Abstract
Seizure disorders are very common and affect 3% of the general population. The recurrent unprovoked seizures that are also called epilepsies are highly diverse as to both underlying genetic basis and clinic presentations. Recent genetic advances and sequencing technologies indicate that many epilepsies previously thought to be without known causes, or idiopathic generalized epilepsies (IGEs), are virtually genetic epilepsy as they are caused by genetic variations. IGEs are estimated to account for ~15-20% of all epilepsies. Initially IGEs were primarily considered channelopathies, because the first genetic defects identified in IGEs involved ion channel genes. However, new findings indicate that mutations in many non ion channel genes are also involved in addition to those in ion channel genes. Interestingly, mutations in many genes associated with epilepsy affect GABAergic signaling, a major biological pathway in epilepsy. Additionally, many antiepileptic drugs work via enhancing GABAergic signaling. Hence, the review will focus on the mutations that impair GABAergic signaling and selectively discuss the newly identified STXBP1, PRRT2, and DNM1 in addition to those long-established epilepsy ion channel genes that also impair GABAergic signaling like SCN1A and GABAA receptor subunit genes. GABAergic signaling includes the pre- and post- synaptic mechanisms. Some mutations, such as STXBP1, PRRT2, DNM1, and SCN1A, impair GABAergic signaling mainly via pre-synaptic mechanisms while those mutations in GABAA receptor subunit genes impair GABAergic signaling via post-synaptic mechanisms. Nevertheless, these findings suggest impaired GABAergic signaling is a converging pathway of defects for many ion channel or non ion channel mutations associated with genetic epilepsies.
Keywords: Ion channels, non ion channels, mutations, GABAergic signaling, epilepsy, vesicles
Introduction
Epilepsy is a common neurologic disorder, and the causes are highly heterogeneous. Genetic generalized epilepsies (GGEs) refer to epilepsy syndromes previously classified as idiopathic generalized epilepsies (IGEs) (Scheffer et al., 2017), which have been associated with variations in multiple genes based on recent genetic advances (Klassen et al., 2011). The incidence of epilepsy (recurrent unprovoked seizures) in children and adolescents ranges from 50 to 100/100,000 (Hauser, 1994). The general frequency of IGEs is estimated to be 15–20% of all epilepsies (Jallon and Latour, 2005). GGEs are a group of neurological disorders which is common in both the pediatric population and andadults (Hauser, 1994). GGEs include several different epilepsy syndromes that vary in clinical severity from relatively benign childhood absence epilepsy (CAE), which may remit with age, to more severe juvenile myoclonic epilepsy (JME), and generalized epilepsy with febrile seizures plus (GEFS+). A subpopulation of GGEs are associated with severe recurrent seizures and cognitive decline that have been referred to as epileptic encephalopathies, which are often refractory to existing treatments and have poor developmental outcome. Epileptic encephalopathies include severe myoclonic epilepsy in infancy (SMEI) or Dravet syndrome, West syndrome or infantile spasms, Ohtohara syndrome, and Lennox-Gastaut syndrome.
Many ion channel and non ion channel gene mutations have been identified in various epilepsies including epileptic encephalopathy (Merwick et al., 2012). The identified ion channel genes include both voltage gated and ligand gated ion channel gene mutations. The voltage gated ion channels affected by mutations include but are not limited to SCN1A, SCN2A, SCN3A, SCN8A, SCN1B, KCNB1, KCNQ2, KCNQ3, Cav3.1, Cav3.2 and Cav3.3. As for the ligand gated ion channels, the genes include ChRNA4 and ChRNB2 as well as mutations in GABAA receptors including GABRA1, GABRB1–3, GABRG2 and GABRD (Anderson et al., 2002;Macdonald et al., 2010). To date, most functional studies of epilepsy genetic mutations have been focused on ion channel genes. Mutation of ion channels that cause either a “gain of function” in excitatory neurotransmission or a “loss of function” in inhibitory neurotransmission could impair the balance of excitation and inhibition, leading to disinhibition and hyper-excitability in the brain.
In addition to mutations in ion channel genes, many mutations in non ion channel genes have also been associated with various kinds of epilepsies. This has changed our traditional view of epilepsy as channelopathies with defects in ion channels. Many non ion channel genes that are associated with epilepsy are still unfamiliar to the field of epilepsy research. The biological function of these genes or how these genes make the brain epileptic is not clear. These genes include but not limited to PCDH19, CDKL5, STXBP1, STX1B, DNM1, PRRT2, CHD2, IQSE2, FOXG1, ALG3, RELN, etc. There is no doubt that the list of genes associated with epilepsy is still growing. This suggests an urgent need for defining biological functions of these genes and their roles in epileptogenesis.
Impaired GABAergic signaling is a converging pathway of pathophysiology in genetic epilepsy
Whether the mutations are in ion channel genes or non ion channel genes, they are likely to cause defects in possible common converging pathways that are critical for seizure generation. Among all the newly identified epilepsy mutations, ion channel genes continue to have the most frequent occurrence on the list. This suggests the prominent role of ion channels in the pathogenesis of epilepsy. Activation of neurotransmitter receptor ion channels at synapses promotes synapse plasticity during brain development. Consequently, impaired ion transport may affect neural excitability and brain development, resulting in epilepsy and other neurodevelopmental disorders. Further, synapse formation and normal function are essential in the signaling and the formation of neural networks. Genes related to synapse formation and function are also closely related to epilepsy and other neurodevelopmental disorders like autism and mental disability (Delahanty et al., 2011). Similarly, some non ion channel genes in the pathways of cell growth (Guo et al., 2013), transcriptional regulation, protein kinase modulation, cell metabolism, and cell-cell interaction may also participate in synapse formation and function while defects in these genes may lead to the genesis of epilepsy (Lubin, 2012;Scharfman and Brooks-Kayal, 2014).
GABAergic signaling is an established pathway for seizure generation. Not surprisingly, many mutations in both ion channel and non ion channel genes have been identified to impair GABAergic signaling. Here we will summarize different genes that impair GABAergic signaling and have been associated with epilepsy. The mechanisms by which these gene mutations impair GABAergic signaling include pre- and post-synaptic mechanisms. The epilepsy genes that impair GABAergic signaling via the pre-synaptic mechanisms include but are not limited to STXBP1, STX1B, DNM1, and PRRT2. These genes encode proteins that are involved in vesicle fusion machinery and vesicle release. The defects in the vesicle fusion machinery affect the pre-synaptic vesicle release. Failure or impaired release of key neurotransmitters would profoundly impair the corresponding neurotransmission and synaptic activity.
GABAergic neurotransmission
The cardinal aspects of GABAergic neurotransmission include pre-synaptic neurotransmitter gamma amino butyric acid (GABA) release and the GABAA receptor post-synaptic mechanisms (Figure 1). GABA is released by GABAergic interneurons that provide much of the inhibition in the cerebral cortex, hippocampus, striatum and amygdala. Impaired interneuron function has been established as an underlying cause for epilepsy via multiple preclinical animal models (Powell, 2013). Along the same line, much effort has been taken to rescue interneuron function to treating epilepsy. For example, it has been reported that GABA progenitor cells grafted into the adult epileptic brain attenuated seizures and comorbidities in mice (Hunt et al., 2013;Hunt and Baraban, 2015).
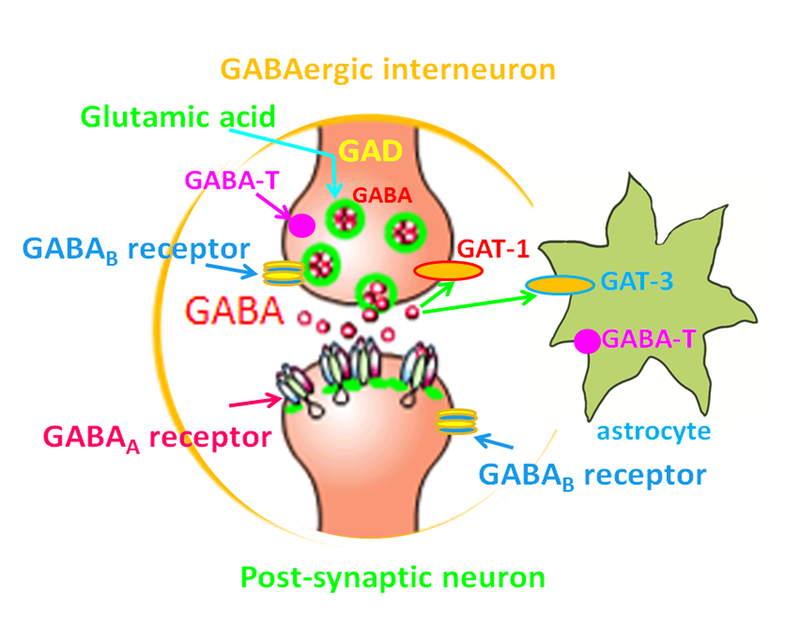
Figure 1.
