Abstract
Context
Maternal hypertensive disorders during pregnancy are suggested to have an impact on offspring obesity risk. However, little is known about the prospective association of rise in maternal blood pressure within normal range during pregnancy with offspring obesity risk.
Objective
We aimed to clarify the associations of diastolic and systolic blood pressure during pregnancy among normotensive women with offspring obesity risk.
Design
Prospective cohort study.
Setting
Southeast China.
Participants
Up to 2013, 88,406 mother-child pairs with anthropometric measurements of the offspring between 4-7 years of age were included in the present analysis.
Main outcomes measured
Offspring overweight/obesity risk.
Results
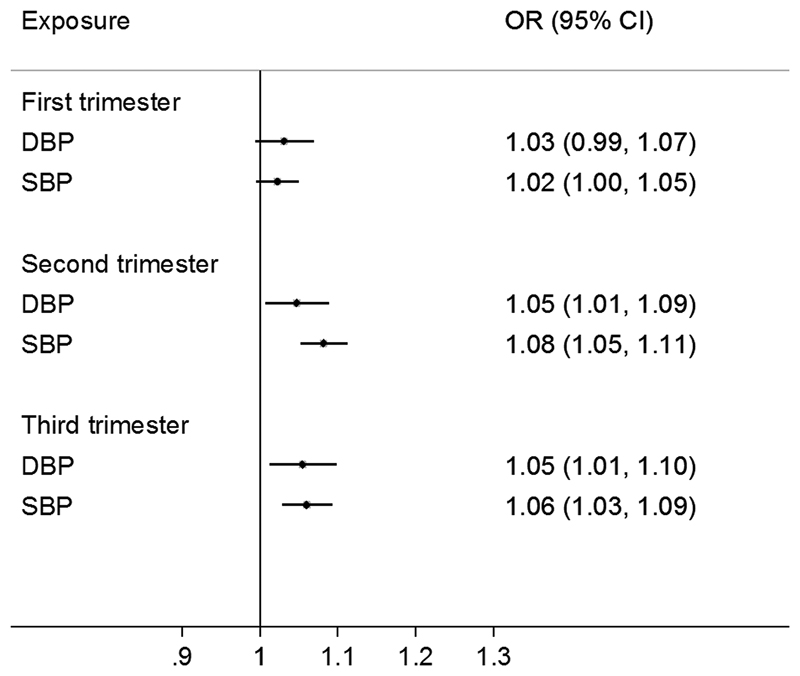
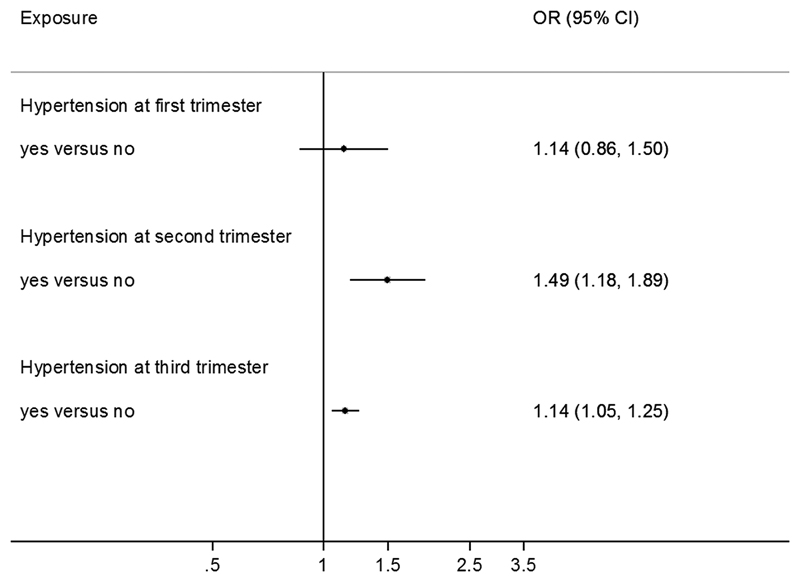
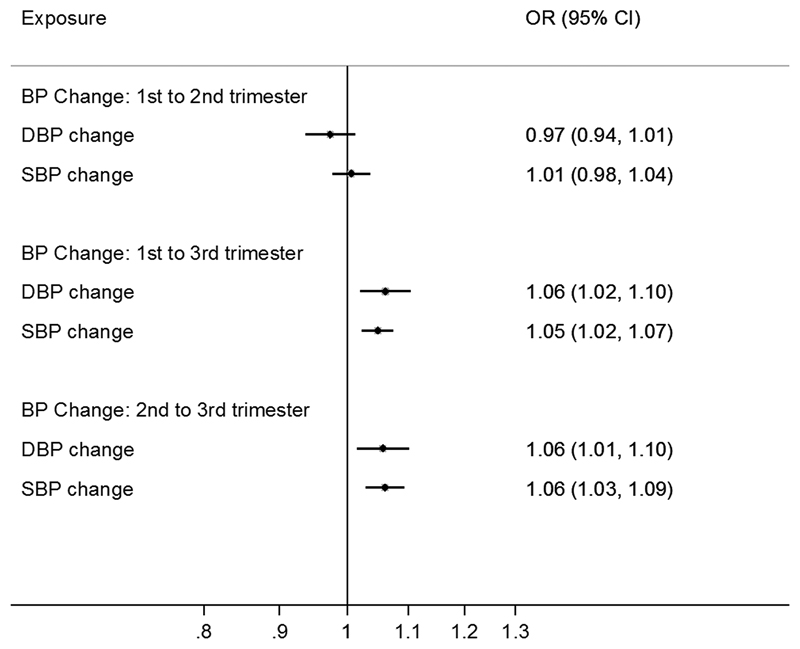
Among normotensive women, second and third trimester diastolic and systolic blood pressure were positively associated with offspring overweight/obesity risk: odds ratios per 10 mmHg higher second and third trimester diastolic blood pressure were: 1.05 (95% confidence interval: 1.01, 1.09) and 1.05 (1.02, 1.10), respectively; and for systolic blood pressure: 1.08 (1.05, 1.11) and 1.06 (1.03, 1.09). Each 10 mmHg greater rise in blood pressure between first to third trimester was associated with higher risk of offspring overweight/obesity, with diastolic: 1.06 (1.01, 1.10) and systolic: 1.05 (1.02, 1.07). Among all women (combining normotensive and hypertensive women), maternal hypertension in the second and third trimester was associated with 49% and 14% higher risk of offspring overweight/obesity respectively.
Conclusions
These results suggest that rise in maternal blood pressure during pregnancy and pregnancy hypertension, independent of maternal body size prior to pregnancy, are risk factors for offspring childhood obesity.
Keywords: pregnancy, blood pressure, offspring, overweight, obesity
Introduction
The prevalence of childhood obesity is increasing globally during the past two decades, especially among developing countries, such as China. In 2010, the age-adjusted prevalence of obesity and overweight/obesity was 8.1% and 19.2%, respectively (by % weight-for-height standards), among Chinese children of 7-18 years old in the Chinese National Surveys on Students’ Constitution and Health (1). There is strong evidence that childhood obesity