criteria; AKI SCr+UO: acute kidney injury defined using serum creatinine criteria or urine output criteria.
Biomarkers from PICU days 1 to 3 for predicting AKI development
Table 3 shows the AUC’s for biomarkers to predict AKI, when measured on PICU days 1, 2 and 3, if AKI had not yet occurred. Results differed by biomarker and PICU day. Of note, on PICU days 2 and 3, only 2–3 AKISCr subjects were available for analysis. On PICU day 1, IL-18 predicted AKI with AUC 0.82 (detailed, Table 3); NGAL and LFABP predicted AKI with AUC’s< 0.7 (Table 3). AKISCr+UO prediction was poor for all 3 PICU day 1 biomarkers (AUC 0.43–0.65, Table 3). For NGAL and LFABP, AKISCr prediction was substantially higher when measured on PICU days 2 or 3 (e.g., AUC 0.9 for day 2 NGAL; AUC 0.77 for day 2 LFABP). A similar phenomenon was seen (i.e., better performance of biomarkers) for predicting AKISCr+UO when measured on PICU days 2 or 3, vs. day 1 (Table 3).
Table 3.
Comparison of AKI vs. non-AKI biomarker concentrations from days 1 to 3 of PICU admission, with associated area under the curve (AUC) for AKI prediction.
|
Biomarker PICU Day£ |
AKISCr | AKISCr+UO | |||||
|---|---|---|---|---|---|---|---|
| No AKI (n=63) | AKI (n=8) £ | AUC (95% CI) | No AKI (n=41) | AKI (n=11) £ | AUC (95% CI) | ||
| NGAL (ng/mg cr) |
Day 1 | 0.10 (0.81) | 2.11 (6.01)§ | 0.69 (0.51–0.88) | 0.14 (1.33) | 0.28 (3.21) | 0.53 (0.34–0.73) |
| Day 2 | 0.16 (0.48) | 15.53 (29.69)§ | 0.90 (0.75–1.00) | 0.23 (0.59) | 0.38 (30.30) | 0.60 (0.29–0.90) | |
| Day 3 | 0.21 (0.51) | 0.70 (68.67) | 0.74 (0.42–1.00) | 0.21 (0.51) | 2.42 (36.61) | 0.66 (0.29–1.00) | |
| IL-18 (pg/mg cr) |
Day 1 | 0.81 (1.90) | 3.06 (18.60)* | 0.82 (0.68–0.96) | 0.74 (1.96) | 1.64 (10.19) | 0.65 (0.45–0.84) |
| Day 2 | 0.79 (3.37) | 3.28 (1.58) | 0.74 (0.63–0.85) | 0.97 (4.17) | 2.61 (10.41) | 0.63 (0.38–0.88) | |
| Day 3 | 0.87 (1.76) | 10.33 (9.25)§ | 0.83 (0.64–1.00) | 0.96 (1.76) | 5.71 (24.31) | 0.69 (0.35–1.00) | |
| LFABP (ng/mg cr) |
Day 1 | 0.26 (1.20) | 0.23 (20.45) | 0.59 (0.33–0.86) | 0.28 (1.64) | 0.20 (0.68) | 0.43 (0.22–0.63) |
| Day 2 | 0.10 (0.46) | 0.71 (1.04) | 0.77 (0.54–1.00) | 0.12 (0.92) | 0.80 (1.12) | 0.67 (0.43–0.90) | |
| Day 3 | 0.08 (0.21) | 0.12 (1.26) | 0.69 (0.42–0.96) | 0.08 (0.20) | 0.41 (0.84) | 0.76 (0.56–0.97) | |
Biomarker concentrations are expressed as Median (interquartile range).
On PICU day 1, 8 subjects with AKISCr were available for analysis (had not yet developed AKI and had a urine specimen available); On PICU days 2 and 3, only 3 and 2 AKI subjects were available for analysis. On PICU day 1, 11 subjects with AKISCr+UO were available for analysis; 6 and 4 AKISCr+UO subjects respectively were available for PICU days 2 and 3.
indicates p-value for difference between AKI vs. non-AKI groups is between 0.05 and 0.07.
indicates p-value for difference between AKI vs. non-AKI groups is <0.05.
Biomarker associations with prolonged AKI
Thirteen subjects had biomarkers available on the first day of AKISCr attainment (Day 1 AKISCr); 7 (54%) had AKISCr duration ≥48 hours. Biomarker concentrations from Day 1 AKISCr were higher in patients with AKISCr duration ≥48 hours (statistically significant for LFABP, Figure 3A). IL-18 and LFABP predicted AKISCr ≥48 hours (AUC’s= 0.74 and 0.83, respectively), whereas AUC for NGAL was 0.69 (detailed, Figure 3A). Fourteen of 33 subjects with AKISCr+UO (and biomarkers available on Day 1 AKISCr+UO) had AKISCr+UO duration ≥48 hours. Figure 3B, shows biomarkers were overall higher in subjects with prolonged AKISCr+UO, but AUC’s for predicting prolonged AKISCr+UO were lower than AUC’s for predicting prolonged AKISCr (Figure 3B).
