Abstract
To detect the horizontal pattern of phylogenetic structure shown by alpine plants, we measured phylogenetic structure using net related index (NRI) and net nearest taxon index (NTI), and analyzed the phylogenetic structure patterns of alpine plants along longitude, latitude and environmental gradients in the Hengduan Mountains Region (HDMR). Our results show that: 1) the phylogenetic structure tended to cluster with increasing latitude and longitude; 2) for NRI, latitude was closer related than longitude, while for NTI, longitude was closer related than latitude, though they both not significantly relate to NTI. The phylogenetic structure tended towards overdispersion in the southern HDMR, with good climate conditions of higher mean annual temperature and more mean annual precipitation. In contrast, with harsh climate conditions of lower mean annual temperature and less mean annual precipitation, the increasing environmental stress led to phylogenetic clustering in the northern HDMR. The results highlighted that in the alpine region of HDMR, environmental filters and geographical isolation had a great effect on the latitudinal and longitudinal alpine species distribution, respectively.
Keywords: Environmental filter, Geographical isolation, Hengduan Mountains Region, Horizontal pattern, NRI, NTI
1. Introduction
The phylogenetic relatedness of species in a community has been employed to explore the underlying factors that structure species diversity patterns along ecological gradients (Webb, 2000, Bryant et al., 2008, Kluge and Kessler, 2011, Loidi et al., 2015). For example, elevation has been found to play a role in shaping community structure in communities of bacteria, which indicated that environmental filtering played a great role in shaping community structure (Wang et al., 2012). Similar phylogenetic patterns have been found in many other communities along environmental gradients, like fern (Kluge and Kessler, 2011), alpine plants (Li et al., 2014b), and ants (Machac et al., 2011). Latitudinal gradients in species diversity have long been recognized, but the factors which play a role in this are not clearly understood. One idea is that environmental factors play a major role in filtering species. Another is that evolutionary events such as extinction, speciation, and dispersal may also be involved.
Phylogenetic structure can be used to measure the mean degree of genetic relatedness among coexisting species in communities on the local scale (Webb et al., 2002). Phylogenetic overdispersion denotes that species in communities are more distantly related than expected, while phylogenetic clustering indicates that species is more closely related than expected. According to this assumption, communities with equal species richness can be assembled with species that are either closely- or distantly-related phylogenetic background (Webb et al., 2002).
When climate changes dramatically, or species disperse into different environments, species must survive in the new climate or go extinct. Assuming phylogenetic niche conservatism (Wiens et al., 2010), only a few lineages with the suitable ecological traits (e.g. cold tolerance) can survive the harsher (e.g. colder) climatic conditions (Ricklefs, 2006). Therefore, the plants that survive such environmental filters are often related, which results in a tendency for phylogenetic clustering along ecological gradients.
The alpine plant region is the zone above the treeline (about 4300 m in HDMR) and below the nival belt (about 5200 in HDMR) (Grabherr et al., 2003, Xu et al., 2014). The typical alpine region is composed of alpine shrub at low elevations, with alpine meadows above and then alpine screes at high elevations in HDMR. The alpine area is one of the harshest zones on the planet as a result of low temperature, limited precipitation, lack of pollinators, fluctuating weather, strong winds and short growing periods (Korner, 2003, Hodkinson, 2005).
The HDMR is located in the eastern edge of the Qinghai–Tibetan plateau. It is an important differentiation center of many temperate plant species and a center of distribution and speciation for many alpine taxa (Sun, 2002, Sun and Li, 2003), which are basically temperate in nature, and many large genera of the north temperate flora contain numerous and diversified species or complexes, such as Aconitum, Arenaria, Corydalis, Delphinium, Gentiana, liglaria, Pedicularis, Primula, Rhododendron, Salix, Saussurea, and Saxifraga. Among the subnival belt flora in the Hengduan Mountains, the north temperate elements make up about 50% (Xu et al., 2014). This indicates a close flora relationship between this region and north temperate area. Many alpine plants may spread to other areas (like north places), because alpine plants are able to adapt to cold climate, they can easy distribute into the north area along the mountain chains. This is especially true for species that originated in the north temperate area when the climate gets warm. This may lead to non-random phylogenetic patterns along the latitudinal or longitudinal gradients.