is associated with many related health problems in adulthood, including obesity, type 2 diabetes and cardiovascular disease (2). In addition, childhood obesity leads to many childhood and adolescent comorbidities, such as hypertension, early puberty, menstrual irregularities, polycystic ovary syndrome and asthma (3). To curb the epidemic of the childhood obesity, it is crucial to identify its potential risk factors, as potential targets for prevention. Thus far, a variety of risk factors for childhood obesity have been identified, including parental obesity, birth weight, rapid weight gain during infancy, breastfeeding, short sleeping duration of the children and genetic variations (3, 4). However, little is known about the role of maternal pregnancy blood pressure (BP) in the development of offspring overweight and obesity in childhood.
Hypertensive disorders during pregnancy (including preeclampsia and gestational hypertension) are associated with impaired foetal growth and higher risk of multiple adverse birth outcomes (5, 6), and are weakly associated with higher offspring BMI or obesity risk in later life (7, 8). Although a few studies have reported the association of pregnancy BP with adverse birth outcomes (5, 9–11), to the best of our knowledge, no studies have examined the prospective association of maternal BP among a general population of pregnant women with offspring obesity risk. In addition, little is known about the association of change in BP during pregnancy with offspring obesity risk. As hypertensive disorders of pregnancy affect only less than 10% of pregnancies (12), investigation of the influence of BP during pregnancy on the offspring obesity risk among the majority of the pregnant women without hypertensive disorders is potentially of greater impact. Given the increasing public health concerns on childhood obesity, it is of interest to examine whether maternal pregnancy BP is an independent risk factor of offspring obesity. If established, it may further our understanding about the aetiology of childhood obesity and inform relevant prevention strategies.
Therefore, the primary aim of the present study was to investigate the association between repeated measures of maternal BP during pregnancy (in the first, second and third trimesters) with risk of offspring overweight/obesity at pre-school ages among normotensive women in the Jiaxing Birth Cohort, China.
