Abstract
Aims
This study was designed to utilize frequency-domain optical coherence tomography (FD-OCT) for assessment of plaque characteristics and vulnerability in patients with acute coronary syndrome (ACS) compared to stable coronary artery disease (SCAD).
Methods and results
We enrolled 48 patients; divided into an ACS-group (27 patients) and SCAD-group (21 patients) according to their clinical presentation. Hypertension and diabetes mellitus were more prevalent in SCAD group. Patients with ACS showed higher frequency of lipid-rich plaques (96.3% vs. 66.7%, P = .015), lower frequency of calcium plaques (7.4% vs. 57.1%, P < .001), and fibrous plaques (14.8% vs. 81%, P < .001) when compared with SCAD patients. The TCFA (defined as lipid-rich plaque with cap thickness <65 μm) identified more frequently (33.3% vs. 14.3%, P = .185), with a trend towards thinner median fibrous cap thickness (70 (50–180) µm vs. 100 (50–220) µm, P = .064) in ACS group. Rupture plaque (52% vs. 14.3%, P = .014), plaque erosion (18.5% vs. 0%, P = .059) and intracoronary thrombus (92.6% vs. 14.3%, P < .001) were observed more frequently in ACS group, while cholesterol crystals were identified frequently in patients with SCAD (0.0% vs. 33.3%, P = .002).
Conclusion
The current FD-OCT study demonstrated the differences of plaque morphology and identified distinct lesion characteristics between patients with ACS and those with SCAD. These findings could explain the clinical presentation of patients in both groups.
Keywords: Frequency-domain optical coherence tomography, Plaque characteristics, Clinical presentation
Abbreviations: %AS, percent area stenosis; ACS, acute coronary syndrome; ECG, electrocardiogram; EF, ejection fraction; FCT, fibrous cap thickness; FD-OCT, frequency-domain optical coherence tomography; ICC, intra-class correlation; IVUS, intravascular ultrasound virtual histologic; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; MFCT, minimum fibrous cap thickness; MLCSA, minimum luminal cross sectional area; NSTE-ACS, non-ST-elevation acute coronary syndrome; RCA, right coronary artery; SAP, stable angina pectoris; SCAD, stable coronary artery disease; STEMI, ST elevation myocardial infarction; TCFAs, thin cap fibroatheromas
1. Introduction
An important goal of research in coronary atherosclerosis is to understand the pathophysiology of atherosclerosis and to identify features of plaque vulnerability. The mechanism responsible for the sudden conversion of a stable disease to unstable life-threatening condition is usually plaque disruption with thrombus formation. The ability to detect and monitor vulnerable plaque is keenly sought to define its natural history and support studies of progression and regression.1
A number of imaging modalities have been proposed to identify specific areas of plaque vulnerability, intravascular ultrasound virtual histologic (IVUS) analysis studies have provided insight into the spatial distribution of ruptured coronary plaques2, 3 but could not identified significant differences in plaque composition between the patients who presented with acute coronary syndrome (ACS) and those with stable coronary artery disease (SCAD).4
Frequency-domain Intracoronary optical coherence tomography (FD-OCT) is a reliable and reproducible imaging modality, which provides superior resolution allowing more detailed analysis of plaque characterization, the purpose of the present study was to demonstrate the difference in plaque composition among a cohort of Egyptian patients presented with ACS compared to patients with SCAD by FD-OCT.
2. Materials and methods
2.1. Study population
In this observational, prospective study, 48 patients were recruited with 27 patients presenting with ACS [ST elevation myocardial infarction, STEMI (n = 22), non-ST-elevation acute coronary syndrome, NSTE-ACS (n = 5)] and 21 patients with SCAD, they underwent coronary angiography at Aswan Heart Center and Kobry El Kobba cardiac center between June 2014-May 2015.
