Abstract
Land surface models and dynamic global vegetation models typically represent vegetation through coarse plant functional type groupings based on leaf form, phenology, and bioclimatic limits. Although these groupings were both feasible and functional for early model generations, in light of the pace at which our knowledge of functional ecology, ecosystem demographics, and vegetation-climate feedbacks has advanced and the ever growing demand for enhanced model performance, these groupings have become antiquated and are identified as a key source of model uncertainty. The newest wave of model development is centered on shifting the vegetation paradigm away from plant functional types (PFTs) and towards flexible trait-based representations. These models seek to improve errors in ecosystem fluxes that result from information loss due to over-aggregation of dissimilar species into the same functional class. We advocate the importance of the inclusion of plant hydraulic trait representation within the new paradigm through a framework of the whole-plant hydraulic strategy. Plant hydraulic strategy is known to play a critical role in the regulation of stomatal conductance and thus transpiration and latent heat flux. It is typical that coexisting plants employ opposing hydraulic strategies, and therefore have disparate patterns of water acquisition and use. Hydraulic traits are deterministic of drought resilience, response to disturbance, and other demographic processes. The addition of plant hydraulic properties in models may not only improve the simulation of carbon and water fluxes but also vegetation population distributions.
Keywords: Hydraulic traits, Land-surface modeling, Whole-plant hydraulic strategy, Trait-based models, Demographic models, Plant functional type
1. The role of vegetation in the global land surface energy and water budgets
Feedbacks between vegetation, weather, and climate are critical to the function of the Earth system (Pitman, 2003, Bonan, 2008, Quillet et al., 2010). Forested ecosystems provide a carbon sink of 2.3–2.5 Pg-C yr−1 (Le Quere et al., 2013), while releasing ∼62,000 km3 yr−1 water to the atmosphere (Jasechko et al., 2013). Carbon uptake and transpirational water loss are regulated dynamically through stomatal aperture, which, in many cases, is governed by water status and plant hydraulic properties (Sperry, 2000, Buckley, 2005, Skelton et al., 2015). Transpiration from plants has been shown to be the largest component of the terrestrial water cycle and is an integral component of latent heat flux – the energy component that is expended evaporating water (Schlesinger and Jasechko, 2014). Changes to transpiration, and therefore latent heat flux, affect the partitioning of the surface energy balance. Incoming solar radiation is redistributed at the surface through different heat and radiative fluxes. Vegetation at the land surface strongly affects this energy redistribution. As transpiration occurs, the surface of the leaf cools due to the latent heat of vaporization, altering its long-wave emissivity through the Stefan–Boltzmann Law and thereby modifying the radiation budget (Strengers et al., 2010). Alternatively, when stomata are closed, most of the incoming solar energy that is absorbed by leaf surfaces turns into heat, which increases sensible heat flux to the atmosphere, and drives stronger emissions of long-wave radiation. Root water uptake from different depths alters the hydrologic cycle through changes in soil water content, groundwater availability, and transpiration (Fatichi et al., 2016). Moisture recycling through transpiration is integral to the rewetting of the boundary layer and continental precipitation patterns, particularly in tropical regions (Hesslerova and Pokorny, 2010, Lee and Boyce, 2010, Aemisegger et al., 2014). Transpiration has been shown to drive cloud formation (de Arellano et al., 2014). Furthermore, vegetation canopy structure and leaf area index (LAI) determine the fraction of precipitation which is intercepted and evaporated, and that which reaches the soil as throughfall (Peng et al., 2014). Canopy roughness exerts strong controls over boundary layer turbulence and atmospheric mixing (Maurer et al., 2015). At a longer time scale, vegetation processes affect soil development and biogeochemical processes (Chapin, 2003). These and other vegetation-climate feedbacks necessitate the inclusion of an adequate representation of vegetation dynamics within hydrologic and climate models (Bonan et al., 2003).
2. PFTs and beyond
Land surface models (LSMs) and dynamic global vegetation models (DGVMs), typically indicated as terrestrial biosphere models (e. g. Fisher et al., 2014), are types of models that handle the surface energy budget for the atmospheric, hydrological, or combined Earth-system models of which they are often a component. The land surface serves as the upper boundary for hydrological models, and the lower boundary for weather and climate models. LSMs explicitly resolve the effects of vegetation and its interactions with climate while representing the vegetation as a static set of parameters. DGVMs likewise represent vegetation using static parameters at the short time scales, but dynamically evolve the vegetative state over time following plant ecological dynamics of establishment, growth, competition, and mortality. Both types of models, and thus, most weather, climate, hydrology, and Earth system models which employ them, generally cluster vegetation into groups on the basis of relatively few characteristics such as aboveground live biomass permanence, leaf longevity, and leaf form (Running et al., 1994). DGVMs and LSMs typically define plant functional types (PFTs) through bioclimatic properties and static physical properties, such as plant height and leaf phenology (Boulangeat et al., 2012, Pappas et al., 2016). These PFTs provide a basic and practical means of classification for modeling of vegetation-climate interactions at large spatial and temporal scales (Kucharik et al., 2006, Moorcroft, 2006, Quillet et al., 2010, Yang et al., 2015). The use of PFTs to model vegetation dynamics originated from the need to categorize plants by ‘ecophysiognomic traits’ into groups having similar structure and function (Box, 1981, Box, 1995, Box, 1996).
PFTs have since provided a feasible method for classification of global vegetation into a small, finite number of clusters with assumed similar ecological behaviors on the basis of a relatively small number of traits. Generally, models employ between 10 and 20 different PFTs, with various combinations of these characteristics, to represent thousands plant species (Poulter et al., 2011). However, specific models may use more or fewer PFTs depending on the intended model application. For a global general circulation model, Box (1996) suggested 15 major plant types. The current, broadly used Community Land Model (CLM) version 4.5 (which is the LSM component of NCAR's Earth system model – CESM) also relies on 15 basic PFTs (Table 1), with additional crop functional types available under specific irrigation schemes or with a particular crop sub-model (Lawrence et al., 2011, Oleson et al., 2013). Kleidon et al. (2007) found that a minimum of eight discrete vegetation classes was required to prevent the formation of multiple steady-states in the SimBA dynamic vegetation model. In contrast, Euskirchen et al. (2009) chose to use 39 different PFTs to test predictions regarding the migration of arctic plant communities under future climate scenarios using the Terrestrial Ecosystem Model.
Table 1.
International Geosphere-Biosphere Programme (IGBP) land cover types (Loveland et al., 2000) and basic plant functional types used by CLM4.5 (Oleson et al., 2013).
| IGBP Land cover types | CLM 4.5 Plant functional types |
|---|---|
| Needleleaf evergreen tree | Needleleaf evergreen tree (temperate) |
| Broadleaf evergreen tree | Needleleaf evergreen tree (boreal) |
| Needlleleaf deciduous tree | Needleleaf deciduous tree (boreal) |
| Broadleaf deciduous tree | Broadleaf evergreen tree (tropical) |
| Mixed forests | Broadleaf evergreen tree (temperate) |
| Closed shrublands | Broadleaf deciduous tree (tropical) |
| Open shrublands | Broadleaf deciduous tree (temperate) |
| Woody savannas | Broadleaf deciduous tree (boreal) |
| Savannas | Broadleaf evergreen shrub (temperate) |
| Grasslands | Broadleaf deciduous shrub (temperate) |
| Persistent wetlands | Broadleaf deciduous shrub (boreal) |
| Croplands | C3 arctic grass |
| Urban/built | C3 grass |
| Cropland/vegetation mosaic | C4 grass |
| Snow/ice | C3 unmanaged rainfed crop |
| Barren/sparse | |
| Water |
Global distributions of plant functional types are derived from remotely sensed land-cover data sets (DeFries et al., 1995, DeFries et al., 2000, Jung et al., 2006). Satellite data is used to differentiate vegetation types in term of deciduousness, leaf form (needle or broad), and LAI (Bonan et al., 2002, Moorcroft, 2006, Quillet et al., 2010). The International Geosphere-Biosphere Programme (IGBP) developed the first broadly used map of land cover classes at a 1 km resolution from Normalized Difference Vegetation Index (NDVI) data from the Advanced Very High Resolution Radiometer (AVHRR) 1992–1993 dataset (Loveland et al., 2000) (Table 1). Recent reclassifications using newer satellite tools such as MODIS (1 and 0.5 km resolution), SPOT4-VEGETATION (1 km), and ENVISTAT-MERIS (0.3 km) have improved the resolution of these maps both spatially and temporally (Lawrence and Chase, 2007, Poulter et al., 2011). The 17 land cover classifications from the IGBP were developed in keeping with the logic of Running et al. (1994) that plant classification should be independent of climate zone. Therefore, the IGBP assessment of vegetative cover focused only on aspects of biomass permanence, leaf longevity, and leaf type. Climatologic limits are imposed on PFTs through merger with Köppen-Geiger climate zones (Kottek et al., 2006, Peel et al., 2007, Poulter et al., 2011) or through the prescription of ‘climate envelopes’ or temperature ranges where specific PFTs can exist (Sitch et al., 2003). While these PFT distribution maps are widely accepted and heavily relied upon by models, uncertainties still remain (Sun et al., 2008). For example, in a study using the LPJmL model, Poulter et al. (2011) found that large uncertainties in key ecosystem fluxes, 30% of gross primary productivity (GPP) and 20% of evapotranspiration, expressed as latent heat flux (LE), resulted from land cover uncertainties.
The heterogeneity of Earth's vegetation and its critical role in mediating biosphere-atmosphere exchanges make it one of the largest sources of uncertainty in predictions of climate change (Friedlingstein et al., 2006, Moorcroft, 2006, Denman and Brasseur, 2007, Scheiter et al., 2013, Wullschleger et al., 2014, Musavi et al., 2015, Yang et al., 2015, Matthes et al., 2016). A recent, large and still evolving body of work has drawn into question the capabilities of the current plant classification scheme to accurately reflect the biodiversity of vegetative ecophysiological functionalities. The coarse resolution at which PFTs are defined and vegetation is resolved has been shown to lead to errors in simulations of carbon flux (Van Bodegom et al., 2012, Pavlick et al., 2013, Matthes et al., 2016), evapotranspiration (Poulter et al., 2011, Matheny et al., 2014a, Matthes et al., 2016), and nitrogen cycling (Ostle et al., 2009). Current PFTs define plant traits that govern these processes as static point estimates that lack the flexibility to reflect any sort of inter- or intra-specific, spatial, or temporal variation or plasticity (As discussed in Prentice et al., 2007, Van Bodegom et al., 2012, Scheiter et al., 2013, Wullschleger et al., 2014). Reich et al. (2007) demonstrated that only 33–66% of the variability of five key leaf functional traits of 2021 species could be attributed to their PFT classification. Likewise, Kattge et al. (2011) demonstrated that up to 75% of variation for certain traits occurred within a given PFT. Recently, Wullschleger et al. (2014) ascertained that the plant traits most useful in determining functional behaviors in terms of carbon, water, and nutrient use, and therefore most relevant for determining more meaningful PFT groups, are plant size, permanence of structure (woody vs. herbaceous), architecture (the ratio of water loss to carbon uptake surface areas), leaf form, carbon metabolism (C3, C4, or CAM), reproduction, and phenology. The assimilation of physiologically diverse species into the same PFT results in the inability of current PFTs to capture variations between species' carbon metabolisms and water use efficiencies (WUE) (Reichstein et al., 2014, Musavi et al., 2015). This type of over-aggregation has been identified as a critical source of information loss, which may obfuscate species-level traits and responses to environmental forcing and competition (Clark et al., 2011, Scheiter et al., 2013, Matheny et al., 2014a). Frequently, competition dynamics are only resolved between PFTs rather than between individuals comprising a functional group (As discussed in Sato et al., 2007, Fisher et al., 2010; Quillet et al., 2010, Scheiter et al., 2013). Multiple studies have shown that more variation can exist between the species comprising a single PFT than among different PFTs (e. g. Wright et al., 2005, Anderegg, 2015). However, encouraging results from Bonan et al. (2012) demonstrated that by using observed trait values for maximum carboxylation rate (Vcmax) with existing PFTs and a multilayer canopy structure, among other improvements, they were able to increase the accuracy of GPP as predicted by CLM 4. Furthermore, the inclusion of trait variation within PFT classifications by Verheijen et al. (2013) enhanced the accuracy of predictions of carbon assimilation and vegetation distributions.
In keeping with these findings, the current sweeping challenge for DGVMs and LSMs is to shift the PFT paradigm from its current representation of over-aggregated static parameters to a more flexible trait-based classification scheme (Pavlick et al., 2013, Scheiter et al., 2013, Fyllas et al., 2014, Reichstein et al., 2014, van Bodegom et al., 2014, Enquist et al., 2015, Musavi et al., 2015, Sakschewski et al., 2015, Yang et al., 2015, Pappas et al., 2016). Classically defined PFTs are themselves based in plant traits, albeit a small number. However, this fact creates a false dichotomy in terms of a naming convention. Following the distinction made by Pappas et al. (2016), we refer to models that employ probabilistic representations of trait diversity using stochastic approaches and coordinated whole-plant trait spectra as ‘trait-based’ in contrast to those which employ the conventional PFT definitions.
