Abstract
This review is an outlook for sorghum as a feed grain for broiler chickens based on a survey of relevant stake-holders and recent research outcomes. Australian grain sorghum production will probably continue to generate a harvest in the order of 2.5 × 106 t of which some 7.9 × 105 t will be used as a feed grain for poultry and pigs. Feed grains are included primarily to provide energy from starch, but energy utilisation by broiler chickens offered sorghum-based diets is relatively inferior, because of incomplete starch digestion. Kafirin, the dominant protein fraction, ‘non-tannin’ phenolic compounds and phytate are 3 ‘starch extrinsic’ factors in sorghum that compromise starch digestibility and energy utilisation in broiler chickens offered sorghum-based diets. Kafirin concentrations in 6 sorghum varieties were negatively correlated with metabolizable energy to gross energy (ME:GE) ratios (r = −0.891; P < 0.02) or the efficiency of energy utilisation in broiler chickens. Importantly, kafirin proportions of sorghum protein may be increasing with time in Australia. If so, this represents a fundamental challenge to sorghum breeders which presumably could be met by the development of sorghum varieties with different characteristics, especially in relation to the γ- and β-kafirin fractions. White sorghum varieties contain lower polyphenol concentrations which should be advantageous as concentrations of total phenolic compounds were negatively correlated to ME:GE ratios (r = −0.838; P < 0.04) in 6 sorghum varieties. It would be desirable if more white varieties were to become available. It is suggested that responses to exogenous phytase in birds offered sorghum-based diets would be more robust if sorghum were to contain lower concentrations of kafirin and phenolic compounds. Paradoxically, while better sorghum varieties almost certainly could be developed, it may not necessarily follow that they will command a price premium from poultry and pig producers.
Keywords: Kafirin, Phenolic compounds, Phytate, Poultry, Sorghum, Starch
1. Introduction
Australian grain sorghum production averaged 2.75 × 105 t through the 1960s but increased to an average of 1.035 × 106 t in the 1970s. This considerable increase almost certainly reflects the demand of the then emerging beef feedlot industry. However, the Australian sorghum harvest has effectively plateaued out with an average crop of 2.046 × 106 t from 2000 to 2016 inclusive on the basis of United States Department of Agriculture data (www.indexmundi.com). The sorghum harvest has ranged from 1.282 × 106 t (2013) to 3.790 × 106 t (2007) during this period. In 2012, the Queensland Department of Agriculture and Fisheries stated that the sorghum trade is completely deregulated and the sorghum produced is used almost exclusively for feed, especially cattle, pigs and poultry, and this totals around 1.4 × 106 t. Also the department asserted that a significant market exists for sorghum in the pet food industry and there is a substantial export market for sorghum but it is not used for human consumption.
Nearly a decade ago, Wylie (2008) suggested that there is the potential to increase average sorghum yields by 1 t/ha, which coupled with a 50% increase in the area grown, would result in a sorghum harvest of 4 × 106 t. It was claimed that this would be a modest projection for Australian sorghum production by 2012, compared to average production over the past 5 years of 2 × 106 t. Despite the bullish forecast of 4 × 106 t, the actual 2012 Australian sorghum harvest was 2.230 × 106 t which reflects the status quo.
The Australian chicken-meat industry is perhaps the single largest ‘customer’ for grain sorghum. Based on Australian Chicken Meat Federation data, the industry processes 630 × 106 birds annually, each bird consumes in the order of 4.34 kg of feed which represents a demand of about 2.7 × 106 t for total feed and approximately 1.6 × 106 t for feed grains, which are the main component of broiler diets. Wheat and sorghum are the 2 feedstuffs that provide the majority of this foundation. Wheat is produced throughout most grain regions of Australia while sorghum is grown primarily in southern Queensland and northern New South Wales. Wheat is the dominant feed grain in southern Australia and usually attracts a premium to sorghum in the regions where sorghum is grown.
More than a decade ago, Hughes and Brooke (2005) concluded that sorghum is regarded as a relatively consistent source of energy in comparison to wheat but with the caveat that anecdotal evidence suggests that some commercial broiler flocks respond poorly to sorghum-based diets. Such concerns about the nutritive value of sorghum for broiler chickens prompted investigations (Bryden et al., 2009a, Perez-Maldonado and Rodrigues, 2009) and reviews (Gidley et al., 2011a, Selle, 2011) in quests to identify the underlying problems. A review of the implications of sorghum in broiler chicken nutrition by Selle et al. (2010a) preceded numerous research papers arising from a sequence of projects that investigated various aspects of sorghum as a feed grain for chicken-meat production. Some of this work is summarised in a book chapter (Selle et al., 2013a) and in 2 review papers (Liu et al., 2013, Liu et al., 2015).
Theoretically, the Australian chicken-meat industry could absorb the majority of the sorghum crop in a ‘normal’ year, quite apart from the potential demand from pork producers and the beef feedlot industry. However, this is not the case as quite large quantities of grain sorghum may be exported, especially to China. Thus, Australian pig and poultry producers are somewhat reluctant to purchase sorghum to meet their feed grain requirements. The purpose of this review is to generate an outlook for sorghum as a feed grain for Australian chicken-meat production. The outlook is based on an amalgamation of both a survey of personnel involved in the sorghum growing, pig and poultry industries in Australia and the outcomes of recent research generated by the Poultry Research Foundation. The ‘open access’ papers arising from this research are listed in Table 1. The primary objective is to identify the steps that should be taken so that the inclusions of grain sorghum in diets for broiler chickens are increased, perhaps substantially, which would be to the mutual advantage of both parties; the grain sorghum and chicken-meat industries.
Table 1.
Open access publications arising out of the ‘sorghum starch’ project.
| Publication | Digital object identifier |
|---|---|
| Khoddami A, Truong HH, Liu SY, Roberts TH, Selle PH (2015). Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broilers. Animal Feed Science and Technology 210, 190–199. | http://dx.doi.org/10.1016/j.anifeedsci.2015.09.029 |
| Liu SY, Truong HH, Khoddami A, Moss AF, Thomson PC, Roberts TH, Selle PH (2016). Comparative performance of broiler chickens offered ten equivalent diets based on three grain sorghum varieties as determined by response surface mixture design. Animal Feed Science and Technology 218, 70–83. | http://dx.doi.org/10.1016/j.anifeedsci.2016.05.008 |
| Liu SY, Fox G, Khoddami A, Nielsen KA, Truong HH, Moss AF, Selle PH (2015). Grain sorghum: a conundrum for chicken-meat production. Agriculture 5, 1224–1251. | http://dx.doi.org/10.3390/agriculture5041224 |
| Selle PH, Truong HH, McQuade LR, Moss AF, Liu SY (2016). Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient digestibility and digestive dynamics in broiler chickens. Animal Nutrition 2, 303–311. | http://dx.doi.org/10.1016/j.aninu.2016.08.001 |
| Selle PH, Truong HH, Khoddami A, Moss AF, Roberts TH, Liu SY (2016). The impacts of hammer-mill screen size and grain particle size on the performance of broiler chickens offered diets based on two red sorghum varieties. British Poultry Science. | http://dx.doi.org/10.1080/00071668.2016.1257777 |
| Truong HH, Neilson KA, McInerney BV, Khoddami A, Roberts TH, Liu SY, Selle PH (2015). Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or reground mash following steam-pelleting at 65 or 97 °C conditioning temperatures. Animal Nutrition 1, 220–228. | http://dx.doi.org/10.1016/j.aninu.2015.08.002 |
| Truong HH, Khoddami A, Moss AF, Liu SY Selle PH (2016). The potential of RVA starch pasting profiles to gauge the quality of sorghum as a feed grain for chicken-meat production. Animal Nutrition. | http://dx.doi.org/10.1016/j.aninu.2016.11.001 |
| Truong HH, Cadogan DJ, Liu SY, Selle PH (2016). Addition of sodium metabisulphite and microbial phytase individually and in combination, to a sorghum-based diet for broiler chickens from 7 to 28 days post-hatch. Animal Production Science 56, 1484–1491. | http://dx.doi.org/10.1071/AN14841 |
| Truong HH, Neilson KA, McInerney BV, Khoddami A, Roberts TH, Cadogan DJ, Liu SY, Selle PH (2016). Comparative performance of broiler chickens offered nutritionally-equivalent diets based on six diverse, ‘tannin-free’ sorghum varieties with quantified concentrations of phenolic compounds, kafirin, and phytate. Animal Production Science 57, 828–838. | http://dx.doi.org/10.1071/AN16073 |
| Truong HH, Neilson KA, McInerney BV, Khoddami A, Roberts TH, Liu SY, Selle PH (2016). Sodium metabisulphite enhances energy utilisation in broiler chickens offered sorghum-based diets with five different grain varieties. Animal Feed Science and Technology 219, 159–174. | http://dx.doi.org/10.1016/j.anifeedsci.2016.06.016 |
2. Grain sorghum survey outcomes
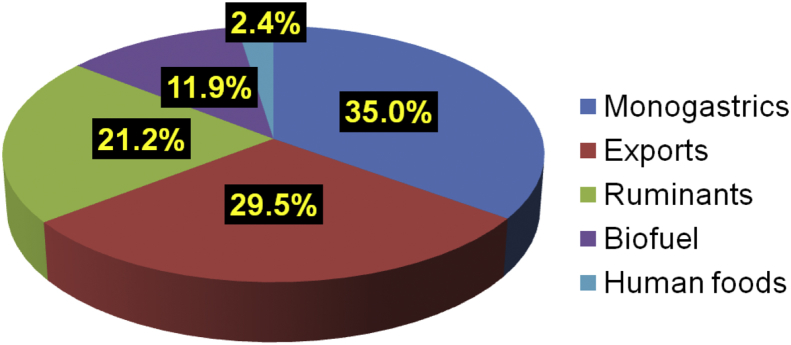
A total of 31 people responded to a survey of 17 questions with opportunities to comment via SurveyMonkey. The respondents consisted in the main of ‘coal-face’ poultry nutritionists, personnel involved in various aspects of grain sorghum production and swine nutritionists. Given a notional average sorghum crop of 2 × 106 t, the respondents estimated that 7 × 105 t would be absorbed by the poultry and pig industries (monogastrics), 4.24 × 105 t by ruminants or essentially feedlot cattle (ruminants), 5.9 × 105 t by exports, and 2.38 × 105 t by biofuel production as illustrated in Fig. 1. The survey respondents forecast that the annual sorghum harvest in ‘5 to 10 years' time’ would be 2.530 × 106 t which does not represent a tangible increase on current production. It was considered that the 2 market segments most likely to expand will be the use of sorghum as a feed grain by the poultry and pig industries and the export trade to China. Dry land cotton, maize and mung beans were seen as the alternative crops most likely to compete with sorghum but the majority view was that sorghum plantings would expand, albeit slightly. Crops that could find their way into the human food chain were considered to be at an advantage. In the main, respondents predicted that if improved sorghum varieties were to be developed, there would be either some or even pronounced increases in sorghum purchases by the chicken-meat industry. Nevertheless, the likelihood that chicken-meat producers would be prepared to pay a price premium for improved sorghum varieties drew conflicting opinions. On one hand, 21% of respondents did not think producers would be prepared to pay a premium; by contrast, 38% of respondents felt that producers would meet a price premium. The development of highly suitable sorghum varieties for inclusion in pig and poultry should result in higher market prices but this eventuality is somewhat problematic.
Fig. 1.
Estimated usage of a notional 2 × 106 t Australian grain sorghum harvest.
