Abstract
The complete regeneration of the periodontal tissues following periodontal disease remains an unmet challenge, and has presented clinicians with a remarkably difficult clinical challenge to solve given the extensive research in this area and our current understanding of the biology of the periodontal tissues. In particular as clinicians we look for treatments that will improve the predictability of the procedure, improve the magnitude of the effect of treatment, and perhaps most importantly in the long term would extend the indications for treatment beyond the need for single enclosed bony defects to allow for suprabony regeneration, preferably with beneficial effects on the gingival soft tissues. A rapid development in both innovative methods and products for the correction of periodontal deficiencies have been reported during the last three decades. For example, guided tissue regeneration with or without the use of bone supplements has been a well-proven treatment modality for the reconstruction of bony defects prior to the tissue engineering era. Active biomaterials have been subsequently introduced to the periodontal community with supporting dental literature suggesting that certain factors should be taken into consideration when undertaking periodontal regenerative procedures. These factors as well as a number of other translational research issues will need to be addressed, and ultimately it is vital that we do not extrapolate results from pre-clinical and animal studies without conducting extensive randomized clinical trials to substantiate outcomes from these procedures. Whatever the outcomes, the pursuit of regeneration of the periodontal tissues remains a goal worth pursuing for our patients. The aim of the review, therefore is to update clinicians on the recent advances in both materials and techniques in periodontal regenerative procedures and to highlight the importance of both patient factors and the technical aspects of regenerative procedures.
Keywords: Periodontal regeneration, Osseous defects, Guided tissue regeneration, Biomaterials, Enamel matrix derivatives, Case selection
1. Introduction
The general goals of periodontal therapy include: 1. The primary and secondary prevention of periodontal disease by controlling infection and inflammation and 2. The maintenance and improvement of the health, function, comfort and aesthetics of all supporting structures and tissues (gingivae, periodontal ligament [PDL], cementum and alveolar bone).
A number of “so called” pathological entities may necessitate special attention, either because they are considered to be areas of minoris resistentiae, e.g., intrabony and interradicular defects (Papapanou and Tonetti, 2000), or because of perceived aesthetic concerns and/or pain to the patients, e.g., marginal tissue recession defects (Chabanski and Gillam, 1997). Therefore the ultimate goal for periodontal treatment is the regeneration of the lost periodontal tissues. The reconstructive surgery has been one of the most dynamic therapeutic procedures in periodontology for the past three decades, and yet, the ultimate goal of regeneration of the periodontal supporting tissues remains unpredictable and challenging.
2. Osseous defects
Based on clinical observations and observations on human skulls bony defects as a result to periodontal disease can be classified as:
-
•
Suprabony or supracrestal: when the base of the pocket is located coronal or occlusal to the bone crest,
-
•
Infrabony or subcrestal: when the apical end of the pocket is located below the bone crest. An infrabony defect may be subdivided to intrabony defect when the subcrestal component involves the root surface of only one tooth and crater when the defect affects the root surfaces of two adjacent teeth on an equal extent (Goldman and Cohen, 1958).
An intrabony defect therefore can be sub classified, with respect to the number of remaining bony walls, in three categories: the 1-wall, 2-wall and 3-wall defects (Goldman and Cohen, 1958).
The furcation involvements may also be included in the group of periodontal bony defects. One of the earliest classification systems for interradicular defects was introduced by Glickman (1953), which was taking into consideration both the horizontal and vertical dimension of bone loss. Tarnow and Fletcher (1984) also suggested a classification for the evaluation of the vertical component for each type of horizontal type of furcation defect, measured from the fornix of the furcation area.
Another classification as proposed by Hamp et al. (1975) is currently the most commonly used classification due to its clinical simplicity:
-
•
Degree I: Horizontal bone loss not exceeding the one third of the tooth width
-
•
Degree II: Horizontal bone loss exceeding the one third of the width of the tooth but not involving the total width of the furcation area
-
•
Degree III: “Through-and-through” bone destruction
The clinical management of intrabony and furcation defects may involve a variety of reconstructive periodontal surgical methods and materials.
3. Biologic foundation of periodontal reconstructive treatment
For the successful reconstruction of periodontal tissues, the methods that are to be utilised should respect the natural sequence of biological events that occur during the periodontal healing and/or mimic the process of tooth and periodontal tissues embryologic development. Melcher (1976) postulated on the importance of the different potential of the four components of the periodontal apparatus in periodontal healing and how the attachment of periodontal tissues to the root surface was determined by the first type of cells that would attach to the root surface.
A number of animal studies reported that the rapid epithelial downgrowth (Stahl et al., 1972) on root surfaces inhibited the connective tissue attachment (Caton and Nyman, 1980). In addition, when dental implants were placed in contact with root tips in monkeys, periodontal support consisting of cementum and connective tissue fibers occurred even on titanium surfaces provided that the implants were placed in contact with vital PDL fibers (Buser et al., 1990a).
The above mentioned experiments and other associated studies (Bowers et al., 1989; Caton and Nyman, 1980, McCulloch, 1985, Stahl et al., 1983) appeared to establish that the bone, the epithelial and gingival connective tissues did not have any regenerative capacity and that the stem cells, the immature progenitor cells residing in the PDL, were the cells with the potential for periodontal regeneration.
4. Guided tissue regeneration
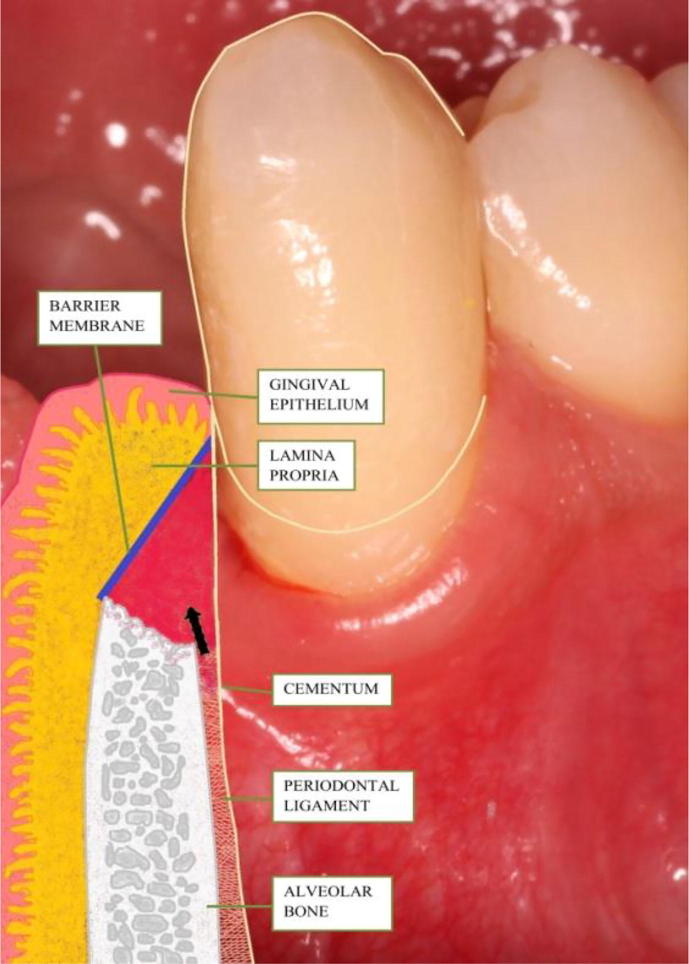
The term guided tissue regeneration (GTR) includes procedures attempting to regenerate lost periodontal tissues when barrier materials are used to exclude the epithelial and connective gingival tissues from the root and bone surface (Fig. 1).
Fig. 1.
Schematic illustration of the use of GTR in regeneration of periodontal tissues.
