Significance
Transcription factors have been intensively examined to decipher how they regulate cellular decisions, but there are few in-depth studies of these factors across traits, environments, and genetic backgrounds. Here, we analyze the Saccharomyces cerevisiae Ste12 protein, a transcription factor essential for both mating and invasion in many fungal species. Generating thousands of variants in the Ste12 DNA-binding domain, we scored each variant for its activity in promoting both mating and invasion. We found altered DNA-binding patterns of exceptional variants that result in yeast that lose their mating efficiency, but gain increased competence in invasion. This surprising malleability in transcription factor function has implications for understanding the evolution of pathogenicity in fungi.
Keywords: yeast, transcription factor, fungal mating, fungal invasion, evolutionary trade-off
Abstract
Few mechanisms are known that explain how transcription factors can adjust phenotypic outputs to accommodate differing environments. In Saccharomyces cerevisiae, the decision to mate or invade relies on environmental cues that converge on a shared transcription factor, Ste12. Specificity toward invasion occurs via Ste12 binding cooperatively with the cofactor Tec1. Here, we determine the range of phenotypic outputs (mating vs. invasion) of thousands of DNA-binding domain variants in Ste12 to understand how preference for invasion may arise. We find that single amino acid changes in the DNA-binding domain can shift the preference of yeast toward either mating or invasion. These mutations define two distinct regions of this domain, suggesting alternative modes of DNA binding for each trait. We characterize the DNA-binding specificity of wild-type Ste12 to identify a strong preference for spacing and orientation of both homodimeric and heterodimeric sites. Ste12 mutants that promote hyperinvasion in a Tec1-independent manner fail to bind cooperative sites with Tec1 and bind to unusual dimeric Ste12 sites composed of one near-perfect and one highly degenerate site. We propose a model in which Ste12 alone may have evolved to activate invasion genes, which could explain how preference for invasion arose in the many fungal pathogens that lack Tec1.
Transcription factors interact with DNA, with cofactors, and with signaling proteins to allow cells to respond to changes in their environment. Despite the requirement to manage these multiple levels of regulation, most eukaryotic transcription factors possess a single DNA-binding domain. Distinct responses must therefore be mediated by diversity in cofactors, organizations of binding sites, and conformational changes in the transcription factor itself. For example, human GCM1 gains a novel recognition sequence when paired with the ETS family factor ELK1 (1); auxin-responsive transcription factors regulate expression differentially depending on the arrangement of their binding sites (2); and the heat-shock factor Hsf1 senses increased temperature by changing its conformation, which allows it to bind a unique recognition sequence (3).
We sought to investigate the features of a single transcription factor that uses distinct cofactors, binding sites, and environmental inputs to mediate a cellular decision. The Ste12 protein of the yeast Saccharomyces cerevisiae governs the choice of mating or invasion. Each of these traits contributes to cellular fitness: mating of haploid yeast cells is required for meiotic recombination, and invasion allows a cell to forage for nutrients or to penetrate tissues, a characteristic of pathogenic fungi. Mating is initiated by binding of the appropriate pheromone, which activates an evolutionarily conserved G protein-coupled mitogen-activated protein kinase (MAPK) pathway (4, 5) (Fig. 1A). In contrast, invasion is initiated in response to increased temperature and limited nutrient availability (6–8). The shared protein kinase Ste11, a client of the chaperone Hsp90 (9), likely contributes to the environmental sensitivity of both traits.
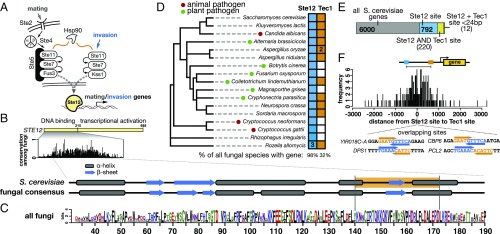
Fig. 1.
Conservation of Ste12 and Tec1 and their DNA-binding sites. (A) The yeast mating and invasion pathways contain shared signaling components, and both depend on Ste12 for activation of distinct regulatory programs. (B) S. cerevisiae Ste12 protein has a nonconserved transcriptional activation domain, as well as a highly conserved DNA-binding domain. The secondary structure of this domain is predicted to contain a pattern of α-helices (gray boxes) interspersed with β-sheets (blue arrows) that are conserved among all fungal species. The region of the DNA-binding domain chosen for mutagenesis is shaded in orange. (C) A logo plot of the conserved portion of the DNA-binding domain of Ste12 generated from 1,229 fungal species. (D) A phylogeny of fungal species selected for those with functionally tested STE12 genes (except for the basal fungi Rhizophagus irregularis and Rozella allomycis, shown as outgroups). Pathogenic species are indicated with circles and colored according to plant (green) or animal (red) hosts. All fungal species were queried for the presence of a STE12 (blue) or TEC1 (orange) gene, and filled squares indicate presence of either gene; numbers inside boxes indicate species with multiple gene copies. Results for all species are shown below each gene’s column. (E) Proportional bar chart showing the number of S. cerevisiae genes that contain Ste12 sites (blue), Tec1 and Ste12 sites (yellow), and adjacent sites fewer than 24 bases from each other (green). (F) Histogram showing frequencies of spacing between all Ste12 and Tec1 binding sites in the S. cerevisiae genome. Negative values indicate that the Tec1 site is 5′ of the Ste12 site, and positive values the converse.
The two pathways converge on Ste12, which interacts differentially with cofactors to activate either mating or invasion. For mating, Ste12 can bind at pheromone-responsive genes as a homodimer or with the cofactors Mcm1 or Matα1 (10–13). The consensus DNA-binding site of Ste12 is TGAAAC, known as the pheromone response element (PRE) (11). For invasion, Ste12 and its cofactor Tec1 are both required to activate genes that mediate filamentation (8, 14, 15). Some of these genes contain a Ste12 binding site near a Tec1 consensus sequence of GAATGT, an organization for heterodimeric binding known as a filamentation response element (FRE). An alternative model, however, posits that expression of invasion genes is driven by a complex of Ste12 and Tec1, acting solely through Tec1 binding sites (15). An environmental component of trait specificity has been shown in fungi that are animal pathogens; upon recognition of host body temperature (37 °C) (6), Cryptococcus neoformans has decreased mating efficiency but increased ability to invade (16). Although trait preference in S. cerevisiae depends on Ste12, the role of this protein in regulating mating and invasion in response to increased temperature is unknown.
Because the highly conserved Ste12 DNA-binding domain ultimately enacts the choice between mating and invasion, here we sought to precisely define its DNA-binding specificity and examine the consequences of mutations and increased temperature on the balance of these traits. We reveal distinct organizational preferences for both homodimeric binding sites and cooperative heterodimeric binding sites with Tec1. Furthermore, single amino acid changes suffice to shift the preference of yeast cells toward one or the other trait; they also suffice to confer dependence on the chaperone Hsp90 and responsiveness to higher temperature. Some hyperinvasive separation-of-function mutations are independent of the cofactor Tec1, thought to be essential for invasion. We show that these Ste12 variants bind to homodimeric Ste12 binding sites in which one site is highly degenerate. Such a binding preference provides a plausible mechanism to activate the expression of invasion genes in a Tec1-independent manner, and provides a model for the regulation of these genes in the many fungal species that have no copy of TEC1.
Results
STE12 Is Present in Nearly All Fungal Genomes, but Most Lack a TEC1 Ortholog.
