Abstract
Background
Despite its recommendation by the current guidelines, the role of long-term oral beta-blocker therapy has never been evaluated by randomized trials in uncomplicated ST-segment elevation myocardial infarction (STEMI) patients without heart failure, left ventricular dysfunction or ventricular arrhythmia who underwent primary percutaneous coronary intervention (PCI).
Methods and results
In a multi-center, open-label, randomized controlled trial, STEMI patients with successful primary PCI within 24 hours from the onset and with left ventricular ejection fraction (LVEF) ≥40% were randomly assigned in a 1-to-1 fashion either to the carvedilol group or to the no beta-blocker group within 7 days after primary PCI. The primary endpoint is a composite of all-cause death, myocardial infarction, hospitalization for heart failure, and hospitalization for acute coronary syndrome. Between August 2010 and May 2014, 801 patients were randomly assigned to the carvedilol group (N = 399) or the no beta-blocker group (N = 402) at 67 centers in Japan. The carvedilol dose was up-titrated from 3.4±2.1 mg at baseline to 6.3±4.3 mg at 1-year. During median follow-up of 3.9 years with 96.4% follow-up, the cumulative 3-year incidences of both the primary endpoint and any coronary revascularization were not significantly different between the carvedilol and no beta-blocker groups (6.8% and 7.9%, P = 0.20, and 20.3% and 17.7%, P = 0.65, respectively). There also was no significant difference in LVEF at 1-year between the 2 groups (60.9±8.4% and 59.6±8.8%, P = 0.06)
Conclusion
Long-term carvedilol therapy added on the contemporary evidence-based medications did not seem beneficial in selected STEMI patients treated with primary PCI.
Trial registration
CAPITAL-RCT (Carvedilol Post-Intervention Long-Term Administration in Large-scale Randomized Controlled Trial) ClinicalTrials.gov.number, NCT 01155635.
Introduction
The long-term oral beta-blocker therapy has been recommended as class I in patients with heart failure (HF), left ventricular (LV) dysfunction, or ventricular arrhythmia after ST-segment elevation myocardial infarction (STEMI) in both the American Heart Association and the American College of Cardiology (AHA/ACC), and the European Society of Cardiology (ESC) guidelines, although randomized controlled trials supporting this recommendation were conducted before the widespread use of primary PCI for STEMI[1,2]. For uncomplicated STEMI patients without HF, LV dysfunction or ventricular arrhythmia, long-term oral beta-blocker therapy is also recommended as class I in the AHA/ACC guideline and class Ⅱa in the ESC guideline[3,4]. However, this recommendation is basically an extrapolation from the trial results in STEMI patients with HF, LV dysfunction or ventricular arrhythmia. There is no prospective trial investigating the role of long-term oral beta-blocker therapy in uncomplicated STEMI patients who underwent primary PCI. In the contemporary STEMI patients, the role of long-term oral beta-blocker therapy has been investigated only in observational studies which reported conflicting results[5–11]. Therefore, we conducted a prospective randomized trial evaluating the efficacy of long-term oral beta-blocker therapy in preventing cardiovascular events in STEMI patients with preserved LV function who underwent primary PCI.
Materials and methods
Study design
Carvedilol Post-Intervention Long-Term Administration in Large-scale Randomized Controlled Trial (CAPITAL-RCT) is a prospective multi-center, open-label, randomized controlled trial in 67 centers in Japan (see Acknowledgments) to investigate whether the long-term use of a beta-blocker (carvedilol) could improve long-term outcomes in STEMI patients undergoing primary PCI. Patients were eligible for the trial if they underwent successful primary PCI within 24 hours after the onset of STEMI and had preserved left ventricular ejection fraction (LVEF ≥40%) as assessed by echocardiography. Patients were ineligible for the trial if they had reduced LVEF (LVEF<40%), prior cardioverter defibrillator implantation, or contraindications to beta-blocker therapy such as unstable hemodynamic status, bradyarrhythmias, symptomatic HF, and severe bronchial asthma and/or chronic obstructive lung diseases.
After screening for eligibility and obtaining written informed consent, the enrolled patients were randomly assigned in a 1-to-1 ratio to the carvedilol group or to the no beta-blocker group within 7 days after primary PCI. Randomization was performed centrally through the electronic data capture system with a stochastic minimization algorithm to balance treatment assignment. Carvedilol was selected as the specific beta-blocker in the present study, because this agent was tested in the most recent trial evaluating the role of long-term oral beta-blocker therapy in STEMI patients (CAPRICORN trial)[2], and was the most widely used beta-blocker for STEMI patients in Japan[9]. In the carvedilol group, carvedilol was to be started from low doses and up-titrated to the target dose of 20mg daily, though the initial dose and titration of carvedilol was at the discretion of the attending physicians. The administration of other standard medications for STEMI patients such as aspirin, thienopyridines, statins, and inhibitors of the renin angiotensin system were also left to their decision.
Baseline and follow-up data were collected by the clinical research coordinators belonging to the participating centers, or the academic research organization (Research Institute for Production Development, Kyoto, Japan) (see Acknowledgments). Follow-up information on vital status, clinical events, and prescription of beta-blockers was collected by either a hospital visit or telephone contact with the patient or the referring physician at 3-month, 1-year, and final follow-up. Persistent discontinuation of carvedilol was defined as withdrawal lasting at least 2 months. Echocardiographic follow-up was recommended at 3-month and 1-year.