Figure 3. Association of biomarkers from the first day of AKI attainment with AKI duration at least 48 hours.
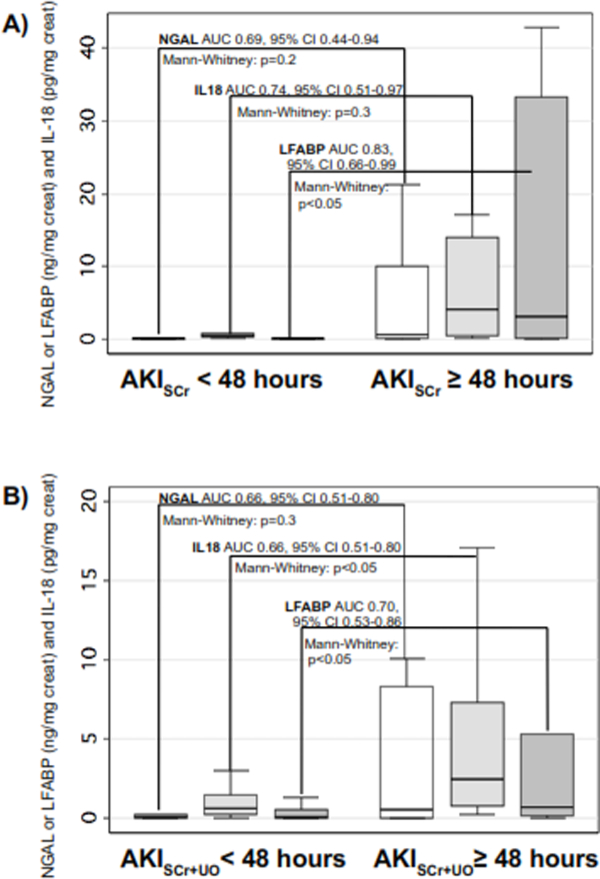
Boxplots of biomarker concentrations (y-axis) measured on the first PICU day the patient fulfilled criteria for AKI, in subjects with AKI duration (fulfilling criteria for AKI) less than vs. greater than or equal to 48 hours (indicated on x-axis). Within graph statistics shown are the p-value for biomarker concentration differences between the two groups (Mann-Whitney U test) and the area under the receiver operating characteristic curve for each biomarker to predict AKI duration at least 48 hours (with lower and upper 95% confidence interval). A) AKI defined using serum creatinine criteria. B) AKI defined using both serum creatinine or urine output criteria. Abbreviations: NGAL: neutrophil gelatinase associated lipocalin; IL-18: interleukin-18; LFABP: liver fatty acid binding protein; AUC: area under the curve; AKISCr: acute kidney injury defined using serum creatinine criteria; AKISCr+UO: acute kidney injury defined using serum creatinine criteria or urine output criteria.
DISCUSSION
This is one of the first multi-centre studies evaluating AKI biomarkers in non-cardiac PICU children. A urine collection protocol to obtain early PICU admission urine with deferred consent, was feasible. Ability of biomarkers to predict AKI was highly variable and modest.
The fact that AKI occurs early in PICU complicates biomarker research. To measure biomarkers during the short therapeutic AKI window (before SCr rise), collecting urine in the first 1–3 PICU days is critical but not easy. This problem may explain the paucity of PICU AKI biomarker studies. We proposed a protocol-based urine collection, with later eligibility screening, to maximize recruitment in AKI biomarker studies. Barriers often related to differences in site practice (Table 1). Facilitators generally related to open communication and clear, labour-minimizing actions (e.g., pre-labeled tubes). Deferred consent has mainly been limited to studies involving time sensitive, life-threatening treatments(25); we propose that to move PICU-AKI research forward, deferred consent may help obtain needed specimens in patients most likely to develop AKI. We demonstrate such a study is feasible and provide insights on successful performance, at least in the Canadian setting. Despite our protocol, however, several patients were excluded from analysis due to early AKI occurrence and/or lacking specimens. Of note, we obtained ethics approval for deferred consent, only by not handling urine in any way before obtaining consent. Although biomarkers have been shown to be stable under conditions of our study(26), urine would ideally be processed immediately, before deferred consent occurs. This may be feasible in other settings or countries.