All patients underwent full clinical history taking, laboratory workup, diagnostic coronary angiography, and then OCT imaging of the target stenosis was performed. In ACS group, STEMI was defined as continuous chest pain that lasted >30 min, with the following criteria; ST-segment elevation >0.1 mV in >2 contiguous leads or new left bundle-branch block on the 12-lead electrocardiogram (ECG), and elevated cardiac markers (troponin T/I). The NSTE-ACS was defined as ischemic symptoms in the absence of ST-segment elevation on the ECG with elevated cardiac markers.
Stable coronary artery disease was defined as typical exertional chest pain relieved by rest, glyceryl trinitrate administration, or both, with positive exercise ECG stress testing or abnormal myocardial perfusion scintigraphy.
Exclusion criteria were cardiogenic shock, hemodynamic or electrical instability, renal insufficiency (serum creatinine level > 1.5 mmol/l), left main coronary artery, and extremely tortuous or heavy calcified vessel because of the potential difficulty in performing and interpreting the OCT findings. The study was approved by the local Ethics Committee and all patients gave written informed consent before any cardiac procedure.
2.2. OCT image acquisition and analysis
In patients with stable angina pectoris (SAP), those with single vessel disease and lesion with significant diameter stenosis and/or in the territory of scintigraphic reversible defects was selected as the target lesion. In ACS patients, the culprit lesion was identified by echocardiographic wall motion abnormalities, electrocardiographic findings and angiographic lesion morphology. In STEMI patients, manual aspiration thrombectomy was performed to clear the infarct related artery from the occlusive thrombus, using Export® AP Aspiration Catheter (Medtronic, USA). After intracoronary administration of nitroglycerin (0.2 mg), OCT imaging of the target stenosis was obtained using the commercially available FD-OCT C7XR system and the DragonFly catheter (St Jude Medical system, Lightlab Imaging Inc., Westford, Massachusetts).
The image-catheter was positioned in the target vessel, distal to the culprit lesion and automated pullback was performed at 20 mm/s while the blood was removed by (non-occlusive OCT- technique) injection of iso-osmolar contrast through the guiding catheter, then images were digitally stored for offline analysis. OCT measurements were performed using the proprietary software for offline analysis (lightLab Imaging) by two different observers to allow for inter-observer variability.
Fibrous plaque was defined as a homogeneous, highly backscattering (signal-rich) region, overlying a lipid core, lipid plaque as a signal-poor region diffusely bordered by overlying signal rich bands. Lipid-rich plaque; characterized by fibrous cap thickness <400 mm over a lipid core extending for >90°; both features in ≥10 consecutive frames. Calcium was identified as a signal-poor region with sharply delineated borders.5, 6
For ruptured plaques, the measurement of fibrous cap thickness (FCT) was assessed both at the non-ruptured site (where there is no communication between the lipid core and the lumen) and at the thinnest part of the residual fibrous cap at the rupture site. Thin-cap fibroatheroma (TCFA) was defined as Lipid arc >90° and the thinnest fibrous cap <65 µm. Macrophages were defined as a signal-rich, distinct or confluent punctate regions that exceed the intensity of background speckle noise and neo-vessels as a non-signal tubule-luminal structures without connection to the vessel lumen, recognized on ≥3 consecutive cross-sectional images.7
Plaque rupture was identified by the presence of fibrous cap discontinuity/disruption and cavity formation in the context of the plaque that communicates with lumen and plaque erosions as the presence of intracoronary thrombus over the luminal surface of plaque in the absence of detectable overlying fibrous cap discontinuity and without cavity formation. Thrombus defined as an irregular mass protruding into the lumen (mural thrombus) or a luminal mass that is not connected to the vessel wall.7
For assessment of lesion severity, minimum luminal cross sectional area (MLCSA) was estimated as the cross section with the smallest lumen area. Reference luminal cross section area was defined as the frame with the largest normal lumen within 0.5 mm proximal or distal to the lesion and before any side branch. Percent area stenosis (%AS) based on luminal reference: (reference lumen – MLA)/reference lumen × 100.5
2.3. Statistical analysis
Data was analyzed using SPSS (version 21 SPSS Inc., Chigago, Illinois). Continuous data was presented as median (range) because they show abnormal distribution. Categorical data was presented as number (percentage). Comparison between groups was performed using Mann Whitney test for quantitative variables and Fisher’s exact test for qualitative ones. Intra-class correlation coefficient (ICC) was conducted to measure the inter-rater consistency for quantitative variables. P value <.05 was used to denote statistical significance.
