Abstract
Introduction
Robotic-assisted surgery (RAS) has emerged as an alternative minimally invasive surgical option. Despite its growing applicability, the frequent need for pneumoperitoneum and Trendelenburg position could significantly affect respiratory mechanics during RAS. AVATaR is an international multicenter observational study aiming to assess the incidence of postoperative pulmonary complications (PPC), to characterise current practices of mechanical ventilation (MV) and to evaluate a possible association between ventilatory parameters and PPC in patients undergoing RAS.
Methods and analysis
AVATaR is an observational study of surgical patients undergoing MV for general anaesthesia for RAS. The primary outcome is the incidence of PPC during the first five postoperative days. Secondary outcomes include practice of MV, effect of surgical positioning on MV, effect of MV on clinical outcome and intraoperative complications.
Ethics and dissemination
This study was approved by the Institutional Review Board of the Hospital Israelita Albert Einstein. The study results will be published in peer-reviewed journals and disseminated at international conferences.
Trial registration number
NCT02989415; Pre-results.
Keywords: robotic surgery, general anesthesia, mechanical ventilation, postoperative pulmonary complications
Strengths and limitations of this study.
This will be the first study to assess the incidence of postoperative pulmonary complications and the ventilatory practice in patients undergoing general anaesthesia for robotic surgery.
This is a multinational, multicenter, prospective, observational, rather than retrospective, study and should enhance our understanding of the incidence of postoperative pulmonary complications and the ventilatory practice in this group of patients.
Ventilatory variables will be measured at the critical points of the surgery, to assess the impact of surgical positioning and of the pneumoperitoneum in respiratory mechanics.
Due to the absence of standardisation on the definition of postoperative pulmonary complications, some complications will not be addressed.
Introduction
Minimally invasive surgery is increasingly being used due to its association with reduced surgical trauma and postoperative pain, low bleeding complication rates, shorter hospital length of stay and increased patient satisfaction.1 2 Robotic-assisted surgery (RAS) has emerged as an alternative minimally invasive surgical option, providing increased ergonomics, magnification of the surgical field, greater amplitude of movement and higher precision.3
Despite the growing applicability of RAS, the need of pneumoperitoneum and steep head-down (Trendelenburg) position could have a marked influence on patients respiratory mechanics. Indeed, this could lead to increased intra-abdominal pressure and cephalic elevation of the diaphragm, decreasing the compliance of the respiratory system and tidal volume, as well as increasing the plateau and peak pressure.4–8
Postoperative pulmonary complication (PPC) usually occur between 5 and 7 days after surgery and include the development of respiratory events, such as acute respiratory failure, acute respiratory distress syndrome (ARDS), pneumonia, prolonged or unplanned mechanical ventilation, reintubation, hypoxemia, atelectasis, bronchospasm, pleural effusion, pneumothorax and respiratory depression.9 Approximately, 5% of patients submitted to surgery develop at least one PPC during the follow-up, resulting in longer hospital length of stay and higher mortality rates.10 The changes in respiratory mechanics induced by the surgical positioning and by the degree of pneumoperitoneum during RAS could increase the risk of PPC in this group of patients. Nevertheless, there are currently insufficient data to guide the best ventilatory strategy during RAS, with some reports suggesting that ventilatory parameters should be adjusted to maintain normocapnia, despite the associated need for high tidal volumes.8 11
The aim of the AVATaR study is to investigate the incidence of PPC, characterise current ventilatory practices and evaluate the association between ventilatory parameters and outcomes in surgical patients undergoing general anaesthesia for RAS.
Methods
Design
This is an international multicenter prospective observational study designed in accordance with the declaration of Helsinki, registered at www.clinicaltrials.gov (trial identification number NCT02989415).
Patient and public involvement
Patients or public were not involved in the study design.
Patient eligibility
Consecutive patients undergoing mechanical ventilation for general anaesthesia for RAS will be consecutively included during a period of 1 month, to be defined by each of the participating centres. The need for informed consent is determined by the Institutional Review Board of each participating centre or country, following local regulations.
Inclusion and exclusion criteria
Patients fulfilling the following inclusion criteria are included: (1) age ≥18 years and (2) surgical procedures performed under general anaesthesia for RAS, including head and neck, chest, cardiac and abdominal surgeries. Patients submitted to procedures during pregnancy or outside the operation room are excluded.