Flexible trait-based representations, like the one put forward by Scheiter et al. (2013), rely on ecological theory and environmental filtering to evolve a composition of various trait combinations. In this review, we will focus specifically on the need for representation of plant hydraulic traits within the context of reimagined PFTs and trait-based modeling schemes. The integral role of transpiration from vegetation, as mediated through plant hydrodynamics, in both the carbon cycle and the global energy budget, makes it a priority for model improvement (Jasechko et al., 2013, Matheny et al., 2014a, Schlesinger and Jasechko, 2014, Christoffersen et al., 2016). However, impacts and feedbacks on transpiration from predicted increases in the variability of precipitation and the number of droughts and disturbances add complexity to this challenge (Allen et al., 2010, Dai, 2013, Fatichi and Ivanov, 2014). Baldocchi and Meyers (1998) showed that simulations of carbon and water fluxes using the classic PFT definitions in CANVEG were only well adapted during times of non-limiting soil moisture and dry leaves, and that additional parameterization was necessary to capture the behaviors of water-stressed and water-logged scenarios. Missing behaviors of water storage and transport within vegetation compartments (Baldocchi and Meyers, 1998), and within-PFT variation in hydraulic traits (Anderegg, 2015) makes current vegetation schemes poorly suited to replicate the effects of drought on vegetation.
It is understood that traits such as relative growth rate, seed dispersal capacity, and mortality are the primary determinants of demography (Perez-Harguindeguy et al., 2013, Yang et al., 2015). However, a number of hydraulic functional traits have been shown to influence establishment and mortality (Pockman and Sperry, 2000, McDowell et al., 2008, Poorter et al., 2010, Anderegg and Meinzer, 2015, Anderegg et al., 2016). For instance, run-away cavitation is a common cause of mortality in seedlings (Williams et al., 1997, Pratt et al., 2014); ring porous wood has been suggested to be an adaptation to resist damage to the conductive system during freeze–thaw cycles (Taneda and Sperry, 2008); and leaf hydraulic strategy has been hypothesized to exert a strong influence on species' ability to survive drought (McDowell et al., 2013, Bartlett et al., 2014). The difference between a plant's minimum xylem water potential and the potential at which dysfunction occurs, defined as the hydraulic safety margin, has been related to mortality risk during drought (Anderegg et al., 2016). This margin of hydraulic safety has also been shown to correlate with regional precipitation predictability, while cavitation vulnerability has been shown to correlate with species distributions along soil moisture gradients (Pockman and Sperry, 2000). Li et al. (2015) demonstrated that the hydraulic properties of conductance and leaf water potential were good indicators of species abundance. Wood density, another trait associated with drought susceptibility (Hoffmann et al., 2011) as well as a number of additional plant hydraulic properties (Pratt et al., 2007, Chave et al., 2009, Lachenbruch and McCulloh, 2014, Anderegg and Meinzer, 2015), has been successfully employed by the current trait-based demographic models TFS (Fyllas et al., 2014) and LPJmL-FIT (Sakschewski et al., 2015).
3. Hydraulic functional traits and strategies
Functional traits are, by definition, those traits that exert strong influence over how an organism performs in terms of growth, survival, and reproduction (McGill et al., 2006, Violle et al., 2007). Hydraulic functional traits at the leaf, stem, and root levels determine a plant's ability to acquire, circulate, and use water. The whole-plant hydraulic strategy, which we define as the syndrome of emergent phenotypical hydraulic functional traits that occur at all three levels (leaf, stem, and root), is critical to understanding the timing and magnitude of transpiration fluxes and species' responses to drought and disturbance (Ford et al., 2007, McDowell et al., 2008, Matheny et al., 2014b, von Allmen et al., 2014, Gaines et al., 2016, Matheny et al., 2016). It is common for co-existing species to employ contrasting whole-plant hydraulic strategies which result in distinct, species-specific patterns of transpiration and growth (e.g. McCulloh et al., 2012, Kocher et al., 2013, Thomsen et al., 2013, Martinez-Vilalta et al., 2014, Matheny et al., 2014b, Meinzer et al., 2014, Anderegg, 2015). In spite of these hydraulic differences, many standard PFT classifications group such species into the same class. The incorporation of whole-plant hydraulic functional traits and strategies into revised or expanded definitions of PFTs will improve model uncertainties due to over-aggregation (Pappas et al., 2015). Coordination of hydraulic functional traits may occur through tradeoffs defined within the plant economics spectrum (Wright et al., 2004, Chave et al., 2009, Freschet et al., 2010, Freschet et al., 2012, Liu et al., 2010, Reich, 2014) and potentially through the proposed hydraulic safety-efficiency tradeoff (Manzoni et al., 2013, Skelton et al., 2015). However, recent work from Gleason et al. (2016) suggests that there may only be a weak tradeoff between xylem safety and efficiency, evincing the large number of species that employ combinations of low efficiency and low safety traits. It is possible that these traits may be trading off with other, non-hydraulic traits in ways that are not yet clear (Gleason et al., 2016). Nonetheless, nature presents a gamut of combinations of leaf, stem, and root hydraulic functional traits. The following section will focus on the key emergent hydraulic traits at each of the three organ levels, summarized in Table 2.
Table 2.
A non-exhaustive list of plant hydraulic traits within each axis (leaf, stem, and root) of the whole-plant hydraulic strategy, the scales at which they are typically measured and reported, and the variables that exert the most influence over each trait.
| Trait description | Measurement scale | Potential influencing variables |
|---|---|---|
| Leaf level | ||
| Maximum stomatal conductance | Leaf, canopy | PAR, VPD, temperature, CO2 concentration, regulation strategy |
| Slope of stomatal response to leaf water potential | Leaf, canopy | VPD, CO2 concentration, regulation strategy |
| Cuticular conductance | Leaf, canopy | Leaf form, cuticle thickness |
| Stem level | ||
| Maximum xylem conductance | Stem, branch | Xylem architecture, aquaporin activity, seasonality, plant age, growth history |
| Xylem vulnerability curve | Stem, branch | Xylem architecture, aquaporin activity, seasonality, plant age, growth history |
| Fraction of active xylem | Stem, branch | Xylem architecture, seasonality, plant age, growth history |
| Saturated xylem water content | Stem, branch | Xylem architecture, wood density |
| Wood density | Stem, branch | Xylem architecture |
| Root level | ||
| Maximum depth | Root, tree | Plant age, soil type, soil moisture/groundwater availability, nutrient environment |
| Vertical distribution of root density | Tree, stand | Plant age, soil type, soil moisture/groundwater availability, nutrient environment |
| Maximum root xylem conductance | Root | Xylem architecture, aquaporin activity, seasonality, plant age, growth history |
| Mycorrhizal activity | Root, tree | Nutrient environment, hyphal density, soil moisture, soil temperature |
4. Leaf traits
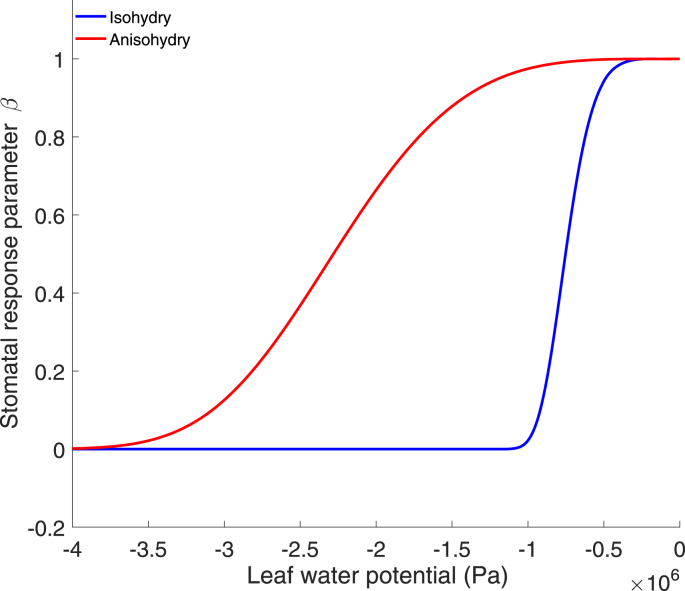
At the leaf level, transpiration is dynamically regulated by the stomata as a response to both internal and external conditions (Ball et al., 1987, Collatz et al., 1991, Tardieu, 1993, Leuning, 1995). Stomatal conductance has been hypothesized to be optimized in order to maximize the amount of carbon uptake while minimizing water loss (Cowan and Farquhar, 1977, Baldocchi, 1994, Katul et al., 2010), yet in some circumstances, e.g. nocturnal transpiration (Bucci et al., 2016), stomata appear to function in ways that are not driven by carbon uptake or optimized for prevention of water loss. Two distinct schemes for the regulation of stomatal conductance in response to leaf water potential are broadly recognized: (1) isohydry, a cavitation-risk adverse strategy through which leaf water potential is maintained by strict control of stomatal openness; (2) anisohydry, a cavitation-risk prone strategy through which high stomatal openness is maintained via tolerance of highly negative leaf water potentials (Stocker, 1956, Tardieu and Simonneau, 1998, Martinez-Vilalta et al., 2014), see illustration in Fig. 1. The majority of plants operate stomata along a range of regulation schemes on a virtual continuum between these two extremes (Klein, 2014). The degree of (an)isohydry is defined through the curve describing the relationship between leaf water potential and stomatal conductance (Fig. 1). However, as this relationship is hard to measure, it is typically determined through a comparison of either the extent of change between predawn- and midday-leaf water potential as a function of soil water potential (Martinez-Vilalta et al., 2014), or the rate of change in stomatal conductance between times when vapor pressure deficit (VPD) is low and when VPD is high (Oren et al., 1999). However, it has recently been shown that the degree of isohydricity can be determined through remotely sensed vegetative optical depth, a proxy for leaf water potential, as measured from the AMSR-E satellite (Konings and Gentine, 2016). Differences between plants' leaf hydraulic strategies are theorized to be of key importance in determining species' resilience to different types of drought (McDowell et al., 2008, Binks et al., 2016), although leaf traits alone may not provide a sufficiently complete picture of drought mortality (Rowland et al., 2015, Matheny et al., 2016).
Fig. 1.
Theoretical figure demonstrating the differences between anisohydric (red) and isohydric (blue) stomatal regulation and leaf water potential. β represents the fraction of actual stomatal conductance, as a proportion of the reference stomatal conductance when soil moisture and vapor pressure deficit (VPD) are non-limiting for transpiration. Lines represent idealized curves that describe stomatal response to declining leaf water potential.
5. Xylem traits
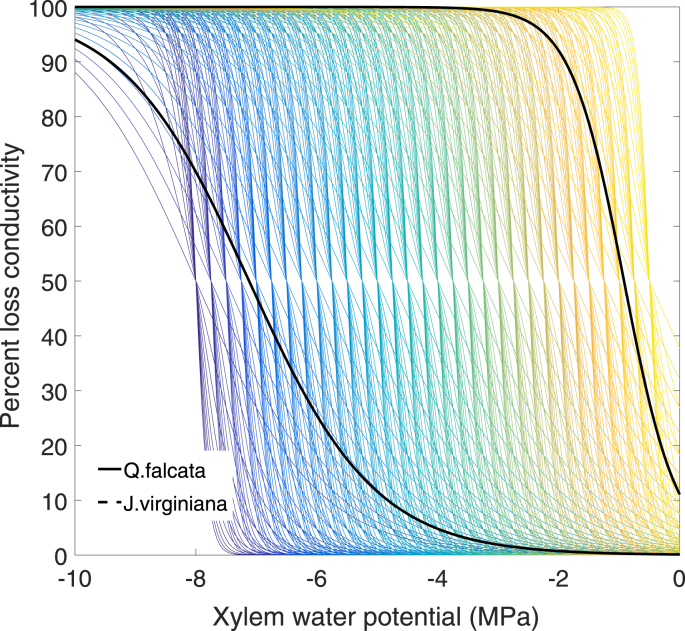
At the branch and stem levels, plants are typically classified on the basis of xylem architectures: ring porous, characterized by a relatively small number very large diameter xylem vessels in the early-wood; diffuse porous, having a relatively even distribution of xylem vessels throughout the sapwood; and conifers (also referred to as “non-porous” (Phillips et al., 1996, Oren et al., 1999)) which transport water through tracheids rather than vessels. In addition to vessel diameter and number, hydraulic conductance is also a function of several other interconnected traits such as vessel length, pit membrane flexibility and size, aquaporin activity, ionic concentrations within the sap, and the ability to refill embolized vessels, among others (Tognetti et al., 1998, Wagner et al., 1998, Tyree and Zimmermann, 2002, Brodribb and Holbrook, 2006, Olano et al., 2013, Hacke, 2014, Klein et al., 2014, Lachenbruch and McCulloh, 2014, Anderegg, 2015). The xylem architecture and physiology determine the functional relationships between xylem water potential and xylem conductivity (or loss thereof) (Fig. 2). Several studies have demonstrated that the large conduits of ring-porous species are much more vulnerable to embolism than the typically smaller conduits employed by diffuse-porous trees (Pockman and Sperry, 2000, Li et al., 2008, Taneda and Sperry, 2008), nonetheless, this is not always the case (Sperry et al., 1994). Following the logic of the hypothesized safety-efficiency tradeoff theory, it would be expected that trees having larger xylem vessels, which are generally more conductive to water but also more vulnerable to cavitation, would tend to employ an isohydric stomatal regulation strategy to avoid damage to the conductive system (Bovard et al., 2005, Bush et al., 2008, Taneda and Sperry, 2008). While xylem composed of smaller diameter conduits would require less stringent stomatal regulation to maintain ‘safe’ levels of water potential, it would also be less conductive in ideally wet conditions. Even so, multiple examples of anisohydric ring-porous species exist (Thomsen et al., 2013, Martinez-Vilalta et al., 2014). Such counter-intuitive combinations of hydraulic traits among organs may be mitigated by additional traits or behaviors such as large capacitance for stem water storage, deep or efficient rooting systems, and drought deciduousness (Brooks et al., 2010, Hoffmann et al., 2011, Kocher et al., 2013, Matheny et al., 2015). Therefore, for the purpose of modeling plant water dynamics, importance should be placed on the emergent functional properties of xylem hydraulic conductance and capacitance, rather than contributing traits.
