Significance
Refractory organic matter found in volatile-rich asteroidal materials essentially comprise the elements C, H, O, N, and S, which are thought to be important building blocks for life. Characterizing the origin(s) of these organics thus constitutes a key step to constrain the origin of life on Earth and appraise the habitability potential of other worlds. However, how and where these organics formed are still highly debated. In this study, we have determined the oxygen isotope composition of refractory organics from two families of carbonaceous chondrites. These data suggest that these organics formed in the nascent Solar System, possibly through chemical reactions occurring in the disk surrounding the young Sun.
Keywords: carbonaceous chondrites, organic matter, oxygen isotopes, protosolar nebula, secondary ion mass spectrometry
Abstract
Dust grains of organic matter were the main reservoir of C and N in the forming Solar System and are thus considered to be an essential ingredient for the emergence of life. However, the physical environment and the chemical mechanisms at the origin of these organic grains are still highly debated. In this study, we report high-precision triple oxygen isotope composition for insoluble organic matter isolated from three emblematic carbonaceous chondrites, Orgueil, Murchison, and Cold Bokkeveld. These results suggest that the O isotope composition of carbonaceous chondrite insoluble organic matter falls on a slope 1 correlation line in the triple oxygen isotope diagram. The lack of detectable mass-dependent O isotopic fractionation, indicated by the slope 1 line, suggests that the bulk of carbonaceous chondrite organics did not form on asteroidal parent bodies during low-temperature hydrothermal events. On the other hand, these O isotope data, together with the H and N isotope characteristics of insoluble organic matter, may indicate that parent bodies of different carbonaceous chondrite types largely accreted organics formed locally in the protosolar nebula, possibly by photochemical dissociation of C-rich precursors.
Type 1–2 carbonaceous chondrites (CCs) contain several weight percent (wt%) carbon that mostly occurs as small patches of organic matter (OM) dispersed in the fine-grained matrix (1). Because this OM possibly played a key role in the development of life on the early Earth, its molecular structure and its chemical and isotopic compositions have been extensively investigated (see ref. 2 and references therein for a recent review). Despite this profusion of structural, chemical, and isotopic information, the question of whether CC OM formed in the cold interstellar medium (e.g., refs. 3–5), formed in the protosolar nebula (PSN) (e.g., refs. 6–9), or is a product of organic synthesis during hydrothermalism on CC parent bodies (e.g., ref. 10) remains highly debated, notably because the extent of chemical and isotopic alteration of OM during secondary processes on CC parent bodies is unclear (11–15).
Oxygen is the third most abundant element in the Solar System and has three stable isotopes, 16O, 17O, and 18O. Because different fractionation laws govern interplanetary and intraplanetary processes (e.g., ref. 16), the oxygen three-isotope system can provide information that cannot be accessed using other two-isotope systems of light elements such as H, C, and N. In planetary bodies, variations of the 17O/16O and 18O/16O ratios almost always obey the mass-dependent relationship δ17O ∼ 0.52 × δ18O, while oxygen isotope abundance variations between Solar System gas and solids are primarily governed by the mass-independent relationship δ17O ∼ 1.0 × δ18O (15). [This δ-notation represents deviations in parts per 1,000 (‰) of the 17,18O/16O ratios relative to those of the standard mean ocean water (SMOW), according to the equation δ17,18O = [(17,18O/16O)sample/(17,18O/16O)SMOW − 1] × 1,000.] Much of our understanding of how our Solar System formed and evolved is thus based on O isotope studies of meteoritic materials (e.g., refs. 16 and 17), and this should apply to CC OM since it contains ∼10–25 wt% O (11, 12).
However, determining the O isotope composition of OM is challenging since it tends to be intimately mixed with O-rich silicates and oxides at nanoscale to microscale in carbonaceous chondrites (e.g., ref. 18). Acid maceration used to isolate the insoluble OM (IOM) fraction from whole-rock samples removes most of the silicates but is less effective at dissolving sulfides and some refractory O-bearing oxides such as chromite, spinel, hibonite, or corundum. Bulk pyrolysis O isotope analysis of IOM is thus susceptible to contamination by residual mineral inclusions. To constrain the triple O isotope composition of CC IOM, we integrate here high spatial resolution secondary ion mass spectrometry (SIMS) data obtained using NanoSIMS with high-precision 17,18O/16O isotope ratios obtained using large geometry multicollector IMS 1270/80 ion probes (referred to as L-SIMS in the following). For each L-SIMS O isotope analysis, measurement of 28Si, 32S, and 56Fe16O intensities allowed a first-order filtering of data for which the O signals were largely affected by contamination by residual silicate and/or oxide phases (see Materials and Methods for details). The results presented here thus provide high-precision triple O isotope estimates for IOM residues isolated from two emblematic CC falls, the Ivuna-type (CI) Orgueil meteorite and the Mighei-type (CM) Murchison meteorite.
