Abstract
Inoculation of alfalfa seedlings with root growth promoting microorganisms under semi-arid climate condition may improve biomass production and nutritive value. The current study aimed to investigate the effect of inoculation of alfalfa seedlings with Piriformospora indica (Pi) and co-inoculating Pi with Glomus intraradices (Gi + Pi) or Sinorhizobium meliloti (Sm + Pi) on hay yield, chemical composition, molecular structures by Fourier transformed infrared (FTIR) spectroscopy, in situ ruminal degradability and in vitro gas production. Seedlings were grown in experimental pots in a greenhouse until first cut and then transferred outside and cut a further 4 times. Biomass yield was similar across the treatments. Acid detergent fiber (ADF) concentration was higher in Pi than in control hay, and ADF decreased further with co-inoculation (P < 0.05). The ether extract (EE) concentration was lower for Pi and Gi + Pi compared with control hay, and control, Pi and Gi + Pi hays had lower EE concentration compared with Sm + Pi (P < 0.05). The FTIR spectroscopic vibration peak height ratio related to proteins (amide 1 + amide 2): total carbohydrate ratio was lower for the inoculation treatments compared with control hay (P < 0.05). In situ ruminal degradability of dry matter and organic matter were higher for hay of inoculated and co-inoculated seedlings than for control hay (P < 0.05). In conclusion, hay of alfalfa seedlings inoculated and co-inoculated with root growth promoting microorganisms had improved nutritional value compared with hay from non-treated alfalfa seedlings, and co-inoculation was the most effective, however, changes were relatively minor.
Keywords: Alfalfa hay, FTIR spectroscopy, Nutrient availability, Piriformospora indica, Ruminal degradability
1. Introduction
Alfalfa (Medicago sativa L.) is grown throughout the world and is the predominant legume forage source fed to dairy cows (Yari et al., 2014). Alfalfa produces high forage yield, and has high nutrient level, digestibility, and unique proportion of structural to non-structural carbohydrates (Elizalde et al., 1999, Yari et al., 2012a, Yari et al., 2012b). Alfalfa is native to warmer temperate climates, but has been adapted to various environmental conditions (Avci et al., 2013). However, the nutritional value (e.g., crude protein content, digestibility) and dairy cow performance on alfalfa grown in arid climate is often lower than for alfalfa grown in temperate regions around the world (Yari et al., 2012a, Yari et al., 2012b, Yari et al., 2014). The limited moisture availability and the high sun light intensity are primary reasons for more lignification of hay cell-walls from alfalfa grown in semi-arid regions (Van Soest, 1994). Lignin interferes with the digestion of cell-wall polysaccharides and decreases total tract digestibility and net energy value of alfalfa hay for ruminants (Elizalde et al., 1999, Yu et al., 2003).
Plant root growth promoting microorganisms can affect plant biochemical pathways (Ghabooli et al., 2013, Ghaffari et al., 2016), which might affect the nutritional value of forage produced. Over 90% of land plants form a ubiquitous symbiotic relationship between Arbuscular mycorrhizal fungus (Smith and Reed, 1997) and plant roots, which enhances the plant nutrition and growth (Augé, 2001) and therefore plants productivity (Sylvia et al., 1993). An experimental model for this group of fungi is Piriformospora indica, a versatile mycorrhiza-like fungus belonging to the order Sebacinales which can infect various taxonomically unrelated hosts (Peškan-Berghöfer et al., 2004, Oelmuller et al., 2009). This root fungus microorganism can increase plant growth and biomass by promoting root growth and might increase also plant resistance against biotic and abiotic environmental stresses such as drought (Varma et al., 1999, Oelmuller et al., 2009, Unnikumar et al., 2013). Glomus intraradices is an arbuscular mycorrhizal fungus used as a soil inoculant in agriculture and is one of the best mycorrhizal varieties of fungi available for myco-forestry (Kristek et al., 2005). The presence of G. intraradices and other endophyte fungus like P. indica in roots might have activated some mechanism for transpiration control, probably mediated by the enhancement of the abscisic acid (an inhibitor of stomatal opening), given that arbuscular mycorrhizal fungi apparently can produce abscisic acid (Scambato et al., 2010).
Sinorhizobium meliloti is a nitrogen-fixing α-proteobacterium that establishes root nodule symbiosis with legume plants, providing ammonia to their hosts and receiving nutrients from them (Jones et al., 2007). In free life or in symbiosis, these bacteria have to deal with adverse environmental conditions such as droughts, rain or floods, which cause severe changes in their extracellular osmolality (Paul, 2013).
The objectives of this study were to determine the effect of inoculation and co-inoculation of alfalfa seedlings with plant-growth-promoting microorganisms (G. intraradices, P. indica and S. meliloti) on forage hay yield, hay chemical composition, molecular structures by Fourier transformed infrared spectroscopy (FTIR), in situ ruminal degradability and in vitro gas production kinetics.
2. Materials and methods
2.1. Experimental design
This study was conducted at Malayer University (Malayer, Iran; latitude 34°30′N, longitude 48°85′E, altitude 1,550 m). The experiment was arranged in a completely randomized design with 4 treatments: alfalfa seedlings without inoculation (control, C), alfalfa seedling inoculated with P. indica alone (Pi), or co-inoculated with G. intraradices (Gi + Pi) or with S. meliloti (Sm + Pi). Each treatment was assigned randomly to 4 biological replicates.
2.2. Preparation of bio-inoculants
P. indica was cultured on complex medium (CM) and grown for 28 days (Peškan-Berghöfer et al., 2004). Spores were isolated in dH2O containing 0.05% tween-20 using a spreader and miracloth (Merck Biosystem, UK) and collected by centrifugation (1,207 × g at room temperature for 7 min). Finally, the spore concentration was adjusted to around 5 × 105 spores/mL using a hemocytometer and a microscope.
The inoculum of G. intraradices was multiplied on the roots of corn (Zea mays) in pot culture (grown on pre-sterilized pot mixture to eliminate contamination with other microbes in the medium). The plants were allowed to grow for 3 months after which the roots were severed and the substrate containing spores, hyphae and root segments were used for inoculating alfalfa seedlings (approximately 1,200 spores per 100 g). The S. meliloti inoculant used in the current study was strain A-11-8, supplied by Department of Biotechnology, Isfahan University of Technology (Isfahan, Iran). The strain was cultured in yeast mannitol suspension for 2 days at 28 ± 1 °C. At the time of inoculation, the suspension contained 108 to 109 cells/mL.