As the target of two different MAPK signaling cascades, Ste12 acts to define pathway specificity at the level of transcription (Fig. 1A). This role in both mating and invasion appears to be conserved in other fungal species, and presumably derives from properties of its DNA-binding domain, the most conserved segment of the protein (Fig. 1B) (17). Although the Ste12 DNA-binding domain has no match outside of the fungal kingdom, several residues as well as the overall predicted secondary structure are conserved in fungi (Fig. 1 B and C). We addressed the cooccurrence of Ste12 and Tec1 by examining 1,229 fungal species for the presence or absence of these two genes. Nearly every fungal species contains a copy of STE12 (97.7% of species), while less than one-third (31.7%) appear to have a copy of TEC1 (SI Appendix, Fig. S1). Furthermore, even among fungal pathogens with a characterized role for Ste12 in invasion, we found several examples in which a TEC1 gene is not present (Fig. 1D). Therefore, fungal species must have evolved Tec1-independent strategies to regulate mating and invasion.
We examined the extent to which Ste12 and Tec1 binding at adjacent sites within filamentation response elements (FREs) could explain invasion-specific activation by Ste12 in S. cerevisiae. Cooperative binding at adjacent (within 24 bp) Ste12 and Tec1 binding sites (FREs) has been demonstrated in vivo and in vitro (18), although most invasion genes do not contain FREs (15). For all promoter sequences in S. cerevisiae, we assessed the frequency and spacing of sites, and identified putative FRE sites (<24 bp). We found 792 unique promoters with matches (P < 1e−4) to a Ste12 binding site, with 220 (27.8%) of these also having a Tec1 binding site (Fig. 1E). The median distance between Ste12 and Tec1 sites is 360 bp, and only 12 pairs of sites (5%) are within 24 bp of each other (Fig. 1F). Among these 12 pairs, 4 have overlapping motifs consisting of a tail-to-tail arrangement, with the Tec1 motif overlapping the 3′ end of the Ste12 motif (Fig. 1F, Inset); this organization is more likely than nonoverlapping sites to occur by chance and may not be functional, although overlapping sites have been observed in cooperative binding of mammalian transcription factors (1). The small number of sites organized for cooperative binding with Tec1 contrasts with the hundreds of genes up-regulated under invasion conditions (19, 20), as well as with the dozens of genes bound by Ste12 under invasion conditions (57 genes unique to invasion, 100 overall) (14). Thus, the model of Ste12 and Tec1 cooperatively binding to FREs within invasion genes cannot alone account for the broad transcriptional response during invasion observed in S. cerevisiae. Specificity may derive from instances of long-range looping interactions, or binding of Ste12 to DNA indirectly through its interaction with Tec1 bound at Tec1 consensus sequences (15). However, given the absence of Tec1 in many species, this model is also unlikely to be applicable in fungi more broadly.
Ste12 and Tec1 Preferentially Bind DNA in Defined Spacings and Orientations.
Because the yeast genome contains few FREs, we sought to determine at high-resolution the in vitro DNA-binding preferences of Ste12 and Tec1. We used high-throughput systematic evolution of ligands by exponential enrichment (HT-SELEX), a method that determines a protein’s DNA-binding specificity by isolating and sequencing bound fragments present in a large, random pool of sequences (1, 21, 22). For the pool of DNA, we used fragments with a random sequence of 36 bp, which allowed us to capture instances of Ste12 and Tec1 binding sites in all orientations and at every possible spacing of 24 bp or fewer. In the Ste12 sample, we captured monomeric or homodimeric instances of the expected Ste12 binding site of TGAAAC, with dimeric sites found 20-fold more frequently than monomeric sites (SI Appendix, Fig. S2C). Many transcription factors exhibit strong preferences for spacing and orientation of sites, and this preference is conserved within transcription factor families (1, 22). Ste12 showed a strong preference (33% of 2,229,168 bound output sequences) for tail-to-tail binding sites with a 3-bp spacer (Fig. 2A, Top). While the most enriched sequences contained two perfect TGAAAC sites with this spacing (Fig. 2B, Top), most of bound sequences contained one perfect TGAAAC site paired with a mismatched site. This strong spacing preference had not been identified in previous protein-binding microarray studies, which used shorter target sequences (23), but is consistent with the known binding of Ste12 as a homodimer (24). We found two instances of homodimeric sites with a perfectly spaced TGAAAC pair in the S. cerevisiae genome: in the promoters of GPA1, encoding the α-subunit of the G protein involved in pheromone response, and of STE12 itself (Fig. 2C). Gpa1 is a component of the initial signaling point after pheromone sensing, and Ste12 is the ultimate transcription target of the MAPK signaling cascade, and both are among the most highly induced genes in response to pheromone (25). Elsewhere in the genome, two sites spaced 3-bp apart, in which one site is perfect and one is mismatched, are found within several pheromone-regulated genes. This configuration occurs in 29 genes in the S. cerevisiae genome, and 12 of the 50 most pheromone-induced genes contain these sites (examples in Fig. 2C). To analyze the ability of these sites to drive Ste12-dependent expression, we used a reporter assay in yeast. The in vitro preferences for the dimeric sites, as well as for the base flanking the TGAAAC motifs, correlated with in vivo activation (SI Appendix, Fig. S2 A and B).
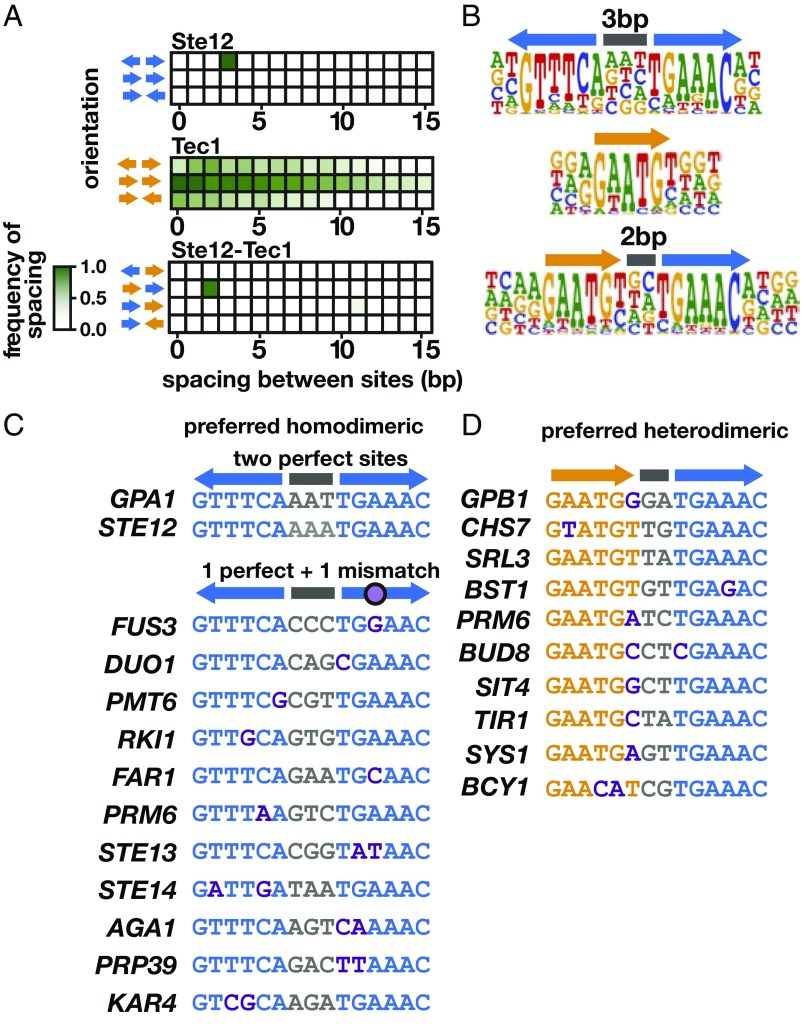
Fig. 2.