The study protocol (S1 Text) was approved by the institutional review board at each participating center (Juntendo University Shizuoka Hospital, Tenri Hospital, Kurashiki Central Hospital, Shiga Medical Center for Adults, Kyoto University Hospital, Yokohama City University Medical Center, Kobe City Medical Center General Hospital, Toyohashi Heart Center, National Hospital Organization Kyoto Medical Center, Osaka Red Cross Hospital, Tsuchiya General Hospital, Nagoya Daini Red Cross Hospital, Nozaki Tokushukai Hospital, Nagoya Tokushukai General Hospital, Ogaki Municipal Hospital, Kawakita General Hospital, Chikamori Hospital, Yotsuba Circulation Clinic, Hoshi General Hospital, New Tokyo Hospital, Shimada Municipal Hospital, Hiroshima City Hiroshima Citizens Hospital, Showa University Hospital, Gunma Cardiovascular Center, Kindai University Nara Hospital, Yamaguchi University Hospital, Toyama Prefectural Central Hospital, Hitachi General Hospital, National Cerebral and Cardiovascular Center, Saitama Cardiovascular and Respiratory Center, Yamagata Prefectural Central Hospital, Shizuoka City Shizuoka Hospital, Tokai University Hospital, Nagoya City East Medical Center, Kokura Memorial Hospital, Higashisumiyoshi Morimoto Hospital, Nara Prefectural Seiwa Medical Center, Tsukazaki Hospital, Kansai Electric Power Hospital, Mitsui Memorial Hospital, Fujioka General Hospital, Nihon University Itabashi Hospital, Iida Municipal Hospital, Otsu Red Cross Hospital, Fukuoka Wajiro Hospital, Fukuoka Tokushukai Hospital, Tachikawa Medical Center Tachikawa General Hospital, Miyazaki Medical Association Hospital, Saiseikai Kumamoto Hospital, Rakuwakai Otowa Hospital, Sakakibara Memorial Hospital, Wakayama Medical University Hospital, Hirosaki University Hospital, Maizuru Kyosai Hospital, Shiga University Of Medical Science Hospital, Saitama Medical Center Jichi Medical University, Japanese Red Cross Wakayama Medical Center, Toho University Ohashi Hospital, Tsukuba Medical Center Hospital, Kyoto Chubu Medical Center, Saga-ken Medical Center Koseikan, Hyogo Prefectural Amagasaki General Medical Center, Kindai University Hospital, Seirei Mikatahara General Hospital, Teikyo University Hospital, Kanazawa Cardiovascular Hospital, Kobe University Hospital). Written informed consent was obtained from all the study patients. The trial was registered with ClinicalTrials.gov.number, NCT 01155635.
Primary and secondary endpoints
The primary endpoint was a composite of all-cause death, myocardial infarction (MI), hospitalization for acute coronary syndrome (ACS), and hospitalization for HF.
The secondary endpoints included the individual components of the primary endpoint as well as cardiac death, non-cardiac death, stroke, vasospastic angina, major bleeding, definite stent thrombosis (ST), target-lesion revascularization (TLR), and any coronary revascularization. Also, we evaluated 3 composite endpoints including cardiac death/MI/ACS/HF, cardiovascular death/MI/stroke, and death/MI/stroke/ACS/HF/any coronary revascularization. Death was regarded as cardiac in origin unless obvious non-cardiac causes could be found. MI and ST were defined according to the Academic Research Consortium definitions[12]. Stroke was defined as ischemic or hemorrhagic stroke requiring hospitalization with symptoms lasting >24 hours. ACS was diagnosed by clinical symptoms, electrocardiographic changes indicative of myocardial ischemia, and elevation of cardiac biomarkers. Unstable angina was adjudicated only in the presence of the angiographically evident culprit lesion. Hospitalizaton for HF was defined as hospitalization due to worsening heart failure requiring intravenous drug therapy. Major bleeding was defined as moderate or severe bleeding according to the Global Utilization of Stereptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) classification[13]. Any coronary revascularization was defined as either PCI or CABG for any reason. Scheduled staged PCI procedures performed within 3 months of the index primary PCI were not regarded as the follow-up events, but were included in the index procedure. An independent clinical event committee adjudicated both the primary and secondary endpoints in a fashion blinded to the assigned treatment group (S1 and S2 Texts).
Statistical analysis
The statistical analysis and reporting of the current study were conducted in accordance with the CONSORT guidelines (S2 Text). Categorical variables were expressed as number and percentage, which were compared with the chi-square test. Continuous variables were expressed as mean ± standard deviation (SD) or median with inter-quartile range (IQR) based on their distributions. The appearance of distribution was judged by illustrating the listed variables in histogram. If it was a bell-shaped appearance, the variables were judged to follow normal distribution. Continuous variables were compared using the Student’s t-test or Wilcoxon rank sum test based on their distributions, accordingly. Clinical outcomes were analyzed according to the intention-to-treat principle (ITT). The cumulative incidences of clinical events were estimated by the Kaplan-Meier method and compared by the log-rank test. Effect of the carvedilol group relative to the no beta-blocker group was assessed by the Cox proportional hazard model, and was expressed as hazard ratio (HR) with 95% confidence interval (CI). Proportional hazard assumption for the assigned group was assessed on the plots of log (time) versus log [-log (survival)] stratified by the assigned group, and the assumptions were verified to be acceptable. We only evaluated the first event for each outcome measure of interest. As a sensitivity analysis, we compared the baseline characteristics and clinical outcomes between the 2 groups of patients who received high-dose (> = 10mg) versus low-dose (<10mg) carvedilol.
The initial study protocol had sought to investigate the effect of carvedilol on mortality, because all-cause death was the primary endpoint in most of the previous trials evaluating the role of long-term oral beta-blocker therapy in STEMI patients[14–16]. With the assumption of 8.7% all-cause mortality at 3-year in STEMI patients undergoing primary PCI in a previous Japanese study, a total of 7460 patients would yield 90% power to detect superiority with a relative 23% reduction of all-cause mortality in the carvedilol group at a level of one-sided typeⅠerror of 0.025[8]. A total of 7600 patients were to be enrolled considering possible dropout during follow-up. However, due to the slow enrollment, the study protocol was modified in February 2013, changing the primary endpoint to a composite of all-cause death, MI, hospitalization for ACS, and hospitalization for HF, and reducing the target number of patients to 1300. Sample size re-calculation was based on an estimated primary event rate of 30.1% at 6-year in STEMI patients with a power of 80% to detect a relative 23% reduction of the primary endpoint in the carvedilol group as compared with the no beta-blocker group. However, the study was prematurely terminated with enrollment of 801 patients due to the slow enrollment.
All statistical analyses were conducted using JMP 12.0 (SAS Institute Inc, Cary, NC) and SAS 9.4 (SAS Institute, Inc, Cary, NC) software. All reported P values were two-sided and P values <0.05 were regarded as statistically significant.
