Abstract
Steroid sulfatase (STS), a desulfating enzyme that converts steroid sulfates to hormonally active steroids, plays an important role in the homeostasis of sex hormones. STS is expressed in the adipose tissue of both male and female mice, but the role of STS in the development and function of adipose tissue remains largely unknown. In this report, we show that the adipose expression of Sts was induced in the high-fat diet (HFD) and ob/ob models of obesity and type 2 diabetes. Transgenic overexpression of the human STS in the adipose tissue of male mice exacerbated the HFD-induced metabolic phenotypes, including increased body weight gain and fat mass, and worsened insulin sensitivity, glucose tolerance, and energy expenditure, which were accounted for by adipocyte hypertrophy, increased adipose inflammation, and dysregulation of adipogenesis. The metabolic harm of the STS transgene appeared to have resulted from increased androgen activity in the adipose tissue, and castration abolished most of the phenotypes. Interestingly, the transgenic effects were sex specific, because the HFD-fed female STS transgenic mice exhibited improved metabolic functions, which were associated with attenuated adipose inflammation. The metabolic benefit of the STS transgene in female mice was accounted for by increased estrogenic activity in the adipose tissue, whereas such benefit was abolished upon ovariectomy. Our results revealed an essential role of the adipose STS in energy homeostasis in sex- and sex hormone–dependent manner. The adipose STS may represent a therapeutic target for the management of obesity and type 2 diabetes.
Our results revealed an essential role of the adipose STS in energy homeostasis in sex- and sex hormone–dependent manner.
In addition to reproduction, estrogens and androgens are also implicated in many physiological functions, including energy homeostasis (1, 2). Postmenopausal women have an increased risk of developing metabolic syndrome (3). Human subjects lacking the estrogen receptor α (ERα) or aromatase, the primary enzyme converting androgens to estrogens, are prone to insulin resistance and obesity (4, 5). In contrast, estrogen replacement therapies ameliorate metabolic disorders and decrease abdominal fat gain in women (3). In rodents, mice deficient of ERα or aromatase develop obesity and insulin resistance (6, 7), whereas estrogens administration improves insulin sensitivity in high-fat diet (HFD)–fed female mice (8) and ob/ob mice (9). In general, estrogens play a beneficial role in improving energy homeostasis in both males and females. Although the effects of estrogens on adipose tissue have been reported, many of the previous studies have focused on the systemic effect of estrogens on certain peripheral tissues such as the liver, brain, and pancreatic β cells. The adipose tissue specific role of estrogens in energy homeostasis needs to be further clarified.
The role of androgens in energy homeostasis has also been suggested. Unlike estrogens, the effect of androgens on energy metabolism shows sexual dimorphism. In females, excess androgenic activity in the liver, skeletal muscle, pancreatic β cells, and metabolic centers of the hypothalamus synergize to worsen metabolic function, inflammation, visceral adiposity, and type 2 diabetes (10–12). In males, a low testosterone predisposes them to diabetes and hyperglycemia (13, 14). It has been reported that androgens, through the activation of androgen receptor (AR), can inhibit hepatic lipogenesis, promote hepatic lipid oxidation, improve insulin sensitivity, and prevent hepatic steatosis in males (15). Androgens improve pancreatic β-cell function and increase insulin secretion, so an androgen supplement has been used for the prevention of type 2 diabetes in hypoandrogenic men (16). In contrast, testosterone deficiency promotes insulin resistance in the skeletal muscle at least in part via an AR-dependent mechanism (17–19). Testosterone deficiency has been linked to visceral obesity in men, and there is an inverse correlation between total serum testosterone and the amount of visceral adipose tissue (20), but these androgen effects were suggested to be indirectly mediated by the AR actions in the skeletal muscle (21, 22). The direct effect of androgens on adiposity and adipose tissue energy homeostasis remains unclear.
The steroid sulfonation and desulfation pathways play an important role in the chemical and functional homeostasis of steroids. Steroid sulfatase (STS) is a desulfation enzyme that converts the hormonally inactive steroid sulfates, such as androgen sulfates and estrogen sulfates, to hormonally active steroids (23). In both humans and rodents, the circulating concentrations of steroid hormone sulfates are substantially higher than their parent hormones, acting as a reservoir for regenerating active steroid hormones by the STS-mediated desulfation (23). Knowing sex hormones can affect energy homeostasis, we hypothesized that STS may play a role in energy metabolism, likely in a tissue specific manner. We previously reported that transgenic (Tg) overexpression of STS in the liver elicited metabolic benefits in both the male and female mice but via distinct mechanisms. In female Tg mice, the metabolic benefit was mediated by increased hepatic estrogen activity, whereas the protective effect in males may have been accounted for by decreased inflammation in the white adipose tissue (24). However, the specific role of STS in the adipose tissue has not been reported.
In this study, we attempted to dissect the sex- and adipose tissue–specific role of STS in energy homeostasis. Our results showed that adipose overexpression of STS aggravated HFD-induced metabolic phenotype in male mice in an androgen-dependent manner and protected female mice from metabolic harm in an estrogen-dependent manner, respectively. The current study illustrated the direct effects of androgens and estrogens on adipose tissue development, inflammation, and energy homeostasis.
Research Design and Methods
Generation of STS Tg mice, diet and drug treatment, body composition analysis, and indirect calorimetry
The tetracycline response element (TetRE)–STS Tg mice expressing the HA-tagged human STS cDNA under the control of the TetRE (24) and the fatty acid binding protein 4 (aP2)–tetracycline transcriptional activator Tg mice expressing the tetracycline transcriptional activator under the control of the adipose specific aP2 gene promoter (25) were generated in our laboratory as previously described. The TetRE-STS/aP2-tetracycline-transcriptional activator (tTA) double Tg, termed aP2-STS Tg mice, were generated by cross-breeding the TetRE-STS mice with the aP2-tTA mice and maintained in the C57BL/6J background. When necessary, doxycycline (DOX; 2 mg/mL) was given in drinking water 1 week before HFD treatment until the end of the experiment. HFD (catalog no. S3282) with 60% of total calories coming from animal fat was purchased from Bio-serv (Frenchtown, NJ). The HFD started when mice were 6 weeks old and lasted for 20 weeks, so the mice were 26 weeks old at the end of their HFD exposure. Body composition analysis by EchoMRI (Houston, TX) and indirect calorimetry by Oxymax Indirect Calorimetry System (Columbus Instruments, Columbus, OH) were performed as we have previously described (26, 27).
Study approval
The Central Animal Facility of the University of Pittsburgh is fully accredited by Association for Assessment of Laboratory Animal Care. All procedures were performed in accordance with relevant federal guidelines and with the approval of the University of Pittsburgh International Animal Care and Use Committee.
