Abstract
The gaze of other dogs and humans is informative for dogs, but it has not been explored which factors predict face-directed attention. We used image presentations of unfamiliar human and dog heads, facing the observer (portrait) or facing away (profile), and measured looking time responses. We expected dog portraits to be aversive, human portraits to attract interest, and tested dogs of different sex, skull length and breed function, which in previous work had predicted human-directed attention. Dog portraits attracted longer looking times than human profiles. Mesocephalic dogs looked at portraits longer than at profiles, independent of the species in the image. Overall, brachycephalic dogs and dogs of unspecified breed function (such as mixed breeds) displayed the longest looking times. Among the latter, females observed the images for longer than males, which is in line with human findings on sex differences in processing faces. In a subsequent experiment, we tested whether dog portraits functioned as threatening stimuli. We hypothesized that dogs will avoid food rewards or approach them more slowly in the presence of a dog portrait, but found no effect of image type. In general, older dogs took longer to approach food placed in front of the images and mesocephalic dogs were faster than dogs of other skull length types. The results suggest that short-headed dogs are more attentive to faces, while sex and breed function predict looking times through complex interactions.
Introduction
In comparative research, the dog has become a favourable subject for studying gazing behaviours. Gaze-directed attention might even pre-date domestication: gaze following, both in distant space and around barriers, is present in the wolf, dogs’ closest relative (Range and Virányi 2011), and eye contact with a human experimenter can be trained, albeit with more effort than in the dog (Gácsi et al. 2009a). The morphology of the head and eyes make them relevant cues for predicting conspecific behaviour (Ueda et al. 2014), but in wolves eye contact is used mostly to signal threat (Schenkel 1967) suggesting that the eyes and gaze of conspecifics are aversive stimuli for canines in the wild.
On the other hand, it appears that dogs preferentially follow the gaze of humans if eye-contact was established first (Téglás et al. 2012); that they use alternated gazing to ‘show’ a human the location of hidden toys (Miklósi et al. 2000) and that they can distinguish human emotional facial expressions (Nagasawa et al. 2011; Ted Ruffman and Morris-Trainor 2011; Turcsán et al. 2014). Visual cues in general play a pivotal role in dog-human communication, like the pointing gesture (Soproni et al. 2002). Dogs can follow pointing gestures better than chimpanzees, humans’ closest living relatives (Miklósi and Soproni 2006). Miklósi et al. (2003) suggested dogs’ attention towards the human gaze may explain dog-wolf differences in learning to follow human pointing (so far only one study has found wolves to perform better than dogs (Udell et al. 2008)). Although there is some support for this capacity being an extension of the predatory (motor) sequence (Udell et al. 2014) i.e. orienting towards and subsequently following the prey, the human gaze may as yet play a crucial role in creating the necessary context for dogs to interpret pointing as a communicative signal and distinguish it from similar, but unintentional movements. Kaminski and Nitzschner provided an extensive review (2013)) – one common finding is that dogs preferentially respond to pointing after eye-contact has been established with the experimenter. Another recent argument that the visibility of the human face and eyes signal communicative intention to dogs, comes from a study showing dogs’ facial expressions are preferentially displayed in response to people facing them upfront (Kaminski et al. 2017). A special role for the human gaze as a signal in dog-human communication could be the result of dogs’ adaptation to life with humans during their unique domestication process (Hare and Tomasello 2005; Miklosi 2014) (though see Udell et al. (2010) for an alternative account).
The relationship between sex and attentiveness to the eyes and gaze is relevant to cross-species comparisons, as some studies have suggested better female performance in the social cognitive domain may persist across mammalian species (de Waal 1996; Bartal et al. 2011). Research on canine visual abilities, however, has not explored sex differences yet.
Previous work had also shown that dog’s responses to human cues (such as pointing) and their perceived trainability vary with breed characteristics like their cephalic index (skull length) (Gácsi et al. 2009b; Helton 2009) and breed function (Gácsi et al. 2009b; Udell et al. 2014).
Skull length might have an impact due to associated differences in the position of the eyes, allowing for binocular vision and the density of retinal ganglion cells (McGreevy et al. 2004). So far it has been demonstrated, that trainability and responsiveness to pointing vary with skull length (Gácsi et al. 2009b; Helton 2009). ‘Cooperative worker’ breeds (i.e. gundogs and herding dogs which work with continuous visual contact of their human partner) were found to be better at following human cues than ‘independent worker’ breeds which work without human visual contact (e.g. sled dogs, hounds, guarding dogs) (McKinley and Sambrook 2000; Gácsi et al. 2009b; Wobber et al. 2009). The success of cooperative dog breeds in following human pointing might be specifically related to the demands of human-dog cooperation, but alternatively they could be due to a more persistent predatory response (Udell et al. 2014) i.e. to fixate and follow moving stimuli, since pointing implies movement and directionality.
How these breed dimensions could affect gazing, previously implicated as an explanation for dogs’ competence in following human pointing (Miklósi et al. 2003), has not been explored before.
Based on findings in the human literature we can also expect responsiveness to the eyes and gaze to change with age, either through change in perceptual processes that specifically affect face perception (Owsley et al. 1981; Thomas et al. 2007) or due to a decline in the ability to quickly separate visual and auditory signals (Chan et al. 2014). A comparison with dogs would be relevant as they have been argued a suitable model animal for studying human aging (Cummings et al. 1996; Adams et al. 2000; Szabó et al. 2016). This could affect orientation to socially relevant visual cues and explain socially inappropriate behaviour in the elderly (Henry et al. 2009; Slessor et al. 2010).
Previous work using two-dimensional images of dogs and humans, as well as still facial expressions, suggests the features visible in pictures resemble the real stimulus sufficiently to elicit corresponding behavioural responses in the dog (Ted Ruffman and Morris-Trainor 2011; Somppi et al. 2012; Törnqvist et al. 2015). In the present study, we investigated how sex, skull length, breed function, and age could predict the responses of dogs to images of human and dog faces, shown either as facing the observer (portrait, both eyes are visible) or a side (profile) view corresponding to averted gaze. We operationalized attention as the duration of looking time dogs displayed toward still images of human and dog portraits and profiles. In accordance with the findings discussed above, we expected that younger dogs, female individuals, as well as dogs of cooperative breeds and brachycephalic dogs, will look longer at the images, specifically the portraits, which could attract attention due to either their role in dog-human communication or as threatening cues. To test more specifically if the images were perceived as threatening, considering the aversive nature of eye contact in wolves (Schenkel 1967), we measured approach latency to food rewards placed in front of the pictures, expecting, in particular, longer approach times for food rewards placed in front of dog images.
