Abstract
Apoptotic cells contain nuclear autoantigens that may initiate a systemic autoimmune response. To explore the mechanism of antibody binding to apoptotic cells, 3H9, a murine autoantibody with dual specificity for phospholipids and DNA, was used. H chain mutants of 3H9 were constructed, expressed as single-chain Fv (scFv) in Escherichia coli, and assessed for binding to phosphatidylserine, an antigen expressed on apoptotic cells. Both 3H9 and its germline revertant bound to dioleoyl phosphatidylserine in ELISA, and binding was enhanced by β2 glycoprotein I (β2GPI), a plasma protein that selectively binds to apoptotic cells. Higher relative affinity for DOPS-β2GPI was achieved by the introduction of Arg residues into the 3H9 H chain variable region at positions previously shown to mediate DNA binding. Specificity of the two structurally most diverse scFv for apoptotic cells was shown by flow cytometry, and two populations of scFv-bound cells were identified by differences in propidium iodide staining. The results suggest that, in autoimmunity, B cells with Ig receptors for apoptotic cells and DNA are positively selected, and that the antibodies they produce have the potential to affect the clearance and processing of apoptotic cells.
Cells undergoing apoptosis package autoantigens, including nuclear proteins and DNA, in vesicles located beneath the surface of the plasma membrane (1). The contents of such surface vesicles may serve to enforce tolerance or, under a different set of circumstances, they may induce an autoimmune response (2–4). However, it is not obvious how autoantigens from apoptotic cells might activate the adaptive immune system, given that recognition and clearance of dying cells by mononuclear scavenger cells, such as monocytes and macrophages, is rapid and noninflammatory (5).
One of the earliest signals for the uptake of apoptotic cells by phagocytes is the exposure of phosphatidylserine on the outer membrane leaflet, a consequence of the loss of membrane asymmetry in apoptotic cells (6). Phagocyte recognition of apoptotic cells occurs by several alternative mechanisms, including binding via the phosphatidylserine receptor (7), class A scavenger receptors (8), vitronectin receptors (9), macrosialin (10), CD14 (11), and CD36 (12). Moreover, serum proteins such as β2 glycoprotein I (β2GPI; refs. 13 and 14), and C1q, the first component of complement (15), bind apoptotic cells and enhance their uptake.
An important clue linking cell death to the onset of autoimmunity is provided by autoantibodies that bind apoptotic cells (16) and recognize surface epitopes that include complexes of phospholipid and β2GPI (17–19). The role of such autoantibodies is not known, although it has been postulated that they may preserve tissue homeostasis by enhancing the removal of dead or dying cells (20). In autoimmunity, it is possible that autoantibodies to apoptotic cells arise secondary to an increased load of apoptotic cells (18, 21), a decreased capacity for apoptotic cell removal (22, 23), or other as yet unknown stimuli. A detailed structural analysis of antibodies that bind to the apoptotic cell surface may distinguish among possible mechanisms responsible for the induction of B cells with this specificity.
To explore mechanisms of antibody binding to apoptotic cells, we expressed previously constructed site-directed mutants of 3H9, a murine anti-DNA and anti-phospholipid antibody (Fig. 1; refs. 24 and 25) as single-chain Fv (scFv) and tested for binding to dioleoyl phosphatidylserine (DOPS) and DOPS-β2GPI complexes. In addition, the two structurally most diverse 3H9 variants were examined for binding to apoptotic Jurkat cells. Our results identify H chain mutations that increase the relative affinity for DOPS and DOPS-β2GPI and show that somatic mutations enhance binding to DOPS-β2GPI and apoptotic cells.
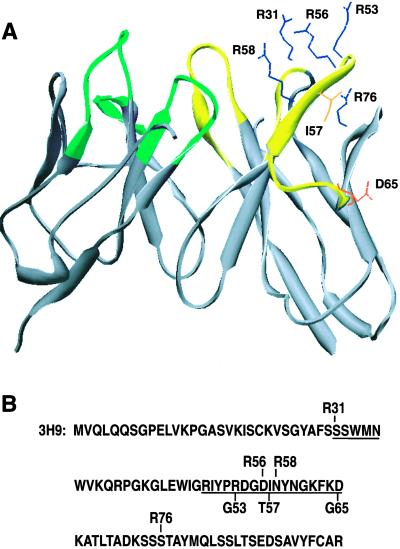
Figure 1.
