Abstract
Background
Psoriasis (PSO) is an immune-mediated inflammatory disease associated with metabolic and cardiovascular comorbidities. It is now known that resolution of inflammation is an active process locally controlled by specialized pro-resolving mediators, (SPMs), named resolvins (Rv), protectins (PD) and maresins (MaR).
Objective
It is unknown whether these potent lipid mediators (LM) are involved in PSO pathophysiology and if skin and blood have disease specific SPMs phenotype profiles.
Methods
We used liquid chromatography-tandem mass spectrometry (LC-MS-MS)-based LM metabololipidomics to obtain skin and peripheral blood LM profiles from PSO compared to healthy subjects. Some LM were tested in cell culture experiments with corresponding gene expression and protein concentration analyses.
Results
The levels of several LM were significantly elevated in lesional PSO skin compared to non-lesional and skin from healthy subjects. Particularly, RvD5, PDx and aspirin-triggered (AT) forms of lipoxin (LX) were present only in lesional PSO skin whereas protectin D1 was present in non-lesional PSO skin. To determine specific roles of SPMs on skin-related inflammatory cytokines, RvD1 and RvD5 were incubated with human keratinocytes. RvD1 and RvD5 reduced the expression levels of IL24 and S100A12 whereas only RvD1 significantly abrogated IL-24 production by keratinocytes.
Conclusions
These findings suggest that an imbalance between locally produced pro-resolution and pro-inflammatory lipid mediators identified in PSO skin and blood compartments might play a role in PSO pathophysiology. Moreover, some of the psoriasis related cytokines can be modified by specific SPMs and involved mechanisms support investigation of targeting novel pro-resolving lipid mediators as a therapy for PSO.
Keywords: Inflammation, psoriasis, resolution, lipid mediators, essential polyunsaturated fatty acids, DHA
INTRODUCTION
Psoriasis (PSO) is an immune-mediated inflammatory skin disease1 associated with metabolic dysfunction and cardiovascular disease (CVD)2. Shared pathogenic mechanisms3 between psoriasis and comorbidities rooted in chronic systemic inflammation including cardiovascular disease and diabetes mellitus4 which in turn affect mortality rates5. Given that the precise understanding of psoriasis pathophysiology is lacking, the disease frequently recurs despite advances in treatment. Thus, searching for new pharmacological targets in biological pathways beyond cells and cytokines is warranted.
The process of inflammation and resolution involves omega-6 and omega-3 fatty acid derived mediators with arachidonic acid (AA) playing a critical role in the onset of the inflammatory cascade. AA is an omega-6 polyunsaturated fatty acid (PUFA), that gives rise to eicosanoid production. The biosynthesis of these mediators is dependent upon cyclooxygenases (COX-1, COX-2) or lipoxygenases (5-LOX, 12-LOX, 15-LOX) activity. These eicosanoids include prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs). Inhibition of COX-2 prostanoid production by acetylsalicylic acid (aspirin; ASA) switches the enzymatic activity from a prostaglandin endoperoxide synthase to a lipoxygenase that leads to the production of endogenous aspirin-triggered (AT) lipid mediators including lipoxins, which also have anti-inflammatory and pro-resolving actions6. These lipid signaling mediators are involved in a wide range of pathophysiological processes by modulating inflammatory responses adequate to the applied biostimuli and milieu7.
The inflammatory response is normally a self-limiting process that is now known to be an active combination of complex bioactive events resulting in resolution8. Research on resolution of inflammation and PUFAs has uncovered that enzymatic conversion of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) leads to the biosynthesis of potent mediators called specialized pro-resolving lipid mediators (SPMs)9. These bioactive mediators, which include resolvins (Rv), protectins (PD) and maresins (MaR), have anti-inflammatory and immunomodulating actions in many animal models with potential to treat human inflammatory diseases10, 11.
Studies are lacking to understand the direct actions of SPMs in psoriasis skin and peripheral blood from the same sample of participants. Prior studies made attempts to profile psoriasis skin12, 13 or peripheral blood14 for the presence of eicosanoids. However, these efforts predated the discovery of the new families of SPMs. Thus, understanding the biology of SPMs and their function in psoriasis may reveal new approaches that can be utilized for expanding understand treatment of the disease.
This report summarizes the first use of rigorous lipid mediator metabololipidomics to identify SPMs in human skin and peripheral blood of psoriasis compared to healthy participants. We hypothesized that lesional psoriasis skin may be a greater source of the targeted lipid mediators compared to non-lesional psoriasis and healthy skin given that SPM limit neutrophil tissue infiltration and stimulate resolution (7, 9, 11). Indeed, among identified bioactive mediators which were abundant in psoriasis lesional skin and peripheral blood, RvD5, PDx, AT-LXA4 and AT-LXB4 were only present in lesional skin. Altogether, this study documents the LM-SPM profiles in healthy and disease skin and identified a role for SPMs in counter-regulating pro-inflammatory cytokines specifically involved in skin psoriasis.
MATERIALS AND METHODS
Study population
Seven consecutive psoriatic and 7 healthy subjects were included in the study (age range 21 – 65 years) with effective size of 5 psoriasis individuals for the final LC-MS-MS–based metabololipidomics lesional skin analysis. All the enrolled subjects were Caucasians as part of an NIH clinical study (13H-0065). A diagnosis of generalized plaque psoriasis was confirmed and quantified by a dermatologist using the Psoriasis Area Severity Index (PASI) score. Corresponding healthy volunteers were consecutively recruited to undergo the same testing as the psoriasis subjects. All included study participants were exempt of any systemic anti-psoriatic treatments or topical therapy within 2 weeks before biopsy. Supplementation with omega-3 fatty acids was also taken into account for the final data interpretation. At baseline, 3 mm punch biopsies were obtained under local anesthesia from psoriatic acute plaque lesions and unaffected skin. Biopsy sites were selected based on active plaques and varied between subjects. However, biopsies of unaffected and control skin were predominantly from buttocks. Financial compensation was provided to study participants. Study approval was granted by the National Heart, Lung and Blood Institute institutional review board in keeping with the Declaration of Helsinki. All study participants submitted written informed consent prior to enrollment.
Blood collection
Peripheral blood from the same study enrolled subjects was collected in either serum separator or EDTA tubes and centrifuged for 20 min at 2400 rpm. Obtained serum and plasma were immediately stored at −80°C until analysis. Different blood fractions have been analyzed because the presence of targeted metabolites in these two compartments is unknown.
LC-MS-MS-based lipidomics of psoriasis skin and blood
Samples were taken to solid-phase extraction (SPE) using Isolute C18 SPE 3 mL cartridges (Biotage, USA), as in Colas et al.15. Briefly, internal standards (d8-5-HETE, d5-RvD2, d5-LXA4, d4-LTB4, d4-PGE2; 500 pg each; Cayman Chemicals, USA) were added along with four volumes of methanol before SPE, and covered on ice for 30-60 minutes to allow for protein precipitation. During SPE, 6 mL of water was eluted through each cartridge, followed by elution of 6 mL of hexane. Lipid mediators were collected by elution and collection of 6 mL of methyl formate. Methyl formate fractions from SPE were analyzed by a liquid chromatography-tandem mass spectrometry system, QTrap 5500 (AB Sciex) equipped with a Shimadzu LC-20AD HPLC (Tokyo, Japan). A Poroshell 120 EC-18 column (100 mm × 4.6 mm × 2.7 μm; Agilent Technologies, USA) was kept in a column oven maintained at 50°C, and lipid mediators (LMs) were eluted in a gradient of methanol/water/acetic acid from 55:45:0.01 (v/v/v) to 98:2:0.01 at 0.5 mL/min flow rate. In order to monitor and quantify the levels of targeted LMs, multiple reaction monitoring (MRM) was used with MS/MS matching signature ion fragments for each molecule (at least six diagnostic ions; ~0.1 pg limits of detection) and standard curves (r2>0.98 for each lipid mediator and pathway marker).
