Abstract
Aim
The aim of the present study was to explore the adjunctive use of Acacia arabica gel in the treatment of chronic periodontitis.
Methods
Single centre, randomised, triple blind, controlled trial on mild to moderate chronic periodontitis patients; Group I (SRP + Acacia arabica, n = 40) and Group II (SRP + placebo, n = 40); were analysed for clinical improvements in periodontal pocket depth (PPD) and clinical attachment levels (CAL) at baseline, 15 and 90 days on application of gels. Gingival index and plaque index were assessed as secondary parameters.
Results
Statistically significant PPD reduction (p < .05) and CAL gain (p < .05) was observed with use of Acacia arabica gel. The reduction in sites with moderate PPD was observed more among Group I than Group II and the difference was statistically significant (p = .001). Secondary outcome variables; Plaque Index and Gingival Index showed better resolution with Acacia arabica gel.
Conclusion
Acacia arabica leads to better clinical outcomes in patients with mild to moderate chronic periodontitis with effective antiplaque and anti-gingivitis action. It may be recommended adjunct to SRP for maintenance in patients with mild to moderate chronic periodontitis.
Keywords: Acacia gum, Anti plaque, Anti gingivitis, Periodontitis, Periodontal pocket, Scaling and root planing, Treatment
1. Introduction
Chronic periodontitis is an inflammatory disease of the periodontium which is multifactorial. Bacterial plaque is the major etiological factor. Bacteria and their endotoxins play a significant role in periodontal breakdown (Cobb, 1996).
Thorough subgingival debridement is the cornerstone of non-surgical periodontal therapy in controlling subgingival microflora. Its effectiveness decreases as the probing pocket depth (PPD) increases, especially as PPD exceeds 5 mm (Cobb, 1996). Supragingival plaque play a contributory role in increasing subgingival bacterial populations (Mousques et al., 1980, Braatz et al., 1985, Sbordone et al., 1990, Pedrazzoli et al., 1992). Effective supragingival plaque control is important in controlling the quantity, composition and rate of subgingival plaque formation and maturation (Dahlen et al., 1992, Hellstrom et al., 1996). It is difficult to achieve long term control over inflammatory periodontal diseases (Lindhe and Nyman, 1984, Knowles et al., 1979, Axelsson and Lindhe, 1981).
Various adjunctive therapies such as chemotherapeutic agents, local bisphosphonates (Akram et al., 2016), statins (Pradeep and Thorat, 2010), lasers and photodynamic therapy (Akram et al., 2016 Apr 1, Abduljabbar et al., 2017) have been employed along with mechanical plaque control regimen (Mandel, 1988). These therapies have shown variable but promising results.
Natural herbal products have been tested for their antiplaque and antibacterial activity in periodontal diseases. Acacia arabica (AA), commonly used in India as chewing stick (‘Babul’ or ‘Kikar’ datun) is one of such plant. The gum of AA has been used by many communities in daily oral hygiene regimen (Tyler et al., 1977). The composition consists of arabica which is a complex mixture of calcium, magnesium and potassium salts of arabic acid. Other constituents are tannins, cyanogenic glycosides, oxidases, peroxidases and pectinases with documented individual antimicrobial properties (Kirtikar and Basu, 1984). In vitro study provides evidence for the antibacterial and antiprotease activities of AA (Clark et al., 1993).
The gold standard adjunct to scaling and root planing (SRP) is chlorhexidine (CHX) (Addy, 1986). Clinical trials comparing AA gum with chlorhexidine have proved its equivalence in plaque inhibition, microbial count reduction and gingivitis resolution without associated adverse effects of CHX (Pradeep et al., 2010, Pradeep et al., 2012). Therefore long term use of AA can be recommended.
The lacuna in evidence which remains is whether AA gum through inhibition of supragingival plaque would be effective against control of chronic periodontitis. The other hypothetical question is whether certain sites inaccessible to SRP and mechanical plaque control may show better resolution through an adjunctive local application of AA. Clinical trial on the effect of AA on chronic periodontitis is a novel study to the best of our knowledge.
This study was conducted with the objective to analyse the adjunctive effect of AA on clinical parameters in mild to moderate chronic periodontitis patients.
2. Methods
2.1. Study design
Pilot, triple-blind, placebo-controlled randomised clinical trial.
Study is approved by Institutional ethics committee of King George’s Medical University’s (KGMU). The present study is in accordance to the Declaration of Helsinki as revised in 2013.
The trial was registered with the Primary Registries in the WHO Registry Network (CTRI/2013/09/004013). Changes in the trial design after ethical approval and trial registration was limited to additional blinding of the statistician.
Participants meeting the eligibility criteria were selected and randomised from the patients coming to the outpatient department of Periodontology, KGMU.
Eighty subjects of both genders aged 18–70 years were recruited in the period from 22nd February 2012 to July 2014. Clinical data collection was completed by November 2014.