Western blot analysis
Protein samples were isolated using RIPA buffer and resolved by electrophoresis on 10% SDS-polyacrylamide gels. Upon the transferring of proteins to polyvinylidene difluoride membranes, the membranes were probed with primary antibodies. The primary antibodies used include: anti-HA (1:1000 dilution; RRID: AB_2314619; catalog no. 2367) (28), antitotal Akt (1:1000 dilution; RRID: AB_329827; catalog no. 9272) (29), antiphospho-Akt (serine 473) (1:1000 dilution; RRID: AB_329825; catalog no. 9271) (30), and anti-Irs1 (1:1000 dilution; RRID: AB_330333; catalog no. 2382) (31) from Cell Signaling; antiphospho-Irs1 (tyrosine) (1:1000 dilution; RRID: AB_297058; catalog no. ab10321) (32) from Abcam; anti-Pparγ (1:1000 dilution; RRID: AB_2136584; catalog no. MAB1857) (33) from R & D Systems; anti-Erk1 (1:1000 dilution; RRID: AB_2140110; catalog no. sc-94) (34) and antiphospho-Erk (1:1000 dilution; RRID: AB_627545; catalog no. sc-7383) (35) from Santa Cruz Biotechnology; and anti-β-actin (1:5000 dilution; RRID: AB_476692; catalog no. A1978) (36) from Sigma-Aldrich. Detection was achieved by using an ECL system from Amersham (Piscataway, NJ).
STS enzymatic activity
Tissues were minced with scissors in ice-cold 25 mM Tris-HCl buffer, pH 7.5, (1:10 w/v for adipose tissue; 1:20 w/v for liver) and homogenized with a Tissue Tearor (BioSpec) using three 30-second bursts. Protein concentrations in the homogenates were determined using a Pierce BCA assay (ThermoFisher). 3H-estrone sulfate (53 Ci/mmol; Perkin Elmer-NEN) was diluted in 50 mM Tris-HCl buffer and 100 µL (140, 000 dpm) were added to all assay tubes. Radioinert estrone sulfate (Sigma) was dissolved in ethanol and then diluted into 50 mM Tris-HCl buffer and 100 µL was added to achieve a final concentration of 10 µM. Experimental compounds were dissolved in ethanol and then diluted in 50 mM Tris-HCl buffer. The assay tubes containing steroids were preincubated for 5 minutes at 37°C in a water bath. The assay was initiated by addition of homogenates (300 µL) to the tubes. Control samples (background) received 300 µL of buffer in place of homogenates. After 30 minutes of incubation at 37°C, 3 mL of toluene was added to the tubes. The tubes were vortexed for 1 minute and centrifuged at 1500g for 10 minutes. Two 500-µL aliquots of the organic phase were removed from each sample and added to 4 mL of scintillation cocktail (Ultima Gold; Perkin Elmer) in scintillation vials. Vials were placed in a Packard Tri-Carb scintillation counter for determination of product formation, with 50% efficiency for 3H. Each sample was run in duplicate.
Serum and liver tissue chemistry
Serum levels of triglycerides (catalog no. 2100-430 from Stanbio), cholesterol (catalog no. 1010-430 from Stanbio), and insulin (catalog no. 90080 from Crystal Chem) were measured using commercial assay kits.
Gene expression analysis
RNA was extracted by using the Trizol Reagent (Invitrogen). One microgram of RNA from each sample was reverse transcribed into cDNA using reverse transcription (Superscript II; Invitrogen) and oligoDT (Invitrogen). Quantitative RT-PCR was performed with an ABI Prism 7900 Thermal Cycler (Applied Biosystems) using SYBR Green detection reagent. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping control gene. Relative gene expression was calculated using the ΔΔCT method, where fold difference was calculated using the expression 2−ΔΔCT. The primer sequences are provided in Supplemental Table 1.
Glucose tolerance test and insulin tolerance test
For glucose tolerance test (GTT), mice received an intraperitoneal injection of d-glucose at 2 g/kg body weight after a 12-hour fasting. For insulin tolerance test (ITT), mice received an intraperitoneal injection of insulin at 0.5 units/kg body weight after a 6-hour fasting.
Histology and immunohistochemistry
Tissues were fixed in 4% formaldehyde, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for general histology. For immunofluorescence, tissue sections were deparaffinized and rehydrated, followed by preincubated in blocking buffer (PBS containing 5% normal donkey serum and 0.3% Triton X-100) for 60 minutes. Tissue sections were then incubated with diluted primary antibody overnight at 4°C and fluorochrome-conjugated secondary antibody for 1 to 2 hours at room temperature in dark the next day. Antibodies used include goat antimouse CD68 (M-20) polyclonal antibody (1:250 dilution; RRID: AB_2074854; catalog no. sc-7084; Santa Cruz Biotechnology) (37). Histomorphometric analysis on insulin-stained pancreatic sections was performed using ImageJ from the National Institutes of Health (Bethesda, MD), and the percent of islet area per total pancreatic area was calculated.
UPLC tandem mass spectrometry analysis of tissue levels of estrogens
UPLC tandem mass spectrometry was carried out with a Waters Acquity UPLC system connected with the Xevo TQ triple quadrupole mass spectrometer as previously described (24).
Statistical analysis
Data are expressed as the means ± SD. One-way ANOVA, followed by Tukey post hoc test, was performed using GraphPad Prism (San Diego, CA). P values <0.05 were considered statistically significant.
Results
Adipose induction of STS in obese mice, and creation of Tg mice expressing STS in the adipose tissue
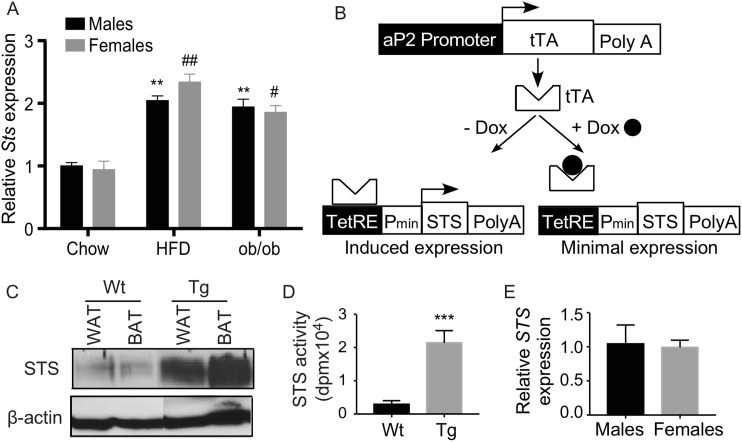
HFD feeding and the leptin deficient ob/ob mice are two commonly used mouse models of obesity and type 2 diabetes. We found the expression of the endogenous Sts was induced in the epididymal white adipose tissue (epi-WAT) in both models and in both sexes (Fig. 1A). To understand the functional relevance of the adipose STS induction and to determine the metabolic effect of STS in vivo, we generated tetracycline-inducible STS Tg mice overexpressing the human STS gene in the adipose tissue (Fig. 1B). The Tet-off Tg system was composed of two Tg lines with one line (TetRE-STS) expressing STS under the control of a minimal cytomegalovirus promoter fused to TetRE (24), and the other line (aP2-tTA) expressing tTA in the adipose tissue under the control of the aP2 gene promoter (25). In mice carrying both transgenes (aP2-STS), tTA would bind to TetRE and induce the expression of STS, whereas treatment with DOX would dissociate tTA from TetRE and thus silence the expression of STS. The overexpression of the Tg STS in epi-WAT and brown adipose tissue was confirmed by Western blotting (Fig. 1C). The transgene expression was undetectable in nontargeting tissues, including the liver and skeletal muscle (data not shown). The adipose expression of the transgene was also confirmed at the enzymatic level by using the tritium-labeled estrone sulfate as substrate (Fig. 1D). The adipose expression of the transgene was comparable between the male and female Tg mice (Fig. 1E).