2.1. Value of sorghum relative to wheat
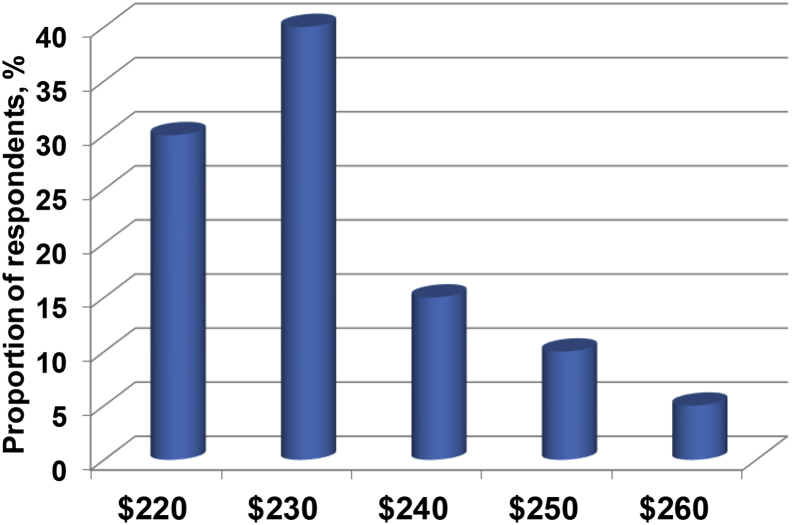
Respondents were asked to value sorghum relative to wheat, given a wheat price of $250/t. As shown in Fig. 2, sorghum was valued at from $220 to $260/t with an average value of $232/t. Nevertheless, 70% of respondents indicated that they would prefer to pay less than the mean outcome. The fact that wheat can notionally command a price premium of $18 (or more) over sorghum essentially stems from the usually higher protein contents of wheat. On a global basis, the protein content of sorghum is 92 g/kg as opposed to 117 g/kg for wheat according to AMINODat 5.0 data. This is somewhat ironic in that broiler chicks offered iso-nitrogenous diets based on relatively low protein sorghum outperformed their counterparts on relatively high-protein sorghum as demonstrated by Truong et al. (2015a). As discussed later, the superior growth performance of birds offered low protein sorghum was attributed to the lower dietary kafirin concentrations in the sorghum-based diet.
Fig. 2.
Estimated value of grain sorghum relative to wheat at $250/t.
2.2. Negative general factors
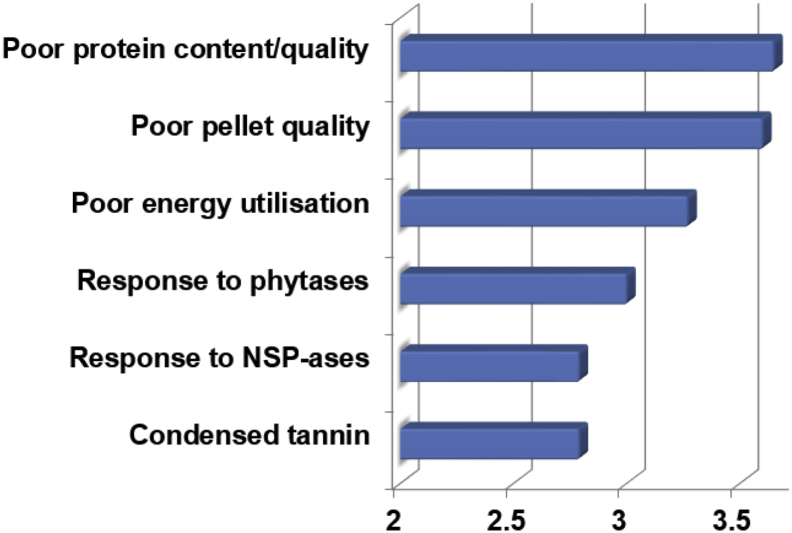
Ranking of importance of general factors considered to be negatively influencing the performance of broiler chickens offered sorghum-based diets are illustrated in Fig. 3. In descending order, substandard content/quality of sorghum protein and poor pellet quality of sorghum-based diets were considered to be the most important factors followed by inadequate energy utilisation and then modest responses to phytate degrading and non-starch polysaccharide (NSP) degrading feed enzymes. In Australia, the average protein content of sorghum is 89 g/kg as opposed to 118 g/kg for wheat according to AMINODat 5.0; this suggests wheat typically contains 33% more crude protein than sorghum. The substandard protein quality of grain sorghum was reviewed by Selle (2011) with the suggestion that any improvements in sorghum protein quality may also enhance energy utilisation. Poor pellet quality of sorghum-based diets stems from the relatively high sorghum starch gelatinisation temperatures (Taylor and Dewar, 2001). The lack of response to NSP-degrading feed enzymes in birds offered sorghum-based diets is predictable because sorghum is a ‘non-viscous’ grain with low soluble NSP contents. In the survey, 48% of respondents ‘strongly agreed’ with the statement that pigs and poultry are advantaged by inclusions of NSP-degrading feed enzymes in wheat-based, as opposed to sorghum-based, diets. However, sorghum certainly contains phytate (Doherty et al., 1982, Selle et al., 2003), so the modest responses to exogenous phytase in birds offered sorghum-based diets are not due to low substrate levels. Interestingly, 22% of respondents ‘strongly agreed’ that it is more advantageous to include phytases in wheat-based diets in comparison to sorghum-based diets.
Fig. 3.
Ranking of importance of general factors on the x-axis considered to be negatively influencing the performance of broiler chickens offered sorghum-based diets. NSP = non-starch polysaccharides.
2.3. Negative specific factors
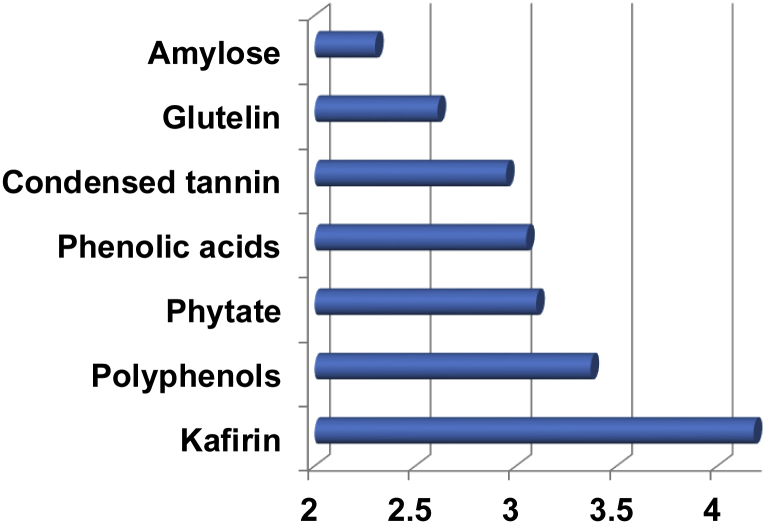
Ranking of importance of specific factors considered to be negatively influencing the performance of broiler chickens offered sorghum-based diets are illustrated in Fig. 4. In descending order, kafirin, polyphenols, phytate and phenolic acids were thought to be the most important factors followed by condensed tannin, glutelin and amylose. Kafirin is the dominant protein fraction in sorghum representing some 55% of total protein and is present in sorghum endosperm as spherical protein bodies. The majority view of respondents was that kafirin impedes starch/energy utilisation (68%) and that this is attributable to biophysical and/or biochemical interactions between kafirin and starch (52%). Also, the majority of respondents (59%) believed that kafirin was a poorly digestible protein and a minority (24%) felt that kafirin had an inferior amino acid profile. Sorghum contains higher concentrations of polyphenols that alternative feed grains (Bravo, 1998); some polyphenolic compounds are red pigments and for this reason red varieties contain more polyphenols than white sorghums. A total of 15 Australian sorghums contained an average of 2.41 g/kg phytate-P (or 8.55 g/kg phytate) which represented 82.7% of the total P content of 2.92 g/kg (Selle et al., 2003). On the basis of this survey, sorghum contained somewhat more phytate than barley, maize or wheat. The dominant phenolic acid in sorghum is ferulic acid which, unlike polyphenolic compounds, is not confined to sorghum. That condensed tannin was considered to be a negative factor is a surprising outcome and it appears that the stigma associated with high tannin, ‘bird-proof’ sorghums partially persists.
Fig. 4.
Ranking of importance of specific factors on the x-axis considered to be negatively influencing the performance of broiler chickens offered sorghum-based diets.
2.3.1. Condensed tannin
Condensed tannin is a polyphenolic compound that may be present in sorghum and its anti-nutritive properties have been well documented (Nyachoti et al., 1997). Indeed, on the basis of the Australian study by McClymont and Duncan (1952), condensed tannin could be described as a toxic factor in poultry. Survey respondents considered condensed tannin was exerting some negative effects but they were also asked a specific question as to whether or not condensed tannin was present in contemporary Australian sorghum crops. The most consistent opinion was that condensed tannin “may be present in local sorghums but levels are negligible”.
Thus it would seem that some respondents consider that negligible condensed tannin levels are still capable of compromising broiler performance. Despite indications to the contrary (Perez-Maldonado and Rodrigues, 2009), the authors are entirely satisfied that condensed tannin is not present in contemporary Australian sorghum crops. This conclusion is based on negligible condensed tannin concentrations in 6 sorghum cultivars determined by vanillin/HCl assays (Khoddami et al., 2015) and negative quantal Clorox bleach tests (Waniska et al., 1992) in more than 50 sorghum cultivars.
2.4. ‘Red vs. white’ sorghums
A 60% majority of respondents strongly agreed with the statement that white sorghums are superior feed grains to red sorghums as opposed to 36% of respondents who believed that white and red sorghums are comparable. That only small quantities of white sorghums are grown, probably less than 5% of the national crop, was attributed to the lack of any financial incentive to grow white sorghum, its relative susceptibility to weather damage and the availability of only one white variety (Liberty). There is the belief that white sorghums are more likely to be down-graded because mould and discolouration are more evident than in red varieties. Nevertheless, white sorghums axiomatically contain less polyphenolic compounds than red sorghums; this is of interest given their perceived superiority as a feed grain for pigs and poultry.
2.5. Biofuel
According to the Australian Broadcasting Commission (ABC) in January 2017, the biorefinery in Dalby will benefit from a Queensland Government mandate which ensures that ethanol-blended E10 fuel makes up at least 30% of petrol available for sales in that state. The ABC indicated that the Dalby plant will process 2 × 105 t of grain sorghum annually which is in agreement with the 2.38 × 105 t forecasted in this survey. The majority of respondents anticipate that the amount of sorghum diverted from the food chain for bioethanol production will increase moderately. However, it was obvious that this is a highly emotive issue based on the respondents' comments. These ranged from “good news for the sorghum industry as there is now a sustainable supply chain” to a “waste of sorghum”. Another respondent opined that the “mandated ethanol use is a government (i.e., taxpayer) subsidy to grain farmers to the detriment of livestock producers and domestic consumers”. Irrespective of the ethical and ecological merits of converting food and feed grains into biofuel, the practice will impact on the outlook for sorghum.
