Abstract
Background/Aims
Recent genomic medicine initiatives underscore the importance of including diverse participants in research. Considerable literature has identified barriers to and facilitators of increasing diversity, yet disparities in recruiting and retaining adequate numbers of participants from diverse groups continue to limit the generalizability of clinical genomic research.
Methods
The North Carolina Clinical Genomic Evaluation by Next-gen Exome Sequencing (NCGENES) study employed evidence-based strategies to enhance participation of under-represented minority patients. Here we evaluate the impact of our efforts by systematically analyzing the “cascade” of attrition of participants throughout study interactions.
Results
Although successful in recruiting a cohort that included ~30% non-Caucasian patients overall, the study still enrolled and retained a lower proportion of minorities compared to the pool of eligible patients who were nominated. We evaluated sociodemographic characteristics and related variables as potential factors associated with attrition throughout these phases of the study.
Conclusions
These results suggest that varied approaches will be needed to increase participation in genomic medicine research. Our findings highlight factors to consider when developing strategies to address this critical need. Failing to include a broad range of populations in research studies will exacerbate existing disparities in the translation of genomic sequencing to medical care.
Keywords: genomic medicine, recruitment, enrollment, retention, diversity
INTRODUCTION
The National Institutes of Health 1993 Revitalization Act [1] promotes detection and reduction of health disparities through research on uptake and responses to tests and treatments [2] and thereby guides changes to health policy and clinical care to advance social justice. Despite substantial efforts, failure to achieve robust inclusion of minorities is well documented throughout clinical research portfolios [3], creating deficits in scientific knowledge, which in turn biases or limits conclusions that can be drawn and undermines efforts to reduce health disparities. Lack of diversity may have additional implications for genomic research, where understanding variation across populations is critical for accurate interpretation of findings [4–5]. The Precision Medicine Initiative (now referred to as the “All of Us” cohort; https://allofus.nih.gov/) aims to generate data about individual variation in predisposition to disease and treatment responses, and proposes to recruit participants who “broadly reflect” the diversity of the U.S.
An extensive literature has identified barriers to inclusion in research [3,6] including individual and system-level barriers [7–8]. Common barriers include distrust of the medical care system and researchers, potential for stigma and discrimination, and lack of access to information, which can be related to language barriers and low literacy. Logistical barriers related to location of clinical research sites, day/time restrictions on when research interactions occur, and out-of-date contact information are also common [3,6,9–10]. Enrollment of minority participants can be facilitated by developing study designs and participation benefits that are informed by participant expectations, such as improved health care access and adequate remuneration [3,6,11]. Johnson and colleagues identified several factors associated with increased enrollment and retention of African American adults in genomic research, including the use of informal contacts for recruitment and employment of recruiters of like ancestry [4]. Considering potential barriers and facilitators, some factors may be more amenable to modification by the study team than others.
The North Carolina Clinical Genomic Evaluation by Next-generation Exome Sequencing (NCGENES) study explored the implementation of genome-scale sequencing in adult and child patients with conditions suspected to have a genetic cause. NCGENES participants underwent exome sequencing with focused analysis of clinically relevant genes and disclosure of diagnostic results and medically actionable secondary findings. Adult patients and caregivers of child or cognitively impaired adult patients also completed telephone surveys and questionnaires that assessed their understanding of and responses to genomic results. In order to identify challenges to the clinical implementation of this testing in diverse groups, a major aim of the study was to describe if and how perceptions, use, and knowledge of testing results differed among previously under-represented North Carolina populations.
NCGENES employed specific, evidence-based strategies to enhance enrollment of under-represented minority patients. To evaluate this goal, we analyzed a cascade of four study events; nomination, approached for recruitment, enrollment, and retention. We then investigated sociodemographic characteristics and factors potentially associated with each step of this cascade. We anticipated that these evidence-based strategies would improve the representativeness of participants in genomic research, and highlight the importance of systematically monitoring recruitment and retention of study participants in order to achieve a diverse study sample.
METHODS
Procedures
The study examined all nominated patients to identify where attrition occurred at each stage of the cascade (described in depth in Figure 1). Nominations could occur through a clinical encounter during which the provider notified the potential participant about the study, or through review of independently maintained clinic databases or relevant diagnostic codes in medical records data. Eligible participants were either notified about the study directly by their clinical provider, or through a mailed “opt-out” letter that described the study and provided a postcard that could be returned to decline any further contact from the study. For the present analysis, participants were classified as either “adults” (cognitively intact patients aged 18 or older who provided consent, underwent sequencing, and completed surveys about their own understanding of and experiences with genomic sequencing) or “caregivers” (parents or guardians aged 18 or older who provided consent for a child or cognitively impaired adult patient to undergo sequencing, and who completed surveys about parental understanding of and experiences with genomic sequencing). Retention corresponded with enrolled participants who completed the study activities described in Figure 1. Telephone surveys accounted for two of the four required study activities (content detailed in Supplementary Table 1). The Institutional Review Boards at the University of North Carolina and Vidant Medical System approved all procedures and assessments.
Figure 1.