Results
Study population
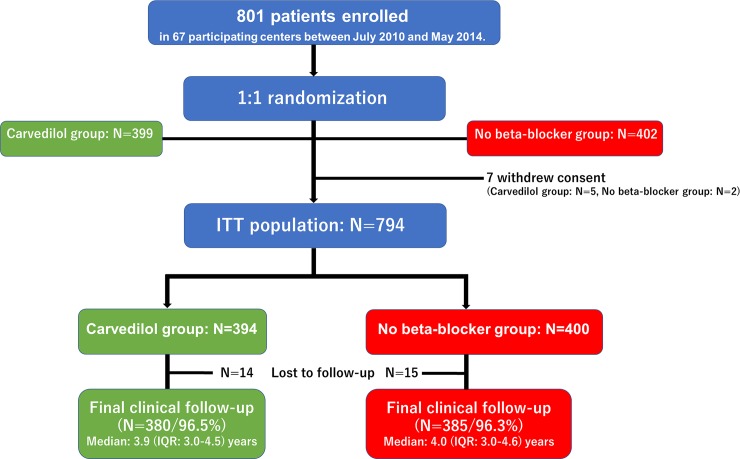
Between August 2010 and May 2014, a total of 801 STEMI patients were randomly assigned to the carvedilol group (N = 399) or to the no beta-blocker group (N = 402). After exclusion of 7 patients who withdrew consent, the ITT population consisted of 794 patients (carvedilol group: N = 394, and no beta-blocker group: N = 400) (Fig 1).
Fig 1. Study flow chart.
ITT = intention-to-treat; IQR = interquartile range.
The baseline clinical, angiographic and procedural characteristics were well balanced between the 2 groups (Table 1). The study population represented low-risk STEMI patients as reflected by relatively young age, low Killip score, short door-to-balloon time, preserved left ventricular systolic function. Anterior STEMI accounted for nearly 40% of patients. Stents were used in almost 95% of patients with nearly identical prevalence of bare-metal and drug-eluting stents. Complete revascularization was achieved in approximately 80% of patients. The contemporary evidence-based medications such as dual antiplatelet therapy, statins, and inhibitors of the renin angiotensin system were prescribed in the majority of patients (Table 1).
Table 1. Baseline characteristics and medications at discharge.
| Variables | Carvedilol group | No beta-blocker group | |
|---|---|---|---|
| N = 394 | N = 400 | ||
| Clinical characteristics | |||
| Age | 63.9±11.2 | 64.5±11.3 | |
| >75years | 59(15%) | 64(16%) | |
| Male | 327(83%) | 312(78%) | |
| Body mass index | 24.0±3.3(N = 388) | 23.9±3.2(N = 392) | |
| <25.0kg/m2 | 250/387(64%) | 250/392(64%) | |
| Hypertension | 239(61%) | 234(59%) | |
| Dyslipidemia | 191(48%) | 211(53%) | |
| Diabetes mellitus | 98(25%) | 81(20%) | |
| Treated with insulin therapy | 6(1.5%) | 12(3.0%) | |
| Current smoking | 186(47%) | 188(47%) | |
| Prior myocardial infarction | 16(4.1%) | 9(2.3%) | |
| Prior stroke | 18(4.6%) | 19(4.8%) | |
| Peripheral artery disease | 11(2.8%) | 9(2.3%) | |
| Prior PCI | 20(5.1%) | 21(5.3%) | |
| Family history of coronary artery disease | 68(17%) | 81(20%) | |
| eGFR (ml/min/1.73m2) | 72.4±21.5 | 72.8±20.9 | |
| Hemodialysis | 2(0.5%) | 1(0.3%) | |
| Left ventricular ejection fraction | 58.1±8.6(N = 390) | 58.0±8.9(N = 398) | |
| Hemoglobin (g/dl) | 13.2±1.9 | 13.0±2.0 | |
| COPD | 5(1.3%) | 7(1.8%) | |
| Malignancy | 29(7.4%) | 23(5.8%) | |
| Presentation and charcteristics of STEMI | |||
| Systolic blood pressure (mmHg) | 121±21(N = 390) | 121±20(N = 398) | |
| Diastolic blood pressure (mmHg) | 70.2±14.8(N = 390) | 71.1±14.5(N = 398) | |
| Heart rate (bpm) | 74.8±14.3(N = 382) | 73.2±13.9(N = 394) | |
| Location of STEMI | |||
| Anterior | 157(40%) | 153(38%) | |
| Inferior/Posterior | 203(52%) | 209(52%) | |
| Lateral | 34(8.6%) | 38(9.5%) | |
| Killip class | |||
| Ⅰ/Ⅱ | 388(98%) | 393(98%) | |
| Abnormal Q wave at admission | 126(32%) | 111(27%) | |
| Door to balloon time (minutes) | 60(54–90) | 66(54–102) (N = 399) | |
| Total ischemic time (hours) | 3.8(2.5–6.2) (N = 392) | 3.5(2.5–6.1) (N = 397) | |
| Peak CK | 1893(921–3427) (N = 393) | 2088(1057–3282) | |
| Angiographic and procedural characteristics | |||
| Infarct-related artery | |||
| LAD | 164(42%) | 172(43%) | |
| RCA | 185(47%) | 185(46%) | |
| LCX | 45(11%) | 43(11%) | |
| Extent of coronary artery disease | |||
| Single-vessel disease | 259(66%) | 264(66%) | |
| Two-vessel disease | 102(26%) | 90(23%) | |
| Three-vessel disease | 33(8.4%) | 46(12%) | |
| LMCA lesion | 9(2.3%) | 12(3.0%) | |
| Primary PCI | |||
| Stent use | 376(95%) | 382(96%) | |
| BMS use | 203/376(54%) | 212/382(56%) | |
| DES use | 176/376(46%) | 174/382(46%) | |
| EES use | 110/376(29%) | 108/382(28%) | |
| BES use | 22/376(5.9%) | 14/382(3.7%) | |
| SES use | 4/376(1.1%) | 6/382(1.6%) | |
| ZES use | 14/376(3.7%) | 14/382(3.7%) | |
| Maximal stent diameter (mm) | 3.2±0.5 | 3.2±0.5 | |
| 3(3–3.5) | 3(3–3.5) | ||
| Total stent length (mm) | 24.5±13.8 | 25.4±13.0 | |
| 23(18–28) | 23(18–28) | ||
| Thrombus aspiration | 330(84%) | 322(81%) | |
| Distal protection | 74(19%) | 69(17%) | |
| Temporary pacemaker | 33(8.4%) | 37(9.3%) | |
| IABP use | 20(5.1%) | 24(6.0%) | |
| Staged PCI | |||
| Staged PCI for non-infarct-related artery | 73/393(20%) | 89/399(22%) | |
| Target lesion | |||
| LAD | 40(10%) | 50(13%) | |
| RCA | 16(4.1%) | 21(5.3%) | |
| LCX | 37(9.4%) | 39(9.8%) | |
| LMCA | 2(0.5%) | 4(1.0%) | |
| Complete revascularization | 326/393(83%) | 326/399(82%) | |
| Medication at discharge | |||
| Aspirin | 388(98%) | 393(98%) | |
| Thienopyridine | 379(96%) | 377(94%) | |
| Clopidogrel | 370(94%) | 367(92%) | |
| Cilostazole | 5(1.3%) | 6(1.5%) | |
| Statin | 340(86%) | 345(86%) | |
| ACE-I/ARB | 296(75%) | 316(79%) | |
| ACE-I | 163(41%) | 186(47%) | |
| ARB | 133(34%) | 131(33%) | |
| Calcium channel blocker | 54(14%) | 49(12%) | |
| Aldosterone antagonist | 38(9.6%) | 35(8.8%) | |
| Nitrate | 44(11%) | 43(11%) | |
| Nicorandil | 63(16%) | 70(18%) | |
| Warfarin | 13(3.3%) | 9(2.3%) | |
| PPI | 303(77%) | 314(79%) | |
| H2 blocker | 51(13%) | 50(13%) | |
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, and categorical variables as number (percentage). Number of patients evaluated was indicated for the variables with missing information.
