Abstract
STUDY QUESTION
What is the recommended assessment and management of women with polycystic ovary syndrome (PCOS), based on the best available evidence, clinical expertise and consumer preference?
SUMMARY ANSWER
International evidence-based guidelines, including 166 recommendations and practice points, addressed prioritized questions to promote consistent, evidence-based care and improve the experience and health outcomes of women with PCOS.
WHAT IS KNOWN ALREADY
Previous guidelines either lacked rigorous evidence-based processes, did not engage consumer and international multidisciplinary perspectives, or were outdated. Diagnosis of PCOS remains controversial, and assessment and management are inconsistent. The needs of women with PCOS are not being adequately met and evidence practice gaps persist.
STUDY DESIGN, SIZE, DURATION
International evidence-based guideline development engaged professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all stages. Appraisal of Guidelines for Research and Evaluation (AGREE) II-compliant processes were followed, with extensive evidence synthesis. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework was applied across evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Governance included a six continent international advisory and a project board, five guideline development groups, and consumer and translation committees. Extensive health professional and consumer engagement informed guideline scope and priorities. Engaged international society-nominated panels included pediatrics, endocrinology, gynecology, primary care, reproductive endocrinology, obstetrics, psychiatry, psychology, dietetics, exercise physiology, public health and other experts, alongside consumers, project management, evidence synthesis and translation experts. In total, 37 societies and organizations covering 71 countries engaged in the process. Twenty face-to-face meetings over 15 months addressed 60 prioritized clinical questions involving 40 systematic and 20 narrative reviews. Evidence-based recommendations were developed and approved via consensus voting within the five guideline panels, modified based on international feedback and peer review, with final recommendations approved across all panels.
MAIN RESULTS AND THE ROLE OF CHANCE
The evidence in the assessment and management of PCOS is generally of low to moderate quality. The guideline provides 31 evidence based recommendations, 59 clinical consensus recommendations and 76 clinical practice points all related to assessment and management of PCOS. Key changes in this guideline include: (i) considerable refinement of individual diagnostic criteria with a focus on improving accuracy of diagnosis; (ii) reducing unnecessary testing; (iii) increasing focus on education, lifestyle modification, emotional wellbeing and quality of life; and (iv) emphasizing evidence based medical therapy and cheaper and safer fertility management.
LIMITATIONS, REASONS FOR CAUTION
Overall evidence is generally low to moderate quality, requiring significantly greater research in this neglected, yet common condition, especially around refining specific diagnostic features in PCOS. Regional health system variation is acknowledged and a process for guideline and translation resource adaptation is provided.
WIDER IMPLICATIONS OF THE FINDINGS
The international guideline for the assessment and management of PCOS provides clinicians with clear advice on best practice based on the best available evidence, expert multidisciplinary input and consumer preferences. Research recommendations have been generated and a comprehensive multifaceted dissemination and translation program supports the guideline with an integrated evaluation program.
STUDY FUNDING/COMPETING INTEREST(S)
The guideline was primarily funded by the Australian National Health and Medical Research Council of Australia (NHMRC) supported by a partnership with ESHRE and the American Society for Reproductive Medicine. Guideline development group members did not receive payment. Travel expenses were covered by the sponsoring organizations. Disclosures of conflicts of interest were declared at the outset and updated throughout the guideline process, aligned with NHMRC guideline processes. Full details of conflicts declared across the guideline development groups are available at https://www.monash.edu/medicine/sphpm/mchri/pcos/guideline in the Register of disclosures of interest. Of named authors, Dr Costello has declared shares in Virtus Health and past sponsorship from Merck Serono for conference presentations. Prof. Laven declared grants from Ferring, Euroscreen and personal fees from Ferring, Euroscreen, Danone and Titus Healthcare. Prof. Norman has declared a minor shareholder interest in an IVF unit. The remaining authors have no conflicts of interest to declare. The guideline was peer reviewed by special interest groups across our partner and collaborating societies and consumer organizations, was independently assessed against AGREE-II criteria, and underwent methodological review. This guideline was approved by all members of the guideline development groups and was submitted for final approval by the NHMRC.
Keywords: polycystic ovary syndrome, guideline, evidence-based, assessment, management, GRADE
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting reproductive aged women, with a prevalence of between 8 and 13% depending on the population studied and definitions used (Teede et al., 2010; Azziz et al., 2016). PCOS is complex with reproductive, metabolic and psychological features (Teede et al., 2010; Azziz et al., 2016). Clinical practice in the assessment and management of PCOS is inconsistent, with key evidence practice gaps, whilst women internationally have highlighted delayed diagnosis and dissatisfaction with care (Teede et al., 2014; Dokras et al., 2017; Gibson-Helm, et al., 2017). Current guidelines either are limited in breadth, do not follow rigorous best practice in development, have not involved consumers, or are outdated (Teede et al., 2011; Legro et al., 2013; Conway et al., 2014; Goodman et al., 2015; Balen et al., 2016), resulting in inconsistent guidance for clinicians and women alike. To address these identified gaps, here we summarize the development process and recommendations from the first international evidence-based guideline for the assessment and management of PCOS, bringing together extensive consumer engagement and international collaboration with leading societies and organizations, multidisciplinary experts, and primary care representatives. In this process, the guideline development groups (GDG) unanimously supported the Rotterdam diagnostic criteria (Group, 2004) for adult women.
This comprehensive evidence-based guideline builds on prior high-quality guidelines and culminates from a rigorous, Appraisal of Guidelines for Research and Evaluation-II (AGREE-II)-compliant, evidence-based guideline development process. It provides a single source of international evidence-based recommendations (EBR) to guide clinical practice with the opportunity for adaptation in relevant health systems. Together with an extensive translation program, the aim is to reduce worldwide variation in care and promote high-quality service provision to improve health outcomes and quality of life in women with PCOS. The guidelines are supported by a multifaceted international translation program with co-designed resources to enhance the skills of health professionals and empower women with PCOS, with an integrated comprehensive evaluation program.
What Does This Mean for Adolescents and Women With PCOS?
This guideline aims to optimize the evidence-based, consistent care that meets the needs and improves the quality of life of women with PCOS. The guideline and translation program were developed with full consumer participation at all stages, targeting areas and outcomes of priority for women with PCOS. The aim is to support women and their healthcare providers to optimize diagnosis, assessment and management of PCOS. There is an emphasis on partnership in care and empowerment of women with PCOS. Personal characteristics, preferences, culture and values are considered. With effective translation, the guideline and health professional and consumer resources will address gaps and priorities identified by women with PCOS and will promote vital future research.
Materials and Methods
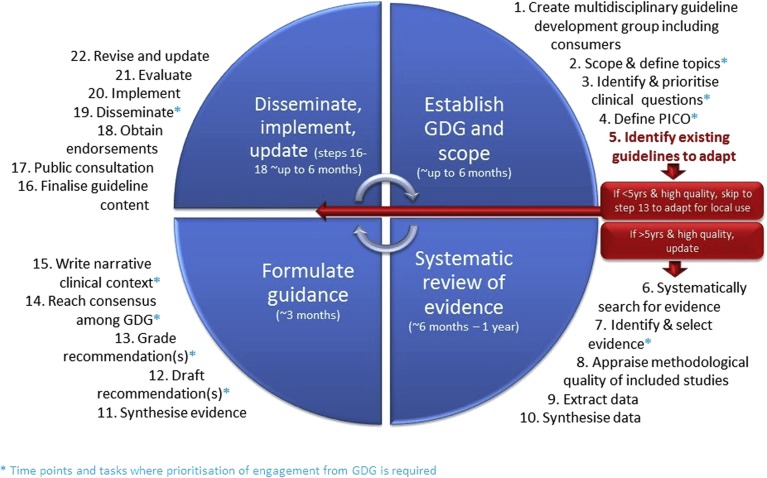
Best practice evidence-based guideline development methods were applied and are detailed in the full guideline and the technical reports and outlined in Figure 1 and available at https://www.monash.edu/medicine/sphpm/mchri/pcos (Misso and Teede, 2012). The process aligns with all elements of the AGREE-II tool for quality guideline assessment (Brouwers et al., 2010). This involved extensive evidence synthesis and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework covering evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength (GRADE working group). Evidence synthesis methods are outlined in the full guideline and followed best practice (NHMRC, 2007, 2009; Brouwers et al., 2010; GRADE working group). Categories include evidence-based or consensus recommendations with accompanying clinical practice points (Table I).