Cell culturing
The cells, media and related detaching kit were from PromoCell, Germany. Primary normal human epidermal keratinocytes (HNEK) were cultivated in complete keratinocyte growth media-2. Cells were used for 8 passages, subcultivated according to the manufacturer’s protocol using the Detach Kit and plated at a density of 1×104 cells per cm2. Cells were grown to confluence for 5-7 days under normal cell culture conditions (37 °C, 5% CO2) and seeded at the same density according to the experiment. Treatment included vehicle (0.1% ethanol), TNFα (10 ng/ml; R&D Systems, USA), RvD1/RvD5 (10 nM; Cayman Chemicals, USA) or TNFα + RvD1 and RvD5. Combination of Rvs with TNFα was done with 15 min RvD1 and RvD5 preincubation followed by TNFα treatment. Targeted concentration of 10 nM RvD1and RvD5 was used based on the published data reviewed elsewhere8, 10. All the treatments have been done in triplicates.
Measurement of IL-24 and S100A12
Cell lysate and supernatant from the cultured HNEK were used for estimating IL-24 and S100A12 concentrations by ELISA Kits (Biomatik, USA) at different time points, 3 h, 14 h and 24 h. ELISA Kits were used for measuring serum levels of IL-24 (RayBiotech, USA) and S100A12 (Elabscience, USA) in the enrolled subjects.
Gene expression analysis
After 6 h of experimental treatment HNEK were collected in TRIzol (Invitrogen) with further RNA isolation using a Direct-zol RNA MiniPrep kit (Zymo Research Corp, USA) according to the manufacturer’s protocol. RNA had an A260:A230 ratio greater than 1.7; an A260:A280 ratio of approximately 2.1 ± 0.1. Complementary DNA was created using a RT2 First Strand Kit (Qiagen, USA). Expression of each gene was measured individually by RT-PCR with SYBR Green (Qiagen, USA) assays by the Roche LightCycler 96 (Roche Diagnostics, USA) with GAPDH gene used for normalization. Relative expression of the genes was calculated by the comparative CT (ΔΔCT) method16. Standard error of the mean was calculated with the REST 2009 software from Qiagen.
Statistical analysis
Statistical analyses were done with mean ± SEM or median and interquartile range for continuous variables and frequencies (N, %) for categorical variables. Data were analyzed with a single 2-tailed unpaired / paired Student’s t test for parametric variables and the Mann-Whitney t test for non-parametric variables. The Pearson’s Chi-square test was used for categorical variables. Differences between cell treated groups were assessed by two-way ANOVA and paired two-tailed t-test for single comparisons. Analysis was performed using GraphPad Prism 6.02 (GraphPad Software Inc., La Jolla, CA, USA) and P ≤ 0.05 were considered statistically significant. Logistic regression analysis for PASI score with different bioactive metabolites and pathway precursors identified in psoriasis skin was performed by Stata/IC 12.0 (StataCorp LP, College Station, TX, USA). Principal component analysis was done by using SIMCA 13.0.3 (Umetrics, Umea, Sweden) with mean centering and unit variance scaling.
RESULTS
Study population
Demographic and clinical characteristics of the psoriasis (n=7) and corresponding healthy (n=7) study populations are presented in Table I. Recruitment scheme and study design were based on an ongoing prospective observational study17. Enrolled subjects were middle-aged Caucasians (mean age: psoriasis 42.29 years vs. healthy volunteers 41.71 years) without known history of tobacco or alcohol use. Half of the each group participants had a past medical history of hyperlipidemia (n=3, 43%). Complete lipid profiling showed no abnormalities between the groups. Psoriasis patients had median PASI score of 7.7 (interquartile range [IQR] 6.6–12.3), consistent with moderate-to-severe psoriasis. Average psoriasis disease duration in this cohort was 17.29 years. Most psoriasis subjects were on topical therapy (71.43%) with biologic management in 28.57% of cases. Only one psoriasis patient was on 500 mg of fish oil per day before enrollment. All study participants had 2 weeks of wash out period. More than half of the patients had associated psoriatic arthritis (57.14%). Additionally, high-sensitivity C-reactive protein (hsCRP) levels were significantly higher in the psoriasis subjects, compared to healthy volunteers (3.77 mg/L vs. 1.0 mg/L, correspondingly, P=0.002). Both psoriasis and healthy groups were at low risk for cardiovascular disease by Framingham risk score (FRS, median psoriasis 1, IQR 1–6; healthy volunteers1, IQR 1–7).
Table I.
Demographic and clinical characteristics of the study groups
| Parameter | PSO (N=7) | Healthy (N=7) | P |
|---|---|---|---|
|
| |||
| Demographics and medical history | |||
|
| |||
| Age (years) | 42.29 ± 6.38 | 41.71 ± 4.98 | 0.47 |
| Male sex, n (%) | 3 (43) | 5 (71) | 0.28 |
| Ethnicity, whites (%) | 7 (100) | 7 (100) | 1.00 |
| Body mass index (kg/m2) | 27.94 ± 1.43 | 27.16 ± 2.26 | 0.39 |
| Hypertension, n (%) | 1 (14) | 2 (28) | 0.52 |
| Hyperlipidemia, n (%) | 3 (43) | 3 (43) | 1.00 |
| Type 2 diabetes mellitus, n (%) | 1 (14) | 0 (0) | 0.30 |
| Current tobacco use, n (%) | 0 (0) | 0 (0) | 1.00 |
| Current alcohol use, n (%) | 0 (0) | 0 (0) | 1.00 |
|
| |||
| Clinical and laboratory values | |||
|
| |||
| Systolic blood pressure (mmHg) | 120.0 ± 4.70 | 114.3 ± 5.19 | 0.22 |
| Diastolic Blood Pressure (mmHg) | 70.57 ± 2.13 | 71.86 ± 5.04 | 0.41 |
| Total cholesterol (mg/dl) | 189.3 ± 14.95 | 186.1 ± 13.32 | 0.44 |
| Triglycerides (mg/dl) | 112.4 ± 17.07 | 170.0 ± 68.11 | 0.21 |
| HDL cholesterol (mg/dl) | 57.14 ± 3.85 | 56.00 ± 5.64 | 0.44 |
| LDL cholesterol (mg/dl) | 109.7 ± 11.06 | 97.00 ± 9.30 | 0.20 |
| ApoA1 (mg/L) | 164.0 ± 7.05 | 153.6 ± 6.87 | 0.16 |
| ApoB (mg/L) | 94.43 ± 8.30 | 81.71 ± 6.88 | 0.26 |
| ApoB / ApoA 1 | 0.76 ± 0.18 | 0.54 ± 0.05 | 0.14 |
| FRS (IQR) | 1 (1–6) | 1 (1–7) | 0.94 |
| hsCRP (mg/L) (IQR) | 3.77 (3.12–10.4) | 1 (0.7–1.5) | 0.002 |
| Glucose (mg/dl) | 105.30 ± 12.19 | 92.50 ± 3.32 | 0.18 |
| Insulin (mg/dl) | 10.63 ± 2.44 | 20.27 ± 12.15 | 0.23 |
| WBC (cells/L) | 6.46 ± 0.82 | 11.93 ± 6.88 | 0.22 |
|
| |||
| Psoriasis Severity and Treatment | |||
|
| |||
| Disease duration (years) | 17.29±3.93 | ||
| PASI score (IQR) | 7.7 (6.6–12.3) | ||
| Psoriatic arthritis, n (%) | 4 (57.14) | ||
| Topical therapy, n (%) | 5 (71.43) | ||
| Biologic therapy, n (%) | 2 (28.57) | ||
| Systemic therapy, n (%) | 0 (0) | ||
FRS, Framingham Risk Score; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein; PASI, psoriasis area and severity index; PSO, psoriasis; WBC, white blood cell. Data are mean±SE or median (Interquartile Range) for parametric and non-parametric variables respectively and as N (%) for categorical variables. P values were derived from a single unpaired 2-tailed t test for parametric variables and the Mann-Whitney test for non-parametric variables. The Pearson’s Chi-square test was used for categorical variables. P<0.05 was considered statistically significant.
