Significance
Neuropathic pain is a widespread problem that is undermanaged by currently available analgesic drugs. An antagonist of the type II angiotensin II receptor (AT2R) reduces pain behaviors related to neuropathy, suggesting that angiotensin receptor signaling is involved in this pain. We find that AT2R expression is detected not in sensory neurons themselves, but in macrophages that infiltrate the site of nerve injury. Inducible depletion of peripheral macrophages attenuates mechanical and cold pain hypersensitivity related to neuropathy, as does transplantation of AT2R-null bone marrow into an otherwise WT recipient. Our observations provide powerful evidence that neuropathic pain is dependent upon angiotensin signaling, macrophages, and the AT2R-mediated downstream signaling therein.
Keywords: neuropathic pain, angiotensin, AT2R, macrophage, chemogenetics
Abstract
Peripheral nerve damage initiates a complex series of structural and cellular processes that culminate in chronic neuropathic pain. The recent success of a type 2 angiotensin II (Ang II) receptor (AT2R) antagonist in a phase II clinical trial for the treatment of postherpetic neuralgia suggests angiotensin signaling is involved in neuropathic pain. However, transcriptome analysis indicates a lack of AT2R gene (Agtr2) expression in human and rodent sensory ganglia, raising questions regarding the tissue/cell target underlying the analgesic effect of AT2R antagonism. We show that selective antagonism of AT2R attenuates neuropathic but not inflammatory mechanical and cold pain hypersensitivity behaviors in mice. Agtr2-expressing macrophages (MΦs) constitute the predominant immune cells that infiltrate the site of nerve injury. Interestingly, neuropathic mechanical and cold pain hypersensitivity can be attenuated by chemogenetic depletion of peripheral MΦs and AT2R-null hematopoietic cell transplantation. Our study identifies AT2R on peripheral MΦs as a critical trigger for pain sensitization at the site of nerve injury, and therefore proposes a translatable peripheral mechanism underlying chronic neuropathic pain.
Neuropathic pain is caused by diseases or lesions affecting the somatosensory nervous system. Often intractable in nature, neuropathic pain is estimated to affect ∼3–17% of the chronic pain population (1). The etiology of neuropathic pain is complex, therefore presenting a formidable challenge to its effective management. It is associated with drugs used in cancer chemotherapy, diabetic neuropathy, postherpetic neuralgia (PHN), traumatic injury, and trigeminal neuralgia, among others (2, 3). The lack of a precise mechanistic understanding of neuropathic pain, which is poorly managed by existing drugs, has undoubtedly hampered the development of effective analgesics (3–6). However, angiotensin II (Ang II) type 2 receptor (AT2R) antagonists have recently proven efficacious in preclinical models of neuropathic, inflammatory, and cancer pain (7), and the AT2R antagonist EMA401 has shown effective pain relief in PHN patients in a phase II clinical trial (8). However, the site of action for this antagonist remains controversial: prior reports suggested that Ang II acts directly on dorsal root ganglia (DRG) neurons to induce peripheral pain sensitization (9–12), whereas activation of sensory neuron AT2R by a bacterial mycolactone toxin has been reported to be analgesic in mice (13). Recent transcriptome analysis in human and rodent sensory ganglia and neurons show a lack of AT2R gene (Agtr2) expression therein, which raises important questions about the tissue/cell target underlying the analgesic action of AT2R antagonism (14–18).
The major effector of the renin-angiotensin system (RAS), Ang II is generated from angiotensinogen (Agt) and angiotensin I by renin and angiotensin-converting enzyme (ACE), respectively (19). The regulation of blood pressure by Ang II has been well-established, with most of these physiological actions ascribed to type 1 receptor (AT1R) signaling; however, the role of AT2R has remained more enigmatic (19). Expression of AT2R in the brain has been reported contributing to a number of functions, such as regulation of drinking behavior and motor activity (19–21). Recent findings have also suggested that AT2R in peripheral sensory neurons is involved in pain modulation (7). Signaling originating from Gαs-coupled AT2R in sensory neurons was shown to elicit peripheral pain sensitization (9, 10). In contrast, activation of Gαi/o-coupled AT2R in sensory neurons by a mycolactone toxin was shown to induce analgesia in mice (13). Interestingly, a recent follow-up study demonstrated that the AT2R antagonist EMA401 was unable to prevent the mycolactone toxin’s effect on sensory neurons in vitro (22). This raises the possibility that the analgesic actions of EMA401 could result from targeting nonneuronal AT2R, or could be entirely independent of AT2R antagonism. This further emphasizes that establishing the tissue/cell target of angiotensin signaling in chronic neuropathic pain states is essential for further therapeutic developments.
Using the spared nerve injury (SNI) model of experimental neuropathy (23) and the complete Freund’s adjuvant (CFA) model (24) of inflammation in mice, we corroborate the effectiveness of AT2R antagonism selectively in neuropathic pain hypersensitivity. Elevated levels of Ang II are detected specifically in injured sciatic nerves. Our observations in Agtr2GFP reporter mice suggest the absence of its expression on any cell type in the DRG. Instead, we report that Agtr2 expression is detected on macrophages (MΦs) that infiltrate the site of peripheral nerve injury. Accordingly, chemogenetic depletion of peripheral MΦs, and transplantation of AT2R-null bone marrow into WT recipients were both able to attenuate neuropathic mechanical and cold pain hypersensitivity. These findings describe the critical role for MΦs expressing AT2R at the site of nerve injury in the development of neuropathy-induced chronic pain hypersensitivity.
Results
AT2R Activation Is Critical for Nerve Injury-Induced Mechanical and Cold Pain Hypersensitivity.
