Significance
Identifying biological targets in major depressive disorder (MDD) is a critical step for development of effective mechanism-based medications. The epigenetic agent acetyl-l-carnitine (LAC) has rapid and enduring antidepressant-like effects in LAC-deficient rodents. Here, we found that LAC levels were decreased in patients with MDD versus age- and sex-matched healthy controls in two independent study centers. In subsequent exploratory analyses, the degree of LAC deficiency reflected both the severity and age of onset of MDD. Furthermore, the lowest LAC levels were found in patients with treatment-resistant depression, whereby history of emotional neglect and being female predicted decreased LAC levels. These translational findings suggest that LAC may serve as a candidate biomarker to help the diagnosis of a clinical endophenotype of MDD.
Keywords: epigenetic, glutamate, treatment-resistant depression, childhood trauma, mGlu2
Abstract
The lack of biomarkers to identify target populations greatly limits the promise of precision medicine for major depressive disorder (MDD), a primary cause of ill health and disability. The endogenously produced molecule acetyl-l-carnitine (LAC) is critical for hippocampal function and several behavioral domains. In rodents with depressive-like traits, LAC levels are markedly decreased and signal abnormal hippocampal glutamatergic function and dendritic plasticity. LAC supplementation induces rapid and lasting antidepressant-like effects via epigenetic mechanisms of histone acetylation. This mechanistic model led us to evaluate LAC levels in humans. We found that LAC levels, and not those of free carnitine, were decreased in patients with MDD compared with age- and sex-matched healthy controls in two independent study centers. Secondary exploratory analyses showed that the degree of LAC deficiency reflected both the severity and age of onset of MDD. Moreover, these analyses showed that the decrease in LAC was larger in patients with a history of treatment-resistant depression (TRD), among whom childhood trauma and, specifically, a history of emotional neglect and being female, predicted the decreased LAC. These findings suggest that LAC may serve as a candidate biomarker to help diagnose a clinical endophenotype of MDD characterized by decreased LAC, greater severity, and earlier onset as well as a history of childhood trauma in patients with TRD. Together with studies in rodents, these translational findings support further exploration of LAC as a therapeutic target that may help to define individualized treatments in biologically based depression subtype consistent with the spirit of precision medicine.
Major depressive disorder (MDD) is among the leading causes of illness and disability worldwide (1, 2). MDD is a severe and life-threatening disease, which is also associated with other major illnesses, such as diabetes, cardiovascular disorders, and Alzheimer’s disease (3, 4). A known risk factor for MDD is childhood trauma, which occurs at alarmingly high rates and has been associated with poorer responses to available antidepressant medications as well as with treatment-resistant depression (TRD) (5). The pathophysiology of MDD remains poorly understood, with a consequent lack of biological targets that can guide the development of diagnostics and improved therapeutics (6, 7).
In rodent models, epigenetic agents such as histone deacetylase inhibitors and the acetylating molecule acetyl-l-carnitine (LAC, Fig. 1A) have been shown to promote rapid antidepressant responses (8–15). Converging evidence from our group and others has shown that supplementation of LAC exerts rapid antidepressant actions, at least in part, by acetylating histones to regulate the expression of key genes important for synaptic plasticity, including the proneurogenic molecule brain-derived neurotrophic factor (BDNF) and a critical regulator of synaptic glutamate release, the metabotropic glutamate receptor of class-2, mGlu2 (10, 16–18). In several animal models, LAC supplementation has been shown to ameliorate glutamatergic dysfunction and associated neuronal atrophy in brain regions such as the hippocampus and medial amygdala (13, 16, 19–21). LAC is an endogenous short-chain acetyl ester of free carnitine that crosses the blood–brain barrier (17, 22, 23). Notably, we found that these animals that rapidly responded to LAC supplementation (10–12, 19) show an endogenous decrease in LAC in plasma and in mood regulatory brain regions (i.e., the hippocampus and prefrontal cortex) (13, 17). Furthermore, the deficiency in LAC was associated with insulin resistance (IR), which was ameliorated by supplementation of LAC (19).