Figure 1.
Adipose induction of STS in obese mice, and creation of Tg mice expressing STS in the adipose tissue. (A) The mRNA expression of mouse Sts in the epididymal WAT of chow-fed wild-type (Wt) mice, HFD-fed Wt mice, and chow-fed ob/ob mice. (B) The schematic representation of the Tet-off STS Tg system. (C) The expression of STS protein in the epi-WAT and brown adipose tissue (BAT) of the Wt and Tg mice was measured by Western blotting. (D) The adipose STS enzymatic activity was determined by estrone sulfate conversion assay and was normalized against protein concentrations. (E) The adipose expression of the STS transgene in female and male Tg mice was measured by real-time PCR. n = 4 mice per group. * or #, P < 0.05; ** or ##, P < 0.01; ***P < 0.001, compared with (A) chow of the sex, or (D) or compared with the Wt. Pmin, minimal human cytomegalovirus promoter; PolyA, polyacrylamide; SV40 polyA, simian virus 40 polyadenylation signal.
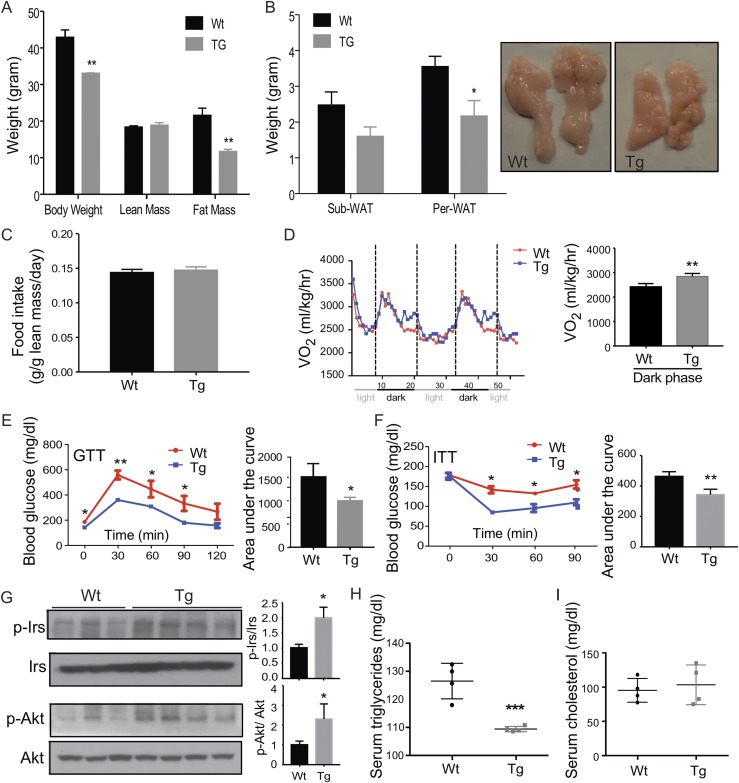
Adipose overexpression of STS aggravates HFD-induced adiposity, insulin resistance, and glucose intolerance in male mice
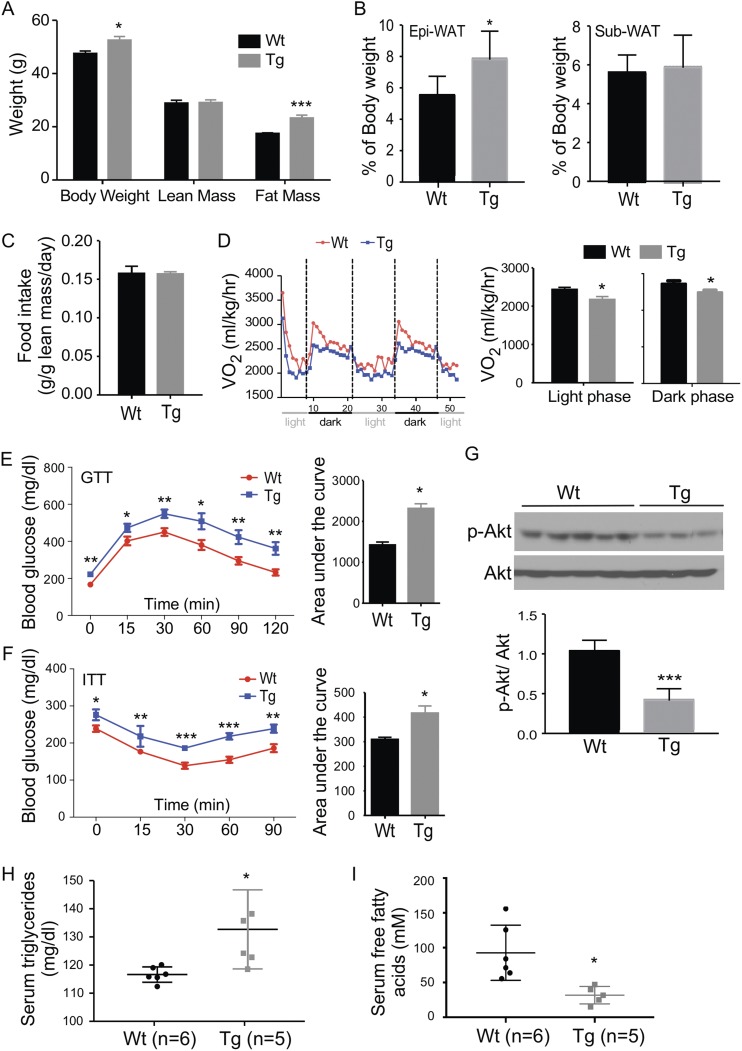
When challenged with HFD for 20 weeks, the male Tg mice showed a substantial increase in body weight compared with the male wild-type (Wt) littermates (Fig. 2A). Body composition analysis by magnetic resonance imaging revealed that the gain of body weight in the Tg males was largely accounted for by the increase of fat mass, whereas the lean mass was not affected (Fig. 2A). Further necropsy analysis showed that it was the mass of epi-WAT, but not the subcutaneous fat, that showed a substantial increase when presented as a ratio of the fat mass to the body weight (Fig. 2B), although the subcutaneous fat had a comparable expression of the STS transgene (data not shown). The body weight gain was achieved without substantial changes in food intake (Fig. 2C). Visceral (intra-abdominal) adipose tissue (mainly epi-WAT in males and perigonodal WAT in females) mass correlates with the development of insulin resistance, whereas the total or subcutaneous tissue mass does not (38). For these reasons, we primarily focused on epi-WAT in this study.
Figure 2.