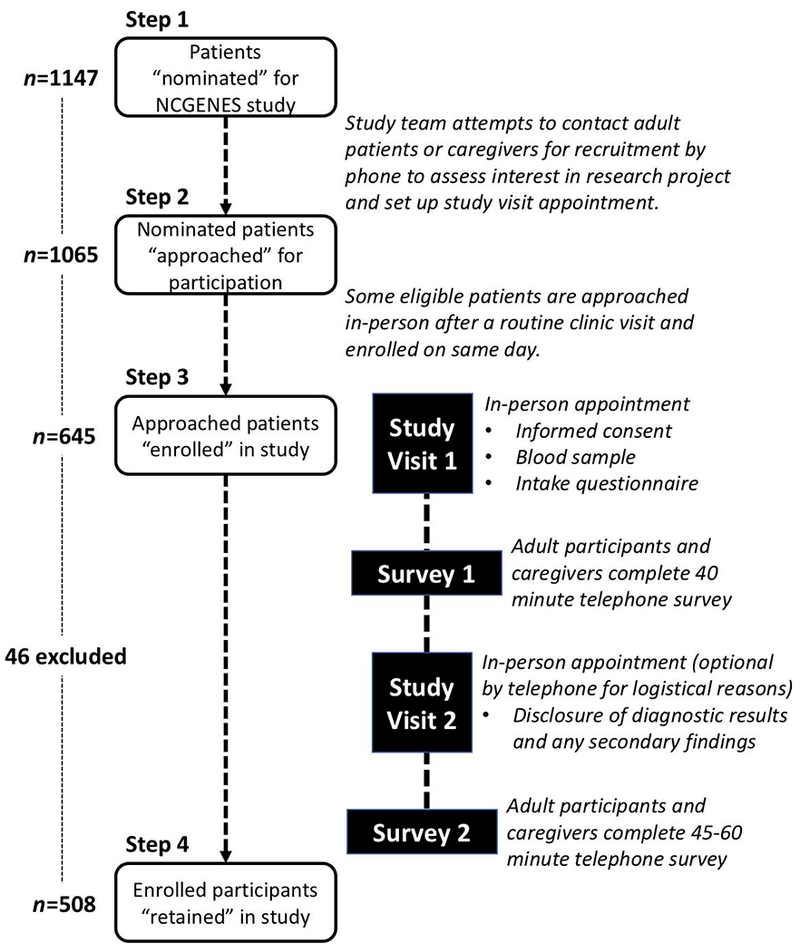
Study steps in the cascade of participant nomination, enrollment, and retention.
Potential predictors of enrollment and retention
We evaluated sociodemographic factors, medical factors, travel distance, and the timing of recruitment and enrollment as possible predictors of participants’ attrition throughout the study.
Sociodemographic characteristics:
For nominated patients, clinical records were used to obtain data regarding race, ethnicity, sex, and age. The present analysis did not include the age of minors or their caregivers. We used the following racial categories for adult and child patients and for caregivers: African American, White, or Other (including people who reported more than one racial background). Ethnicity was categorized as either Hispanic/Latino or non-Hispanic/Latino. We obtained educational level (collapsed into less than or equal to high school graduate or greater than high school graduate) and income data from the post-enrollment questionnaire; this information was only available for those who enrolled in the study and completed this study questionnaire. Annual income was reported on a 10 point scale ranging from ‘1’ (less than $15,000) to ‘10’ ($135,000 or more).
Medical characteristics:
Nominating clinicians identified patients as potential participants based on diagnoses, clinical features, or symptoms suggestive of a single gene condition. This information was supplemented and confirmed during the enrollment visit. Participants were then assigned to broad diagnostic categories: Cardiogenetic Disorders, Hereditary Cancer, Intellectual Disability, Neuromuscular Disorders, Hematology, Ophthalmology, etc. Exome sequencing diagnostic results were categorized as “positive,” “negative,” and “uncertain.” A positive result showed one or more gene variants that explained the health concern; a negative result showed no explanatory gene variants; and uncertain results included those in which the health concern was not fully explained by the results or in which there was uncertainty due to the interpretation of the variants.
Physical functioning:
We assessed patients’ physical functioning using validated scales to evaluate whether there was a link between physical status and participation throughout the study. Adult patient participants self-reported their current level of physical functioning using an adapted version of the Karnofsky Performance Status scale [12] with an 8 point response scale ranging from 1 (ability to carry on normal activity) to 8 (severe disability and hospitalization). Caregivers reported functioning for children or cognitively impaired adults using the Functional Status Questionnaire – General [13], which includes 14 questions assessing the frequency of behaviors indicating functioning (eat well, sleep well, act moody, seem unusually difficult) in the past two weeks on a scale from 0 (“Never or rarely”) to 2 (“Almost always”). Responses were reverse coded as appropriate and the mean was calculated for all items. A higher score indicated worse health status. Because patient functioning was assessed using different measures for adult versus child patients or cognitively impaired adults, we calculated a z-score to standardize raw scores from the two measures and combined them into a single patient functioning variable.
Travel distance:
The travel distance in miles from participants’ homes to their study site (UNC or Vidant) was calculated using ArcGIS online (Esri, Redlands, California). All participants’ home locations were geocoded using their zip code centroid.