Steps and data collection
Local investigators at each participating centres screen all patients submitted to mechanical ventilation during general anaesthesia for RAS during a predefined period of 1 month. PPC will be collected on day 0 (end of surgery until 11:59 pm) and on postoperative days 1, 2, 3, 4 and 5 (each day goes from 00:00 to 23:59). Data collection is finished on the day of hospital discharge or on day 5, for patients who remain hospitalised (figure 1). The start date for each participating centre is flexible and is determined together with the study coordinator.
Figure 1.
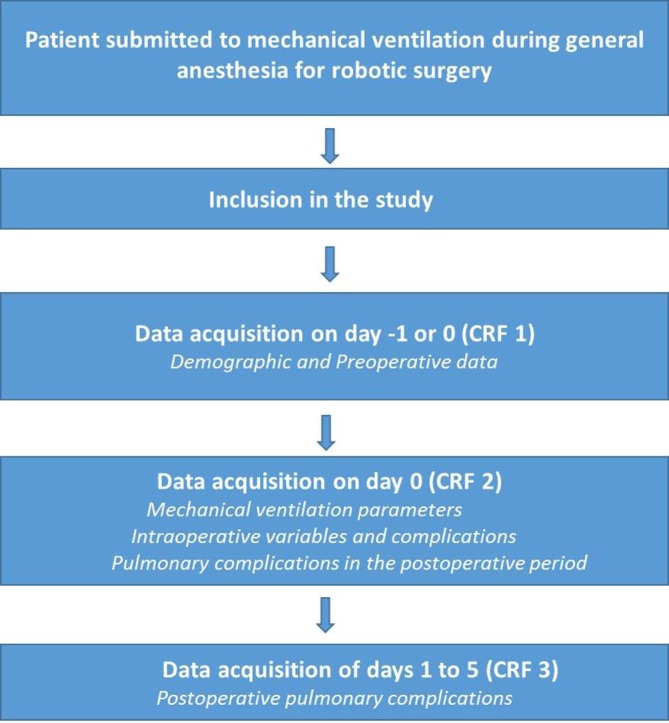
Study flow chart.
Outcomes
The primary outcome is the incidence of PPC, defined as a collapse composite endpoint of: unplanned need for oxygen therapy (defined as supplementary oxygen used due to PaO2 <60 mm Hg or SpO2 <92% in room air in individuals with no prior pulmonary disease or SpO2 <88% in individuals with prior pulmonary disease), development of acute respiratory failure (defined as PaO2 <60 mm Hg or SpO2 <92%, despite treatment with oxygen or need for non-invasive ventilation or unplanned continuous mechanical ventilation), development of pneumonia (defined by the presence of a new or progressive radiographic infiltrate in addition to at least two of the five clinical characteristics: fever >38°C, leucocytosis or leucopenia (leucocyte counte > 12.0 x 10^9/L or < 4.0 x 10^9/L, respectively),12 development of ARDS (defined according to Berlin criteria)13 and/or development of pneumothorax (defined as the presence of air between the visceral and parietal pleura; diagnosis can be made by clinical examination and chest X-ray).14
Secondary outcomes include practice of ventilation, severe PPC (excluding the unplanned need for oxygen), intraoperative complications (including desaturation (SpO2 <92% for 3 min or more), need for unplanned recruitment manoeuvres, need for ventilatory pressure reduction, hypotension (defined as systolic blood pressure <90 mm Hg or mean arterial pressure <65 mm Hg for 3 min or more or need of vasoactive drugs for correction), need for unplanned vasoactive drugs (need for vasoactive drugs not planned before and/or continuous infusion) and/or acute new arrhythmia (atrial fibrillation, sustained ventricular tachycardia, supraventricular tachycardia and/or ventricular fibrillation)), need for unplanned mechanical ventilation after surgery (including reintubation), need for intensive care unit admission, hospital length of stay and hospital mortality.
Study organisation
The steering committee includes the principal investigator, the coordinating investigator and experts in ventilatory support in surgical patients, all of whom contributed to the design and revisions of the original study protocol. The coordinating investigator is responsible for administrative management and communication with the local investigators and provided assistance to the participating clinical sites in study management, record keeping and data management. Local investigators provided structural and scientific leadership. They guaranteed the integrity of data collection and ensure timely completion of the case report forms.