Methods
Ethical Statement
This study on dogs complies with the current laws of Hungary. According to the corresponding definition by law (‘1998. évi XXVIII. Törvény’ 3.§/9. — the Animal Protection Act), non-invasive studies on dogs do not currently require any special permission in Hungary. We confirm that the procedures comply with national and EU legislation. Owners provided written consent to their participation. Our Consent Form was based on the Ethical Codex of the Hungarian Psychologists (2004). We took special care to ensure that the consent process was understood completely by the participant. In the Consent Form participants are informed about the identity of the researchers, the aim, procedure, location, expected time commitment of the experiment, the handling of personal and research data, and data reuse. The information included the participant's right to withdraw their consent at any time. Participants could easily (and without penalty) decline to participate and could ask not to use or delete data collected during the experiments.
Subjects
The owners of 38 family dogs (20 males, 18 females, 1-15 years old, mean age = 5.7 years) volunteered to participate in the study. For each breed related category (i.e. skull length, breed function) we recruited dogs from breeds of different size (see Table 1), i.e. the sample was balanced for size. Each dog participated in two conditions without delay. In a pilot study we observed that dogs looked less at the images after having received food, therefore the foraging situation condition followed the spontaneous looking condition. Only the spontaneous reaction of the animals was of interest, therefore there was no pre-training. Using the classification of Gácsi et al. (2009b) the dogs were characterized along two dimensions. First, the breed function – cooperative or independent work breeds, i.e. cooperative dogs rely on visual feedback from the human partner during work, independent dogs do not, see above. Some breeds and mixed-breed dogs could not be reliably characterized, so they were listed under ‘unspecified’. Second, dogs were also classified according to their skull length, defined by the skull-index (width/length*100 (Evans and De Lahunta 2013)) as brachycephalic (≈81), mesocephalic (≈52) and dolichocephalic (≈39). In most anatomic investigations to date (Schmidt et al. 2011; Georgevsky et al. 2013; Stone et al. 2016) the width to length ratio is usually given as varying between 50 and 60 for mesocephalic dogs. Dogs with values above and below this range are identified as brachycephalic or dolichocephalic respectively. Each of these categories was represented by at least 4 male and 4 female dogs (Table 1).
Table 1. Name, sex, breed, skull length, breed function and age of the dogs.
| Name | Sex | Breed | Skull Length | Breed Function | Age (years) | |
|---|---|---|---|---|---|---|
| 1 | Áfonya | Female | Mix | Mesocephalic | Unspecified | 2 |
| 2 | Bruni | Male | Mix | Mesocephalic | Unspecified | 10.5 |
| 3 | Connor | Male | French bulldog | Brachycephalic | Unspecified | 1.5 |
| 4 | Csele | Female | Mudi | Mesocephalic | Cooperative | 5 |
| 5 | Daisy | Female | Staffordshire terrier | Brachycephalic | Independent | 8 |
| 6 | Dorka | Female | Labrador retriever | Mesocephalic | Cooperative | 2 |
| 7 | Ebola | Female | Boxer | Brachycephalic | Unspecified | 9 |
| 8 | Foltos | Female | Beagle | Mesocephalic | Independent | 8 |
| 9 | Freddie | Male | Dachshound | Dolichocephalic | Independent | 4 |
| 10 | Fruzsi | Female | Dachshound | Dolichocephalic | Independent | 9 |
| 11 | Hummer | Male | Mix | Mesocephalic | Unspecified | 4 |
| 12 | Jacko | Male | Boxer | Brachycephalic | Unspecified | 9 |
| 13 | Joker | Male | Parson russel terrier | Mesocephalic | Independent | 1 |
| 14 | Kamilla | Female | Labrador retriever | Mesocephalic | Cooperative | 8 |
| 15 | Koda | Male | Siberian husky | Mesocephalic | Independent | 2 |
| 16 | Lajos | Male | French bulldog | Brachycephalic | Unspecified | 1 |
| 17 | Lili | Female | Mix | Mesocephalic | Unspecified | 2 |
| 18 | Liza | Female | Mix | Mesocephalic | Unspecified | 2.5 |
| 19 | Manfréd | Male | Dachshound | Dolichocephalic | Independent | 9 |
| 20 | Mangó | Male | Golden retriever | Mesocephalic | Cooperative | 14.5 |
| 21 | Mása | Female | Boxer | Brachycephalic | Unspecified | 2.5 |
| 22 | Matyi | Male | Mix | Mesocephalic | Unspecified | 7.5 |
| 23 | Maya | Female | Cairn terrier | Mesocephalic | Independent | 1 |
| 24 | Miró_1 | Male | Golden retriever | Mesocephalic | Cooperative | 5.5 |
| 25 | Miró_2 | Male | Beagle | Mesocephalic | Independent | 4.5 |
| 26 | Mixi | Female | Foxterrier | Dolichocephalic | Independent | 9 |
| 27 | Mogyoró | Male | Mix | Mesocephalic | Unspecified | 6 |
| 28 | Molly | Female | Mix | Mesocephalic | Unspecified | 5 |
| 29 | Odie | Male | Beagle | Mesocephalic | Independent | 6 |
| 30 | Pötyi | Female | Sheltie | Dolichocephalic | Cooperative | 9 |
| 31 | Samantha | Female | Barzoi | Dolichocephalic | Independent | 8 |
| 32 | Scooby | Male | Border collie | Mesocephalic | Cooperative | 12.5 |
| 33 | Sophie | Female | Westie | Mesocephalic | Independent | 9 |
| 34 | Szuszi | Female | Pug | Brachycephalic | Unspecified | 4 |
| 35 | Twister | Male | Boxer | Brachycephalic | Unspecified | 4.5 |
| 36 | Walter | Male | Golden retriever | Mesocephalic | Cooperative | 3.5 |
| 37 | Zozito | Male | Barzoi | Dolichocephalic | Independent | 3.5 |
| 38 | Zufi | Male | Barzoi | Dolichocephalic | Independent | 3.5 |
Dogs were assessed for their vision loss by dropping a cotton ball in front of them (part of the standard veterinary examination for visual impairment) and presenting a food pellet on a plate 2 meters in front of the dog; dogs with poor vision were excluded from testing. Five dogs, older than 11 years, were dropped from the original sample (N = 43) because of poor vision.