Fig. 2.
An illustration of possible xylem conductance curves as defined by three traits (parameters): xylem water potentials at 50 and 88% loss conductivity and a third shape parameter. Observed conductivity response curves from Q. falcata, a ring porous species, and J. virginiana, a diffuse porous species, were reproduced on the basis of data provided in Maherali et al. (2006).
Field-measured hydraulic conductances of ring and diffuse porous species have been shown to be of comparable magnitudes during moisture-limiting conditions, which have caused the largest, early-wood vessels of the ring porous species to cavitate (Hacke et al., 2006). In this circumstance, the much smaller late-wood vessels of ring porous trees may act as an emergency “high safety, low efficiency” back-up system (Sperry and Sullivan, 1992, Woodcock, 1994, Taneda and Sperry, 2008). This seasonality of conduit sizes within ring porous wood contributes to the long-term temporal variability of hydraulic conductance, while the effects of diurnal embolism are reflected in shorter-term declines of conductance (Sperry et al., 1993, Chuang et al., 2006, Anderegg, 2015). The timing and magnitude of hydraulic trait variability has been shown to fluctuate by individual, life stage and history, population, species, and geographic location (Anderegg, 2015). Vulnerability to embolism may be a plastic trait and can also vary by underlying genetic variability among individuals of the same species and population (Pockman and Sperry, 2000). Differences in conductance and embolism vulnerability within each organ play a key role in the avoidance/tolerance of water stress (Tyree and Ewers, 1991). The hydraulic segmentation hypothesis of Tyree and Zimmermann (2002), through which a plant may sacrifice distal organs (i.e. leaves and roots) or conductivity within distal organs in favor of protecting the larger carbon and time investment of stem xylem from embolism, may provide one of the mechanisms behind behaviors such as drought deciduousness and succulent root decoupling from extremely dry soil (North and Nobel, 1992, Pockman and Sperry, 2000).
6. Root and rhizosphere traits
In addition to xylem conductance within the roots, the rhizosphere holds several other contributing properties, which determine plant water acquisition and use. Such traits include rooting depth and vertical distribution, root length and diameter distribution, efficiency of water extraction, and mycorrhizal interactions (Canadell et al., 2007, Allen, 2009, Reichstein et al., 2014, Wullschleger et al., 2014). Rooting depth is central to a plant's ability to acquire water, and deep roots have been shown to buffer plants with vulnerable leaf and stem strategies from hydraulic impairment (Miller et al., 2010, Matheny et al., 2016). While mesic tree species tend to root at depths between 1 and 5 m (Lyford and Wilson, 1964, Lyford, 1980, Canadell et al., 1996, Jackson et al., 1996, He et al., 2013), extreme cases in more xeric environments have evidenced root depths in excess of 20 m (Jackson et al., 1999). Varied root depths within a single ecosystem allow access to distinct water storage pools with differing seasonal availabilities. This type of ‘root niche separation’ has been postulated to increase the ecosystem resilience of tropical forest to drought in the Amazon (Ivanov et al., 2012). Brooks et al. (2010) demonstrated that the efficient rooting systems of Douglas fir trees have the ability to extract ‘tightly bound’ water from clayey soils, which enables them to persist in water limited regions. Other species' ability to reverse flow in portions of the root system allows for the hydraulic lifting of deeply stored water to surface soil layers to supplement the available water pool for transpiration (Dawson, 1993). Hydraulic lift has been shown to contribute significantly to a tree's subsequent day's transpiration (Horton and Hart, 1998), and also to fuel processes of ecological significance such as maintenance of mycorrhizal hyphae and fine roots (Neumann and Cardon, 2012). Symbiotic interactions with mycorrhizal fungi have been shown to increase plant-water availability by acting as an extension of absorptive surface area (Ruiz-Lozano and Azcón, 1995, Kaya et al., 2003). Yet these symbioses tend to be highly species specific and may be marginal in some cases (Parniske, 2008, Smith and Read, 2008).
The below-ground nature of these traits makes them difficult to study or determine from remote sensing, and therefore the majority of root system traits are underrepresented in the literature as well as in trait databases (Kattge et al., 2011, Wullschleger et al., 2014). Freschet et al. (2010) proposed that the plant economics spectrum may allow the inference of below-ground traits from leaf and stem traits, although the authors note that the root traits were the least well-coordinated among organs. Taneda and Sperry (2008) noted that several genera of ring porous trees tended to have deeper rooting systems (Nilsen et al., 1983, Burns and Honkala, 1990, Miller et al., 2010). Similarly, Hoffmann et al. (2011) found that species having both dense wood and anisohydric stomatal regulation were more likely to root at shallower depths. In keeping with the theory of the coordination of hydraulic traits on a whole-plant scale, Franks et al. (2007) put forward the concept of an ‘isohydrodynamic’ strategy, such that a virtually constant gradient of water potential is maintained from roots to leaves in spite of the stomatal regulation strategy (isohydric or anisohydric). Providing LSMs and DGVMs with a platform or basis for the incorporation of these defining whole-tree hydraulic strategies or an integrated perspective of emergent organ-specific hydraulic traits and their governing tradeoffs is a critical step forward in reconsidering the PFT framework.
7. Creating a framework for the incorporation of hydraulic traits
Inclusion of plant-trait observations at the tissue and organism scales into LSMs and DGVMs will help improve our ability to simulate ecosystem responses to land use and climate change (McIntyre and Lavorel, 2007, Quillet et al., 2010, Boulangeat et al., 2012). Following the framework of Reichstein et al. (2014) and Musavi et al. (2015), for each trait, which is represented by the model as a parameter in a function, there exists a multi-dimensional continuum of potential values, as demonstrated in Fig. 2. While it is overly simplistic to prescribe a single set of values to represent these traits, as is done by current PFT classifications, it remains impractical to allow traits to vary across such a continuum (Fig. 2), as there is no feasible way to measure all variation at all scales between and within individuals, species, and environments in time. Therefore, practical model applications must still employ some type of binning for each trait with a measure of allowable variation within each bin. These bins will include relatively similar species/sizes and/or growth stages. In order for this type of binning scheme to improve upon the current PFT system, bins must be established at a level across which hydraulic traits are conserved. Scaling up from the bin level to the plot and ecosystem levels could be done by weighting species' properties within each bin by their relative abundance and/or leaf area index (LAI) in a bottom up approach (Musavi et al., 2015) in order to aggregate traits themselves to the top level. This approach combines the efficiency and practicality of the original PFT classification system while providing the biophysical observational grounding and flexibility that was lacking in the previous methodology.
The multiscale time dependence of hydraulic traits, and by extension the emergent ecosystem-level water-use efficiency, highlights the requirement that trait-based parameterizations should be flexible rather than fixed, as is the case with the current PFT scheme. This type of adaptability will be a critical advantage for the next generation of DGVMs and LSMs (Poulter et al., 2011, Van Bodegom et al., 2012, Scheiter et al., 2013). As discussed in the previous sections, hydraulic conductivity within an individual organism changes at both the fast (intra-daily) and slow (monthly, seasonal, and annual) time scales (Hacke, 2014, Trifilo et al., 2014). Surmounting these challenges may still require use of a second proposed methodology: the incorporation of mechanistic process-based models (e.g. FETCH (Bohrer et al., 2005, Mirfenderesgi et al., 2016), ExpertN (Janott et al., 2011, Bittner et al., 2012), SPAC (Gentine et al., 2015), root model described by Siqueira et al. (2008)), in order to capture dynamic responses to these traits, which are expressed as variable parameters (Powell et al., 2013). These process-based plant hydraulics models simulate water flow through part or all of the plant vascular system, using the Richards equation (Bohrer et al., 2005, Siqueira et al., 2008, Janott et al., 2011) or Darcy's law (Gentine et al., 2015) for porous media flow. In this type of framework, traits such as hydraulic conductance and, in some models, capacitance are determined at relatively short time steps (e.g. 1–30 min) as functions of water pressures within the plant vascular system. Other traits, such as wood density and leaf area index can be adjusted or allowed to vary at longer, i.e. annual or seasonal, time scales. These vegetation hydrodynamics sub-models have the potential to operate at the bin-level and calculate vegetation soil water withdrawal, stomatal conductance, and transpiration mechanistically on the basis of the whole-plant hydraulic strategy. These model outputs could then be statistically scaled to the patch- or ecosystem-level on the basis of leaf or basal area or other similar metrics following Matheny et al. (2014b).
Trait similarities such as the standard/typical shape of cavitation vulnerability curves (Fig. 2) across ring porous tree species (Sperry and Saliendra, 1994, Hacke et al., 2006, Taneda and Sperry, 2008), and trait relationships, such as that between WUE and cellulose carbon isotope composition (Farquhar and Richards, 1984, Werner et al., 2012), will help constrain the potentially infinite new parameter spaces. Furthermore, the increasing wealth of species- and leaf-level trait data across broad spatial scales will help to facilitate this transition to trait based modeling. Large repositories of trait data such as the BIEN database (Enquist et al., 2009), the xylem water potential database (Choat et al., 2012), the wood density database (Chave et al., 2009), the vessel anatomy database (Zanne et al., 2010), the leaf turgor loss point database (Bartlett et al., 2012, Bartlett et al., 2014, Maréchaux et al., 2016), the GLOPNET leaf trait database (Wright et al., 2004, Reich et al., 2007), the GlobResp leaf respiration database (Atkin et al., 2015), the light response database of Niinemets et al. (2015), and the herbivory defense/response database (Moles et al., 2013) have recently been established. Many of these data resources are available through the TRY global plant trait database (Kattge et al., 2011). These archives, when used in tandem with the mathematical and statistical advancements of model-data fusion techniques (Wang et al., 2009, Peng et al., 2011, Keenan et al., 2012, Dietze et al., 2013), will provide modelers with physically realistic distributions of trait possibilities and combinations for use in regional and global scale simulations.
In an effort to help characterize and quantify trait variability (temporally, spatially, and intra- and inter-specifically) and its empirical and functional relationships with environmental conditions for use in modeling scenarios, special emphasis should be placed on expanding the measurement record across temporal scales and maintaining the integrity of metadata provided for traits recorded in these and other databases. At present, most trait measurements are carried out as snapshots during relatively few growing seasons. A more complete temporal record will enhance our understanding of trait variability with time. Metadata of interest may include the tissue/organ on which the measurement was made, the number of individuals sampled and the variation between individuals, time of year/season, geographic location, mean annual or seasonal meteorological variables (e.g. incoming solar radiation, temperature, humidity, rainfall, soil moisture), and other data of interest surrounding the measurement such as if the year/season was exceptionally dry or hot, or if a specific pest or blight was present. Currently, only some of the trait databases provide some of the metadata needed for forming a predictive description of trait variability across ecosystems and climate.
8. Climate change, biodiversity, and ecosystem demography
The effects of climate change on global vegetation distributions are expected to be seen though the shifting of geographic ranges in response to rising temperatures, changes to precipitation patterns and variability, and extreme weather (Allen et al., 2010, Choat et al., 2012, Soudzilovskaia et al., 2013). The realistic representation of vegetative responses to these phenomena as well as to natural disturbances is necessary for modeling the outcomes of climate and land use change (Shellito and Sloan, 2006, Purves et al., 2008, Quillet et al., 2010, Gough et al., 2013, Matheny et al., 2014a). While trait-based models will not provide solutions for all scenarios, specifically those involving insect attack (Kurz et al., 2008, Herms and McCullough, 2014, Anderegg et al., 2015) or grazing (Yu et al., 2011, Wullschleger et al., 2014), they do provide a necessary step forward in simulating carbon and water fluxes (Scheiter et al., 2013, Reichstein et al., 2014, Pappas et al., 2016). DGVMs and LSMs using standard PFTs and climate envelopes have been criticized for their lack of ability to accurately represent the mechanisms for species coexistence and biodiversity (McMahon et al., 2011, Boulangeat et al., 2012). However, the recent incorporation of plant traits and coexistence mechanisms into DGVMs and, more specifically, demographic models which give additional focus to the incorporation of processes such as recruitment, growth, and mortality of vegetation, provides a feasible avenue for this advancement as demonstrated by aDGVM2 (Scheiter et al., 2013), JeDi (Pavlick et al., 2013), CLM-ED (Fisher et al., 2015), LPJmL-FIT (Sakschewski et al., 2015), and LM3-PPA (Weng et al., 2015).