3H9 residues that were subjected to site-directed mutagenesis. (A) Model of the 3H9 combining site highlighting sidechains exchanged by mutagenesis. Ile-57 (I57), Asp-65 (D65), and Arg-53 (R53) were reverted to germline, and individual Arg residues were introduced at positions 31 (R31), 56 (R56), 58 (R58), and 76 (R76). The structure was simplified to a ribbon diagram, except for the sidechains affected by mutagenesis. A view was chosen to illustrate the clustering of Arg residues that arise by recurrent somatic replacement mutations in autoantibodies to DNA and phospholipids. (B) Sequence of the 3H9 H chain indicating the position of mutated amino acid residues. Germline reversions are shown below the sequence of 3H9, whereas forward mutations are indicated above the sequence. Underlined amino acids are part of CDR1 or CDR2.
Experimental Procedures
Comparative ELISA.
Anti-phospholipid scFv-expression vectors were constructed, and scFv expression and purification were performed as described (26). Details of these procedures and scFv purification results can be found in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org.
Immulon 2 microtiter plates (Dynex Technologies, Chantilly, VA) were coated with DOPS (Sigma) at 10 μg/ml in ethanol and dried for 16 h under vacuum. Plates were blocked with PBS containing 0.5% gelatin (PBS/gel) or PBS/gel with 10 μg/ml purified human β2GPI (Crystal Chem, Chicago). Serial dilutions of scFv in PBS/gel were applied to wells, incubated for 1 h, and unbound scFv were removed by washing. Bound scFv were detected with alkaline phosphatase-conjugated human serum IgG (Jackson ImmunoResearch). After washing and the addition of para-nitrophenyl phosphate (Sigma), absorbance was measured at 405 nm.
The inhibition assays were carried out with microtiter plates coated with DOPS and blocked with PBS/gel containing 10 μg/ml β2GPI. DOPS vesicles were prepared by drying under vacuum and hydrating the lipid in PBS by vortexing. Vesicles were incubated with 200 μg/ml β2GPI for 1 h at room temperature. In parallel, 9 μg/ml R53G/I57T/D65G or 2 μg/ml D56R/S76R were incubated with increasing amounts of DOPS-β2GPI vesicles, calf-thymus DNA (Sigma) digested to an average of 2 kb with S1 nuclease (Boehringer Mannheim) or PBS as a control. After a 1-h incubation, vesicles were removed from solution by centrifugation for 30 min at 14,000 × g. The supernatant containing unbound scFv was applied to the wells containing DOPS-β2GPI, and scFv were incubated for 1 h. DNA-scFv complexes were not removed from solution before binding to DOPS-β2GPI. Unbound scFv were removed by washing, and bound scFv were detected as described above.
Flow Cytometry.
Jurkat cells were harvested from culture, resuspended at a density of 106 cells per ml in RPMI medium 1640 (Mediatech, Herndon, VA) containing 10% FBS and 1.0 μM staurosporine (Sigma) and treated for 16 h at 37°C to induce apoptosis. After treatment, 5 × 105 cells were divided into aliquots, placed into tubes, and washed with 4.0 ml of ice-cold Hanks' balanced salt solution (Mediatech) containing 1.0 mM CaCl2, 3% FBS, and 0.02% NaN3. Washed cells were incubated with 10 μg/ml of D56R/S76R, R53G/I57T/D65G, or 4VH/1VL (27) scFv for 15 min on ice and washed twice as above, followed by staining with FITC-conjugated annexin V (BD Biosciences, San Diego) and allophycocyanin-conjugated rabbit IgG (Molecular Probes), as recommended by the manufacturers. Before analysis on a FACSCalibur (BD Biosciences), cells were stained with 5 μg/ml of propidium iodide (PI). Thirty thousand events were collected per sample and analyzed with FLOWJO software (Treestar, San Carlos, CA).
Statistical Analysis.
Dose-response curves were generated with GRAPHPAD PRISM software (GraphPad, San Diego). Curve midpoints were determined by nonlinear regression with a variable slope and constant maximal absorbance value. Student's t test was used to determine the statistical significance of the differences between binding-curve midpoints.
Molecular Modeling.