The HDMR is famous as a species refugium rich in species diversity (Li and Li, 1993, Myers et al., 2000). Many species can move in from the north when the climate changes dramatically. For example, when temperature has decreased quickly in the past. It can also be a source for species that move out when the climate gets warmer, like during interglacial period. Therefore, this large area provides an excellent natural laboratory to investigate phylogenetic structure of species assemblages and the role of environmental factors (like temperature) play in shaping these assemblages along the longitudes and latitudes.
Here, we examine phylogenetic structure along horizontal directions. We tested two predictions: (1) communities of alpine plants with relatively favorable conditions (southern HDMR) would be phylogenetically overdispersed, and (2) communities of alpine plants with relatively harsh climates (northern HDMR) would be phylogenetically clustered. We also examined whether similar patterns of phylogenetic structure were exhibited along a longitudinal direction due to geological barriers (rivers). Finally, we examined whether key environmental factors (mean annual temperature and annual rainfall) played a role in shaping alpine plant communities.
2. Material and methods
2.1. Study area
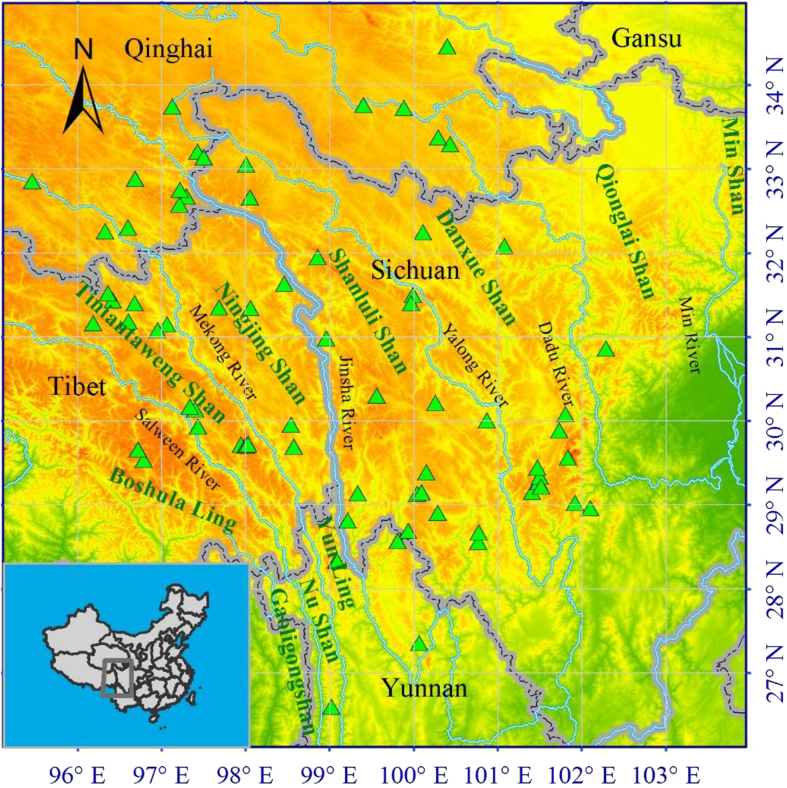
The Hengduan Mountain region (HDMR) extends between 24°40′–34°00N and 96°20′–104°30′E, covering ca. 364,000 km2, and includes western and northwestern Yunnan, western Sichuan, southeastern Tibet, southeastern Qinghai and southwestern Gansu (Li, 1987) (Fig. 1). Seven mountain chains and six rivers make up the main geographic features across the region from north to south (Li, 1989). These geographic features make it easier for alpine plants to disperse along a north–south axis, or latitudinal gradient, rather than an east–west, or longitudinal gradient, as mountain chains provide corridors, while rivers in valleys act as barriers to alpine plant dispersal. The HDMR is also greatly affected by the Indian and Pacific Ocean Monsoon circumfluence, resulting in a distinct wet season from mid-May to October and a dry season from October to mid-May the next year (Zhang, 1989).
Fig. 1.
Terrain and location of the Hengduan Mountains region, the boundary, and the 89 study sites for latitudinal and longitudinal analysis (Triangles). The major mountain chains and rivers are shown.