GABA signaling. In GABAergic interneurons, the neurotransmitter GABA is synthesized from glutamic acid, the principal excitatory neurotransmitter via glutamic acid decarboxylase (GAD). GABA is catabolized by GABA transaminase (GABA-T) which is a membrane bound enzyme expressed by neurons and glia. GABA is released from vesicles in pre-synaptic terminals and activates GABA receptors which include GABAA receptors and GABAB receptors. GABAA receptors hyperpolarize neurons via Cl- influx. The released GABA is taken up by GABA transporters (GAT-1 and GAT-3) back into pre-synaptic compartments of neurons or into astrocytes.
GABA
GABA is the major inhibitory neurotransmitter while glutamate is the major excitatory neurotransmitter in the brain. Both neurotransmitters work together to control many neuronal processes including the overall brain excitation. It has been established that glutamic acid decarboxylase (GAD) converts glutamate to GABA. There are two isoforms of GAD, GAD65 and GAD67 that synthesize GABA in the brain. After released from pre-synaptic terminals, GABA is taken up by GABA transporters. These transporters are widely expressed in neuronal (mainly GAT-1) and glial (mainly GAT-3) cells throughout the brain. Inside the cell, GABA is degraded by GABA transaminase to succinic semialdehyde, and inhibition of this enzyme by the antiseizure drug (ASD) like vigabatrin increases GABAergic neurotransmission. Because of the critical role of GAD in synthesizing GABA, it has been proposed that GAD65 loss of function may preferentially decrease the pre-synaptic reserve pool of GABA and decrease tonic GABA inhibition, leading to increased seizure susceptibility (Kash et al., 1997). Although no human GAD mutations have been found to consistently cause epilepsy, mutations in co-factors that are necessary for GAD65 function have been linked with early life seizures, as occurs in pyridoxine-dependent epilepsy (Kure et al., 1998). GABA acts through fast chloride-permeable ionotropic GABAA receptors and also through slower metabotropic G-protein-coupled GABAB receptors. Since there is no mutation that has been identified in GABAB receptors associated with epilepsy up to date, this review will only focus on GABAA receptors.
GABAA receptors
GABAA receptors are the primary mediators of fast inhibitory synaptic transmission in the central nervous system and have been repeatedly documented to play a critical role in animal models of seizures (Banerjee et al., 1998;Cohen et al., 2003;Evans et al., 1994;Feng et al., 2001;Kapur and Macdonald, 1997;Karle et al., 1998;Kohling et al., 2000;Poulter et al., 1999). These inhibitory receptors are hetero-pentomeric protein complexes composed of multiple subunits that form ligand gated, anion-selective channels. GABAA receptors are modulated by barbiturates, benzodiazepines, zinc, ethanol, anesthetics, and neurosteroids. GABAA receptors are formed by the assembly of multiple subunit subtypes (α1-α6, β1-β3, γ1-γ3, δ, ε, π, θ, and ρ1-ρ3). These GABAA receptor subunits are each encoded by a different gene and form chloride ion channels when assembled in a complete receptor. In the brain, GABAA receptors most commonly contain two α subunits, two β subunits, and a γ or δ subunit. The most common GABAA receptor is the α1β2γ2 subtype, but multiple subtype combinations exist. They vary in different brain regions and cell types and during different times in development. Subunit composition of GABAA receptor plays a major role in determining the intrinsic properties of each channel, including affinity for GABA, kinetics, conductance, allosteric modulation, probability of channel opening, interaction with modulatory proteins, and subcellular distribution.
Antiseizure drugs (ASDs) that take effect via enhancing GABAergic signaling
Potentiation of inhibitory neurotransmission mediated by GABA remains a key mechanism of ASD action as many ASDs are designed to work via modulating GABAA receptors or enhance GABAergic signaling. Additionally, some ASDs work via other mechanisms like limitation of sustained repetitive neuronal firing via blockade of voltage-dependent sodium channels or blockade of glutamatergic excitatory neurotransmission (Meldrum, 1996;Taylor and Meldrum, 1995). Neurons that use GABA as their neurotransmitter represent only a small fraction of neurons in regions that are essential to epileptic activity (Houser, 2014), such as the neocortex, hippocampus and amygdala. Parvalbumin interneurons have been shown to be associated with epileptic activity (Ma and Prince, 2012;Rubinstein et al., 2015). These inhibitory connections are vital in restraining the natural tendency of recurrently connected excitatory neurons to undergo the transition through positive feedback into synchronized epileptiform discharges.
GABAA receptors that contain α1–3, 5 subunits and γ2 subunits are sensitive to benzodiazepines and γ2 subunits are critical for clustering the receptors at synapses contributing to phasic inhibition (Alldred et al., 2005). Phasic inhibition refers to the effects of GABA released at GABAergic synapses that binds to post-synaptic receptors located at the synaptic cleft, in contrast to extra- or peri-synaptic receptors that are activated by ambient GABA, which is referred as tonic inhibition. Phasic inhibition is primarily related to increased conductance when chloride channels open and hyperpolarization of post-synaptic membrane potential when chloride influx occurs. By contrast, δ-subunit-containing GABAA receptors are not present in synapses but in extra- or peri-synaptic regions. GABAA receptors that contain δ subunit can be potentiated by neurosteroids (Bianchi and Macdonald, 2003). This feature has been proposed to treat epilepsy. For example, Ganaxolone, a synthetic analog of allopregnanolone, has been proven to be beneficial for refractory focal epilepsy and infantile spasms (Broomall et al., 2014;Goodkin and Kapur, 2009;Rogawski et al., 2013). A recent phase II study indicates Ganaxolone reduces partial-onset seizures frequency (Sperling et al., 2017).
It is known that drugs blocking GABAA receptors, such as bicuculline and pentylenetetrazol, can cause seizures. This effect has been widely used in experimental animal models to study epilepsy. Conversely, enhancement of GABAA receptor-mediated inhibition is an effective antiepileptic approach that remains as a key mechanism for epilepsy drug discovery. Indeed, the first effective epilepsy treatment, bromide, works via enhancing GABAergic signaling (Krall et al., 1978). It has been demonstrated that bromide enhances GABA-activated currents in cultured neurons (Suzuki et al., 1994). Many ASDs have been developed because of their effect on GABAergic signaling potentiation. These drugs, including benzodiazepines, phenobarbital, felbamate, and topiramate, enhance the function of GABAA receptors. These drugs also include vigabatrin, tiagabine, gapapentin and valproate. Tigabine increases the level of GABAby blocking GAT-1, and hence, is classified as a GABA reuptake inhibitor (Rekling et al., 1990). Vigabatrin inhibits GABA-transaminase and increases brain GABA content (Petroff and Rothman, 1998). Valproate and gabapentin increase GABA synthesis and turnover (Loscher, 1989); both valproate and gabapentin have a range of activities that overlaps with those of drugs that are known to interact with GABA systems. In summary, these ASDs enhance GABAergic signaling either by enhancing GABAA receptor function or increasing GABA level in the synaptic cleft.
Mutations in genes that impair GABAergic signaling via pre-synaptic mechanisms
There are several epilepsy genes that may impair GABAergic signaling via pre-synaptic mechanism. It is possible that many other epilepsy genes whose biologic function is currently unknown may also be involved in this mechanism. Below we will discuss the genes that may directly or indirectly impair GABAergic signaling via pre-synaptic mechanisms.
SCN1A mutations
Mutations in SCN1A are one of the main causes of genetic epilepsy (Anderson et al., 2002;Oliva et al., 2012). Loss of function mutations in SCN1A account for 80% of the most severe kind of epilepsy, Dravet syndrome (Marini et al., 2011). Sodium channel mutations that are associated with accelerated recovery from inactivation and increased sodium channel activity (i.e., those that produce a gain of function) can lead to enhanced seizure susceptibility, as in the epilepsy syndrome GEFS+ (Spampanato et al., 2001). The missense mutations in SCN1A are generally associated with relatively milder epilepsy syndromes like GEFS+ (Escayg and Goldin, 2010) while the loss of function mutations of SCN1A are associated with more severe epilepsy syndromes like Dravet syndrome (Meisler and Kearney, 2005). Although there is still some controversy over the findings in human-patient-derived induced pluripotent stem cells in which increased sodium current in both bipolar- and pyramidal-shaped neurons was observed (Isom, 2014;Liu et al., 2013), it is generally believed that mutations in SCN1A impair GABAergic interneuron activity (Escayg and Goldin, 2010;Kalume et al., 2007;Kalume et al., 2013;Yu et al., 2006). Reduced firing of inhibitory neurons would affect GABA release. This is consistent with enhancing GABAA receptor function by clobazam or other analogs that attenuate the seizure severity and rescue related comorbidity like autistic traits in a Scn1a knockout mouse model (Han et al., 2012).