3. Grain sorghum as a feed grain for chicken-meat production
Our contention is that the quality of sorghum as a feed grain for chicken-meat production is better than its perceived value; nevertheless, scope for improvement remains. The primary reason for including sorghum in diets for broiler chickens is for the provision of energy which is mainly derived from its starch component. However, the utilisation of starch/energy in sorghum by poultry is suboptimal. Black et al. (2005) reported that the amount of energy required to generate 1 kg live-weight gain in chickens offered a sorghum-based diet exceeded that of a wheat-based diet (20.9 vs. 19.8 MJ apparent metabolism energy [AME]/kg gain) to a significant extent. Moreover, the digestibility of sorghum starch is inferior to that of maize on the basis of both in vitro (Giuberti et al., 2012) and in vivo (Liu et al., 2014a, Truong et al., 2016a) data. The inadequacy of sorghum starch digestibility is evident in Table 2, where a mean distal ileal coefficient of only 0.866 was recorded in 5 studies. The average amylose proportion of starch was 30.7% with a range from 26.4% to 37.9% in 13 sorghum grains (Truong et al., 2017). However, the amylose to amylopectin ratio in these sorghums was not related to starch digestibility coefficients in poultry. In a review of wheat, Wiseman et al. (2000) expressed the opinion that it is not starch per se that is poorly utilised in some wheat samples but other inherent factors in wheat are responsible for reducing starch digestibility. This principle seems to be at least equally applicable to sorghum so consideration was given to other ‘starch extrinsic’ factors inherent in sorghum including kafirin, phenolic compounds and phytate, that may be compromising starch/energy utilisation in broilers offered sorghum-based diets.
Table 2.
Apparent starch digestibility coefficients in 4 small intestinal segments of broiler chickens offered diets based on 8 grain sorghum varieties from 7 to 28 days post-hatch in 5 feeding studies.1
| Item | PJ | DJ | PI | DI |
|---|---|---|---|---|
| Mean | 0.657 | 0.768 | 0.838 | 0.866 |
| Minimum | 0.476 | 0.704 | 0.807 | 0.793 |
| Maximum | 0.734 | 0.830 | 0.897 | 0.918 |
| Number of observations | 12 | 15 | 12 | 15 |
PJ = proximal jejunum; DJ = distal jejunum; PI = proximal ileum; DI = distal ileum.
Data derived from Liu et al., 2016, Selle et al., 2016a, Truong et al., 2016a, Truong et al., 2016b, Truong et al., 2016c.
3.1. Kafirin
The survey respondents clearly identified kafirin as the most important negative factor inherent in grain sorghum. Kafirin is the dominant protein fraction in sorghum that is present as discrete protein bodies in sorghum endosperm and sits in close proximity to starch granules. Both kafirin protein bodies and starch granules are embedded in the glutelin protein matrix of the endosperm. The prevailing view is that kafirin impedes starch utilisation (Taylor, 2005). Presumably this is via biophysical and/or biochemical protein–starch interactions (Rooney and Pflugfelder, 1986), although dissenting opinions have been expressed (Gidley et al., 2011b).
The real possibility that kafirin compromises starch/energy utilisation in sorghum-based diets was seen as a critical issue. This is because there is some evidence that the proportion of kafirin of total protein is increasing in Australian sorghum crops (Selle, 2011), probably as an inadvertent outcome of sorghum selection programs. Kafirin proportions of sorghum protein increase at the expense of glutelin with elevating sorghum protein contents on the basis Taylor et al. (1984) data. The accurate quantification of kafirin in sorghum was a real breakthrough and the methodology has been described in detail (Truong et al., 2015a).
As reviewed by Liu et al. (2015), a meta-analyses of relevant studies with non-significant experimental leverages demonstrated that kafirin concentrations significantly depressed metabolizable energy to gross energy (ME:GE) ratios (P < 0.04) and tended to depress N-corrected AME (AMEn) (P < 0.075) in broiler chickens offered sorghum-based diets (Table 3). Both AME and AMEn are absolute values but ME:GE ratios effectively express the efficiency of energy utilisation. Thus, it was demonstrated by this meta-analysis that kafirin does compromise energy utilisation efficiency.
Table 3.
The relationship between dietary concentrations of kafirin and energy utilisation in broiler chickens offered sorghum-based diets.1
| Item | Observations (n) | Experiment leverage (P) | Dietary kafirin leverage (P) | Whole model |
|---|---|---|---|---|
| ME:GE ratio | 10 | 0.109 | 0.034 | R2 = 0.71 |
| P = 0.045 | ||||
| AMEn | 13 | 0.485 | 0.013 | R2 = 0.70 |
| P = 0.074 |
ME = metabolizable energy; GE = gross energy; AMEn = N-corrected apparent metabolizable energy.
Adapted from Liu et al. (2015).
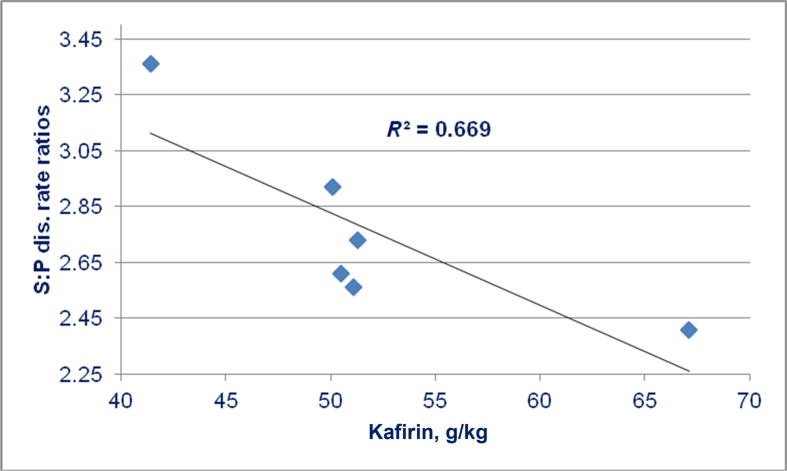
Truong et al. (2016b) evaluated 6 sorghum varieties in which kafirin concentrations ranged from 41.4 to 67.1 g/kg or from 46.2% to 51.4% kafirin as a proportion of sorghum protein. Kafirin concentrations were significantly negatively correlated to ME:GE ratios (r = −0.891; P < 0.02) and N retention (r = −0.887; P < 0.025). Also, as illustrated in Fig. 5, kafirin concentrations were negatively correlated (r = −0.818; P < 0.05) with starch:protein disappearance rate ratios in the distal ileum. This shows that as kafirin in sorghum increased less starch relative to protein was effectively being absorbed at the end of the small intestine. Thus, kafirin was impeding starch disappearance, or glucose absorption, to a greater extent than protein disappearance and amino acid absorption.
Fig. 5.
Linear relationship (r = −0.818; P < 0.05) between kafirin concentrations in 6 sorghum varieties and starch:protein (S:P) disappearance (dis.) rate ratios in the distal ileum of chicks offered sorghum-based diets (adapted from Truong et al., 2016b).
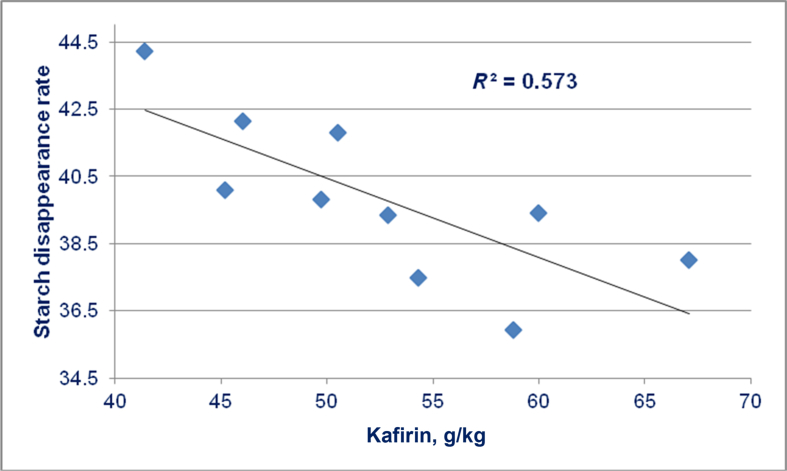
Liu et al. (2016) evaluated 3 sorghum varieties by ‘nutritional geometry’ or an equilateral triangle response surface mixture design involving 10 dietary treatments. Kafirin concentrations in the 10 sorghum-based diets tended to be negatively correlated (r = −0.607; P = 0.063) to weight gains of chicks offered these diets. However, kafirin concentrations were significantly negatively correlated to ME:GE ratios (r = −0.801; P < 0.005), AMEn (r = −0.658; P < 0.04) and starch disappearance rates in the distal jejunum (r = −0.759; P < 0.02). The significant negative linear relationship (r = −0.757; P < 0.02) between kafirin concentrations in 10 sorghum-based diets and starch disappearance rates g/(bird·d) in the distal ileum of chickens is shown in Fig. 6. This demonstrates that kafirin in sorghum was impeding starch disappearance, or glucose absorption, along the small intestine.
Fig. 6.
Linear relationship (r = −0.757; P = 0.011) between kafirin concentrations in 10 sorghum-based diets and starch disappearance rates g/(bird·d) in the distal ileum of chickens (adapted from Liu et al., 2016).
As previously mentioned, 2 red sorghums harvested on the Liverpool Plains of New South Wales in 2009 with protein contents of 99.4 g/kg (Sorghum #3) and 116.3 g/kg (Sorghum #5) were compared by Truong et al. (2015a). Broilers offered Sorghum #3-based diets significantly (P < 0.001) outperformed their Sorghum #5 counterparts in weight gain by 3.75% (1,334 vs. 1,223 g/bird), feed conversion ratio (FCR) by 4.81% (1.524 vs. 1.601), AME by 1.06 MJ/kg (13.61 vs. 12.55 MJ/kg), ME:GE ratio by 4.81% (0.806 vs. 0.769), N retention by 5.6 percentage units (63.6% vs. 58.0%) and AMEn by 1.03 MJ/kg (12.38 vs. 11.35 MJ/kg). Instructively, there were no real differences between the 2 sorghums for starch content (624 vs. 620 g/kg), amylose (26.4% vs. 27.2%), phytate (8.33 vs. 8.51 g/kg), total phenolic compounds (3.52 vs. 3.59 mg gallic acid equivalent [GAE]/g) and total phenolic acids (545 vs. 538 μg/g). However, Sorghum #3 contained 50.7 g/kg kafirin but Sorghum #5 contained 61.5 g/kg or 21.3% more kafirin. Moreover, the iso-nitrogenous diets contained either 56.9 or 74.8 g/kg kafirin, thus diets based on Sorghum #5 contained 31.5% more kafirin. It was concluded that the superiority of Sorghum #3 stemmed from its lower kafirin concentrations relative to Sorghum #5.
Salinas et al. (2006) reported that kafirin as a proportion of sorghum protein was negatively correlated to true ME and AMEn in poultry. However, it may be deduced from the Salinas et al. (2006) study that absolute kafirin concentrations were not significantly correlated to these parameters of energy utilisation. By contrast, our research group has generated unequivocal evidence that absolute kafirin concentrations have negative impacts on energy utilisation in birds offered sorghum-based diets. The precise mechanisms whereby kafirin compromises energy utilisation have yet to be identified although it is thought that kafirin partially prevents swelling of starch granules and starch gelatinisation and impedes access of amylase to its substrate. Taylor and Emmambux (2010) proposed that disulphide cross-linkages between the cysteine rich periphery (β-kafirin and γ-kafirin) of protein bodies and starch granule-associated proteins are involved. Earlier, Hamaker and Bugusu (2003) showed that disulphide-mediated polymerisation of kafirin results in sheet-like protein structures in which starch is embedded following wet-cooking of sorghum which should impede starch digestion.