Adipose overexpression of STS aggravates HFD-induced adiposity, insulin resistance, and glucose intolerance in male mice. All mice are males. Mice were fed with HFD for 20 weeks before analysis. Mice were analyzed for (A) body weight and body composition, (B) fat mass, (C) food intake, (D) oxygen consumption, (E) GTT, and (F) ITT. The quantifications of the GTT and ITT results are shown as the areas under the curve. (G) Western blot analysis of Akt phosphorylation in epi-WAT. Shown below is the densitometric quantification of the Western blotting results. The serum levels of (H) triglycerides and (I) free fatty acids. Results are expressed as mean ± SD. n = 4 mice per group, except those labeled in (H) and (I). *P < 0.05; **P < 0.01, compared with the Wt.
Metabolic cage analysis showed the oxygen consumption was significantly decreased in HFD-fed Tg males during both the light and dark phases (Fig. 2D). When the whole-body insulin sensitivity was evaluated, the Tg males showed fasting hyperglycemia and worse performance in the GTT (Fig. 2E) and ITT (Fig. 2F). Histological analysis of pancreatic sections showed no difference in islet size and total islet area in HFD-fed Wt and Tg males (Supplemental Fig. 1), suggesting that it was not the defect in β cells that accounted for the whole-body insulin resistance in Tg males. To directly assess the insulin sensitivity in the adipose tissue, we harvested and assessed epi-WAT for the phosphorylation of protein kinase B (Akt) by Western blotting. A decreased Akt phosphorylation was observed in epi-WAT, further suggesting insulin resistance in this tissue (Fig. 2G). The Tg males also showed an increased serum level of triglycerides (Fig. 2H), a decreased serum level of free fatty acids (Fig. 2I), but the serum level of cholesterol was not affected (data not shown).
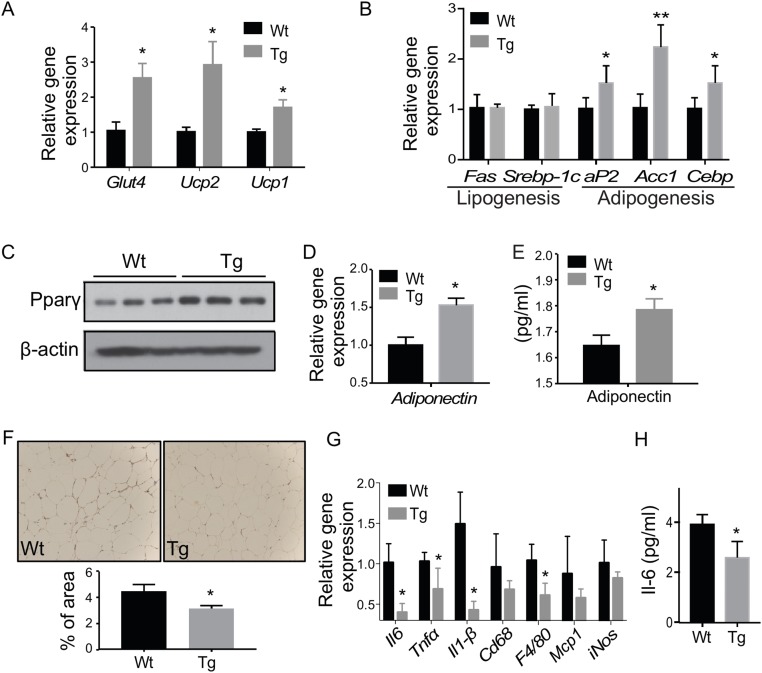
Adipose overexpression of STS decreases lipolysis and adipogenesis and aggravates HFD-induced adipose and systemic inflammation in males
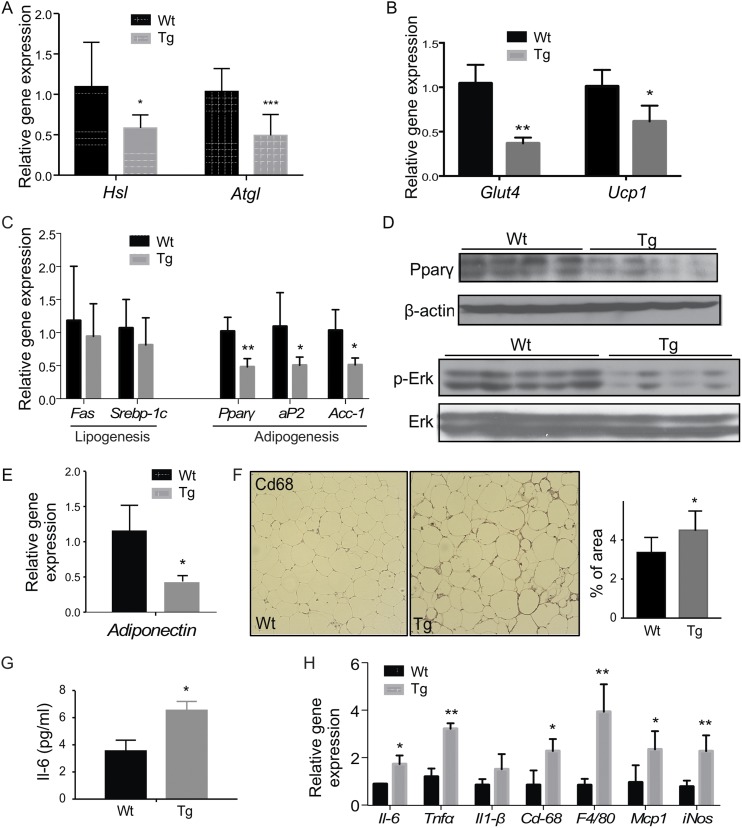
When the gene expression in epi-WAT was profiled, the Tg male showed a decreased expression of two key lipolytic lipases, hormone sensitive lipase and adipose triglyceride lipase (Fig. 3A), which was consistent with the increased epi-WAT fat mass and reduced level of serum free fatty acids. Additionally, the expression of the glucose uptake transporter Glut4 and uncoupling protein 1 responsible for energy expenditure was downregulated in the epi-WAT of the Tg males (Fig. 3B). There were no changes in the expression of lipogenic genes, but the expression adipogenic genes or genes indicative of adipogenesis was significantly decreased in the epi-WAT of Tg males (Fig. 3C). The downregulation of peroxisome proliferator activated receptor γ (PPARγ) and phosphorylated Erk was confirmed by Western blotting (Fig. 3D). PPARγ is a master regulator of adipogenesis (39), whereas the activation of Erk1/2 is required for the cell proliferation in the early phase of adipogenesis (40). Consistent with the inhibition of adipogenesis, the epi-WAT expression of the beneficial adipokine adiponectin was decreased in the Tg males (Fig. 3E), which may have contributed to the worse metabolic function in Tg males. In contrast, the expression of genes involved in lipid droplet formation (Supplemental Fig. 2A) and the fatty acid uptake transporter Cd36 (Supplemental Fig. 2B) was not affected by the transgene.
Figure 3.
Adipose overexpression of STS decreases lipolysis and adipogenesis and aggravates HFD-induced adipose and systemic inflammation in males. Mice are the same as described in Fig. 2. The epi-WAT expression of genes responsible for (A) lipolysis, (B) glucose uptake and energy expenditure, and (C) lipogenesis and adipogenesis was measured by real-time PCR. (D) The protein level of PPARγ and ERK1/2 was measured by Western blotting. (E) Epi-WAT expression of adiponectin. (F) Immunostaining of Cd68. Shown on the right is the quantification. The (G) serum level of IL-6 and (H) adipose expression on proinflammatory genes and macrophage marker genes. Results are expressed as mean ± SD; n = 4 mice per group. *P < 0.05; **P < 0.01; ***P < 0.005, compared with the Wt.