Historically various types of membranes have been introduced for the maintenance of the space between the defect and the root surface in order to enable repopulation of the cells of PDL and the proliferation and establishment of both PDL and bone structures. The barrier materials in GTR must therefore fulfill five main criteria: 1. Tissue integration, 2. Cell occlusivity, 3. Clinical manageability, 4. Space-making ability maintained long enough for both PDL and bone cells to proliferate into the defect and 5. Biocompatibility (non-toxic, non-antigenic and induce no or little inflammation) (Scantlebury, 1993).
GTR can be performed with the use of nonresorbable and bioresorbable membranes. The use of non- resorbable membranes has been gradually phased out for the following reasons: (1) a second surgical procedure four to six months following the initial procedure is necessary for the removal of the non-resorbable membrane which can be traumatic for the patient and a risk for the disruption of healing of the newly formed regenerated tissues; (2) the soft tissue that grows apically on the outside surface of the membrane can potentially be an area of weakness where both inflammation and marginal tissue recession may subsequently occur and (3) specific types of non-resorbable membranes have been associated with early and spontaneous exposure to the oral environment (Selvig et al., 1992).
4.1. Membranes
Six resorbable and autologous materials have been successfully tested for their safety and efficacy in GTR procedures:
4.1.1. Collagen barriers
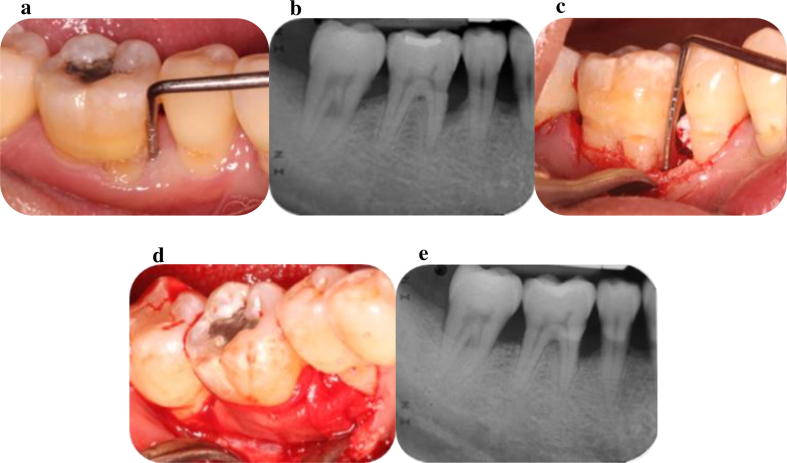
This type of membrane has been widely used in clinical practice (i.e. Bio-Gide®, Geistlich, Wolhusen, Switzerland) due to its attractive biologic and physical properties as well as its commercial availability (Fig. 2a–e). Type I collagen is the predominant component of collagen membrane(s), and it would appear therefore to mimic the natural consistency of the periodontal tissues. Several investigators have suggested that collagen membranes may have a positive role in the regeneration of periodontal tissues (Pitaru et al., 1987). Cross-link techniques have been used in order to improve the characteristics of these membranes, as for example to prolong their resorption rate, which may vary from six to eight weeks to six to eight months. Additional studies however, are required for the improvement of their properties as it has been demonstrated that there are limitations in providing/maintaining the wound space, together with the observation that the amount of regeneration following their use may be both limited and unpredictable (Tatakis et al., 1999).
Fig. 2.
a. Deep persisting periodontal pocket mesial of the LR6; b. Radiographic signs of angular bone loss mesial of the LR6 and furcation involvement; c. Alveolar bone defects as revealed following the elevation of a buccal flap; d. Placement of xenograft and collagen membrane in the defects (Bio-Oss® and Bio-Gide® respectively); e. Radiographic signs of bone fill mesial and at the furcation area of LR6.
4.1.2. Cargile membranes
They are derived from bovine intestines (ox caecum) and their resorption rates appear to range from 30 to 60 days. No significant clinical attachment level (CAL) gain and difficult handling characteristics however have been reported with the use of cargile membranes (Card et al., 1989).
4.1.3. Polylactic, polyglycolic and polyglactin copolymer acid barriers
The poly (α-hydroxy) acids are synthetic materials. Their degradation in the human body by hydrolysis has been shown to result in products that are metabolized through the citric acid cycle (Krebs cycle). These materials have been associated with a localised decrease of pH and inhibitory effects in osteogenesis. In addition, due to their slow degradation rate the barrier material can persist in human body for four to six years and may stimulate a late localised foreign-body reaction (Tatakis et al., 1999). The cross-linking and/or the addition of lactide and/or glycolide (i.e. VICRYL® Periodontal Mesh copolymer; Johnson & Johnson NJ, USA; Fleisher et al., 1988) may however result in their faster degradation. Only a limited number of studies have demonstrated any clinical efficacy of this group of materials in GTR procedures (Bremm et al., 2004, Magnusson et al., 1988, Stavropoulos and Karring, 2004).
4.1.4. Oxidized cellulose mesh barriers
These barrier membranes are made of a resorbable haemostatic dressing material which has been observed to have encouraging effects in GTR procedures (Galgut, 1990). However, it appears to provide with limited wound space and as such cell exclusion may be high. The acidic nature of the material may also be responsible for the delayed healing of the bone tissue following its use (Tatakis et al., 1999). Additional and well designed clinical studies of this material are therefore essential prior to inclusion as barrier membrane in GTR procedures.
4.1.5. Autogenous periosteal barrier membranes
The use of connective tissue with periosteum collected from the host’s palate combined with autogenous bone chips would appear to be a promising combination for use in GTR procedures, as studies have demonstrated superior bone levels’ gain and less post-operative marginal tissue recession when compared with open flap debridement (OFD) alone (Paolantonio et al., 2010). More studies than the existing case report studies are needed for the investigation of these autogenous membranes.
4.1.6. Laminar bone allograft membranes
The use of this barrier membrane in combination with particulate demineralized freeze-dried bone allograft (DFDBA) may also be of promise as demonstrated in a randomized clinical trial (RCT) in patients with twelve pairs of Class II mandibular molar furcation lesions (Scott et al., 1997). However, further studies with much higher power are necessary for conclusive evidence on the use of this material as membrane barrier in GTR procedures.
4.2. Bone replacement grafts
Various types of grafting materials have been introduced in reconstructive periodontal treatment based on their ability to facilitate the reconstruction of the lost supporting apparatus through the following mechanisms:
-
•
Osteoneogenesis (contain bone-forming cells)
-
•
Osteoconduction (serve as a scaffold and space provision for bone formation)
-
•
Osteoinduction (contain bone-inducing substances [Brunsvold and Mellonig, 1993]).
4.2.1. Autografts
Historically Hegedus (1923) published six successfully treated cases of “advanced pyorrhea” with the transplantation of autogenous bone from the tibia to the jaws. Several types of autogenous grafts have been proposed in the literature:
4.2.1.1. Cortical bone chips
(Nabers, 1984, Nabers and O’Leary 1965): One of the problems with this material was its potential for sequestration and the (average) large particle size of the chips. As a result other types of autogenous grafts were preferred (Mellonig, 1992).
4.2.1.2. Osseous coagulum and bone blend (Robinson, 1969)
Compared to open flap debridement alone the use of this autograft in self-contained defects appears to result in improved levels of clinical attachment after healing.
4.2.1.3. Intraoral cancellous bone and marrow
Conflicting outcomes from a number of studies on this material make any interpretation of the results difficult. For example one study by Rosenberg (1971) reported that there was more than 50% bone fill following implantation of the material whereas a study by Renvert et al. (1985) reported that there was just limited difference between grafted and non-grafted areas, with a more favourable result in deep sites.
4.2.1.4. Extra-oral cancellous bone and marrow (Schallhorn, 1968)
Due to a number of reported problems with the use of this type of autogenous graft its use in everyday periodontal practice has limited. Both ankylosis and root resorption may occur following the use of an iliac graft as well as high morbidity associated with the donor site (Dragoo and Sullivan, 1973, Ellegaard et al., 1976).