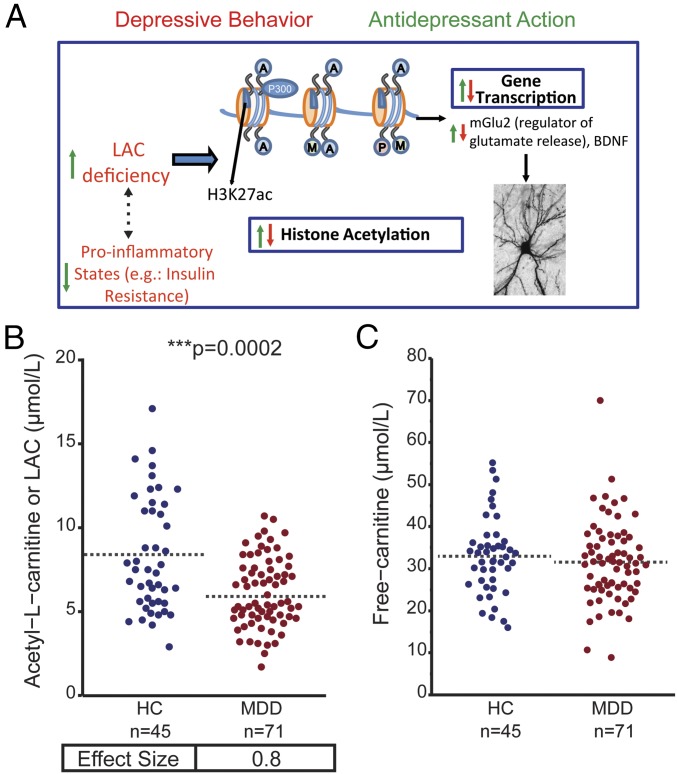
Fig. 1.
Decreased Acetyl-l-carnitine (LAC) Levels in patients with MDD compared with HC. (A) Schematic model featuring our innovative framework: The endogenously produced molecule acetyl-l-carnitine (LAC) is critical for hippocampal function and several behavioral domains. In rodents with depressive-like traits, LAC levels are markedly decreased and accompanied by abnormal hippocampal glutamatergic function, decreased expression of the neurotrophic factor BDNF, and dendritic plasticity as well as by systemic inflammation, including insulin resistance. LAC supplementation rescues those deficits and induces rapid and lasting epigenetic antidepressant-like effects via acetylation of histones. (B and C) Plasma LAC (B) and free-carnitine (C) concentrations in HC and in patients with MDD in acute depressive episodes during study participation as assessed by ultraperformance liquid chromatography–electrospray–tandem mass spectrometry (UPLC-MS/MS). See also SI Appendix, Figs. S1 and S3 *Significant comparisons with HC. ***P < 0.001 in Student’s two-tailed t tests (α = 0.05). Dashed bars indicate group mean.
Therefore, the animal models provide a conceptual platform that is consistent with a known role of glutamatergic dysfunction, altered trophic environment, and proinflammatory states in humans with depression (3, 21, 24–31). Here, using such a biological target- and mechanistically driven approach, we evaluated the role of LAC in MDD in humans.
Results
LAC Levels Differ Between Healthy Controls and Patients with MDD.
In a sample of 116 participants, no difference was observed between healthy controls (HC) (n = 45) and patients with MDD (n = 71) with respect to demographic characteristics, including age and sex (SI Appendix, Tables S1 and S2). All patients were in an acute depressive episode during study participation.
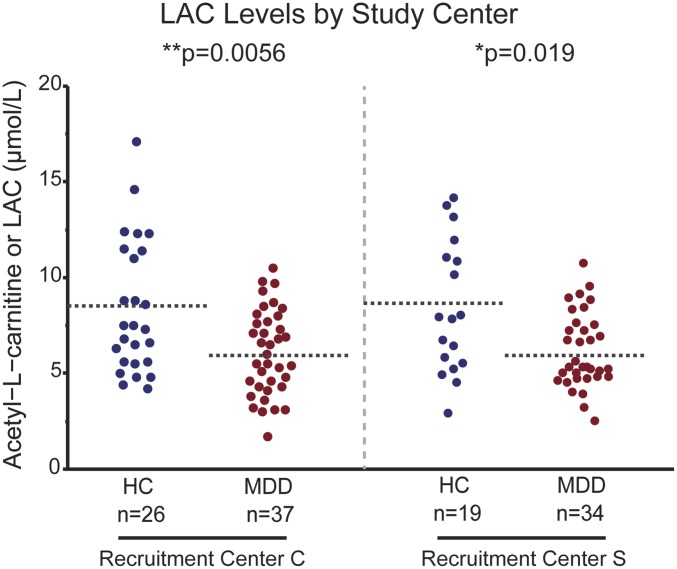
LAC levels (Fig. 1A) in plasma, as the most accessible biospecimen in a clinical setting, were measured by UPLC-MS/MS (ultraperformance liquid chromatography–electrospray–tandem mass spectrometry) and ESI-MS/MS (stable isotope dilution electrospray–tandem mass spectrometry) as previously described (10, 32). LAC differed significantly between HC and patients with MDD with mean concentrations lower in the MDD group (Fig. 1B, P < 0.0001, effect size = 0.8; HC: 8.3 μmol/L ± 0.4, MDD: 6.1 μmol/L ± 0.3; SI Appendix, Fig. S1). No significant difference was observed in free-carnitine concentrations between HC and patients with MDD (Fig. 1C). The association between MDD and LAC held when stratifying by sex (SI Appendix, Fig. S2). Furthermore, LAC levels were similarly decreased in patients with MDD compared with age- and sex-matched HC in both independent study centers [Weill Cornell (C) and Mount Sinai School of Medicine (S)] (Fig. 2).