2.2. Data sources
We generated a database for this study using surveys and previous work carried out since the 1950s, including a comprehensive scientific expedition to the Qinghai–Xizang Plateau by the Chinese Academy of Sciences, an expedition to assess the Biodiversity of the HDMR supported by China and United States, the database of Biodiversity of the Hengduan Mountains and adjacent areas of south-central China (http://hengduan.huh.harvard.edu), and Chinese Virtual Herbarium (http://www.cvh.org.cn). The database includes information on species identity, genus, family, and latitude and longitude coordinates for each entry. Species present with fewer than 20 individuals and specimens without coordinates were not retained for analysis, as we considered these species to have low relative importance. This simplification is unlikely to affect the results. There were 89 sites left for the further analysis (Fig. 1).
2.3. Environmental variables
To examine the relationship between phylogenetic indices of each site and climate, represented by mean annual precipitation and mean annual temperature, we extracted mean annual precipitation and mean annual temperature for each site from the WorldClim v1.4 database (<www.worldclim.org>; (Hijmans et al., 2005)) using ArcGIS 9.3 (ESRI, 2008).
2.4. Constructing phylogenies and assessing the phylogenetic structure
A phylogenetic tree was generated using the informatics tool Phylomatic (Webb and Donoghue, 2005) following Zanne et al. (2014). Our phylogenetic tree included 882 angiosperm species in the alpine region (Fig. S1). Branch lengths were estimated according to Zanne et al. (2014), combining DNA maker data with a molecular phylogeny (32,223 species) for land plants. As a result, our tree was more accurate than the APG III system tree (Angiosperm Phylogeny Group, 2009), which estimated branch length using the BLADJ algorithm implemented in Phylocom (Webb et al., 2008).
We estimated the phylogenetic structure of the sites using two indices with the picante package (Kembel et al., 2010): NRI, based on mean phylogenetic distance (MPD); and NTI, based on mean nearest taxon distance (MNTD) (Webb et al., 2008, Webb et al., 2011). MPD refers to the average phylogenetic relatedness between all possible pairs of taxa in one site. In contrast, MNTD represents the mean phylogenetic relatedness between each taxon and its nearest relative in the site. Based on MPD and MNTD, we calculated NRI and NTI indices, which were defined as follows:
We calculated the NRI and NTI for each site based on presence/absence. Then, we compared those NRI and NTI values to 9999 randomly calculated NRI and NTI values for each site, using our phylogeny to represent the entire species pool of alpine angiosperm plants in the HDMR. A two-tailed significant test was used to assess whether the observed NRI/NTI values differed significantly from zero at P = 0.05. Observed NRI and NTI values of less than 250 than zero indicated significant overdispersion, whereas values more than 9750 greater than zero was assumed to indicate significant clustering. These analyses were performed in R software (R Core Team, 2014).
2.5. Analyses
We related the degree of latitude and longitude to the phylogenetic structure (NRI, NTI) of each site using linear models, and checked the differences of the phylogenetic structure between the northern and southern HDMR divided by 30°N in R software (R Core Team, 2014). Then, variance partitioning was used to examine the contribution of independent effects and joint effects of latitude and longitude on phylogenetic structure (Qian and Ricklefs, 2012).
3. Results
We examined 89 alpine sites in the Hengduan Mountain regions, with a total of 2371 specimen, which belonged to 882 alpine species, 231 genera and 53 families (APG III, 2009, Table S1).