The structure of the 3H9 scFv containing all forward mutations to Arg was modeled with the combined algorithm for antibody framework alignment and complementarity-determining region (CDR)-loop homology modeling, as described by Martin et al. (28). The optimized model coordinates were displayed with the Swiss-Pdb Viewer (http://www.expasy.ch/spdbv/mainpage.html).
Results
Role of H Chain Somatic Mutations in Phosphatidylserine Recognition.
After activation of a B cell, clonal expansion can be traced by features of antigen selection, including isotype switching and the accumulation of somatic mutations that increase the relative affinity for the antigen. By comparison to germline variable (V) genes, we have determined that the anti-DNA and anti-phospholipid autoantibody 3H9 is encoded by an immunodominant J558 VH gene used repeatedly in autoantibodies from murine models of systemic lupus erythematosus (29). The 3H9 H chain acquired three somatic replacement mutations in CDR2: Gly to Asp at position 65, Thr to Ile at position 57, and Gly to Arg at position 53 (Fig. 1). The role of these mutations in DNA binding has been tested (24). To evaluate the role of mutations in shaping the antibody response to phosphatidylserine, the mutations were reverted to germline either individually or as a group, expressed as scFv fusion proteins in Escherichia coli, and purified (see Fig. 5).
All scFv, except for the Arg to Gly revertant (R53G), exhibited detectable binding to DOPS, although 3H9 bound with low relative affinity (Fig. 2A). Individual reversions of the Ile at position 57 (I57T) or the Asp at position 65 (D65G) resulted in a 3-fold increase in relative affinity compared with 3H9. The combined reversion of all three mutations also resulted in a 3-fold increase in relative affinity (Table 1). These results indicate that although the germline-encoded VH is suitable for binding to phosphatidylserine, the somatic mutations that occurred during clonal expansion of 3H9 decreased this affinity. However, the mutation to Arg at position 53, a later event in the evolution of the B cell clone toward 3H9 (30), resulted in an increased affinity for DOPS.
Figure 2.
Phosphatidylserine ELISA of 3H9 and variants. (A and B) Binding of purified scFv to DOPS in solid phase ELISA. (C and D) Binding to DOPS-β2GPI complexes. Absorbance readings corresponding to the indicated concentrations of scFv were taken at 405 nm. (A and C) Summary of results for 3H9 (●) and its revertants: R53G (■), I57T (▾), D65G (▴), and R53G/I57T/D65G (♦). (B and D) Summary of results for 3H9 with forward mutations to Arg: S31R (○), D56R (□), N58R (▵), S76R(⋄), and D56R/S76R (▿). The means and SE of duplicate samples from one representative experiment are shown.
Table 1.
Binding of scFv to DOPS or DOPS-β2GPI
| Antibody | DOPS ± SD* | DOPS-β2GPI ± SD* |
|---|---|---|
| 3H9 | 31.33 ± 0.18 | 20.09 ± 0.16‡ |
| R53G | ND§ | ND§ |
| I57T | 11.78 ± 0.11† | 9.59 ± 0.12†‡ |
| D65G | 12.56 ± 0.15† | 9.08 ± 0.23†‡ |
| R53G/I57T/D65G | 11.72 ± 0.12† | 8.85 ± 0.11†‡ |
| S31R | 9.55 ± 0.08† | 3.44 ± 0.25†‡ |
| D56R | 8.36 ± 0.08† | 3.17 ± 0.26†‡ |
| N58R | 11.83 ± 0.12† | 6.75 ± 0.20†‡ |
| S76R | 10.30 ± 0.14† | 5.89 ± 0.11†‡ |
| D56R/S76R | 5.62 ± 0.22† | 2.01 ± 0.30†‡ |
Concentrations of scFv (μg/ml) that give 50% maximal binding are listed.
Significant change from 3H9 (P < 0.05).
Differences in binding between DOPS and DOPS-β2GPI are significant (P < 0.05).
Not detected.
In addition to Arg-53, Arg residues at other sites within the CDR1, CDR2, and one unique location in the third framework region of anti-DNA H chain V genes create or enhance binding to DNA (29). Notably, Arg residues have also been observed at the same positions within the combining site of antibodies with dual reactivity with DNA and phospholipids (31, 32). To examine the role of Arg mutations in binding to DOPS, we introduced Args at the equivalent sites within the H chain of 3H9 (Fig. 2B). The single Arg mutations at positions 31 (S31R), 56 (D56R), 58 (N58R), and 76 (S76R) resulted in a 3- to 4-fold increase in relative affinity compared with 3H9 (Table 1). In addition, the combination of D56R and S76R raised the relative affinity for DOPS 6-fold above 3H9 and 2-fold above each respective mutation alone. These results demonstrate that each of the Args individually participates in binding to DOPS, and that at least two of the Args can be combined to produce an additive increase in DOPS binding.