4.2.2. Allografts
The bone allogeneic grafts are usually procured within twelve hours of the donor’s death and placed in tissue banks. Four types of allogeneic grafts have been used in periodontal reconstructive therapy:
4.2.2.1. Frozen iliac allograft
has demonstrated favourable results. However, the need for extensive cross-matching to decrease the possibility of disease transmission and graft rejection has limited the widespread use of it in periodontal treatment (Rosen et al., 2000).
4.2.2.2. Freeze-dried bone allograft (FDBA)
This type of graft has been reported to be effective as a scaffold over which new bone may form (Goldgerg and Stevenson, 1987).
4.2.2.3. Demineralized freeze-dried bone allograft (DFDBA)
It has been reported that hydrochloric acid and freeze-drying of cortical bone graft may expose the morphogenetic proteins in the bone matrix, and therefore enhance its osteogenic potential (Urist and Mikulski, 1979). DFDBA has been considered to be one of the “gold standard” grafts in periodontal regeneration, with favourable outcomes (Libin et al., 1975, Pearson et al., 1981, Rosen et al., 2000).
The risk of disease transmission has always been a concern with the use of allografts. However, this may be minimal if the graft is harvested and processed according to the standards and guidelines of established bodies (e.g., American Association of Tissue Banking) (Mellonig, 1995). In addition, human studies have shown no immune reaction (antigenicity) following treatment with both FDBA and DFDBA (Quattlebaum et al., 1988).
4.2.3. Xenografts
These grafts demonstrate osteoconductive properties and have been considered to be risk free of disease transmission. Xenografts are available in two types:
4.2.3.1. Bovine derived bone replacement grafts
Bovine bone is processed for the elimination of its organic part leaving a hydroxyapatite “skeleton” of a microporous structure of cortical and cancellous bone, similar to that of human body. It has been suggested that this type of graft acts as an osteoconductive scaffold and enables bone growth with subsequent integration with host’s bone (Nasr et al., 1999). Bio-Oss® (Geistlich, Wolhusen, Switzerland) is the most well-known and commercially available product in this category (Fig.2) and has been associated with the successful management of intrabony and interradicular defects (Richardson et al., 1999, Taheri et al., 2009).
4.2.3.2. Coralline calcium carbonate
Biocoral® (Inoteb, Saint Gonnery, France) is a resorbable material of calcium carbonate, obtained from a natural coral and it is composed primarily of anagonite (>98% CaCO3). The porosity of the material (>45%) is similar to natural bone and does not appear to require transformation to a carbonate phase, thus allowing both rapid resorption and bone replacement (Nasr et al., 1999). A number of studies have demonstrated promising results with the use of Biocoral® in human bony defects (Gao et al., 1997).
There may however be a risk of rejection of regenerative treatment with the use of xenografts by patients due to cultural and religious reasons.
4.2.4. Alloplastic grafts
These materials function primarily as bone fillers (3rd World Workshop in Periodontics 1996). Synthetic grafts are available in particles of 300–500 μm of diameter and may offer the advantages of unlimited quantity, no risk for disease transmission and no additional surgical site. Alloplastic grafts can be divided in four main categories:
4.2.4.1. Polymers
HTR® (Bioplant, Norwalk, CT) is a commercially available synthetic bone of this category. It is a composite of polymethylmethacrylate, polyhydroxylethylmethacrylate and calcium hydroxide and may be considered to be a non-resorbable material. Human studies have demonstrated favourable clinical results with the use of HTR® and have reported new bone growth deposition on its hydrophilic particles (Stahl et al., 1990, Yukna, 1990). Other studies however, have failed to demonstrate any significant clinical efficacy for this material (Shahmiri et al., 1992).
4.2.4.2. Tricalcium phosphate (TCP)
The most commonly used form of this material is β-tricalcium phosphate (β-TCP) which may be considered to be a partially resorbable material that initially acts as a scaffold for bone formation. A number of studies have indicated beneficial results in periodontal reconstruction with the use of tricalcium phosphate (Cutright et al., 1972, Stein et al., 2009). Other studies however, have highlighted the tendency of the particles of this material to be encapsulated by fibrous connective tissue (Baldock et al., 1985).
4.2.4.3. Hydroxyapatite
This is the dominant mineral component of human bone. Synthetic hydroxyapatite has been introduced in three types (Nasr et al., 1999): (i) the dense type, which is a result of high temperature process, is non porous and dense (Meffert et al., 1985, Yukna, 1989); (ii) the porous type, which is a result of the hydrothermal conversion of the calcium carbonate exoskeleton of the natural coral into the calcium phosphate hydroxyapatite. The porosity of this material facilitates the bone ingrowth into the pores; (iii) the resorbable type, which is a result of a low temperature process. Its slow resorption rate allowed prolonged osteoconductive action (Ricci et al., 1992). A combination of hydroxyapatite (60%) and β-TCP (40%) is commercially available (Straumann® BoneCeramic). However, long-term studies for the efficacy of this combined product are currently lacking.
4.2.4.4. Bioactive glass
This material is composed of CaO, Na2O, SiO2, P2O5 and may be resorbable or non-resorbable, depending on the relative proportion of its compounds. It would appear to bond to bone through the development of a double layer of silica gel and calcium-phosphorous. It has been suggested that the material promotes the adsorption of proteins by osteoclasts to form an extracellular bone matrix (Nasr et al., 1999). There are two main types of commercially available bioactive glasses, the PerioGlas® (NovaBone® Dental) and BioGran® (BIOMET 3i™). Data from the published literature appeared to suggest that bioactive glass was efficacious in the treatment of intrabony defects (Lovelace et al., 1998, Mengel et al., 2006, Subbaiah and Thomas, 2011). However, a histological study of human defects reported that following the use of bioglass, only limited bone formation was observed and, in the most of the samples, evidence of any new cementum and inserting collagen fibers formation was lacking (Nevins et al., 2000). It is possible that different brands of bioglasses vary in their efficacy and therefore this assumption should be investigated in well conducted RCTs (Sohrabi et al., 2012).
5. Active biomaterials
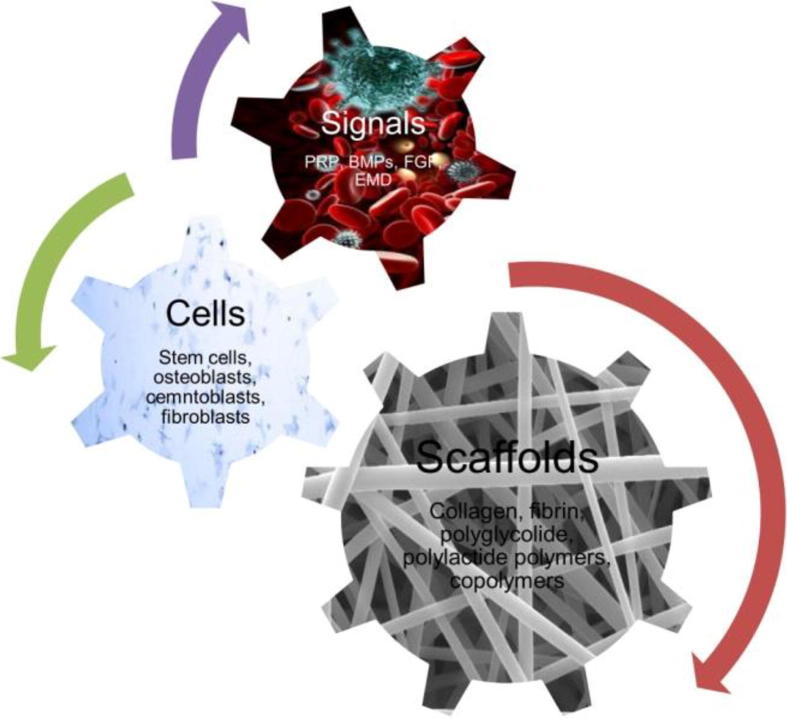
A relatively new discipline, the tissue engineering in periodontal therapy has been developed in part due to a growing body of evidence regarding the biologic functions of the human body. The biological basis for this innovation was based on the concept that certain factors were able to regulate both, the differentiation and function of progenitor cells within the healing wound area (periodontal tissues) which would lead to a more favourable outcome of new bone, cementum and PDL formation (Fig. 3).