Within trait-based DGVMs and demographic models, ecosystem properties serve to ‘filter’ plants with different suites of traits to select for species or hypothetical proxy species most fit for that environment (Sterck et al., 2011, Fisher et al., 2015). These models may be able to more closely reflect trait diversity, species co-existence, and ecosystem productivity than previous generations of DGVMs (Fisher et al., 2015). Driving model demographics with plant traits may promote an improved understanding of trait- and bio-diversity, interactions between biotic and abiotic factors, and their influence on ecosystem function (Loreau et al., 2001, Hooper et al., 2005, Cadotte et al., 2009). Paleoclimatological studies reveal that vegetation responses to past climate shifts have resulted from a combination of species competition and migration (Thuiller et al., 2008, Huntley et al., 2010, Quillet et al., 2010). As warming continues and bioclimatic limits shift (Box, 1981, Harrison et al., 2010), trait-based demographic models will be better suited to replicate future species distributions and abundances without the need for pre-parameterized climate envelopes (Fisher et al., 2015).
Plant functional traits, both hydraulic and otherwise, are responsible for feedbacks between reproduction, growth, and success (Sterck et al., 2011). Therefore, trait-based models must account for tradeoffs among traits (as reviewed in Scheiter et al., 2013, Fisher et al., 2015) to prevent one or two unrealistic ‘overly optimal’ cohort species, or Darwinian demons (Law, 1979), from out-competing all others (Scheiter et al., 2013). Angert et al. (2009) demonstrated that tradeoffs between relative growth rate and intrinsic WUE are strong influencers of demographic response in desert species typically classified within the same traditional PFT. Tradeoffs between specific traits within the hydraulic system (e.g. storage versus strength, and conductance versus hydraulic safety) have been directly related to plant height, growth, and survival among various co-existing rainforest species (Poorter et al., 2010). While biological mechanisms for coexistence and community assembly have been well studied (e.g. Chesson, 2000, Clark et al., 2010), using trait tradeoffs as a means to simulate coexistence and biodiversity in models is still a challenge area. Some key tradeoff surfaces may not be readily apparent or easily studied (Fisher et al., 2015), while others may not be ubiquitous (Moncrieff et al., 2015). Missing relevant tradeoffs may leave models unable to explain particular patterns of species distributions with respect to available resources (Sterck et al., 2011). The continued study and development of a global, whole-plant economics spectrum is integral for the advancement of trait and tradeoff-based demographic models (Reich, 2014). Within this context, the ability to account for intra- and inter-specific trait variation and adaptation will be essential (Messier et al., 2010, Albert, 2015, Anderegg, 2015). Currently, trait parameterization schemes assume that both traits and tradeoffs will continue to be valid under future climate scenarios (Clark and Gelfand, 2006). However, the synthesis efforts of Franks et al. (2014) reveal that both genetic adaptation and phenotypic plasticity are presently occurring within plant communities. Many of the present trait-based models also show a strong dependence on the initial parameterization or the starting composition of an ecosystem, which is a representation of the consequences of past disturbances and management histories on the ecosystem. Therefore, another key challenge for the use of these models in the prediction of future ecosystems will be the use of smart initialization methods and adequate maps of current plant trait distributions. Nonetheless, the adaptive trait selection methodology developed and employed by aDGVM2 (Scheiter et al., 2013) may lay the foundation for future model structures to account for trait variability.
9. Conclusions – a path forward
As we demonstrate above, current PFT classifications, which represent thousands of plant species on the basis of relatively few, assumed-static parameters, have been identified as a critical source of uncertainty in land surface and dynamic global vegetation models (Reich et al., 2007, Wullschleger et al., 2014, Musavi et al., 2015, Yang et al., 2015). Model characterizations of vegetation affect simulations of the surface energy balance and the carbon, water, and nitrogen cycles (Ostle et al., 2009, Matheny et al., 2014a, Matthes et al., 2016). Plant hydraulic traits are integral to the regulation of transpiration and growth through controls on root water uptake and stomatal conductance (Anderegg and Meinzer, 2015). Traits at the root, stem, and leaf levels interact to define the syndrome of emergent phenotypical hydraulic traits we term the whole-plant hydraulic strategy. The incorporation of whole-plant hydraulic strategy into new trait-based representations of vegetation in models will serve to improve simulations of carbon and water fluxes, particularly in cases of drought and disturbance (McDowell et al., 2013, Matheny et al., 2014b). Whole-plant hydraulic strategy has the potential to be incorporated into models in several ways with varying degrees of complexity and computational load.
Here, we presented two alternatives: The first alternative centers on the integration of whole-plant hydraulic strategies into bins of similar properties through the use of combined leaf-xylem-root hydrology strategies. Conceptually, this calls for expanding the current PFTs to include the combination of hydraulic strategies as defining properties for each bin of species. However, this approach may also suffer problems similar to those associated with over-aggregation found within existing PFT paradigms. The second approach for the incorporation of whole-plant hydraulic strategy requires the use of mechanistic plant hydrodynamic sub-models to simulate the functional behaviors of statistically scalable representative individuals. While this method provides greater opportunity to capture behaviors resulting from long- and short-term trait variability, it will require additional computational resources.
Both proposed incorporation schemes present ways by which observational plant trait data from newly established databanks can be utilized to inform practical model parameterizations grounded in reality. Models incorporating trait-based representations of plant hydraulic properties may be better able to capture demographic responses to climate change if modelers and ecologists can successfully identify the appropriate tradeoff surfaces within the plant economics spectrum (e. g. Chave et al., 2009). To test the potential for model improvements through the incorporation of the whole-plant hydraulic strategy and its practical applicability at multiple temporal and spatial scales, modelers should seek to include the discussed as well as other strategies within a board variety of trait-based land surface and dynamic vegetation models. Recent work from Xu et al. (2016) using redefined hydraulic trait specific PFTs and a mechanistic simulation of plant water dynamics, among other improvements, within the Ecosystem Demography model version 2 (ED2) to replicate vegetation dynamics in a tropical forest can provide an excellent starting point for future analyses.
Acknowledgements
We thank Simone Fatichi and two anonymous reviewers for their helpful insights and thoughtful consideration of this work. Funding for this study was provided by the U.S. National Science Foundation Hydrological Science grant 1521238, and the U.S. Department of Energy's Office of Science Office of Biological and Environmental Research, Terrestrial Ecosystem Sciences Program Award No. DE-SC0007041, and Ameriflux Management Project Core Site Agreement No. 7096915. Support for A.M. Matheny was provided by The Ohio State University Presidential Fellowship and the P.E.O. Scholar Award. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies.
(Editor: Sergey Volis)
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
References
- Aemisegger F., Pfahl S., Sodemann H., Lehner I., Seneviratne S.I., Wernli H. Deuterium excess as a proxy for continental moisture recycling and plant transpiration. Atmos. Chem. Phys. 2014;14:4029–4054. [Google Scholar]
- Albert C.H. Intraspecific trait variability matters. J. Veg. Sci. 2015;26:7–8. [Google Scholar]
- Allen C.D., Macalady A.K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., Kitzberger T., Rigling A., Breshears D.D., Hogg E.H., Gonzalez P., Fensham R., Zhang Z., Castro J., Demidova N., Lim J.-H., Allard G., Running S.W., Semerci A., Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010;259:660–684. [Google Scholar]
- Allen M.F. Water Relations in the Mycorrhizosphere. In: Luttge U., Beyschlag W., Budel B., Francis D., editors. Progress in Botany. Springer-Verlag; Berlin & Heidelberg, Germany: 2009. pp. 257–276. [Google Scholar]
- Anderegg W.R.L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2015;205:1008–1014. doi: 10.1111/nph.12907. [DOI] [PubMed] [Google Scholar]
- Anderegg W.R.L., Hicke J.A., Fisher R.A., Allen C.D., Aukema J., Bentz B., Hood S., Lichstein J.W., Macalady A.K., McDowell N., Pan Y.D., Raffa K., Sala A., Shaw J.D., Stephenson N.L., Tague C., Zeppel M. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015;208:674–683. doi: 10.1111/nph.13477. [DOI] [PubMed] [Google Scholar]
- Anderegg W.R.L., Klein T., Bartlett M., Sack L., Pellegrini A.F.A., Choat B., Jansen S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5024–5029. doi: 10.1073/pnas.1525678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg W.R.L., Meinzer F.C. Wood anatomy and plant hydraulics in a changing climate. In: Hacke U., editor. Functional and Ecological Xylem Anatomy. Springer International Publishing; Switzerland: 2015. pp. 235–253. [Google Scholar]
- Angert A.L., Huxman T.E., Chesson P., Venable D.L. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11641–11645. doi: 10.1073/pnas.0904512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin O.K., Bloomfield K.J., Reich P.B., Tjoelker M.G., Asner G.P., Bonal D., Bonisch G., Bradford M.G., Cernusak L.A., Cosio E.G., Creek D., Crous K.Y., Domingues T.F., Dukes J.S., Egerton J.J.G., Evans J.R., Farquhar G.D., Fyllas N.M., Gauthier P.P.G., Gloor E., Gimeno T.E., Griffin K.L., Guerrieri R., Heskel M.A., Huntingford C., Ishida F.Y., Kattge J., Lambers H., Liddell M.J., Lloyd J., Lusk C.H., Martin R.E., Maksimov A.P., Maximov T.C., Malhi Y., Medlyn B.E., Meir P., Mercado L.M., Mirotchnick N., Ng D., Niinemets U., O'Sullivan O.S., Phillips O.L., Poorter L., Poot P., Prentice I.C., Salinas N., Rowland L.M., Ryan M.G., Sitch S., Slot M., Smith N.G., Turnbull M.H., VanderWel M.C., Valladares F., Veneklaas E.J., Weerasinghe L.K., Wirth C., Wright I.J., Wythers K.R., Xiang J., Xiang S., Zaragoza-Castells J. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015;206:614–636. doi: 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- Baldocchi D. An analytical solution for coupled leaf photosynthesis and stomatal conductance models. Tree Physiol. 1994;14:1069–1079. doi: 10.1093/treephys/14.7-8-9.1069. [DOI] [PubMed] [Google Scholar]
- Baldocchi D., Meyers T. On using eco-physiological, micrometeorological and biogeochemical theory to evaluate carbon dioxide, water vapor and trace gas fluxes over vegetation: a perspective. Agric. For. Meteorol. 1998;90:1–25. [Google Scholar]
- Ball J.T., Woodrow I.E., Berry J.A. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. Prog. Photosynth. Res. 1987;4:221–224. [Google Scholar]
- Bartlett M.K., Scoffoni C., Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol. Lett. 2012;15:393–405. doi: 10.1111/j.1461-0248.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- Bartlett M.K., Zhang Y., Kreidler N., Sun S.W., Ardy R., Cao K.F., Sack L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014;17:1580–1590. doi: 10.1111/ele.12374. [DOI] [PubMed] [Google Scholar]