Figure 1.
The steps in developing an evidence-based guideline. GDG = guideline development group; PICO = P: patient, problem or population, I: intervention, C: comparison, control or comparator, O: outcome. Reprinted with permissions from Misso and Teede (2012).
Table I.
Categories of recommendations in the PCOS guideline.
| EBR | Evidence-based recommendations are made where evidence is sufficient to inform a recommendation made by the guideline development group. |
| CCR | Clinical consensus recommendations are made in the absence of adequate evidence on PCOS. These are informed by evidence in other populations and are made by the guideline development group, using rigorous and transparent processes. |
| CPP | Clinical practice points are made where evidence was not sought and are made where important clinical issues arose from discussion of evidence-based or clinical consensus recommendations. |
Terms include ‘should’, ‘could’ and ‘should not’ are informed by the nature of the recommendation (evidence or consensus), the GRADE framework, and quality of the evidence and are independent descriptors reflecting the judgment of the multidisciplinary GDG, including consumers. They refer to overall interpretation and practical application of the recommendation, balancing benefits and harms. ‘Should’ is used where benefits of the recommendation exceed harms, and where the recommendation can be trusted to guide practice. ‘Could’ is used where either the quality of evidence was limited or the available studies demonstrate little clear advantage of one approach over another, or the balance of benefits to harm was unclear. ‘Should not’ is used where there is either a lack of appropriate evidence, or the harms may outweigh the benefits.
The GRADE of the recommendation is determined by the GDG based on comprehensive structured consideration of all elements of the GRADE framework (GRADE working group), including desirable effects, undesirable effects, balance of effects, resource requirements and cost effectiveness, equity, acceptability and feasibility, and includes:
*Conditional recommendation against the option;
**conditional recommendation for either the option or the comparison;
***conditional recommendation for the option; and
****strong recommendation for the option.
Quality of the evidence is categorized according to the number and design of studies addressing the outcome; judgments about the quality of the studies and/or synthesized evidence, such as risk of bias, inconsistency, indirectness, imprecision and any other considerations that may influence the quality of the evidence; key statistical data; and classification of the importance of the outcomes (Table II). The quality of evidence reflects the extent of confidence in an estimate of the effect to support a particular recommendation (GRADE working group) and was largely determined by the expert evidence synthesis team.
Table II.
Quality (certainty) of evidence categories.*
| High | ⊕⊕⊕⊕ | Very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | ⊕⊕⊕○ | Moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | ⊕⊕○○ | Limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect. |
| Very Low | ⊕○○○ | Very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. |
*Adapted from the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) (GRADE working group).
GRADE acknowledges that evidence quality is a continuum; any discrete categorization involves a degree of arbitrariness. Nevertheless, the advantages of simplicity, transparency and clarity outweigh these limitations (GRADE working group).
Results
The recommendation table (Table III) applies the category, descriptive terms, GRADE of the recommendations and the quality of the evidence. The full guideline is available at https://www.monash.edu/medicine/sphpm/mchri/pcos. The full version of the guideline outlines the clinical need for the question, the clinical question, the evidence summary, the recommendation and practice points, and a summary of the justification developed by the GDGs using the GRADE framework, refined by extensive international peer review. The comprehensive evidence reviews, profiles and GRADE frameworks supporting each recommendation, can be found at https://www.monash.edu/medicine/sphpm/mchri/pcos in the Supplementary Technical Reports. The peer review feedback, administrative report on guideline development and disclosure of interest process and declarations can be found at https://www.monash.edu/medicine/sphpm/mchri/pcos. Here we present the clinical topic area recommendations and practice points (Table III). This summary, the full guideline and technical reports are supported by a comprehensive co-designed translation program to optimize dissemination and impact with resources freely available at https://www.monash.edu/medicine/sphpm/mchri/pcos.
Table III.
Recommendations and practice points. © Monash University on behalf of the NHMRC Centre for Research Excellence in PCOS, 2018.
| Category (see Table I for definition) | Recommendation | GRADE and quality |
|---|---|---|
| 1. Screening, diagnostic assessment, risk assessment and life-stage | ||
| Irregular cycles and ovulatory dysfunction | ||
| CCR | Irregular menstrual cycles are defined as:
|
**** |
| When irregular menstrual cycles are present a diagnosis of PCOS should be considered and assessed according to the guidelines | ||
| CCR | In an adolescent with irregular menstrual cycles, the value and optimal timing of assessment and diagnosis of PCOS should be discussed with the patient, taking into account diagnostic challenges at this life stage and psychosocial and cultural factors | **** |
| CPP | For adolescents who have features of PCOS but do not meet diagnostic criteria, an ‘increased risk’ could be considered and reassessment advised at or before full reproductive maturity, 8 years post menarche. This includes those with PCOS features before combined oral contraceptive pill (COCP) commencement, those with persisting features and those with significant weight gain in adolescence. | |
| CPP | Ovulatory dysfunction can still occur with regular cycles and if anovulation needs to be confirmed serum progesterone levels can be measured. | _ |
| Biochemical hyperandrogenism | ||
| EBR | Calculated free testosterone, free androgen index or calculated bioavailable testosterone should be used to assess biochemical hyperandrogenism in the diagnosis of PCOS. |
|
| EBR | High-quality assays such as liquid chromatography–mass spectrometry (LCMS) and extraction/chromatography immunoassays, should be used for the most accurate assessment of total or free testosterone in PCOS. |
|
| EBR | Androstenedione and dehydroepiandrosterone sulfate (DHEAS) could be considered if total or free testosterone are not elevated; however, these provide limited additional information in the diagnosis of PCOS. |
|
| CCR | Direct free testosterone assays, such as radiometric or enzyme-linked assays, preferably should not be used in assessment of biochemical hyperandrogenism in PCOS, as they demonstrate poor sensitivity, accuracy and precision. | |
| CPP | Reliable assessment of biochemical hyperandrogenism is not possible in women on hormonal contraception, due to effects on sex hormone-binding globulin and altered gonadotrophin-dependent androgen production. | _ |
| CPP | Where assessment of biochemical hyperandrogenism is important in women on hormonal contraception, drug withdrawal is recommended for 3 months or longer before measurement, and contraception management with a non-hormonal alternative is needed during this time. | _ |
| CPP | Assessment of biochemical hyperandrogenism is most useful in establishing the diagnosis of PCOS and/or phenotype where clinical signs of hyperandrogenism (in particular hirsutism) are unclear or absent. | _ |
| CPP | Interpretation of androgen levels needs to be guided by the reference ranges of the laboratory used, acknowledging that ranges for different methods and laboratories vary widely. Normal values are ideally based on levels from a well phenotyped healthy control population or by cluster analysis of a large general population considering age and pubertal specific stages. | _ |
| CPP | Where androgen levels are markedly above laboratory reference ranges, other causes of biochemical hyperandrogenism need to be considered. History of symptom onset and progression is critical in assessing for neoplasia, however, some androgen-secreting neoplasms may only induce mild to moderate increases in biochemical hyperandrogenism. | _ |
| Clinical hyperandrogenism | ||
| CCR | A comprehensive history and physical examination should be completed for symptoms and signs of clinical hyperandrogenism, including acne, alopecia, and hirsutism and, in adolescents, severe acne and hirsutism. | **** |
| CCR | Health professionals should be aware of the potential negative psychosocial impact of clinical hyperandrogenism. Reported unwanted excess hair growth and/or alopecia should be considered important, regardless of apparent clinical severity. | **** |