Obesity is commonly associated with chronic and low-grade inflammation primarily originating from excess adipose tissue, which often leads to elevated circulating levels of proinflammatory cytokines such as IL-6 and TNF-α (41). We then analyzed the epi-WAT local and systemic inflammation to determine whether the transgene aggravated fat tissue inflammation contributed to obesity-associated insulin resistance in the males. Immunostaining of Cd68, a macrophage marker gene, showed that the epi-WAT of Tg males had a substantially increased number and size of the crownlike structures compared with the Wt males (Fig. 3F). The average size of adipocytes in the epi-WAT of the Tg males was larger than that of the Wt mice (Supplemental Fig. 2C), a sign of adipocyte hypertrophy. Both the serum level of IL-6 (Fig. 3G) and the adipose expression of proinflammatory marker genes (Fig. 3H) were elevated in Tg males, which may have contributed to adipocyte hypertrophy, because the cytokine levels positively correlate with the adipocyte size (41).
The Tg effect was tissue specific because the hepatic expression of genes involved in gluconeogenesis (Supplemental Fig. 2D), lipogenesis (Supplemental Fig. 2E), and inflammation (Supplemental Fig. 2F) was not affected in the Tg males. The expression of genes involved in fatty acid oxidation in the skeletal muscle was not affected either (Supplemental Fig. 2G). The metabolic effect was also transgene dependent, because silencing of the transgene by treating the Tg males with DOX (Supplemental Fig. 3A) normalized the body weight and body composition (Supplemental Fig. 3B), oxygen consumption (Supplemental Fig. 3C), GTT and ITT (Supplemental Fig. 3D), and expression of genes involved in lipolysis and glucose uptake (Supplemental Fig. 3E), adipogenesis (Supplemental Fig. 3F), and inflammation (Supplemental Fig. 3G).
The metabolic phenotype in aP2-STS Tg males is androgen dependent
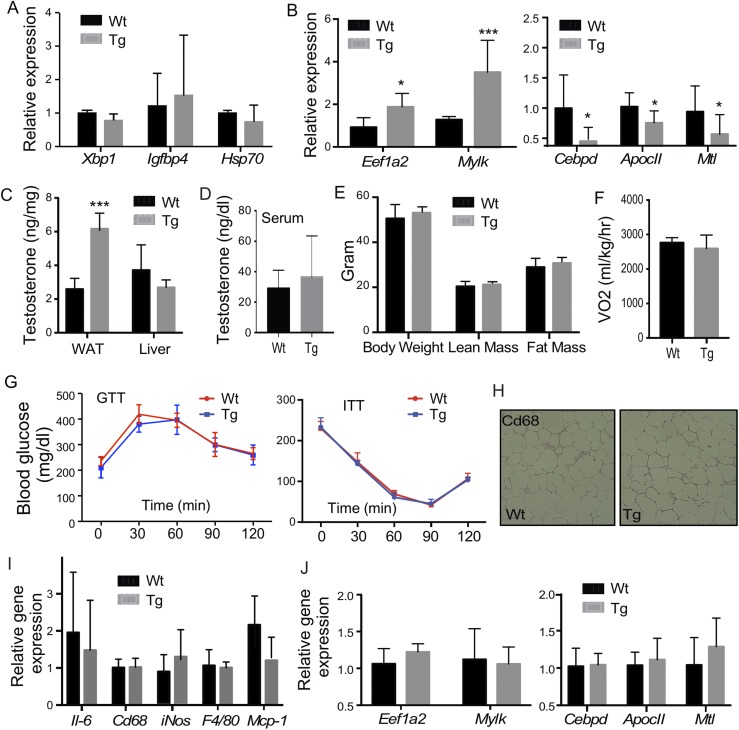
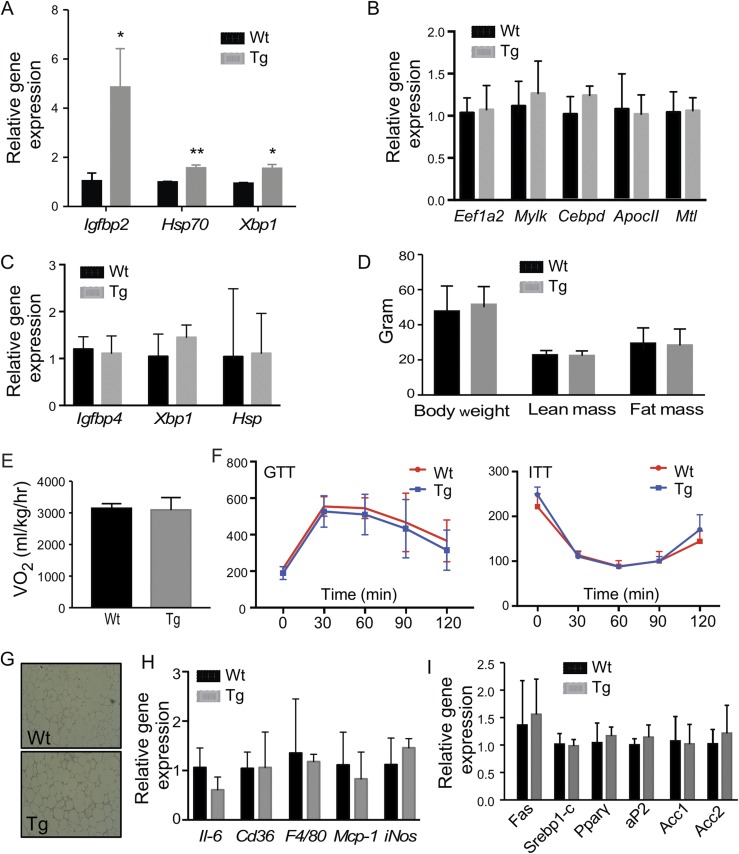
Because the primary function of STS is to desulfonate and reactivate estrogens and androgens, and estrogens and androgens are known to regulate the metabolic functions in rodents and humans (1, 2), we went on to determine whether the aggravation of metabolic function in the Tg males was due to increased estrogenic or androgenic activity in the adipose tissue. In HFD-fed mice, adipose expression of a panel of estrogen-responsive genes was not different between the Wt and Tg males (Fig. 4A). The adipose concentrations of estrone and estradiol in the male mice were low and beyond the detection limit in most of the samples (data not shown). When the androgen activity in the epi-WAT was measured, we found the expression of genes that are positively regulated by androgens, such as Eefia2 and Mylk (42), were increased in Tg males, whereas the expression of genes known to be suppressed by androgens, such as Cebpd, Apoc2, and Mtl (42), were significantly decreased in Tg males (Fig. 4B). The gene regulation was adipose specific, because the expression of androgen responsive genes was not affected in the liver and skeletal muscle of the Tg males (Supplemental Fig. 4). Consistent with the pattern of gene regulation, the tissue level of testosterone in the epi-WAT, but not the liver, was elevated in the Tg males (Fig. 4C), but the circulating level of testosterone was not affected (Fig. 4D).