Identification of SPMs in human psoriasis and healthy skin
We hypothesized that inflamed skin, known to associate with psoriasis catabasis, might affect the local bioactive lipid mediator metabolome8, 18. To this end, we performed a complete LC-MS-MS-based lipid mediator metabololipidomics profiling of tissue samples from psoriasis lesional and non-lesional skin of each individual in comparison to healthy skin from volunteers. Identification and quantification of lipid mediators were performed in accordance with published criteria, matching retention times (RTs) on liquid chromatography (LC) and tandem mass spectrometry (MS-MS) fragmentation spectra (Figure 1, 2; Table II)15. Unbiased differences between individual lipid mediators and the investigated groups were assessed using principal component analysis (PCA) software (Figure 3). PCA analysis indicated 2 outliers in the lesional skin and 1 in the healthy participants groups which were outside of the score plot 95% confidence interval and were subsequently removed from the final analysis. The 2 principal components of the score plot (Figure 3A) revealed a distinguished clustering between lesional skin compared to cumulative results from non-lesional and healthy skin. The corresponding loading plot (Figure 3B) indicated an association between lesional and non-lesional skin with specific lipid mediators, namely RvD5, AT-LXA4 and AT-LXB4 with lesional skin.
Figure 1.
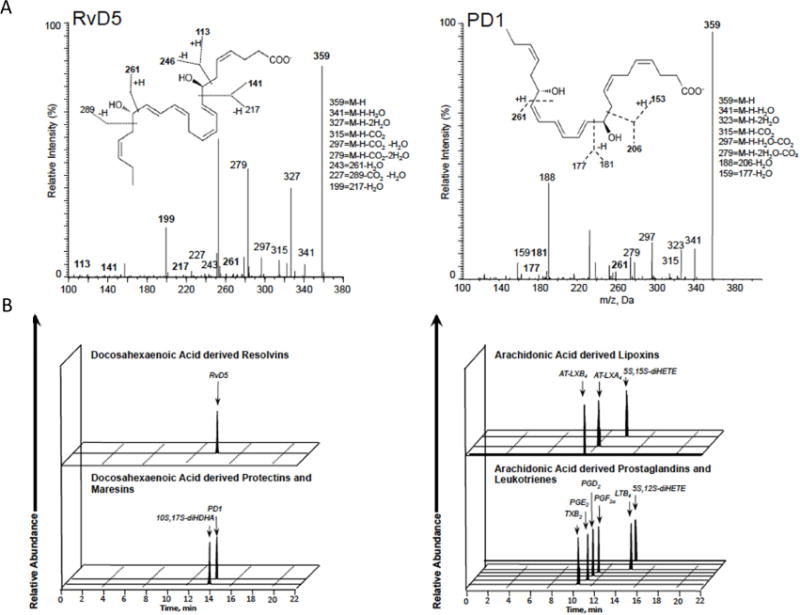
Lipid mediator levels were assessed following solid phase extraction by liquid chromatography tandem mass spectrometry–based (LC-MS-MS–based) metabololipidomics (see Methods for details). (A) MS/MS spectra utilized for identification and prominent diagnostic ions listed in inset. Refer to Table I for patient demographics and Table II for quantification of bioactive lipid mediators. (B) Representative multiple reaction monitoring (MRM) traces for the identified lipid mediators in human psoriasis and healthy skin donors.
Figure 2.
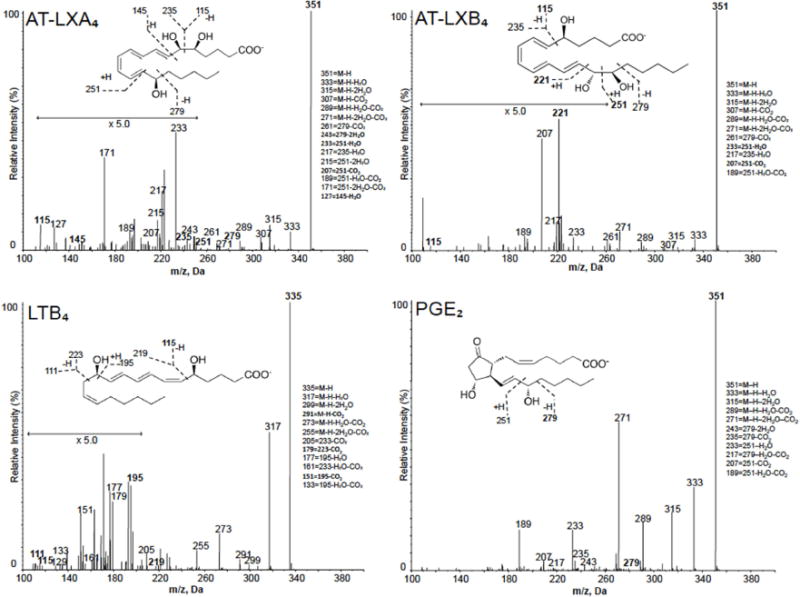
Lipid mediator levels were assessed following solid phase extraction by liquid chromatography tandem mass spectrometry–based (LC-MS-MS–based) metabololipidomics (see Methods for details). MS/MS spectra utilized for identification and prominent diagnostic ions listed in inset. Refer to Table I for patient demographics and Table II for quantification of bioactive lipid mediators.
Table II.
Bioactive SPM and pathway precursors identified in psoriasis and healthy skin
| Bioactive LM / Pathway Precursors | Lipid mediator levels (pg/100mg) | ||||
|---|---|---|---|---|---|
| DHA Metabolome | Lesional Skin | Non-Lesional Skin | *P | Healthy | †P |
| RvD5 | 45.1 (23.4–177.5) | – | – | – | – |
| PD1 | 0.4 (0.2–2.3) | 2.4 (0.8–4) | 0.24 | – | – |
| 10S,17S-diHDHA | 3.3 (1.7–11.5) | – | – | – | – |
| 17-HDHA | 32 (15.7–68.8) | 0.4 (0.2–0.7) | 0.002 | 1.2 (0.4–2.0) | 0.003 |
| 14-HDHA | 39.6 (21.2–41.5) | 0.4 (0.3–1.4) | 0.002 | 1.1 (0.6–1.6) | 0.005 |
| 7-HDHA | 13.5 (6.6–23.5) | 0.4 (0.3–0.9) | 0.002 | 0.3 (0.2–0.5) | 0.02 |
| 4-HDHA | 4.6 (2.5–7.8) | 0.8 (0.6–0.9) | 0.002 | 0.9 (0.8–1.0) | 0.001 |
| DHA | 40341 (25739–45611) | 4511 (1328–9987) | 0.003 | 10197 (3530–13256) | <0.001 |
| EPA Metabolome | |||||
| 18-HEPE | 4.1 (3–7) | 0.3 (0.2–0.5) | 0.002 | 0.4 (0.2–0.6) | <0.001 |
| 15-HEPE | 28.3 (13–47) | 0.5 (0.2–0.9) | 0.002 | 2.1 (1.5–2.4) | 0.004 |
| 12-HEPE | 47.1 (39.8–121.3) | 0.5 (0.3–2) | 0.002 | 1.5 (0.9–1.8) | 0.03 |
| 5-HEPE | 5.2 (2.9–7.7) | 0.7 (0.4–1) | 0.002 | 0.3 (0.2–0.6) | 0.002 |
| EPA | 2217.8 (1490–3255) | 219.2 (107–322) | 0.002 | 469 (319–945) | 0.048 |
| AA Metabolome | |||||
| AT-LXA4 | 3 (2.6–5.1) | – | – | – | – |
| AT-LXB4 | 4.4 (2.7–15.1) | – | – | – | – |
| 5S,15S-diHETE | 4.9 (2.3–7.8) | 0.4 (0.2–1) | 0.002 | 0.5 (0.3–0.6) | 0.004 |
| 5S,12S-diHETE | 14.4 (6.2–16) | 0.7 (0.7–0.7) | 0.13 | – | – |
| LTB4 | 9.6 (7.3–13) | 0.6 (0.6–0.6) | 0.13 | – | – |
| PGE2 | 15.8 (10–26.6) | 1.4 (0.9–6.2) | 0.02 | 7.3 (6.2–8.6) | 0.18 |
| PGD2 | 2 (1.4–2.8) | 0.4 (0.3–1.9) | 0.047 | 0.7 (0.6–1.2) | 0.11 |
| PGF2α | 3.2 (1.9–6.5) | 0.6 (0.6–0.6) | 0.13 | 1 (0.8–1.1) | 0.14 |
| TXB2 | 10 (0.4–15.1) | 5 (5–5) | 0.51 | 4.4 (0.5–38.5) | 0.44 |
| 15-HETE | 452 (326–1043) | 4.4 (3.6–9.7) | 0.002 | 32 (25–74) | 0.03 |
| 12-HETE | 1989 (1494–2197) | 4.4 (3.2–31.4) | 0.002 | 15 (13–26) | 0.01 |
| 5-HETE | 41.7 (29.2–79.7) | 1.6 (1.1–2.5) | 0.002 | 1.4 (1.0–2.5) | <0.001 |
| AA | 14623 (13375–18264) | 3056 (1980–6981) | 0.01 | 5019 (3797–8614) | <0.001 |
Quantification of bioactive lipid mediators (LM) and pathway precursors were assessed by liquid chromatography tandem mass spectrometry–based (LC-MS-MS–based) metabololipidomics. Results are expressed as pg/100mg tissue. Detection limit was approximately 0.1 pg; – denotes below limits along with the following metabolites: RvD1-D4, RvD6, AT-RvD1, AT-RvD3, AT-PD1, 22-OH-PD1, Mar1, 7S, 14S-diHDHA, 4S, 14S-diHDHA, RvE1-E3, LXA4, LXB4, 20-OH-LTB4, 20-COOH-LTB4. *P compared between lesional vs. non-lesional skin. †P compared between lesional vs. healthy skin. Data are median (Interquartile Range) for non-parametric variables. P values were derived from Mann-Whitney test for non-parametric variables. P<0.05 was considered statistically significant.