3.1. Phylogenetic structure along latitudinal and longitudinal gradients
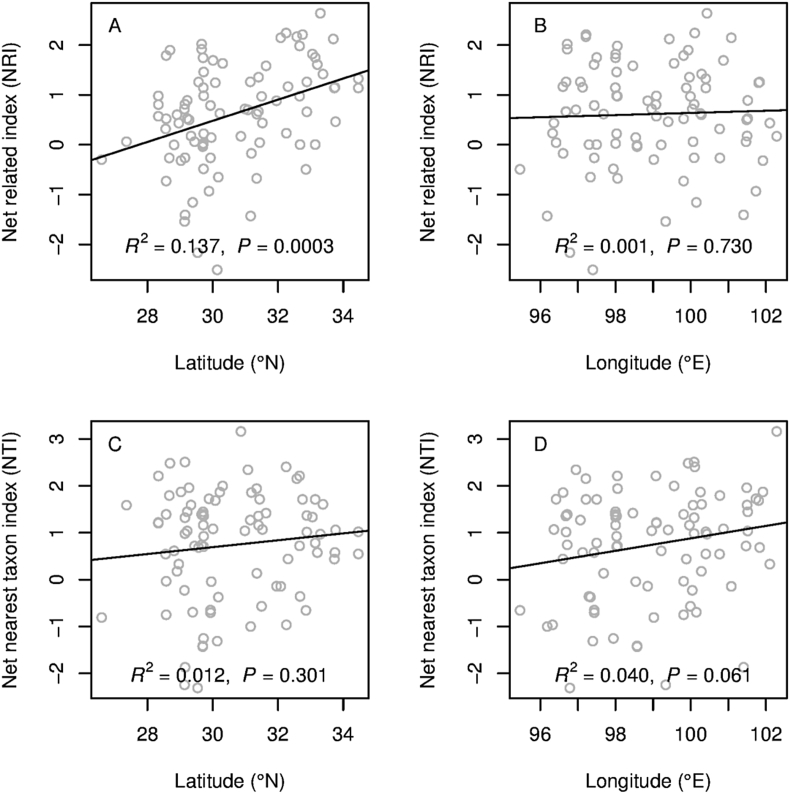
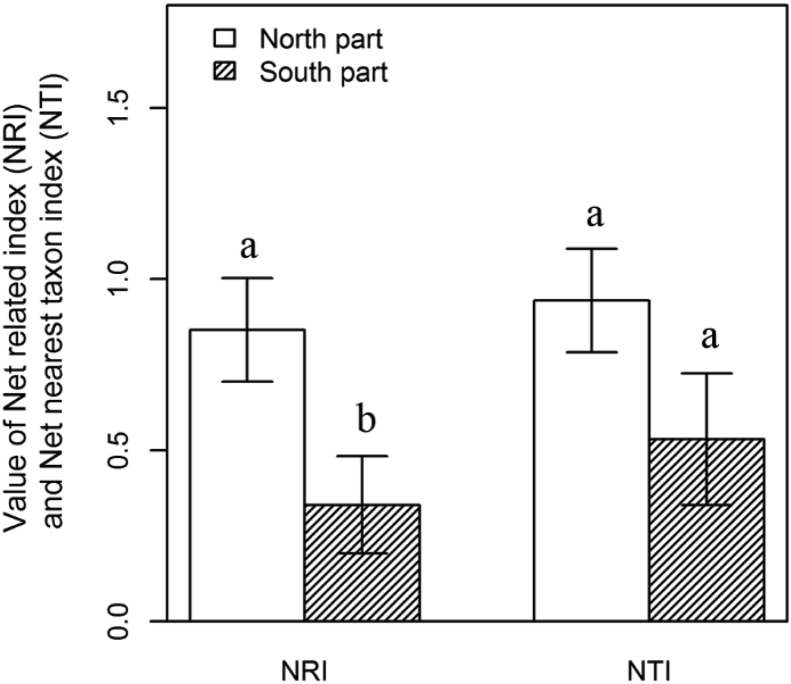
Our analyses showed that 21 (23.6%) sites showed phylogenetic overdispersion and 68 (76.4%) sites showed phylogenetic clustering among 89 alpine sites. Only 2 sites showed significantly phylogenetic overdispersion and 17 sites were significantly phylogenetic clustering. Our analyses also showed that plant tended to become clustered at higher latitudes (Fig. 2A and C): NRI values significantly increased along the latitudinal gradient (Fig. 2A), while NTI values also increased along the latitudinal gradient, but not significantly (Fig. 2C). NRI values were not significantly correlated with the longitudinal gradient (Fig. 2B), while NTI showed a marginally significant increase with increasing longitude (Fig. 2D). The Net related index (NRI) showed a significant difference (P = 0.016) between the northern and southern HDMR, and Net nearest taxon index (NTI) didn't showed the significant difference (P = 0.099) between the two parts of HDMR (Fig. 3). Assuming niche conservatism, this result indicated phylogenetic clustering is consistent with the importance of environmental filtering in structuring community diversity.
Fig. 2.
Variation in community phylogenetic relatedness along latitudinal and longitudinal gradients as measured with the NRI (A and B) and the NTI (C and D). Positive index values indicate phylogenetic clustering, and negative values indicate phylogenetic overdispersion.
Fig. 3.
Comparison of Net related index (NRI) and Net nearest taxon index (NTI) between the northern and southern HDMR by 30°N. The Letters indicate significant differences (α = 0.05) between the northern and southern HDMR.