Antibody Binding to DOPS Is Enhanced by β2GPI.
Antibodies to phospholipids often show enhanced binding to complexes of anionic phospholipids and β2GPI (33). To evaluate the role of β2GPI in the binding of our scFv to DOPS, we performed the binding assays in the presence of human β2GPI (Fig. 2 C and D).
The I57T, D65G, and germline revertants, as well as 3H9, bound to DOPS-β2GPI with significantly higher relative affinity than to DOPS alone, indicating that the binding of these variants is enhanced by β2GPI (Table 1). The R53G mutant demonstrated no detectable binding to either DOPS-β2GPI or DOPS, confirming that the change to Arg at position 53 in 3H9 plays an important role in the recognition of DOPS-β2GPI as well as DOPS.
Each of the forward mutants bound significantly better to DOPS-β2GPI than to DOPS. In addition, the combination of D56R and S76R mutations showed a greater increase in relative binding compared with 3H9 in the presence of β2GPI than in its absence. The results suggest that the combining sites of 3H9 and seven of eight variants conform more precisely to the complex between β2GPI and DOPS than to the phospholipid alone. Experiments with whole serum instead of purified β2GPI indicate that β2GPI is the main serum protein that enhances the binding of 3H9 and its variants to DOPS (data not shown).
Effects of DNA on Antibody Recognition of Phosphatidylserine-β2GPI Complexes.
Our data show that 3H9 and its variants share specificity for DOPS-β2GPI as well as DNA. To define further the dual binding specificity of these antibodies, we carried out competition experiments with DOPS-β2GPI vesicles and DNA (Fig. 3) with both the high-affinity D56R/S76R scFv and the germline revertant R53G/I57T/D65G scFv that binds poorly to dsDNA. The highest concentration of DNA (100 μg/ml) was able to completely inhibit the binding of both antibodies to DOPS-β2GPI, and lower concentrations showed approximately equivalent levels of inhibition for both antibodies.
Figure 3.
Inhibition of DOPS-β2GPI binding by DNA or DOPS-β2GPI vesicles. D56R/S76R (□, ■) or R53G/I57T/D65G (○, ●) were incubated with increasing concentrations of DNA (open symbols) or DOPS-β2GPI vesicles (filled symbols) before incubation on DOPS-β2GPI-coated ELISA plates. The means and SE of triplicate samples from one representative experiment are shown.
In contrast, DOPS-β2GPI vesicles inhibited 45% of R53G/I57T/D65G and 80% of D56R/S76R binding to DOPS-β2GPI (Fig. 3). These results are consistent with DOPS-β2GPI ELISA results in that D56R/S76R had higher relative affinity for DOPS-β2GPI and was more sensitive to inhibition by lower concentrations of vesicles. The fact that DOPS-β2GPI vesicles did not completely inhibit binding to DOPS-β2GPI bound to the ELISA plate may suggest that the conformation of the DOPS-β2GPI complex in vesicles is not identical to the conformation on the ELISA plate, and that the antibodies prefer the antigen as it is presented on the solid support.
Antibody Recognition of Apoptotic Cells.
To evaluate the effects of somatic mutations on antibody recognition of apoptotic cells, we assessed binding of the two most diverse mutants, R53G/I57T/D65G and D56R/S76R, to Jurkat cells treated with staurosporine. Flow cytometry data first were gated according to forward and side scatter to exclude cell fragments and debris (Fig. 4A). Apoptotic cells were identified by staining with annexin V (Fig. 4B), and annexin V-positive and negative cells were analyzed separately to determine the extent of scFv binding and PI staining (Fig. 4C). Only annexin V-positive cells were bound by the R53G/I57T/D65G and D56R/S76R scFv, indicating that the 3H9 variants do not bind to the surface of viable cells. In contrast, the 4VH/1VL control scFv (27) did not bind above background levels to either apoptotic or viable cells.
Figure 4.