Figure 3.
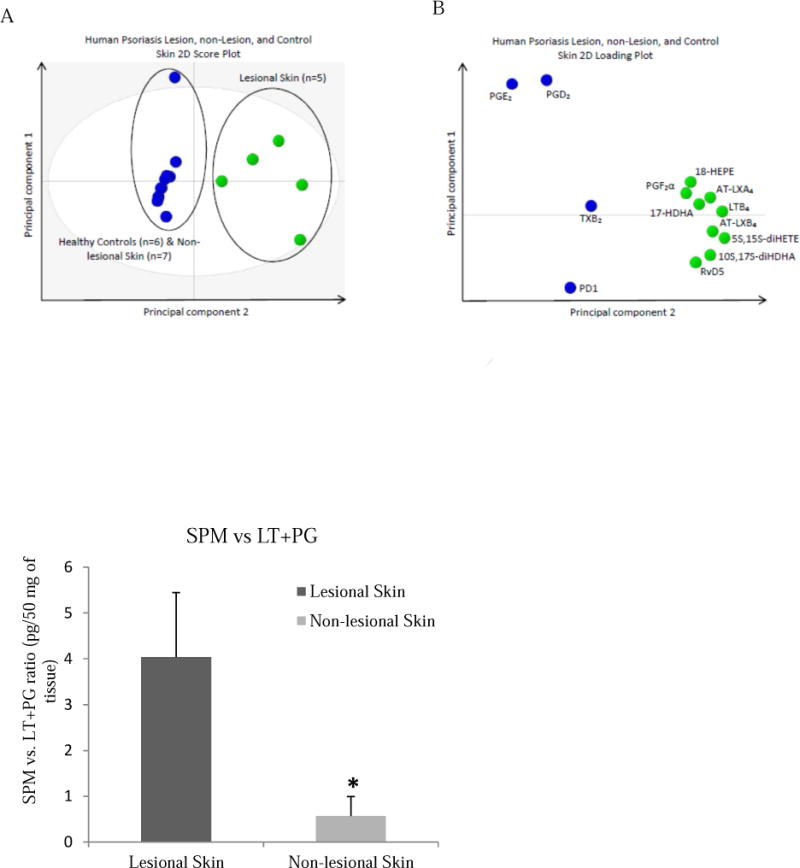
(A) 2D score plot of human lesional skin samples from psoriasis patients (green circles, n=5) compared with human non-lesional skin samples from psoriasis patients and skin from healthy volunteers (blue circles, n=13). 95% confidence interval is denoted by the gray ellipse. (B) 2D loading plot of lipid mediators identified in human lesional, non-lesional, and healthy skin samples. Green circles denote lesional skin patients, blue circles non-lesional and healthy control skin. (C): Ratio of SPMs vs. PG and LT in psoriasis lesional skin compared to non-lesional skin, P<0.05.
In the skin of psoriasis subjects the DHA derived bioactive metabolome represented 30.8% of pathway markers with presence of all the DHA derived SPM, and monohydroxy containing products 4-, 7-, 14-, and 17-HDHA (Table II). These were significantly more abundant in lesional compared to non-lesional skin with predominant accumulation of 14-HDHA (39.6 pg/100 mg tissue vs. 0.4 pg/100 mg tissue, correspondingly, P=0.002). Interestingly, other potent DHA derived mediators, RvD5 (45.1, IQR 23.4–177.5 pg/100 mg tissue) and PD1 as well as its double dioxygenation isomer 10S,17S-diHDHA a.k.a. PDx (3.3, IQR 1.7–11.5 pg/100 mg tissue) were only detected in psoriasis lesional skin (Table II). However, both lesional and non-lesional psoriasis skin had detectable levels of PD1. The concentration of DHA was significantly higher in lesional skin compared to adjacent non-lesional skin (40341 pg/100 mg tissue vs. 4511 pg/100 mg tissue, correspondingly, P=0.003) and skin from the healthy volunteers (40341 pg/100 mg tissue vs. 10197 pg/100 mg tissue, correspondingly, P<0.001).
The EPA bioactive metabolome of the psoriasis skin was ~ 19.2% of the total lipid mediators identified, and exhibited a similar pattern of metabolites and mediators with differences between lesional and non-lesional skin as observed for the DHA metabolome. All EPA derived bioactive products SPM and monohydroxy-, as 5-, 12-, 15-, and 18-HEPE, were present with higher prevalence of 12-HEPE in the lesional skin compared to non-lesional (47.1 pg/100 mg tissue vs. 0.5 pg/100 mg tissue, correspondingly, P=0.002). E-series resolvins were not identified in the skin among all the groups (Table II). Overall, the total concentration of EPA was higher in lesional skin compared to non-lesional skin from the same subjects (2217.8 pg/100 mg tissue vs. 219.2 pg/100 mg tissue, correspondingly, P=0.003) and skin from the healthy volunteers (2217.8 pg/100 mg tissue vs. 469 pg/100 mg tissue, correspondingly, P<0.001).
Identified AA bioactive metabolome represented ~ 50% of the 45 identified lipid mediators, with higher accumulation of all the AA-derived autacoids in lesional skin (Figure 3C, Table II). We identified lipoxygenase products, namely 5-, 12-, and 15-HETE in higher amounts in lesional skin with significant prevalence of 12- and 15-HETE compared to non-lesional skin (1989 pg/100 mg tissue vs. 4.4 pg/100 mg tissue, correspondingly, P=0.002). These results are in agreement with the earlier reported results using different methods which show that 12-HETE is a major product in normal human epidermis19, whereas dermis is more responsible for 15-HETE generation20. Among measured prostanoids, we observed a significantly higher concentration of PGE2 in lesional skin than in non-lesional skin counterparts (15.8 pg/100 mg tissue vs. 1.4 pg/100 mg tissue, correspondingly, P=0.02), which is also in line with earlier published results21. Lipoxin signature profiles were absent in all study groups. Both aspirin-triggered forms of LXA4 and LXB4 were identified only within lesional skin. Also, LTB4 and its double dioxygenation isomer, 5S, 12S-diHETE, were only presented in skin of psoriasis patients with a higher concentration in lesional plaque than in unaffected skin (9.6 pg/100 mg tissue vs. 0.6 pg/100 mg tissue for LTB4 and 14.4 pg/100 mg tissue vs. 0.7 pg/100 mg tissue for 5S,12S-diHETE, correspondingly, P=0.1). Concentration of AA was the highest in lesional skin compared to non-lesional (14623 pg/100 mg tissue vs. 3056 pg/100 mg tissue, P=0.01) and healthy volunteers (14623 pg/100 mg tissue vs. 5019 pg/100 mg tissue, P<0.001). Overall, in skin of healthy subjects, the bioactive metabolome was equally shared by 26.3% of DHA and 26.3% of EPA pathway markers with 47.4% of AA eicosanoids.