Setting and procedure
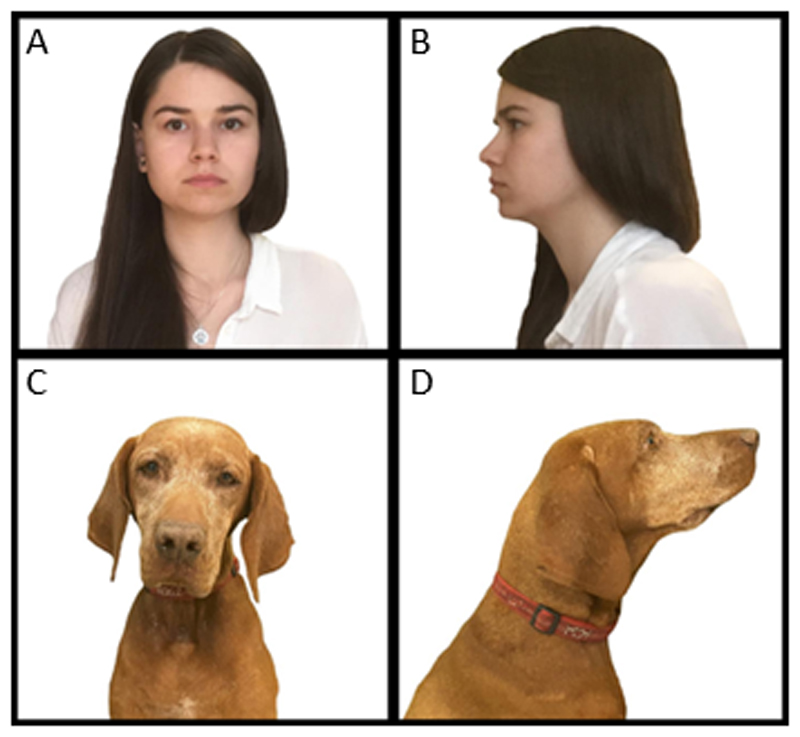
A set of 20 pictures showing 20 faces was used in both conditions. The set contained four types of images. All images were taken from the web and edited to match in luminance and contrast features. Women’s faces were shown due to previous findings suggesting dogs are more likely intimidated by men (Bálint et al. 2016). The sex of the dogs was unknown. Five women portraits, five women profiles, five dog portraits, five dog profiles, all with a closed mouth, 20 in total, 90 cm tall on white background, were used as test-stimuli. The size was chosen to make the relevant cues, like eye-orientation, easy to spot. Faces with closed mouths were chosen to control against emotional expressions being an alternative explanation to eye-orientation (Darwin 1872) (Figure 1). The pictures were projected, at a rate of 120 Hz and with a 1024x768 resolution, on a screen on the wall for 15 seconds each; they were presented in a pre-determined pseudo-random order, chosen to avoid more than two similar pictures in succession e.g. two portraits. Every picture defined a trial, the end of which was signalled by a blank slide. The dogs were accompanied by their owner, who sat 4 meters away from the screen, seated in front of the experimenter and behind their dog. Owners were not informed about the hypotheses of the study and were instructed to remain motionless, silent and not look at the dog during the trials.
Figure 1.
Four types of image were used as test-stimuli. Human portrait (A), human profile (B), dog portrait (C) and dog profile (D).
Both conditions consisted of 10 trials on which 10 of the 20 pictures were presented. In total, a session lasted from 10 to 30 minutes maximum. The image of a bouncing yellow ball and a clicking sound was used to attract the dog’s attention to the screen between the trials. A camera placed under the screen, pointing in the direction of the dog, was used to capture screen-directed looking.
Spontaneous Looking Condition
The dog was kept on a tight leash by the owner in front of him/her, facing toward the canvas during the whole condition.
Duration of looking time: We measured the percentage of time the dog spent looking at the screen during the picture projection, i.e. the total looking time (in seconds) over the 15 second stimulus presentation.
Foraging Situation Condition
The experimenter placed a bowl and in it one piece of dry commercial dog food on the floor, 30 cm away from the centre of the canvas. The placement of food in the bowl was performed while making sure the dog is attending the procedure and looking at the experimenter. Owners were instructed to let the dog off the leash at the beginning of each stimulus presentation, and call their dog back after the trial was over.
Approach latency: We measured the approach time (in seconds), i.e. the latency to approach the bowl containing the food in front of the canvas, from the moment when the picture appeared. The maximum duration for a trial was determined by the maximum duration of the image presentation – 15 seconds.
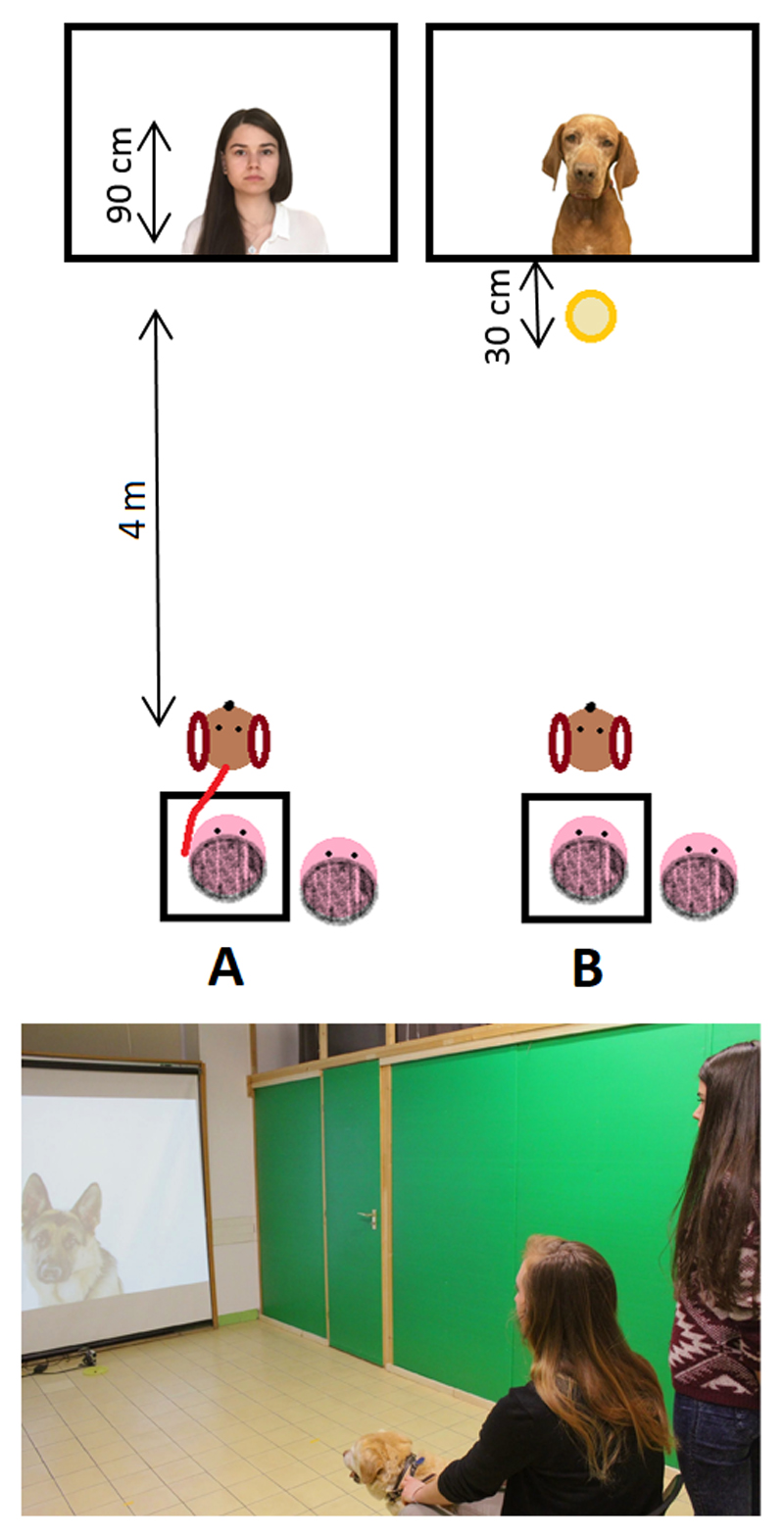
Figure 2 shows the set-up of both conditions.