Syntaxin Binding Protein 1 (STXBP1) mutations
A mutation in STXBP1, a gene also known as Munc18–1, was initially discovered as a cause for Ohtahara Syndrome (Saitsu et al., 2008). Ever since, it has been associated with many other epilepsy syndromes in early childhood and became one of the most prominent genes for epileptic encephalopathy (Stamberger et al., 2016). STXBP1 is a main part of the synaptic fusion machinery, which includes syntaxin, synaptobrevin, and SNAP25—the three main components (Ma et al., 2013). By binding to syntaxin, STXBP1 protein orchestrates the assembly of the other components. Syntaxin enables vesicles to fuse with the plasma membrane. In cells, the so-called minimal fusion machinery provides the final “push” for the vesicle to fuse with the membrane through proteins that twist around each other and pull the vesicle close enough to fuse.
A STXBP1 knockout mouse model has been developed. In STXBP1 heterozygous knockout mice, the reduction of readily releasable vesicles was greater in GABAergic neurons than glutamatergic neurons (Toonen et al., 2006). This thus suggests the contribution of GABAergic signaling in epilepsy associated with STXBP1 mutations. However, the biologic functional study of STXBP1 is still very limited. It is reported that deletion of Munc18–1 in mice results in widespread neurodegeneration that remains poorly characterized. It has been demonstrated that the early stages of spinal motor circuit formation—including motor neuron specification, axon growth and pathfinding, and mRNA expression—are unaffected in Munc18–1(−/−) mice. This indicates that the role of STXBP1 in synaptic activity is dispensable for early nervous system development (Law et al., 2016). A study in human embryonic stem (ES) cells (Patzke et al., 2015) indicated that heterozygous STXBP1 mutations lower the levels of Munc18–1 protein and its binding partner, the t-SNARE-protein Syntaxin-1, by approximately 30% and decrease spontaneous and evoked neurotransmitter release by nearly 50%. This suggests that heterozygous STXBP1 mutations cause early epileptic encephalopathy specifically through a pre-synaptic impairment.
DNM1 mutations
DNM1 is a GTPase and plays an important role in pinching off the vesicle from the plasma membrane. De novo mutations in DNM1 are a cause of severe epileptic encephalopathy like infantile spasms (Allen et al., 2013). Two recent publications have characterized the functional consequences of DNM1 mutations and find that the seizure phenotype is largely due to the deleterious effects of DNM1 mutations in GABAergic interneurons, while behavioral locomotor phenotypes may be due to the effect of the mutation in pyramidal cells (Asinof et al., 2016;Asinof et al., 2015). The loss of DNM1 in inhibitory neurons resulted in early onset lethal seizures with age at death ranging from postnatal 15–27 days old.
The mouse model of DNM1, referred to as the fitful mouse, is a spontaneous mouse mutant that was eventually found to have point mutation in DNM1 in the middle domain of the DNM1 protein. The mouse DNM1 gene has an alternatively spliced exon, which means that two variants of DNM1 are produced from a single gene. Previous studies (Asinof et al., 2015;Boumil et al., 2010) suggest that the homozygous fitful mouse best recapitulates the heterozygous situation in humans given this alternative exon. Homozygous Dnm1 fitful mice develop severe seizures, ataxia, and usually die before the age of 14 days, while heterozygous DNM1 mice only have a mild epilepsy phenotype that starts at the age of 2–3 months.
The findings indicate that when the wild-type DNM1 is deleted from GABAergic interneurons the mice develop an epilepsy phenotype. The affected neurons seem to be parvalbuminergic neurons, a subclass of GABAergic interneurons. Gene deletion in these cells alone is capable of producing the same epilepsy phenotype as the deletion in all cells. When expressed in other interneuron subsets, the epilepsy phenotype is milder. But when DNM1 was deleted from glutamatergic neurons and not GABAergic neurons, the animals with deletions in glutamatergic neurons did not develop seizures. However, these animal show abnormal locomotor, exploratory, and repetitive behaviors, suggesting that the glutamatergic gene deletion may in part be responsible for the non-epilepsy phenotypes in humans, such as developmental delay and autism. It is likely that different neuronal cell types in DNM1 encephalopathy may be responsible for different aspects of the disease. The effect of the mutation in GABAergic interneurons is responsible for the epilepsy phenotype (Asinof et al., 2015). In summary, this suggests selective deletion of DNM1 in GABAergic neuron is sufficient for seizure generation. The DNM1 mutations in patients with epileptic encephalopathies act in a dominant-negative manner which results in less efficient vesicle endocytosis. GABAergic interneurons may be particularly prone to disruptions of this function given their fast firing frequency.
PRRT2 mutations
Proline-rich transmembrane protein 2 (PRRT2 protein) is a pre-synaptic transmembrane protein and a key component of the calcium-dependent neurotransmitter release machinery (Valente et al., 2016). It is thought that PRRT2 protein interacts with the vesicle cycle in several ways. First, it may act as one component of the SNARE complex itself. Secondly, it is involved in vesicle recycling. Third, it may have a role in regulating the pre-synaptic ion channels that trigger the vesicle release. Mutations in PRRT2 protein are associated with benign familial infantile seizures (BFIS), paroxysmal kinesogenic dyskinesia (PKD) (Chen et al., 2011), infantile convulsions with choreoathetosis (ICCA) (Heron et al., 2012;Scheffer et al., 2012), and other atypical phenotypes. It is believed that PRRT2 protein interacts with members of the SNARE complex, namely SNAP25. The SNARE complex is involved in synaptic vesicle fusion and forms the minimal fusion machinery that allows synaptic vesicles to fuse with the plasma membrane. Both vesicles and the pre-synaptic membrane are lipid bilayers that repel each other. The synapse uses torsion of the proteins in the SNARE complex to overcome this repulsion – basically, the vesicle is pulled so close to the membrane that it eventually fuses. The detailed role of PRRT2 protein in modulating this process is unknown.
The mouse model of PRRT2 knockout recapitulates the neurological diseases associated with PRRT2 mutations (Michetti et al., 2017). This suggests haploinsufficiency of PRRT2 underlies the pathophysiology of PKD, ICCA, and seizures associated with PRRT2 mutations (Michetti et al., 2017). Although normal at birth, PRRT2 knockout mice display paroxysmal movements at the onset of locomotion that persist into adulthood. In addition, adult PRRT2 knockout mice present abnormal motor behaviors characterized by wild running and jumping in response to audiogenic stimuli and reduced seizure threshold. Patch-clamp electrophysiology in hippocampal and cerebellar slices revealed specific effects in the cerebellum, where PRRT2 is highly expressed, consisting of a higher excitatory strength at parallel fiber-Purkinje cell synapses during high frequency stimulation. The results show that the PRRT2 knockout mouse reproduces the motor paroxysms present in the human patients carrying PRRT2 mutations. Although a recent study indicates that PRRT2 mutation results in a decrease in the frequency of vesicle release probability (Valente et al., 2016), it is unclear how PRRT2 mutation differentially affects inhibitory vs excitatory neurons. It is likely that mutations in PRRT2, as well as other genes that affect pre-synaptic mechanisms as mentioned above, affects both excitatory and inhibitory neurons. However, it is the effect on GABA neurotransmitter release that influences the epilepsy phenotype.
Mutations in other genes involved in impairing GABAergic signaling via pre-synaptic mechanisms
Mutations in other less-studied genes may also impair GABAergic signaling via pre-synaptic mechanisms. Since the initial discovery of STXBP1 in Ohtahara syndrome, several other genes coding for proteins in the pre-synaptic fusion machinery have been identified as genes for human epilepsies. In addition to those aforementioned mutations, STX1B and SNAP25 have also been found to be mutated in patients with genetic epilepsies. The molecular defects of STX1B (Schubert et al., 2014) and SNAP25 (Rohena et al., 2013) may also involve pre-synaptic vesicle release. This emerging picture of impaired pre-synaptic vesicle release suggests that disruption of the regular function of pre-synaptic proteins may results in epilepsy. This mechanism may be counterintuitive given that global impairment of neurotransmitter release should primarily affect the excitatory neurons given the prominent presentation of pyramidal neurons in the brain. However, the epilepsy phenotype may be more related to the impairment of neurotransmitter release in GABAergic interneurons resulting from the mutations. Consequently, this would result in overall reduced inhibition of neurotransmission, leading to a brain state more tilted toward convulsion.