Flow cytometric analysis of scFv binding to apoptotic cells. (A) Staurosporine-treated Jurkat cells were gated according to forward and side scatter to exclude cell fragments and debris. (B) Cells were gated further into annexin V-positive and -negative populations. (C) Both annexin V-positive and -negative cells were analyzed to determine the extent of scFv binding and PI staining. Annexin V-positive cells (Left) are bound by D56R/S76R and R53G/I57T/D65G (germline), although some annexin V-positive cells fail to bind scFv. Two populations of scFv-bound cells can be distinguished based on PI staining. The mean fluorescence intensity was 1,583 for D56R/S76R, 246 for R53G/I57T/D65G, and 10 for 4VH/1VL. Minimal reactivity was observed with cells incubated with the 4VH/1VL control scFv or with the secondary reagent alone, demonstrating that D56R/S76R and R53G/I57T/D65G are specific for apoptotic cells. No binding to annexin V-negative cells was detected (Right).
Among annexin V-positive cells, two populations of scFv-bound cells were distinguished based on PI staining intensity (Fig. 4C). These results indicate that the scFv used here preferentially recognize cells in the later phases of apoptosis. Consistent with the ELISA results, the mean fluorescence intensity for D56R/S76R was approximately 6-fold higher than for R53G/I57T/D65G and over 100-fold higher than for the control scFv (Fig. 4C). We observed a 2-fold decrease in MFI for the binding of D56R/S76R to cells cultured and washed in the absence of serum (data not shown), suggesting a role for β2GPI in the binding to apoptotic cells.
Because the scFv reacted only with cells that were bound by annexin V, a sensitive marker for phosphatidylserine, it is likely that variants of 3H9 bind to a cell-surface epitope composed of, or similar in structure to, phosphatidylserine and β2GPI. Interestingly, 5–10% of the annexin V-positive cells did not bind the R53G/I57T/D65G and D56R/S76R scFv. The lack of antibody binding to a subset of annexin V-positive cells may suggest differences in the accessibility or arrangement of phosphatidylserine. Such variations have been proposed to coincide with changes in lipid phase (34). Therefore, autoantibodies to phosphatidylserine-β2GPI may represent valuable probes for structural transitions that occur on the cell surface during apoptosis.
Discussion
To investigate the possible interaction of B cells with apoptotic cells, we have analyzed structural requirements for autoantibody binding to phosphatidylserine, an anionic phospholipid expressed on the apoptotic cell surface (5, 6). Our results establish that the mouse germline encodes antibodies specific for phosphatidylserine (Fig. 2A), and that specificity for DOPS provides a possible explanation for the binding of these antibodies to apoptotic cells (Fig. 4C). Binding to DOPS is enhanced by β2GPI (Table 1), a plasma protein that rapidly associates with anionic phospholipids on the membrane of apoptotic cells (13, 17). Complex formation between β2GPI and anionic phospholipids is associated with a structural transition in β2GPI (35) and correlates with increased immunogenicity (36). The fact that 3H9 and its variants show higher relative affinity for DOPS-β2GPI than for DOPS alone suggests that some B cell receptors may recognize a protein–phospholipid complex that constitutes a unique structural feature of apoptotic cells.
What might be the function of B cells whose surface receptors bind to apoptotic cells? Shaw et al. (20) observed that T15 antibodies, long known for their protective role in responses to bacterial phosphorylcholine epitopes, also bind to apoptotic cells and suggested that B cells with this specificity may serve “housekeeping” functions by removing cellular debris. Thus, it is possible that immature B cells expressing VH3H9 participate in the removal of apoptotic cell remnants. However, we know that VH3H9 plays a dominant role in the binding to DNA and phospholipids (37–39), that most VL cannot block this binding (38), and that editing of VH and/or VL genes becomes obligatory. This principle is demonstrated by the fact that recombinase-deficient preB cells expressing the 3H9 VH and VL transgenes die by apoptosis (16). Thus, if immature B cells participate in the uptake of apoptotic cell remnants in the bone marrow, at the same time, they must be undergoing receptor editing to ablate self-reactivity.
What can be deduced from the pattern of somatic mutations in 3H9? The two earliest replacement mutations, Gly at position 65 to Asp and Thr at position 57 to Ile, greatly reduced the binding to DOPS-β2GPI, as seen from the comparison between the triple revertant and R53G (Fig. 2C). This observation may be related to the fact that central tolerance is largely intact in MRL/lpr mice, as shown by studies with facultative self-antigens (40, 41). In 3H9 transgenic mice bred on the MRL/lpr genetic background, selection pressures result in an oligoclonal B cell expansion (42, 43), despite the abundant participation of the transgenes in the primary repertoire. Thus, a rare event may have been required for the 3H9 clone to expand.