Figure 4.
The metabolic phenotype in aP2-STS Tg males is androgen dependent. Mice are the same as described in Fig. 2. Shown are (A) adipose expression of estrogen-responsive genes, (B) adipose expression of androgen-responsive genes, (C) adipose and liver levels of testosterone, and (D) the serum levels of testosterone. Male mice were castrated before being fed with HFD for 20 weeks. Shown are (E) body weight and body composition, (F) oxygen consumption, (G) GTT and ITT, (H) immunostaining of CD68, (I) adipose expression of proinflammatory genes and macrophage marker genes, and (J) adipose expression of androgen responsive genes. Results are expressed as mean ± SD; n = 4 mice per group for all panels, except (B) and (C): Wt, n = 6; Tg, n = 5. *P < 0.05; **P < 0.01; ***P < 0.005, compared with the Wt.
To further evaluate whether the Tg phenotype was androgen dependent, we performed castration on 4-week-old males to remove the primary source of androgen. The castrated mice were then challenged with HFD for 20 weeks. Castration had little effect on the expression of the transgene (data not shown), but it abolished the Tg phenotypes in body weight gain and fat mass gain (Fig. 4E), oxygen consumption (Fig. 4F), GTT and ITT (Fig. 4G), and adipose inflammation at the histological (Fig. 4H) and inflammatory gene expression (Fig. 4I) levels. The regulation of androgen-responsive genes was normalized upon castration as expected (Fig. 4J). These results suggested that the adverse metabolic effects in Tg males were androgen dependent.
Adipose overexpression of STS improves metabolic function in HFD-fed females
Interestingly, the adipose STS Tg effect was sex specific, because the phenotypes in Tg females were totally opposite to those observed in Tg males. Specifically, the Tg females showed a reduced body weight gain, which was due to reduced fat mass (Fig. 5A). The mass of perigonodal adipose tissue (peri-WAT), but not the subcutaneous WAT, was decreased in Tg females (Fig. 5B). The food intake was not affected by the transgene (Fig. 5C). The oxygen consumption in the dark phase was significantly increased (Fig. 5D). The Tg females also showed a significantly lower fasting glucose level and better performance in GTT (Fig. 5E) and ITT (Fig. 5F). The peri-WAT levels of phosphorylated Irs-1 and Akt were increased in the Tg females (Fig. 5G), further suggesting an improved insulin sensitivity. Histological analysis of the pancreas showed no difference in total islet area and islet size (data not shown). The Tg females also showed a reduced serum level of triglycerides (Fig. 5H), but no change in the serum cholesterol (Fig. 5I).
Figure 5.
Adipose overexpression of STS improves metabolic functions in HFD-fed females. All mice are females. Mice were fed with HFD for 20 weeks before analysis. Mice were analyzed for (A) body weight and body composition, (B) fat mass, (C) food intake, (D) oxygen consumption, (E) GTT, and (F) ITT. The quantifications of the GTT and ITT results are shown as the areas under the curve. (G) Western blot analysis of Irs-1 and Akt phosphorylation in epi-WAT. Shown on the right are the densitometric quantifications of the Western blotting results. The serum levels of (H) triglycerides and (I) cholesterol. Results are expressed as mean ± SD; n = 4 mice per group. *P < 0.05; **P < 0.01, compared with the Wt. sub-WAT, subcutaneous WAT.
Adipose overexpression of STS increases energy expenditure and adipogenesis and ameliorates HFD-induced adipose and systemic inflammation in females
The Tg females showed increased expression of glucose uptake transporter Glut4 and uncoupling proteins in the peri-WAT (Fig. 6A). The expression of adipogenic genes was increased in the peri-WAT of Tg females (Fig. 6B), opposite to the suppression in the Tg males. The induction of PPARγ was confirmed at the protein level by Western blotting (Fig. 6C). Both the peri-WAT expression (Fig. 6D) and circulating level of adiponectin (Fig. 6E) were elevated in the Tg females. Consistent with improved metabolic functions and insulin resistance, Tg females showed attenuated HFD-induced adipose and systemic inflammation, as evidenced by decreased crownlike structures (Fig. 6F), decreased expression of proinflammatory genes (Fig. 6G), and a decreased circulating level of Il-6 (Fig. 6H).
Figure 6.
Adipose overexpression of STS increases energy expenditure and adipogenesis and ameliorates HFD-induced adipose and systemic inflammation in females. Mice are the same as described in Fig. 5. (A) Adipose expression of genes responsible for energy uptake and expenditure, and (B) lipogenesis and adipogenesis was measured by real-time PCR. (C) The protein level of PPARγ was measured by Western blotting. The (D) adipose mRNA expression of adiponectin and (E) the serum level of adiponectin. (F) Immunostaining of Cd68. Shown below is the quantification. Adipose expression of (G) proinflammatory genes and macrophage marker genes and (H) the serum level of IL-6. Results are expressed as mean ± SD; n = 4 mice per group. *P < 0.05; **P < 0.01, compared with the Wt.
The metabolic benefit in female Tg mice is estrogen dependent
In understanding the metabolic benefit of the Tg females, we found the expression of estrogen-responsive genes (Fig. 7A), but not the androgen-responsive gene (Fig. 7B), was induced in the peri-WAT. To further determine whether the metabolic benefit was estrogen dependent, we eliminated the primary source of endogenous estrogens from 4-week-old prepubertal female mice by ovariectomy before challenging them with HFD for 20 weeks. Upon ovariectomy, the expression of estrogen responsive genes in the peri-WAT of Tg females no longer showed changes (Fig. 7C), whereas the expression of the STS transgene was not affected (data not shown). Ovariectomy completely abolished the metabolic benefit of the STS transgene in body weight and body composition (Fig. 7D), oxygen consumption (Fig. 7E), GTT and ITT (Fig. 7F), adipose inflammation at the histological (Fig. 7G) and inflammatory gene expression levels (Fig. 7H), and the expression of adipogenic genes (Fig. 7I). These data showed that the metabolic benefit of Tg females was mediated through the estrogen signaling pathway.
Figure 7.
The metabolic benefit in female Tg mice is estrogen dependent. Mice are the same as described in Fig. 5. Shown are adipose expression of (A) estrogen-responsive genes and (B) androgen-responsive genes as measured by real-time PCR. Female mice were ovariectomized before being fed with HFD for 20 weeks. Shown are (C) adipose expression of estrogen-responsive genes, (D) body weight and body composition analysis, (E) oxygen consumption, (F) GTT and ITT, (G) immunostaining of CD68, (H) adipose expression of proinflammatory genes and macrophage marker genes, and (I) genes involved in adipogenesis and lipogenesis. Results are expressed as mean ± SD; n = 4 mice per group. *P < 0.05; **P < 0.01, compared with the Wt.