To investigate the prognostic clinical value of the SPMs detected in skin we also conducted a regression analysis. The psoriasis severity score (PASI) was not correlated with any of the detected SPMs. However, this observation deserves further study by increasing biospecimen number and more sensitive psoriasis disease clinical parameters.
Identification of SPMs in psoriasis and healthy peripheral blood
Although psoriasis clinically manifests by specific local skin changes, there is a significant systemic inflammatory component. Hence, we further analyzed plasma and serum from this patient cohort to understand compartment distribution and provide context for skin analysis. This analysis was exploratory without any adjustment for multiple testing.
Expression of EPA, DHA and AA pathway markers in the detected bioactive metabolome for peripheral blood had a similar trend as for psoriasis skin with slight difference for the healthy participants (Supplemental Table I, II). There were, however, some differences in the specific lipid mediators and SPM identified between serum and plasma of the analyzed groups. For instance, the DHA-derived SPM, RvD1, was identified in both psoriasis and healthy peripheral blood along with PD1. Other DHA pathway markers, as 7-, and 14-HDHA were significantly decreased in psoriasis serum compared to healthy volunteers, whereas a difference in plasma did not show the same level of significance. Only difference observed in psoriasis plasma was 10S, 17S-diHDHA (PDx), which was present in plasma of psoriasis and healthy subjects (Supplemental Table II).
EPA metabolome consisted of 5-, 12-, 15-, 18-HEPE markers identified in serum and plasma of both groups with more significant changes of 18-HEPE in serum. Interestingly, LXA4 and its aspirin-triggered form (AT-LXA4), were identified in plasma of both groups with only AT-LXA4 serum signature. Moreover, the omega 20-hydroxy form of LTB4, 20-OH-LTB4, was present in serum from both groups. Other AA metabolites, 12- and 15-HETE, were significantly changed in serum compartment. Although, differences in each identified metabolite between groups were less significant in peripheral blood than reported for skin biospecimens, plasma replicated changes attributed to skin SPMs profiles.
RvD1 and RvD5 actions on inflammatory genes expression and some of the related protein concentration
Based on the results from the skin LC-MS-MS profile, we interrogated the actions of RvD1 and RvD5 to regulate some of the important inflammatory genes related to psoriasis pathogenesis and SPMs biosynthesis in primary human keratinocytes. In analyses that included the following genes, CCL2, CXCL8, CXCL10, IFN-γ, IL17C, NF-kβ, ALOX15, the most significant changes appeared in IL-1β, IL24, PLA2, PTGS1, S100A12 and TNFα (Supplemental Figure 1; Figure 4 A, B; Figure 5 A, B). Since biological actions of RvD5 on IL-24 and S100A12 in psoriasis were not known, we performed further analysis on these specific cytokines in comparison RvD1 that has been more widely investigated (9).
Figure 4.
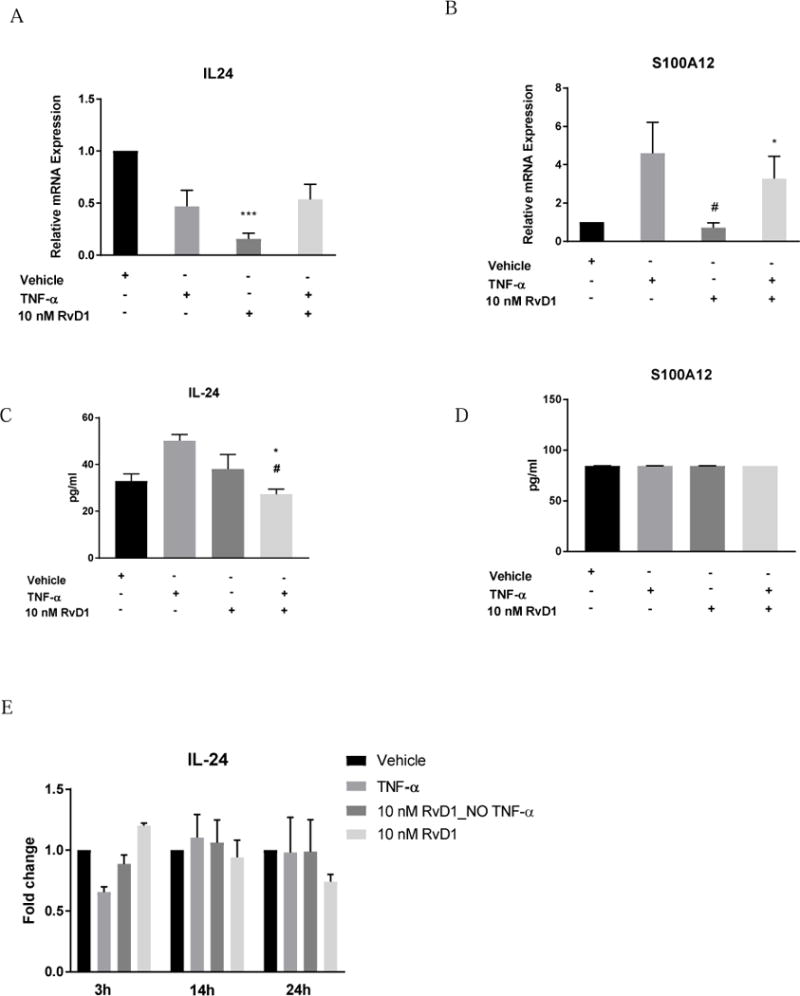
(A, C) Relative levels of IL24 mRNA expression and protein concentration in HNEK cells under RvD1 treatment. (B, D) Relative levels of S100A12 mRNA expression and protein concentration in HNEK cells under RvD1 treatment. (E) IL-24 concentration fold changes under RvD1 treatment at different time points. Values represent the mean ± SEM. * - P<0.05 compared to vehicle; # - P<0.01 compared to TNFα group.
Figure 5.