Somatic diversification mechanisms, such as V gene hypermutation and receptor editing, have the capacity to drastically alter the specificity of functionally rearranged Ig V genes. The L chain of 3H9, encoded by Vκ4/5Jκ4, may itself be the product of receptor editing by secondary VJ rearrangement, as Vκ4/5 genes are frequent editors in VH3H9 H chain-only transgenic mice (44). We suggest that receptor diversification may have reduced the affinity of a 3H9 precursor for apoptotic cells, thus freeing it from central tolerance and allowing its exit from the bone marrow. In support of this idea, recent results from R53G/I57T/D65G transgenic mice indicate that the 3H9 germline transgene also imposes stringent negative selection on B lymphocyte development and results in vigorous VL-receptor editing (M.W., unpublished results). Because R53G/I57T/D65G has a greater relative affinity for DOPS-β2GPI than for DNA (Fig. 2C; ref. 24 and unpublished results), it is possible that binding to apoptotic cells provides a signal for negative selection of developing B cells that is perhaps as powerful as the binding to dsDNA.
In the periphery, the 3H9 clone may have encountered apoptotic cell remnants in the context of a dendritic cell. Studies by MacPherson and coworkers have shown transport of apoptotic cells to lymph nodes by dendritic cells (45) and suggested a role for the association between newly emergent B cells and dendritic cells in the programming of isotype switching and antibody secretion (46). Such interactions may have selected for the replacement of Gly-53 with Arg and reinstated binding to DOPS-β2GPI (Fig. 2C). Alternatively, selection for binding to DNA or nucleoproteins may have provided a mechanism for recovering specificity for DOPS-β2GPI. In either case, the Gly-53 to Arg mutation greatly increased the relative affinity for ssDNA, dsDNA (24), and DOPS-β2GPI (Fig. 2C), thus endowing 3H9 with dual specificity for DNA-protein complexes and apoptotic cells.
Dual specificity may have allowed the 3H9 clone to gain access to a variety of autoantigens, such as DNA, nucleosomes, and ribonucleoproteins, that are sequestered in blebs and apoptotic bodies of dying cells (1). Any B cell capable of binding and, possibly, internalizing such packets of autoantigens may have the potential to present a range of nuclear antigens to helper T cells. The initial interaction with a helper T cell then may determine the direction in which B cell specificity may evolve (47), the nature of the retained replacement mutations, and the further course of affinity maturation.
Because Arg at position 53 of 3H9 plays a pivotal role in the dual specificity for DNA and DOPS-β2GPI, we decided to examine the role of additional Arg residues at positions 31 of CDR1, 56 and 58 of CDR2, and 76 of FWR3. Each of those positions has been the site of somatic mutations to Arg in autoantibodies to DNA and phospholipids (31, 32). Previously, we reported that mutations to Arg at those positions raise the relative affinity of 3H9 for DNA (24). Here, we demonstrate that each mutation also increased the relative affinity for DOPS-β2GPI (Fig. 2D). In addition, inhibition experiments indicate that 56R and 76R serve equally well for DNA binding as for DOPS-β2GPI binding (Fig. 3). Based on these results, we propose that B cells capable of binding to both the apoptotic cell surface and nuclear antigens have a higher probability of being selected than cells that are monospecific for only one of these antigens.
The characteristic of dual specificity is unusual because antibody specificity is generally focused toward the selecting antigen. Nevertheless, in autoimmunity, different combinations of autospecificities have been observed. Autoantibodies have been described that, in addition to binding to DNA, bind to RNP or cytoskeletal elements (37), to subnucleosome particles (48), to proteins involved in hnRNA splicing (49), or to the hinge domain of the IgG molecule (50). In each case, dual specificity is thought to provide the B cell with a selective advantage because it widens the spectrum of autoantigens that can be taken up and presented. However, Ig receptors to apoptotic cell-surface epitopes and DNA are unique in that they provide B cells with access to nuclear antigens, regardless of whether the antigens are sequestered in apoptotic blebs or not.