Fig. 3.
Tissue engineering in periodontics.
Three basic elements have been investigated in order to manipulate the sequence of events that may lead to complete periodontal healing: (1) stem/progenitor cells, (2) conductive scaffolds and (3) signaling molecules (Chen and Jin, 2010).
5.1. Stem cells
The term “stem cells” refers to clonogenic, undifferentiated cells that are capable of self-renewal and multi-lineage differentiation depending on their intrinsic signals that can be regulated by extrinsic factors (Lin et al., 2008). In general terms the molecular signals which can control differentiation of cells include soluble factors (e.g., hormones and cytokines), matrix molecules, direct cell to cell contacts and external stimuli (e.g., mechanical stimulation) (Hughes, 1995).
Stem cells are categorized in embryonic and adult (somatic) cells. Hematopoietic stem cells from bone marrow have been identified first and were already in use for therapeutic purposes. Bone marrow stromal cells (BMSSCs) or mesenchymal stem cells (MSCs) are a different population of stem cells that has been identified in the adult body. It appears that all the tissues with tendency for renewal contain at least a small number of stem cells (Lin et al., 2008).
Seo et al. (2004) isolated MSCs in human PDL and as a result laid the foundation for the development of novel strategies for periodontal reconstruction. A key determinant of the regenerative potential of the PDL was the ability of stem cells to undergo specialised osteoblast and cementoblast differentiation, resulting in new bone and cementum formation.
5.2. Conductive scaffolds
The wound healing process occurs within in a three-dimensional environment, the extracellular matrix (ECM) that facilitates the molecule regulation of the cells activity. It is therefore evident that, in regenerative procedures an artificial tissue-engineering scaffold is an essential prerequisite in order to facilitate bone formation, etc. (Chen and Jin, 2010). A matrix may assist the penetration, attachment, proliferation, differentiation and in-growth of cells that are necessary for regeneration and inhibit the infiltration of undesirable cells on the healing site. The biomaterial scaffold, like the natural biological tissues should be viscoelastic and have an “ideal” porosity for cell in-growth, adequate surface area (bioavailability), adequate mechanical strength and favourable degradation (biodegradable) properties (Ahmed et al., 2008). Biomaterial scaffolds may be fabricated from either natural materials (i.e., collagen and fibrin) or from synthetic materials (i.e., polyglycolide and polylactide polymers and copolymers). These materials may also be designed with a microstructure that has the ability to release molecules to induce and accelerate the periodontal regeneration cascade events (Chen and Jin, 2010). Further research however is required for the development of the ideal conductive scaffold for periodontal regeneration, based on tissue engineering techniques.
5.3. Signaling molecules/growth factors
The carefully controlled coordinated expression of a range of growth factors directs the osteoblastic commitment of MSCs, the proliferation and clonal amplification of progenitor cells, and ultimately the production and release of bone related ECM. Additionally, the activity of a number of these growth factors is further regulated by the production of antagonistic inhibitor molecules which may block their activities (Hughes et al., 2006). In view of the importance of these factors during bone formation, there is continuing research into their therapeutic potential to stimulate tissue regeneration, as briefly discussed below:
5.3.1. Platelet-rich plasma
Platelet-rich plasma (PRP) is an autologous concentration of platelets in plasma, developed by gradient density centrifugation of patient’s blood. PRP contains many growth factors, e.g., platelet- derived growth factor (PDGF) and transforming growth factor-β (TGF-β) (Del Fabbro et al., 2011). Two recent multicenter RCTs on the use of recombinant human PDGF (rhPDGF) in combination with β-TCP in intrabony defects demonstrated that the adjunctive use of rhPDGF resulted in an improvement in CAL gain and greater reduction in probing depths (PD) compared to the sole use of β-TCP (Jayakumar et al., 2011, Nevins et al., 2005). Investigators have also reported encouraging results from the use of PRP in combination with bovine derived xenograft in the treatment of intrabony defects (Ouyang and Qiao, 2006). However, in the treatment of furcation defects (Class II), PRP gel appeared to have only a limited role (Pradeep et al., 2009). Although there is no convincing evidence for its use in periodontal regeneration procedures it would appear that PRP can be advantageous if used as an adjunct to grafting procedures in the treatment of intrabony defects, but not in combination with GTR. PRP has also been used for bone augmentation procedures in implantology (Zeckner et al., 2003).
5.3.2. Bone morphogenetic proteins
Bone Morphogenetic Proteins (BMPs) form a unique group of proteins within the Transforming Growth Factor beta (TGF-β) superfamily. BMPs demonstrate chemotactic properties and, depending on their concentration gradient, they may also function as mitogenic factors or induce the differentiation of mesenchymal progenitor cells into chondroblasts and osteoblasts. BMPs may therefore have a regulatory effect on bone morphogenesis (Sykaras and Opperman, 2003). Further in vivo experimentation may therefore help to precisely determine the therapeutic significance of these molecules
5.3.3. Cell-binding peptide
This is a synthetic clone of a specific acid sequence of Type I collagen, which has been reported to be involved in the binding of cells, e.g., osteoblasts and fibroblasts (Yukna et al., 2000). PepGen P-15® is a product that has been tested in conjunction and compared to an organic bovine-derived hydroxyapatite bone matrix and demonstrated positive effects on periodontal regeneration (Yukna et al., 2000). Further studies however are necessary before more definitive conclusions can be made on P-15® peptide’s efficacy.
5.3.4. Fibroblast growth factor
Fibroblast growth factor-2 (FGF-2) is a member of the heparin binding growth factor family and has been demonstrated to promote the proliferation and attachment of endothelial and PDL cells in wound healing. In a recent multicenter RCT, the use of FGF-2 produced by genetic recombination that transformed Escherichia coli with the human gene FGF-2 was evaluated in the treatment of vertical bony defects resulting in improved CAL (Kitamura et al., 2011).
5.3.5. Enamel matrix derivatives (EMD)
EMD were developed to induce regeneration by mimicking the process that occurs during the development of the root and the periodontal tissues. Specifically they mimic the critical point in tooth development when the inner cells of Hertwig’s epithelial root sheath secrete enamel matrix proteins which are subsequently deposited onto the root surface initiating a cascade of actions that would eventually lead to cementum creation, PDL and bone formation. However, the underlying mechanisms for the precise role of EMD on the cellular and molecular level are not well understood even though products containing EMD are commercially available (Emdogain®, Straumann, Basel, Switzerland) with approximately fifteen years of supportive clinical and histological data.