3.2. Relative contribution of latitude and longitude to phylogenetic structure
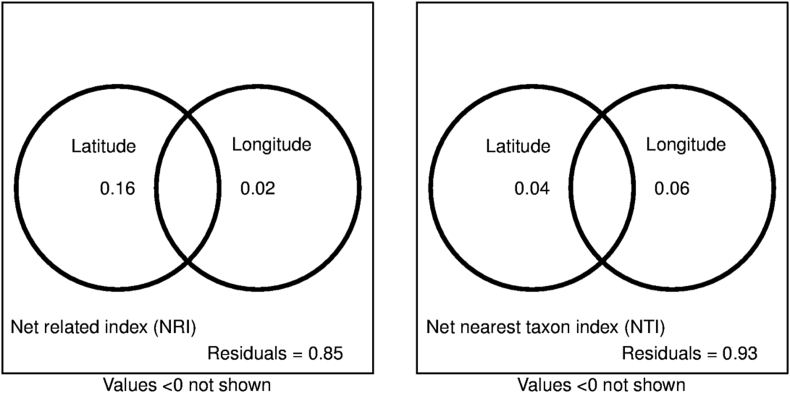
Latitude and longitude are more correlated to NRI than NTI (Fig. 4, Table S2), the unique effects of latitude and longitude to NRI were 16% and 2%, respectively. While the unique effects of latitude and longitude to NTI were low, individually explaining 4% and 6%, there were negative interactions between the effects of latitude and longitude on NRI and NTI.
Fig. 4.
Variation partitioning of NTI and NRI values for alpine plant communities into the independent effects of latitude, longitude, and their overlaps.
3.3. Climate along latitude and longitude
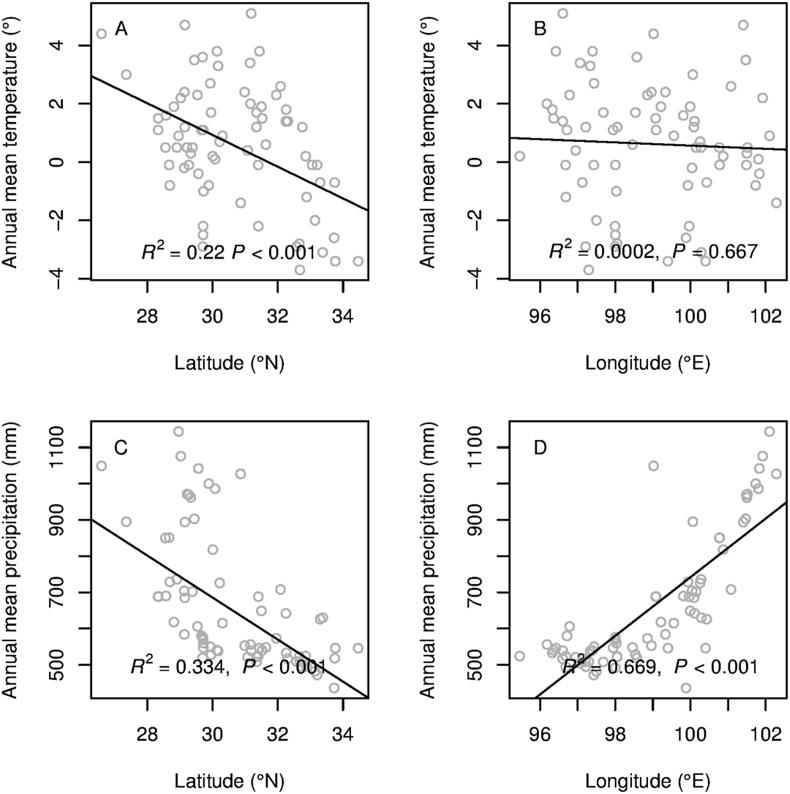
Annual mean temperature and precipitation both decreased significantly with increasing latitude (Fig. 5A and C). Annual mean temperature showed no significant difference along longitude (Fig. 5B), while the annual mean precipitation significantly increased with the increasing longitude (Fig. 5D).
Fig. 5.
Latitudinal and longitudinal gradients in temperature and rainfall.
4. Discussion
In HDMR, we found that mean annual temperature and precipitation both decrease along a latitudinal gradient, which indicates that environmental pressure in the region increases from south to north. Therefore, if plants spread from south to north, for example, from Yunnan to Sichuan province, they will be exposed to gradual environmental filtering during the dispersal process.