| CCR | Standardized visual scales are preferred when assessing hirsutism, such as the modified Ferriman Gallwey score (mFG) with a level ≥4–6 indicating hirsutism, depending on ethnicity, acknowledging that self-treatment is common and can limit clinical assessment. (See recommendations on ethnic variation.) | **** |
| CCR | The Ludwig visual score is preferred for assessing the degree and distribution of alopecia. | **** |
| CPP | There are no universally accepted visual assessments for evaluating acne. | |
| CPP | The prevalence of hirsutism is the same across ethnicities, yet the mFG cut-off scores for defining hirsutism and the severity of hirsutism varies by ethnicity. | _ |
| CPP | As ethnic variation in vellus hair density is notable, over-estimation of hirsutism may occur if vellus hair is confused with terminal hair; only terminal hairs need to be considered in pathological hirsutism, with terminal hairs clinically growing >5 mm in length if untreated, varying in shape and texture and generally being pigmented. | _ |
| Ultrasound and polycystic ovarian morphology (PCOM) | ||
| CCR | Ultrasound should not be used for the diagnosis of PCOS in those with a gynecological age of <8 years (<8 years after menarche), due to the high incidence of multi-follicular ovaries in this life stage. | **** |
| CCR | The threshold for PCOM should be revised regularly with advancing ultrasound technology, and age-specific cut off values for PCOM should be defined. | **** |
| CCR | The transvaginal ultrasound approach is preferred in the diagnosis of PCOS, if sexually active and if acceptable to the individual being assessed. | **** |
| CCR | Using endovaginal ultrasound transducers with a frequency bandwidth that includes 8 MHz, the threshold for PCOM on either ovary, a follicle number per ovary of ≥20 and/or an ovarian volume ≥10 ml on either ovary, ensuring no corpora lutea, cysts or dominant follicles are present. | *** |
| CPP | If using older technology, the threshold for PCOM could be an ovarian volume ≥10 ml on either ovary. | _ |
| CPP | In patients with irregular menstrual cycles and hyperandrogenism, an ovarian ultrasound is not necessary for PCOS diagnosis; however, ultrasound will identify the complete PCOS phenotype. | _ |
| CPP | In transabdominal ultrasound reporting is best focused on ovarian volume with a threshold of ≥10 ml, given the difficulty of reliably assessing follicle number with this approach. | _ |
| CPP | Clear protocols are recommended for reporting follicle number per ovary and ovarian volume on ultrasound. Recommended minimum reporting standards include:
|
_ |
| CPP | There is a need for training in careful and meticulous follicle counting per ovary, to improve reporting. | _ |
| Anti-Müllerian hormone (AMH) | ||
| EBR | Serum AMH levels should not yet be used as an alternative for the detection of PCOM or as a single test for the diagnosis of PCOS. |
|
| CPP | There is emerging evidence that with improved standardization of assays and established cut off levels or thresholds based on large scale validation in populations of different ages and ethnicities, AMH assays will be more accurate in the detection of PCOM. | _ |
| Ethnic variation | ||
| CCR | Health professionals should consider ethnic variation in the presentation and manifestations of PCOS, including:
|
**** |
| Menopause life stage | ||
| CCR | Postmenopausal persistence of PCOS could be considered likely with continuing evidence of hyperandrogenism. | *** |
| CCR | A diagnosis of PCOS postmenopause could be considered if there is a past diagnosis of PCOS, a long-term history of irregular menstrual cycles and hyperandrogenism and/or PCOM, during the reproductive years. | *** |
| CPP | Postmenopausal women presenting with new-onset, severe or worsening hyperandrogenism including hirsutism, require investigation to rule out androgen-secreting tumors and ovarian hyperthecosis. | _ |
| Cardiovascular disease risk (CVD) | ||
| CCR | All those with PCOS should be offered regular monitoring for weight changes and excess weight, in consultation with and where acceptable to the individual woman. Monitoring could be at each visit or at a minimum 6–12 monthly, with frequency planned and agreed between the health professional and the individual. | **** |
| CCR | Weight, height and ideally waist circumference should be measured and BMI calculated with the following considered:
|
**** |
| CCR | All women with PCOS should be assessed for cardiovascular risk factors and global CVD risk. | **** |
| CCR | If screening reveals CVD risk factors including obesity, cigarette smoking, dyslipidemia, hypertension, impaired glucose tolerance, and lack of physical activity, women with PCOS should be considered at increased risk of CVD. | **** |
| CCR | Overweight and obese women with PCOS, regardless of age, should have a fasting lipid profile (cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol and triglyceride level at diagnosis). Thereafter, frequency of measurement should be based on the presence of hyperlipidemia and global CVD risk. | **** |
| CCR | All women with PCOS should have blood pressure measured annually, or more frequently based on global CVD risk. | **** |
| CPP | Health professionals need to be aware that CVD risk in women with PCOS remains unclear pending high-quality studies, however prevalence of CVD risk factors is increased, warranting consideration of screening. | _ |
| CPP | Consideration needs to be given to the significant differences in CVD risk across ethnicities (See Ethnic variation) when determining frequency of risk assessment. | _ |
| Gestational diabetes, impaired glucose tolerance and type 2 diabetes | ||
| CCR | Health professionals and women with PCOS should be aware that, regardless of age, the prevalence of gestational diabetes, impaired glucose tolerance and type 2 diabetes (5-fold in Asia, 4-fold in the Americas and 3-fold in Europe) are significantly increased in PCOS, with risk independent of, yet exacerbated by, obesity. | **** |
| CCR | Glycemic status should be assessed at baseline in all women with PCOS. Thereafter, assessment should be every one to three years, influenced by the presence of other diabetes risk factors. | **** |
| CCR | An oral glucose tolerance test (OGTT), fasting plasma glucose or HbA1c should be performed to assess glycemic status. In high-risk women with PCOS (including a BMI > 25 kg/m2 or in Asians >23 kg/m2, history of impaired fasting glucose, impaired glucose tolerance or gestational diabetes, family history of diabetes mellitus type 2, hypertension or high-risk ethnicity), an OGTT is recommended. | **** |
| CCR | A 75-g OGTT should be offered in all women with PCOS preconception when planning pregnancy or seeking fertility treatment, given the high risk of hyperglycemia and the associated comorbidities in pregnancy. If not performed preconception, an OGTT should be offered at <20 weeks gestation, and all women with PCOS should be offered the test at 24–28 weeks gestation. | **** |
| Obstructive sleep apnea (OSA) | ||
| CCR | Screening should only be considered for OSA in PCOS to identify and alleviate related symptoms, such as snoring, waking unrefreshed from sleep, daytime sleepiness and the potential for fatigue to contribute to mood disorders. Screening should not be considered with the intention of improving cardiometabolic risk, with inadequate evidence for metabolic benefits of OSA treatment in PCOS and in general populations. | **** |
| CCR | A simple screening questionnaire, preferably the Berlin tool, could be applied and if positive, referral to a specialist considered. | *** |
| CPP | A positive screen raises the likelihood of OSA, however, it does not quantify symptom burden and alone does not justify treatment. If women with PCOS have OSA symptoms and a positive screen, consideration can be given to referral to a specialist center for further evaluation. | _ |
| Endometrial cancer | ||
| CCR | Health professionals and women with PCOS should be aware of a 2- to 6-fold increased risk of endometrial cancer, which often presents before menopause; however, absolute risk of endometrial cancer remains relatively low. | *** |
| CPP | Health professionals require a low threshold for investigation of endometrial cancer in women with PCOS or a history of PCOS, with investigation by transvaginal ultrasound and/or endometrial biopsy recommended with persistent thickened endometrium and/or risk factors including prolonged amenorrhea, abnormal vaginal bleeding or excess weight. However, routine ultrasound screening of endometrial thickness in PCOS is not recommended. | _ |
| CPP | Optimal prevention for endometrial hyperplasia and endometrial cancer is not known. A pragmatic approach could include COCP or progestin therapy in those with cycles longer than 90 days. | _ |
| 2. Prevalence, screening, diagnostic assessment and treatment in emotional wellbeing | ||
| Quality of life | ||
| CCR | Health professionals and women should be aware of the adverse impact of PCOS on quality of life. | **** |