Figure 2.
In the Spontaneous Looking Condition dog and owner (seated on a chair, depicted here as a rectangular box) were positioned 4 meters away from the center of a canvas, facing it. A bouncing ball was projected to capture the dog‘s attention and once it looked in the direction of the wall a new image, 90 cm tall, was projected, starting a new trial (A). In the Foraging Situation Condition a bowl of food was placed 30 cm in front of the center of the canvas and the dog was allowed to approach when a new image was projected (B), defining a new trial.
Each session was video-recorded. The owners were instructed to provide no feedback during a trial, and if they talked to the dog or petted it, the trial was repeated (this happened six times for a total of five dogs) or, if noticed only later during coding – excluded (14 trials of 10 dogs). If more than 3 trials had to be excluded the dog’s data was discarded. Following these criteria one dog was removed from the data in the Spontaneous Looking Condition. In the Foraging Situation Condition two other dogs were excluded because they couldn’t be motivated to approach the food reward.
A trained observer using Solomon Coder software (beta 091110, developed by András Péter (copyright 2006–2008) at the Department of Ethology, Eötvös Loránd University, Budapest, Hungary) coded the digital video footage. A second coder, naïve to the hypothesis of the study, coded a random selection of the video material (≈30% of the trials). These were 155 trials for measuring looking time, respectively 76 trials for approach latency. We analysed this sample using intra-class correlations to establish the inter-rater reliability. We found robust reliability for looking time duration (N = 155; ICC = 0.991, P < 0.001 average measure for absolute agreement, 2-way random model) and approach time (N = 76; ICC = 0.999, P < 0.001, average measure for absolute agreement, 2-way random model).
Statistical Analysis
Statistical analysis was performed using SPSS IBM Statistic version 22. Generalized Linear Mixed Models (GLMMs) with identity function, fit by residual maximum likelihood (REML), were calculated for the dependent variables of each condition, i.e. duration of looking time in the Spontaneous Looking Condition and approach time in the Foraging Situation Condition. A Kolmogorov-Smirnov test indicated that looking time was not normally distributed. The variable was therefore log-transformed, in accordance with recommendations in the literature (Csibra et al. 2016), in order to fulfil the assumptions of normality and homogeneity of variances. To account for any remaining deviation from normality, a robust estimation for the model assumptions was chosen. A Satterthwaite approximation for estimating the degrees of freedom was also applied, as the data were not perfectly balanced across conditions. In each model we included as fixed factors: age (in years), sex (male or female), picture type (human portrait or profile, dog portrait or profile), cooperativeness of breed (cooperative, independent, or unspecified; see Gácsi et al. (Gácsi et al. 2009b)), and skull length (brachy-, meso-, or dolichocephalic). We further tested the interactions sex with breed function (2-way interaction) and sex with skull length (2-way interaction), as well as picture type with each of the other predictors, to establish how age, sex, breed function and skull length specifically affect selectivity for the presented stimuli. The model was optimized with backwards elimination. Pairwise post-hoc comparisons for the fixed factors retained in the final model were obtained. For pairwise analysis between categorical factors, in the absence of interaction, a type III test was used to test the significance of the estimates; in the presence of an interaction, estimated marginal means were calculated instead. Main effects of variables involved in interactions were listed and interpreted in the results and discussion if their effect could not be reduced to the interference of the interacting variable. All results are reported with standard errors in the Supplementary Information.
Results
Spontaneous Looking Condition
Results of the GLMM are shown in Table 2, post-hoc tests are shown in Table S1.
Table 2. P-values and related parameters for the main effects and interactions on looking time in the spontaneous looking condition.
| Factors | df | F | p-value |
|---|---|---|---|
| picture type | 3,240 | 2.893 | 0.036 |
| sex | 1,229 | 2.153 | 0.144 |
| breed function | 2,239 | 15.708 | < 0.001 |
| skull length | 2,254 | 21.946 | < 0.001 |
| picture type*skull length | 6,236 | 2.376 | 0.03 |
| sex*breed function | 2,244 | 5.5 | 0.005 |
The effect of picture type was significant (GLMM, F3,240 = 2.893, p = 0.036). Overall dog portraits attracted longer looking times than human profiles (estimates M ± SE, ln of duration: 2.4 ± 0.1 versus 2.1 ± 0.1, t1,272 = 2.437, p = 0.015).
Breed function predicted looking times (GLMM, F2,239 = 15.708, p < 0.001). Overall dogs classified as ‘unspecified’ displayed the shortest looking times (estimates M ± SE, ln of duration: 1.9 ± 0.1, p ≤ 0.001).
Skull length predicted looking times (GLMM, F2,254 = 21.946, p < 0.001), which were overall longest for brachycephalic dogs (estimates M ± SE, ln of duration: 2.7 ± 0.1, p < 0.001); also mesocephalic dogs looked longer at the images than dolichocephalic dogs (estimates M ± SE, ln of duration: 2.1 ± 0.1 versus 1.9 ± 0.1, t1,246 = 2.844, p = 0.005).
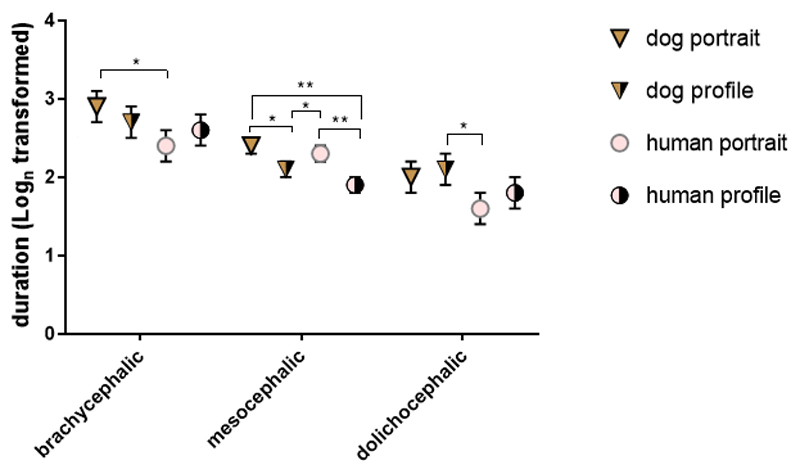
We found two interactions. Picture type interacted with skull length (GLMM, F6,236 = 2.376, p = 0.03, Figure 3). In mesocephalic dogs (N = 22) portraits elicited longer looking times than profiles, whether the image displayed a human or a dog face. There was no significant difference between human and dog faces of the same orientation (portrait or profile). Brachycephalic dogs (N = 8) looked significantly longer at dog portraits than at human portraits. Dolichocephalic dogs (N = 8) looked longer at dog profiles than at human portraits.