3. Results
3.1. Baseline clinical characteristics
We enrolled 48 patients in this study, twenty seven (56.25%) patients presented with ACS-group and 21 (43.75%) patients with SCAD-group. Baseline clinical characteristics are shown in Table 1.
Table 1.
Baseline clinical characteristics.
| Variables | ACS group (n = 27) | SCAD group (n = 21) | P value |
|---|---|---|---|
| Age, y | 48 (27–62) | 55 (33–75) | 0.009 |
| Male | 24 (88.9) | 20 (95.2) | 0.621 |
| Hypertension | 3 (11.1) | 11 (52.4) | 0.003 |
| Diabetes | 9 (33.3) | 14 (66.7) | 0.04 |
| Smoking | 19 (70.4) | 10 (47.6) | 0.143 |
| Family history | 2 (7.4) | 0 (0.0) | 0.497 |
| LV EF% | 48 (25–61) | 55 (40–75) | 0.006 |
Data are presented as median and range or number and (%), EF: ejection fraction.
Most of the recruited patients were males (92%), with no statistical significant difference between the two groups (P = .621). ACS patients were significantly younger with median age of (48 (27–62) vs 55 (33–75) yeas, P = .009). SCAD patients had a significantly higher frequency of hypertension and diabetes mellitus compared to ACS patients (P < .003 and P < .04) respectively.
3.2. Angiographic and OCT image analysis
FD-OCT was performed in all patients without any complications. The angiographic findings and OCT-derived plaque characteristics and measurements are summarized in Table 2. The majority of ACS patients were STEMI patients (81.25%), and the distribution of plaques among the three major coronary arteries was not different between the 2 groups, the left anterior descending (LAD) artery was the most frequently culprit vessel compared to left circumflex artery (LCX) and right coronary artery (RCA) in both groups.
Table 2.
Angiographic and OCT findings of culprit lesions.
| Variables | ACS group (n = 27) | SCAD group (n = 21) | P value |
|---|---|---|---|
| Culprit vessel | |||
| LAD | 22 (81.5) | 17 (81) | 1.0 |
| LCX | 4 (14.8) | 2 (9.5) | 0.683 |
| RCA | 1 (3.7) | 2 (9.5) | 0.574 |
| Culprit lesion component | |||
| Lipid plaque | 26 (96.3) | 14 (66.7) | 0.015 |
| Fibrous plaque | 4 (14.8) | 17 (81) | <0.001 |
| Calcium plaque | 2 (7.4) | 12 (57.1) | <0.001 |
| TCFA | 9 (33.3) | 3 (14.3) | 0.185 |
| Macrophages | 12 (44.4) | 9 (42.9) | 1.0 |
| Cholesterol crystals | 0 (0) | 7 (33.3) | 0.002 |
| Neovascularization | 6 (22.2) | 10 (47.6) | 0.122 |
| Thrombus | 25 (92.6) | 3 (14.3) | <0.001 |
| Plaque Rupture | 14 (52) | 3 (14.3) | 0.014 |
| Plaque Erosion | 5 (18.5) | 0 (0) | 0.059 |
Data are presented as number and (%), LAD: left anterior descending coronary artery, LCX: left circumflex coronary artery, RCA: right coronary artery, TCFA: thin-cap fibroatheroma.
Patients with ACS presented with significant higher frequency of lipid-rich plaques (96.3% vs. 66.7%, P = .015), lower frequency of calcium plaques (7.4% vs. 57.1%, P < .001) and fibrous plaques (14.8% vs. 81%, P < .001), Fig. 1.
Fig. 1.