Identification of the DNA-binding preferences of Ste12 and Tec1 by HT-SELEX. (A) Heatmap showing relative frequencies of the possible orientations and spacings of the primary 6-mer selected in Ste12 (TGAAAC, Top) and Tec1 (GAATGT, Middle) binding reactions. The Bottom heatmap shows the frequency of each respective 6-mer in the cobinding sample containing both proteins. In the cobinding sample, we excluded sequences with homodimeric Ste12 sites, which were present at a 30-fold higher level than heterodimeric sites. The single dark green box in the Top and Bottom heatmaps indicates the most frequent orientation and spacing of sites; white boxes are at most 30% as frequent as the maximum. No frequent dimeric site organizations were observed for Tec1 (Middle). (B) Full motifs identified by Autoseed software (1). (C) Instances of the Ste12 homodimeric motif, with sites oriented tail-to-tail with a 3-bp spacer, were used to query native yeast promoters. Two pheromone-induced genes, GPA1 and STE12, contain two perfect sites, while most other genes contain a perfect site paired with a site containing one or two mismatches, with the 3-bp spacing intact. (D) A similar search of yeast promoters using the preferred Ste12 and Tec1 heterodimeric site identified a set of invasion-associated genes, as well as those not previously linked to invasion.
HT-SELEX with Tec1 protein recapitulated the known binding site of (A/G)GAATGT (Fig. 2B, Middle) (26). We found that the first base of this motif showed a strong preference for a purine base, and that the final T showed less specificity than the rest of the motif. Unlike with Ste12, we did not observe any spacing and orientation preferences for two Tec1 sites, indicating that bound sequences with multiple Tec1 binding sites are likely the result of independent binding events (Fig. 2A, Middle).
We next conducted HT-SELEX in the presence of both Ste12 and Tec1 to identify patterns of cooperative binding events between the two proteins. The DNA-binding domain of Ste12 (1–215) is sufficient for cooperative binding with Tec1 (1–280) in vitro (26, 27). We detected bound sequences containing sites for both proteins, with the highest abundance preference being a tail-to-head orientation of Tec1 and Ste12 sites separated by 2 bp (Fig. 2 A and B, Bottom, and SI Appendix, Fig. S3). Several invasion genes in the S. cerevisiae genome (GPB1, BST1, PRM6, BUD8, and SIT4) contain this type of heterodimeric site, although other genes with this binding site organization (CHS7, SRL3, TIR1, SYS1, and BCY1) have not been previously associated with invasion (Fig. 2D). This arrangement is similar to homodimeric Ste12 sites, except that one Ste12 site is replaced by a Tec1 site, suggesting that a similar protein interaction surface may be used for this heterodimeric binding mode of Ste12, as is used for the homodimeric binding mode. However, even in this sample, the pool of bound sequences was dominated by two Ste12 sites with 3-bp spacing, suggesting that Ste12 has higher affinity to two PRE sites than to an FRE requiring binding with Tec1.
Mutations in the DNA-Binding Domain of Ste12 Separate Mating and Invasion Functions.
The organization of DNA-binding sites selected by Ste12 alone or in combination with Tec1 suggested that Ste12 balances the expression of mating and invasion genes by its mode of binding to DNA. We sought to determine whether this balance could be shifted by mutations within the Ste12 DNA-binding domain. We conducted deep mutational scanning (28) of a segment of this domain by generating ∼20,000 protein variants over 33 amino acids, including single-, double-, and higher-order mutants, and subjected yeast cells carrying this variant library to selection for either mating or invasion (Fig. 3 A and B and SI Appendix, Figs. S4 and S5A). As a control, we employed selection for a third trait, response to osmotic stress, which shares upstream pathway components but does not involve Ste12. Mating selections and osmotic stress selections were carried out in the BY4741 strain background. However, as this strain contains a flo8 mutation that prevents invasion, we carried out invasion selections in a related strain, Σ1278b (29), which has been used for large-scale invasion phenotyping (30). Although Σ1278b and BY4741 have many genetic differences, Ste12 and Tec1 and their essential roles in balancing mating and invasion are conserved between the two strains.
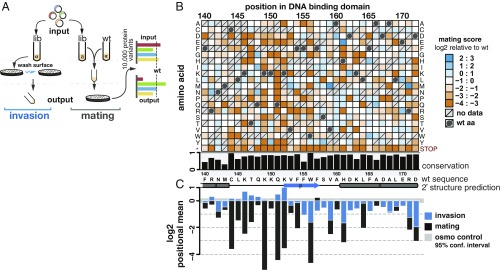
Fig. 3.
Deep mutational scanning identifies Ste12 DNA-binding domain mutants with altered mating and invasion function. (A) Two yeast populations were transformed with the same STE12 variant library. For assaying invasion, Σ1278b MATα cells (n = 400,000 per replicate, three biological replicates) were plated on selective media and grown. Selection was performed by washing colonies from plate surfaces and collecting cells embedded in the agar for sequencing. For assaying mating, BY4741 MATa ste12Δ cells were mated to MATα cells, and diploids (n = 500,000 per replicate, three biological replicates) were selected, scraped from the agar, and sequenced. In both cases, input variant frequencies were defined before selection. (B) The effects of single amino acid substitutions within Ste12’s DNA-binding domain on mating ability are shown. On the x axis, the wild-type Ste12 sequence is shown, along with its predicted secondary structure (helices shown as tubes) and conservation. Conservation was determined as a fraction of identity among 1,229 fungal species. On the y axis, amino acid substitutions are shown. Variants increasing mating efficiency are in shades of blue, and variants decreasing mating efficiency are in shades of orange based on log2 enrichment scores relative to wild-type. Dark gray circles indicate the wild-type Ste12 residue, and crosses indicate missing data. Ste12 variants showed comparable expression levels (SI Appendix, Fig. S6). (C) Positional mean scores for single amino acid substitutions are shown for mating (black) and invasion (blue), excluding stop codons. Gray horizontal bar indicates confidence interval for experimental noise determined from selection for Ste12-independent high osmolarity growth.
We expected that most STE12 mutations would affect mating and invasion similarly, because of the conservation of the Ste12 DNA-binding domain and its requirement for both traits. Indeed, we found positions in which almost any amino acid substitution was highly deleterious to both mating and invasion (Fig. 3 B and C and SI Appendix, Fig. S5). We identified sites that were more sensitive or less sensitive, on average, to mutation, by calculating a positional mean score from all mutations tested at that site. Each mutation was tested in triplicate, and the experimental error was calculated for each mutant individually such that the SE of the positional mean represents the variability among different amino acid substitutions, rather than experimental noise (Fig. 3C and SI Appendix, Fig. S4E). Conservation only partially explained these results, as some of the most deleterious positions are invariant among fungi (W156, C144), whereas others are not (K149, Q151, K152). Differing expression levels among STE12 variants were not predictive for either mating or invasion phenotypes (SI Appendix, Fig. S6). Because invasion was measured in a different strain background than mating for each variant, we verified that strain background did not account for the trait differences. We selected five variants that conferred differential mating and invasion phenotypes [K149E, K150I, K150A, K152L, and (see below) the double-mutant S177K, Q180R, denoted SKQR], and assayed their mating efficiencies in the Σ1278b background. The mating efficiencies for each variant correlated (r2 = 0.7) between strains, with the average difference for the five variants equal to 21% (SI Appendix, Fig. S6D).