Data collection
Data collection is performed using electronic case report form in the Research Electronic Data Capture (REDCap, USA) via the Internet at the Clinical Research Unit system of the Hospital Israelita Albert Einstein (the case report form is available in the online Supplementary data). The system has the following functions: patient registration, data input, data cleaning, audit trail and data export for statistical analysis. Local investigators enter the data directly into the system. Instructions for using the system are available to investigators at all times. Electronic files are archived in the Hospital Israelita Albert Einstein-based server in a secure and controlled environment to maintain confidentiality. Electronic documents are controlled with password protection according to best practices.
bmjopen-2018-021643supp001.pdf (89.9KB, pdf)
Data management
The goal of the clinical data management plan is to provide high–quality data by adopting standardised procedures to minimise the number of errors and missing data, and consequently, to generate an accurate database for analysis. Remote monitoring is performed to signal early aberrant patterns, issues with consistency, credibility and other anomalies, according to predefined queries created in the system. Any missing and outlier data values are individually revised and completed or corrected whenever possible.
Cleaning and locking of the database
The database will be locked as soon as all data are entered and all discrepant or missing data are resolved—or if all efforts are employed and we consider that the remaining issues cannot be fixed. At this step, the data will be reviewed before database locking. After that, the study database will be locked and exported for statistical analysis. At this stage, permission for access to the database will be removed for all investigators and the database will be archived.
Sample size
All patients submitted to ventilation during general anaesthesia for RAS will be consecutively included during the period of 1 month, in a convenience sample.
Predefined statistical analysis plan
For the primary analyses, only patients undergoing abdominal surgery will be included. Also, patients in whom the surgery was converted to open or laparoscopic will be excluded. This group of patients excluded due to these reasons will be studied in a separate report. Continuous distribution of the data will be assessed by visual inspection of histograms and D’Agostino-Pearson’s normality tests. Baseline characteristics will be expressed as counts and percentages, means and SD or medians and IQR whenever appropriate. Hypothesis tests will be two-sided with a significance level of 5%. We will not adjust p values for multiple comparisons. Analyses will be performed using the R (R Core Team, 2016, Vienna, Austria) programme.
Baseline characteristics
Patients baseline characteristics will be presented as shown in mock table 1.
Table 1.
Characteristics of the included patients
| All patients (n=) | |
| Age, years | Mean±SD |
| Male sex | n/Total (%) |
| BMI, kg/m2 | Mean±SD |
| ASA | Mean±SD |
| 1 | n/Total (%) |
| 2 | n/Total (%) |
| 3 | n/Total (%) |
| 4 | n/Total (%) |
| 5 | n/Total (%) |
| ARISCAT | Mean±SD |
| <26 | n/Total (%) |
| 26–44 | n/Total (%) |
| ≥45 | n/Total (%) |
| Functional status | |
| Independent | n/Total (%) |
| Partially dependent | n/Total (%) |
| Totally dependent | n/Total (%) |
| Comorbidities | |
| Hypertension | n/Total (%) |
| Coronary disease | n/Total (%) |
| Atrial fibrillation/flutter | n/Total (%) |
| Heart failure | n/Total (%) |
| Diabetes mellitus | n/Total (%) |
| COPD | n/Total (%) |
| Asthma | n/Total (%) |
| Smoking | n/Total (%) |
| Obstructive sleep apnoea | n/Total (%) |
| Active neoplasia | n/Total (%) |
| Liver cirrhosis | n/Total (%) |
| Anaemia (Hb <10 g/dL) | n/Total (%) |
| Chronic kidney disease | n/Total (%) |
| Haematological disease | n/Total (%) |
| Use of immunosuppression | n/Total (%) |
| Complications≤30 days before surgery | |
| None | n/Total (%) |
| Respiratory infection | n/Total (%) |
| Use of mechanical ventilation | n/Total (%) |
| Transfusion of blood products | n/Total (%) |
| Vital signs | |
| Respiratory rate, mpm | Mean±SD |
| Heart rate, bpm | Mean±SD |
| Mean arterial pressure, mm Hg | Mean±SD |
| SpO2, % | Mean±SD |
| Laboratory tests | |
| Haemoglobin, g/dL | Mean±SD |
| Leucocytes, x10^9/L | Mean±SD |
| Creatinine, mg/dL | Mean±SD |
| Condition of the procedure | |
| Elective | n/Total (%) |
| Urgency | n/Total (%) |
| Emergency | n/Total (%) |
| Expected duration of surgery | |
| ≤2 hours | n/Total (%) |
| 2–3 hours | n/Total (%) |
| >3 hours | n/Total (%) |
| Incision | |
| Peripheral | n/Total (%) |
| Low abdomen | n/Total (%) |
| High abdomen | n/Total (%) |
| Intrathoracic | n/Total (%) |
| Other | n/Total (%) |
| Surgical procedure | |
| Prostatectomy | n/Total (%) |
| Nephrectomy | n/Total (%) |
| Hysterectomy | n/Total (%) |
| Bariatric | n/Total (%) |
| Sacrocolpopexy | n/Total (%) |
| Cholecystectomy | n/Total (%) |
| Cardiac | n/Total (%) |
| Colorectal | n/Total (%) |
| Hernia | n/Total (%) |
| Head and neck | n/Total (%) |
| Pulmonary resection | n/Total (%) |
| Cystectomy | n/Total (%) |
| Pyloropasty | n/Total (%) |
| Pyeloplasty | n/Total (%) |
| Other | n/Total (%) |
ARISCAT, assess respiratory risk in surgical patients in Catalonia; ASA, American Society of Anesthesiology; BMI, body mass index; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; Hb, haemoglobin; mpm, movements per minute; SpO2, pulse oximetry.