We found that alpine plant communities exhibited more phylogenetic overdispersion or random community assembly in the southern HDMR (mainly in northwest Yunnan), whereas they showed phylogenetic clustering in the northern HDMR (Fig. 2). The value of NRI showed a significant higher in the northern HDMR than in the southern HDMR, while the value of NTI was also showed a marginal significant value (P = 0.099) between the northern HDMR and the southern HDMR (Fig. 3). This is consistent with previous studies, which have shown that communities tend towards phylogenetic clustering along gradients of increasing environmental pressure (Machac et al., 2011). Qian et al. (2013) tested the phylogenetic relatedness along latitudinal gradients of angiosperm trees in North America, and found that tree species assemblages tended to show more phylogenetic clustering at higher latitudes with lower temperatures. The flora of Hengduan mountain is basically temperate, and the north temperate flora is dominant (Li and Li, 1993). This probably implies a close historical flora connection between this region and north temperate area, as many genera co-occur in these regions, like Rhododendon, Pedicularis, lagotis Corydalis, Primula, Aconitum, Delphinium and other plants.
Most plant lineages first evolved when the planet was predominately under tropical environments during the Paleocene (Behrensmeyer et al., 1992, Graham, 1999). In the early Eocene and Mid-Eocene, temperature decreased more quickly at higher latitudes (Zachos et al., 2008, Qian et al., 2014). This climate cooling process forced species at higher latitudes to migrate to warmer places at lower latitudes, adapt to cold climates, or suffer extinction (Qian et al., 2014). Many plants may have spread together to lower latitudes like HDMR and nearby areas. During these processes, plant assemblages likely rearranged along the temperature gradient with the change of latitude.
On the other hand, the Qinghai – Tibetan Plateau uplift afterwards (including HDMR), especially the extensive uplifts around between 30 and 23 Ma, 15 – 7 Ma, and 3.6–1.6 Ma, may have triggered speciation events and adaptive radiation, leading some plant taxa to diversify, such as Caragana (Zhang and Fritsch, 2010), Rheum (Sun et al., 2012), Cyananthus (Zhou et al., 2013), Lagotis (Li et al., 2014a), Corydalis (Perez-Gutierrez et al., 2015), Gentiana (Favre et al., 2016), liglaria (Liu et al., 2006), Pedicularis (Wang et al., 2015), Primula (Ren et al., 2015), Rhododendron (Wen et al., 2014), Saussurea (Wang et al., 2009), Saxifraga (Gao et al., 2015) and other taxa (Wen et al., 2014). When the climate became warmer, many plants may have spread to northern habitats under their demands of dispersal, and they would be filtered by a set of environmental factors along a latitudinal gradient. Only fewer lineages with the amount of suitable ecological traits (e.g. cold tolerance) can survive the environmental barriers to distribute into harsher (e.g. colder) climatic conditions (Ricklefs, 2006). So, the few lineages which remain are closely related plants left due to similar biological traits, resulting in the tendency of phylogenetic clustering (Fig. 2).
The annual mean temperature was relative stable along longitude, while the annual mean precipitation was increased significantly along longitude (from west to east). Surprisingly, plant communities did not show increase phylogenetic clustering with decreased rainfall. One possible explanation is that rainfall is not an important factor in influencing phylogenetic structure along longitude in HDMR. This may be due to geological barriers to dispersal such as rivers. The direction of rivers from north to south in this region make the dispersal along a west–east axis, namely, longitudinally, very difficult for alpine plants whose dispersal mechanisms rely mainly on dry fruits, like capsules, capitulum, siliqua, and follicles (Peng et al., 2014). On the other hand, plants can travel much easier along the crest lines of mountain chains without great difficulty (Fig. 2). Therefore, the pattern of phylogenetic clustering along an increasing latitudinal gradient was much clearer than along increasing longitudinal gradient. The correlation between phylogenetic structure and latitude and longitude was more significant using the NTI. This is most likely because the NTI is more sensitive than the NRI. Because only a small amount of plant can change more evidently the NTI than NRI among the sites, like the plants with strong dispersion capacity can transfer though the great barrier of rivers.
5. Conclusion
In summary, our results showed the pattern of phylogenetic overdispersion in the southern HDMR and clustering tendency in the northern HDMR, that indicated the environmental filtering (especially the mean annual temperatures) affected the lineages present. the pattern of phylogenetic structure along increasing longitude probably related to the barrier of rivers, and needed more research.