2.2. Eligibility criteria
Inclusion: (i) subjects of both gender aged between 18 and 70 years, (ii) in good systemic health condition (iii) with mild to moderate chronic Periodontitis (dentition with at least 30% sites exhibiting attachment loss with pocket depth range of 4–6 mm), having minimum of five natural teeth in each quadrant. Exclusion: (i) Subject with history of tobacco chewing/ smoking, any known clinically significant hematological, endocrine, hepatic, renal, cardiovascular, cerebrovascular or psychiatric disease, (ii) on prohibited medication interfering with the efficacy and safety objectives of the trial, (iii) participant in any clinical study in past 6 months.
Subjects were informed regarding the study’s purpose, duration, implications, potential risks and benefits. Willing participants were asked to sign a written informed consent form.
2.3. Sample size calculation
Power analysis for sample calculation was done prior to subject’s enrolment for the trial. Assuming a 1 mm difference in Clinical attachment level (CAL) and using 0.8 mm as standard deviation power analysis revealed that 28 patients (total 56 patients in both groups) were required in each group for a t-test power level of 85% with significance level of 0.95 (Christodoulides et al., 2008). Recruitment of additional patients accounting noncompliance and follow up dropouts were done.
2.4. Clinical periodontal assessment
Baseline examination consisted of recording Periodontal probing depth (PPD) and CAL (at four sites per tooth: mesial, buccal, distal and lingual); Plaque Index (Loe, 1967); dichotomous recording of bleeding on probing (BOP) (Akram et al., 2017) and Gingival index (GI) (Loe, 1967). Positive reading meant bleeding occurrence within 15 s of pocket probing.
PPD was defined as the distance between the gingival margin (GM) and the bottom of the probeable pocket, to the nearest whole millimetre. CAL was calculated as the distance between the cemento enamel junction (CEJ) and the bottom of the probeable pocket, to the nearest whole millimetre.
All measurements were done using the manual periodontal probe with William’s marking (University of Michigan ‘O’ probe, Hu Friedy, Chicago, IL, USA) and the pressure sensitive probe (FP32, Florida probe Corporation, Gainesville, FL, USA).
Clinical periodontal parameters were assessed at baseline, 15 days and 90 days after SRP.
2.5. Outcome variables
Primary outcome variables are probing pocket depth reduction and clinical attachment level. Secondary outcome variables are bleeding on probing, change in clinical gingival health and change in plaque scores.
2.6. Intraexaminer validity
Periodontal assessment was performed by one calibrated examiner (PR).
The examiner evaluated the subjects on two occasions at 0 and 2 days. Calibration was validated as 90% of the readings were reproduced within a 1.0 mm difference. The same calibration was validated on manual and Florida probe system for intra-examiner reproducibility. These recordings were done by a single investigator (PR).
2.7. SRP and the use of the gel
After baseline recordings, thorough scaling and root planing (SRP) was performed similarly for all patients by a single periodontist (RS). The periodontal scaling was performed on two consecutive sittings using an ultrasonic scaler under saline irrigation followed by root planing using hand instruments.
The area for application of the treatment/ placebo gel was then demarcated. The subjects were randomised into two groups and asked to use the given gel on a set of 2–4 teeth (premolars and molars) in one specified quadrant. These were then termed as the test teeth.
2.8. Blinding
Enrolled participants in both arms, treating periodontologists, study coordinators and analysing statistician were blinded to treatment.
2.9. Randomisation and allocation concealment
AA and placebo gel were supplied as similarly labelled identical plain white tubes pre-coded by the manufacturer intermixed in the supplied box. Box was so sealed as to allow only hand space for tube retrieval and dispensation randomly. An investigator blinded to patient’s clinical data dispensed the tubes (ST).
For further allocation concealment, another investigator (RK) totally unaware of the study design, patient’s condition and other details maintained the records of the codes. New gel tube was provided every 15 days. Same investigator dispensed new gel after matching the tube code.
2.10. Product description and usage protocol
Coded tubes contained either AA gel or placebo. Gels were similar in taste, consistency, colour and texture.
Gel usage instructions were given by investigator involved in active treatment (RS). Patients were instructed to apply the gel on the gingiva overlying the test teeth after tooth brushing, twice daily; morning and evening. Gel was to be massaged over the area for 1 min such that it seeps into the gingival sulcus. Patients were told not to rinse after gel application and avoid eating for an hour post application of gel.
Patients were instructed to bring the tubes on recall visits which were every 15 days to check for compliance. New gel tubes were then dispensed. Patients were asked regarding the general health changes, ulceration, and soreness in treatment application area, taste perturbation or any other adverse events by the treatment examiner (RS). Assessment was also done for any colour changes or staining of the teeth and the gingiva.
2.11. Statistical analysis
Blinded statistician was provided the coded data. Analysis was done between two coded groups. After statistical analysis the codes of the tubes were broken as provided by the manufacturer in the sealed opaque envelope at the time of product delivery. The SRP + AA gel was assigned Group I and SRP + placebo gel was assigned as Group II. Results were further interpreted on this basis.
Statistical analysis consisted of testing the time bound intragroup and intergroup differences between active and placebo group. The data set was tested for normality of distribution.
Qualitative data was assessed using chi square test. Intragroup comparison for continuous variables was done using the student ‘t’ test. Intergroup comparison for the research question; whether the post-test means, adjusted for pre-test scores, differ between the two groups; was done using analysis of covariance (ANCOVA).