2.3. Culture and co-culture of plants and fungi
Before plant inoculation, experimental soil was autoclaved at 121 °C for 30 min, and alfalfa seeds of the cultivar “Hamedani” (Hamedan, Iran) were surface-sterilized with 70% ethanol (vol/vol) for 30 s followed by 6% sodium hypochlorite (NaClO) for 5 min. The seeds where then rinsed with water and germinated for 3 to 4 days. The mock-treated alfalfa seedlings were dipped in sterile water only. For the Pi treatment (n = 4), alfalfa seedlings were inoculated with P. indica by immersing in the spore suspension solution (to 5 × 105 spores/mL) with gentle shaking for 1 to 2 h. The pots assigned to the Gi + Pi treatment (n = 4) were first inoculated with 50 g of G. intraradices fungal inoculum by the layering method (Jackson et al.,1972) and then P. indica inoculated seedlings were placed in pots. For the Sm + Pi treatment (n = 4), S. meliloti inoculation was performed over the next 2 days after planting P. indica inoculated seedling. One milliliter 48-h-old bacterial culture (approximately 108 cells/mL) was added to the soil surrounding the root. Inoculated and non-inoculated seedlings were transferred into pots (20 cm diameter and 100 cm height) on February 2, 2014 and were placed in the greenhouse at 25 °C (day) and 15 °C (night), 50% and 80% relative humidity for day and night, respectively and received natural daylight. Pots were manually watered every 3 days till plants reached the vegetative growth stage and thereafter watered every 8 days.
2.4. Alfalfa harvesting and sample preparation
Alfalfa was harvested for the first time on May 20, 2014 in the greenhouse when plant reached the late bud growth stage. After this first cut, alfalfa plants were moved outside in the ground. Alfalfa plants were again harvested when they reached the late bud stage, which was on June 30, July 27, August 23 and September 13, 2014. Weather conditions during the trial are shown in Table 1. Harvested material was weighed fresh and after oven-drying at 50 °C for 48 h. For each treatment, plant material (i.e., hay) of the first 2 pots was pooled and material of pots 3 and 4 was pooled to generate 2 blocks for further analysis. The dried forage samples (4 alfalfa hay treatments × 5 cuts × 2 pooled blocks = 40) were ground trough a 2-mm screen before determination of in situ degradability and through a 1-mm screen prior to chemical analysis and in vitro gas production incubations. Samples per treatment from different cuts were pooled to 1 sample (4 alfalfa hay treatments × 2 pooled blocks = 8) to generate sufficient material for chemical composition analysis and FTIR spectroscopy scanning (Table 2).
Table 1.
Weather conditions around 5 harvests of alfalfa with or without inoculation of root growth promoting microorganisms in 2014.
| Cut No.1 | Date of harvest | Minimum temperature, °C | Maximum temperature, °C | Sunrise time | Sunset time | Dew point, °C | Rainfall, mL |
|---|---|---|---|---|---|---|---|
| 2 | June 30 | 20 | 35 | 05:14 | 20:33 | 5.0 | 0.0 |
| 3 | July 27 | 23 | 35 | 05:33 | 20:22 | 3.0 | 0.0 |
| 4 | Aug. 23 | 17 | 32 | 06:06 | 19:55 | 1.0 | 0.0 |
| 5 | Sept. 13 | 14 | 32 | 06:23 | 19:26 | −7.0 | 0.0 |
The first cut was harvested in greenhouse and other cuts were harvested out of the greenhouse.
Table 2.
Effect of alfalfa seedling inoculation and co-inoculation with root growth promoting microorganisms on alfalfa hay chemical composition and predicted hay quality characteristics for ruminants.
| Item | Treatments1 |
|||||
|---|---|---|---|---|---|---|
| C | Pi | Gi + Pi | Sm + Pi | SEM | P-value | |
| Mean of stage of maturity2 | 2.4 | 2.2 | 2.2 | 2.3 | 0.12 | 0.35 |
| Chemical composition, % dry matter | ||||||
| Dry matter | 26.3 | 24.5 | 24.0 | 24.6 | 1.75 | 0.45 |
| Organic matter | 89.1 | 89.2 | 88.9 | 88.6 | 0.18 | 0.22 |
| Ash | 10.9 | 10.8 | 11.1 | 11.4 | 0.18 | 0.23 |
| Ether extract | 2.40c | 3.34b | 3.15b | 3.77a | 0.115 | <0.01 |
| Crude protein | 17.2 | 16.5 | 16.4 | 16.6 | 0.34 | 0.39 |
| Neutral detergent fiber | 41.7 | 39.6 | 40.9 | 40.3 | 0.76 | 0.29 |
| Acid detergent fiber | 37.4a | 36.3b | 34.8c | 34.0c | 0.45 | <0.01 |
| Acid detergent lignin | 7.0 | 7.3 | 6.0 | 6.2 | 0.43 | 0.14 |
| Cellulose | 30.4 | 30.0 | 28.6 | 28.0 | 0.58 | 0.07 |
| Hemicellulose | 4.2 | 3.2 | 6.2 | 6.4 | 1.23 | 0.08 |
| Total carbohydrate | 69.5 | 69.4 | 69.4 | 68.2 | 0.44 | 0.20 |
| Non-fiber carbohydrate | 27.8 | 29.8 | 28.5 | 27.9 | 0.89 | 0.42 |
SEM = standard error of the mean.
a, b, c Within a row, means without a common superscript differ (P < 0.05).
C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti.
Stage of maturity at time of cutting was determined based on Kalu and Fick (1981), which indicated that alfalfa plants were between early bud stage and late bud stages of growth when harvested (0 to 9 scales).