The mutational analysis revealed separation-of-function mutations that primarily reduced either mating or invasion. Substitutions with mostly deleterious effects on mating clustered in residues N-terminal to the conserved W156 (Fisher’s exact test P = 0.002, designated region I), while substitutions with increased effects on invasion appeared more frequently in this region (P = 0.0003). Substitutions that increased mating were rare. In contrast, substitutions at some positions, almost all within region I, both increased invasion and decreased mating. We verified separation-of-function mutants, and further explored the apparent structure of mutational effects, with a much larger pool of >20,000 double mutants. Double mutants involving two positions in region I were mostly defective for mating, while those involving two positions in region II were mostly defective for invasion, confirming the bipartite arrangement of the mutagenized segment, split by the central W156 (P = 1.3e-5) (Fig. 4A). Double mutants between regions I and II showed variability in mutational effects, with some pairwise combinations increasing mating and others decreasing mating; the effects on invasion, however, for these same combinations did not follow the same pattern, suggesting epistatic interactions (examples highlighted in Fig. 4A and SI Appendix, Fig. S7). Thus, mutations in the Ste12 DNA-binding domain can impose preference for mating or invasion rather than similarly affecting both traits, suggesting that the DNA-binding domain itself contributes to trait specificity.
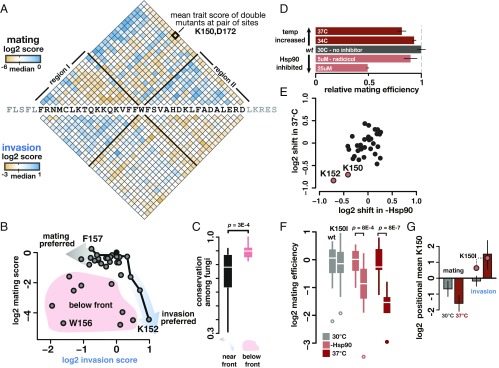
Fig. 4.
Mutations residing in a region of the Ste12 DNA-binding domain increase invasion at the cost of mating, with exceptional mutations doing so in a temperature- and Hsp90-dependent manner. (A) Mean effect of double mutations between all combinations of positions for mating (above wild-type sequence) and invasion (below wild-type sequence). A specific pair of positions is shown in bold. Effects are shown as log2-fold change relative to wild-type and color-coded as in Fig. 3, functional variants in shades of blue, nonfunctional variants are shown in shades of orange. Note difference in scale ranges between mating and invasion. Black lines emanating from W156 represent boundaries of region I, in which mutations primarily reduced mating, and region II, in which mutations primarily reduced invasion. (B) Scatterplot of positional mean scores for both mating and invasion showed inverse relationship, indicating a trade-off between both traits. A point is shown for each of the 33 mutagenized positions of STE12. The trade-off is visualized as a Pareto front (black line) determined empirically from the positional data; positions near the front maintain high values for one trait and minimize costs to the other. Arrows indicate preference for either mating (gray) or invasion (blue). Positions near the front were distinguished from those below the front (shaded in red) by calculating Euclidian distances. (C) Boxplots show conservation for positions near and below the front; positions below the front are significantly more conserved among fungi than those near the front (two-sided t test). (D) Mating efficiency of yeast cells with wild-type Ste12 at increased temperature (dark red) or in the presence of Hsp90 inhibitor radicicol (pale red) is reduced relative to an untreated control (black); error bars represent SEM. (E) The mating efficiency of Ste12 variants at high temperature or with Hsp90 inhibition is shown as the shift in mean effect at each mutated position. Two positions, K150 and K152 (pale red), showed greatest sensitivity to both treatments. (F) K150I was tested in a quantitative assay to validate its temperature-sensitive and Hsp90-dependent mating activity, shown relative to wild-type (left boxplot in each pair) in each treatment (n = 20 for each sample). (G) Ste12 variants at K150 (mean effect) decreased mating (Left) and invasion (Right) at standard temperature (gray bars). At high temperature (red bars), mating further decreased; however, invasion increased. Error bars represent SEM. The K150I variant is individually highlighted for comparison.
To explore the apparent trade-off between mating and invasion in more detail, we used the Pareto front concept (31). This concept, rooted in engineering and economics, defines all feasible solutions for optimizing performance in two essential tasks. Here, we consider all feasible genotypes that affect mating or invasion. We plotted the single mutation mean positional scores for both traits, identifying positions close to the empirically determined Pareto front that show increased invasion and deleterious effects on mating (Fig. 4B and SI Appendix, Fig. S8). We found that this effect is recapitulated in the effects of individual mutants tested in both trait selections at extreme positions on the front (SI Appendix, Fig. S9). Positions well below the Pareto front, including most prominently W156, had deleterious effects on both traits. These positions are significantly more conserved among fungi, consistent with variation at these sites being disfavored given the constraint on Ste12 to maintain both mating and invasion function in the fungal lineage (Fig. 4C). That opposing preference for each trait is better predicted by positional means than every individual mutation suggests that trait preference is encoded in particular functional regions of the Ste12 DNA-binding domain rather than through overall features, like protein stability.
Having established that trait preference can be modulated by mutations in Ste12, we asked whether temperature affected specificity toward mating and invasion, and if that specificity could be altered by mutation. Furthermore, because chaperones maintain protein function at increased temperatures and Hsp90 modulates pheromone signaling in S. cerevisiae (9), we also asked whether mating or invasion changed in the presence of radicicol, a pharmacological inhibitor of Hsp90. We confirmed that mating is modulated by temperature and by Hsp90 function by mating cells expressing wild-type Ste12 at increased temperature or in the presence of radicicol (Fig. 4D). We then subjected cells containing the Ste12 variant library to a mating selection at 37 °C or a mating selection in the presence of radicicol. Most Ste12 variants responded to increased temperature or Hsp90 inhibition as did wild-type Ste12 (SI Appendix, Fig. S10). However, there were two positions, K150 and K152, in which mutations resulted in highly temperature-responsive and Hsp90-dependent mating (Fig. 4E). This pair of lysines resides within the mutagenized segment that modulates mating and invasion specificity. We validated the temperature and Hsp90 effect on a variant (K150I) that conferred mating at near wild-type levels in the absence of heat or radicicol treatment (Fig. 4F). However, in the presence of heat or radicicol treatment, mating of cells with the K150I variant was severely decreased, indicating that this mutation led to buffering by Hsp90. Thus, Hsp90 could facilitate a mutational path toward the pathogenic lifestyle by minimizing mating costs at 30 °C and enhancing invasion at 37 °C. To test this idea, we conducted selection for invasion at 37 °C, which yielded results comparable to Hsp90 inhibition for mating. Indeed, the mean effect of all variants at K150 was to increase invasion at high temperature, with a concomitant decrease in mating, and this result was also true of the individually validated variant K150I (Fig. 4G), demonstrating that K150 variants were not simply unstable at high temperature but gained a novel function. Thus, mutations in STE12 can interact with an environmental factor to further bias cellular decision-making toward invasion over mating. Mutation of STE12 could allow S. cerevisiae to mimic the behavior of fungal pathogens like C. neoformans, for which the sensing of the increased body temperature of their animal hosts facilitates the transition toward an invasive lifestyle (7).