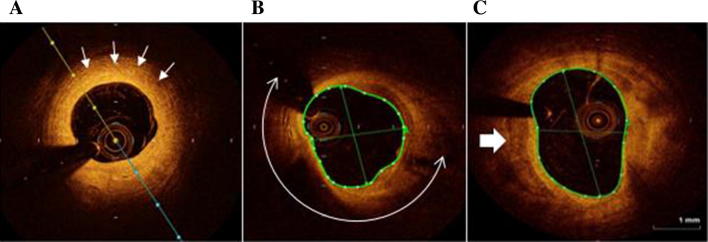
OCT image: examples of plaque composition. A: concentric fibrous plaque (homogeneous, highly backscattering (signal-rich) region). B: lipid-rich plaque (homogeneous, poorly delineated with a lipid arc of more than 90°) C: calcium plaque (signal-poor region with sharply delineated border (white arrow)).
Intracoronary thrombus was observed significantly in patients with ACS (92.6% vs. 14.3%, P < .001), while cholesterol crystals was identified frequently in patients with SCAD (33.3%). Excessive macrophage infiltration was frequently detected in ACS patients (P = 1) while plaque neovascularization was more often present in SCAD patients compared to ACS group (P = .122) with no statistical significance.
In ACS group, the culprit lesion morphology by OCT could not be clearly analyzed in three patients because of excessive residual intraluminal thrombi after aspiration thrombectomy.
FD-OCT images revealed that infarct-related lesion plaque rupture was significantly more prevalent in ACS patients than that observed in target lesion of SCAD patients (52% vs.14.3%, P = .014), and plaque erosion was identified only in ACS patients (Table 2, Fig. 2). Those presented with plaque erosion were younger than those presented with plaque rupture (37 years vs.48 years, P = .4).
Fig. 2.
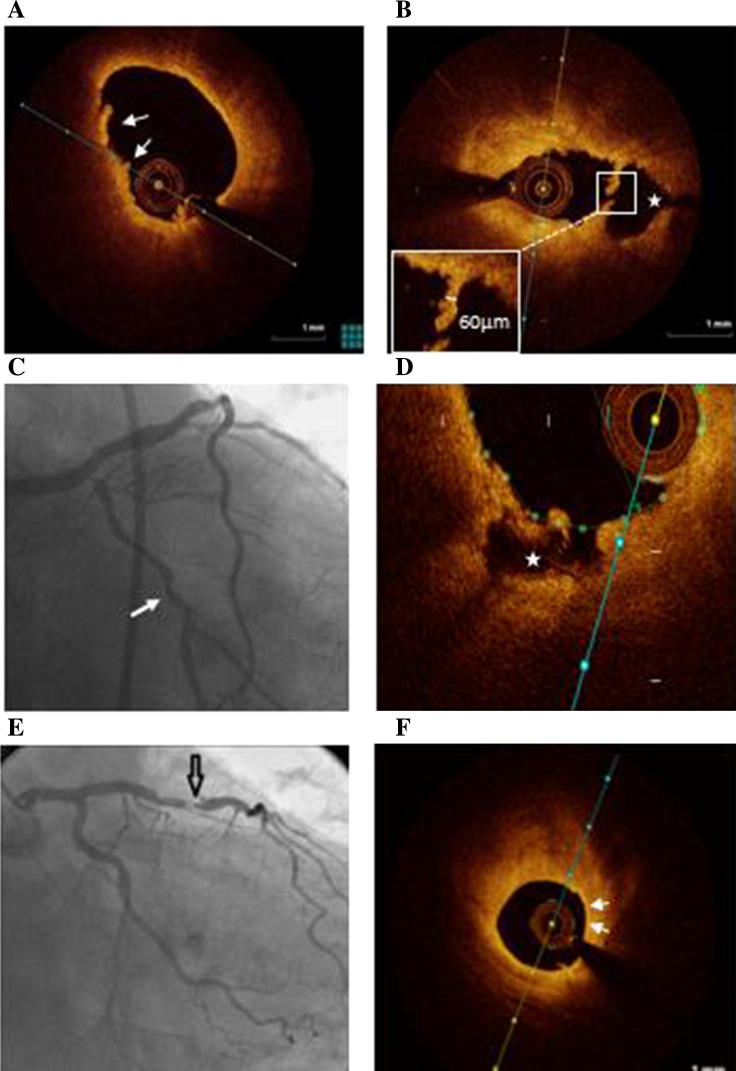
Representative OCT images. A: plaque erosion (luminal thrombus and absence of the endothelium, without evidence of fibrous cap disruption). B: plaque rupture (fibrous cap discontinuity/disruption and cavity formation (*). C: coronary angiography of a patient presented with ACS showing mid-LCX artery lesion. D: OCT image of the LCX artery of the same patient revealed a rupture plaque (cavity = *). E: angiogram showing a tight stenosis in the mid-LAD artery. F: OCT image obtained at the site of the LAD plaque showing a lipid-rich plaque with thin-cap fibroatheroma (arrows).