2.5. Chemical composition analysis and predicted forage quality
Standard procedures described by the Association of Official Analytical Chemists (AOAC, 1991) were used to determine dry matter (DM; method 930.15), ash (AOAC method 942.05), crude protein (CP; AOAC method 984.13) and ether extract (EE; AOAC method 954.02). Neutral detergent fiber (NDF), assayed with heat stable alpha-amylase, and acid detergent fiber (ADF) were determined according to the methods of Van Soest et al. (1991) with the ANKOM A200 Filter Bag Technique (Ankom Technology, Fairport, NY, USA). Acid detergent lignin (ADL) was determined by soaking the ADF filter bag residue in 72% sulphuric acid for 3 h followed by washes with water (AOAC method 973.18). All chemical analyses were performed in duplicate and repeated if required. Hemicellulose (NDF–ADF), cellulose (ADF–ADL), non-fiber carbohydrates (NFC) = 100 × (NDF + CP + EE + ash) and total carbohydrates = 100 × (CP + EE + ash) were calculated according to NRC (2001).
2.6. FTIR scanning of samples
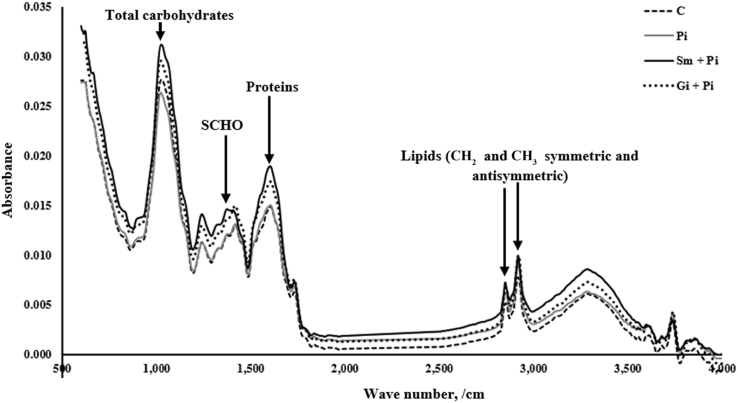
The infra-red (IR) absorbance band of samples was determined using a FTIR spectroscopy (Bruker Tensor 27, Bruker Optics Inc., Billerica, MA, USA) coupled with a universal attenuated total reflectance accessory. The samples were finely ground and pressed uniformly against the diamond surface using a spring-loaded anvil, and the mid-IR spectra recorded from a resolution of 4,000 to 600/cm at 2/cm (Ghasemi et al., 2013). Typical spectral from alfalfa hay samples in the current study and absorbance bands of interest are shown in Fig. 1. Each alfalfa hay sample was scanned 4 times (alfalfa hay treatment × 2 pooled blocks × 2 sub-samples = 16). Regions of IR spectra identified related to proteins (amide 1 + amide 2; 1,483 to 1,708/cm), total carbohydrate (852 to 1,186/cm), structural carbohydrate (1,188.8 to 1,482.9/cm) and lipids (2,770 to 3,000/cm) (Yu et al., 2004, Yari et al., 2013).
Fig. 1.
Typical full spectra of different alfalfa hay samples from alfalfa seedlings inoculated and co-inoculated with root growth promoting microorganisms. C: control; Pi, inoculated with P. indica; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices. SCHO = structural carbohydrates; studied regions were total carbohydrates, 852 to 1,186/cm; SCHO, 1188.9 to 1482.9/cm; proteins (amide 1 + amide 2), 1,483 to 1,708/cm; lipids, 2,770 to 3,000/cm; 4 spectra for each alfalfa hay were used (pooled cuts × 2 pooled blocks × 2 sub-sample).
2.7. In situ ruminal degradability
In situ ruminal incubations were performed as described by Ørskov and McDonald (1979) using 2 ruminal fistulated Mehraban ram lambs (body weight = 35.5 ± 2.5 kg) fed 1 kg DM/day (in g/kg DM; total mixed ration with 120 g chopped alfalfa hay, 540 g chopped wheat straw, 70 g wheat bran, 80 g ground barley, 170 g beet pulp, 10 g mineral-vitamin supplement, 5 g NaCl and 5 g bentonite) twice daily in equal portions. Feeding and animal husbandry of the lambs were according to procedures by the Iranian Council on Animal Care guidelines (ICAC, 1995). Approximately 2 g of each alfalfa samples (5 cuts × 4 treatments × 2 blocks = 40 samples) was placed in Dacron bags (5 cm × 10 cm and pore size of 40 μm). The bags were incubated in the rumen for 12 h (10 bags per incubation). Immediately after retrieval from the rumen, bags were manually washed and oven-dried at 50 °C for 48 h (Yari et al., 2012b). All incubations were performed in 2 runs. The residues in the bags were analyzed for DM and ash as mentioned previously.
2.8. In vitro gas production
A semi-automated system was used for in vitro gas production incubations as described by Theodorou et al. (1994) and Rogerio et al. (1999), with buffered rumen fluid prepared according to Menke and Steinglass (1988). Rumen fluid was collected before the morning feeding from 2 ruminal fistulated ram lambs (same animals as previously mentioned). After collection, ruminal contents were strained through 4 layers of cheese cloth to eliminate large feed particles, and then transported to the laboratory in a pre-warmed thermos.
Each sample (4 treatments × 5 cuts × 2 blocks = 40 samples) was incubated in triplicate vials (125 mL) with 10 mL of rumen liquid and 20 mL of buffer (Menke and Steinglass, 1988) for 96 h at 37.5 °C. Three vials with buffered rumen medium, without sample, were incubated to correct for gas release from the inoculum. Head-space gas accumulation were measured using a pressure transducer (Razi Instruments, Mashhad, Iran), and gas volume was predicted by Boyle's gas law from pressure measurements as: Gp = (Vh/Pa) × Pt, where Gp represents head-space gas volume, Vh, head-space volume (95 mL), Pa represents atmospheric pressure (101,298.77 Pa; Meteorological Office, Mashhad, Iran), and Pt represents pressure transducer reading (Theodorou et al., 1994, Rogerio et al., 1999). Head-space pressure readings were taken at 0, 2, 4, 8, 12, 20, 24, 48, 72 and 96 h after the start of incubation. All incubations were performed into 2 runs. The rate and extent of gas production were determined for each vial by fitting gas production data over time to the nonlinear equation Y = b (1–1/expct), where Y is the volume of gas produced at time t, b is the asymptotic gas production, and c is the fractional rate of gas production (Ørskov and McDonald, 1979). Parameters b and c were calculated using the nonlinear procedure of SAS using iterative least-squares regression (Gauss-Newton method; SAS, 2003). Linear regression equations developed by Menke and Steinglass (1988) were used to estimate organic matter digestibility based on cumulative gas volume at 24 h of incubation and sample CP, fat and ash concentrations.