Figure 3.
Means and standard errors of log-transformed looking times in the Spontaneous Looking Condition for the picture type x skull length interaction.
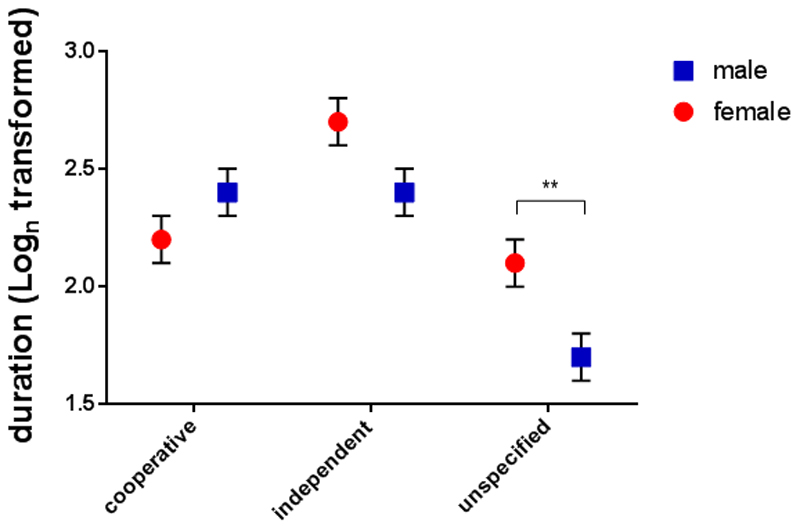
Sex interacted with breed function (GLMM, F2,244 = 5.5, p = 0.005). In breeds classified as ‘unspecified’ (N = 15, mixed breed = 8) females (N = 7) looked significantly longer at the pictures than males, but for cooperative and independent work dogs there was no difference (Figure 4).
Figure 4.
Means and standard errors of log-transformed looking times in the Spontaneous Looking Condition for the sex x breed function interaction.
Age had no effect on the variables.
Foraging Situation Condition
Results of the GLMM are shown in Table 3, post-hoc tests are shown in Table S2.
Table 3. P-values and related parameters for the main effects and interactions on approach latency in the foraging situation condition.
| Factors: | df | F | p-value |
|---|---|---|---|
| Age | 1,295 | 15.835 | < 0.001 |
| Sex | 1,284 | 0.712 | 0.4 |
| skull length | 2,285 | 22.04 | < 0.001 |
| sex*skull length | 2,284 | 28.833 | < 0.001 |
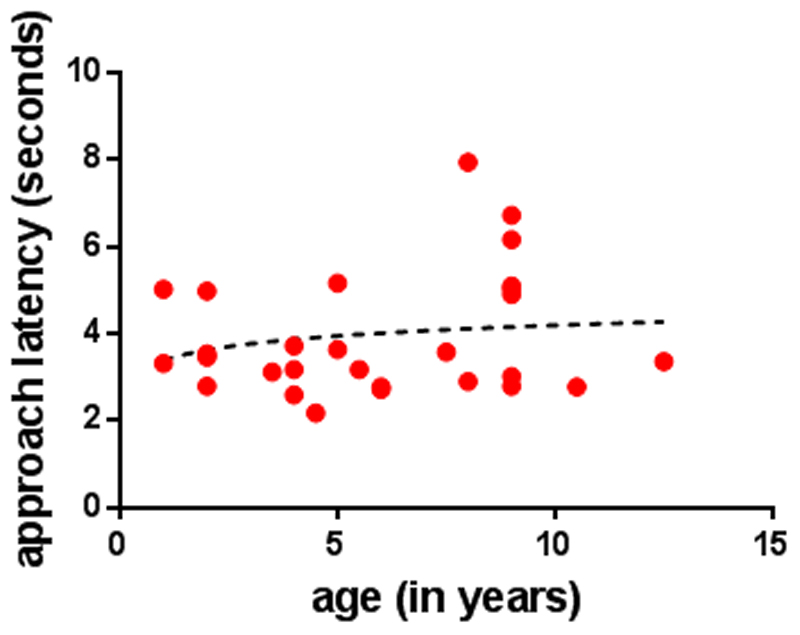
Approach latency significantly increased with age (GLMM, F1,295 = 15.835, p < 0.001, Figure 5).
Figure 5.
Scatter plot of the average approach latency (seconds) and age of the dogs (in years), in the Foraging Situation Condition.
Skull length influenced approach latency (GLMM, F2,285 = 22.04, p < 0.001). Overall mesocephalic dogs approached the fastest (M ± SE, seconds: 3.4 ± 0.1, p < 0.001).
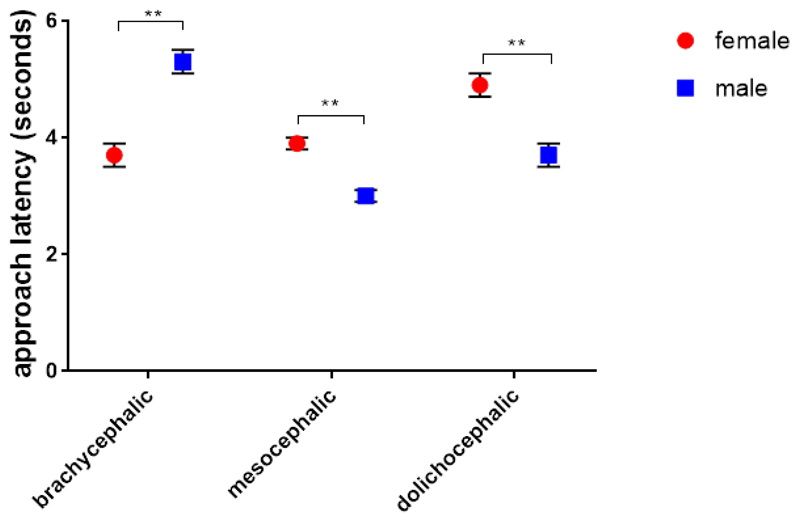
We found one interaction between sex and skull length: males (N = 4) approached the food slower than females among brachycephalic dogs (N = 8). In mesocephalic (N = 22) and dolichocephalic dogs (N = 8) males (N = 16) were faster (GLMM, F2,284 = 28.833, p < 0.001, Figure 6.).