Truong et al. (2016e) recorded the amino acid profiles of kafirin in 2 Australian sorghums varieties. As shown in Table 4, the average amino acid profiles of kafirin in these 2 sorghums are in very close agreement with data reported by Xiao et al. (2014) for 3 sorghum varieties. It is evident that kafirin contains a paucity of basic amino acids, especially lysine, but an abundance of leucine. The abundance of leucine is probably disadvantageous as leucine may depress feed intakes via the mechanistic target of rapamycin (mTOR)-dependent mechanisms (Morrison et al., 2007). The inadequacies of the kafirin amino acid profile are only compounded by the fact that the digestibility of kafirin protein/amino acids is poor because kafirin is hydrophobic and poorly soluble. However, as kafirin constitutes about 15% of total protein in a sorghum-based broiler diet, these inadequacies can be addressed by formulating diets on the basis of digestible amino acids. Thus the poor protein quality of kafirin, and consequently sorghum, is not seen as an insurmountable obstacle. However, the fact that kafirin compromises starch/energy utilisation is a tangible problem which would be costly to rectify by higher dietary inclusions of tallow or vegetable oil.
Table 4.
Mean amino acid profiles of kafirin (g/100 g protein) in 2 Australian sorghums (MP, HP) sorghums in comparison to amino acid profile of one USA sorghum.
| Amino acid | MP and HP sorghums1 | One USA sorghum2 |
|---|---|---|
| Arginine | 2.2 | 2.0 |
| Histidine | 1.9 | 1.6 |
| Isoleucine | 4.1 | 3.0 |
| Leucine | 15.8 | 17.5 |
| Lysine | 0.5 | 0.1 |
| Methionine | 1.2 | 2.1 |
| Phenylalanine | 5.7 | 6.6 |
| Threonine | 2.7 | 2.9 |
| Valine | 4.8 | 3.8 |
| Alanine | 10.1 | 11.8 |
| Aspartic acid | 6.1 | 6.0 |
| Glutamic acid | 24.3 | 28.2 |
| Glycine | 2.1 | 1.4 |
| Proline | 9.5 | 10.2 |
| Serine | 4.2 | 4.3 |
| Tyrosine | 4.7 | 3.6 |
From Truong et al. (2016c).
From Xiao et al. (2014).
Therefore, that kafirin concentrations in Australian sorghum crops may be increasing in recent decades is of real concern. Selection programs have targeted red sorghums with relatively dense or corneous endosperms in a quest to enhance grain weathering resistance (Henzell, 1992). Importantly, it is almost axiomatic that selecting sorghums with hard, corneous endosperms will lead to higher kafirin concentrations as a consequence (Shull et al., 1990, Mazhar and Chandrashekar, 1995). Instructively, the texture or ‘hardness’ of Australian sorghums are relatively high by international standards. Our group determined the texture of 38 sorghum varieties by the Symes particle size index (PSI) method. Under this method, a PSI of <8% corresponds to ‘extra hard, a ‘very hard’ sorghum has a PSI of 8% to 12%, and >12% is considered ‘hard’. The 38 local sorghums had an average PSI of 9.8%. By contrast, in an international survey (de Alencar Figueiredo et al., 2006) the average texture of 117 sorghum samples was an approximate 12.7% PSI. This indicates that Australian sorghums have noticeably harder textures and, arguably, higher kafirin concentrations than sorghums grown overseas.
In comparison to total protein in sorghum, kafirin contains less arginine than sorghum protein (1.06% vs. 3.82%) but more leucine (19.60% vs. 15.19%) from data tabulated by Selle et al., 2010a, Selle et al., 2010b. Initially, Ravindran et al. (1998) and subsequently Bryden et al. (2009b) analysed the concentration and digestibility of 15 amino acids in 6 and 11 local sorghum samples, respectively. Recently, we analysed amino acid concentrations in 8 local sorghums (unpublished data) so it is possible to compare the 3 data-sets. However, as shown in Table 5, arginine contents have linearly decreased (r = −0.714; P < 0.001) but leucine contents have linearly increased (r = 0.721; P < 0.001) over 18 years. Both linear regressions are entirely consistent with the proposal that kafirin is increasing as a proportion of sorghum protein over the past 2 decades.
Table 5.
Arginine and leucine contents of 25 sorghum samples from surveys completed in 1998, 2009 and 2016.
| Item | Arginine, % of 15 amino acids | Leucine, % of 15 amino acids |
|---|---|---|
| 1998 (n = 6) | 4.58b | 15.11a |
| 2009 (n = 11) | 4.04a | 15.58b |
| 2016 (n = 8) | 3.78a | 15.96b |
| SEM | 0.1022 | 0.1123 |
| Significance (P) | <0.001 | <0.001 |
| LSD (P < 0.05) | 0.300 | 0.329 |
| Linear effect | ||
| Correlation coefficient | r = −0.714 | r = 0.721 |
| Significance | P < 0.001 | P < 0.001 |
a,bMean values not sharing a common superscript are significantly different at the 5% level of probability.
Mabelebele et al. (2015) reported the amino acid profiles of 4 South African sorghums, and on the same basis (% of 15 amino acids) as used in Table 5, these sorghums contained 4.21% arginine and 14.22% leucine. By contrast, the 8 Australian sorghums analysed in 2016 contained 3.78% arginine and 15.96% leucine. Both differences suggest, in a very limited number of samples, that kafirin concentrations as a proportion of total protein in our local sorghum varieties are high. As discussed, kafirin is an important limitation to starch/energy utilisation; therefore, sorghum breeding programs should develop new directions to reverse this trend as a priority.
It appears that the formation of disulphide cross-linkages in the periphery of kafirin protein bodies is fundamental to the substandard energy utilisation of broiler chicks offered sorghum-based diets. Therefore, it is noteworthy that Oria et al. (2000) developed a highly digestible sorghum mutant cultivar in which kafirin protein bodies had a unique folded structure. Subsequently, da Silva et al., 2011a, da Silva et al., 2011b evaluated transgenic sorghums in which kafirin synthesis was modified. da Silva et al. (2011b) reported suppression of γ-kafirin synthesis in transgenic sorghum lines which had increased in vitro protein digestibility which was attributed to lesser degrees of disulphide-bonded kafirin polymerisation. Thus it may be possible to develop standard or even high-protein sorghums with either modified protein body structures or with kafirin fractions having more favourable amino acid profiles including reduced numbers of cysteine residues in the β- and γ-fractions. The agronomic viability of such advanced sorghum variants will be critical. Nevertheless, there is recent evidence for allelic diversity of β-, γ- and δ-kafirin genes in grain sorghum genotypes (Laidlaw et al., 2010, Cremer et al., 2014), which suggests that it may be feasible to select sorghums with kafirin protein bodies that are less likely to have negative impacts on starch/energy utilisation.
3.2. Phenolic compounds
The survey respondents identified both polyphenols and phenolic acids as important negative factors inherent in grain sorghum but some confusion appeared to exist. This is not surprising as phenolic compounds are a diverse group of phytochemicals ranging from highly-polymerised inert lignins to simple phenolic acids (Mangan, 1988). Condensed tannin is a polyphenolic compound with tangible anti-nutritive properties but, as discussed, we do not believe it is present in contemporary Australian sorghum crops. However, despite being devoid of condensed tannin, the energy utilisation of sorghum grain is substandard. It is improbable that other phenolic compounds are innocuous and devoid of anti-nutritive properties. For example, Taylor (2005) concluded that grain sorghum cultivars contain higher levels of phenolic compounds than other cereals and red (non-tannin) sorghums are highly pigmented with polyphenols (anthocyanins and anthocyanidins) and, importantly, these phenols bind strongly to starch. Elkin et al. (1996) contended that condensed tannin is only partially responsible for variations in the nutrient quality of grain sorghum. Subsequent data generated by Barros et al. (2012) and Lemlioglu-Austin et al. (2012) provides quite compelling support for this contention. The methodologies used to determine phenolic compound concentrations are thoroughly described in Khoddami et al., 2013, Khoddami et al., 2015. The phenolic acids, including ferulic acid, were quantified in their free, conjugated and bound forms.
Total phenolic compounds were negatively correlated with ME:GE ratios, AMEn and starch disappearance rates in the distal jejunum and distal ileum to significant extents in the Liu et al. (2016) study as shown in Table 6. In addition, total phenolic compounds tended to be negatively correlated with weight gain and N retention and positively correlated with FCR. The negative correlations with starch disappearance rates are noteworthy as the clear implication is that phenolic compounds in sorghum are impeding starch digestion, glucose absorption and consequently energy utilisation in poultry.
Table 6.
Pearson correlations between concentrations of sorghum total phenolics (mg GAE/g) in 10 diets with parameters of growth performance, nutrient utilisation and starch disappearance rates in distal jejunum (DJ) and distal ileum (DI).1
| Item | Weight gain, g/bird | FCR, g/g | ME:GE ratio, MJ/MJ | AMEn, MJ/kg | N retention, % | Starch DJ, g/(bird·d) | Starch DI, g/(bird·d) |
|---|---|---|---|---|---|---|---|
| Total phenolics | |||||||
| Coefficient (r) | −0.629 | 0.521 | −0.784 | −0.744 | −0.578 | −0.756 | −0.754 |
| Significance (P) | 0.051 | 0.123 | 0.010 | 0.014 | 0.080 | 0.011 | 0.012 |
GAE = gallic acid equivalent; AMEn = N-corrected apparent metabolizable energy.
Adapted from Liu et al. (2016).
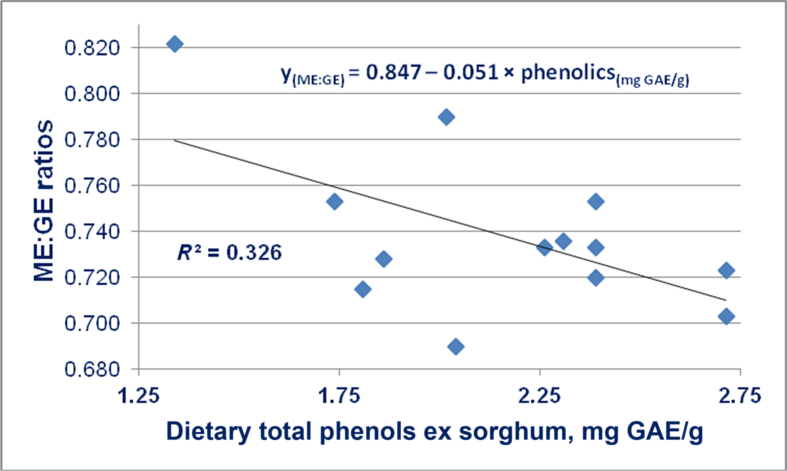
In the Truong et al. (2016b) study, phenolic compounds in 6 sorghum varieties were determined. These included (range of values in parentheses) total phenolics (3.00 to 4.68 mg GAE/g), flavan-4-ols (1.30 to 10.86 absorbance/mL per g), apigeninidin (2.13 to 14.75 μg/g), conjugated ferulic acid (24.5 to 38.43 μg/g) and bound ferulic acid (183.2 to 334.8 μg/g). The Pearson correlations between polyphenols, conjugated and bound phenolic acids in 6 sorghum varieties and parameters of nutrient utilisation, starch digestibilities and disappearance rates are shown in Table 7, Table 8, Table 9, respectively. In respect of ME:GE ratios, there were significant negative correlations with total phenols, flavan-4-ols and apigeninidin (Table 7). Conjugated ferulic and benzoic acids were significantly negatively correlated with ME:GE ratios (Table 8). Similarly, bound ferulic acid tended to be negatively correlated with ME:GE ratios (Table 9). Starch digestibility coefficients in three small intestinal segments were or tended to be negatively correlated with luteolinidin, apigeninidin, total flavonoids, conjugated benzoic, coumaric and vanillic acids and bound ferulic acid. Starch disappearance rates in four small intestinal segments were or tended to be negatively correlated with bound ferulic and syringic acids. In essence, the Truong et al. (2016c) study indicates that various phenolic compounds are negatively influencing efficiency of energy utilisation (ME:GE ratios), starch digestibility coefficients and starch disappearance rates or, effectively, glucose absorption. Also, as shown in Fig. 7, there is a negative linear relationship (r = −0.569; P < 0.05) between dietary levels of total phenolic compounds and ME:GE ratios in broilers offered diets based on 9 ‘tannin-free’ sorghum varieties across 5 feeding studies.