2.9. Statistical analysis
Data of the current study were analyzed using PROC MIXED of SAS 9.2 (SAS, 2003) with the following statistical models:
Yijk = μ + Ti + Cj + Bk + (T × C)ij + eijk ,
Yij = μ + Ti + Bj + eij ,
where Y (Yij and Yijk) is the observation of the dependent variable ij and ijk; μ is the fixed effect of population mean for the variable; Ti is the fixed effect of treatments (i = 4; C, Pi, Gi + Pi and Sm + Pi); Cj is the fixed effect of cut (j = 5), Bk and Bj is the random effect of pooled block (k = 2, i = 2), and (eijk and eij) are the random errors associated with the observation ijk and ij. Model 1 was used to analyze data of forage yield, in situ DM and OM degradability after 12 h and in vitro gas production kinetics. Model 2 was used to analyze data of chemical composition.
The Fisher's protected least significant difference test was used for multiple treatment comparisons using the LSMEAN statement of SAS. For the different statistical tests, significance was declared at P ≤ 0.05 and trends were considered at P ≤ 0.10. The FTIR spectrum per alfalfa hay samples were subjected to univariate analysis by model 2 (using SAS, 2003) and multivariate cluster and principle component analysis (using Statistica v8; Yari et al., 2013).
3. Results
3.1. Forage yield, chemical composition, predicted energy values and forage quality parameters
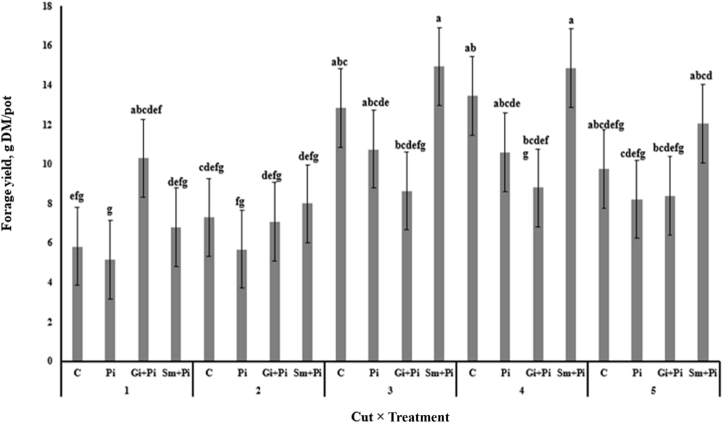
In cut 1, forage hay yield was higher for Gi + Pi treatment compared with Pi treatment. The Sm + Pi treatment had higher hay yield compared with Gi + Pi during cut 3 and 5 (Fig. 2). Hay yield was similar among inoculation treatments in cuts 2 and 5. Wet chemical composition of alfalfa hay was similar among treatments except for EE and ADF (Table 2). The EE concentration was higher in Sm + Pi alfalfa compared with Pi, Gi + Pi and C alfalfa hay. The EE concentration was also higher in Pi and Gi + Pi alfalfa hay than in C alfalfa hay (P < 0.05). The ADF concentration was lower in 3 other treatments compared with the C alfalfa hay (P < 0.05). The ADF concentration was higher in Pi than in Sm + Pi and Gi + Pi alfalfa hays (P < 0.05). Cellulose concentration tended to be reduced and hemicellulose tended to be increased in Sm + Pi and Gi + Pi treatments (P = 0.07 and 0.08, respectively) compared with C and Pi treatments.
Fig. 2.
Effect of alfalfa seedling inoculated and co-inoculated with root growth promoting microorganisms on alfalfa hay forage yield at 5 cuts. C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti. a–g Means with different letter differ at P < 0.05.
3.2. FTIR molecular structures
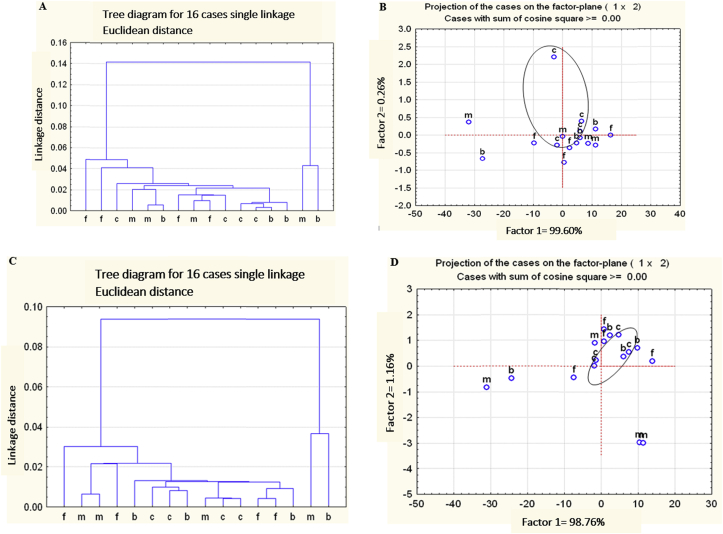
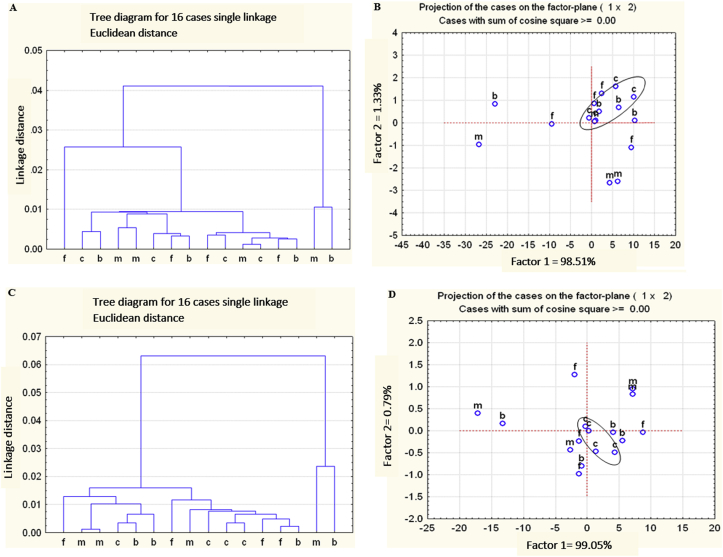
Peak height for total carbohydrate, structural carbohydrate, proteins and lipids were similar among 4 alfalfa hay treatments (Table 3). The Pi, Sm + Pi and Gi + Pi alfalfa hay treatments had higher peak height ratio of proteins to total carbohydrates compared with control alfalfa hay (P < 0.05). Spectra in the region of total carbohydrates (Fig. 3A and B), structural carbohydrates (Fig. 3C), lipids (Fig. 4A), and proteins (amide 1 + amide 2) (Fig. 4C) for the 4 alfalfa hay treatment groups could not be distinguished by cluster analysis and principle component analysis.