PCI = percutaneous coronary intervention; eGFR = estimated glomerular filtration rate; COPD = chronic obstructive pulmonary disease; STEMI = ST-segment elevation myocardial infarction; CK = creatine phosphokinase; LAD = left anterior descending coronary artery; RCA = right coronary artery; LCX = left circumflex coronary artery; LMCA = left main coronary artery; BMS = bare-metal stents; DES = drug-eluting stents; EES = everolimus-eluting stent; BES = biolimus-eluting stent; SES = sirolimus-eluting stent; ZES = zotarolimus-eluting stent; IABP = intra-aortic balloon pumping; ACE-I = angiotensin converting enzyme inhibitors; ARB = angiotensin-receptor blockers; H2 blockers = histamine type-2 receptor blockers.
Administration of beta-blockers
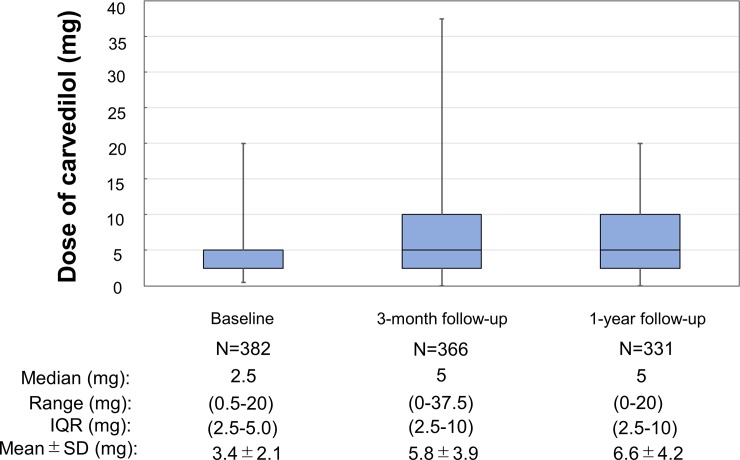
The dose of carvedilol was 3.4 ± 2.1 mg at baseline, which was up-titrated to 5.7 ± 3.9 mg at 3-month, and to 6.6 ± 4.2 mg at 1-year (Fig 2). The number of patients who took the dose of 20 mg (the maximum approved dose) was only 15 (3.8%). Prescription of carvedilol was maintained in 81% at final follow-up in the carvedilol group, while a beta-blocker was started in 14% of patients in the no beta-blocker group (S1 Fig).
Fig 2. Dose of carvedilol at each follow-up.
Among 394 patients in the carvedilol group, 12 patients did not receive the assigned carvedilol treatment; prescription of bisoprolol in 7 patients and no prescription of beta-blocker in 5 patients (protocol violation).
Echocardiographic follow-up
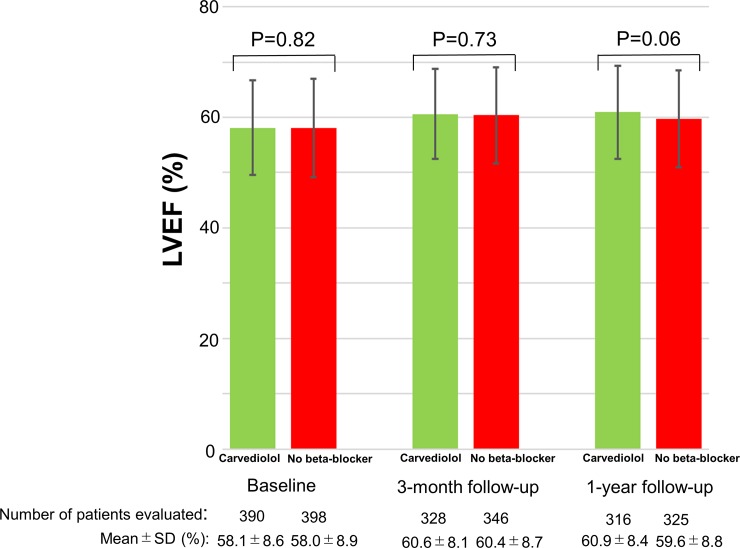
LVEF at baseline was well preserved in both the carvedilol and no beta-blocker groups (58.1 ± 8.6% and 58.3 ± 8.5%, P = 0.84). There was no evidence of adverse LV remodeling with well maintained LVEF at 3-month and at 1-year without any between group differences (Fig 3).
Fig 3. Comparison of LVEF between the carvedilol group and the no beta-blocker group at baseline, at 3-month follow-up, and at 1-year follow-up.
LVEF data was missing in 6 patients (4 patients in the carvedilol group and 2 patients in the no beta-blocker group). IQR = interquartile range; SD = standard deviation, LVEF = left ventricular ejection fraction.
Clinical outcomes
Final clinical follow-up beyond June 2015 was completed in 765 patients (96.4%) (carvedilol group: 380 patients [96.5%], and no beta-blocker group: 385 patients [96.3%], P = 0.88). Median follow-up period was 3.9 (IQR: 3.0–4.6) years in the overall study population (carvedilol group: 3.9 [IQR: 3.0–4.5] years, and no beta-blocker group: 4.0 [IQR: 3.0–4.6] years, P = 0.68).