Intraoperative characteristics
Intraoperative characteristics will be presented as shown in mock table 2.
Table 2.
Intraoperative characteristics
| All patients (n=) | |
| Type of tracheal tube | |
| Simple endotracheal | n/Total (%) |
| Double-lumen endotracheal | n/Total (%) |
| Nasotracheal | n/Total (%) |
| Endobronchial tube | n/Total (%) |
| Endobrochial blocker | n/Total (%) |
| Type of anaesthesia | |
| Total intravenous | n/Total (%) |
| Volatile | n/Total (%) |
| Balanced | n/Total (%) |
| Use of antibiotic prophylaxis | n/Total (%) |
| Use of one-lung ventilation | n/Total (%) |
| Left lung ventilated | n/Total (%) |
| Right lung ventilated | n/Total (%) |
| Use of neuroaxial blockade | n/Total (%) |
| Epidural | n/Total (%) |
| Spinal | n/Total (%) |
| Combined | n/Total (%) |
| Use of Trendelenburg during surgery | n/Total (%) |
| Normal | n/Total (%) |
| Accentuated (≥40° of the bed) | n/Total (%) |
| Reverse | n/Total (%) |
| Surgical conversion | n/Total (%) |
| Conversion to open surgery | n/Total (%) |
| Conversion to laparoscopic | n/Total (%) |
| Use of carbondioxide insufflation | n/Total (%) |
| Abdominal | n/Total (%) |
| Thoracic | n/Total (%) |
| Mediastinum | n/Total (%) |
| Use of opioids | n/Total (%) |
| Short acting | n/Total (%) |
| Long acting | n/Total (%) |
| Both | n/Total (%) |
| Use of neuromuscular blocking agents | n/Total (%) |
| Neuromuscular blockade monitoring | n/Total (%) |
| Reversal of neuromuscular blockade | n/Total (%) |
| Residual curarisation | n/Total (%) |
| Total fluid intake, mL | Mean±SD |
| Crystalloids, mL | Mean±SD |
| Synthetic colloid, mL | Mean±SD |
| Albumin, mL | Mean±SD |
| Urine output, mL | Mean±SD |
| Blood loss, mL | Mean±SD |
| Fluid balance, mL | Mean±SD |
| Temperature at the end of the surgery, °C | Mean±SD |
| Transfusion of blood products | n/Total (%) |
| Red blood cells | n/Total (%) |
| Fresh frozen plasma | n/Total (%) |
| Platelets | n/Total (%) |
| Cryoprecipitate | n/Total (%) |
| Duration of surgery, min | Mean±SD |
| Duration of anaesthesia, min | Mean±SD |
Ventilatory variables
Ventilatory variables and other interventions will be reported hourly for 6 hours and in four specific periods: (1) 5 min after induction and beginning of ventilation (T1); (2) 5 min after CO2 insufflation (T2); (3) 5 min after definitive positioning (immediately before beginning surgery) (T3) and (4) 5 min after removal of pneumoperitoneum and return to supine (T4). Peak, plateau and driving pressure (defined as plateau minus positive-end expiratory pressure (PEEP)) and PEEP levels, tidal volume size, respiratory rate, fraction of inspired oxygen (FiO2), static and dynamic respiratory system compliance, pulse oximetry (SpO2), heart rate, mean arterial pressure and pressure of CO2 insufflation over the four specific periods will be analysed using a mixed model with repeated measures and plotted in an interaction plot (mock table 3).
Table 3.