3. Results
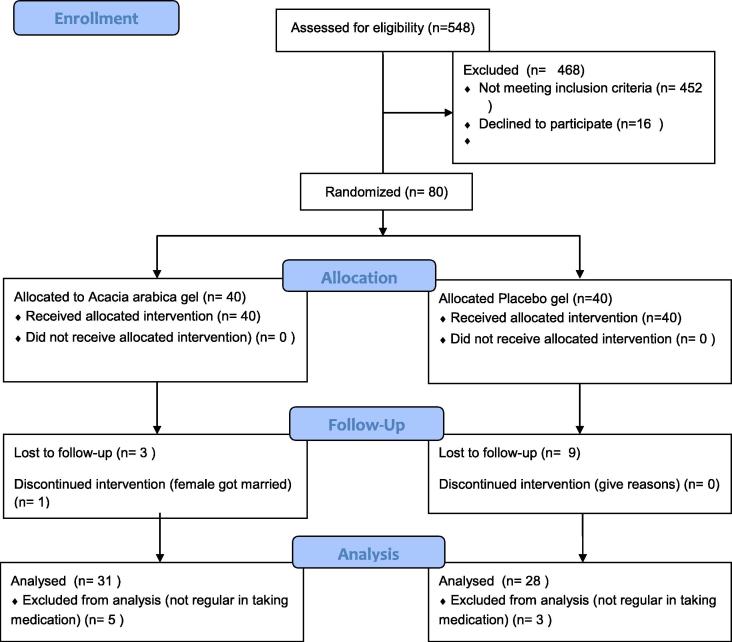
Flow chart for the study is shown in Fig. 1. Thirteen patients were lost to follow up (Group I: 4; Group II: 9) and eight dropped from analysis due to non-compliance of treatment protocol (Group I: 5; Group II: 3).
Fig. 1.
Flow diagram.
Final analysis was done for 59 patients. Group I consisted of 31 patients with 102 teeth assessed at 408 sites. Group II consisted of 28 patients with assessment done for 89 teeth at 356 sites.
Adverse events were not reported by patients or observed by investigators on continuous gel usage. Demographic data is shown in Table 1.
Table 1.
Demographical data.
| Parameters | Group I (N = 31) |
Group II (N = 28) |
P Value |
|---|---|---|---|
| Age (yrs) | *0.037 | ||
| Mean | 33.23 | 39.11 | |
| SD | 09.24 | 11.62 | |
| Range | 18.00–55.00 yrs | 20.00–60.00 yrs | |
| Sex (%) | |||
| Male | 16 (51.6) | 16 (57.1) | 0.670 |
| Female | 15 (48.4) | 12 (42.9) | NS |
NS Not Significant.
Significant
3.1. Primary outcomes
3.1.1. Probing pocket depth
Mean baseline PPD was similar in both the groups (Table 2). Data was analysed in two parts; mild pocket areas: 0–4 mm and moderate pocket sites: 4–6 mm.
Table 2.
Comparison of change in mean percentage of sites with pocket probing depth between two groups.
| Duration in days | Mean percentage of sites with pocket probing depth |
P Value (GROUP I vs II) |
||||
|---|---|---|---|---|---|---|
| Group I (N = 102) |
Group II (N = 085) |
|||||
| % site PPD 0–4 (in mm) |
% site PPD 4–6 (in mm) |
% site PPD 0–4 (in mm) |
% site PPD 4–6 (in mm) |
% site PPD 0–4 (in mm) |
% site PPD 4–6 (in mm) |
|
| Baseline (Mean ± SD) |
52.21 ± 22.55 | 47.79 ± 22.55 | 48.82 ± 26.70 | 51.18 ± 26.70 | 0.355 NS |
0.355 NS |
| 15 (Mean ± SD) |
73.28 ± 24.18 | 26.72 ± 24.18 | 67.06 ± 25.94 | 32.94 ± 25.94 | ||
| 90 (Mean ± SD) |
94.12 ± 11.76 | 05.88 ± 11.76 | 75.29 ± 21.30 | 24.71 ± 21.30 | ||
| Mean Diff. between Baseline – 15 days (P value) |
*21.07 ± 21.90 (0.001) |
*−21.07 ± 21.90 (0.001) |
*18.24 ± 22.62 (0.001) |
*−18.24 ± 22.62 (0.001) |
0.387 NS |
0.388 NS |
| Mean change between groups (B – D15) |
2.83 | 2.83 | ||||
| Mean Diff. between Baseline – 90 days (P value) |
*41.91 ± 23.90 (0.001) |
*−41.91 ± 23.90 (0.001) |
*26.47 ± 27.37 (0.001) |
*−26.47 ± 27.37 (0.001) |
*0.001 | *0.001 |
| Mean change between groups (B – D90) |
15.44 | 15.44 | ||||
NS Not Significant.
Significant.
The change between the groups in mean percentage of sites with PPD 0–4 mm and 4–6 mm was 2.83% respectively which is comparable and non-significant (p = .387).
The reduction in sites with moderate PPD was observed more among Group I than Group II and the difference was statistically significant (p = .001).