| CCR | Health professionals should capture and consider perceptions of symptoms, impact on quality of life and personal priorities for care to improve patient outcomes. | **** |
| CPP | The PCOS quality of life tool (PCOSQ), or the modified PCOSQ, may be useful clinically to highlight PCOS features causing greatest distress, and to evaluate treatment outcomes on women’s subjective PCOS health concerns. | _ |
| Depressive and anxiety symptoms, screening and treatment | ||
| CCR | Health professionals should be aware that in PCOS, there is a high prevalence of moderate to severe anxiety and depressive symptoms in adults; and a likely increased prevalence in adolescents. | **** |
| CCR | Anxiety and depressive symptoms should be routinely screened in all adolescents and women with PCOS at diagnosis. If the screen for these symptoms and/or other aspects of emotional wellbeing is positive, further assessment and/or referral for assessment and treatment should be completed by suitably qualified health professionals, informed by regional guidelines. | **** |
| CCR | If treatment is warranted, psychological therapy and/or pharmacological treatment should be offered in PCOS, informed by regional clinical practice guidelines. | **** |
| CPP | The optimal interval for anxiety and depressive symptom screening is not known. A pragmatic approach could include repeat screening using clinical judgment, considering risk factors, comorbidities and life events. | _ |
| CPP | Assessment of anxiety and or depressive symptoms involves assessment of risk factors, symptoms and severity. Symptoms can be screened according to regional guidelines, or by using simple stepwise approaches (see full guideline for details). | _ |
| CPP | Where pharmacological treatment for anxiety and depression is offered in PCOS, the following need consideration:
|
_ |
| CPP | Factors including obesity, infertility and hirsutism need consideration along with use of hormonal medications in PCOS, as they may independently exacerbate depressive and anxiety symptoms and other aspects of emotional wellbeing. | _ |
| Psychosexual function | ||
| CCR | All health professionals should be aware of the increased prevalence of psychosexual dysfunction and should consider exploring how features of PCOS, including hirsutism and body image, impact on sex life and relationships in PCOS. | **** |
| CCR | If psychosexual dysfunction is suspected, tools such as the Female Sexual Function Index can be considered. | **** |
| Body image | ||
| CCR | Health professionals and women should be aware that features of PCOS can impact on body image. | *** |
| CPP | Negative body image, can be screened according to regional guidelines or by using a stepped approach (see full guideline for details). | _ |
| Eating disorders and disordered eating | ||
| CCR | All health professionals and women should be aware of the increased prevalence of eating disorders and disordered eating associated with PCOS. | ** |
| CCR | If eating disorders and disordered eating are suspected, further assessment, referral and treatment, including psychological therapy, could be offered by appropriately trained health professionals, informed by regional guidelines or by using a stepped approach (see full guideline for details). | ** |
| Information resources, models of care, cultural and linguistic considerations | ||
| CCR | Information and education resources for women with PCOS should be culturally appropriate, tailored and high-quality, should use a respectful and empathetic approach, and promote self-care and highlight peer support groups. | **** |
| CCR | Information and education resources for healthcare professionals should promote the recommended diagnostic criteria, appropriate screening for comorbidities, and effective lifestyle and pharmacological management. | **** |
| CCR | PCOS information should be comprehensive, evidence-based and inclusive of the biopsychosocial dimensions of PCOS across the life-span. | **** |
| CCR | Women’s needs, communication preferences, beliefs and culture should be considered and addressed through provision of culturally and linguistically appropriate co-designed resources and care. | **** |
| CPP | Interdisciplinary care needs to be considered for those with PCOS where appropriate and available. Primary care is generally well placed to diagnose, screen and coordinate interdisciplinary care. | _ |
| CPP | Care needs to be person centered, address women’s priorities and be provided in partnership with those with PCOS and where appropriate, their families. | _ |
| CPP | Guideline dissemination and translation including multimodal education tools and resources is important, with consultation and engagement with stakeholders internationally. | _ |
| 3. Lifestyle | ||
| Effectiveness of lifestyle interventions | ||
| CCR | Healthy lifestyle behaviors encompassing healthy eating and regular physical activity should be recommended in all those with PCOS to achieve and/or maintain healthy weight and to optimize hormonal outcomes, general health and quality of life across the life course. | **** |
| EBR | Lifestyle intervention (preferably multicomponent including diet, exercise and behavioral strategies) should be recommended in all those with PCOS and excess weight, for reductions in weight, central obesity and insulin resistance. |
|
| CPP | Achievable goals such as 5–10% weight loss in those with excess weight yields significant clinical improvements and is considered successful weight reduction within 6 months. Ongoing assessment and monitoring is important during weight loss and maintenance in all women with PCOS. | _ |
| CPP | SMART (Specific Measurable, Achievable, Realistic and Timely) goal setting and self-monitoring can enable achievement of realistic lifestyle goals. | _ |
| CPP | Psychological factors such as anxiety and depressive symptoms, body image concerns and disordered eating, need consideration and management to optimize engagement and adherence to lifestyle interventions. | _ |
| CPP | Health professional interactions around healthy lifestyle, including diet and exercise, need to be respectful, patient-centered and to value women’s individualized healthy lifestyle preferences and cultural, socioeconomic and ethnic differences. Health professionals need to also consider personal sensitivities, marginalization and potential weight-related stigma. | _ |
| CPP | Adolescent and ethnic-specific BMI and waist circumference categories need to be considered when optimizing lifestyle and weight. | _ |
| CPP | Healthy lifestyle may contribute to health and quality of life benefits in the absence of weight loss. | _ |
| CPP | Healthy lifestyle and optimal weight management appears equally effective in PCOS as in the general population and is the joint responsibility of all health professionals, partnering with women with PCOS. Where complex issues arise, referral to suitably trained allied health professionals needs to be considered. | _ |
| CPP | Ethnic groups with PCOS who are at high cardiometabolic risk require greater consideration in terms of healthy lifestyle and lifestyle intervention (see Ethnic Variation). | _ |
| Behavioral strategies | ||
| CCR | Lifestyle interventions could include behavioral strategies such as goal-setting, self-monitoring, stimulus control, problem solving, assertiveness training, slower eating, reinforcing changes and relapse prevention, to optimize weight management, healthy lifestyle and emotional wellbeing in women with PCOS. | **** |
| CPP | Comprehensive health behavioral or cognitive behavioral interventions could be considered to increase support, engagement, retention, adherence and maintenance of healthy lifestyle and improve health outcomes in women with PCOS. | _ |
| Dietary intervention | ||
| CCR | A variety of balanced dietary approaches could be recommended to reduce dietary energy intake and induce weight loss in women with PCOS and overweight and obesity, as per general population recommendations. | **** |
| CCR | General healthy eating principles should be followed for all women with PCOS across the life course, as per general population recommendations. | **** |
| CPP | To achieve weight loss in those with excess weight, an energy deficit of 30% or 500–750 kcal/day (1200–1500 kcal/day) could be prescribed for women, also considering individual energy requirements, body weight and physical activity levels. | _ |
| CPP | In women with PCOS, there is no or limited evidence that any specific energy equivalent diet type is better than another, or that there is any differential response to weight management intervention, compared to women without PCOS. | _ |
| CPP | Tailoring of dietary changes to food preferences, allowing for a flexible and individual approach to reducing energy intake and avoiding unduly restrictive and nutritionally unbalanced diets, are important, as per general population recommendations. | _ |
| Exercise intervention | ||
| CCR | Health professionals should encourage and advise the following for prevention of weight gain and maintenance of health:
|