- Binks O., Meir P., Rowland L., da Costa A.C.L., Vasconcelos S.S., de Oliveira A.A.R., Ferreira L., Christoffersen B.O., Nardini A., Mencuccini M. Plasticity in leaf-level water relations of tropical rainforest trees in response to experimental drought. New Phytol. 2016;211:477–488. doi: 10.1111/nph.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner S., Janott M., Ritter D., Kocher P., Beese F., Priesack E. Functional-structural water flow model reveals differences between diffuse- and ring-porous tree species. Agric. For. Meteorol. 2012;158:80–89. [Google Scholar]
- Bohrer G., Mourad H., Laursen T.A., Drewry D., Avissar R., Poggi D., Oren R., Katul G.G. Finite element tree crown hydrodynamics model (FETCH) using porous media flow within branching elements: a new representation of tree hydrodynamics. Water Resour. Res. 2005;41 [Google Scholar]
- Bonan G.B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- Bonan G.B., Levis S., Kergoat L., Oleson K.W. Landscapes as patches of plant functional types: an integrating concept for climate and ecosystem models. Glob. Biogeochem. Cycles. 2002;16:30. [Google Scholar]
- Bonan G.B., Levis S., Sitch S., Vertenstein M., Oleson K.W. A dynamic global vegetation model for use with climate models: concepts and description of simulated vegetation dynamics. Glob. Change Biol. 2003;9:1543–1566. [Google Scholar]
- Bonan G.B., Oleson K.W., Fisher R.A., Lasslop G., Reichstein M. Reconciling leaf physiological traits and canopy flux data: use of the TRY and FLUXNET databases in the Community Land Model version 4. J. Geophys. Res. Biogeosci. 2012;117:19. [Google Scholar]
- Boulangeat I., Philippe P., Abdulhak S., Douzet R., Garraud L., Lavergne S., Lavorel S., van Es J., Vittoz P., Thuiller W. Improving plant functional groups for dynamic models of biodiversity: at the crossroads between functional and community ecology. Glob. Change Biol. 2012;18:3464–3475. doi: 10.1111/j.1365-2486.2012.02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovard B.D., Curtis P.S., Vogel C.S., Su H.-B., Schmid H.P. Environmental controls on sap flow in a northern hardwood forest. Tree Physiol. 2005;25:31–38. doi: 10.1093/treephys/25.1.31. [DOI] [PubMed] [Google Scholar]
- Box E.O. 1981. Macroclimate and Plant Forms: an Introduction to Predictive Modelling in Phytogeography. Tasks for Vegetation Science; p. Xiii-258. (The Hague, Netherlands; Boston, Mass., USA) [Google Scholar]
- Box E.O. Factors determining distributions of tree species and plant functional types. Vegetatio. 1995;121:101–116. [Google Scholar]
- Box E.O. Plant functional types and climate at the global scale. J. Veg. Sci. 1996;7:309–320. [Google Scholar]
- Brodribb T.J., Holbrook N.M. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant Cell Environ. 2006;29:2205–2215. doi: 10.1111/j.1365-3040.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Brooks R.J., Barnard H.R., Coulombe R., McDonnell J.J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2010;3:100–104. [Google Scholar]
- Bucci S.J., Goldstein G., Scholz F.G., Meinzer F.C. Physiological Significance of Hydraulic Segmentation, Nocturnal Transpiration and Capacitance in Tropical Trees: Paradigms Revisited. In: Goldstein G., Santiago S.L., editors. Tropical Tree Physiology: Adaptations and Responses in a Changing Environment. Springer International Publishing; Cham: 2016. pp. 205–225. [Google Scholar]
- Buckley T.N. The control of stomata by water balance. New Phytol. 2005;168:275–291. doi: 10.1111/j.1469-8137.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- Burns R.M., Honkala B.H. Silvics of North America: 1. Conifer; 2. Hardwoods. In: U.S.D.O. Agriculture, editor. U. S. Forest Service; Washington, D. C: 1990. p. 877. [Google Scholar]
- Bush S.E., Pataki D.E., Hultine K.R., West A.G., Sperry J.S., Ehleringer J.R. Wood anatomy constrains stomatal responses to atmospheric vapor pressure deficit in irrigated, urban trees. Oecologia. 2008;156:13–20. doi: 10.1007/s00442-008-0966-5. [DOI] [PubMed] [Google Scholar]
- Cadotte M.W., Cavender-Bares J., Tilman D., Oakley T.H. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS One. 2009;4:9. doi: 10.1371/journal.pone.0005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadell J., Jackson R.B., Ehleringer J.R., Mooney H.A., Sala O.E., Schulze E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Canadell J.G., Pataki D.E., Pitelka L. Springer; Berlin; New York: 2007. Terrestrial Ecosystems in a Changing World. [Google Scholar]
- Chapin F.S. Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann. Bot. 2003;91:455–463. doi: 10.1093/aob/mcg041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chave J., Coomes D., Jansen S., Lewis S.L., Swenson N.G., Zanne A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. [Google Scholar]
- Choat B., Jansen S., Brodribb T.J., Cochard H., Delzon S., Bhaskar R., Bucci S.J., Feild T.S., Gleason S.M., Hacke U.G., Jacobsen A.L., Lens F., Maherali H., Martinez-Vilalta J., Mayr S., Mencuccini M., Mitchell P.J., Nardini A., Pittermann J., Pratt R.B., Sperry J.S., Westoby M., Wright I.J., Zanne A.E. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–756. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- Christoffersen B.O. Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS v.1-Hydro) Geosci. Model Dev. 2016;9:4227–4255. [Google Scholar]
- Chuang Y.L., Oren R., Bertozzi A.L., Phillips N., Katul G.G. The porous media model for the hydraulic system of a conifer tree: linking sap flux data to transpiration rate. Ecol. Model. 2006;191:447–468. [Google Scholar]
- Clark J.S., Bell D., Chu C.J., Courbaud B., Dietze M., Hersh M., HilleRisLambers J., Ibanez I., LaDeau S., McMahon S., Metcalf J., Mohan J., Moran E., Pangle L., Pearson S., Salk C., Shen Z.H., Valle D., Wyckoff P. High-dimensional coexistence based on individual variation: a synthesis of evidence. Ecol. Monogr. 2010;80:569–608. [Google Scholar]
- Clark J.S., Bell D.M., Hersh M.H., Kwit M.C., Moran E., Salk C., Stine A., Valle D., Zhu K. Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecol. Lett. 2011;14:1273–1287. doi: 10.1111/j.1461-0248.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- Clark J.S., Gelfand A.E. A future for models and data in environmental science. Trends Ecol. Evol. 2006;21:375–380. doi: 10.1016/j.tree.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Collatz G.J., Ball J.T., Grivet C., Berry J.A. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminal boundary layer. Agric. For. Meteorol. 1991;54:107–136. [Google Scholar]
- Cowan I.R., Farquhar G.D. Stomatal function in relation to leaf metabolism and environment. In: Jennings D.H., editor. Integration of Activity in the Higher Plant. Cambridge University Press; New York, NY, USA; Cambridge, England: 1977. pp. 471–505. [Google Scholar]
- Dai A.G. Increasing drought under global warming in observations and models. Nat. Clim. Change. 2013;3:52–58. [Google Scholar]
- Dawson T.E. Hydraulic lift and water use by plants: implications for water-balance, performance and plant-plant interactions. Oecologia. 1993;95:565–574. doi: 10.1007/BF00317442. [DOI] [PubMed] [Google Scholar]
- de Arellano J.V.G., Ouwersloot H.G., Baldocchi D., Jacobs C.M.J. Shallow cumulus rooted in photosynthesis. Geophys. Res. Lett. 2014;41:1796–1802. [Google Scholar]
- DeFries R., Hansen M., Townshend J. Global discrimination of land cover types from metrics derived from AVHRR pathfinder data. Remote Sens. Environ. 1995;54:209–222. [Google Scholar]
- DeFries R.S., Hansen M.C., Townshend J.R.G., Janetos A.C., Loveland T.R. A new global 1-km dataset of percentage tree cover derived from remote sensing. Glob. Change Biol. 2000;6:247–254. [Google Scholar]
- Denman K.L., Brasseur G. Cambridge Univ Press; New York: 2007. Couplings between Changes in the Climate System and Biogeochemistry. [Google Scholar]
- Dietze M.C., Lebauer D.S., Kooper R.O.B. On improving the communication between models and data. Plant Cell Environ. 2013;36:1575–1585. doi: 10.1111/pce.12043. [DOI] [PubMed] [Google Scholar]
- Enquist B., Condit R., Peet R., Schildhauer M., Thiers B. The iPlant Collaborative; 2009. The Botanical Information and Ecology Network (BIEN): Cyberinfrastructure for an Integrated Botanical Information Network to Investigate the Ecological Impacts of Global Climate Change on Plant Biodiversity.www.iplantcollaborative.org/sites/default/files/BIEN_White_Paper.pdf The iPlant Collaborative. Available at: (Accessed 22 August 2013) [Google Scholar]
- Enquist B.J., Norberg J., Bonser S.P., Violle C., Webb C.T., Henderson A., Sloat L.L., Savage V.M. Scaling from traits to ecosystems: developing a general trait driver theory via integrating trait-based and metabolic scaling theories. In: Pawar S., Woodward G., Dell A.I., editors. Advances in Ecological Research. Academic Press; 2015. pp. 249–318. [Google Scholar]
- Euskirchen E.S., McGuire A.D., Chapin F.S., Yi S., Thompson C.C. Changes in vegetation in northern Alaska under scenarios of climate change, 2003–2100: implications for climate feedbacks. Ecol. Appl. 2009;19:1022–1043. doi: 10.1890/08-0806.1. [DOI] [PubMed] [Google Scholar]
- Farquhar G.D., Richards R.A. Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984;11:539–552. [Google Scholar]
- Fatichi S., Ivanov V.Y. Interannual variability of evapotranspiration and vegetation productivity. Water Resour. Res. 2014;50:3275–3294. [Google Scholar]
- Fatichi S., Pappas C., Ivanov V.Y. Modeling plant–water interactions: an ecohydrological overview from the cell to the global scale. Wiley Interdiscip. Rev. Water. 2016;3:327–368. [Google Scholar]
- Fisher J.B., Huntzinger D.N., Schwalm C.R., Sitch S. Modeling the terrestrial biosphere. In: Gadgil A., Liverman D.M., editors. vol. 39. Annual Reviews; Palo Alto: 2014. (Annual Review of Environment and Resources). [Google Scholar]
- Fisher R.A., Muszala S., Verteinstein M., Lawrence P., Xu C., McDowell N.G., Knox R.G., Koven C., Holm J., Rogers B.M., Spessa A., Lawrence D., Bonan G. Taking off the training wheels: the properties of a dynamic vegetation model without climate envelopes, CLM4.5(ED) Geosci. Model Dev. 2015;8:3593–3619. [Google Scholar]
- Fisher R., McDowell N., Purves D., Moorcroft P., Sitch S., Cox P., Huntingford C., Meir P., Woodward F.I. Assessing uncertainties in a second-generation dynamic vegetation model caused by ecological scale limitations. New Phytol. 2010;187:666–681. doi: 10.1111/j.1469-8137.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- Ford C.R., Hubbard R.M., Kloeppel B.D., Vose J.M. A comparison of sap flux-based evapotranspiration estimates with catchment-scale water balance. Agric. For. Meteorol. 2007;145:176–185. [Google Scholar]
- Franks P.J., Drake P.L., Froend R.H. Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ. 2007;30:19–30. doi: 10.1111/j.1365-3040.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- Franks S.J., Weber J.J., Aitken S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014;7:123–139. doi: 10.1111/eva.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet G.T., Aerts R., Cornelissen J.H.C. A plant economics spectrum of litter decomposability. Funct. Ecol. 2012;26:56–65. [Google Scholar]
- Freschet G.T., Cornelissen J.H.C., van Logtestijn R.S.P., Aerts R. Evidence of the 'plant economics spectrum' in a subarctic flora. J. Ecol. 2010;98:362–373. [Google Scholar]
- Friedlingstein P., Cox P., Betts R., Bopp L., Von Bloh W., Brovkin V., Cadule P., Doney S., Eby M., Fung I., Bala G., John J., Jones C., Joos F., Kato T., Kawamiya M., Knorr W., Lindsay K., Matthews H.D., Raddatz T., Rayner P., Reick C., Roeckner E., Schnitzler K.G., Schnur R., Strassmann K., Weaver A.J., Yoshikawa C., Zeng N. Climate-carbon cycle feedback analysis: results from the (CMIP)-M-4 model intercomparison. J. Clim. 2006;19:3337–3353. [Google Scholar]
- Fyllas N.M., Gloor E., Mercado L.M., Sitch S., Quesada C.A., Domingues T.F., Galbraith D.R., Torre-Lezama A., Vilanova E., Ramirez-Angulo H., Higuchi N., Neill D.A., Silveira M., Ferreira L., Aymard G.A., Malhi Y., Phillips O.L., Lloyd J. Analysing Amazonian forest productivity using a new individual and trait-based model (TFS v.1) Geosci. Model Dev. 2014;7:1251–1269. [Google Scholar]