Fig. 2.
LAC levels differ between HC and MDD groups in two independent study centers. Plasma LAC levels were similarly decreased in patients with MDD compared with age- and sex-matched HC in both study centers (C, Cornell; S, Sinai) as assessed by UPLC-MS/MS. *Significant comparisons with HC. **P < 0.01 in Student’s two-tailed t tests (α = 0.05). Dashed bars indicate group mean.
LAC Levels Within the MDD Group.
Within the group of patients with MDD, no difference was observed in LAC concentrations in relation to use of psychotropic medications (SI Appendix, Fig. S3). Given the primary findings that showed a decrease in LAC levels in patients with MDD compared with age- and sex-matched HC in two independent study centers, we evaluated the contribution of clinical characteristics of MDD, such as depression severity and age of onset, on LAC levels by performing exploratory analyses. In subjects with mild MDD no correlation was observed between LAC levels and the severity of the disease using Hamilton Depression-Rating Scale (HDRS-17) (33). Among subjects with moderate to severe MDD, we observed a significant negative correlation between LAC concentrations and severity scores at HDRS-17 (33), whereby the higher the severity the lower the concentrations of LAC (P = 0.04, r = 0.35) (SI Appendix, Fig. S4). This relationship remained significant upon multiple regression analysis controlling for number of past episodes (t = −2.13, P = 0.04) and length of current episode (t = −2.57, P = 0.017) as well as controlling for sex (P < 0.0001) and age (P = 0.0001). Furthermore, prediction model analysis showed that LAC predicted HDRS-17 severity scores among subjects with moderate to severe MDD (P = 0.04, r = 0.35, SI Appendix, Fig. S5). Indeed, the HDRS-17 severity scores statistically inferred from LAC measures were consistent with the rater-administered HDRS-17 severity scores (SI Appendix, Fig. S5). Pearson correlation analysis also showed a positive correlation between LAC concentrations and age of onset of depression (P = 0.04, r = 0.32); that is, earlier age of onset correlated with lower concentrations of LAC (SI Appendix, Fig. S6).
LAC Levels and Treatment-Resistant Depression: Role of Childhood Trauma.
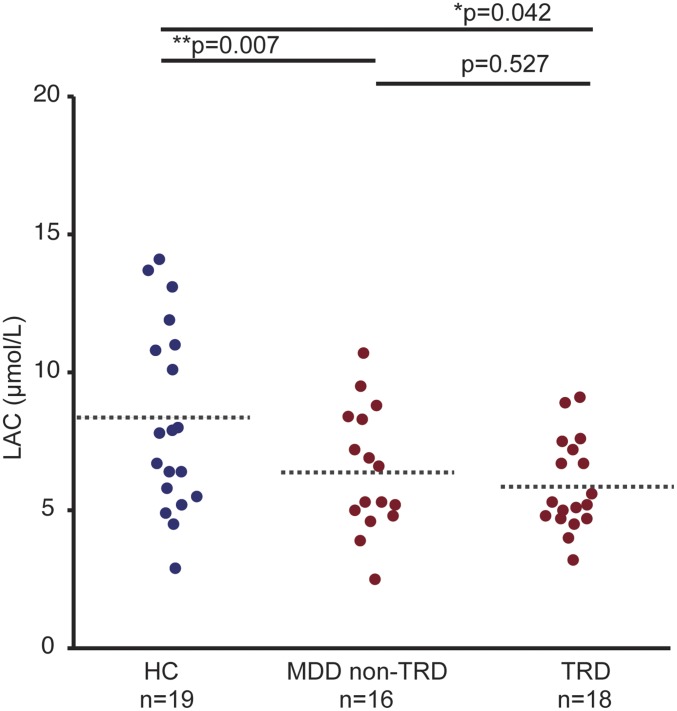
Driven by the findings above showing an LAC deficiency in patients with MDD in two study centers, we evaluated LAC levels across HC subjects and patients with MDD with or without history of TRD. Consistent with lower LAC in patients with MDD that were also characterized by greater severity and earlier onset of the illness, we found that the decrease in LAC was greater in subjects with MDD and a history of TRD, F(2, 53) = 4.3, P = 0.01 (Fig. 3 and SI Appendix, Fig. S7).
Fig. 3.