The mutations that impair GABAergic signaling via post-synaptic mechanisms
As mentioned earlier, the neurotransmitter GABA activates both GABAA and GABAB receptors. Although there are reports that an antibody against GABAB receptor mediates epilepsy opsoclonus-myoclonus syndrome and ataxia (Hoftberger et al., 2013;Kruer et al., 2014), there is no mutation identified in GABAB receptor subunit genes up to date. Thus, we will only focus on GABAA receptor mutations in this review article.
GABAA receptor subunits form a super family that contains 19 subunits. Mutations or variants in several GABAA subunits have been associated with epilepsies. These subunit genes include GABRA1, GABRB1, GABRB2, GABRB3, GABRG2, and GABRD (Ishii et al., 2017a;Johannesen et al., 2016;Kang and Macdonald, 2016;Macdonald et al., 2006;Moller et al., 2017a). Most of these mutations have autosomal dominant inheritance, and thus the patients are heterozygous for the mutation. The seizures and epilepsy syndromes resulting from mutations in these GABAA receptor subunit genes include multiple GE syndromes and vary in severities. These include pure febrile seizures (FS) (Audenaert et al., 2006) and epilepsy syndromes such as CAE (Tanaka et al., 2008), mixed afebrile and febrile seizures (CAE and FS and GEFS+ including Dravet syndrome), and afebrile seizures (Baulac et al., 2001;Dibbens et al., 2004;Harkin et al., 2002;Kananura et al., 2002;Sun et al., 2008;Wallace et al., 2001). The epilepsy mutations include missense and nonsense mutations, as well as insertion or deletion mutations resulting in frame shift mutations in coding regions, and mutations in noncoding regions.
Epilepsy phenotypic heterogeneity of GABAA receptor subunit mutations
There is a great phenotypic heterogeneity of epilepsy syndromes associated with GABAA receptor subunit gene mutations. For example, mutations in GABRA1 have been associated with childhood absence epilepsy (Maljevic et al., 2005), juvenile myoclonic epilepsy (Cossette et al., 2002), and generalized tonic clonic seizures (Lachance-Touchette et al., 2011), as well as Dravet, Ohtahara, and West syndromes (Carvill et al., 2014;Kodera et al., 2016). Studies from mouse models indicate that deletion of GABRA1 is sufficient to cause absence epilepsy (Arain et al., 2012). The knockin mice carrying GABRA1(A322D) displayed absence and myoclonic jerks (Arain et al., 2015). The functional consequence of GABRA1 mutations that are associated with Dravet syndrome has not been characterized.
Mutations in GABRG2 have been associated with a spectrum of seizures and generalized epilepsy syndromes. Phenotypes associated with both missense and nonsense mutations in GABRG2 are variable ranging from mild childhood absence epilepsy and febrile seizures (Baulac et al., 2001;Wallace et al., 2001), to GEFS+ and epileptic encephalopathies like Dravet syndrome(Harkin et al., 2002;Kang and Macdonald, 2016;Shen et al., 2017). The basis for the more severe epilepsy phenotypes with GABRG2 mutations are likely related to the extent of receptor function reduction and the metabolism of the mutant γ2 subunit protein (Kang et al., 2013). This notion is also supported by the comparison of two Gabrg2 loss-of-function mutations in mouse models, which revealed that the mouse with production of the aggregation-prone mutant γ2 subunits had a more severe epilepsy phenotype than the mouse that had simple Gabrg2 haploinsufficiency without the mutant γ2 subunit protein produced (Warner et al., 2016). Protein structure modeling indicates that different mutant γ2 subunits have differing stabilities and interactions with their wild-type subunit binding partners because they adopt different conformations and have different surface hydrophobicities and different tendencies to dimerize. The mutant γ2 subunit associated with the most severe epilepsy phenotype is more likely to form dimers/oligomers than other γ2 mutants, and these oligomers are prone to form ring-like structures (Wang et al., 2016). However, to date, it is unknown if there is a predictable trend for how the type and location of mutation may correlate with disease severity.
Recently, many mutations in GABRB3 have been associated with febrile seizures, absence seizures, autism, infantile spasm, and Lennox-Gaustaut syndrome (Moller et al., 2017b). Some patients are associated with uncharacterized seizures and mental disability (Hamdan et al., 2014). The detailed biological consequences resulting from these mutations are less clear. The functional assays of GABRB3 mutations in an in vitro cell system indicate the mutations reduced receptor trafficking and gating, resulting in a reduced net channel function (Janve et al., 2016). Future study from an in vivo mouse model may shed more light on understanding the defect caused by GABRB3 mutations.
Mutations in GABRB2 and GABRD have also been associated with different epilepsy syndromes. The epilepsy phenotype of GABRB2 mutations include early onset myoclonic encephalopathy or generalized tonic clonic seizures and atypical seizures with intellectual disability (Ishii et al., 2017b;Srivastava et al., 2014). The mutations in GABRD are associated with febrile seizures and juvenile myoclonic epilepsy (Dibbens et al., 2004;Feng et al., 2006).
Impaired trafficking is a major abnormality resulting from GABAA receptor subunit gene mutations
We have demonstrated that loss or impairment of subunit protein on the cell surface is the most common defect for all the missense, nonsense, and other premature termination codon (PTC)-generating GABAA receptor subunit gene mutations, although gating defects has also been identified in some mutations. The reduced cell surface expression could be accompanied by a reduction of total subunit protein or, in some cases, an increased amount of the mutant protein intracellularly. This seems counterintuitive but the mutant protein is not functional, thus explaining the pathophysiology of disease phenotype. We demonstrated that the mutant GABAA receptor subunits (eg. GABRG2(R82Q), GABRG2(R136X), GABRG2(Q390X)) are retained inside the ER, which is the location where the immature GABAA receptor subunit resides once synthesized. With glycosylation studies, we have identified all the mutant subunits that have arrested glycosylation. When coexpressed with the wild-type partnering subunits, the mutant subunits only adopt the ER glycosylation that is the core glycosylation for the immature subunits, while the wild-type subunits have mature glycosylation, suggesting subunit trafficking beyond the trans-Golgi to the cell surface. The mutant subunits with only core glycosylation are retained in the ER, suggesting that they are nonfunctional.
GABAA receptor mutations only cause loss or impaired function of the mutant subunit.
To date most insights into the functional defects are from studies in GABRG2 subunits, although some studies have been carried out in other GABR gene mutations. There are some GABRG2 mutations that may result in a simple loss of function, nearly a simple loss of, or impaired function of the subunit. For example, we have demonstrated that there was no dominant negative suppression by γ2(R136X) subunits on the wild-type partnering subunits like the α1 subunit (Johnston et al., 2014). These mutations often result in a mutant subunit protein that is readily degraded without much interference of the biogenesis and function of the remaining wild-type subunits. Alternatively, nonsense or PTC generating mutations could result in nonsense mediated decay (NMD) that eliminates the mutant allele at the mRNA level if the PTCs occur in an early exon and activate NMD. The functional consequence of these mutations would be similar to the Gabrg2+/− knockout condition, which may represent a simple haploinsufficiency condition. It is interesting that mutations in GABRG2 are more likely to be associated with febrile seizures than other GABAA receptor subunits (Boillot et al., 2015).
GABAA receptor epilepsy mutations cause cellular toxicity in addition to the loss or impaired function of the mutant subunit
Some GABAA receptor mutations may cause severe dominant negative suppression of the wild-type GABAA receptors while some mutations only cause simple haploinsufficiency or mild dominant negative suppression of the wild-type subunits. We have extensively studied GABRG2 nonsense mutations and identified the degradation rate of the mutant protein is likely the modifier of dominant negative suppression and epilepsy phenotype (Kang et al., 2013). Using the nonsense GABRG2 mutations as example, despite loss of function for all the truncated subunits, we have demonstrated that R136X has no dominant negative effect on the remaining α1β2 subunits and Q390X has a strong dominant negative suppression of the wild-type subunits, while W429X has a mild dominant negative effect on the remaining α1β2 subunits (Kang et al., 2009;Kang et al., 2013). Thus the degree of dominant negative suppression of each mutant γ2 subunit varies, likely depending on the specific structural disturbance of each specific mutation (Wang et al., 2016).