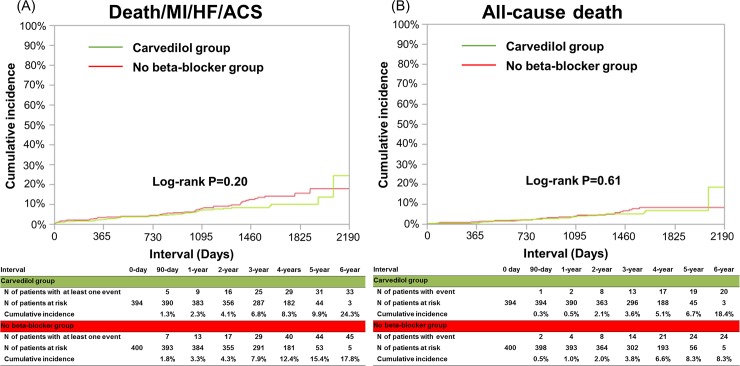
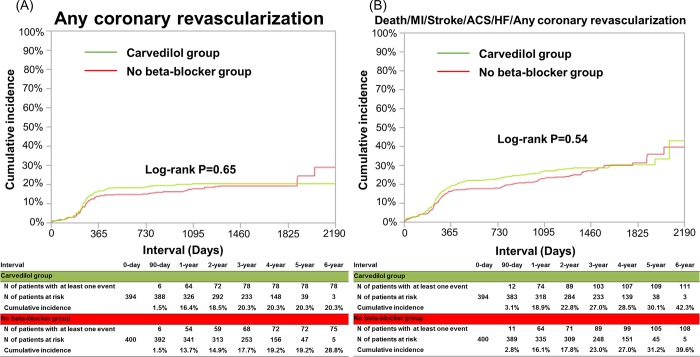
The cumulative 3-year incidence of the primary endpoint was not significantly different between the carvedilol and no beta-blocker groups (6.8% and 7.9%, log-rank P = 0.20, and HR: 0.75, 95%CI: 0.47–1.16, P = 0.20) (Table 2 and Fig 4). The cumulative 3-year incidences of all-cause death and cardiac death were very low and not significantly different between the 2 groups (3.6% and 3.8%, log-rank P = 0.61, and 1.1% and 1.4%, log-rank P = 0.74, respectively) (Table 2 and Fig 4). The cumulative 3-year incidences of any coronary revascularization and a composite of the primary end point or any coronary revascularization were relatively high, but not significantly different between the 2 groups (20.3% and 17.7%, log-rank P = 0.65, and 27.0% and 23.0%, log-rank P = 0.54, respectively) (Table 2 and Fig 5). There were also no significant between-group differences in other secondary endpoints (Table 2).
Table 2. Clinical outcomes.
| Carvedilol group | No beta-blocker group | HR(95%CI) | P value | ||
|---|---|---|---|---|---|
| N of patients with events | N of patients with events | ||||
| (Cumulative 3-year incidence) | (Cumulative 3-year incidence) | ||||
| N = 394 | N = 400 | ||||
| Primary endpoint | |||||
| Death/MI/HF/ACS | 33(6.8%) | 45(7.9%) | 0.75(0.47–1.16) | 0.20 | |
| Secondary endpoint | |||||
| All-cause death | 20(3.6%) | 24(3.8%) | 0.86(0.47–1.55) | 0.61 | |
| Cardiac death | 6(1.1%) | 5(1.4%) | 1.22(0.37–4.23) | 0.74 | |
| Non-cardiac death | 14(2.5%) | 19(2.4%) | 0.76(0.37–1.51) | 0.44 | |
| Myocardial infarction | 7(2.0%) | 10(1.9%) | 0.71(0.26–1.84) | 0.48 | |
| Hospitalization for HF | 5(1.1%) | 10(1.7%) | 0.51(0.16–1.46) | 0.21 | |
| Hospitalization for ACS | 8(2.5%) | 14(2.2%) | 0.58(0.23–1.35) | 0.21 | |
| Stroke | 17(4.0%) | 11(2.1%) | 1.59(0.75–3.50) | 0.22 | |
| Vasospastic angina | 2(0.6%) | 1(0.3%) | 2.03(0.19–43.6) | 0.55 | |
| Major bleeding | 14(2.9%) | 7(1.6%) | 2.07(0.86–5.46) | 0.11 | |
| Definite stent thrombosis | 3(0.8%) | 4(0.8%) | 0.76(0.15–3.45) | 0.72 | |
| Target lesion revascularization | 44(11.3%) | 37(9.1%) | 1.22(0.79–1.90) | 0.36 | |
| Any coronary revascularization | 78(20.3%) | 75(17.7%) | 1.08(0.78–1.48) | 0.65 | |
| Cardiac death/MI/HF/ACS | 19(4.3%) | 28(5.8%) | 0.69(0.38–1.22) | 0.20 | |
| Cardiovascular death/MI/stroke | 27(6.8%) | 26(5.3%) | 1.06(0.62–1.83) | 0.82 | |
| Death/MI/HF/Stroke/ACS/HF/Any coronary revascularization | 108(27.0%) | 111(23.0%) | 1.09(0.83–1.42) | 0.54 | |
Number of patients with event was counted through the entire follow-up period, while the cumulative incidence was indicated at 3-year.
HR = hazard ratio; CI = confidence interval; MI = myocardial infarction; ACS = acute coronary syndrome; HF = heart failure.
Fig 4.
Kaplan-Meier curves for the primary endpoint (a composite of death, MI, hospitalization for ACS, or hospitalization for HF) (A), for all-cause death (B).
Fig 5. Kaplan-Meier curves for any coronary revascularization (A), and for a secondary composite endpoint (a composite of death, MI, stroke, hospitalization for ACS, hospitalization for HF or any coronary revascularization) (B).
MI = myocardial infarction; ACS = acute coronary syndrome; HF = heart failure.
In a sensitivity analysis comparing the 2 groups of patients who received high-dose (> = 10mg: N = 114) versus low-dose (<10mg: N = 214) carvedilol, patients in the low-dose group were older and more often received intra-aortic balloon pumping support than those in the high-dose group (S1 Table). The cumulative 3-year incidences of the primary end point and a composite of the primary endpoint or any coronary revascularization were not significantly different between the high- and low-dose carvedilol groups (2.0% versus 4.0%, log rank P = 0.32, and 22.7% versus 24.7%, log rank P = 0.56, respectively) (S2 Table and S2 Fig).
Discussion
The main finding of this study was that long-term carvedilol therapy added on the contemporary evidence-based medications did not reduce the primary endpoint events (a composite of all-cause death, MI, hospitalization for ACS, and hospitalization for HF) as well as a composite of the primary endpoint or any coronary revascularization in STEMI patients treated with primary PCI.