The LAC deficiency is greater in patients with MDD and history of treatment-resistant depression (TRD). Plasma LAC concentrations across HC, patients with MDD without history of TRD (MDD non-TRD), and patients with MDD and with history of TRD for study center S. F(2, 53) = 4.3, P = 0.01. *Significant comparisons with HC. *P < 0.05, **P < 0.01 in Student’s two-tailed t tests (α = 0.05). Dashed bars indicate group mean. SI Appendix, Fig. S7 also shows a consistency of this stepwise in study center C.
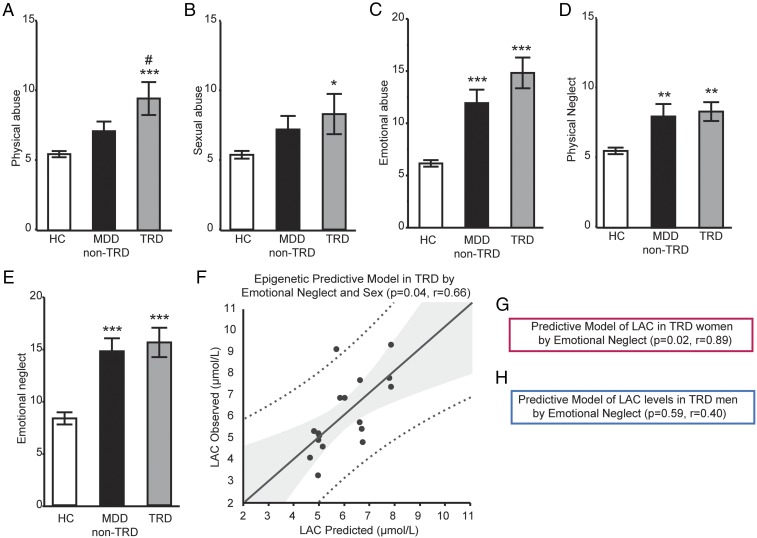
As childhood trauma has been associated with depression severity and treatment resistance, we conducted exploratory analyses to evaluate the contribution of childhood trauma on LAC levels. First, we found that the reported rates of childhood trauma assessed by the Childhood Trauma Questionnaire (CTQ) (34) differed from HC and patients with MDD (Fig. 4). Within the group of patients with MDD, we found that rates of emotional abuse, physical neglect, and emotional neglect were significantly higher either in patients with TRD or in patients with MDD non-TRD compared with HC, while physical and sexual abuse were significantly higher only in patients with TRD versus HC (Fig. 4 A–E): emotional abuse, F(2, 52) = 17.08, P < 0.0001; physical abuse, F(2, 52) = 6.88, P = 0.0023; sexual abuse, F(2, 52) = 2.35, P = 0.1; emotional neglect, F(2, 52) = 13.75, P < 0.0001; physical neglect, F(2, 52) = 6.26, P = 0.0038.
Fig. 4.
LAC as potential moderator of sex-specific effects of childhood trauma in patients with MDD with or without history of treatment-resistant depression (TRD). (A–H) History of childhood trauma as assessed by the Childhood Trauma Questionnaire (CTQ) with individual areas, including psychical abuse (A), sexual abuse (B), emotional abuse (C), physical neglect (D), and emotional neglect (E) in HC (n = 19), in patients with MDD non-TRD (n = 16), and in patients with TRD (n = 18) that reported childhood trauma as assessed using by the CTQ at study center S. Data are presented as mean ± SEM. (F) Multiple regression analysis of LAC by emotional neglect and sex in patients with TRD. In the x axis: LAC concentrations as predicted by the model; in the y axis: LAC concentrations as measured in patients with TRD. (G and H) Models stratified by sex in women (G) and men (H) with TRD. *Significant comparisons with HC; #Significant comparison with MDD non-TRD. *P < 0.05, **P < 0.01, ***P < 0.001 in Student’s two-tailed t tests (α = 0.05) or using multiple regression analysis for the predictive model.
Furthermore, multiple regression analyses showed that LAC levels were predicted by a history of childhood trauma in patients with TRD; that is, emotional neglect by sex interaction predicted LAC levels (P = 0.04, r = 0.66, Fig. 4F). Specifically, models stratified by sex showed emotional neglect as a predictor of LAC concentrations only in women with TRD (P = 0.02, r = 0.89, Fig. 4G), but not in men (P = 0.59, r = 0.40, Fig. 4H). No interaction of LAC with emotional neglect was observed in women HC (P = 0.77) or in women with MDD non-TRD (P = 0.84).
Discussion
We report that a deficiency of the epigenetic agent acetyl-l-carnitine (LAC) occurs in humans who have major depressive disorder. These translational findings are an outgrowth of a mechanistic model in rodents with depressive-like traits, wherein LAC levels are markedly decreased and signal abnormal brain functions as well as metabolic dysregulation (SI Appendix, Fig. S8). Furthermore, hypotheses-generating exploratory analyses reported herein reveal that (i) the degree of LAC deficiency reflected both the severity and age of onset of MDD, and (ii) the LAC deficiency was associated with a history of childhood trauma in patients with treatment-resistant depression (SI Appendix, Fig. S8). These findings compel further research on the potential role of LAC as a candidate biomarker that together with clinical characteristics can aid the diagnosis and identification of a clinical endophenotype of MDD. Furthermore, LAC deficiency may represent an innovative biological therapeutic target in treatment of depression.