Acknowledgements
This work was supported by Major Program of National Natural Science Foundation of China grant no. 31590823 to Hang Sun, the National Natural Science Foundation of China (NSFC) Grant No. 31560063, and Key Disciplines (Ecology) Project of Yunnan Education Department.
(Editor: Victoria Sosa)
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.pld.2016.11.007.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. [Google Scholar]
- Behrensmeyer A.K., Damuth J.D., DiMichele W.A. University of Chicago Press; Chicago: 1992. Terrestrial Ecosystems through Time: Evolutionary Paleoecology of Terrestrial Plants and Animals. [Google Scholar]
- Bryant J.A., Lamanna C., Morlon H. Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11505–11511. doi: 10.1073/pnas.0801920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI, 2008. ArcGIS Desktop 9.3. Redlands, CA.
- Favre A., Michalak I., Chen C.H. Out-of-Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae) J. Biogeogr. 2016;43 preview. [Google Scholar]
- Gao Q.B., Li Y.H., Gornall R.J. Phylogeny and speciation in Saxifraga sect. Ciliatae (Saxifragaceae): evidence from psbA-trnH, trnL-F and ITS sequences. Taxon. 2015;64:703–713. [Google Scholar]
- Grabherr G., Nagy L., Thompson D.B.A. An Outline of Europe's Alpine Areas. In: Nagy L., Grabherr G., Koerner C., Thompson D.B.A., editors. vol. 167. Springer Verlag; Berlin; Heidelberg, New York: 2003. pp. 3–12. (Alpine Diversity in Europe). [Google Scholar]
- Graham A. Oxford University Press; New York: 1999. Late Cretaceous and Cenozoic History of North American Vegetation North of Mexico. [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Hodkinson I.D. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. 2005;80:489–513. doi: 10.1017/s1464793105006767. [DOI] [PubMed] [Google Scholar]
- Kembel S.W., Cowan P.D., Helmus M.R. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Kluge J., Kessler M. Phylogenetic diversity, trait diversity and niches: species assembly of ferns along a tropical elevational gradient. J. Biogeogr. 2011;38:394–405. [Google Scholar]
- Korner C. second ed. Springer; Berlin: 2003. Alpine Plant Life. [Google Scholar]
- Li B.Y. On the boundaries of the Hengduan Mountains. Mt. Res. 1987;5:74–82. [Google Scholar]
- Li B.Y. Geomorphologic regionalization of the Hengduan Mountainous Region. Mt. Res. 1989;7:13–20. [Google Scholar]
- Li G.D., Kim C., Zha H.G. Molecular phylogeny and biogeography of the arctic-alpine genus Lagotis (Plantaginaceae) Taxon. 2014;63:103–115. [Google Scholar]
- Li X.H., Zhu X.X., Niu Y. Phylogenetic clustering and overdispersion for alpine plants along elevational gradient in the Hengduan Mountains Region, southwest China. J. Syst. Evol. 2014;52:280–288. [Google Scholar]
- Li X.W., Li J. A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Acta Bot. Yunnanica. 1993;15:217–231. [Google Scholar]
- Liu J.Q., Wang Y.J., Wang A.L. Radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan plateau. Mol. Phylogenet. Evol. 2006;38:31–49. doi: 10.1016/j.ympev.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Loidi J., Antonio Campos J., Herrera M. Eco-geographical factors affecting richness and phylogenetic diversity patterns of high-mountain flora in the Iberian Peninsula. Alp. Bot. 2015;125:137–146. [Google Scholar]
- Machac A., Janda M., Dunn R.R. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography. 2011;34:364–371. [Google Scholar]
- Myers N., Mittermeier R.A., Mittermeier C.G. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Peng D.L., Ou X.K., Xu B. Plant sexual systems correlated with morphological traits: reflecting reproductive strategies of alpine plants. J. Syst. Evol. 2014;52:368–377. [Google Scholar]
- Perez-Gutierrez M.A., Romero-Garcia A.T., Fernandez M.C. Evolutionary history of fumitories (subfamily Fumarioideae, Papaveraceae): an old story shaped by the main geological and climatic events in the Northern Hemisphere. Mol. Phylogenet. Evol. 2015;88:75–92. doi: 10.1016/j.ympev.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Qian H., Hao Z., Zhang J. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol. 2014;7:154–165. [Google Scholar]