- Gaines K.P., Meinzer F.C., Duffy C.J., Thomas E.M., Eissenstat D.M. Rapid tree water transport and residence times in a Pennsylvania catchment. Ecohydrology. 2016;9:1554–1565. [Google Scholar]
- Gentine P., Guérin M., Uriarte M., McDowell N.G., Pockman W.T. An allometry-based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrology. 2015;9:529–546. [Google Scholar]
- Gleason S.M., Westoby M., Jansen S., Choat B., Hacke U.G., Pratt R.B., Bhaskar R., Brodribb T.J., Bucci S.J., Cao K.F., Cochard H., Delzon S., Domec J.C., Fan Z.X., Feild T.S., Jacobsen A.L., Johnson D.M., Lens F., Maherali H., Martinez-Vilalta J., Mayr S., McCulloh K.A., Mencuccini M., Mitchell P.J., Morris H., Nardini A., Pittermann J., Plavcova L., Schreiber S.G., Sperry J.S., Wright I.J., Zanne A.E. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world's woody plant species. New Phytol. 2016;209:123–136. doi: 10.1111/nph.13646. [DOI] [PubMed] [Google Scholar]
- Gough C.M., Hardiman B.S., Nave L.E., Bohrer G., Maurer K.D., Vogel C.S., Nadelhoffer K.J., Curtis P.S. Sustained carbon uptake and storage following moderate disturbance in a Great Lakes forest. Ecol. Appl. 2013;23:1202–1215. doi: 10.1890/12-1554.1. [DOI] [PubMed] [Google Scholar]
- Hacke U.G. Variable plant hydraulic conductance. Tree Physiol. 2014;34:105–108. doi: 10.1093/treephys/tpu007. [DOI] [PubMed] [Google Scholar]
- Hacke U.G., Sperry J.S., Wheeler J.K., Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Harrison S.P., Prentice I.C., Barboni D., Kohfeld K.E., Ni J., Sutra J.P. Ecophysiological and bioclimatic foundations for a global plant functional classification. J. Veg. Sci. 2010;21:300–317. [Google Scholar]
- He L.L., Ivanov V.Y., Bohrer G., Thomsen J.E., Vogel C.S., Moghaddam M. Temporal dynamics of soil moisture in a northern temperate mixed successional forest after a prescribed intermediate disturbance. Agric. For. Meteorol. 2013;180:22–33. [Google Scholar]
- Herms D.A., McCullough D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. In: Berenbaum M.R., editor. Annual Review of Entomology. Annual Reviews; Palo Alto: 2014. pp. 13–30. [DOI] [PubMed] [Google Scholar]
- Hesslerova P., Pokorny J. Springer; New York: 2010. Effect of Mau Forest Clear Cut on Temperature Distribution and Hydrology of Catchment of Lakes Nakuru and Naivasha: Preliminary Study. [Google Scholar]
- Hoffmann W.A., Marchin R.M., Abit P., Lau O.L. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob. Change Biol. 2011;17:2731–2742. [Google Scholar]
- Hooper D.U., Chapin F.S., Ewel J.J., Hector A., Inchausti P., Lavorel S., Lawton J.H., Lodge D.M., Loreau M., Naeem S., Schmid B., Setala H., Symstad A.J., Vandermeer J., Wardle D.A. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 2005;75:3–35. [Google Scholar]
- Horton J.L., Hart S.C. Hydraulic lift: a potentially important ecosystem process. Trends Ecol. Evol. 1998;13:232–235. doi: 10.1016/s0169-5347(98)01328-7. [DOI] [PubMed] [Google Scholar]
- Huntley B., Barnard P., Altwegg R., Chambers L., Coetzee B.W.T., Gibson L., Hockey P.A.R., Hole D.G., Midgley G.F., Underhill L.G., Willis S.G. Beyond bioclimatic envelopes: dynamic species' range and abundance modelling in the context of climatic change. Ecography. 2010;33:621–626. [Google Scholar]
- Ivanov V.Y., Hutyra L.R., Wofsy S.C., Munger J.W., Saleska S.R., de Oliveira R.C., de Camargo P.B. Root niche separation can explain avoidance of seasonal drought stress and vulnerability of overstory trees to extended drought in a mature Amazonian forest. Water Resour. Res. 2012;48:21. [Google Scholar]
- Jackson R.B., Canadell J., Ehleringer J.R., Mooney H.A., Sala O.E., Schulze E.D. A global analysis of root distributions for terrestrial biomes. Oecologia. 1996;108:389–411. doi: 10.1007/BF00333714. [DOI] [PubMed] [Google Scholar]
- Jackson R.B., Moore L.A., Hoffmann W.A., Pockman W.T., Linder C.R. Ecosystem rooting depth determined with caves and DNA. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11387–11392. doi: 10.1073/pnas.96.20.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janott M., Gayler S., Gessler A., Javaux M., Klier C., Priesack E. A one-dimensional model of water flow in soil–plant systems based on plant architecture. Plant Soil. 2011;341:233–256. [Google Scholar]
- Jasechko S., Sharp Z.D., Gibson J.J., Birks S.J., Yi Y., Fawcett P.J. Terrestrial water fluxes dominated by transpiration. Nature. 2013;496:347–350. doi: 10.1038/nature11983. [DOI] [PubMed] [Google Scholar]
- Jung M., Henkel K., Herold M., Churkina G. Exploiting synergies of global land cover products for carbon cycle modeling. Remote Sens. Environ. 2006;101:534–553. [Google Scholar]
- Kattge J., Diaz S., Lavorel S., Prentice C., Leadley P., Bonisch G., Garnier E., Westoby M., Reich P.B., Wright I.J., Cornelissen J.H.C., Violle C., Harrison S.P., van Bodegom P.M., Reichstein M., Enquist B.J., Soudzilovskaia N.A., Ackerly D.D., Anand M., Atkin O., Bahn M., Baker T.R., Baldocchi D., Bekker R., Blanco C.C., Blonder B., Bond W.J., Bradstock R., Bunker D.E., Casanoves F., Cavender-Bares J., Chambers J.Q., Chapin F.S., Chave J., Coomes D., Cornwell W.K., Craine J.M., Dobrin B.H., Duarte L., Durka W., Elser J., Esser G., Estiarte M., Fagan W.F., Fang J., Fernandez-Mendez F., Fidelis A., Finegan B., Flores O., Ford H., Frank D., Freschet G.T., Fyllas N.M., Gallagher R.V., Green W.A., Gutierrez A.G., Hickler T., Higgins S.I., Hodgson J.G., Jalili A., Jansen S., Joly C.A., Kerkhoff A.J., Kirkup D., Kitajima K., Kleyer M., Klotz S., Knops J.M.H., Kramer K., Kuhn I., Kurokawa H., Laughlin D., Lee T.D., Leishman M., Lens F., Lenz T., Lewis S.L., Lloyd J., Llusia J., Louault F., Ma S., Mahecha M.D., Manning P., Massad T., Medlyn B.E., Messier J., Moles A.T., Muller S.C., Nadrowski K., Naeem S., Niinemets U., Nollert S., Nuske A., Ogaya R., Oleksyn J., Onipchenko V.G., Onoda Y., Ordonez J., Overbeck G., Ozinga W.A., Patino S., Paula S., Pausas J.G., Penuelas J., Phillips O.L., Pillar V., Poorter H., Poorter L., Poschlod P., Prinzing A., Proulx R., Rammig A., Reinsch S., Reu B., Sack L., Salgado-Negre B., Sardans J., Shiodera S., Shipley B., Siefert A., Sosinski E., Soussana J.F., Swaine E., Swenson N., Thompson K., Thornton P., Waldram M., Weiher E., White M., White S., Wright S.J., Yguel B., Zaehle S., Zanne A.E., Wirth C. TRY – a global database of plant traits. Glob. Change Biol. 2011;17:2905–2935. [Google Scholar]
- Katul G., Manzoni S., Palmroth S., Oren R. A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann. Bot. 2010;105:431–442. doi: 10.1093/aob/mcp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C., Higgs D., Kirnak H., Tas I. Mycorrhizal colonisation improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well-watered and water-stressed conditions. Plant Soil. 2003;253:287–292. [Google Scholar]
- Keenan T.F., Davidson E., Moffat A.M., Munger W., Richardson A.D. Using model-data fusion to interpret past trends, and quantify uncertainties in future projections, of terrestrial ecosystem carbon cycling. Glob. Change Biol. 2012;18:2555–2569. [Google Scholar]
- Kleidon A., Fraedrich K., Low C. Multiple steady-states in the terrestrial atmosphere-biosphere system: a result of a discrete vegetation classification? Biogeosciences. 2007;4:707–714. [Google Scholar]
- Klein T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014;28:1313–1320. [Google Scholar]
- Klein T., Yakir D., Buchmann N., Grünzweig J.M. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014;201:712–716. doi: 10.1111/nph.12548. [DOI] [PubMed] [Google Scholar]
- Kocher P., Horna V., Leuschner C. Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits. Tree Physiol. 2013;33:817–832. doi: 10.1093/treephys/tpt055. [DOI] [PubMed] [Google Scholar]
- Konings A.G., Gentine P. Global variations in ecosystem-scale isohydricity. Glob. Change Biol. 2016 doi: 10.1111/gcb.13389. [DOI] [PubMed] [Google Scholar]
- Kottek M., Grieser J., Beck C., Rudolf B., Rubel F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006;15:259–263. [Google Scholar]
- Kucharik C.J., Barford C.C., El Maayar M., Wofsy S.C., Monson R.K., Baldocchi D.D. A multiyear evaluation of a Dynamic Global Vegetation Model at three AmeriFlux forest sites: vegetation structure, phenology, soil temperature, and CO2 and H2O vapor exchange. Ecol. Model. 2006;196:1–31. [Google Scholar]
- Kurz W.A., Dymond C.C., Stinson G., Rampley G.J., Neilson E.T., Carroll A.L., Ebata T., Safranyik L. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- Lachenbruch B., McCulloh K.A. Traits, properties, and performance: how woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant. New Phytol. 2014;204:747–764. doi: 10.1111/nph.13035. [DOI] [PubMed] [Google Scholar]
- Law R. Optimal life histories under age-specific predation. Am. Nat. 1979;114:399–417. [Google Scholar]
- Lawrence D.M., Oleson K.W., Flanner M.G., Thornton P.E., Swenson S.C., Lawrence P.J., Zeng X.B., Yang Z.L., Levis S., Sakaguchi K., Bonan G.B., Slater A.G. Parameterization improvements and functional and structural advances in version 4 of the community land model. J. Adv. Model. Earth Syst. 2011;3:27. [Google Scholar]
- Lawrence P.J., Chase T.N. Representing a new MODIS consistent land surface in the Community Land Model (CLM 3.0) J. Geophys. Res. Biogeosci. 2007;112:17. [Google Scholar]
- Le Quere C., Andres R.J., Boden T., Conway T., Houghton R.A., House J.I., Marland G., Peters G.P., van der Werf G.R., Ahlstrom A., Andrew R.M., Bopp L., Canadell J.G., Ciais P., Doney S.C., Enright C., Friedlingstein P., Huntingford C., Jain A.K., Jourdain C., Kato E., Keeling R.F., Goldewijk K.K., Levis S., Levy P., Lomas M., Poulter B., Raupach M.R., Schwinger J., Sitch S., Stocker B.D., Viovy N., Zaehle S., Zeng N. The global carbon budget 1959–2011. Earth Syst. Sci. Data. 2013;5:165–185. [Google Scholar]
- Lee J.E., Boyce K. Impact of the hydraulic capacity of plants on water and carbon fluxes in tropical South America. J. Geophys. Res. Atmos. 2010;115:13. [Google Scholar]
- Leuning R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995;18:339–355. [Google Scholar]
- Li R., Zhu S., Chen H.Y.H., John R., Zhou G., Zhang D., Zhang Q., Ye Q. Are functional traits a good predictor of global change impacts on tree species abundance dynamics in a subtropical forest? Ecol. Lett. 2015;18:1181–1189. doi: 10.1111/ele.12497. [DOI] [PubMed] [Google Scholar]
- Li Y.Y., Sperry J.S., Taneda H., Bush S.E., Hacke U.G. Evaluation of centrifugal methods for measuring xylem cavitation in conifers, diffuse- and ring-porous angiosperms. New Phytol. 2008;177:558–568. doi: 10.1111/j.1469-8137.2007.02272.x. [DOI] [PubMed] [Google Scholar]
- Liu G.F., Freschet G.T., Pan X., Cornelissen J.H.C., Li Y., Dong M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010;188:543–553. doi: 10.1111/j.1469-8137.2010.03388.x. [DOI] [PubMed] [Google Scholar]
- Loreau M., Naeem S., Inchausti P., Bengtsson J., Grime J.P., Hector A., Hooper D.U., Huston M.A., Raffaelli D., Schmid B., Tilman D., Wardle D.A. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- Loveland T.R., Reed B.C., Brown J.F., Ohlen D.O., Zhu Z., Yang L., Merchant J.W. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int. J. Remote Sens. 2000;21:1303–1330. [Google Scholar]
- Lyford W.H. Development of the root system of northern red oak. Quercus Rubra. Harv. For. Pap. 1980:3–30. [Google Scholar]
- Lyford W.H., Wilson B.F. Development of the root system of Acer rubrum L. Harv. For. Pap. 1964;10:1–17. [Google Scholar]
- Maherali H., Moura C.F., Caldeira M.C., Willson C.J., Jackson R.B. Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant Cell Environ. 2006;29:571–583. doi: 10.1111/j.1365-3040.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- Manzoni S., Vico G., Katul G.G., Palmroth S., Jackson R.B., Porporato A. Hydraulic limits on maximum plant transpiration and the emergence of the safety-efficiency trade-off. New Phytol. 2013;198:169–178. doi: 10.1111/nph.12126. [DOI] [PubMed] [Google Scholar]