Table 7.
Pearson correlations between polyphenols of sorghum and parameters of nutrient utilisation, starch digestibilities and disappearance rates that were either significant (P < 0.05) or approached significance (P < 0.10) in broiler chickens.1
| Polyphenol | Description | Parameter | Correlation coefficient | Significance |
|---|---|---|---|---|
| Flavan-4-ols | Nutrient utilisation | ME:GE ratios | r = −0.919 | P = 0.010 |
| Apigeninidin | r = −0.838 | P = 0.037 | ||
| Total phenols | r = −0.838 | P = 0.037 | ||
| Luteolinidin | N retention | r = −0.731 | P = 0.099 | |
| Apigeninidin | r = −0.832 | P = 0.040 | ||
| 7-methoxy-apigeninidin | r = −0.861 | P = 0.028 | ||
| Flavan-4-ols | AMEn | r = −0.795 | P = 0.059 | |
| Luteolinidin | Starch digestibility | PJ | r = 0.854 | P = 0.031 |
| Apigeninidin | r = −0.765 | P = 0.076 | ||
| Total flavonoids | DJ | r = −0.739 | P = 0.093 |
AMEn = nitrogen-corrected apparent metabolizable energy; PJ = proximal jejunum; DJ = distal jejunum.
Adapted from Truong et al. (2016b).
Table 8.
Pearson correlations between conjugated phenolic acids of sorghum and parameters of nutrient utilisation, starch digestibilities and disappearance rates that were either significant (P < 0.05) or approached significance (P < 0.10) in broiler chickens.1
| Conjugated phenolic acid | Description | Parameter | Correlation coefficient | Significance |
|---|---|---|---|---|
| Vanillic | Nutrient utilisation | AME | r = −0.872 | P = 0.023 |
| Benzoic | ME:GE ratio | r = −0.820 | P = 0.046 | |
| Vanillic | r = −0.773 | P = 0.072 | ||
| Ferulic | r = −0.914 | P = 0.011 | ||
| Benzoic | N retention | r = −0.872 | P = 0.024 | |
| Vanillic | AMEn | r = −0.851 | P = 0.032 | |
| Ferulic | r = −0.752 | P = 0.085 | ||
| Benzoic | Starch digestibility | PJ | r = 0.800 | P = 0.056 |
| Coumaric | PJ | r = 0.941 | P = 0.005 | |
| Vanillic | DI | r = −0.817 | P = 0.047 |
AME = apparent metabolizable energy; ME = metabolizable energy; GE = gross energy; AMEn = N-corrected AME; PJ = proximal jejunum; DI = distal ileum.
Adapted from Truong et al. (2016b).
Table 9.
Pearson correlations between bound phenolic acids of sorghum and parameters of nutrient utilisation, starch digestibilities and disappearance rates that were either significant (P < 0.05) or approached significance (P < 0.10) in broiler chickens.1
| Bound phenolic acid | Description | Parameter | Correlation coefficient | Significance |
|---|---|---|---|---|
| Ferulic | Nutrient utilisation | AME | r = −0.750 | P = 0.086 |
| Ferulic | ME:GE ratio | r = −0.743 | P = 0.090 | |
| Ferulic | AMEn | r = −0.785 | P = 0.064 | |
| Ferulic | Starch digestibility | DJ | r = −0.793 | P = 0.060 |
| Syringic | Starch | PJ | r = −0.761 | P = 0.079 |
| Ferulic | Disappearance | DJ | r = −0.869 | P = 0.025 |
| Ferulic | PI | r = −0.834 | P = 0.039 | |
| Syringic | r = −0.855 | P = 0.030 | ||
| Ferulic | DI | r = −0.821 | P = 0.045 | |
| Syringic | r = −0.930 | P = 0.007 |
AME = apparent metabolizable energy; AMEn = N-corrected AME; PJ = proximal jejunum; DJ = distal jejunum; PI = proximal ileum; DI = distal ileum.
Adapted from Truong et al. (2016b).
Fig. 7.
Linear relationship (r = −0.569; P = 0.042) between dietary levels of total phenolic compounds and ME:GE ratios in broiler chickens based on 13 observations derived from 5 feeding studies (Truong et al., 2015a, Truong et al., 2015b, Truong et al., 2016b, Truong et al., 2016c, Selle et al., 2016c). The 9 sorghum varieties included LVP3, LVP5, FW, Tiger, Block I, HP, Liberty #2, MP and JM. ME = metabolizable energy; GE = gross energy; GAE = gallic acid equivalent.
Phenolic compounds are believed to form complexes readily with starch and are probably more likely to form starch–phenolic complexes with amylose than amylopectin (Tomasik and Schilling, 1998). It appears that phenolic compounds may interact with starch through hydrogen bonds, covalent bonds or chelation via their carboxyl and hydroxyl groups (Yu et al., 2001). Also, Zhu (2015) found that non-covalent interactions between starch and phenolic compounds can influence the nutritional properties of feedstuffs. Kandil et al. (2012) reported that phenolic acids play an important role in the resistance of starch to hydrolysis in a study involving barley, maize, triticale and wheat. Phenolic compounds have been shown to inhibit Na+, K+, -ATPase or the activity of the ‘sodium pump’ (Welsch et al., 1989), which suggests intestinal uptakes of nutrients, including glucose via Na+-dependent transporter systems, would be compromised. In agreement with this suggestion, Thompson et al. (1984) found negative relationships between polyphenol intakes and blood-glucose responses in humans. Thus it would appear that ‘non-tannin’ phenolic compounds have the potential to depress both starch digestion and glucose absorption and, in turn, energy utilisation in birds offered sorghum-based diets.
Truong et al. (2016b) found that conjugated ferulic acid was negatively correlated with ME:GE ratios and also found that flavan-4-ols were negatively correlated with ME:GE ratios to significant extents. Interestingly, flavan-4-ols are not responsible for ‘redness’ in sorghum but they are precursors of red polyphenolic pigments. Therefore, it follows that there would be less flavan-4-ols in white than red sorghums as was the case in a limited number of white varieties in this project.
On the basis of anecdotal evidence, white sorghum varieties are considered to be superior to red sorghum varieties by both pig and poultry nutritionists. Axiomatically, white sorghums will contain less polyphenols than red sorghums and in a limited number of samples we have found that white sorghums also contain less phenolic acids. While speculative, the superiority of white sorghums as a feed grain for poultry may simply be attributed to lesser concentrations of phenolic compounds. Consequently, sorghum breeders should be encouraged to take this difference into consideration.
3.3. Phytate
Phytate (myo-inositol hexaphosphate) is invariably present in feedstuffs of plant origin and survey respondents identified phytate as an important negative factor inherent in grain sorghum. That phytate is an anti-nutritive factor in poultry diets is established (Selle and Ravindran, 2007) and sorghum contains phytate at relative and absolute concentrations that are usually higher than other cereal grains (Selle et al., 2003). Consideration has been given to the reciprocal energy effects of dietary phytate and exogenous phytase (Selle et al., 2012a). However there are experimental data that show phytate negatively impacts on starch utilisation as phytase has been shown to enhance starch digestibility in poultry (Truong et al., 2015c).
In 15 Australian sorghum varieties, the mean phytate-P content was 2.41 g/kg (range: 1.70 to 3.70 g/kg) and phytate-P constituted an average of 82.7% of total P (Selle et al., 2003). In the Liu et al. (2016) study, the 3 sorghum varieties that constituted the apical diets of the equilateral triangle response surface design were Block I, HP and Liberty and their respective phytate-P concentrations were 2.76, 2.19 and 1.39 g/kg. Pearson correlations between sorghum phytate concentrations in 10 diets with parameters of growth performance, nutrient utilisation and starch disappearance rates in the distal jejunum and distal ileum in the Liu et al. (2016) study are shown in Table 10. There were significant negative correlations between sorghum phytate concentrations and ME:GE ratios (r = −0.869; P < 0.005), AMEn (r = −0.633; P < 0.05), starch disappearance rates in the distal jejunum (r = −0.737; P < 0.02) and distal ileum (r = −0.736; P < 0.05) in birds offered 10 sorghum-based diets. Ostensibly, this indicates that phytate was negatively influencing effectively starch absorption and energy utilisation. In the Truong et al. (2016b) study, phytate concentrations in 6 sorghums were negatively correlated (r = −0.839; P < 0.04) with distal ileal starch:protein disappearance rate ratios. Also, phytate concentrations were negatively correlated with starch disappearance rates in the distal jejunum (r = −0.845; P < 0.04) and proximal ileum (r = −0.890; P < 0.02).
Table 10.
Pearson correlations between sorghum phytate concentrations (g/kg) in 10 diets with parameters of growth performance, nutrient utilisation and starch disappearance rates in distal jejunum (DJ) and distal ileum (DI).1
| Item | Weight gain, g/bird | FCR, g/g | ME:GE ratio, MJ/MJ | AMEn, MJ/kg | N retention, % | Starch DJ, g/(bird·d) | Starch DI, g/(bird·d) |
|---|---|---|---|---|---|---|---|
| Phytate | |||||||
| Coefficient (r) | −0.485 | 0.388 | −0.869 | −0.633 | −0.626 | −0.737 | −0.736 |
| Significance (P) | 0.156 | 0.268 | 0.001 | 0.049 | 0.053 | 0.015 | 0.015 |
Adapted from Liu et al. (2016).
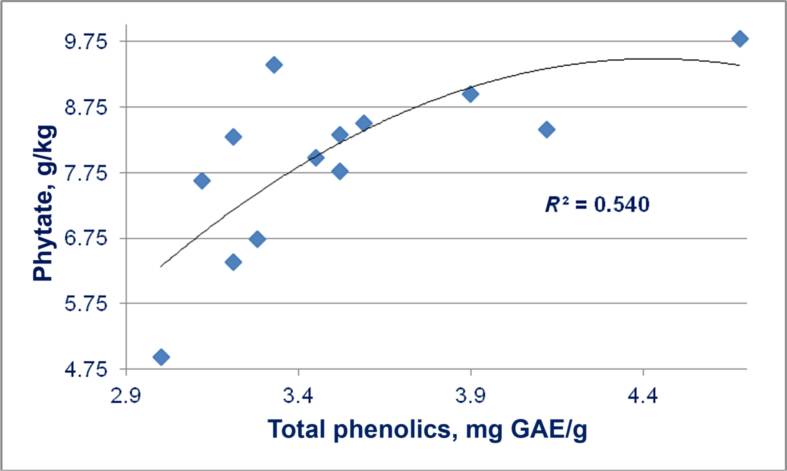
There is a quadratic relationship (r = 0.735; P < 0.025) between concentrations of total phenolic compounds and phytate in 13 sorghum varieties as shown in Fig. 8. This is perhaps not surprising as phenolic compounds and phytate are both mainly located in the aleurone layer of grain sorghum. However, Thompson and Yoon (1984) investigated the effects of polyphenols and phytate on in vitro starch digestibility. The researchers concluded that polyphenols such as tannic acid play a role, although a smaller role compared to phytate, in reducing the in vitro rate of starch digestibility and that different polyphenols influence the rate of digestibility to varying extents. Thus this raises the distinct possibility that phenolic compounds and phytate in tandem are compromising starch digestion and glucose absorption in broiler chicks offered sorghum-based diets and such a combination would be unique across feed grains.