The comparison of mouse models of Gabrg2 knockout and Gabrg2+/Q390X knockin mice has validated the hypothesis from in vitro studies. Gabrg2 knockout mice displayed infrequent absence epilepsy in a seizure prone genetic background or only hyper-anxiety (Crestani et al., 1999;Reid et al., 2013;Warner et al., 2016). By contrast, Gabrg2+/Q390X knockin mice displayed spontaneous seizures, multiple neuropsychiatric comorbidities, and sudden death, featuring the major presentations of Dravet syndrome (Kang et al., 2015). Consistently, data from both in vitro and in vivo models indicate a slight compensatory increase of wild-type subunits in the half gene dose or heterozygous Gabrg2+/− knockout condition but a reduction of wild-type subunits in the dominant negative mutant condition. In addition to impaired channel function, the mutations with dominant negative suppression may also cause neuronal injury or death because of the sustained production and accumulation of the mutant toxic protein. The mutant subunits with dominant negative suppression are likely to form protein aggregates and may disturb cellular homeostasis because of the sustained production of the mutant protein (Kang et al., 2015).
Conclusions
The findings from clinic patients and experimental animal models indicate impaired GABAergic signaling is a common converging pathway underlying multiple epilepsy syndromes associated with these ion channel or non ion channel mutations. This thus suggests that modulating GABAergic signaling remains as an essential therapeutic approach for genetic epilepsy. Although the diagnosis can be highly precise with genetic sequencing, it is unlikely that each specific epilepsy syndrome could have a specific drug developed with the capacity of current technology. It is critical to identify a few common converging pathways that could serve as therapeutic target for more than one epilepsy syndrome. In this scenario, enhancing GABAergic signaling would be an ideal—if not the best—choice for therapeutic target.
Traditionally, there are a few approaches to enhance GABAergic signaling, which include increasing GABA and enhancing GABAA receptor function. One approach to increase GABA levels is via modifying or inhibiting the activity of enzymes and transporters that alter the dynamics of GABA. The examples of ASDs that modify GABA dynamics include valproate, gabapentin, and vigabatrin, which increase cellular GABA by inhibiting GABA-transaminase. Another approach is to selectively increase GABA release. However, there is no ASD available for this specific action. Currently, one of the widely used ASDs, levetiracetam (Keppra), specifically binds to the synaptic vesicle protein SV2A and reduces excitatory neurotransmitter release during trains of high frequency activity. SV2A knockout mice display a severe seizure after the first week indicating that SV2A may regulate signaling cascades involved in seizure generation (Crowder et al., 1999). Keppra has also been reported to affect both glutamate and GABA release (Meehan et al., 2012) but the vesicular release machinery may act differentially in glutamatergic and GABAergic nerve terminals (Janz et al., 1999). In the future, more selective ASDs could be designed to specifically enhance GABA release or reduce glutamate release if the vesicular release mechanisms that differentiate pyramidal cells and interneurons are clearly elucidated.
In addition to traditional ASDs, promoting protein trafficking would be another reasonable approach to enhance GABAergic signaling. Although there is no data available, based on the findings from GABRG2 mutations (Wang et al., 2016;Warner et al., 2016;Xia et al., 2016), promoting GABAA receptor trafficking could attenuate disease phenotype. Potential therapeutic approaches would include increasing wild-type and/or mutant GABAA receptor channel numbers and function, or decreasing the disturbance of the cellular signaling by the presence of the mutant GABAA receptor subunit protein. The drug, whether via direct modulation of the receptor function or increasing receptor trafficking, should be effective in compensating the lost or impaired GABAA channel function. Thus, a combined therapeutic strategy to enhance the wild-type GABAA receptor channel function and eliminate production of mutant protein or promoting protein homeostasis might be beneficial.
Rapid advances in gene editing technology like CRISPR/Cas9 have brought new hope for the treatment of genetic diseases including epilepsies. For example, a recent report on using CRISPR/Cas9-mediated gene editing has successfully ameliorated neurotoxicity and alleviated disease phenotype in a mouse model of Huntington’s disease (Yang et al., 2017). Given that CRISPR/Cas9 can permanently eliminate the expression of targeted genes, use of this approach should be able to remove the mutant allele, thus preventing the production of the mutant protein and attenuating disease phenotype, especially in the condition with a dominant negative mutation like GABRG2(Q390X).
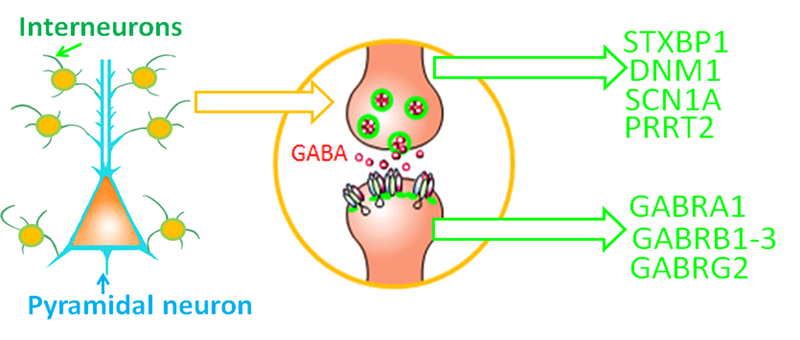
Figure 2.
Mutations via impairing both pre-synaptic GABA neurotransmitter release and post-synaptic GABAA receptor expression and function can affect GABAergic signaling. Synaptic transmission relies on the availability of the neurotransmitter; the release of the neurotransmitter by exocytosis and the binding of the normal functional postsynaptic receptor by the neurotransmitter. (A). Interneurons are the main source of cortical modulation over glutamatergic pyramidal cells (PCs). GABA-releasing interneurons as classified by a complex combination of morphological, connectivity, and intrinsic electrophysiological properties and molecular content are critical for cortical inhibition. (B). Mutations associated with epilepsy could impair both the proteins involving in pre-synaptic GABA release and post-synaptic GABAA receptor function.
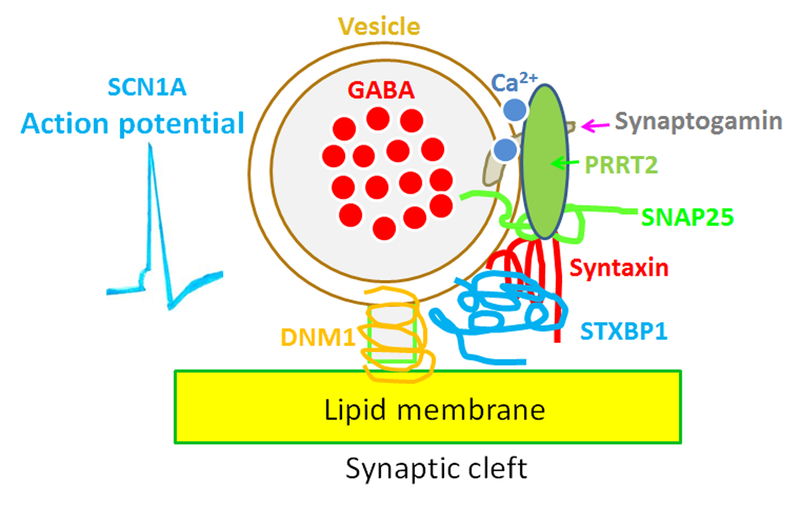
Figure 3.
Diverse defects caused by mutations in different genes impair GABA neurotransmitter release. In a given neuron, opening of sodium channels encoded by SCN1A and influx of Na+ cause neuronal firing in which sodium channels are responsible for the rising phase of action potentials. Calcium enters the axon terminal during an action potential, causing release of the neurotransmitter into the synaptic cleft. Synaptogamin acts as a calcium sensor which binds calcium and activates vesicle fusion. Gene mutations that encode proteins involved in the process of vesicle release include but are not limited to PRRT2, SNAP25, syntaxin, STXBP1 and DNM1. Although the biological function of each gene still requires further study, it has been proposed that these proteins are essential for making up the complicated vesicle release machinery for vesicle docking, fusion and exocytosis.
Table 1.