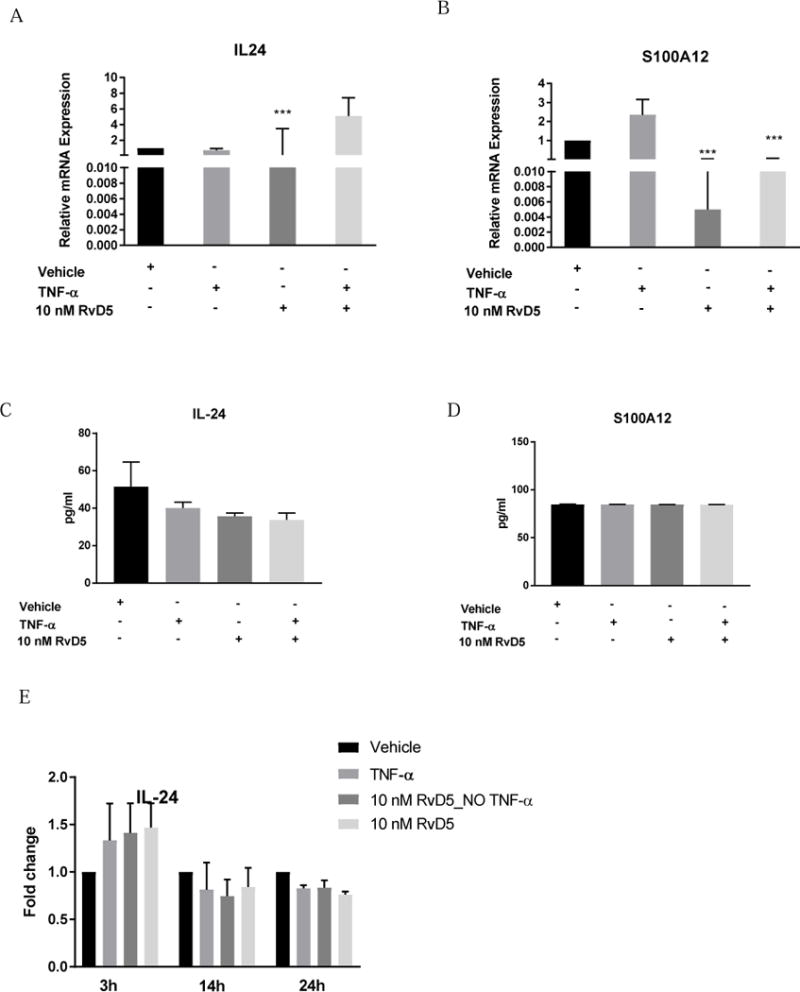
(A, C) Relative levels of IL24 mRNA expression and protein concentration in HNEK cells under RvD5 treatment. (B, D) Relative levels of S100A12 mRNA expression and protein concentration in HNEK cells under RvD5 treatment. (E) IL-24 concentration fold changes under RvD5 treatment at different time points. Values represent the mean ± SEM. * - P<0.05 and *** -P<0.001 compared to vehicle.
The most robust decrease of IL24 and S100A12 expression in human keratinocyte was detected in both RvD1 and RvD5 groups without TNFα (P<0.001), compared to control treatment, Figure 4 A, B; Figure 5 A, B. Based on the preliminary experiments, HNEK lysate appeared to be a better source of IL-24 and S100A12 presence compared to HNEK supernatant. Only preincubation of RvD1 before TNFα stimulation significantly decreased IL-24 concentration in human keratinocytes after 3 hours of incubation, compared to control and TNFα alone groups (P<0.05, P<0.01, correspondingly), Figure 4 C. The same trend of IL-24 decrease was observed for RvD5 without reaching significance, Figure 5 C. However, comparison of IL-24 changes to vehicle treatment at longer incubation times revealed insignificant trend of RvD5 better protective effects than RvD1 treatment (Figure 4 E, Figure 5 E). Additional time points for RvD1 and RvD5 effects on IL-24 can be found in Supplemental Figure 2. Moreover, S100A12 concentration did not change among the RvD1 (Figure 4 D) and RvD5 (Figure 5 D) groups.
Accompanying IL-24 and S100A12 serum measurements showed below the limit of linear quantitation for IL-24 and almost equal levels of S100A12 (0.279 ± 0.04 ng/ml) in both studied groups. Furthermore, although underpowered we still observed a linear relationship of S100A12 with PD1 respectively.
DISCUSSION
Psoriasis is a chronic progressive disease characterized by local skin plaques with a concomitant chronic systemic inflammation. The association of psoriasis with other chronic inflammatory disorders such as CVD at young age make this a public health issue. Although psoriasis progression can be well controlled with immunologic therapies, a lack of precise understanding of its pathophysiology hampers development of novel non-cytokine-based therapies2, 3. There are numerous clinical trials conducted so far on treating subjects with PSO as well as CVD with long-chain omega-3 PUFAs22, 23. However, limited number of study participants, insufficient concentration or purity of the omega-3 supplements and application of low sensitivity clinical diagnostic tools resulted in controversial outcomes. Using targeted lipid mediator metabololipidomics, we identified specialized pro-resolving mediators, abundant in psoriasis lesional skin and peripheral blood with a specific pattern of changes between psoriasis and healthy participants.
As anticipated, AA-derived prostanoids were significantly increased in lesional psoriasis skin and plasma with highest accumulation of PGE2 in the lesions. Besides its cytoprotective role, PGE2 is a key pro-inflammatory mediator responsible for hyperalgesia, enhanced microvascular permeability and cytokine production24. Additionally, PGE2 activates resolution programs and biosynthesis of the SPMs-lipoxins, resolvins, protectins and maresins within inflammatory exudates25. These results are consistent with earlier studies that showed PGE2 is the predominant prostaglandin in the skin21. Accumulation of other AA pathway markers is also consistent with earlier studies that have reported increased AA-derived lipoxygenase products in psoriatic compared to healthy skin. Specifically, 12-HETE is present in normal human epidermis and highly accumulated in psoriasis lesional skin13, whereas 15-HETE is prevalent in the dermis20. The inter-skin origin of these markers can be traced to the human cell types of the converting enzymes. For instance, 12-LOX is highly expressed in platelets and the epidermis, whereas 15-LOX is specific to the reticulocytes (15-LOX-1), leukocytes, and the epidermis (15-LOX-2)26. During skin inflammation, biosynthesis and accumulation of lipoxygenase products, 12-HETE and 15-HETE, in different skin layers may be impaired. These classifications were made independently from the discovery of SPMs. It is known that 12-HETE possesses potent chemotactic activity12, whereas 15-HETE has anti-inflammatory properties19. The observed high concentration of 15-HETE in lesional psoriasis skin suggests its critical role in blunting skin inflammation. LXA4 and LXB4 were near the lower limits of detection in skin and peripheral blood samples from both groups with a trend toward elevation of LTB4 and its double dioxygenation isomer, 5S, 12S-diHETE (P>0.05), in the lesional psoriasis skin and peripheral blood. Along these lines, LTB4 is a potent neutrophil chemoattractant and LTB4 increase dendritic cell motility in the skin by inducing actin filament reorganization and provoking further skin inflammation27, which might lead to a higher concentration of this eicosanoid in the lesional skin. This imbalance in lipoxin-leukotriene homeostasis may explain the abrogated inflammation-resolution profile observed in psoriasis identified in the affected psoriasis skin. From recent studies it is known that aspirin-triggered forms possess potent anti-inflammatory actions28, 29 and presence of these meditators in lesional skin might be a part of compensatory mechanisms triggered by impaired resolution. Interestingly, AT-LXA4 (the 15-R epimer of LXA4) was identified in both serum and plasma of psoriasis and healthy subjects. Of note, two psoriasis patients report 81 mg of aspirin daily, before the 2 weeks wash out prior to this study.
Although, only one psoriasis patient was prescribed fish oil supplement before enrolling in the study, the observed lesional skin DHA bioactive metabolome in lesional skin was markedly higher compared to non-lesional and healthy skin. In particular, the DHA products, 14-HDHA and 17-HDHA, were increased in lesional psoriasis skin. It is shown in an obese mouse model that 17-HDHA treatment reduces inflammatory cytokines adipose tissue expression and improve glucose tolerance30 and reduces pain in arthritis models31. The significant difference in concentrations between DHA and its related precursors in skin might be related to the impaired conversion of DHA to the downstream pathway mediators and its selective release in inflamed tissue. As a result, DHA-derived SPMs, specifically RvD5, PD1 and PDx (10S,17S-diHDHA) were abundant in psoriasis lesional skin. These potent lipid mediators are involved in resolution of inflammation and modulate innate and adaptive immunity32, 33. In the paws of arthritic mice supplemented with an omega-3 PUFA enriched diet, RvD1 and RvD5 were significantly increased34. In balloon injury of the femoral artery in rabbits, RvD1 and RvD5 increase during vascular injury35. PD1 and its isomer PDx exert anti-inflammatory properties by blocking leukocyte infiltration in murine models36, whereas its isomer, PDx, also has glucoregulatory activity37. In our present study RvD5 showed disease specific elevation in lesional psoriasis skin which might be attributed to tissue and cell specificity. It is known that psoriasis skin has peculiar histological architecture characterized by robust neutrophil infiltration into dermis. Such close interaction between keratinocytes and migrated neutrophils might affect the biosynthesis of bioactive mediators and pathway markers from the DHA metabolome.