Fig. 8.
Quadratic relationship (r = 0.735; P = 0.021) between concentrations of total phenolic compounds and phytate in 13 sorghum varieties (Block I, Tiger, JM, Liberty, FW, MP, HP, LVP1, LVP2, LVP3, LVP4, LVP5, LVP6).
Several feeding studies evaluating the effects of feed enzyme additions to sorghum-based broiler diets have been completed (Selle et al., 1999, Selle et al., 2010b, Selle et al., 2012b, Liu et al., 2014a). The impression is that phytase responses in sorghum-based diets are muted in comparison to maize or wheat, which was very evident in the Liu et al. (2014a) study. While speculative, it seems likely that the reasons for the muted responses are that phytase simply cannot address the anti-nutritive properties of kafirin and phenolic compounds in sorghum. If so, it follows that phytase responses would be more robust in broiler diets based on sorghums with lower concentrations of both kafirin and phenolic compounds. This is important given that the inclusion of exogenous phytate-degrading enzymes in poultry and pig diets is a routine procedure in Australia.
4. Reducing agents
Inclusions of the reducing agent sodium metabisulphite in sorghum-based poultry diets was evaluated and reported in a series of 6 papers (Liu et al., 2014b, Selle et al., 2013c, Selle et al., 2014a, Selle et al., 2016a, Truong et al., 2015b, Truong et al., 2016c). The impact of sodium metabisulphite inclusion rates in sorghum-based broiler diets on energy utilisation expressed as AMEn is shown in Table 11. In 4 studies involving conventional sorghum-based diets the average inclusion rate of 2.83 g/kg sodium metabisulphite increased AMEn from 11.58 to 11.89 MJ/kg or by an average response of 0.31 MJ/kg. The median response was 0.37 MJ/kg AMEn, which indicates that the reducing agent has a positive influence on energy utilisation in broiler chickens offered sorghum-base diets.
Table 11.
The impact of sodium metabisulphite inclusion rates in sorghum-based broiler diets on energy utilisation expressed as N-corrected apparent metabolizable energy (AMEn) from a total of 21 observations and 9 grain sorghum varieties.
| Inclusion rate, g/kg | AMEn, MJ/kg DM |
Reference | ||
|---|---|---|---|---|
| Control | Treatment | Response | ||
| 0.25 | 13.32 | 13.61 | 0.29 | Selle et al. (2013c)1 |
| 2.50 | 13.32 | 13.40 | 0.08 | |
| 5.00 | 13.32 | 13.83 | 0.51 | |
| 1.50 | 11.85 | 12.30 | 0.45 | Selle et al. (2014a)2 |
| 2.25 | 11.85 | 12.23 | 0.38 | |
| 3.00 | 11.85 | 12.16 | 0.31 | |
| 3.75 | 11.85 | 12.15 | 0.30 | |
| 4.50 | 11.85 | 12.36 | 0.51 | |
| 5.25 | 11.85 | 12.28 | 0.43 | |
| 1.75 | 11.95 | 11.58 | −0.37 | Truong et al. (2016a)2 |
| 1.75 | 11.34 | 11.79 | 0.45 | Truong et al. (2016c)2 |
| 3.50 | 11.34 | 11.72 | 0.38 | |
| 1.75 | 11.44 | 11.91 | 0.47 | |
| 3.50 | 11.44 | 11.92 | 0.48 | |
| 1.75 | 11.05 | 11.27 | 0.22 | |
| 3.50 | 11.05 | 11.40 | 0.35 | |
| 1.75 | 11.75 | 11.81 | 0.06 | |
| 3.50 | 11.75 | 12.04 | 0.29 | |
| 1.75 | 11.56 | 11.73 | 0.17 | |
| 3.50 | 11.56 | 12.15 | 0.59 | |
| 2.75 | 11.10 | 11.19 | 0.09 | Selle et al. (2016b)2 |
| Mean | Mean | Mean | Mean | |
| 2.83 | 11.58 | 11.89 | 0.31 | |
Atypical ‘sorghum only’ diets.
Conventional sorghum-based diets.
In theory, sodium metabisulphite has 2 modes of action; one is the reduction of disulphide cross-linkages. Selle et al. (2013c) reported that 5.0 g/kg sodium metabisulphite significantly increased free sulphydryl groups (10.03 vs. 1.27 μmol/g protein) and decreased disulphide bonds (18.57 vs. 226.27 μmol/g protein) in sorghum-based diets. Second, sodium metabisulphite and other sulphite reducing agents have the capacity to depolymerise starch via oxidative–reductive reactions (Paterson et al., 1996, Paterson et al., 1997). Increasing sodium metabisulphite inclusions from 0.00 to 5.25 g/kg in sorghum-based diets linearly reduced (r = −0.925; P < 0.001) final rapid visco-analysis (RVA) starch pasting viscosity from 2,567 to 1,935 cP and this marked reduction was attributed to starch depolymerisation (Liu et al., 2014b).
Truong et al. (2016c) reported that 3.50 g/kg sodium metabisulphite increased AMEn by 0.42 MJ/kg from 11.43 to 11.85 MJ/kg in broiler diets based on 5 different sorghum varieties. This ‘energy sparing’ effect could be attributed to either the depolymerisation of starch and/or the reduction of disulphide cross-linkages in protein. In respect of the latter, the periphery of protein bodies consists of the β- and γ-kafirin fractions and both are rich in cystine residues. Therefore, the reduction of disulphide bonds in the periphery of kafirin protein bodies and the mitigation of starch–protein interactions in sorghum endosperm may hold particular relevance (Taylor and Emmambux, 2010). One objective of the Selle et al. (2016a) study was to determine if the ‘energy sparing’ effects of sodium metabisulphite in sorghum-based diets extends to wheat-based diets. On the basis of this study and a preliminary investigation the inclusion of gram per kilogram sodium metabisulphite in wheat-based diets was not promising. This could be seen as support for the concept that reductions of disulphide cross-linkages in kafirin protein bodies induced by sodium metabisulphite are pivotal to the ‘energy sparing’ effects in sorghum-based diets.
5. Grain sorghum particle size
In a series of three separate, but similar, feeding studies (Selle et al., 2012c, Selle et al., 2013b, Selle et al., 2014b) identical diets were offered to broiler chickens based on the same white sorghum (Liberty) variety. This sorghum was ground through hammer-mill screen sizes of 2.0, 3.2 and 6.0 mm prior to being incorporated into complete steam-pelleted diets. Collectively, the outcomes indicated a quadratic relationship between hammer-mill screen size and FCR and for this particular grain sorghum a hammer-mill screen size in the order of 3.75 mm was optimal for FCR.
Therefore, the Selle et al. (2016c) follow-up study consisted of a 2 × 4 factorial array of dietary treatments comprising 2 red sorghum varieties (Tiger and Block I) ground through 4 hammer-mill screen sizes (2.0, 3.2, 4.8, 6.0 mm) prior to incorporation into nutritionally-equivalent diets. The corresponding average geometric mean particle sizes of the ground sorghums were 794, 1,114, 1,362 and 1,405 μm, respectively. The objective was to identify the most appropriate hammer-mill screen size and mean particle size for grain sorghum in poultry diets. However, hammer-mill screen size did not influence weight gain or FCR which was not the anticipated outcome. The 6.0 mm screen size generated significantly higher starch and protein (N) digestibility coefficients in the distal jejunum and distal ileum than the 2.0 mm screen. Indeed, there was a linear correlation (r = 0.720; P < 0.05) between increasing hammer-mill screen sizes and distal ileal starch digestibility coefficients. Moreover, increasing hammer-mill screen size was positively correlated (r = 0.971; P < 0.03) with 7- to-28-day weight gain when Tiger sorghum-based diets were considered separately.
Tiger sorghum proved superior to Block I as significant advantages were observed for FCR, AME, ME:GE ratios, AMEn, distal ileal starch digestibility coefficients and distal jejunal, proximal ileal and distal ileal protein (N) digestibility coefficients. Indicatively, the inferior Block I sorghum contained 31% more kafirin, 17% more phytate, 14% more total phenolic compounds, 58% more flavan-4-ols, 38% more total phenolic acids and 33% more total ferulic acid than Tiger sorghum. It would be instructive to determine if the variation in anti-nutritive factors in these two sorghum varieties were genetically driven or subject to agronomic environmental differences.
The effects of hammer-mill screen size and sorghum particle size on broiler performance were not as expected. It was anticipated that a hammer-mill screen size of less than 4.0 mm would be advantageous based on previous feeding studies with white sorghum. However, the numerically best weight gains and FCR were associated with a hammer-mill screen size of 6.0 mm and a geometric mean particle size in the order of 1,400 μm. Thus an “optimal” hammer-mill screen size was not identified. However, the likelihood is that the optimal hammer-mill screen size and grain particle size for a given sorghum grain is very much a function of grain texture. Then there is the allied problem that determinations of grain sorghum texture are not straightforward.
6. Rapid visco-analysis starch pasting profiles
Starch pasting profiles of feed grains assessed by RVA should be a relatively rapid and accurate indicator of feed grain quality (Selle et al., 2016b). In the Truong et al. (2017) review of this proposal it was found that peak, holding, breakdown and final RVA viscosities were positively correlated with ME:GE ratios to significant extents in a meta-analysis of 5 broiler bioassays. Similarly, peak and breakdown RVA viscosities were positively correlated with AMEn. Also, in the Liu et al. (2016) study peak, holding and breakdown RVA viscosities were positively correlated with ME:GE ratios and AMEn across 10 sorghum-based diets. Importantly, it was also found that concentrations of kafirin and total phenolic compounds in 13 sorghums were negatively correlated with peak and holding RVA viscosities. Therefore, RVA starch pasting profiles do appear to hold promise as a relatively rapid means to assess sorghum quality as a feed grain for chicken-meat production. This potential appears to be linked to quantities of kafirin and total phenolic compounds present in sorghum as it would seem that both factors depress RVA starch viscosities in vitro and, in turn, also depress energy utilisation in birds offered sorghum-based diets.
Promatest protein solubilities of grain sorghums, which may be determined by methods described in Odjo et al. (2012), could be another indicator of feed grain quality. Protein solubility of the 6 red sorghums harvested on the Liverpool Plains ranged from 41.2% to 49.5%. As reported by Khoddami et al. (2015), Promatest protein solubilities of this limited number of sorghums were positively correlated with parameters of energy utilisation including AME (r = 0.874; P < 0.025), ME:GE ratios (r = 0.862; P < 0.03), and AMEn (r = 0.827; P < 0.05). As kafirin is a poorly soluble protein source, there is the implication that these correlations reflect the negative impact of kafirin on starch/energy utilisation on sorghum-based broiler diets. The development of near-infrared spectroscopy (NIR) calibrations to assess protein solubility merits consideration.
7. Outcomes, implications and recommendations
In a review of 11 feeding studies, the mean ileal starch digestibility coefficient for maize-based broiler diets was 0.950 with a range from 0.873 to 0.993 (Truong et al., 2016a). By contrast, the mean ileal starch digestibility coefficient in diets based on 8 grain sorghum varieties was 0.866, ranging from 0.793 to 0.918; thus the digestibility of sorghum starch is clearly inferior to that of maize. While 76% of starch digestion along the small intestine occurred in the proximal jejunum, 13.4% of dietary starch was notionally resistant and this undigested residue passes into the large intestine to fuel hind gut fermentation. This comparison reflects the incomplete starch digestion and poor energy utilisation of sorghum-based diets by broiler chickens.