Intraoperative ventilation in four specific periods
| Five minutes after induction and beginning of ventilation | Five minutes after CO2 insufflation | Five minutes after definitive positioning | Five minutes after desinsufflation and return to supine | |||||
| Patients | P values | Patients | P values | Patients | P values | Patients | P values | |
| Surgical positioning | ||||||||
| Dorsal decubitus | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Ventral decubitus | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Lateral decubitus | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Lithotomy | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Trendelenburg | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Reverse Trendelenburg | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Sitting | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Mode of ventilation | ||||||||
| Pressure controlled | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Volume controlled | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| PCVG | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Other | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Peak pressure, cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Plateau pressure, cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Driving pressure, cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| PEEP, cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Tidal volume, mL/kg PBW | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Respiratory rate, mpm | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| FiO2, % | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Static CRS, mL/cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Dynamic CRS, mL/cmH2O | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Use of recruitment manoeuvres | ||||||||
| Increase in PEEP | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Increase in tidal volume | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Increase in tidal volume and PEEP | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| Manual insufflation with bag | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| CPAP | n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | ||||
| SpO2, % | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| etCO2, mm Hg | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Mean arterial pressure, mm Hg | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Heart rate, bpm | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Pressure of CO2 insufflation, mm Hg | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
bpm, beats per minute; CPAP, continuous positive airway pressure; CRS, respiratory system compliance; etCO2, end-tidal carbon dioxide; FiO2, fraction of inspired oxygen; mpm, movements per minute; PBW, predicted body weight; PCVG, pressure controlled volume guaranteed; PEEP, positive-end expiratory pressure; SpO2, pulse oximetry.
Primary outcome
The number of patients developing a PPC will be reported in absolute numbers and percentages (mock table 4). The impact of ventilatory variables on the development of PPC will be assessed using a generalised linear mixed-effect model. Relevant covariates included in the final multivariable model will be identified as those with p<0.2 in the univariable model (including centre as a random effect), clinical relevance and no statistical association with other relevant variables. In the final model, time of measurement will be included as a fixed effect together with the variables of interest and the centres and patients will be included as random effect. The linearity of each continuous predictor with the log odds outcome will be checked graphically and, if not present, a log-transformation will be performed. Pearson correlation coefficients will be used to assess collinearity between predictors. Since a high collinearity between peak, plateau and driving pressure is expected, the main model will consider the variable with the higher amount of measurements between peak or plateau pressure. Driving pressure will be considered in a sensitivity analysis, excluding PEEP, peak and plateau pressure. Finally, the intraclass correlation coefficient (ICC) will be assessed. The ICC represents the ratio of between-site variance to total variance, ranging from 0 to 1.
Table 4.
Clinical outcomes
| All patients (n=) | |
| Primary outcomes | |
| Postoperative pulmonary complications | n/Total (%) |
| Unplanned need of oxygen | n/Total (%) |
| Acute respiratory failure | n/Total (%) |
| Pneumonia | n/Total (%) |
| ARDS | n/Total (%) |
| Pneumothorax | n/Total (%) |
| Secondary outcomes | |
| Severe postoperative pulmonary complications | n/Total (%) |
| Intraoperative complications | n/Total (%) |
| Desaturation | n/Total (%) |
| Unplanned recruitment manoeuvres | n/Total (%) |
| Need for ventilatory pressure reduction | n/Total (%) |
| Hypotension | n/Total (%) |
| Need for unplanned vasoactive drug | n/Total (%) |
| Acute new arrhythmia | n/Total (%) |
| Unplanned ventilation after surgery | n/Total (%) |
| Reintubation | n/Total (%) |
| New use of mechanical ventilation | n/Total (%) |
| Admission to intensive care unit | n/Total (%) |
| Hospital length of stay | Mean±SD |
| Median (IQR) | Median (IQR) |
| Hospital mortality | n/Total (%) |
ARDS, acute respiratory distress syndrome.
Secondary outcomes
Secondary outcomes will be reported as shown in mock table 4.
Ethics and dissemination
The study will be performed according to the national and international guidelines. The study will not begin at the participating centres until approval has been obtained from the local Institutional Review Board for each participating centre or country, according to local regulation. Prospective written informed consent will be requested before inclusion of all eligible patients. The waiver of consent will follow local guidelines.