The decreased levels of LAC in patients with MDD is particularly important because LAC is an essential molecule for systemic and neural functions (13, 17, 22). LAC plays a central role in the transport of fatty acids into the mitochondria for beta oxidation to sustain energy metabolism in the brain and the rest of the body. LAC also facilitates elimination of oxidative products, provides acetyl groups to regulate expression of neurotrophins and glutamate genes that contain spontaneous glutamate release, and protects from excitotoxicity, therefore interacting with mechanisms that contribute to the pathophysiology of MDD (10, 13, 17, 21). In rodent models with depressive-like traits, LAC levels are markedly decreased and accompanied by hippocampal glutamatergic dysfunction as well as abnormal dendritic plasticity in the hippocampus, among other brain regions (13, 16, 20). LAC supplementation ameliorates these deficits. The specificity of changes in LAC and lack of changes in free carnitine suggest that the relationship between LAC and MDD is independent of potential dietary changes (17, 35). Of interest is also the previously reported positive correlation between peripheral and CNS LAC concentrations (17). Of note, LAC levels were similarly decreased in patients with MDD compared with age- and sex-matched HC in both study sites, providing an initial replication of our results. Furthermore, the use of two different methods to assess LAC in the same 116 samples supports the reliability of our results. Future studies will also be needed to assess whether decreased LAC levels in patients with MDD are sensitive to unhealthy lifestyle choices, such as physical inactivity, poor dietary habits, and lack of adequate sleep.
Furthermore, LAC appears to act as a state-dependent marker, given that all patients were in an acute depressive episode at the time of study participation and that the presence of medications did not influence LAC levels. However, the cross-sectional design of our study does not allow establishing whether the decrease in LAC levels in patients with MDD may also be a trait biomarker. Further studies may help in elucidating trait-dependent LAC levels. In addition, given the high comorbidity of MDD with other psychiatric disorders, future studies with larger cohorts will be needed to investigate whether decreased LAC levels may represent a specific signature for MDD or is a general marker of affective disorders. Moreover, the finding that the decrease in LAC levels in patients with MDD is independent of psychotropic drug treatment raises the possibility that increasing LAC levels may be needed to induce antidepressant effects.
LAC levels were significantly correlated with depression severity and with age of onset of MDD. Although these were exploratory analyses, the strength of such correlations remained after controlling for sex, number of depressive episodes, and duration of the current episode and was independent of use of psychotropic medications. Consistent with these results is the observation of decreased LAC in patients with history of treatment-resistant depression (TRD). The greater decrease in LAC in more severe forms of MDD and in patients with TRD is akin to a “kindling-like” progression of MDD in that earlier age at onset and/or the presence of early life adversity, such as childhood trauma, conveys liability to more severe and treatment-resistant course of illness (35). Our exploratory analyses also revealed that a history of emotional neglect predicted LAC levels in women with TRD, suggesting that LAC may moderate sex-specific effects of childhood adversities in patients with TRD. This finding is consistent with previous epidemiological studies showing sex-specific correlations between childhood trauma and depressive symptoms (36) and that the consequences of neglect differ substantially from those of other traumas (5, 36). Together with findings from previous clinical studies that a history of childhood trauma impairs responses to available antidepressant medications (37, 38), the current findings of LAC deficiency suggest a clinical endophenotype characterized by greater severity, earlier age of onset MDD, a history of treatment-resistant course of the illness, and childhood trauma. Of note, and as a limitation, we had CTQ information only in patients from study center S; therefore, it would be important to ascertain the role of childhood trauma on the LAC deficiency with larger cohorts.