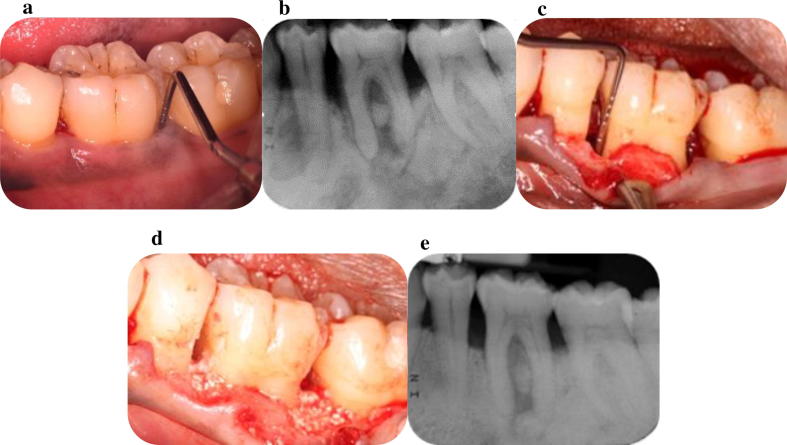
Hammarstrӧm (1997) published one of the first studies that included histological results following the use of EMD in buccal dehiscences in monkeys and reported up to 70% of new cementum and up to 65% bone gain eight weeks following the regenerative procedure. Encouraging results were also published when EMD was applied in intrabony defects’ areas and resulted in partial (66%) bone fill (Heijl et al., 1997; Fig. 4a–e). The use of EMD in the treatment of Class II mandibular furcation defects was also tested and resulted in a higher rate of Class II to Class I conversion (Casarin et al., 2008).
Fig. 4.
a. Deep persisting periodontal pocket mesial and distal of the LL6; b. Radiographic signs of angular bone loss mesial and distal of the LL6 and furcation involvement; c. Alveolar bone defects as revealed following the elevation of a buccal flap; d. Placement of enamel matrix derivatives combined with alloplastic graft (Straumann® Emdogain® PLUS) in the defects; e. Radiographic signs of bone fill mesial, distal and at the furcation area of LL6.
A Cochrane meta-analysis reported that the application of EMD showed statistically significant improvements in both CAL and PD reduction when compared to OFD or placebo (Esposito et al., 2009). One problem in evaluating the potential of EMD is that only short-term studies are currently available. It is therefore essential that further long term studies evaluating the material’s efficacy in regenerative procedures compared to established treatment modalities are initiated in well controlled RCTs.
A number of published clinical studies have reported on root conditioning of the root surfaces with ethylenediaminetetraacetic acid (EDTA) in conjunction with the application of EMD. It was concluded that the adjunctive use of EDTA failed to demonstrate any statistically significant differences in CAL gain and PD reduction in intrabony defects and that the benefits from using this chelating agent in EMD application remained to be clarified in further studies (Parashis et al., 2006, Sculean et al., 2006).
6. Case selection and treatment considerations
The predictability of reconstructive procedures in osseous defects may be dependent upon a variety of associated factors:
6.1. The patient
Socioeconomic and behavioural factors have to be taken into consideration when planning a surgical procedure, as they may affect the ability of the patient to protect and maintain the results of the treatment. For example, cigarette smoking has been demonstrated to prevent new tissue growth and bone formation following GTR in mandibular molars (Machtei et al., 2003). A recent meta-analysis also highlighted the negative effect of smoking in the bone regeneration of intrabony defects (Patel et al., 2012). Diabetic patients may also demonstrate impaired response to periodontal therapy (Grossi et al., 1996). However, evidence regarding the response to regenerative procedures in patients with diabetes is limited at the present time in the published literature.
6.2. The site
Cortellini et al. (1998) in a multicenter RCT evaluated the efficacy of GTR with bioabsorbable barrier membranes, compared to OFD surgery in intrabony defects. The results indicated that GTR with bioabsorbable membranes resulted greater linear amounts of improvement in deep than shallow defects, with similar potential of CAL gains expressed in percentages. Wider defects (≥22°) have been however associated with less beneficial results following reconstructive therapy (Cortellini and Tonetti, 2000, Tsitoura et al., 2004). The number of residual bony walls may be considered as a factor that may subsequently affect the choice of the regenerative method and material. Investigators however have failed to demonstrate any significance of the number of bony walls of a treated defect. In addition, studies have shown that the use of GTR in the treatment of intrabony defects with an unfavorable architecture (one-wall, shallow and wide), may be effective (Aimetti et al., 2005). Class III furcation defects have been considered to be very challenging in reconstructive therapy due to an unfavorable response (Pontoriero and Lindhe, 1995). The existence of root trunk concavities may also influence the outcome of regenerative procedures, with the need to modify the membrane used to allow better adaptation and provide a more satisfactory therapeutic result (Villaça et al., 2004). Bowers et al. (2003), with a prospective study demonstrated that the horizontal and vertical probing depths at the furcation areas need to be evaluated as they have been shown negatively associated with complete closure of the defects. According to the investigators, the root divergence in addition to the interproximal bone height in conjunction to the position of the fornix of the furcation are three parameters that also need to be assessed as they may affect the result of regenerative procedures in furcation defects.
6.3. The procedure and the healing period
Bone defects should be filled by the bone replacement grafts to a realistic level that will allow for primary closure of the flaps. Both the flap adaptation and primary closure are of critical importance in reconstructive therapy, for example in preventing possible exposure and contamination of the regenerative materials. Excellent plaque control during the healing phase is also crucial for a more positive regenerative response (Machtei et al., 1994). A recent in vitro study, based on the rationale of the importance of wound stability during healing, evaluated the efficacy of EMD under biomechanical loading. The results would suggest that occlusal loading may jeopardize the beneficial actions of EMD (Nokhbehsaim et al., 2011).
7. Discussion
Defects concerning the tooth supporting tissues may lead to impairments in the functioning, aesthetics and/or the ability of a person to maintain a stable periodontal status. A rapid development in both innovative methods and products for the correction of periodontal deficiencies has been reported during the last three decades. For example, guided tissue regeneration with or without the use of bone supplements has been a well-proven treatment modality for the reconstruction of bony defects prior to the tissue engineering era. Active biomaterials have been subsequently introduced to the periodontal community with supporting dental literature that has suggested that certain factors should be taken into consideration when undertaking periodontal regenerative procedures. According to Cortellini and Bowers (1995) specific factors such as patient characteristics, the morphology of the defect, and the planned surgical technique may influence the healing response of intrabony defects. The issue of patient compliance, the ability to maintain good oral hygiene and the influence of smoking may also affect the predictability of periodontal regeneration. It is also important to acknowledge that the shape of the defect may be critical, for example, deep and narrow defects have been reported to respond favorably to regenerative procedures (Cortellini and Bowers, 1995). Furthermore, the technical aspect of such procedures should not be ignored for example, flap design, defect debridement, handling of the soft tissues and wound protection, Periodontal regeneration has therefore, been considered as the ultimate and yet unmet goal of modern periodontal treatment. GTR, with and without the use of bone grafting materials appears to be the gold standard on the reconstruction of intrabony and interradicular defects for more than one decade (Needleman et al., 2005). However, with the development of the tissue engineering science various biomaterials have been introduced for clinical use. EMD has the most long-standing evidence in comparison to other biomaterials and demonstrated similar efficacy with the GTR techniques (Esposito et al., 2009). The combination of different materials also seemed to be appealing and in some studies demonstrated encouraging results (Trombelli and Farina, 2008). A number of factors, concerning the patient and the surgical site have to be correctly evaluated prior to the application of any regenerative procedure and to be strictly controlled during the postoperative healing period. The periodontist should therefore, aim for a better understanding of the available materials and techniques so they can apply the developing knowledge in the clinical environment with successful outcomes.
8. Conclusions
The periodontal regeneration of intrabony defects not only involves the experience and skills of the clinicians but also the selection of the suitable regenerative materials and techniques from the dental armamentarium. A variety of surgical techniques and products for regeneration have been available with substantial research evidence reporting on their efficacy. The clinician should make their choice for the best suitable regenerative modality based on general and site specific factors and with respect to the natural healing events occurring post operatively.
Ethical statement
This review article does not require ethical approval.