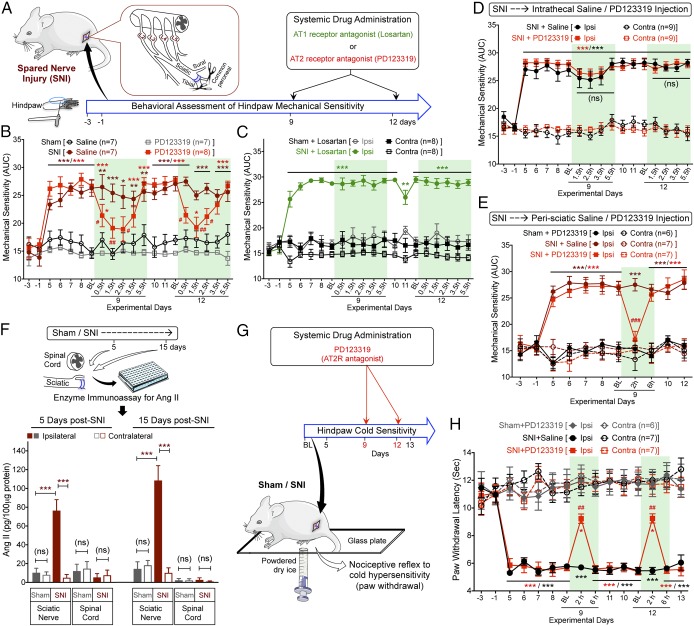
We began by evaluating the ability of AT1R and AT2R antagonism to alleviate nerve injury-induced chronic pain. SNI-induced peripheral neuropathy in mice elicited long-lasting mechanical hypersensitivity when the region being stimulated is innervated by the spared sural nerve: that is, the extreme lateral edge of the plantar surface of the hindpaw (Fig. 1 A and B), as reported previously (23, 25, 26). Instead of mechanical paw withdrawal threshold, the magnitude of total mechanical sensitivity on mouse hindpaws in response to increasing von Frey filament strength was determined, as detailed earlier (27–30) and outlined in SI Appendix, Fig. S1A. Systemic administration of the AT2R antagonist PD123319 dose-dependently attenuated SNI-induced mechanical hypersensitivity in male and female mice to a similar extent (Fig. 1B and SI Appendix, Fig. S1 B and E). However, administration of the AT1R antagonist losartan did not influence SNI-induced mechanical hypersensitivity (Fig. 1C). Administration of PD123319 or losartan alone in sham mice did not alter hindpaw mechanical sensitivity, and no change in mechanical sensitivity was observed in the contralateral hindpaws of SNI mice (Fig. 1 B and C and SI Appendix, Fig. S1C). SNI did not influence hindpaw heat sensitivity in male or female mice (SI Appendix, Fig. S1 D and F), as demonstrated previously (25, 26). We next verified whether AT2R inhibition of SNI-induced mechanical hypersensitivity targets the central or peripheral nervous system. Intrathecal administration of PD123319 did not attenuate mechanical hypersensitivity (Fig. 1D), but peri-sciatic delivery, as with systemic administration (intraperitoneal, i.p.), proved effective (Fig. 1E). Attenuation of SNI-induced mechanical hypersensitivity by PD123319 was independent of any hemodynamic changes, because PD123319 administration did not influence blood pressure, whereas the AT1R antagonist losartan did reduce blood pressure (SI Appendix, Fig. S1G). Vascular permeability of the hindpaw was also unaffected by PD123319, as determined by Evans blue extravasation (SI Appendix, Fig. S1H). Systemic administration of PD123319 did not attenuate mechanical and thermal hypersensitivity induced by chronic hindpaw inflammation with hindpaw CFA injection (SI Appendix, Fig. S2 A–C), suggesting its analgesic efficacy is selective for neuropathic pain.
Fig. 1.
Peripheral AT2R activation mediates neuropathic pain hypersensitivity. (A) Experimental scheme depicting nerve injury-induced neuropathy, pain behavioral assessments, and drug administration timeline in C57BL/6 (B6) mice for B–E (data analysis scheme in SI Appendix, Fig. S1A). (B) Systemic administration of PD123319 (10 mg/kg, i.p.) attenuates SNI-induced mechanical hypersensitivity. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham+saline/PD123319 groups; #P < 0.05 and ##P < 0.01 vs. SNI+saline group. (C) Losartan (10 mg/kg, i.p.) has no effects on SNI-induced mechanical hypersensitivity. Mean ± SEM; **P < 0.01 and ***P < 0.001 vs. sham+losartan-ipsilateral (ipsi) group. (D) Intrathecal PD123319 (30 nmol in 10 μL) does not attenuate SNI-induced mechanical hypersensitivity. Mean ± SEM; ***P < 0.001 vs. contralateral (contra) groups; not significant (ns) vs. SNI+saline-ipsi group. (E) Peri-sciatic PD123319 administration (30 nmol in 10 μL) attenuates SNI-induced mechanical hypersensitivity. Mean ± SEM; ***P < 0.001 vs. contra groups; ###P < 0.001 vs. SNI+saline-ipsi group. (F) SNI elevates Ang II levels in injured mouse sciatic nerve, but not in the spinal cord. Mean ± SEM (n = duplicate tissue samples from six mice per group). ***P < 0.001 vs. respective SNI-contralateral groups; not significant (ns) vs. sham/SNI-contralateral or vehicle groups. (G) Experimental scheme depicting cold hypersensitivity assessment, and drug administration timeline in mice subjected to sham/SNI surgery. (H) Systemic administration of PD123319 (10 mg/kg, i.p.) attenuates SNI-induced cold hypersensitivity. Mean ± SEM; *P < 0.05 and ***P < 0.001 vs. sham+PD123319 group; ##P < 0.01 vs. SNI+saline group. Rectangular boxes in B–E and H denote postdrug administration time points for behavioral assessment.
We next investigated if SNI was associated with changes in Ang II production. Ang II levels were elevated in the ipsilateral sciatic nerve from SNI mice, but not in contralateral or sham-operated mice. Ang II levels were unchanged in the spinal cords of SNI- vs. sham-operated mice (Fig. 1F). Furthermore, hindpaw CFA injection did not lead to any elevation in local Ang II levels (SI Appendix, Fig. S2D). Collectively, these observations suggest that SNI elevates local Ang II levels at the site of injury in the sciatic nerve, which induces AT2R signaling that contributes to mechanical hypersensitivity associated with neuropathic pain.
Given that neuropathic conditions elicit pronounced cold hypersensitivity (31), we assessed hindpaw cold sensitivity in SNI- and sham-operated mice (Fig. 1G). SNI induced significant cold hypersensitivity in ipsilateral hindpaws of SNI mice, which could be attenuated by systemic administration (intraperitoneal) of the AT2R antagonist PD123319 (Fig. 1H). These observations suggest that both mechanical and cold hypersensitivity associated with experimental neuropathy in mice are mediated by peripheral activation of AT2R.
Nerve Injury-Induced Mechanical and Cold Pain Hypersensitivity Is Dependent upon TRPA1.
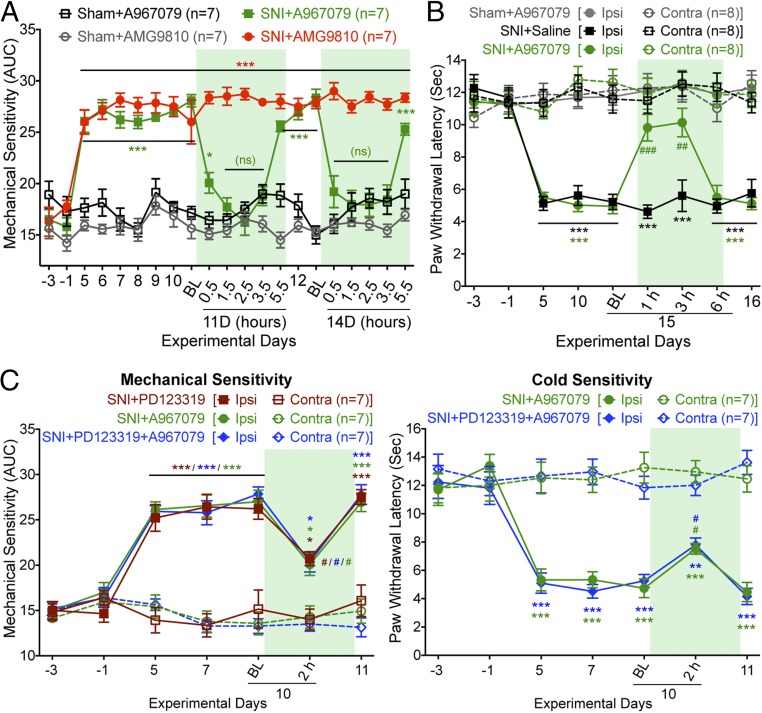
We next investigated the involvement of sensory transient receptor potential (TRP) channels in nerve injury-induced mechanical and cold hypersensitivity. Both TRPV1 and TRPA1 have been implicated in mechanical hypersensitivity (32, 33), and in addition, TRPA1 is involved in cold hypersensitivity in experimental nerve injury/neuropathic pain states in mice (32, 34, 35). Systemic administration of a TRPA1 antagonist (A967079), but not TRPV1 antagonist (AMG9810), attenuated SNI-induced hindpaw mechanical hypersensitivity (Fig. 2A). Administration of a TRPA1 antagonist (A967079) also attenuated SNI-induced hindpaw cold hypersensitivity (Fig. 2B). Systemic coadministration of submaximal dose of AT2R antagonist (PD123319; 3 mg/kg, i.p.) and TRPA1 antagonist (A967079; 10 mg/kg, i.p.) did not lead to any greater attenuation of hindpaw mechanical and cold hypersensitivity than either drug in isolation at that dose (Fig. 2C). This observation indicates that antagonism of AT2R and TRPA1 presumably act in series to attenuate SNI-induced mechanical and cold hypersensitivity.