Time of recruitment:
Most nominees were approached for recruitment via a phone call, and invited to schedule and attend a study enrollment visit. Hematology and ophthalmology clinic patients were usually approached for recruitment and enrolled during a regularly scheduled clinic visit with a genetic counselor associated with the study.
Analyses
Findings are organized separately for adult patients and for caregivers through each stage of the study: nominated participants who were either approached for recruitment versus not approached, enrolled versus not enrolled, and retained versus not retained. Descriptive statistics were used to characterize each variable and its distribution, followed by bivariate associations between potential sociodemographic and related predictors and outcomes at each stage using either chi-square analyses or two-sample t tests, depending on the data type. When more than one factor was associated with the dichotomous outcome at the p < .05 level in bivariate analysis, multivariate logistic regression analyses were conducted with a p-value smaller than 0.05 considered as statistically significant. All of the analyses were carried out using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp, Armonk, NY).
RESULTS
Step 1:Nominated
The first stage of the cascade was the clinicians’ nomination of a potential participant suspected to have a single gene disorder. Study clinicians nominated 1147 adult and child patients. The race and ethnicity of patients nominated for the study closely reflected United States 2010 census data [14] for the state of North Carolina (White 68.5%, African American 21.5%, Hispanic 8.4%). As these individuals were not enrolled, we had no other demographic or medical data; nor did we have any sociodemographic information about caregivers of patients who were nominated but not yet enrolled. A total of 774 adult patients were nominated. They were, on average, just over 45 years old and primarily female (68.6%). The majority were non-Hispanic (90.7%) and White (67.1%). A total of 373 children or cognitively impaired adult patients were nominated. Half (51.7%) were males. The majority were non-Hispanic (82%) and White (77.2%).
Step 2: Approached for recruitment
Study team members approached 1065 of the 1147 patients nominated. Of the 82 nominated patients who were not approached for recruitment, 72 (88%) could not be contacted before the study closed; nine were considered ineligible; contact information was not valid for one. A small percentage of patients (less than 10% of the total number approached) were approached for recruitment on the same day as a regularly scheduled clinic visit.
Adult patients:
Of the 774 adult patients who were nominated, 724 were approached for recruitment (Supplementary Table 2). In bivariate analyses, the mean age of nominees was higher in those who were approached (p = 0.026). There was no difference in the ethnicity, race, and sex of adults who were approached versus not.
Caregivers:
Of the 373 children or adults with impairment who were nominated, 341 caregivers were approached for recruitment (Supplementary Table 3). In bivariate analysis, there were no factors (ethnicity, race, sex) associated with this outcome and multivariate analysis was not conducted.
Step 3: Enrolled
Of the 1065 adult patients and caregivers approached for recruitment (hereafter referred to as “participants.”), 645 completed the initial study visit to enroll in the study. Reasons for not enrolling included lack of interest or poor health (n=187), inability of the study staff to schedule the enrollment visit (n=117), failure to attend a scheduled enrollment visit (“visit incomplete”)(n=104), or patient death prior to enrollment (n=12). Race and ethnicity were not associated with any of these reasons for not enrolling.
Adult participants:
Of the 724 adults approached, 396 enrolled in the study (Table 1). In bivariate analyses, nominated White patients were more likely to enroll than nominated African American patients (p < 0.001). Adults who lived closer to the study site were also more likely to enroll than those who lived further away (p = 0.003). Using logistic regression to investigate the independence of potential predictors of enrollment (Table 2), African American adults were less likely to enroll (OR: 0.36; 95% CI: 0.25-0.51; p < 0.001) than White adults, and adults of other races were also less likely to enroll than Whites (OR: 0.49; 95% CI: 0.24 – 0.996; p = 0.049). In addition, the likelihood of an adult enrolling in the study was reduced by 10% per 30 miles of distance to the enrollment site (OR: 0.90; 95% CI: 0.82-0.98; p = 0.014).
Table 1:
Adult patients approached for recruitment to the NCGENES study (N=724) and either enrolled (N=396) or not enrolled (N=328)
| Approached | Not Enrolled | Enrolled | p value | |
|---|---|---|---|---|
| Ethnicity | ||||
| Hispanic or Latino | 35 | 15 (43%) | 20 (57%) | |
| NOT Hispanic or Latino | 656 | 281 (43%) | 375 (57%) | |
| Missing ethnicity | 33 | 32 | 1 | |
| Race | <0.001 | |||
| White | 482 | 175 (36%) | 307 (64%) | |
| African American | 202 | 130 (64%) | 72 (36%) | |
| Other | 37 | 20 (54%) | 17 (46%) | |
| Missing race | 3 | 3 | 0 | |
| Age, mean (SD) | 45.5 (14.8) | 44.4 (15.0) | 46.3 (14.5) | 0.081 |
| Travel distance, median (range) | 50.9 (0-339.4) | 51.4 (0-339.4) | 31.1 (0–258.1) | <0.001 |
| Sex | ||||
| Female | 502 | 237 (47%) | 265 (53%) | 0.125 |
| Male | 222 | 91 (41%) | 131 (59%) | |
| Time of Recruitment | ||||
| Dedicated Study Appointment | 642 | 311 (48%) | 331 (52%) | <0.001 |
| Regularly Scheduled Clinic Appointment | 80 | 15 (19%) | 65 (81%) | |
| Missing | 2 | 2 | 0 |
Table 2.