*** |
| CCR | Health professionals should encourage and advise the following for modest weight-loss, prevention of weight-regain and greater health benefits:
|
*** |
| CPP | Physical activity includes leisure time physical activity, transportation such as walking or cycling, occupational work, household chores, games, sports or planned exercise, in the context of daily, family and community activities. Daily, 10 000 steps is ideal, including activities of daily living and 30 min of structured physical activity or around 3000 steps. Structuring of recommended activities need to consider women’s and family routines as well as cultural preferences | _ |
| CPP | Realistic physical activity SMART (Specific, Measureable, Achievable, Relevant, Time limited) goals could include 10 min bouts, progressively increasing physical activity 5% weekly, up to and above recommendations. | _ |
| CPP | Self-monitoring including with fitness tracking devices and technologies for step count and exercise intensity, could be used as an adjunct to support and promote active lifestyles and minimize sedentary behaviors. | _ |
| Obesity and weight assessment | ||
| CCR | Health professionals and women should be aware that women with PCOS have a higher prevalence of weight gain and obesity, presenting significant concerns for women, impacting on health and emotional wellbeing, with a clear need for prevention. | *** |
| CCR | All those with PCOS should be offered regular monitoring for weight changes and excess weight (see Cardiovascular Disease Risk). | **** |
| CPP | When assessing weight, related stigma, negative body image and/or low self-esteem need to be considered and assessment needs to be respectful and considerate. Beforehand, explanations on the purpose and how the information will be used and the opportunity for questions and preferences needs to be provided, permission sought and scales and tape measures adequate. Implications of results need to be explained and where this impacts on emotional wellbeing, support provided. | _ |
| CPP | Prevention of weight gain, monitoring of weight and encouraging evidence-based and socio-culturally appropriate healthy lifestyle is important in PCOS, particularly from adolescence. | _ |
| 4. Pharmacological treatment for non-fertility indications | ||
| Pharmacological treatment principles in PCOS | ||
| CPP | Consideration of the individual’s personal characteristics, preferences and values is important in recommending pharmacological treatment. | _ |
| CPP | When prescribing pharmacological therapy in PCOS, benefits, adverse effects and contraindications in PCOS and general populations need to be considered and discussed before commencement. | _ |
| CPP | COCPs, metformin and other pharmacological treatments are generally off label# in PCOS. However, off label use is predominantly evidence-based and is allowed in many countries. Where it is allowed, health professionals need to inform women and discuss the evidence, possible concerns and side effects of treatment. | _ |
| CPP | Holistic approaches are required and pharmacological therapy in PCOS needs to be considered alongside education, lifestyle and other options including cosmetic therapy and counseling. | _ |
| Combined oral contraceptive pills (COCPs) | ||
| EBR | The COCP alone should be recommended in adult women with PCOS for management of hyperandrogenism and/or irregular menstrual cycles. |
|
| EBR | The COCP alone should be considered in adolescents with a clear diagnosis of PCOS for management of clinical hyperandrogenism and/or irregular menstrual cycles. |
|
| EBR | The COCP could be considered in adolescents who are deemed ‘at risk’ but not yet diagnosed with PCOS, for management of clinical hyperandrogenism and irregular menstrual cycles. |
|
| EBR | Specific types or dose of progestins, estrogens or combinations of COCP cannot currently be recommended in adults and adolescents with PCOS and practice should be informed by general population guidelines. |
|
| CCR | The 35 µg ethinyloestradiol plus cyproterone acetate preparations should not be considered first line in PCOS as per general population guidelines, due to adverse effects including venous thromboembolic risks. | * |
| CPP | When prescribing COCPs in adults and adolescents with PCOS:
|
_ |
| Combined oral contraceptive pills in combination with metformin and/or anti-androgen pharmacological agents | ||
| EBR | In combination with the COCP, metformin should be considered in women with PCOS for management of metabolic features where COCP and lifestyle changes do not achieve desired goals. |
|
| EBR | In combination with the COCP, metformin could be considered in adolescents with PCOS and BMI ≥ 25 kg/m2 where COCP and lifestyle changes do not achieve desired goals. |
|
| CPP | In combination with the COCP, metformin may be most beneficial in high metabolic risk groups including those with diabetes risk factors, impaired glucose tolerance or high-risk ethnic groups. | _ |
| EBR | In combination with the COCP, antiandrogens should only be considered in PCOS to treat hirsutism, after 6 months or more of COCP and cosmetic therapy have failed to adequately improve symptoms. |
|
| CCR | In combination with the COCP, antiandrogens could be considered for the treatment of androgen-related alopecia in PCOS. | ** |
| CPP | In PCOS, antiandrogens must be used with effective contraception, to avoid male fetal undervirilisation. Variable availability and regulatory status of these agents is notable and for some agents, potential liver toxicity requires caution. | _ |
| Metformin | ||
| EBR | Metformin in addition to lifestyle, could be recommended in adult women with PCOS, for the treatment of weight, hormonal and metabolic outcomes. |
|
| EBR | Metformin in addition to lifestyle, should be considered in adult women with PCOS with BMI ≥ 25 kg/m2 for management of weight and metabolic outcomes. |
|
| EBR | Metformin in additional to lifestyle, could be considered in adolescents with a clear diagnosis of PCOS or with symptoms of PCOS before the diagnosis is made. |
|
| CPP | Metformin may offer greater benefit in high metabolic risk groups including those with diabetes risk factors, impaired glucose tolerance or high-risk ethnic groups (see Ethnic variation). | _ |
| CPP | Where metformin is prescribed the following need to be considered:
|
_ |
| Anti-obesity pharmacological agents | ||
| CCR | Anti-obesity medications in addition to lifestyle, could be considered for the management of obesity in adults with PCOS after lifestyle intervention, as per general population recommendations. | _ |
| CPP | For anti-obesity medications, cost, contraindications, side effects, variable availability and regulatory status need to be considered and pregnancy needs to be avoided whilst taking these medications. | _ |
| Anti-androgen pharmacological agents | ||
| EBR | Where COCPs are contraindicated or poorly tolerated, in the presence of other effective forms of contraception, antiandrogens could be considered to treat hirsutism and androgen-related alopecia. |
|
| CPP | Specific types or doses of antiandrogens cannot currently be recommended with inadequate evidence in PCOS. | _ |
| Inositol | ||
| EBR | Inositol (in any form) should currently be considered an experimental therapy in PCOS, with emerging evidence on efficacy highlighting the need for further research. |
|
| CPP | Women taking inositol and other complementary therapies are encouraged to advise their health professional. | _ |
| 5. Assessment and treatment of infertility | ||
| Assessment of factors that may affect fertility, treatment response or pregnancy outcomes | ||
| CPP | Factors such as blood glucose, weight, blood pressure, smoking, alcohol, diet, exercise, sleep and mental, emotional and sexual health need to be optimized in women with PCOS, to improve reproductive and obstetric outcomes, aligned with recommendations in the general population. Refer to Lifestyle, Emotional Wellbeing and Diabetes risk sections. | _ |
| CPP | Monitoring during pregnancy is important in women with PCOS, given increased risk of adverse maternal and offspring outcomes. | _ |
| CCR | In women with PCOS and infertility due to anovulation alone with normal semen analysis, the risks, benefits, costs and timing of tubal patency testing should be discussed on an individual basis. | *** |
| CCR | Tubal patency testing should be considered prior to ovulation induction in women with PCOS where there is suspected tubal infertility. | *** |
| Ovulation induction principles | ||
| CPP | The use of ovulation induction agents, including letrozole, metformin and clomiphene citrate is off label in many countries. Where off label use of ovulation induction agents is allowed, health professionals need to inform women and discuss the evidence, possible concerns and side effects. | _ |
| CPP | Pregnancy needs to be excluded prior to ovulation induction. | _ |
| CPP | Unsuccessful, prolonged use of ovulation induction agents need to be avoided, due to poor success rates. | _ |
| Letrozole | ||