Conflict of interest
The authors of this manuscript have no conflict of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Clinical relevance
A large number of surgical techniques and products have been developed for the reconstruction of the lost periodontal tissues. The clinician should be aware of the evidence for supporting each of the available regenerative modalities so they can make the best possible decision for the achievement of a successful outcome in day-to-day clinical practice.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed T.A., Dare E.V., Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. Pert. B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- Aimetti M., Romano F., Pigella E., Pranzini F., Debernardi C. Treatment of wide, shallow and predominantly one-wall intrabony defects with a bioabsorbable membrane: a randomized controlled clinical trial. J. Periodontol. 2005;76:1354–1361. doi: 10.1902/jop.2005.76.8.1354. [DOI] [PubMed] [Google Scholar]
- Baldock W.T., Hutchens L.H, Jr., McFall W.T, Jr., Simpson D.M. An evaluation of tricalcium phosphate implants in human periodontal osseous defects of two patients. J. Periodontol. 1985;56:1–7. doi: 10.1902/jop.1985.56.1.1. [DOI] [PubMed] [Google Scholar]
- Bowers G.M., Chadroff B., Carnevale R., Mellonig J., Corio R., Emerson J., Stevens M., Romberg E. Histologic evaluation of new attachment apparatus formation in humans. Part I. J. Periodontol. 1989;60:664–674. doi: 10.1902/jop.1989.60.12.664. [DOI] [PubMed] [Google Scholar]
- Bowers G.M., Schallhorn R.G., McClain P.K., Morrison G.M., Morgan R., Reynolds M.A. Factors influencing the outcome of regenerative therapy in mandibular Class II furcations: Part I. J. Periodontol. 2003;74:1255–1268. doi: 10.1902/jop.2003.74.9.1255. [DOI] [PubMed] [Google Scholar]
- Bremm L.L., Sallum A.W., Casati M.Z., Nociti F.H., Sallum E.A. Guided tissue regeneration in Class II furcation defects using a resorbable polylactic acid barrier. Am. J. Dent. 2004;17:443–446. [PubMed] [Google Scholar]
- Brunsvold M.A., Mellonig J.T. Bone grafts and periodontal regeneration. Periodontol. 2000. 1993;1:80–91. [PubMed] [Google Scholar]
- Buser D., Warrer K., Karring T. Formation of a periodontal ligament around titanium implants. J. Periodontol. 1990;61:597–601. doi: 10.1902/jop.1990.61.9.597. [DOI] [PubMed] [Google Scholar]
- Card S.J., Caffesse R.G., Smith B.A., Nasjleti C.E. New attachment following the use of a resorbable membrane in the treatment of periodontitis in dogs. Int. J. Periodontics Restorative Dent. 1989;9:58–69. [PubMed] [Google Scholar]
- Casarin R.C., Del Peloso Ribeiro E., Nociti F.H., Jr., Sallum A.W., Sallum E.A., Ambrosano G.M., Casati M.Z. A double-blind randomized clinical evaluation of enamel matrix derivative proteins for the treatment of proximal class-II furcation involvements. J. Clin. Periodontol. 2008;35:429–437. doi: 10.1111/j.1600-051X.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Caton J., Nyman S. Histometric evaluation of periodontal surgery I. The modified Widman flap procedure. J. Clin. Periodontol. 1980;7:212–223. doi: 10.1111/j.1600-051x.1980.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Chabanski M.B., Gillam D.G. Aetiology, prevalence and clinical features of cervical dentine sensitivity. J. Oral Rehabil. 1997;24:15–19. doi: 10.1046/j.1365-2842.1997.00471.x. [DOI] [PubMed] [Google Scholar]
- Chen F.M., Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010;16:219–255. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- Cortellini P., Bowers G.M. Periodontal regeneration of intrabony defects: an evidence-based treatment approach. Int. J. Periodontics Restorative Dent. 1995;15(2):128–145. [PubMed] [Google Scholar]
- Cortellini P., Carnevale G., Sanz M., Tonetti M.S. Treatment of deep and shallow intrabony defects. A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 1998;25:981–987. doi: 10.1111/j.1600-051x.1998.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Cortellini P., Tonetti M.S. Focus on intrabony defects: guided tissue regeneration. Periodontol. 2000. 2000;22:104–132. doi: 10.1034/j.1600-0757.2000.2220108.x. [DOI] [PubMed] [Google Scholar]
- Cutright D.E., Bhaskar S.N., Brady J.M., Getter L., Posey W.R. Reaction of bone to tricalcium phosphate ceramic pellets. Oral Surg. Oral Med. Oral Pathol. 1972;33:850–856. doi: 10.1016/0030-4220(72)90457-4. [DOI] [PubMed] [Google Scholar]
- Del Fabbro M., Bortolin M., Taschieri S., Weinstein R. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J. Periodontol. 2011;82:1100–1111. doi: 10.1902/jop.2010.100605. [DOI] [PubMed] [Google Scholar]
- Dragoo M.R., Sullivan H.C. A clinical and histological evaluation of autogenous iliac bone grafts in humans. II. External root resorption. J. Periodontol. 1973;44:614–625. doi: 10.1902/jop.1973.44.10.614. [DOI] [PubMed] [Google Scholar]
- Ellegaard B., Nielsen I.M., Karring T. Composite jaw and iliac cancellous bone grafts in intrabony defects in monkeys. J. Period. Res. 1976;11:299–310. doi: 10.1111/j.1600-0765.1976.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Esposito M., Grusovin M.G., Papanikolaou N., Coulthard P., Worthington H.V. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD003875.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher N., de Waal H., Bloom A. Regeneration of lost attachment apparatus in the dog using Vicryl absorbable mesh (Polyglactin 910) Int. J. Periodontics Restorative Dent. 1988;8:44–55. [PubMed] [Google Scholar]
- Galgut P.N. Oxidized cellulose mesh used as a biodegradable barrier membrane in the technique of guided tissue regeneration. A case report. J. Periodontol. 1990;61:766–768. doi: 10.1902/jop.1990.61.12.766. [DOI] [PubMed] [Google Scholar]
- Gao T.J., Tuominen T.K., Lindholm T.S., Kommonen B., Lindholm T.C. Morphological and biomechanical difference in healing in segmential tibial defects implanted with Biocoral or tricalcium phosphate cylinders. Biomaterials. 1997;18:219–223. doi: 10.1016/s0142-9612(96)00133-0. [DOI] [PubMed] [Google Scholar]
- Glickman I. first ed. WB Saunders; Philadelphia: 1953. Clinical Periodontology. [Google Scholar]
- Goldgerg V.M., Stevenson S. Natural history of autografts and allografts. Clin. Orthop. Ret. Res. 1987;225:7–16. [PubMed] [Google Scholar]
- Goldman H.M., Cohen W.D. The infrabony pocket: classification ant treatment. J. Periodontol. 1958;29:272–291. [Google Scholar]
- Grossi S.G., Skrepcinski F.B., DeCaro T., Zambon J.J., Cummins D., Genco R.J. Response to periodontal therapy in diabetics and smokers. J. Periodontol. 1996;67(Suppl. 10):1094–1102. doi: 10.1902/jop.1996.67.10s.1094. [DOI] [PubMed] [Google Scholar]
- Hammarstrӧm L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Hamp S.E., Nyman S., Lindhe J. Periodontal treatment of multirroted teeth. Results after 5 years. J. Clin. Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Hegedus Z. The rebuilding of the alveolar process by bone transplantation. Dent. Cosmos. 1923;65:736–742. [Google Scholar]