Subjects and Methods
Study design and participants
The Jiaxing Birth Cohort (JBC) (1999-2013) was initiated in 1999 based on an existing routine health monitoring system in the Jiaxing area (a middle-income area in southeast China), involving more than 0.3 million live mother-child pairs with extensive follow-up information of the children up to 6-7 years old before they started school (13). Women participants living at one of the seven divisions/counties in the Jiaxing area came to register at local clinics before pregnancy or at any stage of pregnancy. Thereafter, participants came to visit the local clinics regularly until the birth (16-28 gestational weeks: once per four weeks; 29-36 gestational weeks: once per two weeks; >36 gestational weeks: once per week). Up to 2013, a total of 338,413 live mother-child pairs were enrolled in the JBC study. For participants who registered in the JBC study during pregnancy, their pre-pregnancy anthropometric measurements were retrieved by linking to a pre-marriage health check database held by the Jiaxing Maternity and Child Health Care Hospital.
Children enrolled in the JBC study visited (with their parents) the local clinics for health checks and anthropometric measurements at ages 1-2 months, 3 months, 6 months, 9 months and 12 months during infancy. In the following stage, children visited the local clinics every 6 months (18, 24, 30, 36 months) until age 36 months. Thereafter, children were asked to visit the clinics once per year before they started school (6-7 years old).
Between 1999 and 2006, a total of 134,680 mother-child (singleton) pairs were enrolled in the JBC study. Mother-child pairs were excluded if they had extreme offspring birth weight (<1500g or >5000g, n=85) or preterm birth (<37 gestational week, n=4425), or no maternal blood pressure record at any of the first, second or third trimester (n=426). Therefore, at baseline, 129,744 mother-child pairs were included in the present study. Up to 2013, 89,185 children had follow-up information between 4-7 years of age (68.7% follow-up rate). For children with multiple follow-ups between 4-7 years of age, the later follow-up visit data was used to maximise the follow-up duration. Children were excluded if they had any missing data on anthropometric measurements (height or weight) at the follow-up visit at age 4-7 years (n=779). Finally, 88,406 mother-child pairs were included in the statistical analyses. The study protocol was approved by the ethics committee of the College of Biosystem Engineering & Food Science at Zhejiang University in China. All participants provided oral informed consent.
Measurement of maternal BP and other key variables
At each clinic visit, seated maternal BP was measured by manual BP monitors in the right arm on a single occasion after 5-10 minutes of resting. At each of the three trimesters, maternal pregnancy hypertension was defined as diastolic blood pressure (DBP) ≥ 90mmHg and/or systolic blood pressure (SBP) ≥ 140mmHg. As we could not separate different hypertensive disorders of pregnancy such as gestational hypertension or preeclampsia (14), this definition (maternal pregnancy hypertension) represents a combination of hypertensive disorders of pregnancy. BP measurements at the first health check (0-12 gestational weeks) were considered as ‘first trimester BP’. BP measurements at a later health check between 13-28 gestational weeks were considered as ‘second trimester BP’. BP measurements at a health check around 37 gestational weeks (≥29 gestational weeks) was considered as ‘third trimester BP’.
At the first health check/recruitment of the participants, maternal demographic characteristics were collected by interview. Maternal anthropometric measurements (weight, height) were taken on site by trained nurses. Maternal BMI was based on pre-pregnancy (n=43,831) or first trimester measurements (n=36,491).
Offspring anthropometric assessment
Body weight and height of the children were measured by trained nurses to the nearest 0.1kg and 0.1cm at each of the follow-up clinic visits. Childhood overweight and obesity were defined according to the international BMI cut-off points by age and sex, as established by the International Obesity Task Force (15).
Statistical analyses
All statistical analyses were performed using STATA version 14 (StataCorp). Initially, logistic regression was used to examine the odds ratio (OR) and 95%CI of offspring overweight/obesity per 10mmHg higher maternal DBP and SBP at first, second and third trimester among normotensive women in three statistical models: Model 1, crude model without adjustment; Model 2, adjusted for maternal age (continuous), menarcheal age (<14y, 14-15y, >15y), education level (<high school, high school, >high school), occupation (farm work/house work, routine job, others), parity (primiparous or multiparous), offspring sex and offspring age at examination (continuous); Model 3, Model 2 plus maternal BMI (continuous) and maternal height (continuous). We included maternal BMI and height in the model 3 to examine the influence of maternal body size on the results in addition to other covariates. Sensitivity analyses were conducted under Model 3 by adopting further potential confounders or inclusion criteria: (3a) with additional covariates: maternal baseline self-reported smoking and drinking status, and family history of hypertension; (3b) only including women with pre-pregnancy BMI data; (3c) including all women with and without hypertensive disorders.