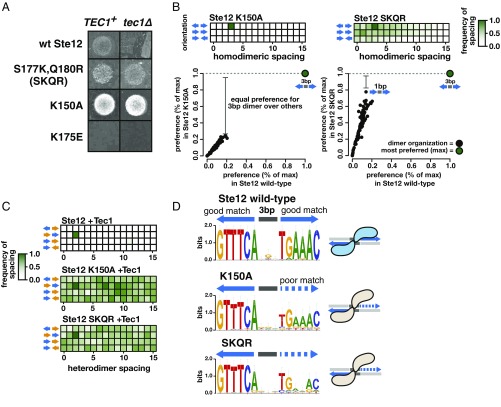
We examined whether natural variation in fungal Ste12 DNA-binding domains includes amino acid residues found to affect S. cerevisiae trait preference, as fungal species have differential capacities for mating and invasion (17, 32). We examined variation in the DNA-binding domain present in species with and without a TEC1 gene (SI Appendix, Fig. S11) and detected variation outside of the mutagenized region that might be expected to benefit invasion. For example, the Ste12 DNA-binding domain of the invasive pathogen Cryptococcus gattii (33) contains two additional positive residues immediately C terminal to region II; such changes are found in many other species that lack Tec1 (SI Appendix, Fig. S11). Introducing these C. gattii-specific residues (S177K, Q180R; denoted SKQR) into the S. cerevisiae Ste12 DNA-binding domain yielded a dominant invasion phenotype (Fig. 5A) with neutral effects on mating (SI Appendix, Fig. S6D) in S. cerevisiae. We introduced a negatively charged residue (K175E) into this same region, which abolished invasion entirely (Fig. 5A). Similar to SKQR, the region I mutation K150A conferred decreased mating and an increase in invasion. The dominant invasion phenotype for each variant suggest that these Ste12 variants engage in altered binding to DNA, altered homodimerization, or altered heterodimerization with Tec1.
Fig. 5.
Ste12 DNA-binding domain mutants that confer invasion independent of the cofactor Tec1 have altered binding specificity. (A) Wild-type Ste12 requires the cofactor Tec1 for invasion; a tec1Δ strain fails to invade. The region I mutant K150A showed a hyperinvasive phenotype with or without Tec1. Introduction of the two positively charged residues found in C. gattii (SKQR) also led to Tec1-independent invasion, whereas introduction of a negative charge (K174E) eliminated invasion. The hyperinvasive Ste12 mutants showed a dominant invasion phenotype, as they were tested in the presence of wild-type Ste12, in the Σ1278b background. (B) Spacing heatmaps for Ste12’s binding site are shown as in Fig. 2A. K150A retains the same preference for tail-to-tail sites with a 3-bp spacer as the wild-type Ste12, but SKQR’s preference is reduced. To visualize this difference, the scatterplots below show frequencies of site organizations relative to the most preferred site (% of max) for each variant (y axis) compared with wild-type Ste12 (x axis), where each point represents one orientation and spacing combination (example of SKQR’s reduced preference is shown: its preference for head-to-tail with 1-bp spacing is ∼78% that of the tail-to-tail with 3-bp spacing). (C) Spacing heatmaps are shown for the K150A and SKQR variants in cobinding reactions with Tec1; unlike wild-type Ste12 (repeated from Fig. 2A, Bottom for clarity), neither variant shows strong preference for any heterodimeric site organization. (D) Logo plots generated from the 50 most highly enriched sequences in HT-SELEX represent ideal sites for each variant alongside wild-type Ste12.
Mutations in Ste12 Can Promote Tec1-Independent Invasion and Alter DNA-Binding Specificity.
Tec1 activates invasion genes, with Ste12 either binding directly to DNA cooperatively with Tec1 or indirectly as part of a complex with Tec1 (14, 15). However, in contrast to both established Ste12 binding modes, the invasion phenotype due to the SKQR variant was independent of Tec1, as was the even stronger invasion phenotype due to the K150A variant (Fig. 5A). Thus, a single mutation in the Ste12 DNA-binding domain might change the binding preference of this domain such that it can be recruited in the absence of Tec1 to binding sites sufficient for invasion.
To understand the relationship between the phenotypes conferred by Ste12 variants and their DNA-binding specificity, we conducted HT-SELEX experiments. We chose the SKQR and K150A variants, each of which showed an altered invasion phenotype, and asked whether they could recognize the homodimeric Ste12 sites favored by the wild-type protein. SKQR showed a greatly reduced (by 45%) preference for two sites separated by 3 bp (Fig. 5B). Consistent with this reduced preference, SKQR conferred reduced activation in vivo from a minimal promoter containing such homodimeric sites (SI Appendix, Fig. S12A). Furthermore, an examination of the most enriched sequences bound in vitro by the SKQR variant showed that it lost specificity for the sequence of the second site, which was also reflected in its in vivo sequence preference (Fig. 5D and SI Appendix, Fig. S12B). In contrast, K150A retained a strong preference for the sites spaced 3-bp apart, nearly identical to wild-type Ste12 (Fig. 5B). However, analysis of its most enriched sequences revealed that, like SKQR, the K150A variant showed reduced specificity for one site of the homodimeric pair (Fig. 5D). The reduced specificity in these variants should expand the number of their recognition sites. Because both of these variants recognized at least one perfect—or near perfect—Ste12 site, we conclude that the primary change to DNA-binding specificity in these variants derives not from loss of specific DNA contacts, but rather from their diminished capacity for symmetric dimeric binding.
We also carried out cobinding SELEX experiments with Tec1, as previously conducted with wild-type Ste12 (reproduced in Fig. 5C, Top). Consistent with their ability to promote Tec1-independent invasion, both SKQR and K150A showed altered patterns of cooperative binding with Tec1. K150A showed no preference for heterodimeric sites with a 2-bp spacer, favored by the wild-type Ste12, in cobinding experiments with Tec1, and SKQR had a greatly reduced preference for these sites (Fig. 5C).
Discussion
Transcription factors maintain their capacity to enact complex regulatory programs over the course of evolution, despite many changes to the genomic locations of their binding sites and to the repertoires of their available cofactors. Nonetheless, by analyzing thousands of mutations in a yeast transcription factor, we found variants that can mediate only one of two alternative programs, and do so in the absence of a requisite cofactor. These variants of the S. cerevisiae Ste12 protein have single amino acid changes that lead to DNA-binding preferences dramatically different from the wild-type protein, as well to an increased ability of yeast to invade, rather than mate, at high temperature. They thus reveal how natural variation in the DNA-binding domain of a transcription factor might affect an environmentally sensitive decision to carry out one developmental process rather than another.