There is no previous prospective trial investigating the role of long-term oral beta-blocker therapy in STEMI patients who underwent primary PCI. In the contemporary STEMI patients, there were several observational studies evaluating the role of long-term oral beta-blocker therapy with conflicting results[5–11]. In a metaanalysis of 10 observational studies including 40873 patients, use of beta-blockers was associated with a reduced risk of all-cause death, but the benefit of beta-blockers in preventing all-cause death was restricted to those with reduced ejection fraction, those with low use proportion of other secondary prevention drugs or those with non-ST-segment elevation myocardial infarction[17]. On the other hands, another metaanalysis of 7 observational studies including 10857 patients suggested that oral beta-blocker therapy is associated with decreased all-cause mortality in patients with STEMI who are treated with primary PCI and who have preserved LVEF[18]. However, there is a possibility of profound selection bias in observational studies by the trend toward avoiding the use of beta-blockers in sicker patients who are deemed to have a poor prognosis. Therefore, the true efficacy of beta-blockers in reducing cardiovascular events could only be addressed by the randomized controlled trials. This is the first randomized trial in the primary PCI era assessing the effect of long-term beta-blocker therapy in STEMI patient undergoing primary PCI, suggesting no significant benefit of a beta-blocker in reducing the cardiovascular events. Regrettably, we could not draw a definitive conclusion from the current study, because we could not deny a moderate effect of carvedilol due to insufficient power of the study for the primary endpoint. However, there are several important implications from our study. First, mortality was the primary endpoint in most of the previous randomized trials of beta-blockers in STEMI patients. However, long-term mortality rate, particularly cardiac death rate, was very low in the present study (annual rate of 1.3% for all-cause death and 0.47% for cardiac death during 3-year after STEMI in the control group). In contrast, the annual mortality rate was 4.4%, and the majority of death was due to cardiovascular causes (91% in the placebo group) in the ß-Blocker Heart Attack Trial (B-HAT), which was conducted between 1978 and 1980 to test clinical benefits of long-term administration of propranolol for patients with acute myocardial infarction[14]. This relatively high cardiovascular mortality was consistently observed in other trials conducted before the primary PCI era[1,14–16]. Recent several studies suggested that the cause of death during long-term follow-up of STEMI patients shifted from cardiac to non-cardiac disease, which coincided with the finding that non-cardiac death accounted for almost two-thirds of all-cause death in the present study[19,20]. Given the extremely low rate of cardiac death in contemporary uncomplicated STEMI patients, it is very unlikely for the long-term beta-blocker therapy to provide a large absolute reduction in mortality. Second, one of the mechanisms for the benefits of the beta-blocker therapy might be the attenuation of myocardial oxygen demand, which might lead to reduction of coronary revascularization procedure. However, there was no significant between groups difference in the rate of any coronary revascularization, which was one of the secondary endpoint with higher incidence. Third, another mechanism for the benefits of the beta-blocker therapy might be the prevention of cardiotoxic effects of catecholamines, leading to the inhibition of the adverse LV remodeling in patients with low LVEF[21,22]. In the current study, serial echocardiographic evaluation was recommended by the protocol, and LVEF was well maintained at 3-month and at 1-year without evidence of adverse LV remodeling regardless of carvedilol administration (Fig 2B). Carvedilol is unlikely to improve the long-term prognosis of STEMI patients without favorable influence on the remodeling process.
Limitations
This trial has several important limitations. First of all, the current trial was underpowered to detect even a moderate difference in the treatment effect for the primary endpoint. The final sample size was reduced due to the slow enrollment, because administration of beta-blockers in STEMI patients is the guideline recommended therapy, and therefore, conducting a randomized trial in this field was very challenging. Nevertheless, we did not find any significant between groups difference in a composite of the primary endpoint or coronary revascularization, which was an adequately powered and clinically relevant endpoint, suggesting that long-term oral carvedilol therapy was not associated with discernible clinical benefits. Second, there was some separation of the Kaplan-Meier curves for the primary endpoint beyond 3-year favoring the carvedilol group. However, in the previous randomized trials, the benefit of beta-blockers in reducing death and/or MI was evident within 1-year[2,14]. Therefore, it is unlikely for carvedilol to have delayed beneficial effects beyond 3-year without any effects during the initial 3 years. Actually, the AHA/ACCF secondary prevention guidelines recommend a 3-year treatment course for beta-blocker in uncomplicated STEMI patients[3]. Third, the open-label trial design is another limitation of the study. However, the initiation of beta-blockers in the no beta-blocker group was acceptably low. Fourth, the mean maintenance dose of carvedilol in the current trial was only 6.3mg/day at 1-year, which might have attenuated the potential benefits of carvedilol. However, a study by Goldberger et al. evaluated the association of the dose of beta-blocker with survival after acute myocardial infarction, demonstrating that the lowest mortality was observed at 25% of the target dose (metoprolol 50 mg/day)[23]. In the sensitivity analysis of the present study, clinical outcomes were not different between the high- and low-dose carvedilol groups. The relationship between the doses of beta-blocker and the magnitude of clinical benefits is still debated[21,24–26]. In our previous report from a Japanese real world registry of 3692 STEMI patients, oral beta-blockers were prescribed only in 44% of patients, and the daily dose of carvedilol was median 5 (interquartile range: 2–10) mg, which was comparable to the dose used in the present study(9). Taken together, the carvedilol dose lower than that used outside Japan is a big limitation to extrapolate the present study results outside Japan. Nevertheless, carvedilol at the dose used in daily Japanese clinical practice seems not to reduce cardiovascular events in uncomplicated STEMI patients treated with primary PCI. Finally, the present study patients could be a highly selected low-risk STEMI population with mean LVEF of 60%. It is unknown whether the present trial result could be applicable to those uncomplicated STEMI patients with lower LVEF. However, in the contemporary registries of STEMI patients treated with primary PCI, the majority of patients have well preserved LVEF and the rates of cardiovascular events such as cardiac death, MI and HF are remarkably low[27–29].
Conclusions
Long-term carvedilol therapy added on the contemporary evidence-based medications did not seem beneficial in selected STEMI patients treated with primary PCI.
Supporting information
(DOCX)
(DOCX)
Kaplan-Meier curves for the cumulative incidences of (A) persistent discontinuation of carvedilol in the carvedilol group and of (B) initiation of beta-blockers in the no beta-blocker group.