Discussion
In this study, we reported the adipose induction of Sts in mouse models of obesity and insulin resistance. Based on our results, the adipose induction of Sts in response to metabolic stress appears to have a sex-specific outcome: it may represent a protective response in females as a consequence of increased estrogen activity in the adipose tissue; whereas in males, the induction may have exacerbated the metabolic harm in an androgen- and inflammation-dependent manner. Under the control of the aP2 gene promoter, the STS transgene had the most obvious effect in epi-WAT, whereas the subcutaneous WAT and brown adipose tissue showed little appreciable microscopic and functional phenotypic changes although the transgene was efficiently expressed in these two fat depots (data not shown). The fat depot–specific effect of the STS transgene remains to be understood.
One of the most interesting observations is the sexually dimorphic effect of STS in the adipose tissue. Sexual dimorphisms have been documented in many aspects of metabolic syndrome, ranging from fat distribution to sex hormone levels. We found that overexpression of STS in the adipose tissue of male mice aggravated HFD-induced obesity, insulin resistance, and inflammation. The worsened metabolic functions in the Tg males likely resulted from induced androgen reactivation and increased androgenic activity in the adipose tissue, whereas castration abolished these adverse effects. These results underscored the importance of adipose androgen signaling in energy homeostasis. It has been suggested that aromatization of testosterone into estrogens is critical to energy homeostasis in males. However, our results showed the STS transgene had little effect on the adipose expression of estrogen-responsive genes and adipose tissue levels of estrogens in Tg males, indicating that the phenotype was specifically caused by increased androgen signaling. The HFD-fed Tg females, on the other hand, exhibited improved metabolic functions that included the relief of insulin resistance and adipose inflammation. The metabolic benefit of STS in female mice was estrogen dependent because the responsive genes of estrogens but not androgens were induced in the adipose tissue. Moreover, ovariectomy abolished the metabolic benefit of the STS transgene, further suggesting that estrogens have mediated the metabolic benefit in Tg females.
Another interesting finding is the sex hormone dependence of the phenotype. The primary function of the STS enzyme is to convert androgen sulfates and estrogen sulfates to hormonally active androgens and estrogens. By prediction, the androgen and estrogen levels should be increased in the adipose tissue of the Tg mice. We have previously reported the role of STS in energy homeostasis through its sulfation of steroids (24, 43). However, our previous work has largely focused on the role of STS and its regulation in estrogen homeostasis (24, 44). The current work focuses on the role of STS in androgen conversion. Generally, androgens are believed to play opposite roles in energy homeostasis in males and females. It has been reported that a deficiency of androgen worsens metabolic functions in males (13, 14), whereas androgen excess worsens metabolic functions in females. Androgens are known to confer metabolic benefits in many tissues in males, including the liver, pancreatic β cells, and skeletal muscle. In the adipose tissue, there is an inverse correlation between total serum testosterone and the amount of visceral adipose tissue (20). On one hand, this could be explained by the conversion of androgens into estrogens by aromatase to provide estrogens in the adipose tissue. Indeed, orchidectomized male rodents treated with either testosterone or estrogen remain lean, whereas those treated with the dihydrotestosterone, which cannot be converted to estrogen, develop obesity (45). This is also true in men for whom testosterone replacement suppresses adiposity, and this effect is blocked in the presence of an aromatase inhibitor (46). On the other hand, most of the reported effects of androgens on adipose tissue in males were believed to be indirectly mediated by AR signaling in the skeletal muscle (21, 22). One of a few studies focusing on the specific role of androgens in adipose tissue is using adipocyte-specific AR knockout mice, in which these mice showed no difference in subcutaneous and epididymal fat mass in either a chow-fed condition or HFD condition (47). AR can also negatively regulate the production of adiponectin (48, 49), a beneficial adipokine that enhances insulin sensitivity and reduces chronic inflammation (50). Consistent with these notions, the adipose expression of adiponectin and the circulating level of adiponectin were decreased in the Tg males, which may have contributed to the metabolic harm of the transgene in this sex. Androgens are known for their antiadipogenic activity (51), which may have helped to explain the decreased expression of adipogenic genes in the adipose tissue of the Tg males. Taken together, our results have demonstrated the direct effects of STS and androgens on the adipose tissue of males.
It is also interesting to note that the sexually dimorphic effect of STS is also tissue specific. We have previously reported that overexpression of STS in the liver of Tg mice alleviated HFD and ob/ob models of obesity and type 2 diabetes in both sexes (24). Interestingly, although the metabolic benefits of hepatic STS were conserved in both sexes, it appeared that STS exerted its metabolic benefit through sex-specific mechanisms. In female mice, STS may have increased hepatic estrogen activity by converting estrogen sulfates to active estrogens and consequently improved the metabolic functions, whereas ovariectomy abolished this protective effect. In contrast, the metabolic benefit of hepatic STS in males may have been accounted for by the male-specific decrease of inflammation in white adipose tissue and skeletal muscle, as well as a pattern of skeletal muscle gene expression that favors energy expenditure. The metabolic benefit of the hepatic STS in male mice was intact upon castration. A few notable differences between the adipose STS and hepatic STS Tg mice include: (1) the adipose STS was protective in females but sensitized males to metabolic stress, whereas the hepatic STS was protective in both sexes; (2) within the male sex, the sensitizing effect of adipose STS was androgen dependent, whereas the protective effect of hepatic STS remains to be defined, because the protective effect was intact upon castration; and (3) also within the male sex, the adipose STS exacerbated adipose inflammation, whereas the hepatic STS attenuated adipose inflammation.
Interestingly, the metabolic effect of the estrogen sulfotransferase (EST) is also tissue- and sex-specific. EST sulfonates estrogens and converts them to hormonally inactive estrogen sulfates. We have previously reported that the induction of hepatic Est is a common feature of several mouse models of type 2 diabetes. Loss of Est in female mice improved metabolic function in ob/ob mice as a result of decreased estrogen deprivation and increased estrogenic activity in the liver. Interestingly, the effect of Est ablation was sex specific; Est ablation in ob/ob males exacerbated the diabetic phenotype, which was accounted for by increased inflammation in the white adipose tissue, as well as decreased islet β-cell mass and failure of glucose-stimulated insulin secretion (26). Interestingly, although the diabetes induction of Est was liver specific, Tg reconstitution of EST in the adipose tissue, but not in the liver, attenuated diabetic phenotype in male ob/ob mice deficient of Est (27). The metabolic benefit of adipose reconstitution of Est was sex specific, because adipose reconstitution of Est in female ob/ob mice deficient of Est had little effect (27).
In summary, this study uncovered a sexually dimorphic and sex hormone–dependent role of STS in adipose inflammation and energy homeostasis. The adipose STS may represent a therapeutic target for the management of obesity and type 2 diabetes. It is encouraging that major progresses have been made in the development of pharmacological modulators of STS (52).
Supplementary Material
Acknowledgments
Li Gao is thanked for her technical assistance. Dr. Robert O’Doherty (University of Pittsburgh) is thanked for the use of metabolic cages at the University of Pittsburgh Center for Metabolism and Mitochondrial Medicine (C3M).
Financial Support: This work was supported in part by National Institutes of Health Grants DK083952 and ES023438 (to W.X.). W.X. is supported in part by the Joseph Koslow Endowed Chair Professorship from the University of Pittsburgh School of Pharmacy.