Results
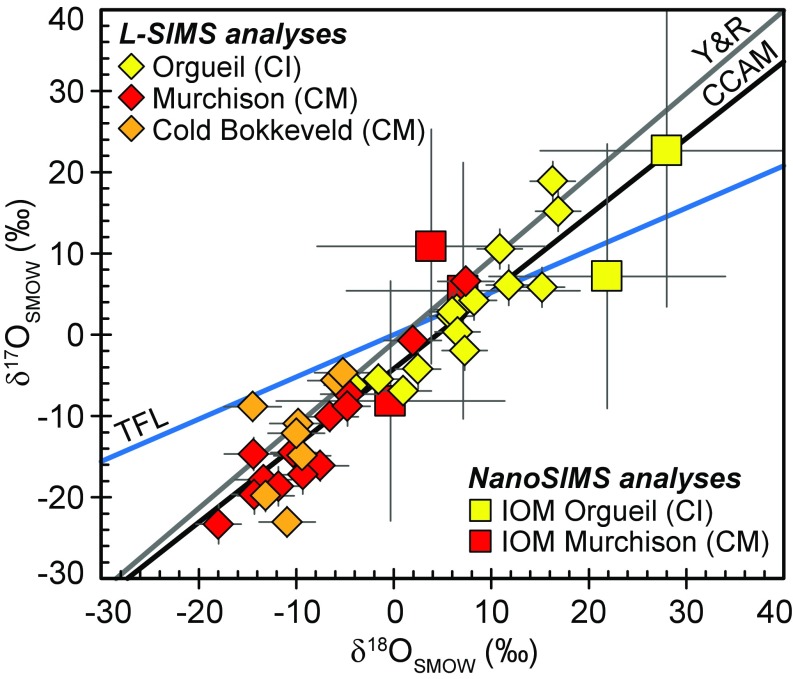
The δ17O and δ18O values measured by L-SIMS in the Orgueil, Murchison, and Cold Bokkeveld IOM residues range between −23.3 ± 2.4‰ and +18.9 ± 2.4‰ and −18.0 ± 2.3‰ and +16.9 ± 2.3‰ (uncertainties reported at 2σ), respectively (Fig. 1 and SI Appendix, Table S1). Least-square regression through all of the data yields a line defined by δ17O = 1.00 (±0.14) × δ18O − 3.78 (±1.35) (95% confidence level, n = 36, r2 = 0.86), which is indistinguishable from the relationship known as the carbonaceous chondrite anhydrous mineral (CCAM) line (δ17O = 0.95 × δ18O − 4.18) (Fig. 1).
Fig. 1.
δ17,18O values obtained in Orgueil, Murchison, and Cold Bokkeveld IOM residues. The terrestrial fractionation line (TFL), the carbonaceous chondrite anhydrous mineral line (CCAM) (19), and the Young and Russell line (Y&R) (20) are also represented.
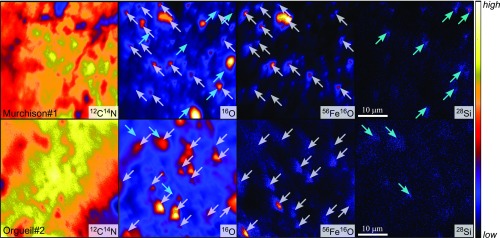
The NanoSIMS data allow determining O isotope ratios with larger uncertainties than those obtained by L-SIMS, but with higher spatial resolution, that is, over region of interests (ROI) that can be selected from ion imaging to minimize the effect of residual oxides and/or silicates, located through analysis of 28Si and 56Fe16O simultaneously with O isotopes. The NanoSIMS analyses obtained over 40-μm2 areas in Murchison and Orgueil IOM residues show little 28Si hot spots but more abundant micrometer-size 16O-enriched areas (Fig. 2 and SI Appendix, Fig. S2). The 16O hot spots are generally associated with 56Fe16O hot spots, suggesting that magnetite and/or chromite are the main mineral phases that have resisted acid maceration treatments.
Fig. 2.