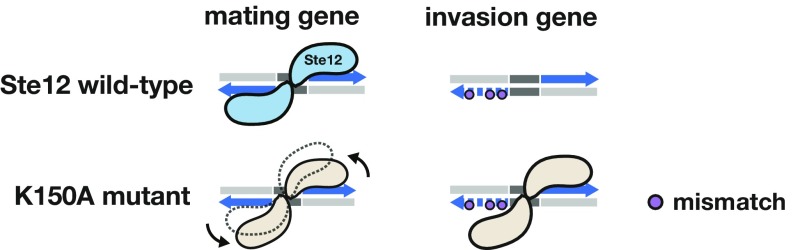
In S. cerevisiae, Ste12 drives expression of mating genes as a homodimer and of invasion genes as a heterodimer with the invasion cofactor Tec1 (8, 18). The single mutations in Ste12 that shift trait preference toward invasion in a dominant and Tec1-independent manner provide a plausible model (Fig. 6) that can explain how fungal species, including many pathogens, without Tec1 or similar cofactors accomplish activation of invasion genes. In mating genes, two properly oriented binding sites facilitate the interaction of two Ste12 proteins; one site tends to be perfect and the other slightly mismatched, thereby likely reducing cooperativity. Under invasion conditions, the Ste12 dimer interface instead allows cooperative interaction between Ste12 and Tec1. We posit that mutations in Ste12 that lead to hyperinvasiveness change this interface such that the conformation of the homodimer is altered and interaction with Tec1 is not possible. In this altered conformation, which may be naturally present in species without Tec1, Ste12 loses its preference for the perfect/near-perfect homodimeric binding sites and instead recognizes a degenerate version of its binding site adjacent to a near-perfect site. The binding mode of these Ste12 mutants exemplifies how transcription factors may access novel binding sites by changes in their recognition of preferred dimeric sites rather than of individual site specificity.
Fig. 6.
A model for novel site recognition by Tec1-independent Ste12 variants. Wild-type Ste12 protein (blue) is compared with the K150A mutant (tan) for binding at a mating gene with two perfect recognition sites (tail-to-tail with a 3-bp spacing, shown in blue) and an invasion gene with a single perfect site along with a degenerate site. Due to its altered dimer interface, the “kinked” mutant K150A is less capable of binding symmetrically at two perfect sites, consistent with its phenotype of reduced mating efficiency. However, the conformation of K150A allows the variant protein to occupy novel sites within invasion genes that contain one perfect or nearly perfect site paired with a degenerate site; wild-type Ste12 is unable to occupy these sites.
Mutations at positions in Ste12 implicated in shifting trait preference toward invasion also conferred dependence on temperature and the chaperone Hsp90. For some fungal pathogens, increased temperatures associated with warm-blooded animals promote invasive growth (7). In the nonpathogenic S. cerevisiae, increased temperature decreases mating and promotes invasion (7). The conserved role of Ste12 in invasion and mating, which can be modified by mutations that affect the environmental sensitivity of this trait choice, suggests that variation in Ste12 may potentiate pathogenic transformation in other species. The temperature- and Hsp90-dependent Ste12 variants do not resemble typical temperature-sensitive mutants in simply losing function under nonpermissive conditions. In response to high temperature or Hsp90 inhibition, these variants failed to promote mating yet conferred an increased ability to invade. These variants thus represent the unusual phenomenon whereby perturbation of Hsp90 or equivalent environmental stress results in a protein gaining a novel function (34–39).
As the ultimate mediators of cellular decisions, transcription factors occupy a predominant position in the signaling networks that drive patterns of gene expression. For this reason, they are susceptible to even subtle mutations that may result in consequential phenotypes. Such effects will be less predictable than the full loss-of-function phenotype resulting from, for example, a failure to bind to DNA. Thus, the type of detailed analysis of missense variants as performed here will be useful in understanding the shifts in phenotype that enable adaptation to novel environments.
Mutations in transcription factors that generate alternative DNA-binding modes have been previously identified. For example, rare coding variants in the homeodomain recognition helices of several transcription factors alter DNA-binding specificity, which likely contributes to associated disease (40). These homeodomain variants bind at sites not dramatically different from the wild-type sites, yet this promiscuity is associated with a deleterious disease phenotype. The demonstration here of a surprising malleability of transcription factor binding stands in stark contrast to the fact that sequences of transcription factors tend to be highly conserved. This study, together with those revealing only weak purifying selection in functional regulatory regions, poses the challenge of reconciling flexible transcription factor activity and often highly variable regulatory regions with the longstanding observation that gene-expression patterns are robust to most perturbations and conserved throughout evolution.
Materials and Methods
HT-SELEX.
We purified fragments of Ste12 (1–215) and Tec1 (1–250) expressed from pGEX-4T-2 vectors. These protein fragments have been used previously and are sufficient for both individual and cooperative binding in vitro. Fragments of each protein, as well as protein variants, were purified using a GST tag and subsequently used for HT-SELEX. SELEX reactions with homogenous and mixed protein populations were performed identically to previous work (1, 21). Briefly, a 50-μL reaction containing purified Ste12 and Tec1 (1:25 molar ratio with DNA), 200-ng nonspecific competitor double-stranded nucleic acid poly (dI/dC), and 100-ng selection ligand (36N) was incubated in binding buffer [140 mM KCl, 5 mM NaCl, 1 mM K2HPO4, 2 mM MgSO4, 20 mM Hepes (pH 7.05), 100 μM EGTA, 1 μM ZnSO4] for 2 h. GST Sepharose (GE) beads were then added to each reaction, incubated for 30 min, and unbound ligand was removed using seven buffer washes. Output reactions were amplified by PCR after each round, and these products were subsequently used to prepare high-throughput sequencing libraries. SELEX motif enrichments were analyzed using Autoseed software (1). Briefly, a pool of binding-selected output sequences was compared against a fully random input sequence to identify sequences, motifs, and orientations enriched relative to the unselected oligo pool.
Generation of STE12 Mutant Libraries.
The STE12 locus from S. cerevisiae strain BY4741, including the intergenic regions, was introduced into the yeast vector pRS415 containing a LEU2 marker (41). Degenerate DNA sequence encoding a 33-amino acid (99 bp) segment of the DNA-binding domain of Ste12 was generated by 2.5% doped oligonucleotide synthesis (Trilink Biotechnologies). Invariant 30-bp sequences were designed on either side of the mutagenized fragment; these flanking sequences contained NotI and ApaI cut sites found in the coding sequence of STE12, and were unique in the STE12 plasmid construct. Both fragment and plasmid were double-digested with NotI and ApaI, and the plasmid library was assembled by standard ligation. STE12 libraries were transformed into electrocompetent Escherichia coli (ElectroMAX DH10B; Invitrogen), and amplified overnight in selective media. Efficiency of ligation was verified by Sanger sequencing across the mutagenized region of 96 transformants (42); no assembly errors were detected, and mutant proportions reflected those expected for doped oligo synthesis at 2.5%. Plasmid libraries were used to transform yeast (BY4741 MATa or Σ1278b MATα) with a deleted endogenous copy of STE12 by high-efficiency lithium acetate transformation (43). The same plasmid library was used to transform yeast (Σ1278b) for invasion selection. Individual point mutations were generated in wild-type STE12 plasmids by site-directed mutagenesis (Q5; New England Biolabs).
Large-Scale Trait Selections.