It is known from animal studies on psoriasis that n-3 PUFAs are protective against psoriasis-like inflammation through the IL-17/IL-23 axis38 and have a promising clinical application for other skin diseases39. It has also been shown that IL24 expression by keratinocytes promotes psoriasis-like skin inflammation40 and contribute to skin specific inflammatory process41. Another pro-inflammatory cytokine, S100A12, is highly increased in human keratinocytes and involved in both psoriasis disease42 and its complications43. In our study, both RvD1 and RvD5 treatment of HNEK significantly decreased IL24 and S100A12 expression, which indicates their skin-specific anti-inflammatory properties. Moreover, keratinocytes pretreatment with RvD1 showed robust IL-24 concentration decrease during the onset of inflammatory response, whereas RvD5 showed the same trend that was not statistically significant. Interestingly, RvD5 treatment tended to have a more continuous protective effect on IL-24 increase compared to RvD1, although changes were not statistically significant. The observed serum levels of IL-24 were near the lower limits of quantitation, and might be attributed to its low presence in circulation as compared to the higher abundance of this cytokine in keratinocytes. The S100A12 concentration did not differ between the treated groups under both RvD1 and RvD5, which might be explained by cell specificity and inflammatory milieu. Additionally, S100A12 serum levels did not differ between the studied groups as well. It has been reported that S100A12 function is Ca2+ dependent and has different activity in proliferating and differentiating keratinocytes44. In our cell culture experiments we did not count the possible effects of additional Ca2+ supplementation shifting our experimental design towards a proliferating keratinocyte profile as present in psoriasis45. The potential application of RvD1 and RvD5 protective role in resolution of skin specific inflammation warrants further functional explanation.
The targeted EPA bioactive metabolome included less analytes identified compared to the DHA and AA LC-MS-MS profiles. Similarly to the AA pathway markers, detected in psoriasis lesional skin compounds included EPA monohydroxy derivatives, specifically 12-HEPE. This observation corresponds to the previous findings that showed baseline levels of 12-HEPE elevation in human skin blister fluid in response to ultraviolet exposure (UVR)46. Pilkington et al. suggested that EPA supplemented in this human UVR-induced skin inflammation model may preferentially be utilized through the 12-LOX pathway resulting in higher 12-HETE:12-HEPE ratio. Our earlier study of atherosclerosis treatment with EPA and DHA in apoE-KO mice showed prevalence of 12-HEPE in the aorta among other monohydroxy acids47. 12-HEPE is less efficient at inducing erythema compared to 12-HETE, following its topical application to human skin48. The marked increase in 12-HETE:12-HEPE ratio in lesional psoriasis skin in our cohort might be determined by human 12- or 15-LOX activity malfunction giving higher dermal leukocytic infiltration observed.
We did not observe the same changes in peripheral blood for some of the mediators and pathway markers and lipid mediators identified in skin likely attributable to tissue specificity. Each tissue and cell type has a specific eicosanoid signature profile which is determined by the related distinct enzymes and pathophysiological condition. Also, available substrates for the SPMs biosynthesis exist as gradients that transport these bioactive clusters upon the milieu demand. Thus, local production of SPMs within the lesional skin may abrogate inflammation and improve psoriasis severity. However, the amounts of SPMs within psoriasis lesions were not produced in high enough concentrations to resolve the inflammation in the skin suggesting that increasing SPMs in psoriasis may represent a goal for new therapeutic interventions.
In this study we utilized an LC-MS-MS-based approach to obtain the LM-SPM profile in human psoriasis skin and peripheral blood compared to healthy volunteers. However, we acknowledge that our study has some limitations such as small number of psoriasis cases and limited mechanistic evidence for the observed changes. Although the number of enrolled subjects was small, by utilizing this state-of-the-art approach, we found an imbalance in pro-resolution and pro-inflammatory local mediators and a robust accumulation of specific mediators in lesional psoriasis skin. Interestingly, non-lesional skin of psoriasis patients had a different distribution of SPMs compared to healthy skin suggesting compensatory mechanisms activated during disease development. The observed findings suggest that locally produced lipid mediators might play a crucial role in controlling local skin inflammation. Although we do not have temporal data on the progression of psoriasis in these patients, it is possible that eicosanoids and SPMs might follow time dependent changes during the disease state. Therefore, targeting these bioactive mediators may facilitate discovery of new therapeutic approaches effectively applied in patients with psoriasis and other diseases with inflammatory component. Further animal and human studies with larger sample size are warranted.
Supplementary Material
Highlights.
Psoriasis skin has disease specific lipid mediators profiles
Lesional psoriasis skin is abundant in pro-inflammatory lipid mediators
Skin-related inflammatory cytokines can be abrogated by the pro-resolving mediators
Specifically, Resolvin D1 and Resolvin D5 might play protective role
Acknowledgments
This study was supported by intramural research funding of National Heart, Lung, and Blood Institute. Studies in the CNS laboratory reported here were supported by the National Institutes of Health (P01GM095467 and GM38765 to C.N.S.). We thank Justin Rodante and the clinical staff of the NHLBI SICMD for obtaining data.
LIST OF ABBREVIATIONS
- AA
Arachidonic Acid
- AT-LXA4
Aspirin-triggered Lipoxin A₄ (5S, 6R, 15(R)-trihydroxy-7E, 9E, 11Z, 13E-eicosatetraenoic acid)
- AT-LXB4
Aspirin-triggered Lipoxin B₄ (5S, 14R, 15R-trihydroxy-6E, 8Z, 10E, 12E-eicosatetraenoic acid)
- AT-PD1
Aspiring-triggered Protectin D1 (10R, 17R-dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid)
- AT-RvD1
Aspirin-triggered Resolvin D1 (7S, 8R, 17R-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
- AT-RvD3
Aspirin-triggered Resolvin D3 (4S, 11R, 17R-trihydroxy-5Z, 7E, 9E, 13Z, 15E, 19Z-docosahexaenoic acid)
- DHA
Docosahexaenoic Acid
- EPA
Eicosapentaenoic Acid
- HDHA
Hydroxydocosahexaenoic acid
- HEPE
Hydroxyeicosapentaenoic acid
- HETE
Hydroxyeicosatetraenoic acid
- HpETE
Hydroperoxyeicosatetraenoic acid
- LC-MS-MS
Liquid chromatography tandem mass spectrometry
- LM
Lipid mediators
- LOX
Lipoxygenase
- LT
Leukotriene
- LTB₄
Leukotriene B₄ (5S,12R-dihydroxy-eicosa-6Z, 8E,10E,14Z-tetraenoic acid)
- LXA₄
Lipoxin A₄ (5S,6R,15S-trihydroxy-eicosa-7E,9E,11Z,13E-tetraenoic acid)
- LXB₄
Lipoxin B₄ (5S,14R,15S-trihydroxy-eicosa-6E,8Z,10E,12E-tetraenoic acid)
- MaR1
Maresin 1 (7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid)
- PDx
Protectin Dx (10(S), 17(S)-dihydroxy-4Z, 7Z, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
- PD1
Protectin D1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid), also known as neuroprotectin D1 (NPD1)
- PG
Prostaglandin
- PGD₂
Prostaglandin D₂ (11-oxo-9α, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid)
- PGE₂
Prostaglandin E₂ (9-oxo-11α,15S-dihydroxy-prosta-5Z,13E-dien-1-oic acid)
- PGF₂α
Prostaglandin F₂α (9α,11α,15S-trihydroxy-prosta-5Z,13E-dienoic acid)
- PUFA
Polyunsaturated fatty acid
- SPM
Specialized pro-resolving mediators
- 20-OH-LTB4
20-hydroxy-Leukotriene B₄ (5S, 12R, 20-trihydroxy-6Z, 8E, 10E, 14Z-eicosatetraenoic acid
- 20-COOH-LTB4
20-carboxy-Leukotriene B₄ (5S, 12R-dihydroxy-6Z, 8E, 10E, 14Z, 20-carboxy-eicosatetraenoic acid
- 22-OH-PD1
22-hydroxy-Protectin D1 (10R,17S,20-trihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid)
- RvD1
Resolvin D1 (7S,8R,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid)
- RvD2
Resolvin D2 (7S,16R,17S-trihydroxy-docosa-4Z,8E,10Z,12E,14E,19Z-hexaenoic acid)
- RvD3
Resolvin D3 (4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoicacid)
- RvD4
Resolvin D4 (4S, 5, 17S-trihydroxy-6E, 8E, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvD5
Resolvin D5 (7S,17S-dihydroxy-docosa-4Z,8E,10Z,13Z,15E,19Z-hexaenoic acid)
- RvD6
Resolvin D6 (4S, 17S-dihydroxy-5E, 7Z, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvE1
Resolvin E1 (5S,12R,18R-trihydroxy-eicosa-6Z,8E,10E,14Z,16E-pentaenoic acid)
- RvE2
Resolvin E2 (5S,18R-dihydroxy-eicosa-6E,8Z,11Z,14Z,16E-pentaenoic acid)
- RvE3
Resolvin E3 (17R,18R-dihydroxy-eicosa-5Z,8Z,11Z,13E,15E-pentaenoic acid)
- TXB₂
Thromboxane B₂ (9α,11,15S-trihydroxythromba-5Z,13E-dien-1-oic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors have declared that no conflict of interest exists
Author contributions
CNS, AVS and NNM designed and performed experiments, analyzed data, and wrote the manuscript. PN and JE performed experiments and analyzed metabololipidomics data. AKD and AC performed statistical analysis. YB, JS, and MPP, provided conceptual expertise and contributed to manuscript preparation.