3.1.2. Clinical attachment level
Clinical attachment level was divided into 3 groups for analysis: CAL between 0–4 mm, between 4–6 mm and >6 mm. Teeth with PPD between 4–6 mm with recession present had CAL >6 mm. Baseline mean percentage of sites with CAL is shown in Table 3.
Table 3.
Comparison of change in mean percentage of sites with cal between two groups.
| Duration in days | Mean percentage of sites with CAL |
P Value (GROUP I vs II) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group I (N = 102) |
Group II (N = 085) |
||||||||
| % site CAL 0–4 |
% site CAL 4–6 |
% site CAL >6 |
% site CAL 0–4 |
% site CAL 4–6 |
% site CAL >6 |
% site CAL 0–4 |
% site CAL 4–6 |
% site CAL >6 |
|
| Baseline (Mean ± SD) |
25.49 ± 24.11 | 44.85 ± 23.43 | 29.66 ± 22.73 | 27.06 ± 24.76 | 45.59 ± 29.42 | 27.35 ± 23.35 | 0.662 NS |
0.851 NS |
0.496 NS |
| 15 (Mean ± SD) |
42.89 ± 30.54 | 48.04 ± 28.51 | 09.07 ± 13.98 | 37.65 ± 26.34 | 56.47 ± 28.12 | 05.88 ± 13.72 | |||
| 90 (Mean ± SD) |
64.22 ± 28.04 | 35.05 ± 27.53 | 00.74 ± 04.24 | 39.20 ± 27.93 | 54.63 ± 30.65 | 06.17 ± 13.42 | |||
| Mean Diff. between Baseline – 15 days (P value) |
*17.40 ± 23.81 (0.001) |
03.19 ± 35.30 (0.393) NS |
*20.59 ± 22.36 (0.001) |
*10.59 ± 15.61 (0.001) |
*10.88 ± 27.13 (0.001) |
*21.47 ± 22.54 (0.001) |
*0.020 | 0.094 NS |
0.789 NS |
| Mean change between groups (B – D15) | 06.81 | −07.69 | −00.88 | ||||||
| Mean Diff. between Baseline – 90 days (P value) |
*38.73 ± 25.52 (0.001) |
*−09.80 ± 35.21 (0.006) |
*28.92 ± 23.00 (0.001) |
*12.65 ± 20.20 (0.001) |
*09.57 ± 27.55 (0.002) |
*22.22 ± 25.22 (0.001) |
*0.001 | 0.961 NS |
0.065 NS |
| Mean change between groups (B – D90) |
26.08 | 00.23 | 06.70 | ||||||
NS Not Significant.
Significant.
Percentage of sites with CAL >6 mm at day 15 and day 90 was comparable for Group I and II with statistically non-significant difference.
The percentage of sites with CAL 4–6 mm, at day 90 in both groups showed a significant reduction. On intergroup comparisons, both the groups had comparable CAL gain in sites with CAL 4–6 mm at day 15 and 90 (p = .094; 0.961).
Mean percentage of sites with CAL 0–4 showed statistically significant gain in Group 1 compared to Group II.
3.2. Secondary variables
3.2.1. Plaque index (PI)
PI was assessed in groups of 0–2 and 2–3 (Table 4).
Table 4.
Comparison of change in mean percentage of sites with plaque index between two groups.
| Duration in days | Mean percentage of sites with Plaque index |
P Value (Group I Vs II) |
||||
|---|---|---|---|---|---|---|
| Group I (N = 102) |
Group II (N = 089) |
|||||
| % site PI 0–2 | % site PI 2–3 | % site PI 0–2 | % site PI 2–3 | % site PI 0–2 | % site PI 2–3 | |
| Baseline (Mean ± SD) |
60.78 ± 29.33 | 35.78 ± 29.54 | 62.08 ± 29.94 | 33.99 ± 29.97 | 0.763 NS |
0.679 NS |
| 15 (Mean ± SD) |
01.47 ± 06.88 | 00.00 ± 00.00 | 00.56 ± 03.73 | 00.00 ± 00.00 | ||
| 90 (Mean ± SD) |
02.21 ± 07.13 | 00.00 ± 00.00 | 42.70 ± 32.68 | 04.21 ± 15.19 | ||
| Mean Diff. between Baseline – 15 days (P value) |
*−59.31 ± 32.42 (0.001) |
*−35.78 ± 29.54 (0.001) |
*−61.52 ± 29.69 (0.001) |
*−33.99 ± 29.97 (0.001) |
0.623 NS |
0.679 NS |
| Mean change between groups (B – D15) |
−2.21 | 01.79 | ||||
| Mean Diff. between Baseline – 90 days (P value) |
*−58.57 ± 30.36 (0.001) |
*−35.78 ± 29.54 (0.001) |
*−19.38 ± 44.55 (0.001) |
*−29.78 ± 30.82 (0.001) |
*0.001 | 0.173 NS |
| Mean change between groups (B – D90) |
39.19 | 06.00 | ||||
By Student‘t’ test.
NS = Not Significant.
By ANCOVA.
Significant.
At the end of 90 days, mean percentage of sites with PI 0–2 and 2–3 showed a change of 58.57% and 35.78% for Group I and 19.38% and 29.78% for Group II respectively from baseline.