EE genes affecting GABAergic signaling and their postulated molecular defects.
| GENE | LOCUS | pre- or post- synaptic mechanism |
POSTULATED MECHANISMS | PHENOTYPES | REFERENCES (for mechanisms) |
|---|---|---|---|---|---|
| SCN1A | 2q24 | pre-synaptic | impaired interneuron activity | GEFS+, DS | Yu et al, 2006 |
| PRRT2 | 16p11.2 | pre-synaptic | impaired neurotransmitter release? | IS,EE, PKD | Valente et al, 2016 |
| STXBP1 | 9q34.11 | pre-synaptic | impaired neurotransmitter release? | GEFS+, DS | Patzke et al., 2015 |
| STX1B | 16p11.2 | pre-synaptic | impaired neurotransmitter release? | FS, epilepsy | Schubert et al, 2014 |
| SNAP25 | 20p12.2 | pre-synaptic | impaired neurotransmitter release? | epilepsy and ID | Rohena et al., 2013 |
| DNM1 | 9q34.11 | pre-synaptic | impaired neurotransmitter release? | IS, LGS, EE | Asinof et al 2015 |
| GABRA1 | 5q34 | post-synaptic | NMD, ERAD | CAE, JME, EE, DS | Kang et al, 2009 |
| GABRB2 | 5q34 | post-synaptic | ERAD, reduced surface expression | EME | Ishii et al, 2016 |
| GABRB3 | 15q12 | post-synaptic | reduced surface expression, gating defect | CAE, IS, LGS, DS | Janve et al, 2016 |
| GABRG2 | 5q34 | post-synaptic | NMD, ERAD,gating defect, neuronal injury | FS, GEFS+,DS,EE | Kang et al, 2016 |
DS=Dravet syndrome, IS=infantile spasm, EE=epileptic encephalopathy
PKD=paroxysmal kinesigenic dyskinesia, ID=intellectual disability
CAE=childhood absence epilepsy, JME=Juvenile myoclonic epilepsy
EME=early myoclonic encephalopathy, FS=febrile seizures
LGS=Lennox Gaustaut syndrome
GEFS+=generalized epilepsy with febrile seizure plus
Highlights.
Impaired GABAergic signaling is a converging pathway for epilepsies associated with mutations in several unrelated genes.
GABAergic signaling could be impaired via pre- and post-synaptic mechanisms.
Mutations in some genes like STXBP1, DNM1, PRRT2 and SCN1A may impair GABA release via pre-synaptic mechanisms.
Mutations in some genes like GABRA1, GABRG2, GABRB2 and GABRB3 impair GABAergic signaling via post-synaptic mechanisms.
Modulating GABAergic signaling remains an essential therapeutic approach for genetic epilepsies.
Acknowledgements:
Research was supported by grants from Citizen United for Research in Epilepsy (CURE), Dravet syndrome foundation (DSF), Dravet.org which is previously named IDEAleague, Vanderbilt Clinical and Translation Science Award and NINDS R01 NS082635 to J.Q.K.
Abbreviations:
- IGEs
idiopathic generalized epilepsies
- GGE
genetic generalized epilepsies
- CAE
childhood absence epilepsy
- JME
juvenile myoclonic epilepsy
- ASDs
antiseizure drugs
Reference List
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B, 2005. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci 25, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AS, Berkovic SF, Cossette P, Delanty N, Dlugos D, Eichler EE, Epstein MP, Glauser T, Goldstein DB, Han Y, Heinzen EL, Hitomi Y, Howell KB, Johnson MR, Kuzniecky R, Lowenstein DH, Lu YF, Madou MR, Marson AG, Mefford HC, Esmaeeli NS, O’Brien TJ, Ottman R, Petrovski S, Poduri A, Ruzzo EK, Scheffer IE, Sherr EH, Yuskaitis CJ, Abou-Khalil B, Alldredge BK, Bautista JF, Berkovic SF, Boro A, Cascino GD, Consalvo D, Crumrine P, Devinsky O, Dlugos D, Epstein MP, Fiol M, Fountain NB, French J, Friedman D, Geller EB, Glauser T, Glynn S, Haut SR, Hayward J, Helmers SL, Joshi S, Kanner A, Kirsch HE, Knowlton RC, Kossoff EH, Kuperman R, Kuzniecky R, Lowenstein DH, McGuire SM, Motika PV, Novotny EJ, Ottman R, Paolicchi JM, Parent JM, Park K, Poduri A, Scheffer IE, Shellhaas RA, Sherr EH, Shih JJ, Singh R, Sirven J, Smith MC, Sullivan J, Lin TL, Venkat A, Vining EP, Von Allmen GK, Weisenberg JL, Widdess-Walsh P, Winawer MR, 2013. De novo mutations in epileptic encephalopathies. Nature 501, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Berkovic S, Dulac O, Gardiner M, Jain S, Laue FM, Lindhout D, Noebels J, Ottman R, Scaramelli A, Serratosa J, Steinlein O, Avanzini G, Bailey-Wilson J, Cardon L, Fischbach R, Gwinn-Hardy K, Leppert M, Ott J, Lindblad-Toh K, Weiss K, 2002. ILAE genetics commission conference report: molecular analysis of complex genetic epilepsies. Epilepsia 43, 1262–1267. [DOI] [PubMed] [Google Scholar]
- Arain F, Zhou C, Ding L, Zaidi S, Gallagher MJ, 2015. The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy. Neurobiol Dis 82, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain FM, Boyd KL, Gallagher MJ, 2012. Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor alpha1 subunit. Epilepsia 53, e161–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asinof S, Mahaffey C, Beyer B, Frankel WN, Boumil R, 2016. Dynamin 1 isoform roles in a mouse model of severe childhood epileptic encephalopathy. Neurobiol Dis 95, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asinof SK, Sukoff Rizzo SJ, Buckley AR, Beyer BJ, Letts VA, Frankel WN, Boumil RM, 2015. Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy. PLoS Genet 11, e1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL, De Jonghe P, 2006. A novel GABRG2 mutation associated with febrile seizures. Neurology 67, 687–690. [DOI] [PubMed] [Google Scholar]
- Banerjee PK, Tillakaratne NJ, Brailowsky S, Olsen RW, Tobin AJ, Snead OC III, 1998. Alterations in GABAA receptor alpha 1 and alpha 4 subunit mRNA levels in thalamic relay nuclei following absence-like seizures in rats. Exp Neurol 154, 213–223. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E, 2001. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 28, 46–48. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL, 2003. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci 23, 10934–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillot M, Morin-Brureau M, Picard F, Weckhuysen S, Lambrecq V, Minetti C, Striano P, Zara F, Iacomino M, Ishida S, An-Gourfinkel I, Daniau M, Hardies K, Baulac M, Dulac O, LeGuern E, Nabbout R, Baulac S, 2015. Novel GABRG2 mutations cause familial febrile seizures. Neurol Genet 1, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumil RM, Letts VA, Roberts MC, Lenz C, Mahaffey CL, Zhang ZW, Moser T, Frankel WN, 2010. A missense mutation in a highly conserved alternate exon of dynamin-1 causes epilepsy in fitful mice. PLoS Genet 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomall E, Natale JE, Grimason M, Goldstein J, Smith CM, Chang C, Kanes S, Rogawski MA, Wainwright MS, 2014. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol 76, 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Moller RS, Hjalgrim H, Cook J, Geraghty E, O’Roak BJ, Petrou S, Clarke A, Gill D, Sadleir LG, Muhle H, von SS, Nikanorova M, Hodgson BL, Gazina EV, Suls A, Shendure J, Dibbens LM, De JP, Helbig I, Berkovic SF, Scheffer IE, Mefford HC, 2014. GABRA1 and STXBP1: Novel genetic causes of Dravet syndrome. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, Guo SL, He J, Chen YF, Zhang QJ, Li HF, Lin Y, Murong SX, Xu J, Wang N, Wu ZY, 2011. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet 43, 1252–1255. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA, 2003. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci 17, 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA, 2002. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet 31, 184–189. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H, 1999. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci 2, 833–839. [DOI] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM, 1999. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A 96, 15268–15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty RJ, Kang JQ, Brune CW, Kistner EO, Courchesne E, Cox NJ, Cook EH Jr., Macdonald RL, Sutcliffe JS, 2011. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol Psychiatry 16, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC, 2004. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet 13, 1315–1319. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL, 2010. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51, 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MS, Viola-McCabe KE, Caspary DM, Faingold CL, 1994. Loss of synaptic inhibition during repetitive stimulation in genetically epilepsy-prone rats (GEPR). Epilepsy Res 18, 97–105. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL, 2006. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors. J Neurosci 26, 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Naritoku DK, Randall ME, Faingold CL, 2001. Modulation of audiogenically kindled seizures by gamma-aminobutyric acid-related mechanisms in the amygdala. Exp Neurol 172, 477–481. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Kapur J, 2009. The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia 50, 2011–2018. [DOI] [PubMed] [Google Scholar]