We investigated the impact of UO AKI criteria on biomarker performance. We confirmed previous pediatric studies showing discrepancy between SCr- and UO-defined AKI(3,24), as shown by a substantial number of patients with AKI by one method, but not the other (Supp. Table 1). Although adult data suggest that UO-defined AKI is associated with clinical outcomes(5,32) little is known on this matter in children. UO criteria are challenging to collect, particularly in patients without urinary catheters. Biomarker performance was worse when including UO criteria. It is possible that a small, temporary UO reduction (i.e., stage 1) may not reflect tubular injury, but rather be a sensitive indicator of temporary volume depletion. It may be that in children, more severe UO reductions are more relevant, in terms of outcome associations or renal tissue injury. These UO criteria were based on AKI definition empirically derived for adults. A pediatric-specific, outcome-based UO definition is needed.
There is little data on biomarkers in non-cardiac surgery patients. Early PICU biomarker diagnostic performance was highly variable. On PICU day 1, where the sample size was highest, IL-18 was the best predictor of AKISCr development (AUC = 0.82), comparable to or better than what was previously reported in cardiac and non-cardiac PICU patients(12,13,16); PICU day 1 NGAL AKISCr prediction was moderate and LFABP performed poorly, unlike some previous reports(12,15,17). There is high diagnostic heterogeneity in the PICU, increasing the likelihood of variability in biomarker performance. Different biomarkers may perform variably by primary disease etiology. For instance, NGAL and IL-18 were shown to be higher in sepsis, possibly since IL-18 is a cytokine and NGAL is neutrophil-derived(16,17). In those studies, NGAL predicted AKI in sepsis, but IL-18 was only diagnostic of AKI without sepsis. Biomarkers appear to be higher in younger children(12), possibly due to tubular immaturity. Biomarkers have also been shown to predict more severe (e.g., stage 2) AKI better than stage 1 AKI(12). It is likely that there is more AKI misclassification in stage 1 AKI (i.e., patients without renal injury, but only reduced GFR due to temporary volume depletion). Our sample size precluded analysis of diagnostic, age or AKI severity subgroups. Future studies should be powered to evaluate other factors potentially affecting biomarkers and control for these in multivariate analyses. Finally, studies in cardiac and non-cardiac surgery patients have shown that early PICU admission clinical variables may be useful for predicting AKI (clinical models predicting AKI with AUC’s from ~0.7 to ~0.8)(12,15). Future studies should evaluate the extent to which adding biomarkers to clinical prediction models significantly improves prediction and ultimately is cost-effective, for future AKI therapeutic trial selection and clinical care.
Our sample size was too small to make strong conclusions on biomarker performance from PICU days 2 or 3. However, AKI prediction was much stronger for NGAL and LFABP on these days. It is possible that after the initial high acuity resuscitation phase, other factors affecting biomarker concentrations (e.g., inflammation, fluid administration) are less prominent after day 1. Early PICU biomarker excretion patterns may be distinct for different biomarkers. Future larger studies should elucidate the extent to which timing of biomarker measurement may impact diagnostic performance (as has been shown in cardiac surgery patients).
LFABP and less so, IL-18, predicted AKI duration >48 hours, supporting this previously shown utility of biomarkers(17). When SCr rises, there is often uncertainty on whether there is mild, temporary SCr rise vs. an early sign of significant AKI (i.e., true cell injury). Biomarkers may identify tissue injury and help make timely decisions on nephrotoxins and fluid management. We used AKI >48 hours to represent prolonged AKI, based previous studies. Future studies may identify alternative “AKI duration” thresholds.
Study limitations included low sample size (described above), and multiple testing. Baseline SCr was missing for ~50% patients and had to be estimated, which may have resulted in AKI misclassification. Lack of baseline SCr in PICU patients is common, highlighting an inherent issue with SCr-based AKI definitions. We did not collect detailed UO criteria after PICU day 7, based on our previously research. Though it is conceivable that some patients may have developed UO-defined AKI later, this proportion would unlikely be high enough to substantially affect results and also, biomarkers may not be useful to predict such delayed UO-AKI.
CONCLUSION
Validating early AKI biomarkers may improve AKI management and trial performance. Better characterization of biomarker performance (timing, subgroups) and more discovery research to identify valid AKI biomarkers in the PICU are needed. An early PICU urine collection protocol, likely critical for PICU AKI biomarker studies, is feasible and beneficial for obtaining early PICU urine specimens.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge PICU head nurses and clinical teams for protocol collections, and study participants.
source of funding
MZ received a research salary award from the Fonds de Recherches du Quebec-Sante. PD is supported by grants from the National Institutes of Health (NIH, P50DK096418). PD is a co-inventor on patents submitted for the use of NGAL as a biomarker of kidney injury.