NanoSIMS images showing the distribution of 12C14N, 16O, 56Fe16O, and 28Si secondary ion species in Murchison and Orgueil IOM acid maceration residues. White and cyan arrows indicate higher 56Fe16O and 28Si intensities, respectively.
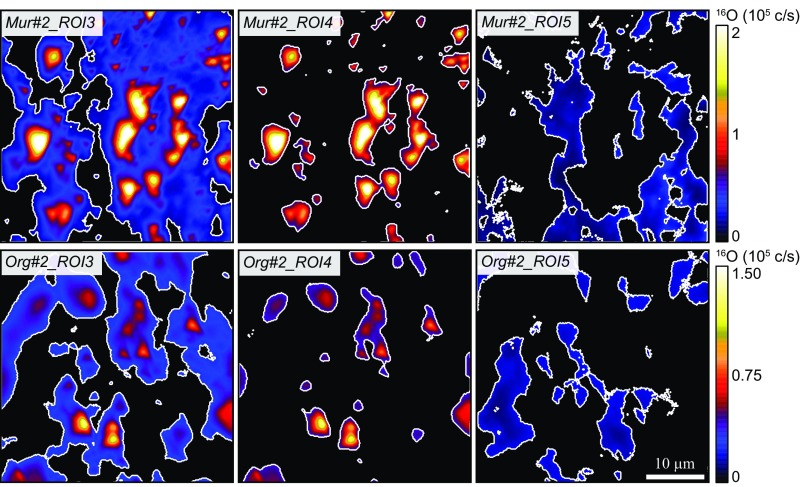
In terms of surface, these 16O-enriched ROIs represent 16–18% and 12–21% of the total 40 × 40-μm areas analyzed in Murchison and Orgueil, respectively, while pure IOM ROIs, defined based on 16O intensity maps (Materials and Methods), comprise 21–31% and 18–20% of the Murchison- and Orgueil-analyzed areas, respectively (Fig. 3 and SI Appendix, Table S2). The processed NanoSIMS data indicate that the δ17,18O values of the residual O-rich inclusions in Murchison tend to be ∼20–40‰ lower than the δ17,18O values obtained for O-rich inclusions in Orgueil (SI Appendix, Fig. S3 and Table S2). The δ17,18O values obtained for Murchison pure IOM areas are around 0 to +10‰, while Orgueil pure IOM areas tend to have higher δ17,18O values around +10 to +30‰ (Fig. 1). Overall, the O isotope composition estimated for Murchison and Orgueil pure IOM using NanoSIMS are roughly consistent with the 17,18O-rich end of the trends defined by Murchison and Orgueil acid residues L-SIMS data (Fig. 1).
Fig. 3.
Examples of the ROIs defined on 16O ion images for one analysis each of Murchison and Orgueil acid residues. ROI#3 (Left) corresponds to intermediate O intensity, ROI#4 (Center) corresponds to O hot spots, and ROI#5 (Right) corresponds to pure IOM (see text for details).
Discussion
Assessing the Level of Contamination from Residuals Microinclusions in IOM.
The main challenge in determining the O isotope composition of IOM isolated from carbonaceous chondrites is related to the presence of residual nanoinclusions to microinclusions that have resisted acid maceration, as shown here by NanoSIMS imaging. The consistency between O isotope values estimated for pure IOM based on high-resolution NanoSIMS analyses and the most 17,18O-rich compositions obtained by L-SIMS for Murchison and Orgueil acid residues, respectively, suggest that the latter provide an accurate estimate for the O isotope composition of Murchison and Orgueil IOM. We thus consider here that the average values calculated from the two most 17,18O-rich compositions measured by L-SIMS on both Murchison and Orgueil provide us with the best estimates for the O isotope compositions of pure IOM end-members in these samples. This yields δ18O = +4.7 ± 7.7‰ (2 SD) and δ17O = +2.9 ± 10.3‰ (2 SD) for Murchison IOM and δ18O = +16.6 ± 0.8‰ (2 SD) and δ17O = +17.0 ± 5.2‰ (2 SD) for Orgueil IOM.