3.2.2. Gingival index
At 90 days, mean percentage of sites with GI 0, 1 and ≥2 showed significant change of 43.14%, 40.93% and 84.07% respectively for Group I and change of 6.74%, 36.80% and 43.54% respectively for Group II (Table 5).
Table 5.
Comparison of change in mean percentage of sites with gingival index between two groups.
| Duration in days | Mean percentage of sites with gingival index ( ± SD) |
P Value (Group I Vs II) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group I (N = 102) |
Group II (N = 089) |
||||||||
| % site GI 0 | % site GI 1 | % site GI ≥2 | % site GI 0 | % site GI 1 | % site GI ≥2 | % site GI 0 | % site GI 1 | % site GI ≥2 | |
| Baseline (Mean ± SD) |
00.00 ± 00.00 | 07.60 ± 18.55 | 92.40 ± 18.55 | 01.69 ± 11.18 | 06.74 ± 19.13 | 91.57 ± 21.63 | 0.155 NS |
0.754 NS |
0.777 NS |
| 15 (Mean ± SD) |
46.81 ± 24.79 | 48.78 ± 23.70 | 04.41 ± 10.20 | 28.65 ± 30.75 | 62.64 ± 31.56 | 08.71 ± 15.12 | |||
| 90 (Mean ± SD) |
43.14 ± 28.40 | 48.53 ± 27.66 | 08.33 ± 16.63 | 08.43 ± 20.62 | 43.54 ± 29.56 | 48.03 ± 32.90 | |||
| Mean Diff. between Baseline – 15 days (P value) |
*46.81 ± 24.79 (0.001) |
*41.18 ± 31.20 (0.001) |
*−87.99 ± 20.73 (0.001) |
*26.96 ± 28.76 (0.001) |
*55.90 ± 40.42 (0.001) |
*−82.87 ± 23.72 (0.001) |
*0.001 | *0.006 | 0.116 NS |
| Mean change between groups (B – D15) |
19.85 | −14.72 | 5.12 | ||||||
| Mean Diff. between Baseline – 90 days (P value) |
*43.14 ± 28.40 (0.001) |
*40.93 ± 34.61 (0.001) |
*−84.07 ± 27.88 (0.001) |
*06.74 ± 17.98 (0.001) |
*36.80 ± 35.17 (0.001) |
*−43.54 ± 37.60 (0.001) |
*0.001 | 0.416 NS |
*0.001 |
| Mean change between groups (B – D90) |
36.4 | 4.13 | 40.53 | ||||||
By Student‘t’ test.
NS = Not Significant.
By ANCOVA.
S = Significant.
4. Discussion
Within its limitations, this triple blind placebo controlled randomised clinical trial provides evidence for improvement in clinical parameters through adjunctive use of AA containing gel in mild to moderate chronic periodontitis patients. Significant probing pocket depth reduction and CAL gain was observed with adjunctive use of AA gel. Secondary parameters of plaque control and BOP showed better outcomes in AA gel group compared to SRP alone.
The shift in pocket probing depths for both the groups from sites with moderate pocket depth 4–6 mm towards sites with mild pocket depths 0–4 mm occurred on day 15 and day 90 (Table 2). This shift was comparable in both the groups at day 15 but more in Group I compared to Group II on day 90 (p = .001). Poor supragingival plaque control may lead to reestablishment of the subgingival microbiota within 40–60 days following subgingival debridement (Sbordone et al., 1990). AA gel has proven efficacy against supragingival plaque (Pradeep et al., 2012, Gazi, 1991, Tangade et al., 2012). An in vitro study by Clark et al. revealed protease activity inhibition in the presence of 0.5% w/v of AA gum sonicates (Clark et al., 1993). Trypsin-like activities of Porphyromonas gingivalis and Prevotella intermedia were found to be highly sensitive to AA gum (Clark et al., 1993). Detectable levels of P gingivalis and P intermedia are associated with chronic periodontal disease progression and their elimination by therapy leads to improved clinical response (Dzink et al., 1988, Christersson et al., 1991, Slots and Listgarten, 1988, Haffajee et al., 1997). Greater PPD reduction in Group I could be attributed to the antiprotease inhibition of P gingivalis and P intermedia by AA gel.
The results of the systematic reviews comparing the use of local adjuncts to SRP in periodontal disease have shown local chemotherapeutics to be modestly effective in PPD reduction than SRP alone (Bonito et al., 2005, Cosyn and Wyn, 2006). Microbiological and biomarker studies may provide further evidence for the clinical efficacy of AA gel seen in the present clinical trial.
The mean percentages of sites with CAL from >6 mm and 4–6 mm showed CAL gain. Mean sites with CAL 0–4 mm showed further CAL gain in both the groups at analysed two time periods. The studies related to the use of local adjuncts to SRP in a systematic review had shown CAL gains to be smaller and statistical significance less common (Bonito et al., 2005). Marginal improvements in CAL were found to be a fraction compared to SRP alone (Bonito et al., 2005).
At 90 day analysis, both groups showed good plaque control (PI 0–2). The mean percentage of sites was significantly more in Group I than group II. Not all patients may respond well to therapy or maintain stable oral hygiene regimen over extended periods of time following successful therapy (Wilson, 2000, Wilson, 2000), explaining the equal distribution in both groups of subjects with poor plaque scores (PI 2–3).