These current translational findings are an extension of preclinical research that shows that in rodents characterized by an LAC decrease and depressive-like phenotypes, supplementation with LAC leads to antidepressant responses seen after 3 d that also last for 14 d after drug withdrawal. Responses to standard antidepressant medications require repeated weeks of administration in the same animal models (10–12, 14, 18, 19). To the best of our knowledge, there is no clinical study to date that tested the efficacy of LAC in patients with MDD. Previous studies showed that LAC treatment is well tolerated and effective in treatment of depressive symptoms, but these studies mainly focused on limited cohorts of elderly patients with dysthymia or fibromyalgia (13, 39, 40). The conceptual framework that we pursue suggests an LAC deficiency as a potential therapeutic target in the pathophysiology of MDD toward the development of more effective precision medicine approaches tailored to specific clinical biobehavioral phenotypes. Indeed, LAC levels, together with clinical characteristics (i.e., MDD severity scores) and developmental history (i.e., age of MDD onset and childhood trauma), may serve to identify specific clinical phenotypes of depression. Such phenotypes may be more likely to benefit from a biologically based treatment with LAC supplementation or augmentation. Within this framework, it is important to emphasize that clinical trials of acute treatment with LAC are needed to validate the current postulate. Furthermore, it will be important to test whether LAC supplementation has preventive effects given its long-lasting antidepressant action in animals and evidence of induction of resilience. Based upon our earlier reported association of decreased LAC with insulin resistance (IR) in animals with depressive-like behaviors (19), it will also be important to investigate the association of LAC deficiency with insulin resistance.
In conclusion, the current findings of LAC deficiency in MDD suggest a possible endophenotype of depression, characterized by history of childhood trauma, greater depression severity, and earlier age at onset. Future prospective, placebo-controlled studies will be needed to address some of the limitations inherent in our cross-sectional cohorts. Further study with larger cohorts is also needed to better understand the role of LAC in clinically distinct populations of patients with depressive disorders.
Methods
The Rockefeller University Institutional Review Board and the respective Institutional Review Boards of the collaborating Institutions (Weill Cornell Medicine, Icahn School of Medicine at Mount Sinai, and Duke University) approved the current study in its entirety.
Participants.
Following an initial phone screen, potential participants were evaluated in person to determine study eligibility. All participants determined to be eligible to join the study provided written informed consent before study enrollment. Study participants, ranging between 20 and 70 y old, were recruited at two independent sites, the Affective Disorders Research Program at Weill Cornell Medicine and the Mood and Anxiety Disorders Program at the Icahn School of Medicine at Mount Sinai. At both study sites, study clinicians or trained coordinators conducted the Structured Clinical Interview for DSM-IV (SCID) or Mini International Neuropsychiatric Interview (MINI) to confirm MDD diagnosis and rule out exclusionary comorbid conditions.
Inclusion and exclusion criteria were similar at both recruitment sites. Inclusion criteria included a primary diagnosis of MDD in a current major depressive episode. Inclusion criteria in the TRD group also included having at least moderate severity and a history of nonresponse to at least two therapeutic trials of an antidepressant according to the Antidepressant Treatment History Form (ATHF) or Antidepressant Treatment Record (ATR) during their lifetime. Exclusion criteria included a presence of neurologic or other physical illness, such as diabetes, alcohol or substance abuse in the last 6 mo, or an unstable medical illness. Current medication use was assessed at screening for all study participants. Participants were free of current substances of abuse as determined by a urine toxicology test at the time of screening. HC were free of lifetime psychiatric illness and significant medical conditions. Participants were free of active infections and systemic illness as confirmed by medical history at the time of study evaluation. Blood samples were obtained via antecubital venous collection using standard techniques and were drawn after a period of fasting (>6 h). Participants were asked not to exercise for >6 h before blood draw.
Clinical and Psychiatric Assessment.
Clinical assessment consisted of a physical examination, including measures of height, weight, and body mass index (BMI). Other data collected included current medication use and history of failed antidepressant trials. Demographic information, including sex, was also recorded from the participants (SI Appendix, Tables S1 and S2). The psychiatric examination at screening included SCID or MINI to confirm MDD diagnosis, and trained raters administered the structured depression-rating scales: 17-item Hamilton Depression-Rating Scale (HDRS-17) and the Montgomery–Asberg Depression Rating Scale (MADRS). Among participants, two were identified as having eating disorders and one as having Crohn’s disease and, therefore, were excluded from the analyses. Cutoff scores of 19 and 34 were used to stratify for depression severity at the HDRS-17 and MADRS, respectively (33). As a result, among the 71 patients with MDD, 28 patients had moderate depression (HDRS-17 < 19, MADRS < 34) and 43 patients had severe depression at the time of study evaluation (HDRS-17 ≥ 19, MADRS ≥ 34). With regard to medication use, 53 patients were free of antidepressant medications at the time of study participation and 18 patients were on psychotropic medications. A subgroup of 18 patients with MDD recruited at Icahn School of Medicine at Mount Sinai reported history of TRD defined by at least two failed antidepressant trials (41, 42). All participants (i.e., HC, MDD non-TRD, and TRD) at Icahn School of Medicine at Mount Sinai completed the Childhood Trauma Questionnaire (CTQ) (43) to assess for childhood traumatic experiences in five specific areas: physical, sexual, and emotional abuse and physical and emotional neglect. Some information about subjects from the Icahn School of Medicine at Mount Sinai included in the current study was previously reported (44).