Table 3.
Effect of alfalfa seedling inoculation and co-inoculation with root growth promoting microorganisms on alfalfa hay Fourier transformed infrared spectroscopy (FTIR) molecular structures.
| Item1 | Treatments2 |
||||||
|---|---|---|---|---|---|---|---|
| C | Pi | Gi + Pi | Sm + Pi | SEM | P-value | ||
| FTIR molecular structures peak height | |||||||
| TCHO | 0.02837 | 0.02581 | 0.03078 | 0.03057 | 0.007246 | 0.95 | |
| SCHO | 0.01193 | 0.01304 | 0.01488 | 0.01495 | 0.003919 | 0.93 | |
| Proteins (amide 1 + amide 2) | 0.01449 | 0.01498 | 0.01856 | 0.01899 | 0.004478 | 0.84 | |
| Lipids | 0.00743 | 0.00852 | 0.00920 | 0.00932 | 0.002659 | 0.95 | |
| FTIR molecular peak height ratios | |||||||
| Proteins: SCHO | 1.2155 | 1.1414 | 1.2893 | 1.3452 | 0.06881 | 0.22 | |
| Proteins:TCHO | 0.5124b | 0.575a | 0.5938a | 0.6247a | 0.02068 | 0.01 | |
| Protein:lipid | 1.9586 | 1.8315 | 2.1191 | 2.1750 | 0.10,450 | 0.13 | |
| SCHO:TCHO | 0.4262 | 0.5044 | 0.4649 | 0.4713 | 0.03066 | 0.38 | |
| Lipids:TCHO | 0.2626 | 0.3118 | 0.2825 | 0.2912 | 0.02074 | 0.30 | |
SEM = standard error of the mean; SCHO = structural carbohydrate; TCHO = total carbohydrate.
a, b Within a row, means without a common superscript differ (P < 0.05).
TCHO, 852 to 1,186/cm; SCHO, 1,188.9 to 1,482.9/cm; Proteins (amide 1 + amide 2), 1,483 to 1,708/cm; lipids (CH2 and CH3, symmetric and antisymmetric functional groups) 2,770 to 3,000/cm; proteins: SCHO, ratio of proteins (amide 1 + amide 2) to SCHO; proteins: TCHO, ratio of proteins (amide 1 + amide 2) to TCHO; proteins: lipids, ratio of proteins (amide 1 + amide 2) to lipids; SCHO: TCHO, ratio of SCHO to TCHO.
C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti.
Fig. 3.
Multivariate analysis of Fourier transformed infrared spectroscopy (FTIR) spectrum of different alfalfa hays from alfalfa seedlings inoculated and co-inoculated with root growth promoting microorganisms. Spectra were in the region related (A, B) total carbohydrates, 852 to 1,186/cm and (C, D) structural carbohydrates, 1,188.9 to 1,482.9/cm of alfalfa hay from control group (c), inoculated with P. indica (f), co-inoculation with P. indica and G. intraradices (m) and co-inoculation of P. indica and S. meliloti (b) by (A, C) cluster analysis and (B, D) principal component analysis; 4 spectra for each alfalfa hay were used (pooled cuts × 2 pooled blocks × 2 sub-sample).
Fig. 4.
Multivariate analysis of FTIR spectrum of different alfalfa hays from alfalfa seedlings inoculated and co-inoculated with root growth promoting microorganisms. Spectra were in the region related to (A, B) lipids, 2,770 to 3,000/cm and (C, D) proteins (amide 1 + amide 2), 1,483 to 1,708/cm, of alfalfa hay from control group (c) inoculated with P. indica (f), co-inoculation with P. indica and G. intraradices (m) and co-inoculation of P. indica and S. meliloti (b) by (A, C) cluster analysis and (B, D) principal component analysis; 4 spectra for each alfalfa hay were used (pooled cuts × 2 pooled blocks × 2 sub-sample).
3.3. In situ ruminal degradability at 12 h and in vitro gas production parameters
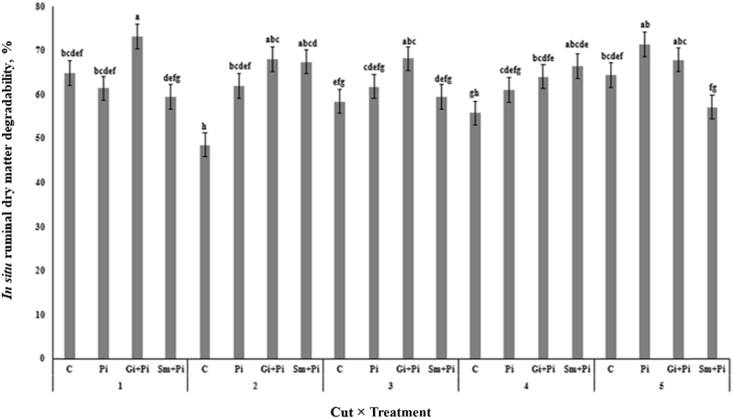
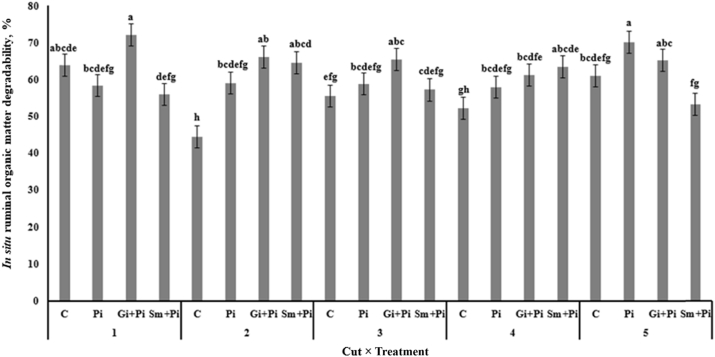
Ruminal in situ DM degradability in cut 1 was higher for Gi + Pi alfalfa hay compared with C, Pi and Sm + Pi alfalfa hays; in cut 2 higher for Pi, Sm + Pi and Gi + Pi alfalfa hays compared with C alfalfa hay; in cut 3 higher for Gi + Pi alfalfa hay compared with Sm + Pi and C alfalfa hay, in cut 4 higher for Sm + Pi and Gi + Pi alfalfa hay compared with C alfalfa hay and in cut 5 higher for Pi and Gi + Pi alfalfa hay compared with Sm + Pi alfalfa hay (Fig. 5). Ruminal in situ OM degradability in cut 1 was higher for Gi + Pi alfalfa hay compared with Pi and Sm + Pi alfalfa hay; in cut 2 higher for Pi, Sm + Pi and Gi + Pi alfalfa hays than C alfalfa hay; in cut 3 higher for Gi + Pi alfalfa hay compared with C alfalfa hay; in cut 4 higher for Sm + Pi and Gi + Pi alfalfa hay compared with C alfalfa hay, and in cut 5 higher for Pi and Gi + Pi alfalfa hay compared with and Sm + Pi alfalfa hay (Fig. 6).