Fig. 2.
Peripheral AT2R/TRPA1 inhibition attenuates neuropathic pain hypersensitivity. (A) TRPA1 antagonist A967079, but not TRPV1 antagonist AMG9810 (30 mg/kg for each, i.p.), attenuates SNI-induced mechanical hypersensitivity. Rectangular boxes denote postdrug administration behavioral assessment time points. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham-A967079/AMG9810 groups; not significant (ns) vs. sham-A967079 group, n = 8 mice per group. (B) TRPA1 antagonist A967079 attenuates SNI-induced cold hypersensitivity. Rectangular box denotes postdrug administration behavioral assessment time point. Mean ± SEM; ***P < 0.001 vs. sham-A967079 group; ##P < 0.01 and ###P < 0.001 vs. SNI-saline group. (C) Coadministration of the AT2R antagonist PD123319 (3 mg/kg, i.p.) and the TRPA1 antagonist A967079 (10 mg/kg, i.p.) does not additively reverse SNI-induced mechanical (Left) or cold hypersensitivity (Right). Rectangular boxes denote postdrug administration behavioral assessment time points. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. respective SNI-contralateral groups; #P < 0.05 vs. 10d-BL time point.
Macrophages Infiltrating the Site of Nerve Injury Express AT2R.
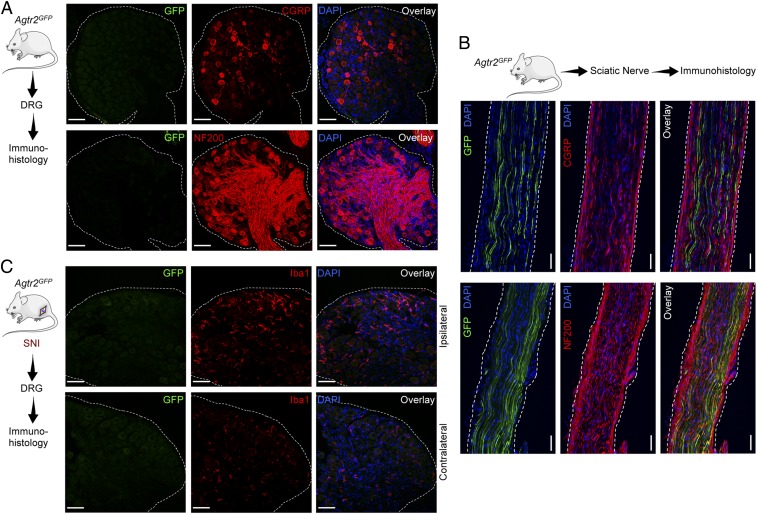
Earlier studies used AT2R antibody staining to show AT2R expression in rodent DRGs, which also exhibited Ang II/AT2R-mediated potentiation of TRPV1 currents (11). However, recent transcriptome analysis showed negligible or zero expression of Agtr2/AGTR2 mRNA in mouse and human DRGs (14–16, 36, 37). Furthermore, we also assessed Agtr2 expression in DRG neurons in Agtr2GFP mice, where GFP expression is driven by the Agtr2 promoter. Immunostaining of DRG sections from Agtr2GFP reporter mice displayed no detectable GFP signal (Fig. 3A). In the sciatic nerves of Agtr2GFP mice, GFP staining is observed only in a subset of NF200+ (myelinated) fibers, but not in CGRP+ (peptidergic nociceptive neuron marker) fibers (Fig. 3B). In accordance with this observation, no GFP signal is observed in neurons or nerve fibers in the superficial laminae of Agtr2GFP mouse spinal cord, where CGRP expression by central terminals of sensory neurons is detectable (SI Appendix, Fig. S3). However, numerous NF200 and NeuN+ somata in deeper laminae of the spinal cord dorsal horn and ventral horn express GFP (SI Appendix, Fig. S3), indicating that a subset of central neurons do express AT2R. In particular, GFP expression on ventral horn neurons with larger somata (an anatomical feature of motor neurons) coincides with the expression of GFP in a subset of NF200+ fibers in the sciatic nerves (38, 39). Furthermore, induction of SNI in Agtr2GFP mice did not lead to any detectable GFP signal in any cell types, including neurons and microglia/ MΦs in DRGs (Fig. 3C). Consistent with prior reports (40–42), increased microglia/MΦs were observed in the ipsilateral DRGs of SNI mice (Fig. 3C). Collectively, our findings argue against AT2R expression and downstream signaling within DRG sensory neurons, as has been proposed earlier (11, 12), thereby suggesting the possible involvement of nonneuronal AT2R.
Fig. 3.
Absence of AT2R gene expression in DRG sensory neurons and microglia/MΦs without or with nerve injury/neuropathy. (A) The Agtr2 gene (coding for AT2R) is not expressed in neurons and nonneuronal cells in mouse DRG, as verified by lack of GFP signal in DRG sections from Agtr2GFP reporter mice, in which the Agtr2 promoter drives GFP expression. DRG sections are stained with CGRP and NF200 antibodies to mark peptidergic and myelinated sensory neurons. (Scale bars, 50 μm.) (B) A subset of sciatic nerve fibers of Agtr2GFP mice are GFP+ (green). Such fibers are CGRP− (red; Upper), and NF200+ (red; Lower) DAPI: blue. (Scale bars, 200 µm.) (C) Seven days following SNI surgery, there is an appreciable increase in Iba1+ cells (red; Center) in ipsilateral vs. contralateral DRG, wherein GFP signal (green; Left) remains negligible. DAPI: blue. (Scale bars, 50 μm.)