LAC Measures by Ultraperformance Liquid Chromatography–Electrospray–Tandem Mass Spectrometry.
LAC and free carnitine in plasma were analyzed using ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) with electrospray ionization in positive ion mode on an Xevo-TQD or a TQD tandem mass spectrometer equipped with Acquity UPLC system (Waters Corp.). For the determination of free l-carnitine and LAC, plasma samples were spiked with [2H3]-free carnitine and acetyl–[2H3]-carnitine internal standards. The total concentration of carnitine (sum of free and acylated carnitine) was determined in a second aliquot of each sample mixed with [2H3]-free carnitine internal standard. These second aliquots were subjected to base hydrolysis of the acylcarnitine species by incubation with 1 mol/L KOH at 65 °C for 15 min, followed by neutralization with 1 mol/L HCl. Protein was precipitated in all aliquots using 0.1% formic acid in acetonitrile and removed by centrifugation. The sample extracts were dried and reconstituted in 0.1% formic acid and 7.5 mmol/L ammonium formate in 18:82 (vol/vol) acetonitrile:deionized water. l-Carnitine and LAC were separated on an Acquity BEH HILIC, 2.1 mm × 100 mm, 1.7-μm column (Waters Corp.) with gradient elution using 0.1% formic acid and 7.5 mmol/L ammonium formate in acetonitrile:deionized water as the mobile phase and detected using selected reaction monitoring. The ratios of signal intensities for the transitions m/z 162 > 103 (free carnitine) and 165 > 103 ([2H3]-carnitine) and m/z 204 > 85 (acetylcarnitine) and 207 > 85 (acetyl–[2H3]-carnitine) were converted to a concentration by means of a calibration curve. Materials: l-carnitine.HCl and LAC hydrochloride (Sigma Chemical Co.); 2H3-l-carnitine.HCl and acetyl–2H3-l-carnitine.HCl (Cambridge Isotope Laboratories, Inc.); all other reagents, solvents, and solvent additives were purchased from Sigma Chemical Co. or VWR. All groups were evenly divided between the experimental plates to account for any interplate variability.
LAC Measures by Stable Isotope Dilution Electrospray–Tandem Mass Spectrometry.
Deuterated internal standards were obtained from Cambridge Isotope Laboratories, Inc. and from Sigma Chemical Co. Three Molars methanolic HCl was obtained from Sigma Chemical Co. General reagents and solvents were obtained from VWR. Plasma acetylcarnitine was analyzed as methyl ester by a semiquantitative method using stable isotope dilution electrospray–tandem mass spectrometry (ESI-MS/MS). Plasma was mixed in an internal standard mixture containing d3-acetylcarnitine, d3-propionylcarnitine, d3-butylcarnitine, d3-octanoylcarnitine, and d3-palmitoylcarnitine. Protein was precipitated by the addition of methanol and removed by centrifugation. An aliquot of the supernatant liquid was dried under nitrogen and methylated by incubation with 3 M HCl in methanol at 50 °C for 15 min. The derivatized extract was dried under nitrogen, reconstituted in methanol:dH2O 85:15 (vol/vol), and analyzed directly by flow injection–MS/MS on a TQD tandem mass spectrometer coupled with an Acquity UPLC system (Waters Corporation). Acetylcarnitine was detected using a precursor ion scan of m/z 99, with a scan range of m/z 200–500. Concentrations were determined from the ratio of ion intensities of acetylcarnitine species to its specified deuterated internal standard, multiplied by the concentration of the standard. All groups were evenly divided between the experimental plates to account for any interplate variability.
Statistical Analysis.
Statistical analyses were conducted using JMP Software from SAS (Statistical Analysis Systems Institute). Two-tailed t tests and χ2 analyses were used to compare, respectively, continuous and categorical demographic and clinical characteristics between HC and MDD subjects. Between- and within-group differences in patient plasma LAC and free-carnitine concentrations were compared using t tests. Within-group Pearson correlations were conducted to examine the relationship between LAC concentrations and depression severity or age of onset. Multiple regression analysis was used to control for other clinical characteristics. A one-way ANOVA followed by post hoc Student’s t tests was used to examine LAC levels upon use of psychotropic medications as well as across HC and subjects with MDD with or without history of TRD. Predictive models were inferred using multiple regression analysis to assess the ability of LAC and CTQ areas to predict the dependent variables, HDRS-17 scores, and LAC concentrations, respectively. Significance was set at 0.05, and data are presented as mean ± SD, unless otherwise specified.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Robertson Foundation (to C.N.) and, partly, by a grant from the Hope for Depression Research Foundation (HDRF) (to B.S.M.). This work was also funded in part by the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the University of California at Irvine, and the Hudson Alpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 8475.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801609115/-/DCSupplemental.