(DOCX)
Kaplan-Meier curves for the cumulative incidences of (A) the composite of all-cause death, myocardial infarction, heart failure hospitalization and emergent hospitalization for acute coronary syndrome and of (B) the composite of all-cause death, myocardial infarction, stroke, heart failure hospitalization, emergent hospitalization for acute coronary syndrome and any coronary revascularization in the high-dose and the low-dose groups.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We deeply appreciate the following co-investigators of the participating centers: Juntendo University Shizuoka Hospital; Satoru Suwa, Tenri Hospital; Yoshihisa Nakagawa, Chisato Izumi, Makoto Miyake, Kurashiki Central Hospital; Kazuaki Kadota, Reo Hata, Shiga Medical Center for Adults; Shigeru Ikeguchi, Yasutaka Inuzuka, Kyoto University Hospital; Takeshi Kimura, Neiko Ozasa, Hiroki Watanabe, Bao Bingyuan, Hiroki Shiomi, Yokohama City University Medical Center; Kiyoshi Hibi, Kobe City Medical Center General Hospital; Yutaka Furukawa, Shuichiro Kaji, Toyohashi Heart Center; Takahiko Suzuki, Mitsuyasu Terashima, National Hospital Organization Kyoto Medical Center; Masaharu Akao, Osaka Red Cross Hospital; Tsukasa Inada, Tsuchiya General Hospital; Yasuhiko Hayashi, Nagoya Daini Red Cross Hospital; Mamoru Nanasato, Nozaki Tokushukai Hospital; Masaaki Okutsu, Takashi Kitao, Nagoya Tokushukai General Hospital; Ryosuke Kametani, Shingo Yoshioka, Ogaki Municipal Hospital; Takahito Sone, Kawakita General Hospital; Youichi Sugimura, Chikamori Hospital; Kazuya Kawai, Yotsuba Circulation Clinic; Mistunori Abe, Yumiko Abe, Hoshi General Hospital; Hironori Kaneko, New Tokyo Hospital; Sunao Nakamura, Shimada Municipal Hospital; Takeshi Aoyama, Hiroshima City Hiroshima Citizens Hospital; Yasuharu Nakama, Showa University Hospital; Youichi Kobayashi, Gunma Cardiovascular Center; Shigeru Oshima, Kindai University Nara Hospital; Manabu Shirotani, Yamaguchi University Hospital; Masafumi Yano, Toyama Prefectural Central Hospital; Kazuo Usuda, Hitachi General Hospital; Yutaka Eki, National Cerebral and Cardiovascular Center: Satoshi Yasuda, Saitama Cardiovascular and Respiratory Center; Makoto Muto, Yamagata Prefectural Central Hospital; Mikiyuki Matsui, Shizuoka City Shizuoka Hospital; Ryuzo Nawada, Tokai University Hospital; Yuji Ikari, Nagoya City East Medical Center; Yoshimasa Murakami, Kokura Memorial Hospital; Kenji Ando, Higashisumiyoshi Morimoto Hospital; Yuji Sakanoue, Nara Prefectural Seiwa Medical Center; Naofumi Doi, Tsukazaki Hospital; Hidetaka Iida, Kansai Electric Power Hospital; Katsuhisa Ishii, Mitsui Memorial Hospital; Kazuhiro Hara, Fujioka General Hospital; Masahiro Inoue, Nihon University Itabashi Hospital; Atsushi Hirayama, Tadateru Takayama, Iida Municipal Hospital; Kazuya Yamamoto, Otsu Red Cross Hospital; Kazuaki Kaitani, Fukuoka Wajiro Hospital; Taro Saito, Fukuoka Tokushukai Hospital; Hidenori Shimomura, Tachikawa Medical Center Tachikawa General Hospital; Masaaki Okabe, Miyazaki Medical Association Hospital; Yoshisato Shibata, Saiseikai Kumamoto Hospital; Koichi Nakao, Rakuwakai Otowa Hospital; Yuji Hiraoka, Sakakibara Memorial Hospital; Tetsuya Sumiyoshi, Wakayama Medical University Hospital; Takashi Akasaka, Hirosaki University Hospital; Ken Okumura, Takumi Higuma, Maizuru Kyosai Hospital; Kimihito Usui, Shiga University Of Medical Science Hospital; Minoru Horie, Saitama Medical Center Jichi Medical University; Shinichi Momomura, Japanese Red Cross Wakayama Medical Center; Takashi Tamura, Toho University Ohashi Hospital; Masato Nakamura, Tsukuba Medical Center Hospital; Yuichi Noguchi, Kyoto Chubu Medical Center; Tetsuya Tatsumi, Saga-ken Medical Center Koseikan; Takanori Yoshida, Hyogo Prefectural Amagasaki General Medical Center; Yoshiki Takatsu, Kindai University Hospital; Shunichi Miyazaki, Seirei Mikatahara General Hospital; Yasushi Wakabayashi, Teikyo University Hospital; Ken Kozuma, Kanazawa Cardiovascular Hospital; Masanobu Namura, Kobe University Hospital; Toshiro Shinke. <Steering Committee>: Takeshi Kimura (principal investigator), Hiroshi Nonogi, Masakiyo Nobuyoshi, Kazuaki Mitsudo, Yasuhiko Hayashi, Koichi Nakao, Tetsuya Sumiyoshi, Takahiko Suzuki, Keiichi Igarashi, Takaaki Isshiki, Masunori Matsuzaki, Kazuo Kimura, Shunichi Miyazaki, Minoru Horie, Kenichi Hirata, Hiroshi Ito, Toyoaki Murohara, Yuji Ikari, Masaharu Ishihara. <Clinical Event Committee>: Masaharu Akao, Yutaka Furukawa, Yoshihisa Nakagawa. <Statistical Analysis>: Takeshi Morimoto. <Data safety monitoring board>: Hisao Ogawa, Tetsu Yamaguchi. <Coordinating center: Research Institute for Production Development (Kyoto, Japan)>: Naoko Okamoto, Miya Hanazawa, Kumiko Kitagawa, Misato Yamauchi, Yumika Fujino, Saori Tezuka, Yuki Sato, Chikako Hibi, Hitomi Sasae, Emi Takinami, Yuriko Uchida, Yuko Yamamoto, Satoko Nishida, Asuka Takahashi, and Yui Kinoshita.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan).