References
- 1.Kuek A, Hazleman BL, Östör AJK. Immune‐mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgraduate Medical Journal. 2007;83:251–260. doi: 10.1136/pgmj.2006.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41–55. doi: 10.1016/j.det.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating Evidence for the Association and Shared Pathogenic Mechanisms between Psoriasis and Cardiovascular–Related Co‐morbidities. The American journal of medicine. 2014;127:1148–1153. doi: 10.1016/j.amjmed.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronckers IMGJ, Paller AS, van Geel MJ, van de Kerkhof PCM, Seyger MMB. Psoriasis in Children and Adolescents: Diagnosis, Management and Comorbidities. Paediatric Drugs. 2015;17:373–384. doi: 10.1007/s40272-015-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2015;7:a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Yacoubian S, Yang R. Anti‐inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang MJ, Spite M. Resolvins: anti‐inflammatory and proresolving mediators derived from omega‐3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN. Resolution phase of inflammation: novel endogenous anti‐inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 12.Camp RD, Cunningham FM, Fincham NJ, et al. Psoriatic skin lesions contain a novel lipid neutrophil chemokinetic compound which is distinct from known chemoattractant eicosanoids. Br J Pharmacol. 1988;94:1043–1050. doi: 10.1111/j.1476-5381.1988.tb11620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L‐hydroxy‐5,8,10,14‐eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A. 1975;72:5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrocher R, Ferrari S, de Gironcoli M, et al. Effect of fish oil supplementation on erythrocyte lipid pattern, malondialdehyde production and glutathione-peroxidase activity in psoriasis. Clin Chim Acta. 1989;179:121–131. doi: 10.1016/0009-8981(89)90158-7. [DOI] [PubMed] [Google Scholar]
- 15.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro- resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Naik HB, Natarajan B, Stansky E, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehncke WH. Etiology and Pathogenesis of Psoriasis. Rheum Dis Clin North Am. 2015;41:665–675. doi: 10.1016/j.rdc.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Kragballe K, Desjarlais L, Duell EA, Voorhees JJ. In vitro synthesis of 12-hydroxy-eicosatetraenoic acid is increased in uninvolved psoriatic epidermis. J Invest Dermatol. 1986;87:47–52. doi: 10.1111/1523-1747.ep12523561. [DOI] [PubMed] [Google Scholar]
- 20.Kragballe K, Pinnamaneni G, Desjarlais L, Duell EA, Voorhees JJ. Dermis-derived 15-hydroxy-eicosatetraenoic acid inhibits epidermal 12-lipoxygenase activity. J Invest Dermatol. 1986;87:494–498. doi: 10.1111/1523-1747.ep12455564. [DOI] [PubMed] [Google Scholar]
- 21.Ziboh VA. Biosynthesis of prostaglandin E 2 in human skin: subcellular localization and inhibition by unsaturated fatty acids and anti-inflammatory drugs. J Lipid Res. 1973;14:377–384. [PubMed] [Google Scholar]
- 22.Millsop JW, Bhatia BK, Debbaneh M, Koo J, Liao W. Diet and psoriasis, part III: role of nutritional supplements. Journal of the American Academy of Dermatology. 2014;71:561–569. doi: 10.1016/j.jaad.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JH, Mozaffarian D. omega-3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: new pieces in a complex puzzle. Heart (British Cardiac Society) 2014;100:530–533. doi: 10.1136/heartjnl-2013-305257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boniface K, Bak-Jensen KS, Li Y, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 26.Elabdeen HR, Mustafa M, Szklenar M, Ruhl R, Ali R, Bolstad AI. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS One. 2013;8:e70838. doi: 10.1371/journal.pone.0070838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada Y, Honda T, Hanakawa S, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med. 2015;212:1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho KJ, Spite M, Owens CD, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano M, Cianci E, Simiele F, Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 30.Neuhofer A, Zeyda M, Mascher D, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62:1945–1956. doi: 10.2337/db12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Petasis NA. Resolvins and Protectins in Inflammation-Resolution. Chemical reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiurchiu V, Leuti A, Dalli J, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Science translational medicine. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norling LV, Headland SE, Dalli J, et al. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1:e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyahara T, Runge S, Chatterjee A, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 37.White PJ, St-Pierre P, Charbonneau A, et al. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med. 2014;20:664–669. doi: 10.1038/nm.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin S, Wen J, Bai XC, et al. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Molecular medicine reports. 2014;9:2097–2104. doi: 10.3892/mmr.2014.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Sun M, Ren J, et al. Gas chromatography-mass spectrometry analysis of effects of dietary fish oil on total fatty acid composition in mouse skin. Scientific reports. 2017;7:42641. doi: 10.1038/srep42641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumari S, Bonnet MC, Ulvmar MH, et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity. 2013;39:899–911. doi: 10.1016/j.immuni.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Jin SH, Choi D, Chun YJ, Noh M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicology and applied pharmacology. 2014;280:199–206. doi: 10.1016/j.taap.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to- severe psoriasis. J Invest Dermatol. 2012;132:2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foell D, Kane D, Bresnihan B, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford, England) 2003;42:1383–1389. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 44.Moroz OV, Burkitt W, Wittkowski H, et al. Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC biochemistry. 2009;10:11. doi: 10.1186/1471-2091-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valdimarsson H, Bake BS, Jonsdotdr I, Fry L. Psoriasis: a disease of abnormal Keratinocyte proliferation induced by T lymphocytes. Immunology today. 1986;7:256–259. doi: 10.1016/0167-5699(86)90005-8. [DOI] [PubMed] [Google Scholar]
- 46.Pilkington SM, Rhodes LE, Al-Aasswad NM, Massey KA, Nicolaou A. Impact of EPA ingestion on COX- and LOX-mediated eicosanoid synthesis in skin with and without a pro-inflammatory UVR challenge–report of a randomised controlled study in humans. Mol Nutr Food Res. 2014;58:580–590. doi: 10.1002/mnfr.201300405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorokin AV, Yang ZH, Vaisman BL, et al. Addition of aspirin to a fish oil-rich diet decreases inflammation and atherosclerosis in ApoE-null mice. J Nutr Biochem. 2016;35:58–65. doi: 10.1016/j.jnutbio.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham FM, Woollard PM, Camp RD. Proinflammatory properties of unsaturated fatty acids and their monohydroxy metabolites. Prostaglandins. 1985;30:497–509. doi: 10.1016/0090-6980(85)90122-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