- Maréchaux I., Bartlett M.K., Gaucher P., Sack L., Chave J. Causes of variation in leaf-level drought tolerance within an Amazonian forest. J. Plant Hydraul. 2016;3:e004. [Google Scholar]
- Martinez-Vilalta J., Poyatos R., Aguade D., Retana J., Mencuccini M. A new look at water transport regulation in plants. New Phytol. 2014;204:105–115. doi: 10.1111/nph.12912. [DOI] [PubMed] [Google Scholar]
- Matheny A.M., Bohrer G., Garrity S.R., Morin T.H., Howard C.J., Vogel C.S. Observations of stem water storage in trees of opposing hydraulic strategies. Ecosphere. 2015;6:165. [Google Scholar]
- Matheny A.M., Bohrer G., Stoy P.C., Baker I., Black A.T., Desai A.R., Dietze M., Gough C.M., Ivanov V.Y., Jassal R., Novick K., Schäfer K., Verbeeck H. Characterizing the diurnal patterns of errors in the prediction of evapotranspiration by several land-surface models: an NACP analysis. J. Geophys. Res. Biogeosci. 2014;119:1458–1473. [Google Scholar]
- Matheny A.M., Bohrer G., Vogel C.S., Morin T.H., He L., Frasson R.P.M., Mirfenderesgi G., Schäfer K.V.R., Gough C.M., Ivanov V.Y., Curtis P.S. Species-specific transpiration responses to intermediate disturbance in a northern hardwood forest. J. Geophys. Res. Biogeosci. 2014;119:2292–2311. [Google Scholar]
- Matheny A.M., Fiorella R.P., Bohrer G., Poulsen C.J., Morin T.H., Wunderlich A., Vogel C.S., Curtis P.S. Contrasting strategies of hydraulic control in two co-dominant temperate tree species. Ecohydrology. 2016 [Google Scholar]
- Matthes J.H., Goring S., Williams J.W., Dietze M.C. Benchmarking historical CMIP5 plant functional types across the Upper Midwest and Northeastern United States. J. Geophy. Res. Biogeosci. 2016;121:523–535. [Google Scholar]
- Maurer K.D., Bohrer G., Kenny W.T., Ivanov V.Y. Large-eddy simulations of surface roughness parameter sensitivity to canopy-structure characteristics. Biogeosciences. 2015;12:2533–2548. [Google Scholar]
- McCulloh K.A., Johnson D.M., Meinzer F.C., Voelker S.L., Lachenbruch B., Domec J.-C. Hydraulic architecture of two species differing in wood density: opposing strategies in co-occurring tropical pioneer trees. Plant Cell Environ. 2012;35:116–125. doi: 10.1111/j.1365-3040.2011.02421.x. [DOI] [PubMed] [Google Scholar]
- McDowell N., Pockman W.T., Allen C.D., Breshears D.D., Cobb N., Kolb T., Plaut J., Sperry J.S., West A., Williams D.G., Yepez E.A. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McDowell N.G., Fisher R.A., Xu C.G., Domec J.C., Holtta T., Mackay D.S., Sperry J.S., Boutz A., Dickman L., Gehres N., Limousin J.M., Macalady A., Martinez-Vilalta J., Mencuccini M., Plaut J.A., Ogee J., Pangle R.E., Rasse D.P., Ryan M.G., Sevanto S., Waring R.H., Williams A.P., Yepez E.A., Pockman W.T. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytol. 2013;200:304–321. doi: 10.1111/nph.12465. [DOI] [PubMed] [Google Scholar]
- McGill B.J., Enquist B.J., Weiher E., Westoby M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- McIntyre S., Lavorel S. A conceptual model of land use effects on the structure and function of herbaceous vegetation. Agric. Ecosyst. Environ. 2007;119:11–21. [Google Scholar]
- McMahon S.M., Harrison S.P., Armbruster W.S., Bartlein P.J., Beale C.M., Edwards M.E., Kattge J., Midgley G., Morin X., Prentice I.C. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol. Evol. 2011;26:249–259. doi: 10.1016/j.tree.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Meinzer F.C., Woodruff D.R., Marias D.E., McCulloh K.A., Sevanto S. Dynamics of leaf water relations components in co-occurring iso- and anisohydric conifer species. Plant Cell Environ. 2014;37:2577–2586. doi: 10.1111/pce.12327. [DOI] [PubMed] [Google Scholar]
- Messier J., McGill B.J., Lechowicz M.J. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 2010;13:838–848. doi: 10.1111/j.1461-0248.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- Miller G.R., Chen X.Y., Rubin Y., Ma S.Y., Baldocchi D.D. Groundwater uptake by woody vegetation in a semiarid oak savanna. Water Resour. Res. 2010;46:14. [Google Scholar]
- Mirfenderesgi G., Bohrer G., Matheny A.M., Fatichi S., Frasson R.P.M., Schäfer K.V.R. Tree level hydrodynamic approach for resolving aboveground water storage and stomatal conductance and modeling the effects of tree hydraulic strategy. J. Geophy. Res. Biogeosci. 2016;121:1792–1813. [Google Scholar]
- Moles A.T., Peco B., Wallis I.R., Foley W.J., Poore A.G.B., Seabloom E.W., Vesk P.A., Bisigato A.J., Cella-Pizarro L., Clark C.J., Cohen P.S., Cornwell W.K., Edwards W., Ejrnaes R., Gonzales-Ojeda T., Graae B.J., Hay G., Lumbwe F.C., Magana-Rodriguez B., Moore B.D., Peri P.L., Poulsen J.R., Stegen J.C., Veldtman R., Zeipel H., Andrew N.R., Boulter S.L., Borer E.T., Cornelissen J.H.C., Farji-Brener A.G., DeGabriel J.L., Jurado E., Kyhn L.A., Low B., Mulder C.P.H., Reardon-Smith K., Rodriguez-Velazquez J., De Fortier A., Zheng Z., Blendinger P.G., Enquist B.J., Facelli J.M., Knight T., Majer J.D., Martinez-Ramos M., McQuillan P., Hui F.K.C. Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 2013;198:252–263. doi: 10.1111/nph.12116. [DOI] [PubMed] [Google Scholar]
- Moncrieff G.R., Scheiter S., Slingsby J.A., Higgins S.I. Understanding global change impacts on South African biomes using dynamic vegetation models. South Afr. J. Bot. 2015;101:16–23. [Google Scholar]
- Moorcroft P.R. How close are we to a predictive science of the biosphere? Trends Ecol. Evol. 2006;21:400–407. doi: 10.1016/j.tree.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Musavi T., Mahecha M.D., Migliavacca M., Reichstein M., van de Weg M.J., van Bodegom P.M., Bahn M., Wirth C., Reich P.B., Schrodt F., Kattge J. The imprint of plants on ecosystem functioning: a data-driven approach. Int. J. Appl. Earth Obs. Geoinf. 2015;43:119–131. [Google Scholar]
- Neumann R.B., Cardon Z.G. The magnitude of hydraulic redistribution by plant roots: a review and synthesis of empirical and modeling studies. New Phytol. 2012;194:337–352. doi: 10.1111/j.1469-8137.2012.04088.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U., Keenan T.F., Hallik L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015;205:973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen E.T., Sharifi M.R., Rundel P.W., Jarrell W.M., Virginia R.A. Diurnal and seasonal water relations of the desert phreatophyte Prosopis glandulosa (honey mesquite) in the Sonoran Desert of California. Ecology. 1983;64:1381–1393. [Google Scholar]
- North G.B., Nobel P.S. Drought-induced changes in hydraulic conductivity and structure in roots of Ferocactus acanthods and Opuntia ficus-indica. New Phytol. 1992;120:9–19. [Google Scholar]
- Olano J.M., Arzac A., Garcia-Cervigon A.I., von Arx G., Rozas V. New star on the stage: amount of ray parenchyma in tree rings shows a link to climate. New Phytol. 2013;198:486–495. doi: 10.1111/nph.12113. [DOI] [PubMed] [Google Scholar]
- Oleson K.W., Lawrence D.M., Bonan G., Drewniak B., Koven C., Levis S., Li F., Riley W., Subin Z.M., Swenson S.C., Thornton P., Bozbiyik A., Fisher R., Heald C.L., Kluzek E., Lamarque J.-F., Lawrence P.J., Leung L.R., Lipscomb W., Muszala S., Ricciuto D.M., Sacks W.J., Sun Y., Tang J., Yang Z.L. 2013. Technical Description of Version 4.5 of the Community Land Model (CLM) p. 420. NCAR Technical Note, NCAR/TN-503+STR. [Google Scholar]
- Oren R., Sperry J.S., Katul G.G., Pataki D.E., Ewers B.E., Phillips N., Schafer K.V.R. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999;22:1515–1526. [Google Scholar]
- Ostle N.J., Smith P., Fisher R., Woodward F.I., Fisher J.B., Smith J.U., Galbraith D., Levy P., Meir P., McNamara N.P., Bardgett R.D. Integrating plant-soil interactions into global carbon cycle models. J. Ecol. 2009;97:851–863. [Google Scholar]
- Pappas C., Fatichi S., Burlando P. Modeling terrestrial carbon and water dynamics across climatic gradients: does plant trait diversity matter? New Phytol. 2016;209:137–151. doi: 10.1111/nph.13590. [DOI] [PubMed] [Google Scholar]
- Pappas C., Fatichi S., Rimkus S., Burlando P., Huber M.O. The role of local-scale heterogeneities in terrestrial ecosystem modeling. J. Geophys. Res. Biogeosci. 2015;120:341–360. [Google Scholar]
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- Pavlick R., Drewry D.T., Bohn K., Reu B., Kleidon A. The Jena Diversity-Dynamic Global Vegetation Model (JeDi-DGVM): a diverse approach to representing terrestrial biogeography and biogeochemistry based on plant functional trade-offs. Biogeosciences. 2013;10:4137–4177. [Google Scholar]
- Peel M.C., Finlayson B.L., McMahon T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007;11:1633–1644. [Google Scholar]
- Peng C.H., Guiot J., Wu H.B., Jiang H., Luo Y.Q. Integrating models with data in ecology and palaeoecology: advances towards a model-data fusion approach. Ecol. Lett. 2011;14:522–536. doi: 10.1111/j.1461-0248.2011.01603.x. [DOI] [PubMed] [Google Scholar]
- Peng H.H., Zhao C.Y., Feng Z.D., Xu Z.L., Wang C., Zhao Y. Canopy interception by a spruce forest in the upper reach of Heihe River basin, Northwestern China. Hydrol. Process. 2014;28:1734–1741. [Google Scholar]
- Perez-Harguindeguy N., Diaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., Bret-Harte M.S., Cornwell W.K., Craine J.M., Gurvich D.E., Urcelay C., Veneklaas E.J., Reich P.B., Poorter L., Wright I.J., Ray P., Enrico L., Pausas J.G., de Vos A.C., Buchmann N., Funes G., Quetier F., Hodgson J.G., Thompson K., Morgan H.D., ter Steege H., van der Heijden M.G.A., Sack L., Blonder B., Poschlod P., Vaieretti M.V., Conti G., Staver A.C., Aquino S., Cornelissen J.H.C. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013;61:167–234. [Google Scholar]
- Phillips N., Oren R., Zimmermann R. Radial patterns of xylem sap flow in non-, diffuse- and ring-porous tree species. Plant Cell Environ. 1996;19:983–990. [Google Scholar]
- Pitman A.J. The evolution of, and revolution in, land surface schemes designed for climate models. Int. J. Climatol. 2003;23:479–510. [Google Scholar]
- Pockman W.T., Sperry J.S. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am. J. Bot. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Poorter L., McDonald I., Alarcón A., Fichtler E., Licona J.-C., Peña-Claros M., Sterck F., Villegas Z., Sass-Klaassen U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010;185:481–492. doi: 10.1111/j.1469-8137.2009.03092.x. [DOI] [PubMed] [Google Scholar]
- Poulter B., Ciais P., Hodson E., Lischke H., Maignan F., Plummer S., Zimmermann N.E. Plant functional type mapping for earth system models. Geosci. Model Dev. 2011;4:993–1010. [Google Scholar]
- Powell T.L., Galbraith D.R., Christoffersen B.O., Harper A., Imbuzeiro H.M.A., Rowland L., Almeida S., Brando P.M., da Costa A.C.L., Costa M.H., Levine N.M., Malhi Y., Saleska S.R., Sotta E., Williams M., Meir P., Moorcroft P.R. Confronting model predictions of carbon fluxes with measurements of Amazon forests subjected to experimental drought. New Phytol. 2013;200:350–365. doi: 10.1111/nph.12390. [DOI] [PubMed] [Google Scholar]
- Pratt R.B., Jacobsen A.L., Ewers F.W., Davis S.D. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol. 2007;174:787–798. doi: 10.1111/j.1469-8137.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- Pratt R.B., Jacobsen A.L., Ramirez A.R., Helms A.M., Traugh C.A., Tobin M.F., Heffner M.S., Davis S.D. Mortality of resprouting chaparral shrubs after a fire and during a record drought: physiological mechanisms and demographic consequences. Glob. Change Biol. 2014;20:893–907. doi: 10.1111/gcb.12477. [DOI] [PubMed] [Google Scholar]
- Prentice I.C., Bondeau A., Cramer W., Harrison S.P., Hickler T., Lucht W., Sitch S., Smith B., Sykes M.T. Dynamic global vegetation modeling: quantifying terrestrial ecosystem responses to large-scale environmental change. In: Canadell J., Pataki D.E., Pitelka L., editors. Terrestrial Ecosystems in a Changing World. Springer; Berlin, Germany: 2007. pp. 175–192. [Google Scholar]