| EBR | Letrozole should be considered first line pharmacological treatment for ovulation induction in women with PCOS with anovulatory infertility and no other infertility factors to improve ovulation, pregnancy and live birth rates. |
|
| CPP | Where letrozole is not available or use is not permitted or cost is prohibitive, health professionals can use other ovulation induction agents. | _ |
| CPP | Health professionals and women need to be aware that the risk of multiple pregnancy appears to be less with letrozole, compared to clomiphene citrate. | _ |
| Clomiphene citrate and metformin | ||
| EBR | Clomiphene citrate could be used alone in women with PCOS with anovulatory infertility and no other infertility factors to improve ovulation and pregnancy rates. |
|
| EBR | Metformin could be used alone in women with PCOS, with anovulatory infertility and no other infertility factors, to improve ovulation, pregnancy and live birth rates, although women should be informed that there are more effective ovulation induction agents. |
|
| EBR | Clomiphene citrate could be used in preference, when considering clomiphene citrate or metformin for ovulation induction in women with PCOS who are obese (BMI is ≥30 kg/m2) with anovulatory infertility and no other infertility factors. |
|
| EBR | If metformin is being used for ovulation induction in women with PCOS who are obese (BMI ≥ 30 kg/m2) with anovulatory infertility and no other infertility factors, clomiphene citrate could be added to improve ovulation, pregnancy and live birth rates. |
|
| EBR | Clomiphene citrate could be combined with metformin, rather than persisting with clomiphene citrate alone, in women with PCOS who are clomiphene citrate-resistant, with anovulatory infertility and no other infertility factors, to improve ovulation and pregnancy rates. |
|
| CPP | The risk of multiple pregnancies is increased with clomiphene citrate use and therefore monitoring needs to be considered. | _ |
| Gonadotrophins | ||
| EBR | Gonadotrophins could be used as second line pharmacological agents in women with PCOS who have failed first line oral ovulation induction therapy and are anovulatory and infertile, with no other infertility factors. |
|
| EBR | Gonadotrophins could be considered as first line treatment, in the presence of ultrasound monitoring, following counseling on cost and potential risk of multiple pregnancy, in women with PCOS with anovulatory infertility and no other infertility factors. |
|
| EBR | Gonadotrophins, where available and affordable, should be used in preference to clomiphene citrate combined with metformin therapy for ovulation induction, in women with PCOS with anovulatory infertility, clomiphene citrate-resistance and no other infertility factors, to improve ovulation, pregnancy and live birth rates. |
|
| EBR | Gonadotrophins with the addition of metformin could be used rather than gonadotrophin alone, in women with PCOS with anovulatory infertility, clomiphene citrate-resistance and no other infertility factors, to improve ovulation, pregnancy and live birth rates. |
|
| EBR | Either gonadotrophins or laparoscopic ovarian surgery could be used in women with PCOS with anovulatory infertility, clomiphene citrate-resistance and no other infertility factors, following counseling on benefits and risks of each therapy. |
|
| CPP | Where gonadotrophins are prescribed, considerations include:
|
_ |
| CPP | Gonadotrophin induced ovulation is only triggered when there are fewer than three mature follicles and needs to be canceled if there are more than two mature follicles with the patient advised to avoid unprotected intercourse. | _ |
| Anti-obesity pharmacological agents | ||
| CCR | Pharmacological anti-obesity agents should be considered an experimental therapy in women with PCOS for the purpose of improving fertility, with risk to benefit ratios currently too uncertain to advocate this as fertility therapy. | * |
| Laparoscopic surgery | ||
| EBR | Laparoscopic ovarian surgery could be second line therapy for women with PCOS, who are clomiphene citrate resistant, with anovulatory infertility and no other infertility factors. |
|
| CCR | Laparoscopic ovarian surgery could potentially be offered as first line treatment if laparoscopy is indicated for another reason in women with PCOS with anovulatory infertility and no other infertility factors. | *** |
| CPP | Risks need to be explained to all women with PCOS considering laparoscopic ovarian surgery. | _ |
| CPP | Where laparoscopic ovarian surgery is to be recommended, the following need to be considered:
|
_ |
| Bariatric surgery | ||
| CCR | Bariatric surgery should be considered an experimental therapy in women with PCOS, for the purpose of having a healthy baby, with risk to benefit ratios currently too uncertain to advocate this as fertility therapy. | * |
| CPP | If bariatric surgery is to be prescribed, the following need to be considered:
|
|
If pregnancy occurs, the following need to be considered:
|
_ | |
| In vitro fertilization (IVF) | ||
| CCR | In the absence of an absolute indication for IVF ± ICSI), women with PCOS and anovulatory infertility could be offered IVF as third line therapy where first or second line ovulation induction therapies have failed. | *** |
| CPP | In women with anovulatory PCOS, the use of IVF is effective and when elective single embryo transfer is used, multiple pregnancies can be minimized. | _ |
| CPP | Women with PCOS undergoing IVF ± ICSI therapy need to be counseled prior to starting treatment including on:
|
_ |
| CCR | Urinary or recombinant follicle stimulation hormone can be used in women with PCOS undergoing controlled ovarian hyperstimulation for IVF ± ICSI, with insufficient evidence to recommend specific follicle stimulating hormone (FSH) preparations. | _ |
| CCR | Exogenous recombinant luteinizing hormone treatment should not be routinely used in combination with follicle stimulating hormone therapy in women with PCOS undergoing controlled ovarian hyperstimulation for IVF ± ICSI. | _ |
| EBR | A gonadotrophin releasing hormone antagonist protocol is preferred in women with PCOS undergoing an IVF ± ICSI cycle, over a gonadotrophin releasing hormone agonist long protocol, to reduce the duration of stimulation, total gonadotrophin dose and incidence of ovarian hyperstimulation syndrome (OHSS). |
|
| CPP | Human chorionic gonadotrophin is best used at the lowest doses to trigger final oocyte maturation in women with PCOS undergoing an IVF ± ICSI cycle to reduce the incidence of OHSS. | _ |
| CPP | Triggering final oocyte maturation with a gonadotropin-releasing hormone (GnRH) agonist and freezing all suitable embryos could be considered in women with PCOS having an IVF/ICSI cycle with a GnRH antagonist protocol and at an increased risk of developing OHSS or where fresh embryo transfer is not planned. | _ |
| CPP | In IVF ± ICSI cycles in women with PCOS, consideration needs to be given to an elective freeze of all embryos. | _ |
| EBR | Adjunct metformin therapy could be used before and/or during follicle stimulating hormone ovarian stimulation in women with PCOS undergoing a IVF ± ICSI therapy with a GnRH agonist protocol, to improve the clinical pregnancy rate and reduce the risk of OHSS. |
|
| CCR | In a GnRH agonist protocol with adjunct metformin therapy, in women with PCOS undergoing IVF ± ICSI treatment, the following could be considered:
|
_ |
| CPP | In IVF ± ICSI cycles, women with PCOS could be counseled on potential benefits of adjunct metformin in a GnRH antagonist protocol to reduce risk of ovarian hyperstimulation syndrome (see above for metformin therapy considerations). | _ |
| CPP | The term in vitro maturation (IVM) treatment cycle is applied to ‘the maturation in vitro of immature cumulus oocyte complexes collected from antral follicles’ (encompassing both stimulated and unstimulated cycles, but without the use of a human gonadotrophin trigger). | _ |
| CCR | In units with sufficient expertise, IVM could be offered to achieve pregnancy and livebirth rates approaching those of standard IVF ± ICSI treatment without the risk of OHSS for women with PCOS, where an embryo is generated, then vitrified and thawed and transferred in a subsequent cycle. | ** |
EBR = evidence based recommendation; CCR = clinical consensus recommendation; CPP = clinical practice point. Evidence quality: ⊕⊕⊕⊕ = high quality; ⊕⊕⊕○ = moderate quality; ⊕⊕○○ = low quality and ⊕○○○ = very low quality evidence. ****Strong recommendation for the option; ***Conditional recommendation for the option; **Conditional recommendation for either the option or the comparison; *Conditional recommendation against the option. Note: Off-label prescribing occurs when a drug is prescribed for an indication, a route of administration, or a patient group that is not included in the approved product information document for that drug by the regulatory body. Prescribing off-label is often unavoidable and common, and does not mean that the regulatory body has rejected the indication, but more commonly there has not been a submission to request evaluation of the indication or that patient group for any given drug.