Increase in number of sites with PI 0–2 suggests anti plaque activity of AA gel. Improvements seen are similar to various studies utilising AA gum as toothpaste, chewing gum, gel and powder (Pradeep et al., 2010, Pradeep et al., 2012, Gazi, 1991, Tangade et al., 2012). PI scores with local application of AA gel are proven to be comparable to local CHX use (Pradeep et al., 2010, Pradeep et al., 2012). The anti-plaque activity of AA may also be related to the decrease in PPD in Group I (Dahlen et al., 1992, Hellstrom et al., 1996).
At the end of 90 days increased sites in Group I showed improved GI status compared to Group II. AA gel is associated with reduction in microbial counts of Streptococcus sanguis, S mitis, S intermedius, S oralis, Actinomyces viscosus and A naeslundii (Pradeep et al., 2012). These are gram positive bacteria associated with gingivitis (Moore and Moore, 1994). Their reduction would result in resolution of gingival inflammation and hence improved GI status (Bernimoulin, 2003). Further, these bacteria are associated with early plaque colonisation (Li et al., 2004). Their reduction may also be the reason for improved PI status in Group I.
Bleeding on Probing showed significant reduction in groups at day 15 which was sustained for Group I at Day 90. Improvement in BOP may be related to decreased plaque accumulation and better gingival status (Pradeep et al., 2010, Pradeep et al., 2012, Gazi, 1991). Study using AA containing toothpaste showed similar reduction in BOP at 28 days re-evaluation (Tangade et al., 2012). Tannins, a constituent of AA gum have astringent and hemostatic properties (Bentley and Trimen, 1983) and may lead to improvement in BOP and GI status. Reduction in number of sites with P gingivalis activity also leads to reduction in BOP (Osamu et al., 2002, Tetsuro et al., 2008).
Mild to moderate periodontitis cases were assessed for localised response of the gel in the present study. The role of supragingival plaque control has been proven to be effective in mild to moderate cases but not on advanced periodontal disease (Kaldahl et al., 1996, Westfelt et al., 1998). In deep pockets, local penetration of the gel was not possible. Other variables in deep pockets may have served as study confounders. Studies have revealed areas in deep pockets to be prone to residual plaque and calculus even after thorough scaling and root planing (Hunter et al., 1984, Breininger et al., 1987, Fleischer et al., 1989, Takacs et al., 1993, Kocher et al., 1998). Non-surgical periodontal therapy alone is considered ineffective in patients with severe periodontitis exceeding PPD >6 mm (Cobb, 1996).
The strengths of the study is that it is the first study utilising AA gum gel for analysis as an adjunct to SRP in control and maintenance of mild to moderate periodontitis patients. This clinical trial was designed according to CONSORT 2010 guidelines considering the suggestions laid by other researchers on trial design in non-surgical periodontal therapy (Jeffcoat, 1992, Pihlstrom, 1997, Cobb, 2002).
The limitations of this study are that it does not assess bone defect fill. Radiographic assessment for defect fill could have been more representative of true periodontal healing. Another limitation is the short follow-up period.
5. Conclusion
Within its limitations, the trial has shown clinical improvement in Probing pocket depth, clinical attachment level, plaque index, gingival index and bleeding on probing. Further studies exploring AA gum as local drug delivery agent and its properties of susbstantivity, microbial inhibitions, effect on inflammatory markers may be beneficial for long term maintenance of periodontitis patients.
Acknowledgments
Acknowledgements
The authors acknowledge Mr. Ashish Agarwal and Prof. Nandlal for their valuable support.
Conflict of interest and source of funding statement
The authors declare that they do not have a conflict of interest. This study was partially supported by Charak Pharmaceuticals (India). The company was responsible for supplying coded, similarly packaged placebo and Acacia arabica gel (GUMTONE). The contribution besides the material supply was none whatsoever. The company was not involved in protocol designing or study content. The Company did not read or approve the manuscript before submission and was not aware of its wording or results. The company was also not involved in data collection, data analysis or data interpretation. The study is registered by R. Singhal in the Central Trial registry of India (CTRI/2013/09/004013).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abduljabbar T., Javed F., Shah A., Samer M.S., Vohra F., Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med. Sci. 2017;32:449–459. doi: 10.1007/s10103-016-2086-5. [DOI] [PubMed] [Google Scholar]
- Addy M. Chlorhexidine compared with other locally delivered antimicrobials. A short review. J. Clin. Periodontol. 1986;13(10):957–964. doi: 10.1111/j.1600-051x.1986.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Akram Z., Abduljabbar T., Kellesarian S.V., Hassan A., Ibrahim M., Javed F., Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br. J. Clin. Pharmacol. 2016;83:444–454. doi: 10.1111/bcp.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram Z., Al-Shareef S.A., Daood U., Asiri F.Y., Shah A.H., AlQahtani M.A., Vohra F., Javed F. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomed. Laser Surgery. 2016 Apr 1;34(4):137–149. doi: 10.1089/pho.2015.4076. [DOI] [PubMed] [Google Scholar]
- Akram Z., Baharuddin N.A., Vaithilingam R.D., Rahim Z.H., Chinna K., Krishna V.G., Saub R., Safii S.H. Effect of nonsurgical periodontal treatment on clinical periodontal variables and salivary resistin levels in obese Asians. J. Oral Sci. 2017;59:93–102. doi: 10.2334/josnusd.16-0127. [DOI] [PubMed] [Google Scholar]
- Axelsson P., Lindhe J. The significance of maintenance care in the treatment of periodontal disease. J. Clin. Periodontol. 1981;8:281–294. doi: 10.1111/j.1600-051x.1981.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Bentley, R., Trimen, H., 1983. Medicinal plants. Indian edition. Churchill Volume II, London, p. 94.