- Purves D.W., Lichstein J.W., Strigul N., Pacala S.W. Predicting and understanding forest dynamics using a simple tractable model. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17018–17022. doi: 10.1073/pnas.0807754105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillet A., Peng C.H., Garneau M. Toward dynamic global vegetation models for simulating vegetation-climate interactions and feedbacks: recent developments, limitations, and future challenges. Environ. Rev. 2010;18:333–353. [Google Scholar]
- Reich P.B. The world-wide 'fast-slow' plant economics spectrum: a traits manifesto. J. Ecol. 2014;102:275–301. [Google Scholar]
- Reich P.B., Wright I.J., Lusk C.H. Predicting leaf physiology from simple plant and climate attributes: a global GLOPNET analysis. Ecol. Appl. 2007;17:1982–1988. doi: 10.1890/06-1803.1. [DOI] [PubMed] [Google Scholar]
- Reichstein M., Bahn M., Mahecha M.D., Kattge J., Baldocchi D.D. Linking plant and ecosystem functional biogeography. Proc. Natl. Acad. Sci. 2014;111:13697–13702. doi: 10.1073/pnas.1216065111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L., da Costa A.C.L., Galbraith D.R., Oliveira R.S., Binks O.J., Oliveira A.A.R., Pullen A.M., Doughty C.E., Metcalfe D.B., Vasconcelos S.S., Ferreira L.V., Malhi Y., Grace J., Mencuccini M., Meir P. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature. 2015;528:119–122. doi: 10.1038/nature15539. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano J.M., Azcón R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995;95:472–478. [Google Scholar]
- Running S.W., Loveland T.R., Pierce L.L. A vegetation classification logic based on remote sensing for using in global biogeochemical models. Ambio. 1994;23:77–81. [Google Scholar]
- Sakschewski B., von Bloh W., Boit A., Rammig A., Kattge J., Poorter L., Penuelas J., Thonicke K. Leaf and stem economics spectra drive diversity of functional plant traits in a dynamic global vegetation model. Glob. Change Biol. 2015;21:2711–2725. doi: 10.1111/gcb.12870. [DOI] [PubMed] [Google Scholar]
- Sato H., Itoh A., Kohyama T. SEIB-DGVM: a new dynamic global vegetation model using a spatially explicit individual-based approach. Ecol. Model. 2007;200:279–307. [Google Scholar]
- Scheiter S., Langan L., Higgins S.I. Next-generation dynamic global vegetation models: learning from community ecology. New Phytol. 2013;198:957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- Schlesinger W.H., Jasechko S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014;189:115–117. [Google Scholar]
- Shellito C.J., Sloan L.C. Reconstructing a lost Eocene paradise, part II: on the utility of dynamic global vegetation models in pre-quaternary climate studies. Glob. Planet. Change. 2006;50:18–32. [Google Scholar]
- Siqueira M.B., Katul G., Porporato A. Onset of water stress, hysteresis in plant conductance, and hydraulic lift: scaling soil water dynamics from millimeters to meters. Water Resour. Res. 2008;44 W01432. [Google Scholar]
- Sitch S., Smith B., Prentice I.C., Arneth A., Bondeau A., Cramer W., Kaplan J.O., Levis S., Lucht W., Sykes M.T., Thonicke K., Venevsky S. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Change Biol. 2003;9:161–185. [Google Scholar]
- Skelton R.P., West A.G., Dawson T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. 2015;112:5744–5749. doi: 10.1073/pnas.1503376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Read D.J. third ed. Elsevier Academic Press Inc; San Diego: 2008. Mycorrhizal Symbiosis. [Google Scholar]
- Soudzilovskaia N.A., Elumeeva T.G., Onipchenko V.G., Shidakov, Salpagarova F.S., Khubiev A.B., Tekeev D.K., Cornelissen J.H.C. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18180–18184. doi: 10.1073/pnas.1310700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J.S. Hydraulic constraints on plant gas exchange. Agric. For. Meteorol. 2000;104:13–23. [Google Scholar]
- Sperry J.S., Alder N.N., Eastlack S.E. The effect of reduced hydraulic conductance on stomatal conductance and xylem cavitation. J. Exp. Bot. 1993;44:1075–1082. [Google Scholar]
- Sperry J.S., Nichols K.L., Sullivan J.E.M., Eastlack S.E. Xylem embolism in ring-porous, diffuse-porous and coniferous trees of northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Sperry J.S., Saliendra N.Z. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ. 1994;17:1233–1241. [Google Scholar]
- Sperry J.S., Sullivan J.E.M. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterck F., Markesteijn L., Schieving F., Poorter L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20627–20632. doi: 10.1073/pnas.1106950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker O. Die Abhängigkeit der Transpiration von den Umweltfaktoren. Pflanze und Wasser/Water Relations of Plants. In: Adriani M.J., Aslyng H.C., Burström H., Geiger R., Gessner F., Härtel O., Huber B., Hülsbruch M., Kalle K., Kern H., Killian C., Kisser J.G., Kramer P.J., Lemée G., Levitt J., Meyer B.S., Mothes K., Pisek A., Ruttner F., Stålfelt M.G., Stiles W., Stocker O., Stocking C.R., Straka H., Thornthwaite W.C., Troll C., Ullrich H., Veihmeyer F.J., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 1956. pp. 436–488. [Google Scholar]
- Strengers B.J., Muller C., Schaeffer M., Haarsma R.J., Severijns C., Gerten D., Schaphoff S., van den Houdt R., Oostenrijk R. Assessing 20th century climate-vegetation feedbacks of land-use change and natural vegetation dynamics in a fully coupled vegetation-climate model. Int. J. Climatol. 2010;30:2055–2065. [Google Scholar]
- Sun W.X., Liang S.L., Xu G., Fang H.L., Dickinson R. Mapping plant functional types from MODIS data using multisource evidential reasoning. Remote Sens. Environ. 2008;112:1010–1024. [Google Scholar]
- Taneda H., Sperry J.S. A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol. 2008;28:1641–1651. doi: 10.1093/treephys/28.11.1641. [DOI] [PubMed] [Google Scholar]
- Tardieu F. Will increases in our understanding of soil-root relations and root signaling substantially alter water flux models? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1993;341:57–66. [Google Scholar]
- Tardieu F., Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998;49:419–432. [Google Scholar]
- Thomsen J., Bohrer G., Matheny A.M., Ivanov V.Y., He L., Renninger H., Schäfer K. Contrasting hydraulic strategies during dry soil conditions in Quercus rubra and Acer rubrum in a sandy site in Michigan. Forests. 2013;4:1106–1120. [Google Scholar]
- Thuiller W., Albert C., Araujo M.B., Berry P.M., Cabeza M., Guisan A., Hickler T., Midgely G.F., Paterson J., Schurr F.M., Sykes M.T., Zimmermann N.E. Predicting global change impacts on plant species' distributions: future challenges. Perspect. Plant Ecol. Evol. Syst. 2008;9:137–152. [Google Scholar]
- Tognetti R., Longobucco A., Raschi A. Vulnerability of xylem to embolism in relation to plant hydraulic resistance in Quercus pubescens and Quercus ilex co-occurring in a Mediterranean coppice stand in central Italy. New Phytol. 1998;139:437–447. [Google Scholar]
- Trifilo P., Barbera P.M., Raimondo F., Nardini A., Lo Gullo M.A. Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiol. 2014;34:109–122. doi: 10.1093/treephys/tpt119. [DOI] [PubMed] [Google Scholar]
- Tyree M.T., Ewers F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991;119:345–360. [Google Scholar]
- Tyree M.T., Zimmermann M.H. Springer-Verlag New York Inc.; 175 Fifth Avenue, New York, NY: 2002. Xylem Structure and the Ascent of Sap. Xylem Structure and the Ascent of Sap. [Google Scholar]
- van Bodegom P.M., Douma J.C., Verheijen L.M. A fully traits-based approach to modeling global vegetation distribution. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13733–13738. doi: 10.1073/pnas.1304551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bodegom P.M., Douma J.C., Witte J.P.M., Ordonez J.C., Bartholomeus R.P., Aerts R. Going beyond limitations of plant functional types when predicting global ecosystem-atmosphere fluxes: exploring the merits of traits-based approaches. Glob. Ecol. Biogeogr. 2012;21:625–636. [Google Scholar]
- Verheijen L.M., Brovkin V., Aerts R., Bonisch G., Cornelissen J.H.C., Kattge J., Reich P.B., Wright I.J., van Bodegom P.M. Impacts of trait variation through observed trait-climate relationships on performance of an Earth system model: a conceptual analysis. Biogeosciences. 2013;10:5497–5515. [Google Scholar]
- Violle C., Navas M.L., Vile D., Kazakou E., Fortunel C., Hummel I., Garnier E. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- von Allmen E.I., Sperry J.S., Bush S.E. Contrasting whole-tree water use, hydraulics, and growth in a co-dominant diffuse-porous vs. ring-porous species pair. Trees. 2014:1–12. [Google Scholar]
- Wagner K.R., Ewers F.W., Davis S.D. Tradeoffs between hydraulic efficiency and mechanical strength in the stems of four co-occurring species of chaparral shrubs. Oecologia. 1998;117:53–62. doi: 10.1007/s004420050631. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Trudinger C.M., Enting I.G. A review of applications of model-data fusion to studies of terrestrial carbon fluxes at different scales. Agric. For. Meteorol. 2009;149:1829–1842. [Google Scholar]
- Weng E.S., Malyshev S., Lichstein J.W., Farrior C.E., Dybzinski R., Zhang T., Shevliakova E., Pacala S.W. Scaling from individual trees to forests in an Earth system modeling framework using a mathematically tractable model of height-structured competition. Biogeosciences. 2015;12:2655–2694. [Google Scholar]
- Werner C., Schnyder H., Cuntz M., Keitel C., Zeeman M.J., Dawson T.E., Badeck F.W., Brugnoli E., Ghashghaie J., Grams T.E.E., Kayler Z.E., Lakatos M., Lee X., Maguas C., Ogee J., Rascher K.G., Siegwolf R.T.W., Unger S., Welker J., Wingate L., Gessler A. Progress and challenges in using stable isotopes to trace plant carbon and water relations across scales. Biogeosciences. 2012;9:3083–3111. [Google Scholar]
- Williams J.E., Davis S.D., Portwood K. Xylem embolism in seedlings and resprouts of Adenostoma fasciculatum after fire. Aust. J. Bot. 1997;45:291–300. [Google Scholar]
- Woodcock D.W. Occurrence of woods with a gradation in vessel diameter across a ring. Iawa J. 1994;15:377–385. [Google Scholar]
- Wright I.J., Reich P.B., Cornelissen J.H.C., Falster D.S., Garnier E., Hikosaka K., Lamont B.B., Lee W., Oleksyn J., Osada N., Poorter H., Villar R., Warton D.I., Westoby M. Assessing the generality of global leaf trait relationships. New Phytol. 2005;166:485–496. doi: 10.1111/j.1469-8137.2005.01349.x. [DOI] [PubMed] [Google Scholar]
- Wright I.J., Reich P.B., Westoby M., Ackerly D.D., Baruch Z., Bongers F., Cavender-Bares J., Chapin T., Cornelissen J.H.C., Diemer M., Flexas J., Garnier E., Groom P.K., Gulias J., Hikosaka K., Lamont B.B., Lee T., Lee W., Lusk C., Midgley J.J., Navas M.L., Niinemets U., Oleksyn J., Osada N., Poorter H., Poot P., Prior L., Pyankov V.I., Roumet C., Thomas S.C., Tjoelker M.G., Veneklaas E.J., Villar R. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wullschleger S.D., Epstein H.E., Box E.O., Euskirchen E.S., Goswami S., Iversen C.M., Kattge J., Norby R.J., van Bodegom P.M., Xu X.F. Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 2014;114:1–16. doi: 10.1093/aob/mcu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Medvigy D., Powers J.S., Becknell J.M., Guan K. Diversity in plant hydraulic traits explains seasonal and inter-annual variations of vegetation dynamics in seasonally dry tropical forests. New Phytol. 2016;121:80–95. doi: 10.1111/nph.14009. [DOI] [PubMed] [Google Scholar]
- Yang Y.Z., Zhu Q.A., Peng C.H., Wang H., Chen H. From plant functional types to plant functional traits: a new paradigm in modelling global vegetation dynamics. Prog. Phys. Geogr. 2015;39:514–535. [Google Scholar]
- Yu Q., Epstein H.E., Walker D.A., Frost G.V., Forbes B.C. Modeling dynamics of tundra plant communities on the Yamal Peninsula, Russia, in response to climate change and grazing pressure. Environ. Res. Lett. 2011;6:12. [Google Scholar]
- Zanne A.E., Westoby M., Falster D.S., Ackerly D.D., Loarie S.R., Arnold S.E., Coomes D.A. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010;97:207–215. doi: 10.3732/ajb.0900178. [DOI] [PubMed] [Google Scholar]