The genesis of this incomplete starch digestibility and suboptimal energy utilisation appears to be related to concentrations of kafirin and a variety of phenolic compounds in ‘tannin-free’ sorghums. Phenolic compounds and phytate concentrations in sorghum well may be related so there is the likelihood that the deleterious impacts of total phenolic compounds are amplified by concentrations of phytate in sorghum. There are indications that both phytate and phenolic compounds retard intestinal uptakes of glucose. The suboptimal energy utilisation in broilers offered sorghum-based diets can be attenuated by the dietary inclusion of the reducing agent, sodium metabisulphite. This positive impact probably stems largely from the reduction of disulphide cross-linkages in the periphery of kafirin protein bodies. If so, the advantages of sodium metabisulphite inclusions in poultry diets could be limited to sorghum-based diets and may not apply to other feed grains. Rapid visco-analysis starch pasting profiles appear to be indicative of the quality of sorghum as a feed grain and Promatest protein solubility may also be predictive in this context.
Broiler diets may be based on a blend of wheat and sorghum and such blends could diminish the gravity of the anti-nutritive properties of grain sorghum. However, in one recent study (Moss et al., 2017), broilers were offered diets containing either 475 g/kg wheat or sorghum or as an equal blend in association with 125 g/kg (ground or whole) barley. The wheat-based diet supported a significantly better FCR (1.362 vs. 1.400) from 7 to 28 days post-hatch while the blend (1.378) was intermediate. The same response patterns were observed for parameters of nutrient utilisation and starch and protein digestibilities. Thus, the equal wheat-sorghum blend diluted the anti-nutritive properties of grain sorghum but they still remained evident in this feeing study.
Our contention is that quality of sorghum as a feed grain for chicken-meat production is somewhat better than the perceived value; nevertheless, the performance of broiler chickens offered sorghum-based diets is open to improvement. Two prime targets in this respect are reductions in concentrations of kafirin and ‘non-tannin’ phenolic compounds. From data generated in Taylor et al. (1984) kafirin, as a proportion of protein, is positively correlated (r = 0.469; P < 0.005) with sorghum protein concentrations. Consequently, as protein contents of grain sorghum increase, kafirin concentrations will also increase in both relative and absolute terms. Therefore, it follows that ‘low-protein’ sorghums are more likely to support better broiler performance than ‘high-protein’ varieties by virtue of lesser kafirin contents as demonstrated by Truong et al. (2015a). The real possibility that kafirin is increasing as a proportion of sorghum protein in Australian crops needs to be reversed by sorghum breeding programs as a priority given confirmation of this proposal. The inclusion of white sorghum varieties in poultry diets would automatically reduce concentrations of ‘non-tannin’ phenolic compounds which should be beneficial. However, white sorghums are not extensively grown in Australia which appears to be related to undesirable agronomic properties. Quite possibly, the development of ‘pink’ sorghum varieties with lesser polyphenol concentrations than red varieties, but better agronomic properties than white varieties, would support better broiler performance.
The inclusion of phytate-degrading feed enzymes in poultry diets is routine but responses generated in sorghum-based diets appear to be muted. While speculative, the likelihood is that broiler chickens offered diets based on sorghums with low or modified kafirin levels coupled with low phenolic compound contents would respond more robustly to exogenous phytases. The premise for this contention is that phytase simply cannot attenuate the anti-nutritive properties of these sorghum components. Another tangible benefit is that such sorghums could result in better pellet quality stemming from lower starch gelatinisation temperatures in sorghums with lesser concentrations of kafirin and phenolic compounds.
Acknowledgements
The funding provided by the Rural Industries Research and Development Corporation Chicken-meat Program for a series of sorghum orientated projects should be gratefully acknowledged; therefore, the authors thank Dr. Vivien Kite and Dr. Kylie Hewson and members of the Advisory Panel. The authors thank Dr. Karlie Nielsen and her colleagues at Australian Proteome Analytical Facility (Macquarie University) very much for her quantifications of kafirin concentrations in grain sorghum. The authors also acknowledge several colleagues for their support and advice including Professor Mingan Choct, Dr. Tim Walker, Mr. Greg Hargreave, Mr. Greg McDonald, Mr. Alan Cruickshank, Dr. Glen Fox and Mr. Denis McGrath of the Feed Grain Partnership. Finally, the authors express their gratitude to the survey respondents.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AMINODat® 5.0 . Evonik Nutrition & Care GmbH; D-63457 Hanau, Germany: 2016. Animal nutritionist's information edge. [Google Scholar]
- Barros F., Awika J.M., Rooney L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J Agric Food Chem. 2012;60:11609–11617. doi: 10.1021/jf3034539. [DOI] [PubMed] [Google Scholar]
- Black J., Hughes R., Nielsen S., Tredrea A., MacAlpine R., Van Barneveld R. The energy value of cereal grains, particularly wheat and sorghum, for poultry. Proc Aust Poult Sci Symp. 2005;17:21–29. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Bryden W., Li X., Ravindran G., Hew L., Ravindran V. RIRDC Publication; Barton, ACT: 2009. Ileal digestible amino acid values in feedstuffs for poultry. [Google Scholar]
- Bryden W., Selle P., Cadogan D., Li X., Muller N., Jordan D. RIRDC Publication; 2009. A review of the nutritive value of sorghum for broilers; p. 9. 077. [Google Scholar]
- Cremer J.E., Liu L., Bean S.R., Ohm J.-B., Tilley M., Wilson J.D. Impacts of kafirin allelic diversity, starch content, and protein digestibility on ethanol conversion efficiency in grain sorghum. Cereal Chem. 2014;91:218–227. [Google Scholar]
- da Silva L.S., Jung R., Zhao Z-y, Glassman K., Taylor J., Taylor J.R.N. Effect of suppressing the synthesis of different kafirin sub-classes on grain endosperm texture, protein body structure and protein nutritional quality in improved sorghum lines. J Cereal Sci. 2011;54:160–167. [Google Scholar]
- da Silva L.S., Taylor J., Taylor J.R.N. Transgenic sorghum with altered kafirin synthesis: kafirin solubility, polymerization, and protein digestion. J Agric Food Chem. 2011;59:9265–9270. doi: 10.1021/jf201878p. [DOI] [PubMed] [Google Scholar]
- de Alencar Figueiredo L.F., Davrieux F., Fliedel G., Rami J.F., Chantereau J., Deu M. Development of NIRS equations for food grain quality traits through exploitation of a core collection of cultivated sorghum. J Agric Food Chem. 2006;54:8501–8509. doi: 10.1021/jf061054g. [DOI] [PubMed] [Google Scholar]
- Doherty C., Faubion J., Rooney L. Semiautomated determination of phytate in sorghum and sorghum products. Cereal Chem. 1982:59. [Google Scholar]
- Elkin R.G., Freed M.B., Hamaker B.R., Zhang Y., Parsons C.M. Condensed tannins are only partially responsible for variations in nutrient digestibilities of sorghum grain cultivars. J Agric Food Chem. 1996;44:848–853. [Google Scholar]
- Gidley M., Flanagan B., Sharpe K., Sopade P. Starch digestion in monogastrics – mechanisms and opportunities. Rec Adv Anim Nutr Aust. 2011;18:207–213. [Google Scholar]
- Gidley M.J., Pluschke A.M., Sopade P.A., Al-Rabadi G.J.S., Sultan A., Gan C.Y. Poultry Research Foundation; Sydney: 2011. Sorghum grain starch digestibility: effects of particle size and enzyme treatment; pp. 139–146. [Google Scholar]
- Giuberti G., Gallo A., Cerioli C., Masoero F. In vitro starch digestibility and predicted glycemic index of cereal grains commonly used in pig nutrition. Anim Feed Sci Technol. 2012;174:163–173. [Google Scholar]
- Hamaker B.R., Bugusu B.A. Workshop on the proteins of sorghum and millets: enhancing nutritional and functional properties for Africa [CD] (Pretoria, South Africa) 2003. Overview: sorghum proteins and food quality. [Google Scholar]
- Henzell R.G. Australian Institute of Agricultural Science; Melbourne: 1992. Grain sorghum breeding in Australia: current status and future prospects; pp. 70–80. [Google Scholar]
- Hughes R., Brooke G. Variation in the nutritive value of sorghum – poor quality grain or compromised health of chickens. Proc Aust Poult Sci Symp. 2005:35–38. [Google Scholar]
- Kandil A., Li J., Vasanthan T., Bressler D.C. Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. J Agric Food Chem. 2012;60:8444–8449. doi: 10.1021/jf3000482. [DOI] [PubMed] [Google Scholar]
- Khoddami A., Truong H.H., Liu S.Y., Roberts T.H., Selle P.H. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Anim Feed Sci Technol. 2015;210:190–199. [Google Scholar]
- Khoddami A., Wilkes M., Roberts T. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw H.K.C., Mace E.S., Williams S.B., Sakrewski K., Mudge A.M., Prentis P.J. Allelic variation of the β-, γ- and δ-kafirin genes in diverse Sorghum genotypes. Theor Appl Genet. 2010;121:1227–1237. doi: 10.1007/s00122-010-1383-9. [DOI] [PubMed] [Google Scholar]
- Lemlioglu-Austin D., Turner N.D., McDonough C.M., Rooney L.W. Effects of sorghum [Sorghum bicolor (L.) Moench] crude extracts on starch digestibility, estimated glycemic index (EGI), and resistant starch (RS) contents of porridges. Molecules. 2012;17:11124–11138. doi: 10.3390/molecules170911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Selle P.H., Cowieson A.J. Strategies to enhance the performance of pigs and poultry on sorghum-based diets. Anim Feed Sci Technol. 2013;181:1–14. [Google Scholar]
- Liu S., Cadogan D., Péron A., Truong H., Selle P. Effects of phytase supplementation on growth performance, nutrient utilisation and digestive dynamics of starch and protein in broiler chickens offered maize-, sorghum-and wheat-based diets. Anim Feed Sci Technol. 2014;197:164–175. [Google Scholar]
- Liu S., Selle P., Khoddami A., Roberts T., Cowieson A. Graded inclusions of sodium metabisulphite in sorghum-based diets: II. Modification of starch pasting properties in vitro and beneficial impacts on starch digestion dynamics in broiler chickens. Anim Feed Sci Technol. 2014;190:68–78. [Google Scholar]
- Liu S.Y., Fox G., Khoddami A., Neilson K.A., Truong H.H., Moss A.F. Grain sorghum: a conundrum for chicken-meat production. Agriculture. 2015;5:1224–1251. [Google Scholar]
- Liu S.Y., Truong H.H., Khoddami A., Moss A.F., Thomson P.C., Roberts T.H. Comparative performance of broiler chickens offered ten equivalent diets based on three grain sorghum varieties as determined by response surface mixture design. Anim Feed Sci Technol. 2016;218:70–83. [Google Scholar]