Consequently, we examined (based on Model 3): (1) the association between change in BP (per 10mmHg rise) during pregnancy with childhood overweight/obesity risk among normotensive women and among all women, adjusting for potential confounders; (2) the association between maternal hypertension during pregnancy at each of the three trimesters with offspring overweight/obesity risk, adjusting for potential confounders; (3) to investigate foetal growth as a potential mediator, we explored the association of maternal BP (per 10 mmHg increase) with offspring birth weight (continuous) among normotensive women, and the difference in birth weight between hypertensive and normotensive women.
To explore potential non-linear relationships, we examined the association (based on Model 3) of maternal DBP and SBP at each trimester with overweight/obesity risk, or offspring birth weight, using restricted cubic spline models (four knots, according to Harrell’s recommendation) (16) among all participants including both hypertensive and normotensive women. Four knots offer an adequate fit of the model and is a good compromise between flexibility and loss of precision caused by overfitting (16), and there was no substantial difference in the shape or non-linear association when we selected 3 or 5 knots.
Interaction between different pregnancy BP and different maternal/infant characteristics (maternal age, BMI, menarcheal age, and offspring sex) on offspring overweight/obesity risk was examined by adding relevant interaction terms to Model 3. We further examined potential meditation by birth weight, and the proportion (% of total effect) mediated by birth weight using the method proposed by Kenny et al.(17) The ‘binary_mediation’ command in STATA was used to estimate the mediation effect for our dichotomous outcome (overweight/obesity compared to normal weight), and bias-corrected 95%CI was calculated via bootstrapping with 500 replications (18). A two-tailed P-value <0.05 was considered statistically significant.
Results
Population characteristics
The mean maternal age at birth of their offspring was 25 y (SD=3.7) and mean maternal BMI was 20.5 kg/m2 (SD=2.6). Mean maternal DBP increased from 68.4mmHg to 69.1mmHg to 75.3mmHg in the first, second and third trimester, respectively, and similarly SBP increased from 105.9 to 108.7mmHg to 115.2mmHg (Supplemental Fig. 1). Mean gestational age at BP measurement in the first, second and third trimester was 9.3wks (SD=2.8), 26.4wks (SD=1.8) and 38.7wks (SD=1.1), respectively. Among the children who were followed up at 4-7 years old (mean age: 5.9y [SD=0.7]), 9.1% were overweight (6.6%) or obese (2.5%). The proportion of children who were overweight/obesity was higher among mothers with younger age, higher BMI, earlier menarcheal age, higher education levels, having a routine job, first pregnancy, or Caesarean–section delivery, and in male offspring (Table 1).
Table 1. Maternal and offspring characteristics by offspring adiposity status at 4-7 years old in the Jiaxing Birth Cohort (n=88450).
| n | Offspring without overweight/obesity (n=80366) | Offspring with overweight/obesity (n=8084) | |
|---|---|---|---|
| Maternal age (years) | |||
| ≤25 | 54427 | 90.7 | 9.3 |
| 26-30 | 23146 | 90.5 | 9.5 |
| 31-35 | 9987 | 92.3 | 7.7 |
| ≥36 | 857 | 92.0 | 8.0 |
| Maternal BMI (kg/m2) | |||
| <18.5 | 16362 | 94.7 | 5.3 |
| 18.5-24.9 | 60057 | 90.5 | 9.5 |
| 25-29.9 | 3421 | 81.4 | 18.6 |
| ≥30 | 482 | 80.1 | 19.9 |
| Maternal menarcheal age (years) | |||
| <14 | 12286 | 88.3 | 11.7 |
| 14-15 | 51556 | 90.8 | 9.2 |
| >15 | 24275 | 92.3 | 7.7 |
| Maternal education | |||
| <High school | 66928 | 91.5 | 8.5 |
| High school | 15637 | 88.7 | 11.3 |
| >High school | 5813 | 88.9 | 11.1 |
| Maternal occupation | |||
| Farm work/housework | 59539 | 91.6 | 8.4 |
| Routine job | 23134 | 89.7 | 10.3 |
| Others | 5703 | 88.1 | 11.9 |
| Maternal parity | |||
| Primiparous | 72959 | 90.4 | 9.6 |
| Multiparous | 15491 | 93.1 | 6.9 |
| Caesarean - section | |||
| No | 23758 | 92.8 | 7.2 |