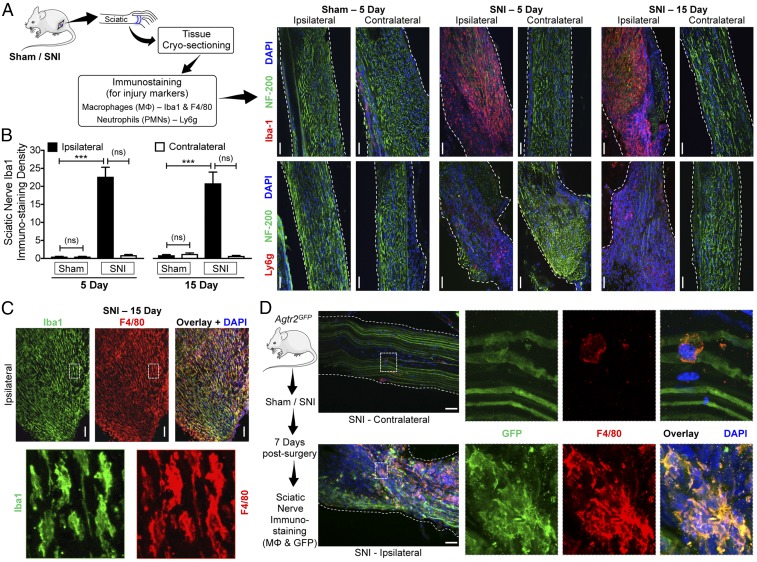
We next investigated the site of nerve injury to obtain histological evidence for the underlying mechanism. SNI induced massive and sustained infiltration of MΦs in both male and female mice, and some level of increase in neutrophil infiltration into the site of nerve injury (Fig. 4 A–C and SI Appendix, Fig. S4). Interestingly, the spared sural nerve fibers, which did not show any loss of nerve fiber staining (with NF200), also did not show any MΦ infiltration. As has been shown previously (43), increased microglial density was observed in the ipsilateral spinal cord dorsal horn of SNI mice, without any detectable neutrophil staining (SI Appendix, Fig. S5). Because AT2R is critical for SNI-induced mechanical and cold hypersensitivity, we next determined whether MΦs infiltrating the injured sciatic nerve express AT2R. Induction of SNI in Agtr2GFP mice showed substantial overlap of F4/80 and GFP immunoreactivity, indicating that MΦs in the vicinity of the nerve injury express Agtr2 (Fig. 4 C and D). However, spinal cord microglia in Agtr2GFP mice do not exhibit any detectable GFP signal after SNI (SI Appendix, Fig. S6). These observations suggest that peripheral MΦs that infiltrate the site of nerve injury, but not spinal cord microglia, express AT2R, and could therefore constitute a target for the analgesic action of AT2R antagonism in neuropathic pain.
Fig. 4.
Peripheral MΦ infiltration and AT2R expression therein are associated with nerve injury/neuropathy. (A) Experimental protocol for identification of injury markers in the sciatic nerve of mice subjected to sham or SNI surgery. (B–D) Massive MΦ infiltration (Iba1-red, Upper row in B; Iba1-green and F4/80-red in C) and considerable neutrophil infiltration (Ly6g-red, Lower row in B) accompany SNI-induced nerve fiber degeneration (decreased NF200 staining; green) in ipsilateral sciatic nerves, 5 and 15 d after SNI. Sections are costained with nuclear marker (DAPI; blue). (Scale bars, 200 μm.) MΦ density in sciatic nerves is quantified in B. Mean ± SEM; ***P < 0.001 vs. respective sham-ipsilateral groups; not significant (ns) vs. contralateral groups (n = 2 sections per mouse, 4 mice per group). Lower row images in C are magnified (630×) views of the areas marked with white dotted boxes in Upper row images. (D) Enhanced infiltration of MΦs that express GFP (F4/80-red, GFP-green and DAPI-blue) in the ipsilateral sciatic nerves from Agtr2GFP is observed 7 d after SNI, indicating AT2R expression in MΦs under nerve injury/neuropathy conditions. (Scale bars, 200 μm.) Right column images are magnified (630×) views of the areas marked with white dotted boxes on Left column images.
Macrophages and AT2R Expression Therein Are Critical for Neuropathic Mechanical and Cold Pain Hypersensitivity.
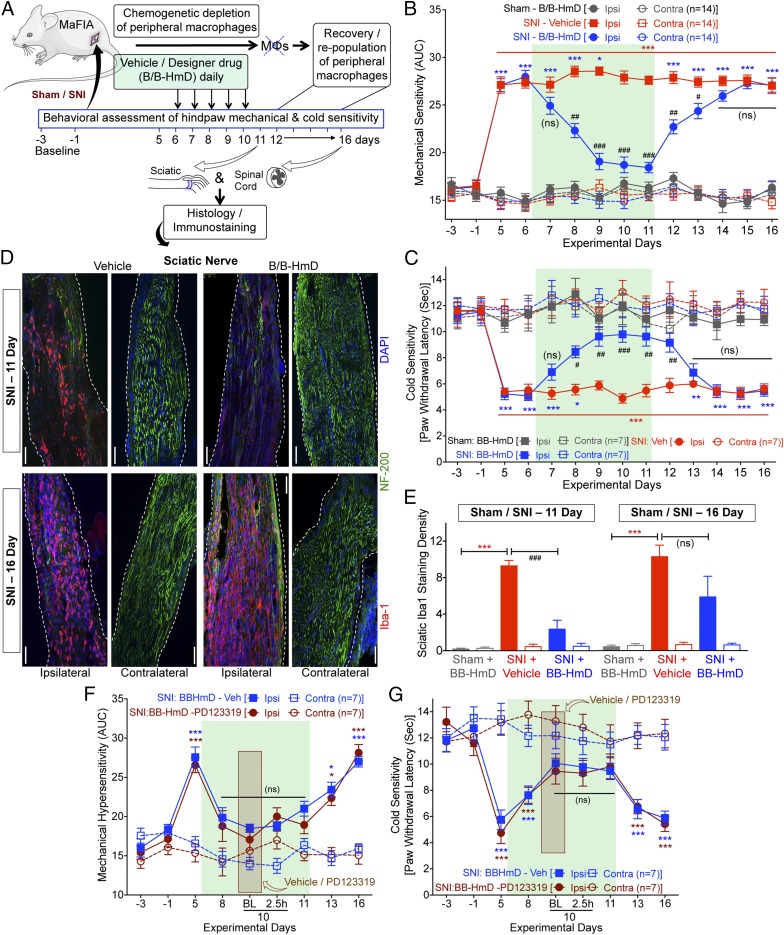
We next verified if peripheral MΦs are critical for neuropathic pain hypersensitivity. We utilized specific chemogenetic depletion of peripheral MΦs (but not in brain, spinal cord, and DRG MΦs/microglia) with designer drug (B/B-HmD) treatment in macrophage Fas-induced apoptosis (MaFIA) mice (Fig. 5A and SI Appendix, Fig. S7). SNI induced robust mechanical and cold hypersensitivity in MaFIA mice, similar to that observed in WT mice (Fig. 5 B and C). Following SNI, chemogenetic MΦ depletion progressively attenuated mechanical and cold hypersensitivity, with no influence on heat sensitivity (Fig. 5 B and C and SI Appendix, Fig. S8A). The attenuating effect of MΦ depletion on mechanical hypersensitivity was observed in male and female MaFIA mice to a similar degree (SI Appendix, Fig. S8 B and C). As seen earlier in WT mice, massive MΦ infiltration was also observed in injured sciatic nerves of MaFIA mice before MΦ depletion. In SNI-MaFIA mice, 5 consecutive days of B/B-HmD administration (days 6–10 after SNI) significantly depleted most peripheral MΦs at the site of injury (Fig. 5 D and E). Interestingly, cessation of MΦ depletion (day 11 after SNI onwards) was associated with progressive redevelopment of mechanical and cold hypersensitivity (Fig. 5 B and C), which was accompanied by repopulation of infiltrating MΦs in the injured sciatic nerves (Fig. 5 D and E). Chemogenetic depletion of MΦs did not influence the increase in MΦ/microglia density in the spinal cord dorsal horn of MaFIA-SNI mice (SI Appendix, Fig. S9). These observations suggest that peripheral MΦs are critical for neuropathic pain hypersensitivity. We verified the specificity of B/B-HmD–mediated peripheral immune cell depletion to monocytes/MΦs by flow cytometry. In line with prior reports (44, 45), compared with vehicle-administered MaFIA mice, B/B-HmD administration led to a significant loss (∼50%) of monocytes/MΦs in the peripheral blood, accompanied by neutrophilia, without any alteration in T or B lymphocyte numbers (SI Appendix, Fig. S7 C and D). This indicates that a 50% reduction in peripheral blood monocyte/MΦs leads to near-complete loss of infiltrating MΦs at the site of nerve injury (Fig. 5 D and E).