GRADE working group. Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines.
© International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018, Helena Teede et al. Monash University (monash.edu/medicine/sphpm/mchri/pcos), 2018, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in PCOS. This image/content is not covered by the terms of the Creative Commons licence of this publication. For permission to reuse, please contact the rights holder.
Discussion
The International Guideline for the Assessment and Management of PCOS and the related translation program aims to provide clinicians with a quality, reliable source of international EBR to guide consistent clinical practice and to empower women with evidence-based information. All recommendations were formulated after an assessment of the best available evidence, multidisciplinary clinical expertise, consumer preferences and structured review by the five GDGs. Detailed methods for stakeholder engagement and guideline development can be found at https://www.monash.edu/medicine/sphpm/mchri/pcos. The guideline provides 166 recommendations: EBR = 31, clinical consensus recommendations (CCR) = 59, and clinical practice points (CPP) = 76. Overall, evidence is of low to moderate quality, requiring significant research expansion in this neglected, yet common condition.
We endorse the Rotterdam PCOS Diagnostic Criteria in adults (two of oligo- or anovulation, clinical and/or biochemical hyperandrogenism, or polycystic ovaries on ultrasound), after exclusion of related disorders. Where both oligo- or anovulation and hyperandrogenism are present, ultrasound is not necessary for diagnosis. In adolescents, both oligo-anovulation and hyperandrogenism are required, with ultrasound not recommended for diagnosis. Ultrasound criteria are refined with advancing technology. Anti-Müllerian hormone levels are not yet adequate for diagnosis of PCOS. Insulin resistance is recognized as a key feature of PCOS, yet clinical measurement is not recommended at the current time. Once diagnosed, assessment and management includes reproductive, metabolic, and psychological features. Education, self-empowerment, multidisciplinary care and lifestyle intervention for prevention or management of excess weight are prioritized. Depressive and anxiety symptoms should be screened, assessed and managed, and health professionals should be aware of other impacts on emotional wellbeing and quality of life. Combined oral contraceptive pills are first-line pharmacological management for menstrual irregularity and hyperandrogenism, with no specific formulation recommended and with low-dose preparations preferred. Metformin is recommended in addition or alone, primarily for management of metabolic features. Letrozole is first-line pharmacological infertility therapy; with clomiphene and metformin both having a role, alone and in combination. In women with PCOS and anovulatory infertility, gonadotrophins are second line. In the absence of an absolute indication for IVF, women with PCOS and anovulatory infertility could be offered IVF third line where other ovulation induction therapies have failed.
The combined effects of the provision of a single source of evidence-based recommendations and a comprehensive international translation and dissemination program will amplify the impact of the guideline and recommendations globally. It will support and build the capability of health professionals to deliver high-quality, evidence-based assessment and management of PCOS and will augment the health literacy and self-management of PCOS health consumers. The guideline recommendations are protected under copyright, however the process for adaption of guideline recommendations to the regional context is available at https://www.monash.edu/medicine/sphpm/mchri/pcos. The guideline and translation program will contribute to early diagnosis and improved health outcomes, and promote best-practice PCOS models of care. The translation program will be inclusive of a range of outputs such as the first evidence-based PCOS APP (AskPCOS), a rigorously developed question prompt list to optimize health professional engagement, health literacy enhancing tools, comprehensive PCOS-related health information; internationally accessible health professional accredited courses, webinars with international expert panels, and e-health information resources available at https://www.monash.edu/medicine/sphpm/mchri/pcos. Most importantly, the guideline and translation of the guideline is expected to improve patient experiences through the provision of timely and accurate diagnosis, accessible evidence-based information and improved multidisciplinary support. Ultimately, this initiative may serve as an exemplar for international collaborative engagement and healthcare impact. Key elements included extensive collaboration, broad stakeholder representation, including consumer partnership, distributive leadership, adequate funding, robust project management and governance, adherence to best practice and integrated comprehensive translation and evaluation.
Acknowledgements
We gratefully acknowledge the contribution of our partners, engaged and collaborating organizations:
The Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Polycystic Ovary Syndrome and the members of this Centre who coordinated this international guideline effort
- Our partner organizations are:
- American Society for Reproductive Medicine (ASRM)
- European Society of Human Reproduction and Embryology (ESHRE)
- Our collaborating and engaged societies and consumer groups:
- Androgen Excess and Polycystic Ovary Syndrome Society (AEPCOS)
- American Pediatric Endocrine Society
- Asia Pacific Paediatric Endocrine Society (APPES)
- Asia Pacific Initiative on Reproduction (ASPIRE)
- Australasian Paediatric Endocrine Group (APEG)
- Australian Diabetes Society (ADS)
- British Fertility Society (BFS)
- Canadian Society of Endocrinology and Metabolism (CSEM)
- Dietitians Association Australia
- Endocrine Society (US Endo)
- Endocrine Society Australia (ESA)
- European Society of Endocrinology (ESE)
- European Society for Paediatric Endocrinology (ESPE)
- Exercise and Sports Science Australia (ESSA)
- Federation of Obstetric and Gynaecological Societies of India (FOGSI)
- Fertility Society Australia (FSA)
- International Society of Endocrinology (ISE)
- International Federation of Fertility Societies (IFFS)
- International Federation of Gynecology and Obstetrics (FIGO)
- Italian Society of Gynaecology and Obstetrics
- Japanese Society for Paediatric Endocrinology (JSPE)
- Latin American Society for Paediatric Endocrinology (SLEP)
- Nordic Federation of Societies of Obstetrics and Gynaecology (NFOG)
- PCOS Challenge
- PCOS Society of India
- Paediatric Endocrine Society (PES)
- Polycystic Ovary Association Australia (POSSA)
- Royal Australasian College of Physicians (RACP)
- Royal Australian College of General Practitioners (RACGP)
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG)
- Royal College of Obstetricians and Gynaecologists (RCOG)
- South African Society of Gynaecology and Obstetrics (SASOG)
- Verity UK
- Victorian Assisted Reproductive Technology Association (VARTA)
Other relevant organizations are welcome to partner in guideline translation once approved.