There is symmetry in the binding specificity of B cell receptors for DNA and apoptotic cells vs. pattern-recognition receptors of the innate immune system. Macrophage scavenger receptors that participate in the clearance of apoptotic cells also bind to nucleic acids and anionic phospholipids (51). However, autoimmune B cells that are specific for apoptotic cell remnants may gain an advantage if they secrete antibodies that opsonize apoptotic cells and divert their uptake from scavenger receptors to Fc receptors. In that way, B cells that react with apoptotic cells may tip the balance from tolerance to autoimmunity.
Supplementary Material
Acknowledgments
We thank Jane Brandt for her contributions to the construction of scFv expression vectors and Dr. Ken Nishimoto for use of his data analysis software. The research was supported by a Lupus Foundation of America Gina Finzi Memorial Fellowship (to B.A.C.), a McGill University Studentship (to P.D.), a Canadian Institutes of Health Research grant (to J.R.), and an institutional grant from the University of Tennessee (to M.Z.R.).
Abbreviations
- β2GPI
β2-glycoprotein I
- scFv
single-chain variable fragment
- DOPS
dioleoyl phosphatidylserine
- PI
propidium iodide
- V
variable
- CDR
complementarity-determining region
References
- 1.Casciola-Rosen L A, Anhalt G, Rosen A. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade F, Casciola-Rosen L, Rosen A. Rheum Dis Clin N Am. 2000;26:215–227. doi: 10.1016/s0889-857x(05)70136-8. [DOI] [PubMed] [Google Scholar]
- 3.Grodzicky T, Elkon K B. Am J Med. 2000;108:73–82. doi: 10.1016/s0002-9343(99)00332-0. [DOI] [PubMed] [Google Scholar]
- 4.Rovere P, Sabbadini M G, Fazzini F, Bondanza A, Zimmeraman V S, Rugarli C, Manfredi A A. Arthritis Rheum. 2000;43:1663–1672. doi: 10.1002/1529-0131(200008)43:8<1663::AID-ANR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Savill J, Fadok V A. Nature (London) 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 6.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 7.Fadok V A, Bratton D L, Rose D M, Pearson A, Ezekewitz R A B, Henson P M. Nature (London) 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 8.Platt N H, Suzuki H, Kurihara Y, Kodama T, Gordon S. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savill J, Dransfield I, Hogg N, Haslett C. Nature (London) 1990;343:170–174. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 10.Sambrano G R, Steinberg D. Proc Natl Acad Sci. USA. 1995;92:1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devitt A, Moffat O D, Raykundalia C, Capra J D, Simmons D L, Gregory C D. Nature (London) 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y, Silverstein R L, Allen J, Savill J. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian K, Schroit A J. J Biol Chem. 1998;273:29272–29277. doi: 10.1074/jbc.273.44.29272. [DOI] [PubMed] [Google Scholar]
- 14.Manfredi A A, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini M G. Arthritis Rheum. 1998;41:215–223. doi: 10.1002/1529-0131(199802)41:2<215::AID-ART5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Navratil J S, Watkins S C, Wisnieski J J, Ahearn J M. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Li H, Suri-Payer E, Hardy R R, Weigert M. J Exp Med. 1998;188:1247–1254. doi: 10.1084/jem.188.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price B E, Rauch J, Shia M A, Walsh M T, Lieberthal W, Gilligan H M, O'Laughlin T, Koh J S, Levine J S. J Immunol. 1996;157:2201–2208. [PubMed] [Google Scholar]
- 18.Levine J S, Subang R, Koh J S, Rauch J. J Autoimmun. 1998;11:413–424. doi: 10.1006/jaut.1998.0235. [DOI] [PubMed] [Google Scholar]