Multivariate analysis for factors associated with adult enrollment
| Adult Patients | Odds ratio (OR) | 95% CI | p value |
|---|---|---|---|
| Race | |||
| White | 1.00 | ||
| African American | 0.36 | 0.25 – 0.51 | <0.001 |
| Other | 0.49 | 0.24 – 0.996 | 0.049 |
| Age | 1.01 | 0.997 – 1.02 | 0.135 |
| Travel distance per 30 miles | 0.88 | 0.80 – 0.97 | 0.008 |
| Time of enrollment | |||
| Dedicated study appointment | 1.00 | ||
| Regularly scheduled clinic appointment | 3.41 | 1.87 – 6.21 | <0.001 |
Caregivers:
Of the 341 caregivers approached, 249 enrolled their dependent child or cognitively impaired adult in the study (Table 3). In bivariate analyses, the patient’s race was a predictor of enrollment (p = 0.007), but ethnicity and sex, travel distance, and method of being approached for recruitment (i.e., during a routine clinic visit compared to other means) were not significantly associated with enrolling in the study.
Table 3:
Caregivers approached for recruitment to the NCGENES study (N=341) and either enrolled (N=249) or not enrolled (N=92)
| Total approached | Not Enrolled | Enrolled | p value | |
|---|---|---|---|---|
| Patient’s Ethnicity | ||||
| Hispanic or Latino | 59 | 17 (29%) | 42 (71%) | 0.747 |
| NOT Hispanic or Latino | 276 | 73 (26%) | 203 (74%) | |
| Missing | 6 | 2 | 4 | |
| Patient’s Race | ||||
| White | 260 | 62 (24%) | 198 (76%) | 0.007 |
| African American | 54 | 24 (44%) | 30 (56%) | |
| Other | 26 | 6 (23%) | 20 (77%) | |
| Missing | 1 | 0 | 1 | |
| Travel distance, median (range) | 56.3 (0 – 306.4) | 52.2 (2.55 – 233.0) | 57.0 (0 – 306.4 ) | 0.930 |
| Patient’s Sex | ||||
| Female | 163 | 42 (26%) | 121 (74%) | 0.714 |
| Male | 178 | 50 (28%) | 128 (72%) | |
| Time of Recruitment | ||||
| Dedicated Study Appointment | 312 | 86 (28%) | 226 (72%) | 0.373 |
| Regularly Scheduled Clinic Appointment | 28 | 5 (18%) | 23 (82%) | |
| Missing | 1 | 1 | 0 |
Travel distance:
One reason for establishing a satellite clinic at Vidant was to facilitate enrollment by not requiring patients to travel to UNC Hospitals, which is ~110 miles from Vidant’s clinic facility. We compared adults approached for recruitment at UNC Hospitals with those from Vidant Medical System to determine if travel distance was a predictor for enrollment. For nominees at Vidant, the median distance from their homes to the enrollment site was 25.9 miles, while for nominees at UNC Hospitals, the median distance was 50.9 miles for adults and 56.3 miles for caregivers. Bivariate analyses of the adult patients at Vidant showed that travel distance from the clinic was not statistically significantly associated with enrollment (p = 0.994). In contrast, bivariate analyses of adult patients from UNC showed a positive association between distance and enrollment (p < 0.001). As noted above, distance was not a significant predictor of enrollment by caregivers, suggesting that they may be more highly motivated.
Enrollment at study visits versus regularly scheduled clinic visits:
The majority of study participants from UNC were enrolled during an NCGENES-specific study visit. However, patients nominated from ophthalmology and hematology clinics (n=80) were usually approached during a routine clinic visit. Comparing only adult patients, enrollment rates of those approached during a regularly scheduled clinic visit were higher (81%) than those who were nominated by their clinician, approached for recruitment by study staff, and then required to attend a separate, study-specific enrollment study visit (52%), meaning that adult patients were more likely to enroll with same-day recruitment (p < 0.001). Among nominees with bleeding disorders, 100% of adults and 84% of caregivers enrolled in the study. Among nominees with retinal disorders, 77% of adults and 78% of caregivers enrolled in the study. In contrast, the enrollment rate was lower among adult nominees with cardiogenetic conditions (41%) and cancer (33%) and among caregivers of child nominees who had structural anomalies (33%) and neuromuscular conditions (50%).
Step 4: Retained throughout study interactions
More than six months after enrollment, sequencing results were returned at a clinic visit, and two weeks later, participants completed the second telephone survey. Of the 645 enrolled participants, 46 participants were excluded because they were part of a pilot phase (n=20), were unable to take the survey for logistical reasons (n=6), or they had received a medically actionable secondary finding and because of study protocol were ineligible for the second survey (n=20). Of the remaining 599 enrolled participants, 508 were retained through completion of the second telephone survey.