TCFAs were identified more frequently (33.3% vs. 14.3%, P = .185) with thinner median fibrous cap thickness (70 (50–180) µm vs. 100 (50–220) µm, P = .064) in ACS patients. Patients with ACS had less MLCSA compared to SCAD group (P = .11), and significant percent area stenosis (0.013). Table 3, summarizes OCT-derived quantitative differences in patients with ACS and SCAD.
Table 3.
OCT based quantitative analysis.
| Variables | ACS group (n = 27) | SCAD group (n = 21) | P value |
|---|---|---|---|
| MFCT (mm) | 70 (50–180) | 100 (50–220) | 0.064 |
| MLCSA (mm2) | 2.1 (1.3–3.5) | 2.5 (1.0–6.1) | 0.11 |
| Ref. LCSA (mm2) | 7.6 (4.7–15.6) | 6.3 (3.0–22.1) | 0.6 |
| Area stenosis% | 71 (40–83) | 57 (14–90) | 0.013 |
Data are presented as median and range, MFCT = Minimum fibrous cap thickness, MLCSA = Minimum luminal cross sectional area.
3.3. Inter-observer variability
Inter-observer variability yielded acceptable concordance. The intra-class correlation coefficient for inter-observer reliability was 0.86 for fibrous cap thickness and MLCSA and was 0.80 and 0.70 for reference CSA and area percent stenosis respectively, Table 4.
Table 4.
Inter-Observer variability.
| ICC | 95% CI | |
|---|---|---|
| FCT | 0.867 | 0.740–0.932 |
| MLCSA | 0.867 | 0.740–0.932 |
| Reference CSA | 0.808 | 0.624–0.902 |
| Area% stenosis | 0.708 | 0.428–0.851 |
ICC = Intra-class correlation, FCT = fibrous cap thickness, MLCSA = Minimum luminal cross sectional area.
4. Discussion
Early post-mortem and angioscopic studies postulate that ACS caused by the disruption or erosion of vulnerable plaques that can lead to subsequent thrombosis and coronary occlusion.8 In addition, histologic studies have suggested that plaque composition play a crucial role in the pathogenesis and clinical presentation of patients with coronary occlusion, independent of the severity of the underlying stenosis.9
Intravascular FD-OCT is a high-resolution imaging modality that offers an opportunity to visualize and assess the coronary plaque morphologies in a variety of clinical settings. Currently, characterization of plaque morphology underlying the thrombotic coronary occlusion responsible for ACS by using OCT provides insight into the pathogenesis of ACS and may have practical implications for management.10
To the best of our knowledge, this is the first study which assesses the difference in plaque features between patients presenting with ACS compared to SCAD in a cohort of Egyptian population using FD-OCT. The main findings are summarized as follows:
-The culprit lesion of patients with ACS as compared with those without ACS exhibit significantly more vulnerable plaque features and a higher lipid-rich plaque burden.
-Also, it showed significantly higher frequency of plaque rupture, thrombosis, and higher frequency of plaque erosions in ACS compared to SCAD patients supporting the previous pathology and OCT studies.