| Yes | 64432 | 90.1 | 9.2 |
| Offspring sex | |||
| Boy | 45654 | 89.9 | 10.1 |
| Girl | 42763 | 91.9 | 8.1 |
| High BP at 1st trimester | |||
| No | 74175 | 90.9 | 9.1 |
| Yes | 506 | 87.4 | 12.6 |
| High BP at 2nd trimester | |||
| No | 85601 | 90.9 | 9.1 |
| Yes | 704 | 83.2 | 16.8 |
| High BP at 3rd trimester | |||
| No | 80179 | 91.1 | 8.9 |
| Yes | 7818 | 89.0 | 11.0 |
| Gestational weeks, wk | 88273 | 39.3 (1.12) | 39.2(1.10) |
| DBP at 1st trimester, mmHg | 74683 | 68.4 (7.2) | 68.9 (7.4) |
| SBP at 1st trimester, mmHg | 74710 | 105.8 (10) | 106.6 (10.3) |
| DBP at 2nd trimester, mmHg | 86320 | 69.0 (7.1) | 69.6 (7.4) |
| SBP at 2nd trimester, mmHg | 86323 | 108.6 (10) | 109.9 (10.4) |
| DBP at 3rd trimester, mmHg | 87993 | 75.2 (8.6) | 76.0 (9.0) |
| SBP at 3rd trimester, mmHg | 88002 | 115.1 (11.4) | 116.5 (11.6) |
| Infant birth weight, g | 88312 | 3328.9 (400.6) | 3443 (423.8) |
SBP, systolic blood pressure; DBP, diastolic blood pressure.
High BP, high blood pressure, which was defined as systolic blood pressure≥140 mmHg or diastolic blood pressure≥90 mmHg.
Maternal BP during pregnancy and offspring overweight/obesity at childhood
Among normotensive women, apparent positive associations between first trimester BP and offspring overweight/obesity risk were attenuated on adjustment for mother’s BMI and height (Model 3). By contrast, second and third trimester maternal DBP and SBP were positively associated with offspring overweight/obesity risk: third trimester DBP: OR per +10mmHg: 1.05 (95%CI: 1.01, 1.10); third trimester SBP: 1.06 (1.03, 1.09) in adjusted models (Model 3) (Figure 1, Table 2). Similarly, maternal hypertension at first trimester was not associated with offspring overweight/obesity risk; while hypertension at second (OR: 1.49; 95%CI: 1.18, 1.89) and third trimester (OR: 1.14; 95%CI: 1.05, 1.25) was associated with higher risk of offspring overweight/obesity (Figure 2). Across both normotensive and hypertensive women, the associations between maternal BP and offspring overweight/obesity risk appeared to be largely linear or J-shaped (Supplemental Fig. 2). In sensitivity analyses, excluding women without pre-pregnancy BMI slightly attenuated the association only with third trimester DBP (Supplemental Table 1). No interaction between DBP, SBP with any maternal characteristic was observed.
Figure 1. Odds ratio (OR) for offspring overweight/obesity per +10mmHg maternal blood pressure at different pregnancy stages among normotensive women.
Logistic regression was performed, adjusting for maternal characteristics (age, menarcheal age, educational level, occupation, parity status, corresponding diastolic or systolic blood pressure at the previous trimesters), offspring sex, age at examination, maternal BMI and height.
Table 2. Association between maternal blood pressure (per +10mmHg) at each pregnancy trimester among normotensive women with offspring overweight/obesity risk at 4-7 years old.
| Diastolic blood pressure | Systolic blood pressure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy stages | Model | n | OR | 95%CI | p | n | OR | 95%CI | p |
| First trimester | Model 1 | 74141 | 1.10 | 1.06, 1.14 | <0.001 | 74141 | 1.07 | 1.05, 1.10 | <0.001 |
| Model 2 | 73754 | 1.12 | 1.08, 1.16 | <0.001 | 73754 | 1.09 | 1.06, 1.12 | <0.001 | |
| Model 3 | 73524 | 1.03 | 0.99, 1.07 | 0.109 | 73524 | 1.02 | 1.00, 1.05 | 0.106 | |
| Second trimester | Model 1 | 85559 | 1.11 | 1.08, 1.15 | <0.001 | 85559 | 1.13 | 1.10, 1.16 | <0.001 |
| Model 2 | 72898 | 1.10 | 1.06, 1.14 | <0.001 | 72923 | 1.12 | 1.09, 1.16 | <0.001 | |
| Model 3 | 72675 | 1.05 | 1.01, 1.09 | 0.02 | 72699 | 1.08 | 1.05, 1.11 | <0.001 | |
| Third trimester | Model 1 | 80142 | 1.11 | 1.07, 1.15 | <0.001 | 80142 | 1.12 | 1.10, 1.15 | <0.001 |
| Model 2 | 66726 | 1.08 | 1.04, 1.13 | <0.001 | 66746 | 1.08 | 1.05, 1.11 | <0.001 | |
| Model 3 | 66520 | 1.05 | 1.01, 1.10 | 0.011 | 66539 | 1.06 | 1.03, 1.09 | <0.001 | |
The analyses were based on women without hypertension at each corresponding trimester.