References
- 1.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 3.Rasgon NL, McEwen BS. Insulin resistance: A missing link no more. Mol Psychiatry. 2016;21:1648–1652. doi: 10.1038/mp.2016.162. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre RS, et al. Metabolic syndrome and major depressive disorder: Co-occurrence and pathophysiologic overlap. Curr Diab Rep. 2009;9:51–59. doi: 10.1007/s11892-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 5.Nemeroff CB. Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89:892–909. doi: 10.1016/j.neuron.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gururajan A, Clarke G, Dinan TG, Cryan JF. Molecular biomarkers of depression. Neurosci Biobehav Rev. 2016;64:101–133. doi: 10.1016/j.neubiorev.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71:454–456. doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 10.Nasca C, et al. 2013l-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors Proc Natl Acad Sci USA 1104804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo SJ, Charney DS. Next generation antidepressants. Proc Natl Acad Sci USA. 2013;110:4441–4442. doi: 10.1073/pnas.1301593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flight MH. Antidepressant epigenetic action. Nat Rev Neurosci. 2013;14:226. doi: 10.1038/nrn3466. [DOI] [PubMed] [Google Scholar]
- 13.Wang SM, et al. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J Psychiatr Res. 2014;53:30–37. doi: 10.1016/j.jpsychires.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, et al. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience. 2015;285:281–291. doi: 10.1016/j.neuroscience.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Madiraju P, Pande SV, Prentki M, Madiraju SR. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics. 2009;4:399–403. doi: 10.4161/epi.4.6.9767. [DOI] [PubMed] [Google Scholar]
- 16.Lau T, Bigio B, Zelli D, McEwen BS, Nasca C. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol Psychiatry. 2017;22:227–234. doi: 10.1038/mp.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettegrew JW, Levine J, McClure RJ. Acetyl-l-carnitine physical–chemical, metabolic, and therapeutic properties: Relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry. 2000;5:616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- 18.Cuccurazzu B, et al. Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the proneurogenic and antidepressant effects of acetyl-l-carnitine. Neuropsychopharmacology. 2013;38:2220–2230. doi: 10.1038/npp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigio B, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci USA. 2016;113:7906–7911. doi: 10.1073/pnas.1603111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasca C, et al. Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron. 2017;96:402–413.e5. doi: 10.1016/j.neuron.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz IB, McEwen B. Effects of carnitine on fatty-acid oxidation by muscle. Science. 1959;129:334–335. doi: 10.1126/science.129.3345.334. [DOI] [PubMed] [Google Scholar]
- 23.Scafidi S, et al. Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem. 2010;114:820–831. doi: 10.1111/j.1471-4159.2010.06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitani H, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Lin KW, Wroolie TE, Robakis T, Rasgon NL. Adjuvant pioglitazone for unremitted depression: Clinical correlates of treatment response. Psychiatry Res. 2015;230:846–852. doi: 10.1016/j.psychres.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasgon NL, et al. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: A pilot study. Sci World J. 2010;10:321–328. doi: 10.1100/tsw.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duman RS, Li N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarate CA, Jr, Machado-Vieira R. Ketamine: Translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry. 2017;22:324–327. doi: 10.1038/mp.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteggia LM, Zarate C., Jr Antidepressant actions of ketamine: From molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, et al. Acetylcarnitine is a candidate diagnostic and prognostic biomarker of hepatocellular carcinoma. Cancer Res. 2016;76:2912–2920. doi: 10.1158/0008-5472.CAN-15-3199. [DOI] [PubMed] [Google Scholar]
- 33. (2018) Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS). Available at www.ids-qids.org/interpretation.html. Accessed June 15, 2018.
- 34.Bernstein DP, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 35.van Vlies N, et al. Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse. Biochem J. 2005;387:185–193. doi: 10.1042/BJ20041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia M, et al. Sex differences in the effect of childhood trauma on the clinical expression of early psychosis. Compr Psychiatry. 2016;68:86–96. doi: 10.1016/j.comppsych.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Williams LM, Debattista C, Duchemin AM, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: Data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry. 2016;6:e799. doi: 10.1038/tp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapczinski F, et al. Allostatic load in bipolar disorder: Implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–692. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Meister R, et al. Comparative safety of pharmacologic treatments for persistent depressive disorder: A systematic review and network meta-analysis. PLoS One. 2016;11:e0153380. doi: 10.1371/journal.pone.0153380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veronese N, et al. Acetyl-l-carnitine supplementation and the treatment of depressive symptoms: A systematic review and meta-analysis. Psychosom Med. 2018;80:154–159. doi: 10.1097/PSY.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 41.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67:16–22. [PubMed] [Google Scholar]
- 42.Russell JM, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65:341–347. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein DP, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 44.Kiraly DD, et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065. doi: 10.1038/tp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.