- Heijl L., Heden G., Svärdström G., Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J. Clin. Periodontol. 1997;24(9 Pt 2):705–714. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Hughes F.J. Cytokines and cell signaling in the periodontium. Oral Dis. 1995;1:259–265. doi: 10.1111/j.1601-0825.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Hughes F.J., Turner W., Belibasakis G., Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol. 2000. 2006;41:48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Rajababu P., Rohini S., Butchibabu K., Naveen A., Reddy P.K. Multi-centre randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with beta-tricalcium phosphate in human intra-osseous periodontal defects. J. Clin. Periodontol. 2011;38:163–172. doi: 10.1111/j.1600-051X.2010.01639.x. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Akamatsu M., Machigashira M., Hara Y., Sakagami R., Hirofuji T. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J. Dent. Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- Libin B.M., Ward H.L., Fishman L. Decalcified, lyophilized bone allografts for use in human periodontal defects. J. Periodonto. 1975;46:51–56. doi: 10.1902/jop.1975.46.1.51. [DOI] [PubMed] [Google Scholar]
- Lin N.H., Menicacin D., Mrozik K., Gronthos S., Bartold P.M. Putative stem cells in regenerating human periodontium. J. Periodontal Res. 2008;53:514–523. doi: 10.1111/j.1600-0765.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Lovelace T.B., Mellonig J.T., Meffert R.M., Jones A.A., Nummikoski P.V., Cochran D.L. Clinical evaluation of bioactive glass in the treatment of periodontal osseous defects in humans. J. Periodontol. 1998;69:1027–1035. doi: 10.1902/jop.1998.69.9.1027. [DOI] [PubMed] [Google Scholar]
- Machtei E.E., Cho M.I., Dunford R., Norderyd J., Zambon J.J., Genco R.J. Clinical, microbiological, and histological factors which influence the success of regenerative periodontal therapy. J. Periodontol. 1994;65:154–161. doi: 10.1902/jop.1994.65.2.154. [DOI] [PubMed] [Google Scholar]
- Machtei E.E., Oettinger-Barak O., Peled M. Guided tissue regeneration in smokers: effect of aggressive anti-infective therapy in Class II furcation defects. J. Periodontol. 2003;74:579–584. doi: 10.1902/jop.2003.74.5.579. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Batich C., Collins B.R. New attachment formation following controlled tissue regeneration using biodegradable membranes. J. Periodontol. 1988;59:1–6. doi: 10.1902/jop.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- McCulloch C.A.G. Progenitor cell populations in the periodontal ligament of mice. Anat. Rec. 1985;211:258–262. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- Meffert R.M., Thomas J.R., Hamilton K.M., Brownstein C.N. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J. Periodontol. 1985;56:63–73. doi: 10.1902/jop.1985.56.2.63. [DOI] [PubMed] [Google Scholar]
- Melcher A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- Mellonig J.T. Autogenous and allogeneic bone grafts in periodontal therapy. Crit. Rev. Oral Biol. Med. 1992;3:333–352. doi: 10.1177/10454411920030040201. [DOI] [PubMed] [Google Scholar]
- Mellonig J.T. Donor selection, testing and inactivation of the HIV virus in freexe-dried bone allografts. Pract Periodontics Aesthet Dent. 1995;7:13–22. [PubMed] [Google Scholar]
- Mengel R., Schreiber D., Flores-de-Jacoby L. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: results of a 5-year clinical and radiological study. J. Periodontol. 2006;77:1781–1787. doi: 10.1902/jop.2006.060029. [DOI] [PubMed] [Google Scholar]
- Nabers C.L. Long-term results of autogenous bone grafts. Int. J. Periodontics Restorative Dent. 1984;4:50–67. [PubMed] [Google Scholar]
- Nabers C.L., O’Leary T.J. Autogenous bone transplants in the treatment of osseous defects. J. Periodontol. 1965;36:5–14. doi: 10.1902/jop.1965.36.1.5. [DOI] [PubMed] [Google Scholar]
- Nasr H.F., Aichelmann-Reidy M.E., Yukna R.A. Bone and bone substitutes. Periodontol. 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Needleman I., Tucker R., Giedrys-Leeper E., Worthington H. Guided tissue regeneration for periodontal intrabony defects – a cochrane systematic review. Periodontol. 2000. 2005;37:106–123. doi: 10.1111/j.1600-0757.2004.37101.x. [DOI] [PubMed] [Google Scholar]
- Nevins M.L., Camelo M., Nevins M., King C.J., Oringer R.J., Schenk R.K., Fiorellini J.P. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int. J. Periodontics Restorative Dent. 2000;20:458–467. [PubMed] [Google Scholar]
- Nevins M., Giannobile W.V., McGuire M.K., Kao R.T., Mellonig J.T., Hinrichs J.E. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J. Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- Nokhbehsaim M., Deschner B., Bourauel C., Reimann S., Winter J., Rath B. Interactions of enamel matrix derivative and biomechanical loading in periodontal regenerative healing. J. Periodontol. 2011;82:1725–1734. doi: 10.1902/jop.2011.100678. [DOI] [PubMed] [Google Scholar]
- Ouyang X.Y., Qiao J. Effect of platelet-rich plasma in the treatment of periodontal intrabony defects in humans. Chin. Med. J. (Engl). 2006;119:1511–1521. [PubMed] [Google Scholar]
- Paolantonio M., Femminella B., Coppolino E., Sammartino G., D’Arcangelo C., Perfetti G., Perinetti G. Autogenous periosteal barrier membranes and bone grafts in the treatment of periodontal intrabony defects of single-rooted teeth: a 12-month reentry randomized controlled clinical trial. J. Periodontol. 2010;81:1587–1595. doi: 10.1902/jop.2010.100094. [DOI] [PubMed] [Google Scholar]
- Papapanou P.N., Tonetti M. Diagnosis and epidemiology of periodontal osseous lesions. Periodontol. 2000. 2000;22:8–21. doi: 10.1034/j.1600-0757.2000.2220102.x. [DOI] [PubMed] [Google Scholar]
- Parashis A.O., Tsiklakis K., Tatakis D.N. EDTA gel root conditioning: lack of effect on clinical and radiographic outcomes of intrabony defect treatment with enamel matrix derivative. J. Periodontol. 2006;77:103–110. doi: 10.1902/jop.2006.77.1.103. [DOI] [PubMed] [Google Scholar]
- Patel R.A., Wilson R.F., Palmer R.M. The effect of smoking on periodontal bone regeneration: a systematic review and meta-analysis. J. Periodontol. 2012;83:143–155. doi: 10.1902/jop.2011.110130. [DOI] [PubMed] [Google Scholar]
- Pearson G.E., Rosen S., Deporter D.A. Preliminary observations on the usefulness of a decalcified, freeze-dried cancellous bone allograft material in periodontal surgery. J. Periodontol. 1981;52:55–59. doi: 10.1902/jop.1981.52.2.55. [DOI] [PubMed] [Google Scholar]
- Pitaru S., Tal H., Soldinger M., Azar-Avidan O., Noff M. Collagen membranes prevent the apical migration of epithelium during periodontal wound healing. J. Period Res. 1987;22:331–333. doi: 10.1111/j.1600-0765.1987.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Pontoriero R., Lindhe J. Guided tissue regeneration in the treatment of degree III furcation defects in maxillary molars. J. Clin. Periodontol. 1995;22:810–812. doi: 10.1111/j.1600-051x.1995.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Pai S., Garg G., Devi P., Shetty S.K. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J. Clin. Periodontol. 2009;36:581–588. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- Quattlebaum J.B., Mellonig J.T., Hensel N.F. Antigenicity of freeze-dried cortical bone allograft in human periodontal osseous defects. J. Periodontol. 1988;59:394–397. doi: 10.1902/jop.1988.59.6.394. [DOI] [PubMed] [Google Scholar]
- Renvert S., Garrett S., Shallhorn R.G., Egelberg J. Healing after treatment of periodontal intraosseous defects. III. Effect of osseous grafting and citric acid conditioning. J. Periodontol. 1985;12:441–455. doi: 10.1111/j.1600-051x.1985.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Ricci J.L., Blumenthal N.C., Spivak J.M., Alexander H. Evaluation of a low-temerature calcium phosphate particulate implant material: physical-chemical properties and in vivo bone response. J. Oral Maxillofac. Surg. 1992;50:969–978. doi: 10.1016/0278-2391(92)90058-8. [DOI] [PubMed] [Google Scholar]