The BY4742 MATα strain was used as the mating partner for the library-transformed BY4741 MATa in all selections and mating assays. Transformed yeast cells were grown to late log-phase in a single 500-mL culture, and cells were harvested to determine plasmid variant frequencies in the input population. The same culture was used to seed 36 independent mating selections for each treatment: 1 million MATa cells with STE12 variants were mixed with 10-fold excess wild-type MATα cells and allowed 5 h to mate (44). Depending on treatment type, cell mixtures were left at 30 °C with DMSO, 30 °C with the Hsp90 inhibitor radicicol, or 37 °C with DMSO. A 5-μM concentration of the Hsp90 inhibitor radicicol (R2146; Sigma-Aldrich) was chosen due to its measureable effect on mating efficiency and lack of pleiotropic growth defects. The temperature of 37 °C was chosen for the similarity of effects on mating between temperature and radicicol treatments. After mating was completed, cell mixtures were plated using auxotrophic markers present only in mated diploids. Plasmids containing STE12 variants were extracted from this output population, as well as from the premating input, for subsequent deep sequencing. Mating selections were repeated in triplicate. For invasion, Σ1278b yeast cells transformed with the Ste12 plasmid library were grown to late log-phase in a single 500-mL culture, diluted (10,000 cells per plate), and plated onto 40 plates of synthetic complete medium lacking leucine (2% agar). Plates were incubated at 30 °C or 37 °C for 72 h to allow for sufficient invasion, as previously described (30). After 3 d, cells were washed from the plate surfaces, enriching for cells embedded in the agar. Agar pucks were removed from plates with a razor. Using the “salsa” blender setting (Hamilton Beach), a coarse slurry was generated and subsequently poured over a vacuum apparatus lined with cheesecloth. The resulting liquid cell suspension was spun down at 5,000 rpm to collect cells for subsequent deep sequencing. Individual invasion assays (as in Fig. 5A) were treated identically, but 10-μL aliquots of OD-normalized cultures were plated to image colonies for each strain. For high osmolarity growth, selections were conducted using the BY4741 MATa library-transformed population grown overnight in media containing 1.5 M Sorbitol, as described previously (45). Populations were sequenced before and after growth to determine enrichment scores that defined the 95% confidence interval used in Fig. 3C. For all trait selections, we chose sample sizes that were at least 10-fold higher than the variant library size, ensuring that each variant would be adequately sampled in each of the three biological replicate selections.
Sequencing and Determination of Trait Scores.
Sequencing was completed on Illumina’s MiSeq or NextSeq platforms. Sequencing libraries were prepared by extracting plasmids from yeast populations (Yeast Plasmid Miniprep II; Zymo Research) before and after selection. These plasmids were used as template for PCR amplification that added adaptor sequences and 8-bp sample indexes to the 99-bp mutagenized region for sequencing (all libraries amplified <15 cycles). Paired-end reads spanning the mutagenized region were filtered to obtain a median of 5 million reads per sample. Using ENRICH software (46), read counts for each variant before and after selection were used to determine mating efficiency and invasion ability of STE12 variants. Briefly, counts for a particular variant in the input and output libraries were normalized by their respective read totals to determine frequency in each, and a ratio of the output and input frequencies determine a variant’s functional score. Finally, enrichment scores were normalized by the enrichment of wild-type Ste12 in each selection. Treatment scores were calculated identically, and in all cases where difference scores are shown, the score represents log2(treated) − log2(untreated).
Calculating Intramolecular Epistasis Scores.
Intramolecular epistasis scores were defined as the deviation of a double mutant’s functional score (Wij) from the multiplied scores of its constituent single-mutants (wi × wj). A negative epistasis score indicates that the deleterious effect of one mutation is increased by the presence of the partner mutation, while a positive epistasis score indicates that the partner mutation decreases the deleterious effects of individual mutations (47).
Quantitative Mating Assay.
Individual variants tested for mating efficiency were treated identically to the large-scale mating selection experiments, except genotypes were scored individually on selective plates for either mated diploids (2N) or both diploids and unmated MATa haploids (2N, 1N). The proportion of mated individuals (mating efficiency) is taken as the ratio of colony counts on diploid to counts on diploid + haploid plates [2N/(2N + 1N)]. Ste12 variants were tested alongside wild-type to determine relative changes in response to treatment.
STE12 Variant RNA-Seq.
RNA was extracted from yeast cells harboring the STE12 mutant library grown under nonselective conditions using acid phenol extraction, as previously described (48). STE12-specific cDNA was created using a gene-specific cDNA primer and SuperScript III (Life Technologies). cDNA was amplified in a manner similar to the plasmids, and prepared for sequencing using Illumina Nextseq.
Large-Scale Analysis of Ste12 Binding Sites in Vivo.
A HIS3 reporter gene was used for testing large populations of binding-site variants (SI Appendix, Fig. S12) in media lacking histidine. Although Ste12 does bind single PREs as a monomer, two sites are needed for signal detection in reporter assays (49). We designed oligonucleotides that maintain the central portion of the native PRE, but randomized six surrounding bases on either side (NNNNNNTTTCAAAATGAAANNNNNN). This library was cloned into a promoter from the same plasmid used in the luciferase assay, at the same position as the native PRE. Strains containing one of four Ste12 protein variants were transformed with the same binding-site library reporter population, and grown overnight in synthetic media lacking histidine with 10 mM of the His3 competitive inhibitor 3-amino-triazole. Three biological replicate selections were conducted for each Ste12 protein variant tested against the binding-site library. Cells were collected and sequenced before and after selections to determine enrichment scores for each binding-site variant. Computational analysis of binding-site enrichment scores was identical to pipeline for Ste12 protein variants. Enrichment of all binding-site variants are shown relative to the enrichments of empty plasmids, which were spiked-in to the binding-site library as control. The 250 binding sites with the highest enrichments were grouped and Weblogo (50) was used to generate base preference plots.
Evolutionary Analysis of Ste12 DNA-Binding Domain Among Fungi.
Using fungal genomes deposited into the National Center for Biotechnology Information and from the fungal sequencing project at the Joint Genome Institute, we created a BLAST database of translated coding sequences and queried with the S. cerevisiae Ste12 protein sequence. Full protein sequences from BLAST hits were aligned using MUSCLE (51) and used to determine conservation at each site in the mutated segment. The secondary structure of S. cerevisiae’s Ste12 DNA-binding domain was determined using Psipred (52).
Data Availability.
High-throughput sequencing reads have been submitted to National Center for Biotechnology Information Sequence Read Archive under accession nos. SRR5408956–SRR5408965. Datasets with calculated enrichment scores for each tested variant in each condition, along with positional mean scores, and scores from binding site library selections are provided in Datasets S1–S5.
Supplementary Material
Acknowledgments
We thank J. Thomas for advice on analysis of fungal variation, and D. Fowler and H. Malik for comments on the manuscript. The work was supported by National Institutes of Health Grant R01GM114166 (to C.Q. and S.F.). M.W.D. was supported by a National Science Foundation Graduate Research Fellowship and a Washington Research Foundation-Hall fellowship. S.F. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The high-throughput sequence reads reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra (accession nos. SRR5408956–SRR5408965).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805882115/-/DCSupplemental.