- Mabelebele M., Siwela M., Gous R.M., Iji P.A. Chemical composition and nutritive value of South African sorghum varieties as feed for broiler chickens. S Afr J Anim Sci. 2015;45:206–213. [Google Scholar]
- Mangan J. Nutritional effects of tannins in animal feeds. Nutr Res Rev. 1988;1:209–231. doi: 10.1079/NRR19880015. [DOI] [PubMed] [Google Scholar]
- Mazhar H., Chandrashekar A. Quantification and distribution of kafirins in the kernels of sorghum cultivars varying in endosperm hardness. J Cereal Sci. 1995;21:155–162. [Google Scholar]
- McClymont G.L., Duncan D.C. Studies on nutrition of poultry. Aust Vet J. 1952;28:229–233. doi: 10.1111/j.1751-0813.1948.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Morrison C.D., Xi X., White C.L., Ye J., Martin R.J. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–E171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A.F., Sydenham C.J., Truong H.H., Liu S.Y., Selle P.H. The interactions of exogenous phytase with whole grain feeding and effects of barley as the whole grain component in broiler diets based on wheat, sorghum and wheat-sorghum blends. Anim Feed Sci Technol. 2017;227:1–12. [Google Scholar]
- Nyachoti C.M., Atkinson J.L., Leeson S. Sorghum tannins: a review. Worlds Poult Sci J. 1997;53:5–21. [Google Scholar]
- Odjo S., Malumba P., Dossou J., Janas S., Béra F. Influence of drying and hydrothermal treatment of corn on the denaturation of salt-soluble proteins and color parameters. J Food Eng. 2012;109:561–570. [Google Scholar]
- Oria M.P., Hamaker B.R., Axtell J.D., Huang C.-P. A highly digestible sorghum mutant cultivar exhibits a unique folded structure of endosperm protein bodies. Proc Natl Acad Sci. 2000;97:5065–5070. doi: 10.1073/pnas.080076297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson L., Mitchell J.R., Hill S.E., Blanshard J. Evidence for sulfite induced oxidative reductive depolymerisation of starch polysaccharides. Carbohydr Res. 1996;292:143–151. [Google Scholar]
- Paterson L.A., Hill S.E., Mitchell J.R., Blanshard J.M.V. Sulphite and oxidative—reductive depolymerization reactions. Food Chem. 1997;60:143–147. [Google Scholar]
- Perez-Maldonado R.A., Rodrigues H.D. RIRDC; 2009. Nutritional characteristics of sorghums from Queensland and New South Wales for chicken meat production. [Google Scholar]
- Ravindran V., Bryden W.L., Hew L. RIRDC; 1998. Digestible amino acids in poultry feedstuffs. [Google Scholar]
- Rooney L., Pflugfelder R. Factors affecting starch digestibility with special emphasis on sorghum and corn. J Anim Sci. 1986;63:1607–1623. doi: 10.2527/jas1986.6351607x. [DOI] [PubMed] [Google Scholar]
- Salinas I., Pró A., Salinas Y., Sosa E., Becerril C.M., Cuca M. Compositional variation amongst sorghum hybrids: effect of kafirin concentration on metabolizable energy. J Cereal Sci. 2006;44:342–346. [Google Scholar]
- Selle P., Ravindran V., Pittolo P., Bryden W. An evaluation of microbial phytase in sorghum-based broiler diets. Proc Aust Poult Sci Symp. 1999:97–100. [Google Scholar]
- Selle P.H., Walker A.R., Bryden W.L. Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Aust J Exp Agric. 2003;43:475–479. [Google Scholar]
- Selle P.H., Ravindran V. Microbial phytase in poultry nutrition. Anim Feed Sci Technol. 2007;135:1–41. [Google Scholar]
- Selle P.H., Cadogan D.J., Li X., Bryden W.L. Implications of sorghum in broiler chicken nutrition. Anim Feed Sci Technol. 2010;156:57–74. [Google Scholar]
- Selle P., Cadogan D., Ru Y., Partridge G. Impact of exogenous enzymes in sorghum-or wheat-based broiler diets on nutrient utilization and growth performance. Int J Poult Sci. 2010;9:53–58. [Google Scholar]
- Selle P. The protein quality of sorghum. Proc Aust Poult Sci Symp. 2011:147–160. [Google Scholar]
- Selle P.H., Cowieson A.J., Cowieson N.P., Ravindran V. Protein–phytate interactions in pig and poultry nutrition: a reappraisal. Nutr Res Rev. 2012:1–17. doi: 10.1017/S0954422411000151. [DOI] [PubMed] [Google Scholar]
- Selle P., Cadogan D., Creswell D., Partridge G. Phytase supplementation of sorghum-based broiler diets with reduced phosphorus levels. Proc Aust Poult Sci Symp. 2012:70. [Google Scholar]
- Selle P., Liu S., Cai J., Cowieson A. Steam-pelleting and feed form of broiler diets based on three coarsely ground sorghums influences growth performance, nutrient utilisation, starch and nitrogen digestibility. Anim Prod Sci. 2012;52:842–852. [Google Scholar]
- Selle P., Liu S., Cowieson A. Sorghum: production, growth habits and health benefits. Nova Science Publishers; Hauppauge, NY: 2013. Sorghum: an enigmatic grain for chicken-meat production; pp. 1–43. [Google Scholar]
- Selle P., Liu S., Cai J., Cowieson A. Steam-pelleting temperatures, grain variety, feed form and protease supplementation of mediumly ground, sorghum-based broiler diets: influences on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Anim Prod Sci. 2013;53:378–387. [Google Scholar]
- Selle P., Liu S., Cai J., Caldwell R., Cowieson A. Preliminary assessment of including a reducing agent (sodium metabisulphite) in ‘all-sorghum’ diets for broiler chickens. Anim Feed Sci Technol. 2013;186:81–90. [Google Scholar]
- Selle P., Liu S., Cai J., Caldwell R., Cowieson A. Graded inclusions of sodium metabisulphite in sorghum-based diets: I. Reduction of disulphide cross-linkages in vitro and enhancement of energy utilisation and feed conversion efficiency in broiler chickens. Anim Feed Sci Technol. 2014;190:59–67. [Google Scholar]
- Selle P.H., Liu S.Y., Khoddami A., Cai J., Cowieson A.J. Steam-pelleting temperatures and grain variety of finely ground, sorghum-based broiler diets. 1. Influence on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Anim Prod Sci. 2014;54:339–346. [Google Scholar]
- Selle P.H., Truong H.H., McQuade L.R., Moss A.F., Liu S.Y. Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient utilisation and digestive dynamics in broiler chickens. Anim Nutr. 2016;2:303–311. doi: 10.1016/j.aninu.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P., Khoddami A., Moss A., Truong H., Liu S. RVA starch pasting profiles may be indicative of feed grain quality. Proc Aust Poult Sci Symp. 2016:255–258. [Google Scholar]
- Selle P.H., Truong H.H., Khoddami A., Moss A.F., Roberts T.H., Liu S.Y. The impacts of hammer-mill screen size and grain particle size on the performance of broiler chickens offered diets based on two red sorghum varieties. Br Poult Sci. 2016:1–10. doi: 10.1080/00071668.2016.1257777. [DOI] [PubMed] [Google Scholar]
- Shull J., Chandrashekar A., Kirleis A., Ejeta G. Development of sorghum (Sorghum bicolor (L.) Moench) endosperm in varieties of varying hardness. Food Struct. 1990;9:8. [Google Scholar]
- Taylor J.R.N., Schussler L., van der Walt W.H. Fractionation of proteins from low-tannin sorghum grain. J Agric Food Chem. 1984;32:149–154. doi: 10.1021/jf00121a036. [DOI] [PubMed] [Google Scholar]
- Taylor J.R.N., Dewar J. Developments in sorghum food technologies. Adv Food Nutr Res. 2001;43:217–264. doi: 10.1016/s1043-4526(01)43006-3. [DOI] [PubMed] [Google Scholar]
- Taylor J.R.N. Non-starch polysaccharides, protein and starch: form function and feed – highlights on sorghum. Proc Aust Poult Sci Symp. 2005:16. [Google Scholar]
- Taylor J.R.N., Emmambux M.N. Review: developments in our understanding of sorghum polysaccharides and their health benefits. Cereal Chem. 2010;87:263–271. [Google Scholar]
- Thompson L.U., Yoon J.H. Starch digestibility as affected by polyphenols and phytic acid. J Food Sci. 1984;49:1228–1229. [Google Scholar]
- Thompson L.U., Yoon J.H., Jenkins D., Wolever T., Jenkins A.L. Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am J Clin Nutr. 1984;39:745–751. doi: 10.1093/ajcn/39.5.745. [DOI] [PubMed] [Google Scholar]
- Tomasik P., Schilling C.H. Complexes of starch with inorganic guests. Adv Carbohydr Chem Biochem. 1998;53:263–343. [Google Scholar]
- Truong H.H., Neilson K.A., McInerney B.V., Khoddami A., Roberts T.H., Liu S.Y. Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or re-ground mash following steam-pelleting at 65 or 97°C conditioning temperatures. Anim Nutr. 2015;1:220–228. doi: 10.1016/j.aninu.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong H.H., Cadogan D.J., Liu S.Y., Selle P.H. Addition of sodium metabisulfite and microbial phytase, individually and in combination, to a sorghum-based diet for broiler chickens from 7 to 28 days post-hatch. Anim Prod Sci. 2015:1484–1491. [Google Scholar]
- Truong H.H., Liu S.Y., Selle P.H. Phytase influences the inherently different starch digestive dynamics of wheat- and maize-based broiler diets. Proc Aust Poult Sci Symp. 2015;26:126–129. [Google Scholar]
- Truong H.H., Liu S.Y., Selle P.H. Starch utilisation in chicken-meat production: the foremost influential factors. Anim Prod Sci. 2016;56:797–814. [Google Scholar]
- Truong H.H., Neilson K.A., McInerney B.V., Khoddami A., Roberts T.H., Cadogan D.J. Comparative performance of broiler chickens offered nutritionally equivalent diets based on six diverse, ‘tannin-free’ sorghum varieties with quantified concentrations of phenolic compounds, kafirin, and phytate. Anim Prod Sci. 2016;57:828–838. [Google Scholar]
- Truong H.H., Neilson K.A., McInerney B.V., Khoddami A., Roberts T.H., Liu S.Y. Sodium metabisulphite enhances energy utilisation in broiler chickens offered sorghum-based diets with five different grain varieties. Anim Feed Sci Technol. 2016;219:159–174. [Google Scholar]
- Truong H.H., Khoddami A., Moss A.F., Liu S.Y., Selle P.H. The potential of rapid visco-analysis starch pasting profiles to gauge the quality of sorghum as a feed grain for chicken-meat production. Anim Nutr. 2017;3:11–18. doi: 10.1016/j.aninu.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniska R.D., Hugo L.F., Rooney L.W. Practical methods to determine the presence of tannins in sorghum. J Appl Poult Res. 1992;1:122–128. [Google Scholar]
- Welsch C.A., Lachance P.A., Wasserman B.P. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698. doi: 10.1093/jn/119.11.1698. [DOI] [PubMed] [Google Scholar]
- Wiseman J., Nicol N., Norton G. Relationship between apparent matabolisable (AME) values and in vivo/in vitro starch digestibility of wheat for broilers. Worlds Poult Sci J. 2000;56:305–318. [Google Scholar]
- Wylie P. Grains Research and Development Corporation; 2008. Managing sorghum for high yields: a blueprint for doubling sorghum production. [Google Scholar]
- Xiao J., Li Y., Li J., Gonzalez A.P., Xia Q., Huang Q. Structure, morphology, and assembly behavior of kafirin. J Agric Food Chem. 2014;63:216–224. doi: 10.1021/jf504674z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vasanthan T., Temelli F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J Agric Food Chem. 2001;49:4352–4358. doi: 10.1021/jf0013407. [DOI] [PubMed] [Google Scholar]
- Zhu F. Interactions between starch and phenolic compound. Trends Food Sci Technol. 2015;43:129–143. [Google Scholar]