References
- 1.Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1993;270: 1589–95. [PubMed] [Google Scholar]
- 2.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357: 1385–1390. 10.1016/S0140-6736(00)04560-8 [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61: 78–140. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33: 2569–2619. 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 5.Yang JH, Hahn J-Y, Song Y Bin, Choi S-H, Choi J-H, Lee SH, et al. Association of Beta-Blocker Therapy at Discharge With Clinical Outcomes in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. JACC Cardiovasc Interv. Elsevier Inc; 2014;7: 592–601. 10.1016/j.jcin.2013.12.206 [DOI] [PubMed] [Google Scholar]
- 6.Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016; i4801 10.1136/bmj.i4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dondo TB, Hall M, West RM, Jernberg T, Lindahl B, Bueno H, et al. β-Blockers and Mortality After Acute Myocardial Infarction in Patients Without Heart Failure or Ventricular Dysfunction. J Am Coll Cardiol. 2017;69: 2710–2720. 10.1016/j.jacc.2017.03.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozasa N, Kimura T, Morimoto T, Hou H, Tamura T, Shizuta S, et al. Lack of Effect of Oral Beta-Blocker Therapy at Discharge on Long-Term Clinical Outcomes of ST-Segment Elevation Acute Myocardial Infarction After Primary Percutaneous Coronary Intervention. Am J Cardiol. Elsevier Inc.; 2010;106: 1225–1233. 10.1016/j.amjcard.2010.06.048 [DOI] [PubMed] [Google Scholar]
- 9.Bao B, Ozasa N, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, et al. β-Blocker therapy and cardiovascular outcomes in patients who have undergone percutaneous coronary intervention after ST-elevation myocardial infarction. Cardiovasc Interv Ther. 2013;28: 139–147. 10.1007/s12928-012-0137-9 [DOI] [PubMed] [Google Scholar]
- 10.Bangalore S, Bhatt DL, Steg PG, Weber MA, Boden WE, Hamm CW, et al. β-blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes. 2014;7: 872–81. 10.1161/CIRCOUTCOMES.114.001073 [DOI] [PubMed] [Google Scholar]
- 11.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308: 1340–9. 10.1001/jama.2012.12559 [DOI] [PubMed] [Google Scholar]
- 12.Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJRM, Schönberger JPAM, et al. Comparison of Coronary-Artery Bypass Surgery and Stenting for the Treatment of Multivessel Disease. N Engl J Med. 2001;344: 1117–1124. 10.1056/NEJM200104123441502 [DOI] [PubMed] [Google Scholar]
- 13.GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329: 673–82. 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 14.beta-blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247: 1707–14. [DOI] [PubMed] [Google Scholar]
- 15.The MIAMI Trial Research Group. Metoprolol in acute myocardial infarction (MIAMI): a randomised placebo- controlled international trial. Eur Hear J. 1985;6: 199–226. [PubMed] [Google Scholar]
- 16.Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304: 801–7. 10.1056/NEJM198104023041401 [DOI] [PubMed] [Google Scholar]
- 17.Huang B-T, Huang F-Y, Zuo Z-L, Liao Y-B, Heng Y, Wang P-J, et al. Meta-Analysis of Relation Between Oral β-Blocker Therapy and Outcomes in Patients With Acute Myocardial Infarction Who Underwent Percutaneous Coronary Intervention. Am J Cardiol. Elsevier Inc.; 2015;115: 1529–1538. 10.1016/j.amjcard.2015.02.057 [DOI] [PubMed] [Google Scholar]
- 18.Misumida N, Harjai K, Kernis S, Kanei Y. Does Oral Beta-Blocker Therapy Improve Long-Term Survival in ST-Segment Elevation Myocardial Infarction With Preserved Systolic Function? A Meta-Analysis. J Cardiovasc Pharmacol Ther. 2015;21: 280–285. 10.1177/1074248415608011 [DOI] [PubMed] [Google Scholar]
- 19.Pedersen F, Butrymovich V, Kelbæk H, Wachtell K, Helqvist S, Kastrup J, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64: 2101–2108. 10.1016/j.jacc.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Shiomi H, Morimoto T, Yaku H, Furukawa Y, Nakagawa Y, et al. Cardiac and Noncardiac Causes of Long-Term Mortality in ST-Segment-Elevation Acute Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. Circ Cardiovasc Qual Outcomes. 2017;10 10.1161/CIRCOUTCOMES.116.002790 [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi Y, Tanimoto S, Aoki J, Nakajima H, Hara K, Tanabe K. Effects of β-blockers on left ventricular remodeling in patients with preserved ejection fraction after acute myocardial infarction. Int J Cardiol. Elsevier Ireland Ltd; 2016;221: 765–769. 10.1016/j.ijcard.2016.07.123 [DOI] [PubMed] [Google Scholar]
- 23.Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, et al. Effect of Beta-Blocker Dose on Survival after Acute Myocardial Infarction. J Am Coll Cardiol. 2015;66: 1431–1441. 10.1016/j.jacc.2015.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori M, Sasayama S, Kitabatake A, Toyo-Oka T, Handa S, Yokoyama M, et al. Low-dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: The Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. Am Heart J. 2004;147: 324–330. 10.1016/j.ahj.2003.07.023 [DOI] [PubMed] [Google Scholar]
- 25.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150: 784–94. 10.1155/2011/543272 [DOI] [PubMed] [Google Scholar]
- 26.Simona T, Mary-Krause M, Funck-Brentano C, Lechat P, Jaillon P. Bisoprolol dose-response relationship in patients with congestive heart failure: A subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II). Eur Heart J. 2003;24: 552–559. 10.1016/S0195-668X(02)00743-1 [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi T, Shiomi H, Morimoto T, Watanabe H, Ono K, Shizuta S, et al. Incidence and Prognostic Impact of Heart Failure Hospitalization During Follow-Up After Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017;119: 1729–1739. 10.1016/j.amjcard.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 28.Fox KAA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297: 1892–900. 10.1001/jama.297.17.1892 [DOI] [PubMed] [Google Scholar]
- 29.Donataccio MP, Puymirat E, Parapid B, Steg PG, Eltchaninoff H, Weber S, et al. In-hospital outcomes and long-term mortality according to sex and management strategy in acute myocardial infarction. Insights from the French ST-elevation and non-ST-elevation Myocardial Infarction (FAST-MI) 2005 Registry. Int J Cardiol. Elsevier Ireland Ltd; 2015;201: 265–270. 10.1016/j.ijcard.2015.08.065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Kaplan-Meier curves for the cumulative incidences of (A) persistent discontinuation of carvedilol in the carvedilol group and of (B) initiation of beta-blockers in the no beta-blocker group.
(DOCX)
Kaplan-Meier curves for the cumulative incidences of (A) the composite of all-cause death, myocardial infarction, heart failure hospitalization and emergent hospitalization for acute coronary syndrome and of (B) the composite of all-cause death, myocardial infarction, stroke, heart failure hospitalization, emergent hospitalization for acute coronary syndrome and any coronary revascularization in the high-dose and the low-dose groups.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.