Adult participants:
Of the 367 enrolled adult participants who were eligible to complete the first and second telephone surveys, 310 were retained through the second telephone survey (Table 4). In bivariate analyses, several factors were significantly associated with being less likely to be retained: African American race (p < 0.001); lower education levels (p < 0.001); and poorer physical functioning (p = 0.005). Bivariate analyses of ethnicity, age, sex, income, diagnostic result, or approach/enrollment method did not show a statistical difference between participants who were retained and those who were not. Using logistic regression to investigate the independence of potential predictors of retention (Table 5), African Americans (OR: 0.31; 95% CI: 0.16 – 0.62; p = 0.001) were less likely to be retained than Whites, and participants with a high school or greater degree were more likely to be retained than those with less than a high school education (OR: 4.10; 95% CI: 2.15 – 7.82; p < 0.001). However, physical functioning was not associated with being retained (OR: 0.84; 95% CI: 0.62 – 1.13; p = 0.247).
Table 4.
Adult participants enrolled in the study (N=367) and either retained (N=310) or not retained (N=57).
| Enrolled | Retained | Not Retained | p value | |
|---|---|---|---|---|
| Ethnicity | ||||
| Hispanic or Latino | 20 | 15 (75%) | 5 (25%) | 0.216 |
| NOT Hispanic or Latino | 346 | 294 (85%) | 52 (15%) | |
| Missing | 1 | 1 | 0 | |
| Race | ||||
| White | 281 | 251 (89%) | 30 (11%) | <0.001 |
| African American | 69 | 45 (65%) | 24 (35%) | |
| Other | 17 | 14 (82%) | 3 (18%) | |
| Age, mean (SD) | 46.6 (14.5) | 46.9 (14.6) | 45.0 (13.6) | 0.379 |
| Sex | ||||
| Female | 246 | 214 (87%) | 32 (13%) | 0.066 |
| Male | 121 | 96 (79%) | 25 (21%) | |
| Education Level | ||||
| High school or less | 80 | 52 (65%) | 28 (35%) | <0.001 |
| Greater than high school | 280 | 254 (91%) | 26 (9%) | |
| Missing | 7 | 4 | 3 | |
| Income, mean (SD) | 4.9 (3.2) | 5.0 (3.1) | 4.4 (3.4) | 0.194 |
| Physical functioning Z score, mean (SD) | 0.014 (1.01) | -0.049 (0.99) | 0.36 (1.06) | 0.005 |
| Diagnostic result | ||||
| Negative | 230 | 194 (84%) | 36 (16%) | 0.626 |
| Positive | 48 | 42 (88%) | 6 (12%) | |
| Uncertain | 70 | 62 (89%) | 8 (11%) | |
| Missing | 19 | 12 | 7 | |
| Time of Enrollment | ||||
| Dedicated Study Appointment | 307 | 260 (85%) | 47 (15%) | 0.845 |
| Regularly Scheduled Clinic Appointment | 60 | 50 (83%) | 10 (17%) |
Table 5.
Multivariate analysis for factors associated with adult retention
| Adult Participants | Odds ratio (OR) | 95% CI | p value |
|---|---|---|---|
| Race | |||
| White | 1.00 | ||
| African American | 0.31 | 0.16 – 0.62 | 0.001 |
| Other | 0.48 | 0.13 – 1.86 | 0.290 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.67 | 0.36 – 1.28 | 0.227 |
| Education | |||
| High School or less | 1.00 | ||
| Greater than High School | 4.10 | 2.15 – 7.82 | <0.001 |
| Physical functioning Z score | 0.84 | 0.62 – 1.13 | 0.247 |
Caregivers:
Of the 232 enrolled caregivers who were eligible to complete the telephone surveys, 198 were retained through the second survey (Table 6). In bivariate analyses, caregivers of White patients were more likely to be retained than caregivers of African American patients (p = 0.013); and caregivers of Hispanic patients were less likely to be retained (p < 0.001) than those of non-Hispanic or Latino patients. With regard to the caregivers themselves, White caregivers were more likely to be retained than African American caregivers (p = 0.005); those with lower education were less likely to be retained (p = 0.024); those with a higher annual income were more likely to be retained (p = 0.029); and those who enrolled at a routine clinic visit were less likely to be retained than those who enrolled at a separate enrollment visit (p = 0.026). Using logistic regression to investigate the independence of potential predictors of retention (Table 7), caregivers were morelikely to be retained when the patient’s ethnicity was non-Hispanic compared to Hispanic (OR: 3.71; 95% CI: 1.26 – 11.0; p = 0.018). Race, income, education, physical functioning, and time of recruitment were not independent predictors.
Table 6.