- Guo D, Zeng L, Brody DL, Wong M, 2013. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. Plos One 8, e64078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Srour M, Capo-Chichi JM, Daoud H, Nassif C, Patry L, Massicotte C, Ambalavanan A, Spiegelman D, Diallo O, Henrion E, Dionne-Laporte A, Fougerat A, Pshezhetsky AV, Venkateswaran S, Rouleau GA, Michaud JL, 2014. De novo mutations in moderate or severe intellectual disability. PLoS Genet 10, e1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA, 2012. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S, 2002. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet 70, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, 1994. The prevalence and incidence of convulsive disorders in children. Epilepsia 35 Suppl 2, S1–S6. [DOI] [PubMed] [Google Scholar]
- Heron SE, Grinton BE, Kivity S, Afawi Z, Zuberi SM, Hughes JN, Pridmore C, Hodgson BL, Iona X, Sadleir LG, Pelekanos J, Herlenius E, Goldberg-Stern H, Bassan H, Haan E, Korczyn AD, Gardner AE, Corbett MA, Gecz J, Thomas PQ, Mulley JC, Berkovic SF, Scheffer IE, Dibbens LM, 2012. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet 90, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de FA, Saiz A, Dalmau J, Graus F, 2013. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 81, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, 2014. Do structural changes in GABA neurons give rise to the epileptic state? Adv Exp Med Biol 813, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Baraban SC, 2015. Interneuron Transplantation as a Treatment for Epilepsy. Cold Spring Harb Perspect Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC, 2013. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci 16, 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Kang JQ, Schornak CC, Hernandez CC, Shen W, Watkins JC, Macdonald RL, Hirose S, 2017a. A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J Med Genet 54, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Kang JQ, Schornak CC, Hernandez CC, Shen W, Watkins JC, Macdonald RL, Hirose S, 2017b. A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J Med Genet 54, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, 2014. “It was the interneuron with the parvalbumin in the hippocampus!” “no, it was the pyramidal cell with the glutamate in the cortex!” searching for clues to the mechanism of dravet syndrome - the plot thickens. Epilepsy Curr 14, 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon P, Latour P, 2005. Epidemiology of idiopathic generalized epilepsies. Epilepsia 46 Suppl 9, 10–14. [DOI] [PubMed] [Google Scholar]
- Janve VS, Hernandez CC, Verdier KM, Hu N, Macdonald RL, 2016. Epileptic encephalopathy de novo GABRB mutations impair GABAA receptor function. Ann Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Sudhof TC, 1999. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24, 1003–1016. [DOI] [PubMed] [Google Scholar]
- Johannesen K, Marini C, Pfeffer S, Moller RS, Dorn T, Niturad CE, Gardella E, Weber Y, Sondergard M, Hjalgrim H, Nikanorova M, Becker F, Larsen LH, Dahl HA, Maier O, Mei D, Biskup S, Klein KM, Reif PS, Rosenow F, Elias AF, Hudson C, Helbig KL, Schubert-Bast S, Scordo MR, Craiu D, Djemie T, Hoffman-Zacharska D, Caglayan H, Helbig I, Serratosa J, Striano P, De JP, Weckhuysen S, Suls A, Muru K, Talvik I, Talvik T, Muhle H, Borggraefe I, Rost I, Guerrini R, Lerche H, Lemke JR, Rubboli G, Maljevic S, 2016. Phenotypic spectrum of GABRA1: From generalized epilepsies to severe epileptic encephalopathies. Neurology 87, 1140–1151. [DOI] [PubMed] [Google Scholar]
- Johnston AJ, Kang JQ, Shen W, Pickrell WO, Cushion TD, Davies JS, Baer K, Mullins JG, Hammond CL, Chung SK, Thomas RH, White C, Smith PE, Macdonald RL, Rees MI, 2014. A Novel GABRG2 mutation, p.R136*, in a family with GEFS+ and extended phenotypes. Neurobiol Dis 64, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA, 2013. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest 123, 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA, 2007. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci 27, 11065–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A, Steinlein OK, 2002. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol 59, 1137–1141. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Macdonald RL, 2016. Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL, 2009. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci 29, 2845–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL, 2013. Trafficking-deficient mutant GABRG2 subunit amount may modify epilepsy phenotype. Ann Neurol 74, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Zhou C, Xu D, Macdonald RL, 2015. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat Neurosci 18, 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL, 1997. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 17, 7532–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle J, Woldbye DP, Elster L, Diemer NH, Bolwig TG, Olsen RW, Nielsen M, 1998. Antisense oligonucleotide to GABA(A) receptor gamma2 subunit induces limbic status epilepticus. J Neurosci Res 54, 863–869. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S, 1997. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A 94, 14060–14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J, 2011. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 145, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera H, Ohba C, Kato M, Maeda T, Araki K, Tajima D, Matsuo M, Hino-Fukuyo N, Kohashi K, Ishiyama A, Takeshita S, Motoi H, Kitamura T, Kikuchi A, Tsurusaki Y, Nakashima M, Miyake N, Sasaki M, Kure S, Haginoya K, Saitsu H, Matsumoto N, 2016. De novo GABRA1 mutations in Ohtahara and West syndromes. Epilepsia 57, 566–573. [DOI] [PubMed] [Google Scholar]
- Kohling R, Vreugdenhil M, Bracci E, Jefferys JG, 2000. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci 20, 6820–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall RL, Penry JK, Kupferberg HJ, Swinyard EA, 1978. Antiepileptic drug development: I. History and a program for progress. Epilepsia 19, 393–408. [DOI] [PubMed] [Google Scholar]
- Kruer MC, Hoeftberger R, Lim KY, Coryell JC, Svoboda MD, Woltjer RL, Dalmau J, 2014. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: the first pediatric case of gamma-aminobutyric acid type B receptor autoimmunity. JAMA Neurol 71, 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure S, Sakata Y, Miyabayashi S, Takahashi K, Shinka T, Matsubara Y, Hoshino H, Narisawa K, 1998. Mutation and polymorphic marker analyses of 65K- and 67K-glutamate decarboxylase genes in two families with pyridoxine-dependent epilepsy. J Hum Genet 43, 128–131. [DOI] [PubMed] [Google Scholar]
- Law C, Schaan PM, Levesque M, Kaltschmidt JA, Verhage M, Kania A, 2016. Normal Molecular Specification and Neurodegenerative Disease-Like Death of Spinal Neurons Lacking the SNARE-Associated Synaptic Protein Munc18–1. J Neurosci 36, 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O’Malley HA, Patino GA, O’Brien JE, Rusconi R, Gupta A, Thompson RC, Natowicz MR, Meisler MH, Isom LL, Parent JM, 2013. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol 74, 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, 2012. Epileptogenesis: can the science of epigenetics give us answers? Epilepsy Curr 12, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Su L, Seven AB, Xu Y, Rizo J, 2013. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 339, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Prince DA, 2012. Functional alterations in GABAergic fast-spiking interneurons in chronically injured epileptogenic neocortex. Neurobiol Dis 47, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ, 2010. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol 588, 1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ, Feng HJ, 2006. GABA(A) receptor mutations associated with generalized epilepsies. Adv Pharmacol 54, 147–169. [DOI] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Suls A, De JP, Zara F, Guerrini R, 2011. The genetics of Dravet syndrome. Epilepsia 52 Suppl 2, 24–29. [DOI] [PubMed] [Google Scholar]
- Meehan AL, Yang X, Yuan LL, Rothman SM, 2012. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia 53, 469–476. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA, 2005. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest 115, 2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, 1996. Update on the mechanism of action of antiepileptic drugs. Epilepsia 37 Suppl 6, S4–11. [DOI] [PubMed] [Google Scholar]
- Merwick A, O’Brien M, Delanty N, 2012. Complex single gene disorders and epilepsy. Epilepsia 53 Suppl 4, 81–91. [DOI] [PubMed] [Google Scholar]