In line with the current study, previous pathological observations, IVUS and OCT studies11, 12 showed that the distribution of TCFAs as indicator of culprit plaque were common in the LAD artery particularly the proximal segment, but distributed relatively less through the LCX and RCA.
Characterization of culprit plaque revealed to be predominantly lipid-rich plaques in ACS patients compared to patients without ACS, this observation is concordant to that reported by Rodriguez-Granillo et al.13 and Li Xin et al.,4 they found that the percentage of lipid core lesions were significantly greater in patients with ACS than in patients with SAP, whereas a converse trend was observed for plaques with a fibrotic content. Similarly, Sebastian et al. showed statistically significant higher frequency of lipid rich plaques in ACS vs. SAP type 2 diabetic patients (p < .001).14
In this study, the prevalence of calcifications and fibrous plaques was significantly higher in SCAD patients compared to ACS patients (p < .001 for both), comparable with our observation, previous IVUS and computed tomographic coronary angiography studies15, 16 demonstrated a higher prevalence of soft plaque with spotty calcification as a marker of vulnerability that cause ACS, while large calcium deposits were frequently observed in SAP.
The location and number of calcium deposits within the vessel wall play an important role for plaque stability. Masato et al., used OCT to investigate the characteristics of coronary calcium in ACS and SAP, they found that the minimum distance between the inner edge of the calcium nodules and the luminal surface was significantly shorter in culprit lesions of ACS group than SAP group.17
Intracoronary thrombosis plays an important role in the pathogenesis and clinical manifestation of ACS patients. In our population, intracoronary thrombus was observed significantly in patients with ACS when compared to SCAD patients (93% vs. 14%, P < .001).
Similarly, previous study reported higher frequency of intracoronary thrombi in AMI patients than stable angina patients.14 Also, thrombus was more frequently observed in rupture and intact fibrous capped lesions compared with the stable lesions by OCT.18
Plaque neovascularization and excessive macrophage accumulation are known to be markers of plaque vulnerability and rupture. In a previous OCT study, both were significantly higher in type 2 diabetes mellitus patients presented with ACS vs. SCAD (p < .001 for both).14 Also, Kitabata et al.19 demonstrated increase of microvessels density in OCT-detected TCFAs in vivo. In our study, neovascularization was seen only in 22% of ACS patients compared to 47% of SCAD patients, while macrophage infiltration was more frequent in ACS patients than SCAD patients but with no significant difference.
At present, OCT might be the best tool to detect TCFA in vivo, it showed superiority to estimate accurately the fibrous cap thickness compared to intravascular ultrasound and coronary angioscopy for patients presenting with AMI20, moreover, Kume et al.21 examined 35 lipid-rich plaques from 38 human cadavers and demonstrated a good correlation of the fibrous cap thickness between OCT and histology (r = 0.90; p < .001).
Several studies22, 23, reported higher frequency of TCFA and thinner FCT in target lesions of patients with ACS (unstable angina and AMI) in comparison to stable angina patients. A comparison of 26 acute STEMI patients with 16 SAP patients showed that STEMI patients had a higher proportion of OCT-TCFA in the culprit lesion (85% vs. 13%, p < .001) and a thinner fibrous cap (57 ± 12 µm vs.180 ± 65 µm, p < .001).24 In this study, in spite of higher frequency of TCFAs in ACS patient, there was no significant difference between the two groups. This result should be interpreted with caution, taking into account the small sample size.
Pathological studies25, 26, as well as in vivo coronary imaging studies22, 27 demonstrated that among the different underlying mechanisms contributing to ACS (plaque rupture, erosion, and calcified nodule) plaque rupture is the most common substrate of ACS.