Caregivers of children or cognitively impaired adult patients enrolled in the study (N=232) and either retained (N=198) or not retained (N=34).
| Enrolled | Retained | Not Retained | p value | |
|---|---|---|---|---|
| Patient’s Ethnicity | ||||
| Hispanic or Latino | 40 | 25 (63%) | 15 (37%) | <0.001 |
| NOT Hispanic or Latino | 188 | 169 (90%) | 19 (10%) | |
| Missing | 4 | 4 | 0 | |
| Patient’s Race | ||||
| White | 186 | 164 (88%) | 22 (12%) | 0.013 |
| African American | 29 | 23 (79%) | 6 (21%) | |
| Other | 16 | 10 (63%) | 6 (37%) | |
| Missing | 1 | 1 | 0 | |
| Caregiver’s Ethnicity | ||||
| NOT Hispanic or Latino | 189 | 165 (87%) | 24 (13%) | 0.120 |
| Hispanic or Latino | 35 | 27 (77%) | 8 (23%) | |
| Missing | 8 | 6 | 2 | |
| Caregiver’s Race | ||||
| White | 172 | 154 (90%) | 18 (10%) | 0.005 |
| African American | 21 | 17 (81%) | 4 (19%) | |
| Other | 31 | 21 (68%) | 10 (32%) | |
| Missing | 8 | 6 | 2 | |
| Patient’s Sex | ||||
| Female | 211 | 179 (85%) | 33 (15%) | 0.747 |
| Male | 21 | 19 (91%) | 2 (9%) | |
| Caregiver Education Level | ||||
| High School or less | 54 | 41 (76%) | 13 (24%) | 0.024 |
| Greater than High School | 172 | 153 (89%) | 19 (11%) | |
| Missing | 6 | 4 | 2 | |
| Household income, mean (SD) | 4.5 (2.9) | 4.7 (3.0) | 3.4 (2.6) | 0.029 |
| Patient’s Physical functioning Z score, mean (SD) | 0.007 (1.002) | −0.037 (0.976) | 0.272 (1.125) | 0.106 |
| Diagnostic result | ||||
| Negative | 117 | 101 (86%) | 16 (14%) | 0.637 |
| Positive | 38 | 33 (87%) | 5 (13%) | |
| Uncertain | 47 | 38 (81%) | 9 (19%) | |
| Missing | 30 | 26 | 4 | |
| Time of Enrollment | ||||
| Dedicated Study Appointment | 210 | 183 (87%) | 27 (13%) | 0.026 |
| Regularly Scheduled Clinic Appointment | 22 | 15 (68%) | 7 (32%) |
Table 7.
Multivariate analysis for factors associated with caregiver retention
| Odds ratio (OR) | 95% CI | p value | |
|---|---|---|---|
| Child’s Ethnicity | |||
| Hispanic or Latino | 1.00 | ||
| NOT Hispanic or Latino | 3.71 | 1.26 – 11.0 | 0.018 |
| Caregiver’s Race | |||
| White | 1.00 | ||
| African American | 0.58 | 0.14 – 2.47 | 0.465 |
| Other | 0.46 | 0.16 – 1.28 | 0.135 |
| Caregiver Education Level | |||
| Yes | 1.00 | ||
| No | 1.03 | 0.34 – 3.06 | 0.964 |
| Household income | 1.05 | 0.89 – 1.27 | 0.623 |
| Child’s Physical functioning Z score | 0.78 | 0.53 – 1.16 | 0.220 |
| Time of Recruitment | |||
| Dedicated Study Appointment | 1.00 | ||
| Regularly Scheduled Clinic Appointment | 0.49 | 0.15 – 1.57 | 0.227 |
DISCUSSION
Translational research is establishing the foundation for genomic sequencing in healthcare [15], necessitating engagement of diverse participants in order to make its application generalizable to the entire population. Many of the NCGENES study aims involved understanding psychosocial implications of genomic sequencing for patients and their families. By successfully enrolling ~30% of the participants from non-white and/or Hispanic demographic groups, we were moderately successful with our recruitment and enrollment strategies. However, there was still attrition across all stages of the study, which is expected in any complex longitudinal study requiring numerous interactions. Our goal in this analysis was to identify factors that differentially impacted attrition, to inform future research designs to achieve more broadly representative samples and results.
The design of NCGENES was intended to emulate a clinical scenario in which exome sequencing was considered to be a potentially useful diagnostic test for a patient with features suggestive of a genetic disorder. Therefore, the primary eligibility criterion was the judgment of the patient’s clinician (e.g., geneticist, genetic counselor, cardiologist, or neurologist) that exome sequencing might provide useful information. Similar to standard medical practice, there was likely variability in how different referring clinicians determined eligibility. Studies have demonstrated bias in the referral to genetic specialty services and/or testing initiated in the primary care or oncology settings [16–18], but this phenomenon has not been well-studied for genetics clinicians. The total population of patients nominated for NCGENES reflected the general population demographics of North Carolina, making it unlikely that significant biases existed at this stage overall, although we cannot rule out systematic differences in nomination practices among individual providers.
Approximately 60% of nominees who were approached for recruitment were enrolled in the study. Half of those who declined to participate cited lack of interest or poor health, one-quarter could not be reached to schedule the enrollment visit, and one-quarter cancelled or were “no-shows” for a scheduled visit. Race emerged as a significant factor accounting for differential enrollment, with African American patients being less likely to enroll. This suggests participation barriers due to the demands of everyday life and the need for alternative recruitment procedures. We found preliminary evidence that convenient enrollment protocols influenced the rate of enrollment independent of race and ethnicity. The subset of patients who were recruited by a genetic counselor during a regularly scheduled clinic visit were more likely to enroll than those who were required to attend a separate study enrollment visit. In addition, the familiarity and existing relationship with the genetic counselor may have played a role [19].