- Michetti C, Castroflorio E, Marchionni I, Forte N, Sterlini B, Binda F, Fruscione F, Baldelli P, Valtorta F, Zara F, Corradi A, Benfenati F, 2017. The PRRT2 knockout mouse recapitulates the neurological diseases associated with PRRT2 mutations. Neurobiol Dis 99, 66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller RS, Wuttke TV, Helbig I, Marini C, Johannesen KM, Brilstra EH, Vaher U, Borggraefe I, Talvik I, Talvik T, Kluger G, Francois LL, Lesca G, de BJ, Blichfeldt S, Chatron N, Holert N, Jacobs J, Swinkels M, Betzler C, Syrbe S, Nikanorova M, Myers CT, Larsen LH, Vejzovic S, Pendziwiat M, von SS, Hopkins S, Dubbs H, Mang Y, Mukhin K, Holthausen H, van Gassen KL, Dahl HA, Tommerup N, Mefford HC, Rubboli G, Guerrini R, Lemke JR, Lerche H, Muhle H, Maljevic S, 2017a. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology 88, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller RS, Wuttke TV, Helbig I, Marini C, Johannesen KM, Brilstra EH, Vaher U, Borggraefe I, Talvik I, Talvik T, Kluger G, Francois LL, Lesca G, de BJ, Blichfeldt S, Chatron N, Holert N, Jacobs J, Swinkels M, Betzler C, Syrbe S, Nikanorova M, Myers CT, Larsen LH, Vejzovic S, Pendziwiat M, von SS, Hopkins S, Dubbs H, Mang Y, Mukhin K, Holthausen H, van Gassen KL, Dahl HA, Tommerup N, Mefford HC, Rubboli G, Guerrini R, Lemke JR, Lerche H, Muhle H, Maljevic S, 2017b. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology 88, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva M, Berkovic SF, Petrou S, 2012. Sodium channels and the neurobiology of epilepsy. Epilepsia 53, 1849–1859. [DOI] [PubMed] [Google Scholar]
- Patzke C, Han Y, Covy J, Yi F, Maxeiner S, Wernig M, Sudhof TC, 2015. Analysis of conditional heterozygous STXBP1 mutations in human neurons. J Clin Invest 125, 3560–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Rothman DL, 1998. Measuring human brain GABA in vivo: effects of GABA-transaminase inhibition with vigabatrin. Mol Neurobiol 16, 97–121. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Brown LA, Tynan S, Willick G, William R, McIntyre DC, 1999. Differential expression of alpha1, alpha2, alpha3, and alpha5 GABAA receptor subunits in seizure-prone and seizure-resistant rat models of temporal lobe epilepsy. J Neurosci 19, 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, 2013. Interneuron development and epilepsy: early genetic defects cause long-term consequences in seizures and susceptibility. Epilepsy Curr 13, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Kim T, Phillips AM, Low J, Berkovic SF, Luscher B, Petrou S, 2013. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology 80, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Jahnsen H, Mosfeldt LA, 1990. The effect of two lipophilic gamma-aminobutyric acid uptake blockers in CA1 of the rat hippocampal slice. Br J Pharmacol 99, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C, 2013. Neuroactive steroids for the treatment of status epilepticus. Epilepsia 54 Suppl 6, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohena L, Neidich J, Truitt CM, Gonzalez KD, Tang S, Devinsky O, Chung WK, 2013. Mutation in SNAP25 as a novel genetic cause of epilepsy and intellectual disability. Rare Dis 1, e26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Han S, Tai C, Westenbroek RE, Hunker A, Scheuer T, Catterall WA, 2015. Dissecting the phenotypes of Dravet syndrome by gene deletion. Brain 138, 2219–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, Okada I, Yoshimura Y, Hirai S, Kumada T, Hayasaka K, Fukuda A, Ogata K, Matsumoto N, 2008. De novo mutations in the gene encoding STXBP1 (MUNC18–1) cause early infantile epileptic encephalopathy. Nat Genet 40, 782–788. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Brooks-Kayal AR, 2014. Is plasticity of GABAergic mechanisms relevant to epileptogenesis? Adv Exp Med Biol 813, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshe SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM, 2017. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58, 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Grinton BE, Heron SE, Kivity S, Afawi Z, Iona X, Goldberg-Stern H, Kinali M, Andrews I, Guerrini R, Marini C, Sadleir LG, Berkovic SF, Dibbens LM, 2012. PRRT2 phenotypic spectrum includes sporadic and fever-related infantile seizures. Neurology 79, 2104–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Siekierska A, Langlois M, May P, Huneau C, Becker F, Muhle H, Suls A, Lemke JR, de Kovel CG, Thiele H, Konrad K, Kawalia A, Toliat MR, Sander T, Ruschendorf F, Caliebe A, Nagel I, Kohl B, Kecskes A, Jacmin M, Hardies K, Weckhuysen S, Riesch E, Dorn T, Brilstra EH, Baulac S, Moller RS, Hjalgrim H, Koeleman BP, Jurkat-Rott K, Lehman-Horn F, Roach JC, Glusman G, Hood L, Galas DJ, Martin B, de Witte PA, Biskup S, De JP, Helbig I, Balling R, Nurnberg P, Crawford AD, Esguerra CV, Weber YG, Lerche H, 2014. Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat Genet 46, 1327–1332. [DOI] [PubMed] [Google Scholar]
- Shen D, Hernandez CC, Shen W, Hu N, Poduri A, Shiedley B, Rotenberg A, Datta AN, Leiz S, Patzer S, Boor R, Ramsey K, Goldberg E, Helbig I, Ortiz-Gonzalez XR, Lemke JR, Marsh ED, Macdonald RL, 2017. De novo GABRG2 mutations associated with epileptic encephalopathies. Brain 140, 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL, 2001. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci 21, 7481–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, Klein P, Tsai J, 2017. Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 58, 558–564. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Cohen J, Pevsner J, Aradhya S, McKnight D, Butler E, Johnston M, Fatemi A, 2014. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am J Med Genet A 164A, 2914–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamberger H, Nikanorova M, Willemsen MH, Accorsi P, Angriman M, Baier H, Benkel-Herrenbrueck I, Benoit V, Budetta M, Caliebe A, Cantalupo G, Capovilla G, Casara G, Courage C, Deprez M, Destree A, Dilena R, Erasmus CE, Fannemel M, Fjaer R, Giordano L, Helbig KL, Heyne HO, Klepper J, Kluger GJ, Lederer D, Lodi M, Maier O, Merkenschlager A, Michelberger N, Minetti C, Muhle H, Phalin J, Ramsey K, Romeo A, Schallner J, Schanze I, Shinawi M, Sleegers K, Sterbova K, Syrbe S, Traverso M, Tzschach A, Uldall P, Van CR, Verhelst H, Viri M, Winter S, Wolff M, Zenker M, Zoccante L, De JP, Helbig I, Striano P, Lemke JR, Moller RS, Weckhuysen S, 2016. STXBP1 encephalopathy: A neurodevelopmental disorder including epilepsy. Neurology 86, 954–962. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang Y, Liang J, Liu X, Ma X, Wu H, Xu K, Qin J, Qi Y, Wu X, 2008. SCN1A, SCN1B, and GABRG2 gene mutation analysis in Chinese families with generalized epilepsy with febrile seizures plus. J Hum Genet 53, 769–774. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kawakami K, Nakamura F, Nishimura S, Yagi K, Seino M, 1994. Bromide, in the therapeutic concentration, enhances GABA-activated currents in cultured neurons of rat cerebral cortex. Epilepsy Res 19, 89–97. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Olsen RW, Medina MT, Schwartz E, Alonso ME, Duron RM, Castro-Ortega R, Martinez-Juarez IE, Pascual-Castroviejo I, Machado-Salas J, Silva R, Bailey JN, Bai D, Ochoa A, Jara-Prado A, Pineda G, Macdonald RL, Delgado-Escueta AV, 2008. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet 82, 1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP, Meldrum BS, 1995. Na+ channels as targets for neuroprotective drugs. Trends Pharmacol Sci 16, 309–316. [DOI] [PubMed] [Google Scholar]
- Toonen RF, Wierda K, Sons MS, de WH, Cornelisse LN, Brussaard A, Plomp JJ, Verhage M, 2006. Munc18–1 expression levels control synapse recovery by regulating readily releasable pool size. Proc Natl Acad Sci U S A 103, 18332–18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente P, Castroflorio E, Rossi P, Fadda M, Sterlini B, Cervigni RI, Prestigio C, Giovedi S, Onofri F, Mura E, Guarnieri FC, Marte A, Orlando M, Zara F, Fassio A, Valtorta F, Baldelli P, Corradi A, Benfenati F, 2016. PRRT2 Is a Key Component of the Ca(2+)-Dependent Neurotransmitter Release Machinery. Cell Rep 15, 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF, 2001. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 28, 49–52. [DOI] [PubMed] [Google Scholar]
- Wang J, Shen D, Xia G, Shen W, Macdonald RL, Xu D, Kang JQ, 2016. Differential protein structural disturbances and suppression of assembly partners produced by nonsense GABRG2 epilepsy mutations: implications for disease phenotypic heterogeneity. Sci Rep 6, 35294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang JQ, 2016. DIfferential molecular and behavioral alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Pourali P, Warner TA, Zhang CQ, Macdonald L, Kang JQ, 2016. Altered GABAA receptor expression in brainstem nuclei and SUDEP in Gabrg2(+/Q390X) mice associated with epileptic encephalopathy. Epilepsy Res 123, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Chang R, Yang H, Zhao T, Hong Y, Kong HE, Sun X, Qin Z, Jin P, Li S, Li XJ, 2017. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest 127, 2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA, 2006. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9, 1142–1149. [DOI] [PubMed] [Google Scholar]