Figure 6.
Means and standard errors of approach latency (seconds) in the Foraging Situation Condition for the sex x skull length interaction.
Picture type and breed function had no effect on approach latency.
Discussion
In the present study we measured dogs’ reaction to facial images of unfamiliar humans or dogs, shown as portraits or profiles, and compared their responses based on sex, skull length, breed and age.
One prediction was that looking time, measured during a Spontaneous Looking Condition, would be higher in the case of human portraits, reasoning that dogs’ gazing behaviour would reflect a preference for eye contact engagement in response to the human images. Eye contact has been found crucial in human-dog bonding and communication (Miklósi et al. 2000; Téglás et al. 2012). In contrast, we expected that dog portraits will elicit avoidance, because the literature suggests that in dog-dog interactions eye-contact signifies threat (Schenkel 1967; Öhman 1986). However, increased looking at images of conspecifics has been reported previously (Somppi et al. 2012) which suggests that aversive stimuli tend to be highly salient and might demand increased attention (Armony and Dolan 2002). To test whether the images were observed out of interest or perceived as aversive, we additionally tested the dogs’ approach behaviour in the presence of the same pictures during a Foraging Situation Condition.
The type of picture used (portrait or profile) influenced looking times differently for dogs of different cephalic index. Only mesocephalic dogs reacted with longer looking times to all portrait images, regardless if humans or dogs were shown. Brachycephalic dogs looked longer at dog portraits than at human portraits, and dolichocephalic dogs looked longer at dog profiles than at human portraits. Across dogs of different skull length, the difference in looking times directed at dog portraits versus human profiles was persistently significant, indicating that these two images were clearly distinguishable for most dogs. Our data, in contrast to our expectations, does not support the notion that dogs prefer to look at human portraits.
Among dogs with ‘unspecified’ breed function, females observed the images longer. Many dogs classified as ‘unspecified’ were mix-breed dogs (8 out of 15, 53.3%). This group showed the shortest looking times overall. Possibly sex differences in gazing disappear in specialized breeding due to a ceiling effect on the possible increase of spontaneous looking responses in dogs.
The finding that females look at faces longer than males is in line with human findings on sex differences in face-directed attention (Connellan et al. 2000; Lutchmaya et al. 2002; Bayliss et al. 2005), which are characterized by reduced gaze following and eye contact initiation in men. Shared mammalian evolution (Decety 2011) could underlie this difference between men and women, but reports of sex effects in the literature are scarce and inconclusive regarding non-human animals (Choleris and Kavaliers 1999). As there was no inanimate control image, nor different effects of picture type for the sexes, the present finding could also reflect general differences in attention between female and male dogs, suggested also by previous work (Müller et al. 2011). Future studies should investigate if these sex differences are specifically social in nature and due to similar biological substrates, as those found in humans (Lutchmaya et al. 2002).
Sex differences in approach behaviour, observed during the foraging situation condition, were strongly associated with skull length, which was previously found to correlate negatively with the density of retinal ganglion cells and therefore affect acuity (McGreevy et al. 2004). Mesocephalic and dolichocephalic females approached the food more slowly than males of the same skull length, but in brachycephalic dogs the relationship with sex was reversed i.e. males approached slower. Questionnaire data on dog personality suggests that females are less bold on average (Kubinyi et al. 2009), which predicts that they would approach the image of an unfamiliar dog slower, but this may interact with the higher trainability of brachycephalic dogs (Helton 2009). Alternatively, the better visual acuity of brachycephalic dogs gives them more certainty to approach novel images, which interferes with the effect of sex on boldness. It would also be interesting to explore in future work how dogs, based on their sex and breed, are expected to behave by the owner. Classical work from human psychology suggests for instance that an individual’s behaviour and performance can be guided by confrontation with stereotypes or bias about that individual’s group/category (Steele and Aronson 1995; Spencer et al. 1999) – a phenomenon known as ‘stereotype threat’.
The study of canine perception and cognition has recently also received attention with regard to age induced changes (Head et al. 2000; Chapagain et al. 2018). The dog has been proposed on several occasions as a model animal for studying human aging (Cummings et al. 1996; Adams et al. 2000). In one of the more recent investigations, border collies of different ages were found to perform differently in sustained attention tasks with a peak at middle age (Wallis et al. 2014). The slowed approach in the Foraging Situation Condition could be either due to above mentioned changes in facial perception (Owsley and Sekuler 1981; Thomas et al. 2007) or due to a decline in sensorimotor functions, which typically accompanies the aging process (Doherty 2003; Wallis et al. 2014).
Overall, our study provided several interesting insights on how dogs process human and conspecific faces. We found that skull length, which was previously shown to indicate quality of vision (McGreevy et al. 2004), attention for visual cues (Gácsi et al. 2009b), and trainability (Helton 2009), is possibly the most relevant breed characteristic to predict dogs’ facial perception as well. Skull length did not only affect how dogs of different sexes would approach food in the presence of the images in the Foraging Situation Condition, but even more importantly, how looking time changed for different picture types. Dogs within and across breed related categories showed looking times dependent on the type of image used, which was an important internal control for the behavioural significance of the images. Moreover, independent of the picture type, brachycephalic skull length predicted longer looking times, suggesting that between dogs of different cephalic index, differences in gazing behaviour might arise from differences in visual processing (McGreevy et al. 2004).
To highlight the effect that human faces might have on the gazing behaviour of dogs, different methods could prove to be more useful, for instance, comparing the ease with which dogs can be trained to approach portraits or profiles of human or dog faces to obtain rewards. Alternatively, using images of familiar faces the dogs were socialized with could reveal differences in looking behaviour more specifically associated with communicative intent.
The data can be interpreted more consistently if we do not assume that looking time measures preference in the present study. Looking times seem instead to have increased with the behavioural salience of the image. Of the dogs for which audio data was available, more than half vocalized and of the presentations which elicited barking 68% were images of dogs (see Video 1 and Supplementary), which also suggests that some, and especially dog pictures had a negative physiological effect. Assuming that vocalizations were caused by the threatening features of dog and/or eye-contact signalling images also offers a plausible explanation for the interactions observed between the type of image and cephalic index of the dogs in the Spontaneous Looking Condition. As brachycephalic dogs have more developed visual capacities – binocular vision and a higher density of retinal ganglion cells (McGreevy et al. 2004), they might more easily distinguish the inherently threatening canine portrait (Schenkel 1967) from a frontal presentation of the human face, which otherwise shares some basic features: for instance both eyes are visible. Within the same frame of interpretation, the looking duration patterns of mesocephalic dogs might reflect their relatively weaker acuity: while portraits were distinguished from profiles, the dog and human portraits did not elicit different responses in this group. Still, overall mesocephalic dogs were fastest to approach rewards placed in front of the pictures. It is possible that relative to mesocephalic dogs, who occupy an intermediate position with regard to visual acuity (McGreevy et al. 2004), brachycephalic dogs were more distracted by the pictures, as they should be able to extract more information from them, while dolichocephalic dogs were possibly less certain and confident to approach as their visual skills are the weakest. Their skull shape is associated with impaired binocular vision and fewer ganglion cells in the retina. Because picture type did not affect approach latency in the present study, future work will need to address this question more specifically.