Author Contributions: Y.B. and W.X. participated in research design. Y.B., M.J., W.G., X.G., M.X., S.R., N.W.G., and K.W.S. conducted experiments. Y.B. and W.X. performed data analysis. Y.B., D.Y., and W.X. wrote or contributed to writing of the manuscript. W.X. acquired funding. Y.B. and W.X. are guarantors; as such, they have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Akt
protein kinase B
- aP2
fatty acid binding protein 4
- AR
androgen receptor
- DOX
doxycycline
- epi-WAT
epididymal white adipose tissue
- ERα
estrogen receptor α
- EST
estrogen sulfotransferase
- GTT
glucose tolerance test
- HFD
high-fat diet
- ITT
insulin tolerance test
- peri-WAT
perigonodal adipose tissue
- PPARγ
peroxisome proliferator activated receptor γ
- STS
steroid sulfatase
- TetRE
tetracycline response element
- Tg
transgenic
- tTA
tetracycline-transcriptional activator
- Wt
wild-type
References
- 1. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL; Rancho Bernardo Study . Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25(1):55–60. [DOI] [PubMed] [Google Scholar]
- 2. Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring). 2015;23(4):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. [DOI] [PubMed] [Google Scholar]
- 4. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 5. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. [DOI] [PubMed] [Google Scholar]
- 6. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97(23):12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 9. Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20(6):1287–1299. [DOI] [PubMed] [Google Scholar]
- 10. Holmäng A, Larsson BM, Brzezinska Z, Björntorp P. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol. 1992;262(6 Pt 1):E851–E855. [DOI] [PubMed] [Google Scholar]
- 11. O’Meara NM, Blackman JD, Ehrmann DA, Barnes RB, Jaspan JB, Rosenfield RL, Polonsky KS. Defects in beta-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76(5):1241–1247. [DOI] [PubMed] [Google Scholar]
- 12. Nohara K, Laque A, Allard C, Münzberg H, Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring). 2014;22(6):1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96(8):2341–2353. [DOI] [PubMed] [Google Scholar]
- 14. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. [DOI] [PubMed] [Google Scholar]
- 15. Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47(6):1924–1935. [DOI] [PubMed] [Google Scholar]
- 16. Palomar-Morales M, Morimoto S, Mendoza-Rodríguez CA, Cerbón MA. The protective effect of testosterone on streptozotocin-induced apoptosis in beta cells is sex specific. Pancreas. 2010;39(2):193–200. [DOI] [PubMed] [Google Scholar]
- 17. Haren MT, Siddiqui AM, Armbrecht HJ, Kevorkian RT, Kim MJ, Haas MJ, Mazza A, Kumar VB, Green M, Banks WA, Morley JE. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl. 2011;34(1):55–68. [DOI] [PubMed] [Google Scholar]
- 18. Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28(7):1636–1642. [DOI] [PubMed] [Google Scholar]
- 19. Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54(4):1000–1008. [DOI] [PubMed] [Google Scholar]
- 20. Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol. 1992;2(5):675–682. [DOI] [PubMed] [Google Scholar]
- 21. Gentile MA, Nantermet PV, Vogel RL, Phillips R, Holder D, Hodor P, Cheng C, Dai H, Freedman LP, Ray WJ. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of beta-catenin. J Mol Endocrinol. 2010;44(1):55–73. [DOI] [PubMed] [Google Scholar]
- 22. Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA. Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology. 2010;151(7):3125–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26(2):171–202. [DOI] [PubMed] [Google Scholar]
- 24. Jiang M, He J, Kucera H, Gaikwad NW, Zhang B, Xu M, O’Doherty RM, Selcer KW, Xie W. Hepatic overexpression of steroid sulfatase ameliorates mouse models of obesity and type 2 diabetes through sex-specific mechanisms. J Biol Chem. 2014;289(12):8086–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wada T, Ihunnah CA, Gao J, Chai X, Zeng S, Philips BJ, Rubin JP, Marra KG, Xie W. Estrogen sulfotransferase inhibits adipocyte differentiation. Mol Endocrinol. 2011;25(9):1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao J, He J, Zhai Y, Wada T, Xie W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009;284(38):25984–25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garbacz WG, Jiang M, Xu M, Yamauchi J, Dong HH, Xie W. Sex- and tissue-specific role of estrogen sulfotransferase in energy homeostasis and insulin sensitivity. Endocrinology. 2017;158(11):4093–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. RRID: AB_2314619.
- 29. RRID: AB_329827.
- 30. RRID: AB_329825.
- 31. RRID: AB_330333.
- 32. RRID: AB_297058.
- 33. RRID: AB_2136584.
- 34. RRID: AB_2140110.
- 35. RRID: AB_627545.
- 36. RRID: AB_476692.
- 37. RRID: AB_2074854.
- 38. Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13(9):795–803. [DOI] [PubMed] [Google Scholar]
- 39. Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. [DOI] [PubMed] [Google Scholar]
- 40. Sale EM, Atkinson PG, Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14(4):674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mothe-Satney I, Filloux C, Amghar H, Pons C, Bourlier V, Galitzky J, Grimaldi PA, Féral CC, Bouloumié A, Van Obberghen E, Neels JG. Adipocytes secrete leukotrienes: contribution to obesity-associated inflammation and insulin resistance in mice. Diabetes. 2012;61(9):2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Calvo E, Martel C, Luu-The V, Labrie F, Tchernof A. Response of the adipose tissue transcriptome to dihydrotestosterone in mice. Physiol Genomics. 2008;35(3):254–261. [DOI] [PubMed] [Google Scholar]
- 43. Bi Y, Shi X, Zhu J, Guan X, Garbacz WG, Huang Y, Gao L, Yan J, Xu M, Ren S, Ren S, Liu Y, Ma X, Li S, Xie W. Regulation of cholesterol sulfotransferase SULT2B1b by hepatocyte nuclear factor 4alpha constitutes a negative feedback control of hepatic gluconeogenesis. Mol Cell Biol. 2018;38(7):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang M, Klein M, Zanger UM, Mohammad MK, Cave MC, Gaikwad NW, Dias NJ, Selcer KW, Guo Y, He J, Zhang X, Shen Q, Qin W, Li J, Li S, Xie W. Inflammatory regulation of steroid sulfatase: A novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. J Hepatol. 2016;64(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Movérare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, Swanson C, Vanderschueren D, Oscarsson J, Gustafsson JA, Ohlsson C. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring). 2006;14(4):662–672. [DOI] [PubMed] [Google Scholar]
- 46. Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, Yeh S, Chang C. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149(5):2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf). 2004;60(4):500–507. [DOI] [PubMed] [Google Scholar]
- 49. Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–2741. [DOI] [PubMed] [Google Scholar]
- 50. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. [DOI] [PubMed] [Google Scholar]
- 51. Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol Endocrinol. 2005;19(10):2437–2450. [DOI] [PubMed] [Google Scholar]
- 52. Potter BVL. Sulfation pathways : Steroid sulfatase inhibition via aryl sulfamates: clinical progress, mechanism and future prospects. J Mol Endocrinol. 2018;61(2):T233–T252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.