Our enrollment of Hispanic and Latino participants representing 9.6% of the total study population, may be attributed to the use of culture-specific, patient-centered approaches [6,20] such as reducing language barriers and being responsive to cultural differences [21–23]. We included a native Spanish-speaker on the study team, who approached eligible participants and scheduled and interpreted their study visits. She also translated all study documents into Spanish to ensure accurate explanations of genetic concepts.
Another step to increase enrollment among minorities was to partner with a community based heart failure clinic that cares for a high proportion of African American patients, the great majority of whom had not previously had genetic testing. The rate of enrollment among African Americans from this clinic was higher than enrollment of African Americans approached in thestudy overall. This success is attributable to familiarity with the clinic facility and team members and reduced travel burden via closer proximity and mileage reimbursement, consistent with studies demonstrating that a trusting relationship with study investigators is an important factor in the successful recruitment and enrollment of research participants [8,19,24–25]. Further, although travel distance did not appear to be a barrier for enrollment at the satellite clinic or for caregivers, it was a barrier for adult nominees at the UNC site, who were less likely to enroll per each additional 30 miles they had to travel.
Thus, similar to prior studies, our data support the conclusion that offering options for enrollment at a greater number of sites, with closer proximity to the study population and with greater flexibility for same-day enrollment, could improve the enrollment rate. A possible negative consequence of same-day recruitment and enrollment, however, is the potential risk that patients may feel pressured into enrolling. To mitigate this concern, the voluntary nature of research participation should be emphasized and options for enrolling at a later time should be offered. It is also notable that caregivers of patients who were enrolled during a same-day visit were less likely to be retained, suggesting that those who enrolled at a separate study visit were more invested in the research project because of the effort they made to attend.
An innovative aspect of our study was including factors related to retention, which is quite important for the interpretation of results. The study employed several retention strategies, including regular telephone reminders to complete study activities, staff continuity, a Spanish speaking staff member who also translated result disclosure visits, and partnership with the community-based cardiology clinic to provide a convenient and familiar environment for disclosure of results. Although some of these strategies represent evidence-based recommendations [4], much of the research about these issues focuses on bolstering recruitment and enrollment rates rather than enhancing study retention [19]. Nearly 85% of the participants in NCGENES were retained through two telephone surveys stretching over a period of greater than 6 months. Our endpoint for retention, the completion of the second telephone survey, was chosen because it included measures of factors such as distress, motivation to change lifestyle behaviors or use of health services, and sharing of results with others. Multivariate analysis found that African American adults and adults with lower education were significantly less likely to be retained, while Hispanic caregivers were less likely to be retained. Loss of these more vulnerable groups could affect the generalizability of our overall results.
These results extend knowledge about factors associated with retention of underrepresented groups in health research, revealing that different factors may be related to retaining adults as compared to retaining caregivers of children or cognitively impaired adult participants. Clearly, easing barriers to recruitment and enrollment do not, by themselves, guarantee successful retention, and our findings document the need to develop a broader range of approaches to achieve maximally representative study populations during all phases of a study.
CONCLUSIONS
The inclusion of diverse groups in all areas of clinical research is necessary if health equity and equal access surrounding genomic testing is to be achieved [26]. Enhancing participant diversity increases our knowledge about the clinical significance of genomic variants from different ancestral populations and allows researchers to understand how the perceptions, knowledge, and use of genomic sequencing results may differ among groups, particularly those that are often underrepresented. If the views of diverse populations are excluded, studies that define preferences about learning different types of genomic findings may create expectations that have limited generalizability, and failure to retain certain groups across the cascade of study activities could bias longitudinal analyses of outcomes and responses to genomic results, leading to policies that fail to reflect the breadth of views in the general population. Conducting analyses to detect differential attrition throughout the stages of a research study can provide beneficial information that aids in rectifying such biases. To the extent that the goal of full representation is achieved in research, policy and clinical practice will more effectively serve the needs of a greater proportion of all patients.
Supplementary Material
Acknowledgements:
Hassan Alhosaini, Ania Berchuck, Kate Foreman, Sonia Guarda, Amanda Henley, Cynthia Khan, Kristy Lee, and Allison O’Neal. This study was supported by NHGRI U01 HG006487. J.S.B. is a Yang Family Biomedical Scholar.