Fig. 5.
Effect of alfalfa seedling inoculation and co-inoculation with root growth promoting microorganisms on in situ 12 h ruminal DM degradability of alfalfa hay at 5 cuts. C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti. a–g Means with different letters differ at P < 0.05.
Fig. 6.
Effect of alfalfa seedling inoculated and co-inoculated with root growth promoting microorganisms on in situ 12 h ruminal organic matter degradability of alfalfa hay at 5 cuts. C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti. a–g Means without a common letter differ at P < 0.05.
Gas production kinetics and predicted potential of nutrient supply (organic matter digestibility) were also similar among the 4 alfalfa hays (Table 4).
Table 4.
Effect of alfalfa seedling inoculation and co-inoculation with root growth promoting microorganisms on alfalfa hay in vitro ruminal fermentation kinetics using gas production method.
| Item1 | Treatments2 |
|||||
|---|---|---|---|---|---|---|
| C | Pi | Gi + Pi | Sm + Pi | SEM | P-value3 | |
| b, mL/g DM | 199 | 203 | 204 | 208 | 16.7 | 0.51 |
| c, /h | 0.068 | 0.070 | 0.071 | 0.065 | 0.0055 | 0.25 |
| Gas 24 h, mL/g DM | 156 | 162 | 163 | 163 | 5.07 | 0.76 |
| Predicted potential nutrient supply | ||||||
| OMD, % | 57.7 | 58.1 | 58.5 | 58.4 | 0.90 | 0.93 |
b, potential of gas production; c, rate of gas production; gas 24 h, cumulative gas production till 24 h of incubation; OMD, predicted organic matter digestibility (Menke and Steinglass, 1988).
C, control; Pi, seedlings inoculated with P. indica; Gi + Pi, co-inoculation of seedlings with P. indica and G. intraradices; Sm + Pi, co-inoculation of seedlings with P. indica and S. meliloti.
The P-value of the effect of cut was 0.05 and 0.003 for b and c, respectively. The effect of treatment × cut interaction was 0.85.
4. Discussions
4.1. Forage yield
Inoculation or co-inoculation of alfalfa seedlings had no effect on forage yield compared with no inoculation of alfalfa seedlings in the current study, while inoculation of barley seedlings with P. indica was found to increase biomass yield (Ghabooli et al., 2013, Ghabooli, 2014). These authors indicated that P. indica changed some biochemical pathways in the plant which promoted higher biomass yield. The lack of response in forage yield in the current study may be due to different alfalfa variety or growth conditions. The current results are, however, not in accordance with findings of Azcon et al. (1979) who reported that alfalfa seed inoculation with mycorrhiza fungus and rhizobium bacterium increased biomass yield. Co-inoculation of those microorganisms with P. indica might be more effective than the combinations tested in the current study. In most of the cases in literature where P. indica improved biomass yield, plants were grown under environmental stress condition (Waller et al., 2005, Ghabooli et al., 2013, Murphy et al., 2014). Under normal growing conditions, the effect of plant inoculations with P. indica on forage biomass yield may be negligible or negative depending on plant species (Augé, 2001, Nadeem et al., 2014). Therefore, inoculation of alfalfa seedlings with P. indica under stress or water deficiency condition warranties further researches.
4.2. Chemical composition and molecular structures of alfalfa hay
Inoculation and co-inoculation of alfalfa seedlings increased EE content in alfalfa hay samples of the current study. The EE fraction consists of components soluble in ether, which mostly consist of lipids and pigment waxes (AOAC, 1991). Therefore, inoculation with root microorganisms as used in the current study with alfalfa seedlings may affect the synthesis of those components. Increased synthesis of waxes may help the plant to protect its organs against environmental stress conditions or diseases (Van Soest, 1994). Co-inoculation of Gi + Pi did not increase EE content beyond inoculation with P. indica only, while co-inoculation of Sm + Pi increased the EE content compared Pi alone. The higher EE content may be due to higher absorption of soil nutrient by alfalfa roots as a result of symbiosis with these endophytic microorganisms. The P. indica has been reported to stimulate the phosphorous and water absorption (Ghaffari et al., 2016), and the synthesis of ATP, cell walls and phospholipids in plants were previously found to increase with increasing phosphorous absorption (Kristek et al., 2005). While EE increased with inoculation, lipid molecular structures investigated using FTIR, were not affected by inoculation, although they increased numerically with (co)inoculation. It seems that the EE fraction might not relate to lipid structures detected by FTIR.