Fig. 5.
Peripheral MΦ infiltration is critical for nerve injury/neuropathy-induced mechanical and cold hypersensitivity. (A–C) Chemogenetic depletion of peripheral MΦs in MaFIA mice with B/B-HmD administration (2 mg/kg/d for 5 d, starting 6 d after SNI) (A), significantly attenuates SNI-induced ipsilateral hindpaw mechanical (B) and cold hypersensitivity (C), which subsequently returns to predepletion levels 3–4 d after the last B/B-HmD administration. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. respective baselines, sham – B/B-HmD-ipsi group or respective SNI – B/B-HmD-contra group; #P < 0.05, ##P < 0.01, ###P < 0.001 and not significant (ns) vs. SNI – vehicle-ipsi group. (D) Histological confirmation of MΦ (Iba1-red) depletion at day 11 after SNI (after fifth B/B-HmD), and repopulation at day 16 after SNI (5 d after final B/B-HmD) in the sciatic nerves (NF200-green) of MaFIA mice, which are quantified in E. (Scale bars, 200 μm.) (E) Mean ± SEM; ***P < 0.001 vs. respective sham – B/B-HmD-ipsi group; ###P < 0.001 and not significant (ns) vs. respective SNI – vehicle-ipsi groups (n = 2 sections per mouse, 4 mice per group). (F and G) Following MΦ depletion, administration of PD123319 on day 10 (red box) has no additional effect on ipsilateral hindpaw mechanical (F) or cold (G) hypersensitivity. Aqua rectangular boxes in B, C, F, and G, and red rectangular boxes in D and E denote postdrug administration behavioral assessment time points. *P < 0.05, and ***P < 0.001 vs. respective SNI-contra group.
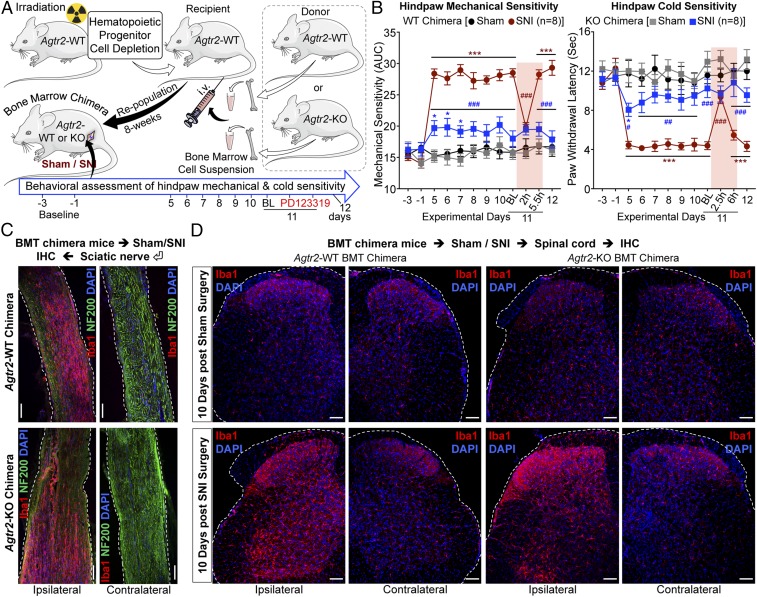
To test further whether the requirement for functional AT2R signaling resides within hematopoietic cells, we irradiated Agtr2-WT recipients and transplanted bone marrow hematopoietic progenitors from Agtr2-WT or Agtr2-KO donors 8 wk before SNI (Fig. 6A). We verified the loss of Agtr2 mRNA expression in the isolated MΦs from the ipsilateral sciatic nerves of Agtr2-KO chimera mice with SNI by qRT-PCR (SI Appendix, Fig. S10A). Agtr2-WT chimeras display similar mechanical and cold hypersensitivity to that seen in Agtr2-WT mice, and retained responsiveness to treatment with PD123319 on postoperative day 11 (Fig. 6B and SI Appendix, Fig. S10 B–D). However, significant attenuation of mechanical and cold hypersensitivity was observed in Agtr2-KO chimeras (Fig. 6B), despite a similar increase in MΦ infiltration into the sciatic nerve and elevation of microglial density in the spinal cord of Agtr2-WT and Agtr2-KO chimeras (Fig. 6 C and D). Taken together, these observations suggest that peripheral MΦ infiltration and the AT2R signaling therein are necessary for SNI/Ang II-induced pain hypersensitivity.
Fig. 6.
AT2R expression in the hematopoietic lineage is critical for nerve injury/neuropathy-induced mechanical and cold hypersensitivity. (A) Schematic showing generation of Agtr2-WT and Agtr2-KO bone marrow chimeras, and subsequent induction of nerve-injury/neuropathy for pain-related behavioral assessment. (B) SNI induces significant mechanical (Left) and cold hypersensitivity (Right) in Agtr2-WT chimeras, which could be attenuated by systemic administration of the AT2R antagonist PD123319 (10 mg/kg, i.p.). In contrast, Agtr2-KO chimeras show significantly attenuated mechanical (Left) and cold hypersensitivity (Right) upon SNI induction, indicating the critical role of MΦ AT2R in the induction and maintenance of neuropathic pain hypersensitivity. Mean ± SEM; *P < 0.05 and ***P < 0.001 vs. Agtr2-WT or Agtr2-KO sham-ipsi groups; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. Agtr2-WT SNI-ipsi group. Rectangular boxes in B denote postdrug administration behavioral assessment time points. (C) Representative confocal microscopic images of sciatic nerve sections from Agtr2-WT and Agtr2-KO bone marrow chimeras 15 d after SNI. Elevated Iba1 expression (red) is observed within the ipsilateral, but not contralateral nerves of Agtr2-WT (Upper) and Agtr2-KO (Lower) bone marrow chimeras. NF200: green; DAPI: blue. (Scale bars, 200 µm.) (D) Microglial proliferation/density (Iba1: red, DAPI: blue) in spinal cord dorsal horn from Agtr2-WT and Agtr2-KO bone marrow chimera mice, 10 d after sham/SNI surgery. (Scale bars, 50 µm.)