- Bernimoulin J.P. Recent concepts in plaque formation. J. Clin. Periodontol. 2003;30(Suppl. 5):7–9. doi: 10.1034/j.1600-051x.30.s5.3.x. [DOI] [PubMed] [Google Scholar]
- Bonito A.J., Lux L., Lohr K.N. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J. Periodontol. 2005;76:1227–1236. doi: 10.1902/jop.2005.76.8.1227. [DOI] [PubMed] [Google Scholar]
- Braatz L., Garrett S., Claffey N., Egelberg J. Antimicrobial irrigation of deep pockets to supplement nonsurgical periodontal therapy. II. Daily irrigation. J. Clin. Periodontol. 1985;12:630–638. doi: 10.1111/j.1600-051x.1985.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Breininger D.R., O’Leary T.J., Blumenshine R.V.H. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J. Periodontol. 1987;58:9–18. doi: 10.1902/jop.1987.58.1.9. [DOI] [PubMed] [Google Scholar]
- Christersson L.A., Zambon J.J., Genco R.J. Dental bacterial plaques: nature and role in periodontal disease. J. Clin. Periodontol. 1991;18:441. doi: 10.1111/j.1600-051x.1991.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Christodoulides N., Nikolidakis D., Chondros P., Becker J., Schwarz F., Rossler R., Sculean A. Photodynamic therapy as an adjunct to non surgical periodontal treatment: a randomised controlled clinical trial. J. Periodontol. 2008;79(9):1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- Clark D.T., Gazi M.I., Cox S.W., Eley B.M., Tinsley G.F. The effects of Acacia arabica gum on the in vitro growth and protease activities of periodontopathic bacteria. J. Clin. Periodontol. 1993;20:238–243. doi: 10.1111/j.1600-051x.1993.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Cobb C.M. Non-surgical pocket therapy: mechanical. Ann. Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- Cobb C.M. Clinical significance of non surgical periodontal therapy: an evidence based perspective of scaling and root planing. J. Clin. Periodontol. 2002;29(Suppl. 2):6–16. [PubMed] [Google Scholar]
- Cosyn J., Wyn I.A. Systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2006;77:257–264. doi: 10.1902/jop.2006.050216. [DOI] [PubMed] [Google Scholar]
- Dahlén G., Lindhe J., Sato K., Hanamura H., Okamoto H. The effect of supragingival plaque control on the composition of the subgingival flora in periodontal pockets. J. Clin. Periodontol. 1992;19:802–809. doi: 10.1111/j.1600-051x.1992.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Dzink J.L., Socransky S.S., Haffajee A.D. The predominant cultivable microbiota of active and inactive lesions destructive periodontal diseases. J. Clin. Periodontol. 1988;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Fleischer H.C., Mellonig J.T., Brayer W.K., Gray J.L., Barnett J.D. Scaling and root planing efficacy in multirooted teeth. J. Periodontol. 1989;60:402–409. doi: 10.1902/jop.1989.60.7.402. [DOI] [PubMed] [Google Scholar]
- Gazi M.I. The finding of antiplaque features in Acacia Arabica type of chewing gum. J. Clin. Periodontol. 1991;18(1):75–77. doi: 10.1111/j.1600-051x.1991.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Haffajee A.D., Cugini M.A., Dibart S., Smith C., Kent R.L., Jr, Socransky S.S. Clinical and microbiological features of subjects with adult periodontitis who responded poorly to scaling and root planing. J. Clin. Periodontol. 1997;24:767–769. doi: 10.1111/j.1600-051x.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom M.K., Ramberg P., Krok L., Lindhe J. The effect of supragingival plaque control on the subgingival microflora in human periodontitis. J. Clin. Periodontol. 1996;23:934–940. doi: 10.1111/j.1600-051x.1996.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Hunter R.D., O’Leary T.J., Kafrawy A.T. The effectiveness of hand versus ultrasonic instrumentation in open flap root planing. J. Periodontol. 1984;55:697–703. doi: 10.1902/jop.1984.55.12.697. [DOI] [PubMed] [Google Scholar]
- Jeffcoat M.K. Principles and pitfalls of clinical trials design. J. Periodontol. 1992;63:1045–1051. doi: 10.1902/jop.1992.63.12s.1045. [DOI] [PubMed] [Google Scholar]
- Kaldahl W.B., Kalkwarf K.L., Patil K.D., Molvar M.P., Dyer J.K. Longterm evaluation of periodontal therapy. II. Incidence of sites breaking down. J Periodontol. 1996;67:103–108. doi: 10.1902/jop.1996.67.2.103. [DOI] [PubMed] [Google Scholar]
- Kirtikar K.R., Basu B.D. Indian medicinal plants. 1984;vol. 2:919–935. [Google Scholar]
- Knowles J.W., Burgett F.G., Nissle R.R., Shick R.A., Morrison E.C., Ramfjord S.P. Results of periodontal treatment related to pocket depth and attachment level. Eight years. J. Periodontol. 1979;50:225–233. doi: 10.1902/jop.1979.50.5.225. [DOI] [PubMed] [Google Scholar]