The ADF content was higher for Pi alfalfa hay than C alfalfa hay, and ADF concentration declined even further with co-inoculation (Gi + Pi and Sm + Pi). The ADF fraction consists mostly of cellulose and lignin (Van Soest et al., 1991) and the lignin fraction tended to be lower for Gi + Pi alfalfa hay than in C hay. Ghabooli et al. (2013) found that inoculation of barley with P. indicai changed the expression pattern in plants related to metabolism of major and minor carbohydrates, which might explain the decreased ADF (and tendency for decreased lignin) with (co)inoculation with P. indica in the current study. Co-inoculation treatments tended to decrease the cellulose and increase the hemicellulose concentration of alfalfa hay, which indicates that the carbohydrate composition changed with root microorganism inoculation.
The FTIR spectra in the region of 1,482.9 to 1,188.9/cm, which mainly relates to structural carbohydrates including cellulose and lignin (Ghasemi et al., 2013), was similar among alfalfa hays as determined by either univariate or multivariate analysis. The CP content was similar among hay of the 4 alfalfa treatments, and univariate and multivariate analysis of FTIR spectra in the protein region were also similar among the alfalfa hays.
4.3. Predicted alfalfa hay quality, digestion and availability
It seems alfalfa hay quality improved with Pi inoculation and improved further with co-inoculation. The decreased ADF content with Pi inoculation and co-inoculation might explain the improved hay quality because ADF concentration has been found to have significant impact on alfalfa hay quality in ruminant (Horrocks and Valentine, 1999, Yu et al., 2003, Yari et al., 2012a, Yari et al., 2012b), and its concentration has a negative relation with forage digestibility (Van Soest, 1994, NRC, 2001). In situ DM degradability in cuts 2 and 4 and in situ organic matter degradability in cuts 2, 3, 4 and 5 of alfalfa hay were increased in the current study by inoculation and to a greater extent by co-inoculation of alfalfa seedlings. This supports the findings of the differences in ADF and predicted alfalfa hay quality characteristics with inoculation and co-inoculation. However, results of inoculation and co-inoculation on ruminal degradability depended on cut number, which might be due to differences in weather condition such as temperature, sunlight duration and intensity and humidity during growth of the plants. These wheatear conditions have been found to affect digestibility (Weir et al., 1960, Van Soest, 1994, Yari et al., 2012a) and might affect the expression of the inoculation and co-inoculation treatments.
The in vitro ruminal gas production method can also be used to predict the potential nutrient supply of feeds for ruminants (Menke and Steingass, 1988, Getachew et al., 2004). Fermentation kinetics and gas accumulation at different times of incubation were similar among the 4 alfalfa hays in the current study and therefore did not support the findings in terms of alfalfa hay quality characteristics and in situ degradability described above. In vitro fermentation characteristics from forages were previously found to be affected mainly by the ratio between nitrogen and organic matter and nitrogen and total carbohydrate (Stefanon et al., 1996, Cone and Van Gelder, 1999). These ratios were similar among 4 alfalfa hays in the current study, which might explain the similar in vitro fermentation characteristics in the current study.
The FTIR peak height ratio of protein to total carbohydrates was lower in C alfalfa hay compared with hay from inoculated and co-inoculated alfalfa seedlings, which suggests higher spectroscopic vibration related to protein relative to carbohydrates for hay of the 3 inoculated seedling groups. Yari et al. (2013) found that alfalfa hay quality and FTIR peak height ratio of protein to total carbohydrates decreased with advancing maturity at which alfalfa hay was harvested.
5. Conclusions
Alfalfa seedlings were inoculated with P. indica alone and co-inoculated with G. intraradices or S. meliloti and then grown under semi-arid field conditions. Inoculation of alfalfa seedlings with P. indica increased EE concentration and decreased acid detergent fiber concentration in alfalfa hays, which they were enhanced even further with co-inoculation. Hay quality characteristics improved by inoculation and co-inoculation of alfalfa seedlings in terms of predicted total digestible nutrient, digestible DM and net energy for lactation, in situ 12 h ruminal degradability of DM and organic matter and FTIR spectroscopic vibration peak height ratio related to molecular structure of proteins (amide 1 + amide 2):total carbohydrate ratio. Overall, inoculation and co-inoculation improved alfalfa hay nutrient profile and quality while maintaining forage biomass yield under semi-arid field condition. However, changes were relatively minor and further research is needed to investigate the benefit of using these root growth promoting microorganisms under a wider range of farming conditions, in different seasons and years including under stress condition such as water deficiency.
Acknowledgment
The authors thank the Malayer University for laboratory support.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Augé R.M. Water relations, drought and VA mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. 2001. [Google Scholar]
- Avci M.A., Ozkose A., Tamkoc A. Determination of yield and quality characteristics of alfalfa (Medicago sativa l.) varieties grown in different locations. J Anim Vet Adv. 2013;12:487–490. [Google Scholar]
- Azcon G., de Aguilar C., Azcón R., Barea J. Endomycorrhizal fungi and Rhizobium as biological fertilisers for Medicago sativa in normal cultivation. Nature. 1979;279:325–327. [Google Scholar]
- Cone J.W., Van Gelder A.H. Influence of protein fermentation on gas production profiles. Anim Feed Sci Technol. 1999;76:251–264. [Google Scholar]
- Elizalde J., Merchen N., Faulkner D. Fractionation of fiber and crude protein in fresh forages during the spring growth. J Anim Sci. 1999;77:476–484. doi: 10.2527/1999.772476x. [DOI] [PubMed] [Google Scholar]
- Getachew G., Robinson P., DePeters E., Taylor S. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim Feed Sci Technol. 2004;111:57–71. [Google Scholar]
- Ghabooli M. Effect of Piriformospora indica inoculation on some physiological traits of barley (Hordeum vulgare) under salt stress. Chem Nat Compd. 2014;50:1082–1087. [Google Scholar]