Discussion
Our findings demonstrate that local Ang II-AT2R activation in peripheral MΦs constitutes a critical trigger for chronic pain hypersensitivity associated with nerve injury/neuropathy. Prior reports suggested that Ang II acts directly on DRG neurons to induce neurite outgrowth and PKA-mediated TRPV1 modulation via Gαs-coupled AT2R, resulting in peripheral pain sensitization (9, 10). Furthermore, activation of Gαi/o-coupled AT2R on sensory neurons by a bacterial mycolactone toxin has been reported to be analgesic in mice (13). Our findings indicate that AT2R antagonism provides effective analgesia in neuropathic, but not inflammatory pain. However, our findings also suggest that DRG neurons do not express AT2R. Instead, AT2R activation in MΦs that infiltrate the site of injury induces persistent neuropathic mechanical and cold pain hypersensitivity. Our findings identify MΦ AT2R as the tissue/cell target underlying the analgesic action of AT2R antagonism for chronic neuropathic pain, and also uncover a translatable peripheral mechanism for such pain.
We demonstrate that Ang II levels are elevated in injured sciatic nerve, and that an AT2R antagonist dose-dependently attenuates mechanical hypersensitivity induced by nerve injury/neuropathy, but not by chronic hindpaw inflammation. Attenuation of both heat and mechanical hypersensitivity by the same AT2R antagonist in CFA-induced chronic inflammation has been shown previously (46). Similar to MΦ infiltration in nerve injury/neuropathy, MΦs and other immune cell infiltration has been well characterized in the CFA-induced model of inflammation (24). Furthermore, accumulation of a wide variety of inflammatory mediators that sensitize multiple pain-transducing receptors/channels, such as TRPs and Nav, are considered to constitute inflammatory thermal and mechanical pain mechanisms (32, 47). This, in combination with our observation that Ang II levels are unchanged in CFA versus saline-injected hindpaws, suggests a lack of AT2R activation at the site of CFA-induced inflammation, which would preclude the effectiveness of AT2R antagonism for inflammatory pain.
With regard to the source of Ang II, mouse and human MΦs have been shown to express the RAS genes Agt, renin, and ACE (48), raising the possibility that the entirety of the RAS required is supplied by MΦs. A scenario where the liver and vasculature are the source of this Agt/Ang II is unlikely, because this would presumably lead to changes in blood pressure, which we show remains unaltered following nerve injury. It is more likely that infiltrating MΦs at the site of nerve injury contribute to the local elevation of Ang II levels. Considerable levels of Agt mRNA have also been detected in mouse and human DRGs, without any detectable renin mRNA, as revealed by RNA-seq data (14–16, 36, 37). Because renin serves as the first rate-limiting enzyme for the generation of Ang II, direct secretion of Ang II by neurons is implausible. One possible scenario is that following nerve injury, sensory nerves secrete Agt, which is then processed by local MΦ-derived renin and ACE to produce Ang II. In depth studies utilizing tissue-specific expression/knockdown of RAS genes are thus needed to determine the precise source of Ang II under multiple experimental and disease-related neuropathic pain conditions.
Prior studies have suggested AT2R expression in DRG neurons, with AT2R antibody staining, Ang II-induced potentiation of capsaicin-mediated Ca2+ influx, and its attenuation by an AT2R antagonist (11, 12). However, our histological analysis utilizing Agtr2GFP show no detectable AT2R expression on sensory neurons under naïve or SNI conditions, clearly implicating nonneuronal AT2R signaling in the development of neuropathic pain. It is important to note that we do observe AT2R expression in a subset of spinal cord ventral horn neurons, possibly in motor neurons that send efferents to the periphery along the sciatic nerve. Because intrathecal administration of an AT2R antagonist did not influence pain hypersensitivity in mice, we speculate that AT2R function in these spinal cord ventral horn neurons is not involved in neuropathic pain states. The Agtr2GFP reporter mouse we utilized is a BAC-transgenic line, and it does not employ expression from the endogenous Agtr2 locus. However, prior studies in the central nervous system detected a high degree of colocalization between GFP immunoreactivity and presence of the Agtr2 transcript (21).
In search of the mechanism underlying the analgesic action of AT2R antagonism, we observed massive MΦ infiltration into the injured sciatic nerve, as well as increased density of microglia in the ipsilateral DRG and spinal cord, consistent with prior observations (43, 49). Chemogenetic depletion of peripheral MΦs (while sparing DRG and spinal cord microglia) in mice attenuated nerve injury-induced mechanical and cold pain hypersensitivity, indicating that peripheral MΦs are an indispensable component. Restoration of mechanical and cold hypersensitivity following repopulation of MΦs at the site of nerve injury strengthens this assertion. Infiltration of MΦs into peripheral nerves and DRGs, as well as microglial activation in spinal cord, have been implicated in multiple inflammatory, neuropathic, and cancer pain conditions. MΦ/microglia-derived inflammatory mediators, growth factors, and spinal modulatory signaling have been suggested as the predominant modulatory factors for peripheral pain sensitization (15, 49–52).
It has been long understood that central sensitization of peripheral nerve injury responses, which serves as a pain signal amplification system in the CNS, is instrumental in the development of persistent neuropathic pain states (51). A large body of literature suggests that microglial activation at DRG and spinal cord levels is a critical driver of nociceptive hypersensitivity and subsequent chronic pain (40–42, 51, 53, 54). Accordingly, effective attenuation of nociceptive hypersensitivity in experimental rodent pain models has been reported, utilizing pharmacological and genetic approaches targeting a number of genes and signaling axes in the spinal cord microglia (15, 51, 53, 54). Our data also show DRG and spinal cord microgliosis, ipsilateral to injury/SNI (Figs. 3C and 6D and SI Appendix, Figs. S3, S5, S6, S7B, and S9). Importantly, this microgliosis persists in situations where peripheral MΦ density has been compromised (B/B-HmD depletion in the MaFIA mouse) (SI Appendix, Figs. S7B and S9) or a lack of AT2R function in MΦs (Agtr2-KO chimerism) (Fig. 6D), both of which result in attenuation of neuropathic pain hypersensitivity. Altogether, these findings lead us to propose that both peripheral and central microglial/MΦ activation are critical components of peripheral and central pain sensitization, respectively. There is a high likelihood that peripheral MΦ signaling triggers the nociceptive excitatory input, which is then amplified by the central microglial/MΦ activation, leading to amplification of this excitatory signal input to drive neuropathic pain. Therefore, targeted depletion or inhibition of microglial/MΦ-derived signals at either level are capable of attenuating neuropathic pain hypersensitivity. However, the full repertoire of molecular and cellular signaling mechanisms linking peripheral MΦ-induced hyperexcitability to DRG/spinal cord microglial/MΦ activation, and how such processes are disrupted by interfering with peripheral MΦ function, remains to be uncovered. We observed that AT2R is not expressed in DRG and spinal cord microglia under naïve or nerve injury conditions (Fig. 3C and SI Appendix, Figs. S3 and S6). Furthermore, it is important to note that AT2R antagonists, including PD123319 and EMA401, do not cross the blood–brain barrier (11, 12). Recent findings suggest the involvement of MΦ infiltration in pain hypersensitivity in rodent models of experimental trigeminal neuropathy and chemotherapy-induced neuropathy (49, 52). This phenomenon, in combination with DRG/trigeminal/spinal cord sensitization pathways, likely maintains persistent neuropathic pain. Furthermore, we observed no sex-specific differences in neuropathic pain hypersensitivity. Sex differences in the contribution of immune cells, including spinal cord microglia, to chronic pain conditions in mice have recently been detailed (55). The observation that Ang II-AT2R–dependent mechanical and cold hypersensitivity does not appear to directly involve microglia in the spinal cord may explain the lack of any sex-related differences in peripheral nerve injury-induced pain hypersensitivity.