Model 1: crude model without adjustment.
Model 2: adjusted for maternal characteristics (age, menarcheal age, educational level, occupation, parity status, and corresponding diastolic or systolic blood pressure at previous trimesters), offspring sex and age at examination.
Model 3: Model 2 plus maternal BMI and height.
Figure 2. Association between maternal hypertension (compared with normotensive women) at each pregnancy trimester with offspring overweight/obesity risk.
Logistic regression was performed, adjusting for maternal characteristics (age, menarcheal age, educational level, occupation, parity status, corresponding hypertensive status at the previous trimesters), offspring sex, age at examination, maternal BMI and height.
Changes in DBP and SBP from the first to third trimester among normotensive women were positively associated with offspring overweight/obesity risk: DBP: OR per +10mmHg BP rise: 1.06 (1.02, 1.10); SBP 1.05 (1.02, 1.07) (Figure 3). Across adjacent trimesters, BP changes from the second to third trimester were positively associated with offspring overweight/obesity risk, but surprisingly no association was seen with BP change from the first to second trimester. In sensitivity analyses, similar results were observed in the larger sample including hypertensive women (Supplemental Fig. 3).
Figure 3. Odds ratio (OR) for offspring overweight/obesity per 10mmHg rise in maternal blood pressure during pregnancy among normotensive women.
Logistic regression was performed, adjusting for maternal characteristics (age, menarcheal age, educational level, occupation, parity status, corresponding diastolic or systolic blood pressure at previous trimesters and change of the diastolic or systolic blood pressure between the previous trimesters (only for the outcome: change in blood pressure between second and third trimester), offspring sex, age at examination, maternal BMI and height.
Potential mediation by birth weight
Among normotensive women, in adjusted models, first trimester DBP and SBP were inversely associated with birth weight. By contrast, second and third trimester SBP (Supplemental Fig. 4) and change in SBP between the first and third trimester were positively associated with birth weight (Supplemental Fig. 5). Conversely, maternal hypertension at each of the three trimesters was associated with lower birth weight (Supplemental Fig. 6).
Across both normotensive and hypertensive women, the associations between second and third trimester DBP and SBP and offspring birth weight were non-linear (Supplemental Fig. 7). In mediation analyses among normotensive women, birth weight explained 24.2%, 9.2% and 5.9% of the associations between second trimester SBP, third trimester SBP and change in SBP from first to third trimester with offspring overweight/obesity risk, respectively (Supplemental Table 2).
Discussion
In this large prospective cohort study, we found that higher second and third trimester (but not first trimester) DBP and SBP among normotensive women, were positively associated with offspring overweight/obesity risk. In addition, changes in DBP and SBP between the first and third trimester were positively associated with offspring overweight/obesity risk among normotensive women. These associations were independent of maternal body size and were only partially mediated by higher offspring birth weight. Maternal hypertension at the second and third trimester (but not first trimester) was also positively associated with offspring overweight/obesity risk, yet being associated with lower offspring birth weight.
To the best of our knowledge, only a few studies have examined the prospective association of maternal hypertensive status with offspring overweight/obesity risk.(7, 8) In the UK ALSPAC study, gestational hypertension, compared with normotension, was associated with a 41% (OR: 1.41; 95%CI: 1.02, 1.95) higher relative risk of offspring obesity at 9 years old (7). In addition, a recent meta-analysis and systematic review suggested that hypertensive disorders of pregnancy were associated with higher adult offspring BMI and risk of overweight/obesity (8). Results from our present study confirm those previous reports (7, 8), and add novel evidence that maternal second and third trimester BP are positively associated with offspring overweight/obesity risk in women without hypertension. The apparent positive association of first trimester BP with childhood overweight/obesity was explained by confounding due to larger maternal size. In particular, change in BP during pregnancy, especially between second and third trimester, was positively associated with offspring adiposity risk. Taken together, our findings suggest that monitoring and control of the BP rise from middle to late pregnancy might be important, not only for pregnancy outcomes, but also for the prevention of childhood obesity.