- 19.Pittoni V, Ravirajan C T, Donohoe S, Machin S J, Lydyard P M, Isenberg D A. Clin Exp Immunol. 2000;119:533–543. doi: 10.1046/j.1365-2249.2000.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw P X, Hörkko S, Chang M-K, Curtiss L K, Palinski W, Silverman G J, Witztum J L. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mevorach D, Zhou J-L, Song X, Elkon K B. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botto M, Dell'Agnola C, Bygrave A E, Thompson E M, Cook H T, Petry F, Loos M, Pandolfi P P, Walport M J. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Koralov S B, Kelsoe G. J Exp Med. 2000;192:1339–1351. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radic M Z, Mackle J, Erikson J, Mol C, Anderson W F, Weigert M. J Immunol. 1993;150:4966–4977. [PubMed] [Google Scholar]
- 25.Radic M Z, Seal S N. Methods Companion Methods Enzymol. 1997;11:20–26. doi: 10.1006/meth.1996.0383. [DOI] [PubMed] [Google Scholar]
- 26.Cocca B A, Seal S N, Radic M Z. Protein Expression Purif. 1999;17:290–298. doi: 10.1006/prep.1999.1148. [DOI] [PubMed] [Google Scholar]
- 27.Seal S N, Hoet R M, Raats J M H, Radic M Z. Arthritis Rheum. 2000;43:2132–2138. doi: 10.1002/1529-0131(200009)43:9<2132::AID-ANR25>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Martin A C, Cheetham J C, Rees A R. Proc Natl Acad Sci USA. 1989;86:9268–9272. doi: 10.1073/pnas.86.23.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radic M Z, Weigert M. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 30.Shlomchik M J, Aucoin A H, Pisetsky D S, Weigert M G. Proc Natl Acad Sci USA. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita Y, Sumida T, Ichikawa K, Maeda T, Yonaha F, Iwamoto I, Yoshida S, Koike T. J Immunol. 1993;151:849–856. [PubMed] [Google Scholar]
- 32.Monestier M, Kandiah D A, Kouts S, Novick K E, Ong G L, Radic M Z, Krilis S A. J Immunol. 1996;156:2631–2641. [PubMed] [Google Scholar]
- 33.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar L, Ortega-Pierres G, Campos B, Fonseca R, Ibáñez M, Wong C, Farfán N, Naciff J M, Kaetzel M A, Dedman J R, Baeza I. J Biol Chem. 1999;274:25193–25196. doi: 10.1074/jbc.274.36.25193. [DOI] [PubMed] [Google Scholar]
- 35.Bouma B, de Groot P G, van den Elsen J M, Ravelli R B, Schouten A, Simmelink M J, Derksen R H, Kroon J, Gros P. EMBO J. 1999;18:5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subang R, Levine J S, Janoff A S, Davidson S M, Taraschi T F, Koike T, Minchey S R, Whiteside M, Rauch J. J Autoimmun. 2000;15:21–32. doi: 10.1006/jaut.2000.0382. [DOI] [PubMed] [Google Scholar]
- 37.Radic M Z, Mascelli M A, Erikson J, Shan H, Weigert M. J Immunol. 1991;146:176–182. [PubMed] [Google Scholar]
- 38.Ibrahim S M, Weigert M, Basu C, Erikson J, Radic M Z. J Immunol. 1995;155:3223–3233. [PubMed] [Google Scholar]
- 39.Seal S N, Monestier M, Radic M Z. Eur J Immunol. 2000;30:3432–3440. doi: 10.1002/1521-4141(2000012)30:12<3432::AID-IMMU3432>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Rathmell J C, Goodnow C C. J Immunol. 1994;153:2831–2842. [PubMed] [Google Scholar]
- 41.Rubio C F, Kench J, Russell D M, Yawger R, Nemazee D. J Immunol. 1996;157:65–71. [PubMed] [Google Scholar]
- 42.Brard F, Shannon M, Prak E L, Litwin S, Weigert M. J Exp Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roark J H, Kuntz C L, Nguyen K A, Mandik L, Cattermole M, Erikson J. J Immunol. 1995;154:4444–4455. [PubMed] [Google Scholar]
- 44.Radic M Z, Erikson J, Litwin S, Weigert M. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang F P, Platt N, Wykes M, Major J R, Powell T J, Jenkins C D, MacPherson G G. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wykes M, Pombo A, Jenkins C, MacPherson G G. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 47.Kaliyaperumal A, Mohan C, Wu W, Datta S K. J Exp Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Losman M J, Fasy T M, Novick K E, Monestier M. Int Immunol. 1992;5:513–523. doi: 10.1093/intimm/5.5.513. [DOI] [PubMed] [Google Scholar]
- 49.Retter M W, Cohen P L, Eisenberg R A, Clarke R H. J Immunol. 1996;156:1296–1306. [PubMed] [Google Scholar]
- 50.Rumbley C A, Voss E W. Clin Exp Immunol. 1995;102:341–348. doi: 10.1111/j.1365-2249.1995.tb03787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson A M. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.