Because dogs, at least in some contexts, rely on our faces to understand our behaviours and intentions (Miklósi et al. 2000, 2003; Nagasawa et al. 2011; Téglás et al. 2012; Müller et al. 2015) it is important to consider for the refinement of dog-human communication, training and welfare, how the animal’s sex and breed characteristics impact facial perception. So far previous work could show that trainability and sensitivity to human pointing are impacted by skull length or breed function (Helton 2009; Udell et al. 2014), but our work to our knowledge is the first to explore how these dimensions of breed categorization affect dogs’ response to faces.
Supplementary Material
Supplementary material 1 (DOCX 175 kb) Data: https://osf.io/3tgrb/
Dogs respond with social behaviours towards dog portraits. Shown in this sequence, examples of barking, growling, averted gaze, yawning, explorative sniffing.
Available at: https://youtu.be/NVxGZGES3sQ
Video abstract: https://youtu.be/pS5j8TMrDZE
Acknowledgements
The authors would like to thank Flóra Szánthó, Tamás Faragó, Fanni Tompai and Antal Dóka for assistance in the lab, Borbála Turcsán, Lisa Wallis and Patrizia Piotti for statistical consultancy, Kauê Machado Costa for useful comments on the manuscript and editing, József Topál, Dóra Szabó, Fanni Lehoczki for general consultancy, Ákos Árokszállási for the randomization script and the owners of the dogs for their time and assistance.
Compliance with Ethical Standards
Funding: This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 680040) and from the Bolyai Foundation of the Hungarian Academy of Sciences.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: This study on dogs complies with the current laws of Hungary. According to the corresponding definition by law (‘1998. évi XXVIII. Törvény’ 3. §/9. — the Animal Protection Act), non-invasive studies on dogs are currently allowed to be done without any special permission in Hungary. We confirm that the procedures comply with national and EU legislation. Owners provided written consent to their participation. Our Consent Form was based on the Ethical Codex of the Hungarian Psychologists (2004).
References
- Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:675–692. doi: 10.1016/S0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40:817–26. doi: 10.1016/S0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Bálint A, Faragó T, Miklósi Á, Pongrácz P. Threat-level-dependent manipulation of signaled body size: dog growls’ indexical cues depend on the different levels of potential danger. Anim Cogn. 2016:1–17. doi: 10.1007/s10071-016-1019-9. [DOI] [PubMed] [Google Scholar]
- Bartal IB-a, Decety J, Mason P, et al. Empathy and Pro-Social Behavior in Rats. Science (80- ) 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss AP, di Pellegrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Q J Exp Psychol. 2005;58:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Chan YM, Pianta MJ, McKendrick AM. Older age results in difficulties separating auditory and visual signals in time. J Vis. 2014;14:1–11. doi: 10.1167/14.11.13. [DOI] [PubMed] [Google Scholar]
- Chapagain D, Range F, Huber L, Virányi Z. Cognitive Aging in Dogs. Gerontology. 2018 doi: 10.1159/000481621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Kavaliers M. Social learning in animals: Sex differences and neurobiological analysis. Pharmacol Biochem Behav. 1999;64:767–776. doi: 10.1016/S0091-3057(99)00141-0. [DOI] [PubMed] [Google Scholar]
- Connellan J, Baron-Cohen S, Wheelwright S, et al. Sex differences in human neonatal social perception. Infant Behav Dev. 2000;23:113–118. doi: 10.1016/S0163-6383(00)00032-1. [DOI] [Google Scholar]
- Csibra G, Hernik M, Mascaro O, et al. Statistical treatment of looking-time data. Dev Psychol. 2016;52:521–536. doi: 10.1037/dev0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Head E, Ruehl W, et al. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872. p. 374. [DOI] [Google Scholar]
- de Waal FB. Macaque social culture: development and perpetuation of affiliative networks. J Comp Psychol. 1996;110:147–154. doi: 10.1037/0735-7036.110.2.147. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Ann N Y Acad Sci. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Evans HE, De Lahunta A. Miller’s Anatomy of the Dog. Elsevier Health Sciences; 2013. [Google Scholar]
- Gácsi M, Györi B, Virányi Z, et al. Explaining dog wolf differences in utilizing human pointing gestures: Selection for synergistic shifts in the development of some social skills. PLoS One. 2009a;4:4–9. doi: 10.1371/journal.pone.0006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gácsi M, McGreevy PD, Kara E, Miklósi Á. Effects of selection for cooperation and attention in dogs. Behav Brain Funct. 2009b;5:31. doi: 10.1186/1744-9081-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgevsky D, Carrasco JJ, Valenzuela M, McGreevy PD. Domestic dog skull diversity across breeds, breed groupings, and genetic clusters. J Vet Behav Clin Appl Res. 2013;9:228–234. doi: 10.1016/j.jveb.2014.04.007. [DOI] [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs? Trends Cogn Sci. 2005;9:439–444. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Head E, Cotman CW, Milgram NW. Canine cognition, aging and neuropathology. Prog Neuro-Psychopharmacology Biol Psychiatry. 2000;24:671–673. doi: 10.1016/s0278-5846(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Helton WS. Cephalic index and perceived dog trainability. Behav Processes. 2009;82:355–358. doi: 10.1016/j.beproc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Henry JD, von Hippel W, Baynes K. Social inappropriateness, executive control, and aging. Psychol Aging. 2009;24:239–244. doi: 10.1037/a0013423. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Hynds J, Morris P, Waller BM. Human attention affects facial expressions in domestic dogs. Sci Rep. 2017;7:12914. doi: 10.1038/s41598-017-12781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski J, Nitzschner M. Do dogs get the point? A review of dog-human communication ability. Learn Motiv. 2013;44:294–302. doi: 10.1016/j.lmot.2013.05.001. [DOI] [Google Scholar]
- Kubinyi E, Turcsán B, Miklósi Á. Dog and owner demographic characteristics and dog personality trait associations. Behav Processes. 2009;81:392–401. doi: 10.1016/j.beproc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behav Dev. 2002;25:327–335. doi: 10.1016/S0163-6383(02)00094-2. [DOI] [Google Scholar]