The AVATaR Steering Committee will publish the study findings, whatever they are. The main manuscript will be submitted by the writing committee on behalf of the research group (AVATaR and the PROVENet investigators). Two to three investigators per centre will be listed as collaborators in the online Supplementary appendix in alphabetical order according to the name of the centre. All efforts will be made to link all collaborators to the final publication in indexed databases.
Supplementary Material
Footnotes
Contributors: VNFQ, LGVdC, RPB, FT, LDB, DSC, UCD, JRG, SNTH, MWH, AK, LABdM, RMM, GHM, IdPP, AT, MFVM, JS, TAM-T, PP, MGdA, MJS and ASN conceived the study and drafted the study design. FT, DSC, MWH, PP, MGdA, MJS and ASN provided administrative support. LDB, AK, IM, GM, GHM, MFVM and TAM-T are the members of the steering committee and are national coordinators. VG, JPC, JS, TNW, SK, LE, L-LC, J-Wl, JAJI, BT and HG are members of the steering committee and are local coordinators.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Institutional Review Board of the Hospital Israelita Albert Einstein, São Paulo, Brazil under reference number 2.047.801.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: AVATaR and PROVE Network investigators: MD Anderson Cancer Center Mauro Bravo - Hospital Israelita Albert Einstein Renato Carneiro de Freitas Chaves Felipe Rothman Bruno Schuind Arantes Dina Mie Hatanaka - Hospital Clinic Barcelona Joan Beltran Concepción Monsalve - Maasstad Hospital Rotterdam Sijgje Maria Droger, RN, MA - Mayo Clinic Atousa Deljou, MD Moldovan Sabov, MD - Hospital Clinico San Carlos Yosef Saleh Rabbu Marina del Barrio de Bonis - University of Dusseldorf Renate Babian Robert Rabenalt - Kliniken Essen-Mitte Wiebke Köhne - Rabin Medical Center, Beilinson Hospital, PetachTikvah and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. Ariel Ronen - Massachusetts General Hospital Aalok V. Agarwala, MD - Consorcio Hospital General Universitario de Valencia Manuel Granell Juan Catala - Tel Aviv Medical Center Idit Matot
References
- 1. Royston CM, Lansdown MR, Brough WA. Teaching laparoscopic surgery: the need for guidelines. BMJ 1994;308:1023–5. 10.1136/bmj.308.6935.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sullivan MJ, Frost EA, Lew MW. Anesthetic care of the patient for robotic surgery. Middle East J Anaesthesiol 2008;19:967–82. [PubMed] [Google Scholar]
- 3. Lee JR. Anesthetic considerations for robotic surgery. Korean J Anesthesiol 2014;66:3–11. 10.4097/kjae.2014.66.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi EM, Na S, Choi SH, et al. Comparison of volume-controlled and pressure-controlled ventilation in steep Trendelenburg position for robot-assisted laparoscopic radical prostatectomy. J Clin Anesth 2011;23:183–8. 10.1016/j.jclinane.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 5. Sharma KC, Brandstetter RD, Brensilver JM, et al. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest 1996;110:810–5. 10.1378/chest.110.3.810 [DOI] [PubMed] [Google Scholar]
- 6. Sahin DA, Haliloglu B, Sahin FK, et al. Stepwise rising CO2 insufflation as an ischemic preconditioning method. J Laparoendosc Adv Surg Tech A 2007;17:723–30. 10.1089/lap.2007.0008 [DOI] [PubMed] [Google Scholar]
- 7. Karapolat S, Gezer S, Yildirim U, et al. Prevention of pulmonary complications of pneumoperitoneum in rats. J Cardiothorac Surg 2011;6:6–14. 10.1186/1749-8090-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danic MJ, Chow M, Alexander G, et al. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robot Surg 2007;1:119–23. 10.1007/s11701-007-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88–105. 10.1097/EJA.0000000000000118 [DOI] [PubMed] [Google Scholar]
- 10. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338–50. 10.1097/ALN.0b013e3181fc6e0a [DOI] [PubMed] [Google Scholar]
- 11. Dal Moro F, Crestani A, Valotto C, et al. Anesthesiologic effects of transperitoneal versus extraperitoneal approach during robot-assisted radical prostatectomy: results of a prospective randomized study. Int Braz J Urol 2015;41:466–72. 10.1590/S1677-5538.IBJU.2014.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Thoracic Society Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 13. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 14. Sharma A, Jindal P. Principles of diagnosis and management of traumatic pneumothorax. J Emerg Trauma Shock 2008;1:34–41. 10.4103/0974-2700.41789 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-021643supp001.pdf (89.9KB, pdf)