- Richardson C.R., Mellonig J.T., Brunsvold M.A., McDonnell H.T., Cochran D.L. Clinical evaluation of Bio-Oss: a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J. Clin. Periodontol. 1999;26:421–428. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- Robinson E. Osseous coagulum for bone induction. J. Periodontol. 1969;40:503–510. doi: 10.1902/jop.1969.40.9.503. [DOI] [PubMed] [Google Scholar]
- Rosen P.S., Reynolds M.A., Bowers G.M. The treatment of intrabony defects with bone grafts. Periodontol. 2000. 2000;22:88–103. doi: 10.1034/j.1600-0757.2000.2220107.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M.M. Free osseous tissue autografts as a predictable procedure. J. Periodontol. 1971;42:195–209. doi: 10.1902/jop.1971.42.4.195. [DOI] [PubMed] [Google Scholar]
- Scantlebury T. 1982–1992: a decade of technology development for guided tissue regeneration. J. Periodontol. 1993;64:1129–1137. doi: 10.1902/jop.1993.64.11s.1129. [DOI] [PubMed] [Google Scholar]
- Schallhorn R.G. The use of autogenous hip marrow biopsy implants for bony crater defects. J. Periodontol. 1968;39:145–147. doi: 10.1902/jop.1968.39.1.145. [DOI] [PubMed] [Google Scholar]
- Scott T.A., Towle H.J., Assad D.A., Nicoll B.K. Comparison of bioabsorbable laminar bone membrane and non-resorbable Eptfe membrane in mandibular furcations. J. Periodontol. 1997;68:679–686. doi: 10.1902/jop.1997.68.7.679. [DOI] [PubMed] [Google Scholar]
- Sculean A., Berakdar M., Willershausen B., Arweiler N.B., Becker J., Schwarz F. Effect of EDTA root conditioning on the healing of intrabony defects treated with an enamel matrix protein derivative. J. Periodontol. 2006;77:1167–1172. doi: 10.1902/jop.2006.050300. [DOI] [PubMed] [Google Scholar]
- Selvig K., Kersten B., Chamberlain A., Wikesjӧ U., Nilveus R. Regenerative surgery of intrabony periodontal defects using ePTFE barrier membranes. Scanning electron microscopic evaluation of retrieved membranes vs. clinical healing. J. Periodontol. 1992;63:974–978. doi: 10.1902/jop.1992.63.12.974. [DOI] [PubMed] [Google Scholar]
- Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shahmiri S., Singh I.J., Stahl S.S. Clinical response to the use of the HTR polymer implant in human intrabony lesions. Int. J. Periodontics Restorative Dent. 1992;12:294–299. [PubMed] [Google Scholar]
- Sohrabi K., Saraiya V., Laage T.A., Harris M., Blieden M., Karimbux N. An evaluation of bioactive glass in the treatment of periodontal defects: a meta-analusis of randomized controlled clinical trials. J. Periodontol. 2012;83:453–464. doi: 10.1902/jop.2011.110347. [DOI] [PubMed] [Google Scholar]
- Stahl S.S., Froum S.J., Kushner L. Healing responses of human teeth following the use of debridement, grafting and citric acid root conditioning. II. Clinical and histologic observations: one year post-surgery. J. Periodontol. 1983;54:325–338. doi: 10.1902/jop.1983.54.6.325. [DOI] [PubMed] [Google Scholar]
- Stahl S.S., Froum S., Tarnow D. Human histologic responses to guided tissue regenerative techniques in intrabony lesions. J. Clin. Periodontol. 1990;17:191–198. doi: 10.1111/j.1600-051x.1990.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Stavropoulos A., Karring T. Long-term stability of periodontal conditions achieved following guided tissue regeneration with bioresorbable membranes: case series results after 6–7 years. J. Clin. Periodontol. 2004;31:939–944. doi: 10.1111/j.1600-051X.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- Stahl S.S., Slavkin H.C., Yamada L., Levine S. Speculations about gingival repair. J. Periodontol. 1972;43:395–402. doi: 10.1902/jop.1972.43.7.395. [DOI] [PubMed] [Google Scholar]
- Stein J.M., Fickl S., Yekta S.S., Hoischen U., Ocklenburg C., Smeets R. Clinical evaluation of a biphasic calcium composite grafting material in the treatment of human periodontal intrabony defects: a 12-month randomized controlled clinical trial. J. Periodontol. 2009;80:1774–1782. doi: 10.1902/jop.2009.090229. [DOI] [PubMed] [Google Scholar]
- Subbaiah R., Thomas B. Efficacy of a bioactive alloplast, in the treatment of human periodontal osseous defects-a clinical study. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e239–244. doi: 10.4317/medoral.16.e239. [DOI] [PubMed] [Google Scholar]
- Sykaras N., Opperman L.A. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J. Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- Taheri M., Molla R., Radvar M., Sohrabi K., Najafi M.H. An evaluation of bovine derived xenograft with and without a bioabsorbable collagen membrane in the treatment of mandibular class II furcation defects. Aust. Dent. J. 2009;54:220–227. doi: 10.1111/j.1834-7819.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Tarnow D., Fletcher P. Classification of the vertical component of furcation involvement. J. Periodontol. 1984;55:283–284. doi: 10.1902/jop.1984.55.5.283. [DOI] [PubMed] [Google Scholar]
- Tatakis D.N., Promsudthi A., Wikesjö U.M.E. Devices for periodontal regeneration. Periodontol 2000. 1999;19:59–73. doi: 10.1111/j.1600-0757.1999.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Trombelli L., Farina R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J. Clin. Periodontol. 2008;35:117–135. doi: 10.1111/j.1600-051X.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- Tsitoura E., Tucker R., Suvan J., Laurell L., Cortellini P., Tonetti M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J. Clin. Periodontol. 2004;31:643–647. doi: 10.1111/j.1600-051X.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Urist M.R., Mikulski A.J. A soluble bone morphogenetic protein extracted from bone matrix with a mixed aqueous and nonaqueous solvent. Proc. Soc. Exp. Biol. Med. 1979;162:48–53. doi: 10.3181/00379727-162-40616. [DOI] [PubMed] [Google Scholar]
- Villaça J.H., Rodrigues D.C., Novaes A.B., Jr., Taba M., Jr., Souza S.L. Root trunk concavities as a risk factor for regenerative procedures of class II furcation lesions in humans. J. Periodontol. 2004;75:1493–1499. doi: 10.1902/jop.2004.75.11.1493. [DOI] [PubMed] [Google Scholar]
- Yukna R.A. Osseous defects responses to hydroxyapatite grafting versus open flap debridement. J. Clin. Periodontol. 1989;16:398–402. doi: 10.1111/j.1600-051x.1989.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Yukna R.A. HTR polymer grafts in human periodontal osseous defects. I. 6-month clinical results. J. Periodontol. 1990;61:633–642. doi: 10.1902/jop.1990.61.10.633. [DOI] [PubMed] [Google Scholar]
- Yukna R.A., Krauser J.T., Callan D.P., Evans G.H., Cruz R., Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. 6-month results. J. Periodontol. 2000;71:1671–1679. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- Zeckner W., Tangl S., Tepper G., Fürst G., Bernhart T., Haas R. Influence of platelet-rich plasma on osseous healing of dental implants: a histologic and histomorphometric study in minipigs. Int. J. Oral Maxillofac. Implants. 2003;28:15–22. [PubMed] [Google Scholar]
Further reading
- Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J. Clin. Periodontol. 1997;24:693–696. doi: 10.1034/j.1600-051x.1997.00693.x. [DOI] [PubMed] [Google Scholar]