The mechanism linking MΦ AT2R activation to persistent excitation of peripheral sensory nerves remains a matter of in depth investigation. Among the pain-transducing receptors, TRPV1 was shown to be directly modulated by AT2R expressed on DRG neurons (11). However, our study did not find evidence of AT2R expression in DRG neurons. Furthermore, TRPA1—but not TRPV1—has been suggested to be involved in nociceptor sensitization underlying neuropathic mechanical and cold pain hypersensitivity in rodent models (32–35). TRPA1 is also shown to be a sensor of cell damage products, including reactive oxygen/nitrogen species (ROS/RNS), which activate the channel in sensory neurons (56–58). Our findings suggest that TRPA1 is involved in AT2R-dependent neuropathic mechanical and cold pain hypersensitivity (Fig. 2). Because peripheral MΦs infiltrate the site of nerve injury in neuropathy, it is plausible that AT2R activation in MΦs serves as a cell damage signal, which subsequently provides pathological activators/modulators of TRPA1. Our parallel study has recently identified such macrophage-to-sensory neuron cell damage signaling. This involves MΦ AT2R activation followed by ROS/RNS production, which then transactivate TRPA1 on sensory neurons to elicit sustained nociceptor excitation (17). Previously, ROS activation of TRPA1 has been shown to sensitize channel activation to mild cold temperatures (59), which presumably constitutes a mechanism for MΦ AT2R-mediated cold hypersensitivity in nerve injury/neuropathy.
Interestingly, a recent study utilizing MΦ depletion in clodronate liposome-treated mice showed a significant delay in the development of SNI-induced tactile hypersensitivity, with only a small/transient delay in cold hypersensitivity, suggesting no involvement of MΦs in neuropathic cold hypersnsitivity (40). Clodronate liposome-treatment leads to depletion of monocytes/MΦs in blood and DRGs (40). However, in our chemogenetic monocyte/MΦ depletion, utilizing MaFIA mice, the DRG microglia/MΦs remain unaffected (SI Appendix, Fig. S7B), and AT2R is expressed only in peripheral monocyte/MΦs that infiltrate the injured sciatic nerve, but not in DRG microglia/MΦs (Figs. 3C and 4D). Additionally, in the above-mentioned study, clodronate liposome-mediated monocyte/MΦ depletion was initiated before the induction of neuropathic injury (SNI), whereas we performed monocyte/MΦ depletion after the establishment of sustained mechanical and cold hypersensitivity (Fig. 5). This may explain the differences in our observation on attenuation of both mechanical and cold hypersensitivity in SNI by peripheral monocyte/MΦ depletion. AT2R has previously been implicated in injury/inflammatory responses, albeit in a largely antiinflammatory capacity (60). Furthermore, increased expression of RAS components, including AT2R, has been shown to accompany the differentiation of MΦs from monocytes (48). Therefore, future studies are needed to identify the role of AT2R activation in MΦ infiltration at the site of nerve injury, and its involvement in the induction versus maintenance of mechanical and cold pain hypersensitivity under specific disease-related neuropathies.
Our findings raise some intriguing possibilities that warrant further exploration. Conditions in which local or circulating RAS components are elevated may be associated with mechanical and cold pain hypersensitivity. An association between hypertension and neuropathy has been observed in diabetes mellitus (61, 62). Furthermore, ACE inhibitors have been demonstrated to impact nerve conduction in human diabetic neuropathy (63, 64). Whether this effect could be ascribed to a reduction in Ang II levels or Ang II-AT2R–mediated pain sensitization remains unclear, but it may be contingent on MΦ accumulation in the vicinity of damaged nerves. A recent report showed an increase in MΦ density in the skin biopsies of diabetic neuropathic patients (65). Consistent with this speculation on RAS involvement in pain, our study defines Ang II-AT2R signaling on peripheral MΦs as an indispensable component for the development of chronic neuropathic pain.
The performance of existing analgesics for neuropathic pain and the success rate of new-generation analgesic development have been suboptimal (66). This necessitates a comprehensive understanding of the pathophysiology and mechanisms underlying neuropathic pain. With the recent success of the AT2R antagonist EMA401 for treatment of neuropathic pain associated with PHN (8), our discovery of MΦ AT2R as a critical peripheral trigger for neuropathic pain sensitization provides a translatable mechanism for pharmacotherapeutic targeting.
Materials and Methods
All experiments involving the use of mice and the procedures followed therein were approved by Institutional Animal Studies Committees of Washington University in St. Louis and The University of Iowa, in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals (67). Details of mice, experimental models of neuropathy and inflammation, behavior tests, blood pressure monitoring, plasma extravasation assay, Ang II enzyme immunoassay, immunohistochemistry and image quantification, irradiation and mouse bone marrow transplantation, FACS, flow cytometry, qPCR, chemicals and reagents, and statistical analyses are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Erica Lantelme, Samantha Kelly, and Sherri Vogt for technical assistance; Drs. Justin Grobe and Nicole Littlejohn for help with Agtr2-WT and Agtr2-KO mouse breeding; Dr. Mathias Leinders for help with intravenous injections in mice and advice on plasma extravasation assays; Drs. Curt D. Sigmund and Justin Grobe for generously providing Agtr2-KO mice (originally generated by Drs. Victor J. Dzau and Richard E. Pratt); and Profs. Donna L. Hammond and Alex S. Evers for their continued support and encouragement for this work. The majority of this study was supported by funds from the Washington University Pain Center and Washington University School of Medicine, Department of Anesthesiology. Additional funding sources that supported this study are: pilot and feasibility NIH Grant P30DK056341 from the Washington University Nutrition Obesity Research Center (to A.J.S.); NIH Grant NS069898 (to D.P.M.); NIH Grant CA171927 (to A.D.M.); NIH Grant HL125805 (to A.D.d.K.); and NIH Grant NS42595 (to R.W.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721815115/-/DCSupplemental.