Plaque rupture characterized by the presence of fibrous cap discontinuity and cavity formation within the plaque, being well visualized on OCT in 50–70% of the culprit lesion of ACS patients. In addition, plaque rupture may be noted in SCAD patients. On the other hand, OCT has a unique ability to detect plaque erosion in 20–30% of the culprit lesion of the ACS population. These erosions characterized by presence of an irregular luminal surface with thrombi15 and had more fibrous plaque and less TCFA and smaller plaque burden compared with plaque rupture.28
The current study showed that plaque rupture was significantly more prevalent in ACS patients when compare with target lesion plaque rupture found in SCAD patients (52% vs.14.3%, P = .014), while plaque erosion was identified only in patients presented with ACS. Furthermore, patients presented with ACS and plaque erosion were younger than those presented with plaque rupture (37 years vs. 48 years, P = .4), this finding was similarly observed in other studies, that found plaque erosions tend to occur in younger patients, particularly, premenopausal women with cigarette smoking being the predominant risk factor.29, 30
Compared with plaques of SCAD patients, those of ACS patients were more vulnerable, had thinner median FCT (70 (50–180) µm vs. 100 (50–220) µm, P = .064), less MLCSA and subsequently more percent stenosis. Bezerra et al.31 found that FD-OCT depicted more severe native coronary disease than IVUS; MLA was 2.33 ± 1.56 mm2 versus 3.32 ± 1.92 mm2, respectively (p < .001). Also, Higuma et al., studied plaque characteristics of STEMI patients using both OCT and IVUS, they found severely narrowed MLCSA and large plaque burden in the majority of the STEMI patients.32 In contrast with our observation, Sebastian et al., failed to show significant MLCSA or more severe stenosis (79.51 ± 7.14 vs. 78.13 ± 8.17, p = .215) at the culprit site in ACS and SCAD diabetic patients.14
4.1. Limitation
The current study has some limitations. First, the sample size was small, and therefore, this study might not represent the whole spectrum of ACS and SCAD Egyptian patients. Second, we excluded patients with hemodynamic instability or cardiogenic shock, so, our result may not reflect the true incidence of plaque disruption in ACS patients. Furthermore, the use of manual aspiration thrombectomy was occasionally required in our ACS STEMI patients prior to OCT study, in spite we used it carefully to avoid mechanical trauma, it may have altered the plaque morphology, particularly plaque rupture. Third, despite the high resolution of OCT, the definition of plaque erosion still debatable, based primarily on absence of endothelial cells after exclusion of a fibrous cap rupture and this could explain lower rate of plaque erosion in our patients.
5. Conclusion
The current FD-OCT study demonstrated the differences of plaque morphology and identified distinct lesion characteristics between patients with ACS and patients with SCAD. These findings could explain the clinical presentation of patients in both groups.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.MacNeill Briain D., Lowe Harry C. Intravascular modalities for detection of vulnerable plaque current status. Arterioscler Thromb Vasc Biol. 2003;23:1333–1342. doi: 10.1161/01.ATV.0000080948.08888.BF. [DOI] [PubMed] [Google Scholar]
- 2.Cheruvu P.K., Finn A.V., Gardner C., Caplan J., Goldstein J. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 3.Pregowski J., Tyczynski P., Mintz G.S., Kim S.W., Witkowski A. Intravascular ultrasound assessment of the spatial distribution of ruptured coronary plaques in the left anterior descending coronary artery. Am Heart J. 2006;151:898–901. doi: 10.1016/j.ahj.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Li Xin-ming, Huang Cong-xin, Wang Tian-song. Comparison of coronary plaque composition among patients with acute coronary syndrome and stable coronary artery disease. Chin Med J. 2008;121:534–539. [PubMed] [Google Scholar]
- 5.Prati Francesco, Regar Evelyn. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Euro Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 6.Prati Francesco, Guagliumi Giulio, Mintz Gary S. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Euro Heart J. 2012;33:2513–2522. doi: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Vito L., Yoon J.H., Kato K., Yonetsu T., Vergallo R., COICO group (Consortium of Investigators for Coronary OCT) Comprehensive overview of definitions for optical coherence tomography-based plaque and stent analyses. Coron Artery Dis. 2014;25:172–185. doi: 10.1097/MCA.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 8.Mizuno K., Satomura K., Miyamoto A., Arakawa K., Shibuya T., Arai T. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992;326:287–291. doi: 10.1056/NEJM199201303260502. [DOI] [PubMed] [Google Scholar]