There is accumulating evidence that high maternal BP or hypertensive disorders of pregnancy are associated with offspring BP (7, 8, 19–21). Epidemiologic studies unequivocally support the positive association between body weight and BP, and between obesity and hypertension (22). It was hypothesized that adiposity is in the causal pathway of maternal gestational hypertensive disorders with offspring high BP (7). This speculation needs confirmation in future studies.
The potential effects of pregnancy hypertensive disorders and normal range BP during pregnancy on higher offspring obesity risk may involve quite different mechanisms. Hypertensive disorders of pregnancy are well-known causes of intrauterine growth restriction (5, 23, 24), and DBP levels higher than 90 mmHg, a threshold commonly used to define hypertensive disorders, are inversely associated with birth weight (9). Higher third trimester umbilical artery vascular resistance, a parameter reflecting the placental dysfunction (25, 26), has been associated with slower foetal growth and a smaller size at birth, but higher childhood BMI (27). The mechanism linking intrauterine growth restriction with later adiposity may include change in foetal adipose tissue morphology and metabolism, altered pathway regulating appetite and modification of hormone and epigenome in foetus (28). Therefore, we postulated that hypertensive disorders during pregnancy may promote childhood overweight/obesity through its effect on intrauterine growth restriction.
High birth weight is also a well-known risk factor of childhood obesity (29). Therefore, we postulated that, among normotensive women, higher birth weight might mediate the positive association between maternal SBP and childhood overweight/obesity. However, the relationship between maternal BP and offspring birth weight is complex. Our findings that first trimester BP was inversely associated with birth weight are consistent with a recent genetic Mendelian randomisation study indicating a causal foetal growth restricting effect of maternal SBP (30). First trimester BP may be more strongly correlated with pre-pregnancy BP and common genetic determinants of non-pregnancy BP than mid-late pregnancy BP. In contrast to first trimester BP and hypertensive disorders, normal range maternal BP during mid-late pregnancy is associated not with intrauterine growth restriction, but with higher offspring birth weight. Our findings are supported by a previous large study of 210,814 mother-infant pairs: maternal DBP after 34 weeks gestation (but not earlier in pregnancy) showed an inverted U-shaped relation with birth weight and perinatal survival, with a maximum birth weight at around DBP of 80 mm Hg (9). The mechanism behind the positive association between normal range BP and higher birth weight is unclear. In the absence of placental vasculature resistance, increasing maternal BP may be advantageous for placental blood flow and foetal growth. However, late pregnancy DBP levels above 80 mmHg appear to be disadvantageous for both short and long-term health outcomes. That previous study did not have data on SBP (8). We found that second trimester SBP was positively associated with birth weight, even at above-normal range SBP levels, and birth weight mediated 24% of the effect of the second trimester SBP on childhood overweight/obesity in our analysis. These results suggested that higher maternal second trimester SBP affected offspring childhood overweight/obesity risk partly through increasing birth weight of the children.
The strengths of this study include its large sample size, prospective design and high follow-up rate. In addition, the JBC study has repeated BP measurements at different stages of pregnancy, and both maternal and offspring demographic and lifestyle are well documented at each visit by trained nurses or doctors. Furthermore, the results of the present study are robust as suggested by a variety of sensitivity analyses.
There are several limitations. First, maternal BP data are based on a single measurement at each visit. Second, we were unable to distinguish between gestational hypertension and preeclampsia due to lack of information on urine protein. However, our primary aim was to examine BP among normotensive women, with pregnancy hypertension as a secondary exposure. Third, the JBC study is based on a single region in Southeast China, and may not necessarily be more broadly representative, although many of our findings are consistent with those seen in other populations. Fourth, we did not adjust for excess weight gain during pregnancy due to the heterogeneity of gestational weeks for the weight measurement among different participants. Fifth, both birth weight and postnatal weight gain might potentially mediate the association between maternal blood pressure and offspring obesity. However, due to limited availability of postnatal weight data up to 4 y, the mediation analysis only focused on birth weight. Finally, there may be other potential confounders in this observational study.
In conclusion, among normotensive women, greater gestational rises in DBP and SBP were associated with higher offspring childhood overweight/obesity risk. This association was partially mediated by higher offspring birth weight. These findings provide new insights into the biologic mechanisms linking to childhood obesity. The 5%-8% increment in the odds of childhood overweight/obesity corresponding to the higher blood pressure at maternal second or third trimester might have important public health implications for the prevention of childhood obesity, given the increasing prevalence of childhood obesity in the past decades in China. These findings also added to the rationale to monitor and limit the BP rise in mid-late pregnancy.