References
- 1.Jolma A, et al. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature. 2015;527:384–388. doi: 10.1038/nature15518. [DOI] [PubMed] [Google Scholar]
- 2.Boer DR, et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014;156:577–589. doi: 10.1016/j.cell.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Hentze N, Le Breton L, Wiesner J, Kempf G, Mayer MP. Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. eLife. 2016;5:e11576. doi: 10.7554/eLife.11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody A, Weiner J, Ramanathan S. Modularity of MAP kinases allows deformation of their signalling pathways. Nat Cell Biol. 2009;11:484–491. doi: 10.1038/ncb1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook JG, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- 6.Beyhan S, Gutierrez M, Voorhies M, Sil A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 2013;11:e1001614. doi: 10.1371/journal.pbio.1001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect JR. Cryptococcus neoformans: The yeast that likes it hot. FEMS Yeast Res. 2006;6:463–468. doi: 10.1111/j.1567-1364.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 8.Madhani HD, Fink GR. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- 9.Louvion JF, Abbas-Terki T, Picard D. Hsp90 is required for pheromone signaling in yeast. Mol Biol Cell. 1998;9:3071–3083. doi: 10.1091/mbc.9.11.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalatan JG, Coyle SM, Rajan S, Sidhu SS, Lim WA. Conformational control of the Ste5 scaffold protein insulates against MAP kinase misactivation. Science. 2012;337:1218–1222. doi: 10.1126/science.1220683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan JW, Kirkman C, Fields S. The yeast STE12 protein binds to the DNA sequence mediating pheromone induction. Proc Natl Acad Sci USA. 1989;86:5703–5707. doi: 10.1073/pnas.86.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errede B, Ammerer G. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 1989;3:1349–1361. doi: 10.1101/gad.3.9.1349. [DOI] [PubMed] [Google Scholar]
- 13.Yuan YO, Stroke IL, Fields S. Coupling of cell identity to signal response in yeast: Interaction between the alpha 1 and STE12 proteins. Genes Dev. 1993;7:1584–1597. doi: 10.1101/gad.7.8.1584. [DOI] [PubMed] [Google Scholar]
- 14.Zeitlinger J, et al. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell. 2003;113:395–404. doi: 10.1016/s0092-8674(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 15.Chou S, Lane S, Liu H. Regulation of mating and filamentation genes by two distinct Ste12 complexes in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:4794–4805. doi: 10.1128/MCB.02053-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Courchesne W. A novel quantitative mating assay for the fungal pathogen Cryptococcus neoformans provides insight into signalling pathways responding to nutrients and temperature. Microbiology. 1998;144:1691–1697. doi: 10.1099/00221287-144-6-1691. [DOI] [PubMed] [Google Scholar]
- 17.Wong Sak Hoi J, Dumas B. Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot Cell. 2010;9:480–485. doi: 10.1128/EC.00333-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhani HD, Fink GR. Combinatorial control required for the specificity of yeast MAPK signaling. Science. 1997;275:1314–1317. doi: 10.1126/science.275.5304.1314. [DOI] [PubMed] [Google Scholar]
- 19.Boer VM, de Winde JH, Pronk JT, Piper MDW. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem. 2003;278:3265–3274. doi: 10.1074/jbc.M209759200. [DOI] [PubMed] [Google Scholar]
- 20.Prinz S, et al. Control of yeast filamentous-form growth by modules in an integrated molecular network. Genome Res. 2004;14:380–390. doi: 10.1101/gr.2020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolma A, et al. Multiplexed massively parallel SELEX for characterization of human transcription factor binding specificities. Genome Res. 2010;20:861–873. doi: 10.1101/gr.100552.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolma A, et al. DNA-binding specificities of human transcription factors. Cell. 2013;152:327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Gordân R, et al. Curated collection of yeast transcription factor DNA binding specificity data reveals novel structural and gene regulatory insights. Genome Biol. 2011;12:R125. doi: 10.1186/gb-2011-12-12-r125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan YL, Fields S. Properties of the DNA-binding domain of the Saccharomyces cerevisiae STE12 protein. Mol Cell Biol. 1991;11:5910–5918. doi: 10.1128/mcb.11.12.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts CJ, et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873–880. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- 26.Heise B, et al. The TEA transcription factor Tec1 confers promoter-specific gene regulation by Ste12-dependent and -independent mechanisms. Eukaryot Cell. 2010;9:514–531. doi: 10.1128/EC.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson KA, et al. Two regulators of Ste12p inhibit pheromone-responsive transcription by separate mechanisms. Mol Cell Biol. 2000;20:4199–4209. doi: 10.1128/mcb.20.12.4199-4209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler DM, Fields S. Deep mutational scanning: A new style of protein science. Nat Methods. 2014;11:801–807. doi: 10.1038/nmeth.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 30.Ryan O, et al. Global gene deletion analysis exploring yeast filamentous growth. Science. 2012;337:1353–1356. doi: 10.1126/science.1224339. [DOI] [PubMed] [Google Scholar]
- 31.Shoval O, et al. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336:1157–1160. doi: 10.1126/science.1217405. [DOI] [PubMed] [Google Scholar]
- 32.Jones SK, Jr, Bennett RJ. Fungal mating pheromones: Choreographing the dating game. Fungal Genet Biol. 2011;48:668–676. doi: 10.1016/j.fgb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser JA, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 34.Sangster TA, Lindquist S, Queitsch C. Under cover: Causes, effects and implications of Hsp90-mediated genetic capacitance. BioEssays. 2004;26:348–362. doi: 10.1002/bies.20020. [DOI] [PubMed] [Google Scholar]
- 35.Jarosz DF, Taipale M, Lindquist S. Protein homeostasis and the phenotypic manifestation of genetic diversity: Principles and mechanisms. Annu Rev Genet. 2010;44:189–216. doi: 10.1146/annurev.genet.40.110405.090412. [DOI] [PubMed] [Google Scholar]
- 36.Jarosz DF, Lindquist S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science. 2010;330:1820–1824. doi: 10.1126/science.1195487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangster TA, et al. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci USA. 2008;105:2963–2968. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karras GI, et al. HSP90 shapes the consequences of human genetic variation. Cell. 2017;168:856–866.e12. doi: 10.1016/j.cell.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahni N, et al. Widespread macromolecular interaction perturbations in human genetic disorders. Cell. 2015;161:647–660. doi: 10.1016/j.cell.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrera LA, et al. Survey of variation in human transcription factors reveals prevalent DNA binding changes. Science. 2016;351:1450–1454. doi: 10.1126/science.aad2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mumberg D, Müller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded Carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 44.Leu JY, Murray AW. Experimental evolution of mating discrimination in budding yeast. Curr Biol. 2006;16:280–286. doi: 10.1016/j.cub.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Posas F, Witten EA, Saito H. Requirement of STE50 for osmostress-induced activation of the STE11 mitogen-activated protein kinase kinase kinase in the high-osmolarity glycerol response pathway. Mol Cell Biol. 1998;18:5788–5796. doi: 10.1128/mcb.18.10.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin AF, et al. 2016. Enrich2: A statistical framework for analyzing deep mutational scanning data. bioRxiv, 10.1101/075150.
- 47.Araya CL, et al. A fundamental protein property, thermodynamic stability, revealed solely from large-scale measurements of protein function. Proc Natl Acad Sci USA. 2012;109:16858–16863. doi: 10.1073/pnas.1209751109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuperus JT, Lo RS, Shumaker L, Proctor J, Fields S. A tetO toolkit to alter expression of genes in Saccharomyces cerevisiae. ACS Synth Biol. 2015;4:842–852. doi: 10.1021/sb500363y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su TC, Tamarkina E, Sadowski I. Organizational constraints on Ste12 cis-elements for a pheromone response in Saccharomyces cerevisiae. FEBS J. 2010;277:3235–3248. doi: 10.1111/j.1742-4658.2010.07728.x. [DOI] [PubMed] [Google Scholar]
- 50.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 53.Grant CE, Bailey TL, Noble WS. FIMO: Scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
High-throughput sequencing reads have been submitted to National Center for Biotechnology Information Sequence Read Archive under accession nos. SRR5408956–SRR5408965. Datasets with calculated enrichment scores for each tested variant in each condition, along with positional mean scores, and scores from binding site library selections are provided in Datasets S1–S5.