- Kocher T., Tersic-Orth B., Plagmann H.C. Instrumentation of furcation with modified sonic scaler inserts. A study on manikins (II) J. Clin. Periodontol. 1998;25:451–456. doi: 10.1111/j.1600-051x.1998.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Li J., Helmerhorst E.J., Leone C.W., Troxler R.F., Yaskell T., Haffajee A.D., Socransky S.S., Oppenheim F.G. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 2004;97:1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Nyman S. Long-term maintenance of patients treated for advanced periodontal disease. J. Clin. Periodontol. 1984;11:504–514. doi: 10.1111/j.1600-051x.1984.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Loe H. The gingival index, the plaque index and the retention index system. J. Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Mandel I.D. Chemotherapeutic agents for controlling plaque and gingivitis. J. Clin. Periodontol. 1988;15:488–498. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Moore W.E., Moore L.V. The bacteria of periodontal diseases. Periodontology. 1994;2000(5):66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Mousques T., Listgarten M.A., Phillips R.W. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J. Periodontal. Res. 1980;15:144–151. doi: 10.1111/j.1600-0765.1980.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Osamu F., Takafumi H., Kenji I., Mayumi M., Katsumasa M. Microbiological markers for prediction and assessment of treatment outcome following non-surgical periodontal therapy. J. Periodontol. 2002;73(11):1253–1259. doi: 10.1902/jop.2002.73.11.1253. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli V., Kilian M., Karring T., Kirkegaard E. Effect of surgical and non-surgical periodontal treatment on periodontal status and subgingival microbiota. J. Clin. Periodontol. 1992;18:598–604. doi: 10.1111/j.1600-051x.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Pihlstrom B.L. Overview of periodontal clinical trials utilising anti infective or host modulating agents. Ann. Periodontol. 1997;2(1):153–164. doi: 10.1902/annals.1997.2.1.153. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Thorat M.S. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J. Periodontol. 2010;81:214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Happy D., Garg G. Short-term clinical effects of commercially available gel containing Acacia arabica: a randomized controlled clinical Trial. Aust. Dent. J. 2010;55:65–69. doi: 10.1111/j.1834-7819.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Agarwal E., Bajaj P., Naik S.B., Shanbhag N., Uma S.R. Clinical and microbiologic effects of commercially available gel and powder containing Acacia arabica on gingivitis. Aust. Dent. J. 2012;57:312–318. doi: 10.1111/j.1834-7819.2012.01714.x. [DOI] [PubMed] [Google Scholar]
- Sbordone L., Ramaglia L., Guletta E., Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J. Periodontol. 1990;61:579–584. doi: 10.1902/jop.1990.61.9.579. [DOI] [PubMed] [Google Scholar]
- Slots J., Listgarten M.A. Bacteroides gingivalis, Bacteroides intermedius, and Actinobacillus actinomycetemcomitans in human periodontal diseases. J. Clin. Periodontol. 1988;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Takacs V.J., Lie T., Perala D.G., Adams D.F. Efficacy of 5 machining instruments in scaling of molar furcations. J. Periodontol. 1993;64:228–236. doi: 10.1902/jop.1993.64.3.228. [DOI] [PubMed] [Google Scholar]
- Tangade P.S., Mathur A., Tirth A., Kabasi S. Anti-gingivitis effects of Acacia arabica-containing toothpaste. Chin. J. Dental Res. 2012;15(1):49–53. [PubMed] [Google Scholar]
- Tetsuro M., Shuji A., Tetsuya R., Hiroshi K., Akihiro Y., Toshihiro A., Tadamichi T. Site-specific development of periodontal disease is associated with increased levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in subgingival plaque. J. Periodontol. 2008;79(4):670–676. doi: 10.1902/jop.2008.070398. [DOI] [PubMed] [Google Scholar]
- Tyler V., Brady L., Robbers J. seventh ed. Lea & Febiger; Philadelphia: 1977. Pharmacognosy; pp. 64–68. [Google Scholar]
- Westfelt E., Rylander H., Dahlén G., Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J. Clin. Periodontol. 1998;25:536–541. doi: 10.1111/j.1600-051x.1998.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Wilson T.G., Jr. Compliance and its role in periodontal therapy. Periodontology. 2000;12:16–23. doi: 10.1111/j.1600-0757.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Wilson T.G., Jr. Supportive periodontal treatment introduction- definition, extent of need, therapeutic objectives, frequency and efficacy. Periodontology. 2000;12:11–15. doi: 10.1111/j.1600-0757.1996.tb00074.x. [DOI] [PubMed] [Google Scholar]