- McGreevy P, Grassi TD, Harman AM. A strong correlation exists between the distribution of retinal ganglion cells and nose length in the dog. Brain Behav Evol. 2004;63:13–22. doi: 10.1159/000073756. [DOI] [PubMed] [Google Scholar]
- McKinley J, Sambrook TD. Use of human-given cues by domestic dogs (Canis familiaris) and horses (Equus caballus) Anim Cogn. 2000;3:13–22. doi: 10.1007/s100710050046. [DOI] [Google Scholar]
- Miklosi A. Dog Behaviour, Evolution, and Cognition. OUP Oxford; 2014. [Google Scholar]
- Miklósi Á, Kubinyi E, Zsófia V, et al. A Simple Reason for a Big Difference: Wolves Do Not Look Back at Humans, but Dogs Do. Curr Biol. 2003;13:763–766. doi: 10.1016/S. [DOI] [PubMed] [Google Scholar]
- Miklósi A, Polgárdi R, Topál J, Csányi V. Intentional behaviour in dog-human communication: An experimental analysis of “showing” behaviour in the dog. Anim Cogn. 2000;3:159–166. doi: 10.1007/s100710000072. [DOI] [Google Scholar]
- Miklósi Á, Soproni K. A comparative analysis of animals’ understanding of the human pointing gesture. Anim Cogn. 2006;9:81–93. doi: 10.1007/s10071-005-0008-1. [DOI] [PubMed] [Google Scholar]
- Müller CA, Mayer C, Dorrenberg S, et al. Female but not male dogs respond to a size constancy violation. Biol Lett. 2011;7:689–691. doi: 10.1098/rsbl.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CA, Schmitt K, Barber ALA, Huber L. Dogs can discriminate emotional expressions of human faces. Curr Biol. 2015;25:601–605. doi: 10.1016/j.cub.2014.12.055. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Murai K, Mogi K, Kikusui T. Dogs can discriminate human smiling faces from blank expressions. Anim Cogn. 2011;14:525–533. doi: 10.1007/s10071-011-0386-5. [DOI] [PubMed] [Google Scholar]
- Öhman A. Face the beast and fear the face: Animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–145. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: face perception. Investig Ophthalmol Vis Sci. 1981;21:362–365. [PubMed] [Google Scholar]
- Range F, Virányi Z. Development of gaze following abilities in wolves (canis lupus) PLoS One. 2011;6 doi: 10.1371/journal.pone.0016888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel R. Submission: Its features and function in the wolf and dog. Integr Comp Biol. 1967;7:319–329. doi: 10.1093/icb/7.2.319. [DOI] [Google Scholar]
- Schmidt MJ, Neumann AC, Amort KH, et al. Cephalometric measurements and determination of general skull type of cavalier king charles spaniels. Vet Radiol Ultrasound. 2011;52:436–440. doi: 10.1111/j.1740-8261.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- Slessor G, Laird G, Phillips LH, et al. Age-related differences in gaze following: Does the age of the face matter? Journals Gerontol - Ser B Psychol Sci Soc Sci. 2010;65 B:536–541. doi: 10.1093/geronb/gbq038. [DOI] [PubMed] [Google Scholar]
- Somppi S, Törnqvist H, Hänninen L, et al. Dogs do look at images: Eye tracking in canine cognition research. Anim Cogn. 2012;15:163–174. doi: 10.1007/s10071-011-0442-1. [DOI] [PubMed] [Google Scholar]
- Soproni K, Miklósi A, Topál J, Csányi V. Dogs’ (Canis familiaris) responsiveness to human pointing gestures. J Comp Psychol. 2002;116:27–34. doi: 10.1037//0735-7036.116.1.27. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Steele CM, Quinn DM. Stereotype Threat and Women’s Math Performance. J Exp Soc Psychol. 1999;35:4–28. doi: 10.1006/jesp.1998.1373. [DOI] [Google Scholar]
- Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. J Pers Soc Psychol. 1995;69:797–811. doi: 10.1037/0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- Stone HR, McGreevy PD, Starling MJ, Forkman B. Associations between domestic-dog morphology and behaviour scores in the dog mentality assessment. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó D, Gee NR, Miklósi Á. Natural or pathologic? Discrepancies in the study of behavioral and cognitive signs in aging family dogs. J Vet Behav Clin Appl Res. 2016;11:86–98. [Google Scholar]
- Ted Ruffman, Morris-Trainor Z. Do dogs understand human facial expressions? J Vet Behav Clin Appl Res. 2011;6:78–79. doi: 10.1016/j.jveb.2010.09.013. [DOI] [Google Scholar]
- Téglás E, Gergely A, Kupán K, et al. Dogs’ gaze following is tuned to human communicative signals. Curr Biol. 2012;22:209–212. doi: 10.1016/j.cub.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, et al. Reduction in White Matter Connectivity, Revealed by Diffusion Tensor Imaging, May Account for Age-related Changes in Face Perception. J Cogn Neurosci. 2007;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnqvist H, Somppi S, Koskela A, et al. Comparison of dogs and humans in visual scanning of social interaction. R Soc open Sci. 2015;2 doi: 10.1098/rsos.150341. 150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcsán B, Szánthó F, Miklósi Á, Kubinyi E. Fetching what the owner prefers? Dogs recognize disgust and happiness in human behaviour. Anim Cogn. 2014 doi: 10.1007/s10071-014-0779-3. [DOI] [PubMed] [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL. Wolves outperform dogs in following human social cues. Anim Behav. 2008;76:1767–1773. doi: 10.1016/j.anbehav.2008.07.028. [DOI] [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol Rev. 2010;85:327–345. doi: 10.1111/j.1469-185X.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- Udell MAR, Ewald M, Dorey NR, Wynne CDL. Exploring breed differences in dogs (Canis familiaris): Does exaggeration or inhibition of predatory response predict performance on human-guided tasks? Anim Behav. 2014;89:99–105. doi: 10.1016/j.anbehav.2013.12.012. [DOI] [Google Scholar]
- Ueda S, Kumagai G, Otaki Y, et al. A comparison of facial color pattern and gazing behavior in canid species suggests gaze communication in gray wolves (Canis lupus) PLoS One. 2014;9:e98217. doi: 10.1371/journal.pone.0098217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis LJ, Range F, Müller CA, et al. Lifespan development of attentiveness in domestic dogs: Drawing parallels with humans. Front Psychol. 2014;5:1–13. doi: 10.3389/fpsyg.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber C, Hare B, Koler-Matznick J, et al. Breed Differences in Domestic Dogs’ (Canis familiaris) Comprehension of Human Communicative Signals. Interact Stud. 2009;10:206–224. doi: 10.1075/is.10.2.06wob. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dogs respond with social behaviours towards dog portraits. Shown in this sequence, examples of barking, growling, averted gaze, yawning, explorative sniffing.
Available at: https://youtu.be/NVxGZGES3sQ
Video abstract: https://youtu.be/pS5j8TMrDZE