References
- 1.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Colloca L, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: Central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21:28. doi: 10.1007/s11916-017-0629-5. [DOI] [PubMed] [Google Scholar]
- 4.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;(4):CD007938. doi: 10.1002/14651858.CD007938.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ, Mannion RJ. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 6.Mathieson S, et al. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376:1111–1120. doi: 10.1056/NEJMoa1614292. [DOI] [PubMed] [Google Scholar]
- 7.Smith MT, Anand P, Rice AS. Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: Preclinical and clinical studies. Pain. 2016;157:S33–S41. doi: 10.1097/j.pain.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 8.Rice ASC, et al. EMA401-003 study group EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: A randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 9.Danser AHJ, Anand P. The angiotensin II type 2 receptor for pain control. Cell. 2014;157:1504–1506. doi: 10.1016/j.cell.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Anand U, et al. Mechanisms underlying clinical efficacy of angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: Clinical tissue and in vitro studies. Mol Pain. 2015;11:38. doi: 10.1186/s12990-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand U, et al. Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2013;17:1012–1026. doi: 10.1002/j.1532-2149.2012.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MT, Woodruff TM, Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor (AT2R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med. 2013;14:1557–1568. doi: 10.1111/pme.12157. [DOI] [PubMed] [Google Scholar]
- 13.Marion E, et al. Mycobacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Goswami SC, et al. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J Pain. 2014;15:1338–1359. doi: 10.1016/j.jpain.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Z, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapio MR, Goswami SC, Gross JR, Mannes AJ, Iadarola MJ. Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Exp Neurol. 2016;283:375–395. doi: 10.1016/j.expneurol.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd AJ, et al. Angiotensin II triggers peripheral macrophage-to-sensory neuron redox crosstalk to elicit pain. J Neurosci. 2018:3542-17. doi: 10.1523/JNEUROSCI.3542-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin K, Baillie GJ, Vetter I. Neuronal cell lines as model dorsal root ganglion neurons: A transcriptomic comparison. Mol Pain. 2016;12:1–17. doi: 10.1177/1744806916646111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 20.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 21.de Kloet AD, et al. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016;221:891–912. doi: 10.1007/s00429-014-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand U, et al. Mycolactone-mediated neurite degeneration and functional effects in cultured human and rat DRG neurons: Mechanisms underlying hypoalgesia in Buruli ulcer. Mol Pain. 2016;12:1–11. doi: 10.1177/1744806916654144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 24.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 2015;112:E6808–E6817. doi: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decosterd I, Allchorne A, Woolf CJ. Progressive tactile hypersensitivity after a peripheral nerve crush: Non-noxious mechanical stimulus-induced neuropathic pain. Pain. 2002;100:155–162. doi: 10.1016/s0304-3959(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 26.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: A behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 27.Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Mickle AD, Shepherd AJ, Loo L, Mohapatra DP. Induction of thermal and mechanical hypersensitivity by parathyroid hormone-related peptide through upregulation of TRPV1 function and trafficking. Pain. 2015;156:1620–1636. doi: 10.1097/j.pain.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd AJ, Mickle AD, Kadunganattil S, Hu H, Mohapatra DP. Parathyroid hormone-related peptide elicits peripheral TRPV1-dependent mechanical hypersensitivity. Front Cell Neurosci. 2018;12:38. doi: 10.3389/fncel.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd AJ, Mohapatra DP. Pharmacological validation of voluntary gait and mechanical sensitivity assays associated with inflammatory and neuropathic pain in mice. Neuropharmacology. 2018;130:18–29. doi: 10.1016/j.neuropharm.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto K, et al. Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration. Mol Pain. 2015;11:69. doi: 10.1186/s12990-015-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mickle AD, Shepherd AJ, Mohapatra DP. Nociceptive TRP channels: Sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals (Basel) 2016;9:E72. doi: 10.3390/ph9040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eid SR, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 35.del Camino D, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoury-Hanold W, et al. Viral spread to enteric neurons links genital HSV-1 infection to toxic megacolon and lethality. Cell Host Microbe. 2016;19:788–799. doi: 10.1016/j.chom.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin K, Deuis JR, Lewis RJ, Vetter I. Transcriptomic and behavioural characterisation of a mouse model of burn pain identify the cholecystokinin 2 receptor as an analgesic target. Mol Pain. 2016;12:1–13. doi: 10.1177/1744806916665366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altmann C, et al. Progranulin promotes peripheral nerve regeneration and reinnervation: Role of notch signaling. Mol Neurodegener. 2016;11:69. doi: 10.1186/s13024-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonanomi D, et al. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell. 2012;148:568–582. doi: 10.1016/j.cell.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobos EJ, et al. Mechanistic differences in neuropathic pain modalities revealed by correlating behavior with global expression profiling. Cell Rep. 2018;22:1301–1312. doi: 10.1016/j.celrep.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011;1405:95–108. doi: 10.1016/j.brainres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Scholz J, et al. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain. 2008;138:130–142. doi: 10.1016/j.pain.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett SH, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 45.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakrabarty A, Liao Z, Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain. 2013;14:1053–1065. doi: 10.1016/j.jpain.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mickle AD, Shepherd AJ, Mohapatra DP. Sensory TRP channels: The key transducers of nociception and pain. Prog Mol Biol Transl Sci. 2015;131:73–118. doi: 10.1016/bs.pmbts.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamura A, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens. 1999;17:537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 49.Old EA, et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest. 2014;124:2023–2036. doi: 10.1172/JCI71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weaver JL, et al. Hematopoietic pannexin 1 function is critical for neuropathic pain. Sci Rep. 2017;7:42550. doi: 10.1038/srep42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 52.Trevisan G, et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain. 2016;139:1361–1377. doi: 10.1093/brain/aww038. [DOI] [PubMed] [Google Scholar]
- 53.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beggs S, Salter MW. The known knowns of microglia-neuronal signalling in neuropathic pain. Neurosci Lett. 2013;557:37–42. doi: 10.1016/j.neulet.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 55.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 57.Macpherson LJ, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viana F. TRPA1 channels: Molecular sentinels of cellular stress and tissue damage. J Physiol. 2016;594:4151–4169. doi: 10.1113/JP270935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyake T, et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun. 2016;7:12840. doi: 10.1038/ncomms12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matavelli LC, Siragy HM. AT2 receptor activities and pathophysiological implications. J Cardiovasc Pharmacol. 2015;65:226–232. doi: 10.1097/FJC.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teunissen LL, et al. Is cardiovascular disease a risk factor in the development of axonal polyneuropathy? J Neurol Neurosurg Psychiatry. 2002;72:590–595. doi: 10.1136/jnnp.72.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarrelli MM, et al. Italian General Practitioner Study Group Arterial hypertension as a risk factor for chronic symmetric polyneuropathy. J Epidemiol Biostat. 2001;6:409–413. doi: 10.1080/135952201753337158. [DOI] [PubMed] [Google Scholar]
- 63.Malik RA, et al. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: Randomised double-blind controlled trial. Lancet. 1998;352:1978–1981. doi: 10.1016/S0140-6736(98)02478-7. [DOI] [PubMed] [Google Scholar]
- 64.Suresha RN, et al. Evaluation of analgesic activity of perindopril in albino mice. J Adv Pharm Technol Res. 2014;5:129–133. doi: 10.4103/2231-4040.137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Üçeyler N, et al. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve. 2017;55:884–893. doi: 10.1002/mus.25240. [DOI] [PubMed] [Google Scholar]
- 66.Percie du Sert N, Rice ASC. Improving the translation of analgesic drugs to the clinic: Animal models of neuropathic pain. Br J Pharmacol. 2014;171:2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th Ed The National Academies Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.