Supplementary Material
Acknowledgements
We thank all the participants involved in the Jiaxing Birth Cohort, and all the staff working on the project.
Funding: This work is supported by the National Basic Research Program of China (973 Program: 2015CB553604); by National Natural Science Foundation of China (NSFC: 81273054); and by the Ph.D. Programs Foundation of Ministry of Education of China (20120101110107). JSZ is supported by the Marie Skłodowska-Curie Fellowships (701708, RG82205, SJAI/051). KKO is supported by the Medical Research Council (Unit Programme number MC_UU_12015/2). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions: The authors’ responsibilities were as follows: JSZ, HL, YG and DL contributed to the research design. JZ, KKO and DL wrote the paper and JSZ performed the statistical analysis for the manuscript. TH, YH and FW contributed to the interpretation and revision of the report. All authors contributed towards critical review of the manuscript during the writing process. All author approved the final version of the report.
Disclosure: The author reports no conflicts of interest in this work.
References
- 1.Sun HP, Ma Y, Han D, Pan CW, Xu Y. Prevalence and trends in obesity among china's children and adolescents, 1985-2010. Plos One. 2014;9 doi: 10.1371/journal.pone.0105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 3.Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation. 2012;126:1770–1779. doi: 10.1161/CIRCULATIONAHA.111.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A, Avon Longitudinal Study of P, Children Study T Early life risk factors for obesity in childhood: Cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker R, Steegers EA, Hofman A, Jaddoe VW. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: The generation r study. Am J Epidemiol. 2011;174:797–806. doi: 10.1093/aje/kwr151. [DOI] [PubMed] [Google Scholar]
- 6.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: The avon longitudinal study of parents and children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoulass JC, Robertson L, Denadai L, Black C, Crilly M, Iversen L, Scott NW, Hannaford PC. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: A systematic review of the literature and meta-analysis. J Epidemiol Community Health. 2016;70:414–422. doi: 10.1136/jech-2015-205483. [DOI] [PubMed] [Google Scholar]
- 9.Steer PJ, Little MP, Kold-Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: Prospective study. BMJ. 2004;329:1312. doi: 10.1136/bmj.38258.566262.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim WY, Lee YS, Tan CS, Kwek K, Chong YS, Gluckman PD, Godfrey KM, Saw SM, Pan A. The association between maternal blood pressures and offspring size at birth in southeast asian women. BMC Pregnancy and Childbirth. 2014;14 doi: 10.1186/s12884-014-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: Findings from a prospective cohort. Hypertension. 2014;64:36–44. doi: 10.1161/HYPERTENSIONAHA.113.02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Zheng JS, Liu H, Jiang J, Huang T, Wang F, Guan Y, Li D. Cohort profile: The jiaxing birth cohort in china. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw203. [DOI] [PubMed] [Google Scholar]
- 14.Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, Militello M, Pedata R. Hypertensive disorders of pregnancy. J Prenat Med. 2009;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New york: Springer; 2001. [Google Scholar]
- 17.West AA, Caudill MA. Applied choline-omics: Lessons from human metabolic studies for the integration of genomics research into nutrition practice. J Acad Nutr Diet. 2014;114:1242–1250. doi: 10.1016/j.jand.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Martin FP, Moco S, Montoliu I, Collino S, Da Silva L, Rezzi S, Prieto R, Kussmann M, Inostroza J, Steenhout P. Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatr Res. 2014;75:535–543. doi: 10.1038/pr.2013.250. [DOI] [PubMed] [Google Scholar]
- 19.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics. 2012;129:e1552–1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 20.Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. doi: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, Macdonald-Wallis C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: Findings from a prospective study. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of the obesity society and the american society of hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananth CV, Peedicayil A, Savitz DA. Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology. 1995;6:391–395. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155:203–209. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]
- 25.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl)(1):S99–107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 26.Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28:67–80. doi: 10.1053/j.semperi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Gaillard R, Steegers EA, Tiemeier H, Hofman A, Jaddoe VW. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: The Generation R study. Circulation. 2013;128:2202–2210. doi: 10.1161/CIRCULATIONAHA.113.003881. [DOI] [PubMed] [Google Scholar]
- 28.Sarr O, Yang K, Regnault TR. In utero programming of later adiposity: The role of fetal growth restriction. J Pregnancy. 2012;2012 doi: 10.1155/2012/134758. 134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, Guo XR. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes Rev. 2011;12:525–542. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 30.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, Cavadino A, Paternoster L, Armstrong LL, De Silva NMG, Wood AR, et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA. 2016;315:1129–1140. doi: 10.1001/jama.2016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.