REFERENCES
- 1.NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research - Amended, October, 2001. (n.d.). Retrieved from https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm
- 2.Corbie-Smith G, Blumenthal C, Henderson G, Garrett J, Bussey-Jones J, Moloney M, ... Darter J (2008). Studying genetic research participants: lessons from the “Learning About Research in North Carolina” study. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 17(8), 2019–2024. 10.1158/1055-9965.EPI-07-2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, … Burchard EG. (2015). Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Medicine, 12(12), e1001918 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson VA, Powell-Young YM, Torres ER, & Spruill IJ (2011). A systematic review of strategies that increase the recruitment and retention of African American adults in genetic and genomic studies. The ABNF Journal: Official Journal of the Association of Black Nursing Faculty in Higher Education, Inc, 22(4), 84–88. [PMC free article] [PubMed] [Google Scholar]
- 5.Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, … Kohane IS (2016). Genetic Misdiagnoses and the Potential for Health Disparities. The New England Journal of Medicine, 375(7), 655–665. 10.1056/NEJMsa1507092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George S, Duran N, & Norris K (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16–31. 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, & Martin MY (2014). Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer, 120 Suppl 7, 1097–1105. 10.1002/cncr.28574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salman A, Nguyen C, Lee Y-H, & Cooksey-James T (2015). A Review of Barriers to Minorities’ Participation in Cancer Clinical Trials: Implications for Future Cancer Research. Journal of Immigrant and Minority Health / Center for Minority Public Health. 10.1007/s10903-015-0198-9 [DOI] [PubMed] [Google Scholar]
- 9.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, … Bass EB (2008). Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer, 112(2), 228–242. 10.1002/cncr.23157 [DOI] [PubMed] [Google Scholar]
- 10.Roundtable on the Promotion of Health Equity and the Elimination of Health Disparities, Board on Population Health and Public Health Practice, Health and Medicine Division, & National Academies of Sciences, Engineering, and Medicine (2016). Strategies for Ensuring Diversity, Inclusion, and Meaningful Participation in Clinical Trials: Proceedings of a Workshop. Washington (DC): National Academies Press (US) Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK384596/ [PubMed] [Google Scholar]
- 11.Nápoles AM, Chadiha LA, & Resource Centers for Minority Aging Research. (2011). Advancing the science of recruitment and retention of ethnically diverse populations. The Gerontologist, 51 Suppl 1, S142–146. 10.1093/geront/gnr019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingard JR, Curbow B, Baker F, & Piantadosi S (1991). Health, functional status, and employment of adult survivors of bone marrow transplantation. Annals of Internal Medicine, 114(2), 113–118. [DOI] [PubMed] [Google Scholar]
- 13.Lewis CC, Pantell RH, & Kieckhefer GM (1989). Assessment of children’s health status. Field test of new approaches. Medical Care, 27(3 Suppl), S54–65. [DOI] [PubMed] [Google Scholar]
- 14.Census U.S. Bureau QuickFacts selected: North Carolina. (n.d.). Retrieved July 12, 2017, from https://www.census.gov/quickfacts/NC
- 15.Green RC, Goddard KAB, Jarvik GP, Amendola LM, Appelbaum PS, Berg JS, … CSER Consortium. (2016). Clinical Sequencing Exploratory Research Consortium: Accelerating Evidence-Based Practice of Genomic Medicine. American Journal of Human Genetics, 98(6), 1051–1066. 10.1016/j.ajhg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal T, Bonner D, Cragun D, Johnson S, Akbari M, Servais L, … Vadaparampil S (2014). BRCA sequencing and large rearrangement testing in young Black women with breast cancer. Journal of Community Genetics, 5(2), 157–165. 10.1007/s12687-013-0166-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cragun D, Bonner D, Kim J, Akbari MR, Narod SA, Gomez-Fuego A, … Pal T (2015). Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast Cancer Research and Treatment, 151(1), 169–176. 10.1007/s10549-015-3374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, … Armstrong K (2016). Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 34(22), 2610–2618. 10.1200/JCO.2015.66.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancey AK, Ortega AN, & Kumanyika SK (2006). Effective recruitment and retention of minority research participants. Annual Review of Public Health, 27, 1–28. 10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
- 20.Zamora I, Williams ME, Higareda M, Wheeler BY, & Levitt P (2015). Brief Report: Recruitment and Retention of Minority Children for Autism Research. Journal of Autism and Developmental Disorders. 10.1007/s10803-015-2603-6 [DOI] [PubMed] [Google Scholar]
- 21.Calderón JL, Baker RS, Fabrega H, Conde JG, Hays RD, Fleming E., & Norris K (2006). An ethno-medical perspective on research participation: a qualitative pilot study. MedGenMed: Medscape General Medicine, 8(2), 23. [PMC free article] [PubMed] [Google Scholar]
- 22.García AA, Zuñiga JA, & Lagon C (2016). A Personal Touch: The Most Important Strategy for Recruiting Latino Research Participants. Journal of Transcultural Nursing: Official Journal of the Transcultural Nursing Society / Transcultural Nursing Society 10.1177/1043659616644958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalela P, Suarez L, Muñoz E, Gallion KJ, Pollock BH, Weitman SD, … Ramirez AG (2014). Promoting Factors and Barriers to Participation in Early Phase Clinical Trials: Patients Perspectives. Journal of Community Medicine & Health Education, 4(281), 1000281 10.4172/2161-0711.1000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, & Corbie-Smith G (2010). The role of race and trust in tissue/blood donation for genetic research. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 12(2), 116–121. 10.1097/GIM.0b013e3181cd6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes TB, Varma VR, Pettigrew C, & Albert MS (2015). African Americans and Clinical Research: Evidence Concerning Barriers and Facilitators to Participation and Recruitment Recommendations. The Gerontologist 10.1093/geront/gnv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullerton SM, Knerr S, & Burke W (2012). Finding a place for genomics in healthdisparities research. Public Health Genomics, 15(3–4), 156–163. 10.1159/000334717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