- Ghabooli M., Khatabi B., Ahmadi F.S., Sepehri M., Mirzaei M., Amirkhani A. Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J Proteomics. 2013;94:289–301. doi: 10.1016/j.jprot.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Ghaffari M.R., Ghabooli M., Khatabi B., Hajirezaei M.R., Schweizer P., Salekdeh G.H. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol Biol. 2016;90:699–717. doi: 10.1007/s11103-016-0461-z. [DOI] [PubMed] [Google Scholar]
- Ghasemi E., Ghorbani G., Khorvash M., Emami M., Karimi K. Chemical composition, cell wall features and degradability of stem, leaf blade and sheath in untreated and alkali-treated rice straw. Animal. 2013;7:1106–1112. doi: 10.1017/S1751731113000256. [DOI] [PubMed] [Google Scholar]
- Horrocks R.D., Valentine J.F. Academic Press; 1999. Harvested forages. [Google Scholar]
- Jones K.M., Kobayashi H., Davies B.W., Taga M.E., Walker G.C. How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICAC, Iranian Council of Animal Care . vol. 1. Isfahan University of Technology; Isfahan, Iran: 1995. (Guide to the Care and Use of Experimental Animals). [Google Scholar]
- Jackson N.E., Franklin R.E., Miller R.H. Effects of vesicular-arbuscular mycorrhizae on growth and phosphorus content of three agronomic crops. Soil Sci Soc Am Proc. 1972;36:64–67. [Google Scholar]
- Kalu B.A., Fick G.W. Quantifying morphological development of alfalfa for studies of herbage quality. Crop Sci. 1981;21:267–271. [Google Scholar]
- Kristek S., Kristek A., Pavlovic H. The influence of mycorrhizal fungi (Glomus sp.) on field pea plant survival and growth in drought caused stress conditions. Plant Soil Environ. 2005;51:385. [Google Scholar]
- Menke K.H., Steingass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 1988;28:7–55. [Google Scholar]
- Murphy B.R., Doohan F.M., Hodkinson T.R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 2014;62:29–39. [Google Scholar]
- Nadeem S.M., Ahmad M., Zahir Z.A., Javaid A., Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv. 2014;32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 7th revised ed. National Academic Science; Washington, DC, USA: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- Oelmuller R., Sherameti I., Tripathi S., Varma A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis. 2009;49:1–17. [Google Scholar]
- 15th ed. 1991. Official methods of analysis of the AOAC International. Arlington, VA. [Google Scholar]
- Ørskov E., McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979;92:499–503. [Google Scholar]
- Paul D. Osmotic stress adaptations in rhizobacteria. J Basic Microbiol. 2013;53:101–110. doi: 10.1002/jobm.201100288. [DOI] [PubMed] [Google Scholar]
- Peškan-Berghöfer T., Shahollari B., Giang P.H., Hehl S., Markert C., Blanke V. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant–microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plantarum. 2004;122:465–477. [Google Scholar]
- Rogerio M.M., Fergus L.M., Mewa S.D., Emyr O., Kulwant S.C., Michael K.T. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol. 1999;79:321–330. [Google Scholar]
- Scambato A.A., Echeverria M., Sansberro P., Ruiz O.A., Menéndez A.B. Glomus intraradices improved salt tolerance in Prosopis alba seedlings by improving water use efficiency and shoot water content. Braz J Plant Physiol. 2010;22:285–289. [Google Scholar]
- Smith S.E., Reed D.J. 2nd ed. Academic Press; London: 1997. Mycorrhizal symbiosis; p. 589. [Google Scholar]
- Stefanon B., Pell A., Schofield P. Effect of maturity on digestion kinetics of water-soluble and water-insoluble fractions of alfalfa and brome hay. J Anim Sci. 1996;74:1104–1115. doi: 10.2527/1996.7451104x. [DOI] [PubMed] [Google Scholar]
- Sylvia D.M., Hammond L.C., Bennett J.M., Haas J.H., Linda S.B. Field response of maize to a VAM fungus and water management. Agron J Biosci. 1993;85:193–198. [Google Scholar]
- Theodorou M.K., Williams B.A., Dhanoa M.S., McAllan A.B., France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol. 1994;48:185–197. [Google Scholar]
- Unnikumar K.R., Sowjanya S.K., Varma A. Piriformospora indica: a versatile root endophytic symbiont. Symbiosis. 2013;60:107–113. [Google Scholar]
- Van Soest P.J. Cornell University Press; 1994. Nutritional ecology of the ruminant. [Google Scholar]
- Van Soest P.J., Robertson J., Lewis B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Varma A., Verma S., Sudah S.N., Franken P. Piriformospora indica, a cultivable plant growth-promoting root endophyte. Appl Environ Microbiol. 1999;65:2741–2744. doi: 10.1128/aem.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller F., Achatz B., Fodor H.B.J., Becker K., Fischer M., Heier T. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir W., Jones L., Meyer J.H. Effect of cutting interval and stage of maturity on the digestibility and yield of alfalfa. J Anim Sci. 1960;19:5–19. [Google Scholar]
- Yari M., Valizadeh R., Naserian A.A., Ghorbani G., Moghaddam P.R., Jonker A. Botanical traits, protein and carbohydrate fractions, ruminal degradability and energy contents of alfalfa hay harvested at three stages of maturity and in the afternoon and morning. Anim Feed Sci Technol. 2012;172:162–170. [Google Scholar]
- Yari M., Valizadeh R., Naserian A.A., Jonker A., Yu P. Modeling nutrient availability of alfalfa hay harvested at three stages of maturity and in the afternoon and morning in dairy cows. Anim Feed Sci Technol. 2012;178:12–19. [Google Scholar]
- Yari M., Valizadeh R., Naserian A.A., Jonker A., Yu P. Protein molecular structures in alfalfa hay cut at three stages of maturity and in the afternoon and morning and relationship with nutrient availability in ruminants. J Sci Food Agric. 2013;93:3072–3080. doi: 10.1002/jsfa.6141. [DOI] [PubMed] [Google Scholar]
- Yari M., Valizadeh R., Naserian A.A., Jonker A., Azarfar A., Yu P. Effects of including alfalfa hay cut in the afternoon or morning at three stages of maturity in high concentrate rations on dairy cows performance, diet digestibility and feeding behavior. Anim Feed Sci Technol. 2014;192:62–72. [Google Scholar]
- Yu P., Christensen D., McKinnon J., Markert J. Effect of variety and maturity stage on chemical composition, carbohydrate and protein subfractions, in vitro rumen degradability and energy values of timothy and alfalfa. Can J Anim Sci. 2003;83:279–290. [Google Scholar]
- Yu P., Mckinnon J.J., Christensen C.R., Christensen D.A. Using synchrotron-based FTIR microspectroscopy to reveal chemical features of feather protein secondary structure: comparison with other feed protein sources. J Agric Food Chem. 2004;52:7353–7361. doi: 10.1021/jf0490955. [DOI] [PubMed] [Google Scholar]