- Qian H., Ricklefs R.E. Disentangling the effects of geographic distance and environmental dissimilarity on global patterns of species turnover. Glob. Ecol. Biogeogr. 2012;21:341–351. [Google Scholar]
- Qian H., Zhang Y.J., Zhang J. Latitudinal gradients in phylogenetic relatedness of angiosperm trees in North America. Glob. Ecol. Biogeogr. 2013;22:1183–1191. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: a Language and Environment for Statistical Computing. [Google Scholar]
- Ren G.P., Conti E., Salamin N. Phylogeny and biogeography of Primula sect. Armerina: implications for plant evolution under climate change and the uplift of the Qinghai-Tibet plateau. BMC Evol. Biol. 2015;15 doi: 10.1186/s12862-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. Evolutionary diversification and the origin of the diversity-environment relationship. Ecology. 2006;87:S3–S13. doi: 10.1890/0012-9658(2006)87[3:edatoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sun H. Evolution of Arctic-tertiary fora in Himalayan-Hengduan Mountains. Acta Bot. Yunnanica. 2002;24:671–688. [Google Scholar]
- Sun H., Li Z.M. Qinghai-Tibet plateau uplift and its impact on Tethys flora. Adv. Earth Sci. 2003;18:852–862. [Google Scholar]
- Sun Y., Wang A., Wan D. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Mol. Phylogenet. Evol. 2012;63:150–158. doi: 10.1016/j.ympev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Li W.T., Liu Y.N. Range-wide multilocus phylogenetic analyses of Pedicularis sect. Cyathophora (Orobanchaceae): implications for species delimitation and speciation. Taxon. 2015;64:959–974. [Google Scholar]
- Wang J.J., Soininen J., He J.Z. Phylogenetic clustering increases with elevation for microbes. Environ. Microbiol. Rep. 2012;4:217–226. doi: 10.1111/j.1758-2229.2011.00324.x. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Susanna A., Von Raab-Straube E. Island-like radiation of Saussurea (Asteraceae: Cardueae) triggered by uplifts of the Qinghai-Tibetan plateau. Biol. J. Linn. Soc. 2009;97:893–903. [Google Scholar]
- Webb C.O. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 2000;156:145–155. doi: 10.1086/303378. [DOI] [PubMed] [Google Scholar]
- Webb, C. O., Ackerley D. D., Kembel S. W., 2011. “Software for the analysis of phylogenetic community structure and character evolution (with phylomatic and ecovolve).” User's Manual Version 4.2. From http://phylodiversity.net/phylocom/.
- Webb C.O., Ackerly D.D., Kembel S.W. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Webb C.O., Ackerly D.D., McPeek M.A. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002;33:475–505. [Google Scholar]
- Webb C.O., Donoghue M.J. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes. 2005;5:181–183. [Google Scholar]
- Wen J., Zhang J.Q., Nie Z.L. Evolutionary diversifications of plants on the Qinghai-Tibetan plateau. Front. Genet. 2014;5:4. doi: 10.3389/fgene.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens J.J., Ackerly D.D., Allen A.P. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- Xu B., Li Z., Min Sun H. Plant diversity and floristic characters of the alpine subnival belt flora in the Hengduan Mountains, SW China. J. Syst. Evol. 2014;52:271–279. [Google Scholar]
- Zachos J.C., Dickens G.R., Zeebe R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451:279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- Zanne A.E., Tank D.C., Cornwell W.K. Three keys to the radiation of angiosperms into freezing environments. Nature. 2014;506:89–92. doi: 10.1038/nature12872. [DOI] [PubMed] [Google Scholar]
- Zhang M.L., Fritsch P.W. Evolutionary response of Caragana (Fabaceae) to Qinghai-Tibetan plateau uplift and Asian interior aridification. Plant Syst. Evol. 2010;288:191–199. [Google Scholar]
- Zhang Y.G. Climatic division of the Hengduan Mountainous Region. Mt. Res. 1989;7:21–28. [Google Scholar]
- Zhou Z., Hong D.Y., Niu Y. Phylogenetic and biogeographic analyses of the Sino-Himalayan endemic genus Cyananthus (Campanulaceae) and implications for the evolution of its sexual system. Mol. Phylogenet. Evol. 2013;68:482–497. doi: 10.1016/j.ympev.2013.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.