- 9.Little W.C., Constantinescu M., Applegate R.J., Kutcher M.A., Burrows M.T. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo Yoshiki, Kubo Takashi, Akasaka Takashi. The use of optical coherence tomography in acute coronary syndrome. Exp Rev Cardiovasc Ther. 2016;14:649–657. doi: 10.1586/14779072.2016.1145054. [DOI] [PubMed] [Google Scholar]
- 11.Kolodgie F.D., Burke A.P., Farb A. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285–292. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Kenichi, Kawasaki Daizo, Masutani Motomaru. OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. J Am Coll Cardiol Img. 2010;3:168–175. doi: 10.1016/j.jcmg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Granillo G.A., McFadden E.P., Valgimigli M. Coronary plaque composition of non-culprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J. 2006;151:1020–1024. doi: 10.1016/j.ahj.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Reith Sebastian, Battermann Simone. Optical coherence tomography derived differences of plaque characteristics in coronary culprit lesions between type 2 diabetic patients with and without acute coronary syndrome. Catheter Cardio Interven. 2014;84:700–707. doi: 10.1002/ccd.25267. [DOI] [PubMed] [Google Scholar]
- 15.Kubo T., Imanishi T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular and coronary angioscopy. J Am Col of Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 16.Meijs M., Meijboom W.B., Bots M. Comparison of frequency of calcified versus non-calcified coronary lesions by computed tomographic angiography in patients with stable versus unstable angina pectoris. Am J Cardiol. 2009;104:305–311. doi: 10.1016/j.amjcard.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Mizukoshi Masato, Kubo Takashi, Takarada Shigeho, Kitabata Hironori. Coronary superficial and spotty calcium deposits in culprit coronary lesions of acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2013;112:34–40. doi: 10.1016/j.amjcard.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 18.Tearney G.J., Yabushita H. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107(1):113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 19.Kitabata H., Tanaka A., Kubo T. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105(12):1673–1678. doi: 10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 20.Hong Myeo.-K., Mintz Gary S. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction. Circulation. 2004;110:928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- 21.Kume T., Akasaka T. Assessment of coronary artery plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172–1175. doi: 10.1016/j.amjcard.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Jang I.K., Tearney G.J. In vivo characterization of coronary atherosclerosis plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motomaru M., Takahiro O. Frequency and predictors of thin cap fibro-atheroma in patients with acute myocardial infarction and stable angina pectoris. J Am Heart Cardiol. 2008;52:787–792. doi: 10.1016/j.jacc.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T., Imanishi T., Kashiwagi M. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am J Cardiol. 2010;105:318–322. doi: 10.1016/j.amjcard.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Naghavi M., Libby P., Falk E., Casscells S.W., Litovsky S., Rumberger J. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies. Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 26.Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(Suppl 8):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 27.Kubo T, Ino Y, et al. Optical coherence tomography imaging in acute coronary syndromes. Cardiol Res Pract. 2011;312978. [DOI] [PMC free article] [PubMed]
- 28.Higuma T., Soeda T., Abe N., Yamada M., Yokoyama H. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:1166–1176. doi: 10.1016/j.jcin.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Farb A., Burke A.P., Tang A.L. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 30.Burke A.P., Farb A., Malcom G.T., Liang Y., Smialek J., Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 31.Bezerra H.G., Attizzani Guilherme F. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. J Am Coll Cardiol Intv. 2013;6:228–236. doi: 10.1016/j.jcin.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Higuma T, Soeda T, Yamada M, Yokota Y, et al. Coronary plaque characteristics associated with reduced TIMI (thrombolysis in myocardial infarction) flow grade in patients with ST-segment–elevation myocardial infarction. A combined optical coherence tomography and intravascular ultrasound study. Circ Cardiovasc Interv. 2016;9:e003913. [DOI] [PubMed]