Appendix
International PCOS Network
Marianne Andersen, Odense University Hospital, Denmark
Ricardo Azziz, State University of New York System Administration, USA
Adam Balen, Leeds Teaching Hospitals, UK
Estifanos Baye, Monash Centre for Health Research and Implementation, Australia
Jacqueline Boyle, Monash Centre for Health Research and Implementation, Australia
Leah Brennan, Australian Catholic University, Australia
Frank Broekmans, University Medical Centre Utrecht, Netherlands
Preeti Dabadghao, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
Luigi Devoto, University of Chile, Faculty of Medicine, Chile
Didier Dewailly, University of Lille, France
Linda Downes, Monash Centre for Health Research and Implementation, Australia
Bart Fauser, University Medical Center Utrecht, Netherlands
Stephen Franks, Imperial College, London, UK
Rhonda M. Garad, Monash Centre for Health Research and Implementation, Australia
Melanie Gibson-Helm, Monash University, Australia
Cheryce Harrison, Monash Centre for Health Research and Implementation, Australia
Roger Hart, The University of Western Australia, Australia
Rachel Hawkes, Verity, UK
Angelica Hirschberg, Karolinska Institutet, Sweden
Kathleen Hoeger, University of Rochester, USA
Femke Hohmann, Huisartsenpraktijk Hohmann & De Vet, Rotterdam, Netherlands
Samantha Hutchison, Monash Health Centre for Research Implementation, Australia
Anju Joham, Monash Centre for Health Research and Implementation, Australia
Louise Johnson, Victorian Assisted Reproductive Treatment Authority, Australia
Cailin Jordan, Genea Hollywood Fertility, Australia
Jayashri Kulkarni, Monash Alfred Psychiatry Research Centre, Australia
Richard S. Legro, Penn State College of Medicine, Hershey, PA, USA
Rong Li, Peking University Third Hospital, China
Marla Lujan, Cornell University, USA
Jaideep Malhotra, Rainbow Hospital, India
Darren Mansfield, Monash University and Monash Health, Australia
Kate Marsh, Northside Nutrition & Dietetics, Australia
Veryan McAllister, Polycystic Ovary Syndrome Association Australia, Australia
Edgar Mocanu, Royal College of Surgeons, Rotunda Hospital, Dublin 1, Ireland
Ben W. Mol, Monash University, Australia
Ernest Ng, Department of Obstetrics & Gynaecology, The University of Hong Kong, Hong Kong
Sharon Oberfield, Columbia University Medical Center, USA
Sasha Ottey, The PCOS Challenge: The National Polycystic Ovary Syndrome Association, USA
Alexia Peña, The Robinson Research Institute at the University of Adelaide, Australia
Jie Qiao, Peking University Third Hospital, China
Leanne Redman, Pennington Biomedical Research Center, USA
Raymond Rodgers, The Robinson Research Institute at the University of Adelaide, Australia
Luk Rombauts, Department of Obstetrics and Gynecology, Monash University, Australia
Daniela Romualdi, Fondazione Policlinico Universitario Agostino Gemelli, Rome
Duru Shah, Gynaecworld, India
Jane Speight, Deakin University, Australia
Poli Mara Spritzer, Federal University of Rio Grande Do Sul, Brazil
Elisabet Stener-Victorin, Karolinska Institutet, Sweden
Nigel Stepto, Victoria University, Australia
Juha S. Tapanainen, University of Helsinki, Helsinki University Hospital, Finland
Eliza C. Tassone, Monash Centre for Health Research and Implementation, Australia
Shakila Thangaratinam, BARC (Barts Research Centre for Women’s Health) Barts and the London School of Medicine and Dentistry, Queen Mary University of London, UK
Mala Thondan, Harp Family Medical Centre, Australia
Chii-Ruey Tzeng, Taipei Medical University Hospital, Taiwan
Zephne van der Spuy, University of Cape Town, South Africa
Eszter Vanky, Dept. of Clinical and Molecular Medicine, Norwegian University of Science and Technology, Norway
Maria Vogiatzi, Children’s Hospital of Philadelphia, University of Pennsylvania, USA
Angela Wan, Monash Centre for Health Research and Implementation, Australia
Chandrika Wijeyaratne, Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Colombo, Sri Lanka
Selma Witchel, Children’s Hospital of Pittsburgh of UPMC, University of Pittsburgh, USA
Jane Woolcock, Women’s and Children’s Hospital Adelaide, Australia
Bulent O. Yildiz, Hacettepe University School of Medicine, Ankara, Turkey
Contributor Information
International PCOS Network:
Marianne Andersen, Ricardo Azziz, Adam Balen, Estifanos Baye, Jacqueline Boyle, Leah Brennan, Frank Broekmans, Preeti Dabadghao, Luigi Devoto, Didier Dewailly, Linda Downes, Bart Fauser, Stephen Franks, Rhonda M Garad, Melanie Gibson-Helm, Cheryce Harrison, Roger Hart, Rachel Hawkes, Angelica Hirschberg, Kathleen Hoeger, Femke Hohmann, Samantha Hutchison, Anju Joham, Louise Johnson, Cailin Jordan, Jayashri Kulkarni, Richard S Legro, Rong Li, Marla Lujan, Jaideep Malhotra, Darren Mansfield, Kate Marsh, Veryan McAllister, Edgar Mocanu, Ben W Mol, Ernest Ng, Sharon Oberfield, Sasha Ottey, Alexia Peña, Jie Qiao, Leanne Redman, Raymond Rodgers, Luk Rombauts, Daniela Romualdi, Duru Shah, Jane Speight, Poli Mara Spritzer, Elisabet Stener-Victorin, Nigel Stepto, Juha S Tapanainen, Eliza C Tassone, Shakila Thangaratinam, Mala Thondan, Chii-Ruey Tzeng, Zephne van der Spuy, Eszter Vanky, Maria Vogiatzi, Angela Wan, Chandrika Wijeyaratne, Selma Witchel, Jane Woolcock, and Bulent O Yildiz
Authors’ roles
Professor Teede, Professor Norman and all listed authors, were members of the project board, and coordinated GDG activities from prioritizing clinical questions, providing clinical input into evidence synthesis, chairing the GDG process and GRADE framework application, finalizing recommendations, responding to feedback and endorsing the guideline. Helena Teede was the guideline development and translation lead and engaged with all GDG meetings, overseeing the process. Joop Laven, Anuja Dokras, Lisa Moran, Terhi Piltonen and Michael Costello chaired the GDGs. Marie Misso led the guideline development and evidence synthesis processes. Robert Norman was the PCOS Centre for Research Excellence co-director, the deputy chair of the International advisory board and the deputy chair of two GDG’s. All other authors, were actively engaged as guideline development group, consumer, translation or international advisory board members or members of the evidence synthesis and translation team, contributed to the article, prioritizing clinical questions, discussing recommendations until voting and consensus, responses to external peer review and approval of the final recommendations across all GDGs.
Funding
The Australian National Health and Medical Research Council (NHMRC) (APP1078444) funded this work, supported by European Society of Human Reproduction and Embryology (ESHRE) and American society for Reproductive Medicine (ASRM).
Conflict of interest
Disclosures of conflicts of interest were declared at the outset and updated throughout the guideline process, aligned with NHMRC guideline processes. Full details of conflicts declared across the guideline development groups are available at https://www.monash.edu/medicine/sphpm/mchri/pcos/guideline in the Register of disclosures of interest. Of named authors, Dr Costello has declared shares in Virtus Health and past sponsorship from Merck Serono for conference presentations. Prof. Laven declared grants from Ferring, Euroscreen and personal fees from Ferring, Euroscreen, Danone and Titus Healthcare. Prof. Norman has declared a minor shareholder interest in an IVF unit. The remaining authors have no conflicts of interest to declare.
References
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BC, Norman RJ, Teede H. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 2016;22:687–708. [DOI] [PubMed] [Google Scholar]
- Brouwers M, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE et al. . AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 2014;171:P1–P29. [DOI] [PubMed] [Google Scholar]
- Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, Teede H. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril 2017;107:1380–1386.e1. [DOI] [PubMed] [Google Scholar]
- Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College or Endocrinology and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evalution and treatment of polycystic ovary syndrome. Endocr Pract 2015;21:1291–1300. [DOI] [PubMed] [Google Scholar]
- GRADE Working Group Grading of Recommendations Assessment, Development and Evaluation (GRADE) Guidelines 2017. http://www.gradeworkinggroup.org/.
- Group REA-SPCW Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Hassan Murad M, Pasquali R, Welt CK. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misso M, Teede H. Evidence based guideline (EBG) development: a practical guide In: Ilic D (ed). Knowledge Transfer: Practices, Types and Challenges. New York: Nova Publishers, 2012. [Google Scholar]
- National Health and Medical Research Council NHMRC Standards and Procedures for Externally Developed Guidelines Australia; 2007. https://www.nhmrc.gov.au/guidelines-publications/nh56.
- National Health and Medical Research Council NHMRC Levels of Evidence and Grades for Recommendations for Developers Of Guidelines Australia; 2009. https://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf.
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede H, Gibson-Helm M, Norman RJ, Boyle J. Polycystic ovary syndrome: perceptions and attitudes of women and primary health care physicians on features of pcos and renaming the syndrome. J Clin Endocrinol Metab 2014;99:E107–E111. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BGA, Wong JLA, Norman RJ, Costello MF, Guideline Development Groups . Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med J Aust 2011;195:S65–S112. [DOI] [PubMed] [Google Scholar]