Footnotes
Conflicts of interest
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Copyright form disclosures: Dr. Palermo received funding from the McGill University Research Bursary Program. Dr. Dart disclosed other support in the form of operating funds that were provided to perform the study at her site. Dr. Devarajan received support for article research from the National Institutes of Health (NIH). Dr. Gottesman received support for article research from the Canadian Institutes of Health Research (CIHR), and his institution received fund from the CIHR. Dr. Majesic received funding from Dr. Morgan.
REFERENCES
- 1.Alkandari O, Eddington KA, Hyder A, et al. : Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 2011;15:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider J, Khemani R, Grushkin C, et al. : Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 2010;38:933–939 [DOI] [PubMed] [Google Scholar]
- 3.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. : Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007;71:1028–1035 [DOI] [PubMed] [Google Scholar]
- 4.Plotz FB, Hulst HE, Twisk JW, et al. : Effect of acute renal failure on outcome in children with severe septic shock. Pediatr Nephrol 2005;20:1177–1181 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl 2012;2:1–138 [Google Scholar]
- 6.American Society of Nephrology Renal Research Report. J Am Soc Nephrol 2005;16:1886–1903 [DOI] [PubMed] [Google Scholar]
- 7.Koyner JL, Sher Ali R, Murray PT: Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Exp Nephrol 2008;109:e109–117 [DOI] [PubMed] [Google Scholar]
- 8.Molitoris BA: Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 2014;124:2355–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monsel A, Zhu YG, Gennai S, et al. : Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014;121:1099–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okusa MD, Molitoris BA, Palevsky PM, et al. : Design of clinical trials in acute kidney injury: a report from an NIDDK workshop--prevention trials. Clin J Am Soc Nephrol 2012;7:851–855 [DOI] [PubMed] [Google Scholar]
- 11.Jo SK, Rosner MH, Okusa MD: Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2007;2:356–365 [DOI] [PubMed] [Google Scholar]
- 12.Parikh CR, Devarajan P, Zappitelli M, et al. : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 2011;22:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanmassenhove J, Vanholder R, Nagler E, et al. : Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 2013;28:254–273 [DOI] [PubMed] [Google Scholar]
- 14.Basu RK, Wong HR, Krawczeski CD, et al. : Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014;64:2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu RK, Wang Y, Wong HR, et al. : Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 2014;9:654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washburn KK, Zappitelli M, Arikan AA, et al. : Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant 2008;23:566–572 [DOI] [PubMed] [Google Scholar]
- 17.Zappitelli M, Washburn KK, Arikan AA, et al. : Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 2007;11:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray PT, Mehta RL, Shaw A, et al. : Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014;85:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarajan P: Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr 2005;17:193–199 [DOI] [PubMed] [Google Scholar]
- 20.Melnikov VY, Faubel S, Siegmund B, et al. : Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 2002;110:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore E, Bellomo R, Nichol A: Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: from the bench to clinical research to clinical practice. Minerva Anestesiol 2010;76:425–440 [PubMed] [Google Scholar]
- 22.Du Y, Zappitelli M, Mian A, et al. : Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol 2011;26:267–274 [DOI] [PubMed] [Google Scholar]
- 23.Haase M, Devarajan P, Haase-Fielitz A, et al. : The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011;57:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slater MB, Anand V, Uleryk EM, Parshuram CS: A systematic review of RIFLE criteria in children, and its application and association with measures of mortality and morbidity. Kidney Int 2012;81:791–798 [DOI] [PubMed] [Google Scholar]
- 25.Jansen TC, Kompanje EJ, Bakker J: Deferred proxy consent in emergency critical care research: ethically valid and practically feasible. Crit Care Med 2009;37:S65–68 [DOI] [PubMed] [Google Scholar]
- 26.Parikh CR, Butrymowicz I, Yu A, et al. : Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis 2014;63:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack MM, Ruttimann UE, Getson PR: Pediatric risk of mortality (PRISM) score. Crit Care Med 1988;16:1110–1116 [DOI] [PubMed] [Google Scholar]
- 28.Lagos-Arevalo P, Palijan A, Vertullo L, et al. : Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol 2015;30:665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009;4:1832–1843 [DOI] [PubMed] [Google Scholar]
- 30.Hoste L, Dubourg L, Selistre L, et al. : A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant 2014;29:1082–1091 [DOI] [PubMed] [Google Scholar]
- 31.Rink N, Zappitelli M: Estimation of glomerular filtration rate with and without height: effect of age and renal function level. Pediatr Nephrol 2015;30:1327–1336 [DOI] [PubMed] [Google Scholar]
- 32.Petaja L, Vaara S, Liuhanen S, et al. : Acute Kidney Injury After Cardiac Surgery by Complete KDIGO Criteria Predicts Increased Mortality. J Cardiothorac Vasc Anesth August 16, 2016. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