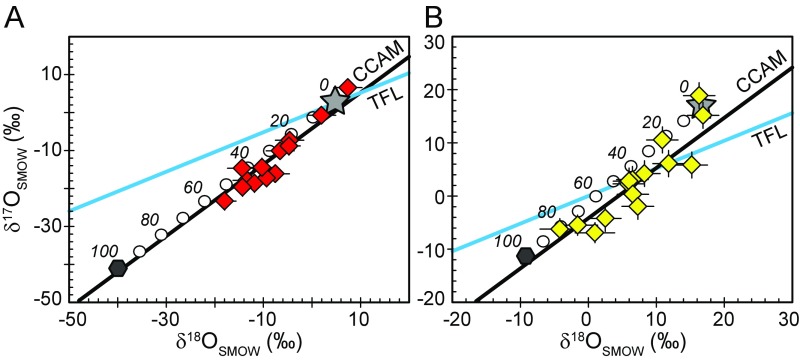
Fig. 4 presents the results of mixing calculations, where O isotope compositions have been calculated for mixed compositions between the IOM δ17,18O values calculated from the 17,18O-rich L-SIMS data for Murchison and Orgueil and the most negative δ17,18O values measured in mineral phases in both Murchison (approximately −40‰ for spinel; ref. 21) and Orgueil (approximately −10‰ for olivine; ref. 22). These calculations show that up to ∼50% and ∼80% contamination of the Murchison and Orgueil O signals, respectively, by 16O-rich residual mineral phases could explain the spread of δ17,18O values measured by L-SIMS (Fig. 4). Such levels of contamination are consistent with NanoSIMS data obtained over 40-μm2 areas selected randomly in Murchison and Orgueil acid residues (SI Appendix, Fig. S3). In addition, these calculations are also consistent with O-rich contaminants in Murchison being characterized by δ17,18O values ∼20–40‰ lower than those of O-rich inclusions in Orgueil (SI Appendix, Fig. S3 and Table S2), even though it is not possible to estimate their true δ17,18O values corrected for NanoSIMS instrumental mass fractionation since their exact mineralogy was not determined.
Fig. 4.
Calculated O isotope values for mixed compositions between the estimated IOM δ17,18O values (gray stars) in (A) Murchison and (B) Orgueil acid residues and the most negative δ17,18O values measured in mineral phases in both Murchison (approximately −40‰ for spinel; ref. 21) and Orgueil (approximately −10‰ for olivine; ref. 22) (dark gray hexagons). White dots represent 10% mixing intervals, and 20% mixing intervals are given on the diagrams from 0 to 100% mineral contribution.
Comparison of Bulk and SIMS-Derived Oxygen Isotope Data.
Because O-rich residual contaminants have lower δ17,18O values than IOM, it is important to note that the pure IOM O isotope compositions calculated from 17,18O-rich L-SIMS analyses provide minimum estimates. The δ18O and δ17O values estimated for Orgueil IOM by L-SIMS (+16.6 ± 0.8‰ and +17.0 ± 5.2‰, respectively) are higher than the bulk δ18O and δ17O values determined by Halbout et al. (23) (+6.0 ± 0.8‰ and +3.4 ± 0.4‰, respectively), which may indicate that their O isotope ratios were also affected by contamination issues. On the other hand, the δ18O value estimated for Orgueil IOM by L-SIMS is in good agreement with the bulk δ18O value of +14.5 ± 0.6‰ determined by Alexander et al. (11). For Murchison IOM, the δ18O value calculated from L-SIMS analyses of +4.7 ± 7.7‰ (2 SD) is lower than the bulk IOM δ18O values of +13.8 ± 1.6‰ (n = 2, 2 SD) determined by Alexander et al. (11, 12). These authors take advantage of the oxidation of sulfides in air over several days to gradually remove them from acid residues (11). Modification of the O isotope composition of organic O-bearing chemical functions during such oxidation of acid residues in air may be a possibility to explain the discrepancy between bulk and L-SIMS δ18O values obtained for Murchison IOM, since atmospheric O2 has a δ18O value of approximately +23 to 24‰ (24, 25). It is also possible that our L-SIMS δ18O estimate for Murchison IOM does not correspond to pure IOM but to IOM still contaminated by minute amounts of O-rich residual inclusions. At this stage, it is not possible to favor one of these two hypotheses over the other. Interestingly, δ18O values obtained on CM chondrite bulk IOM are characterized by much larger variations (from −3.7‰ in Essebi to +14.4‰ in Murchison, both meteorites being observed falls) compared with other chondrite types (11, 12), which may either be related to variable contamination issues of bulk analyses or indicate that CM chondrites accreted IOM with variable O isotope compositions. Clearly, further bulk and in situ investigations are required to fully explore this issue.
Triple Oxygen Isotope Constraints on the Origin of Carbonaceous Chondrite IOM.
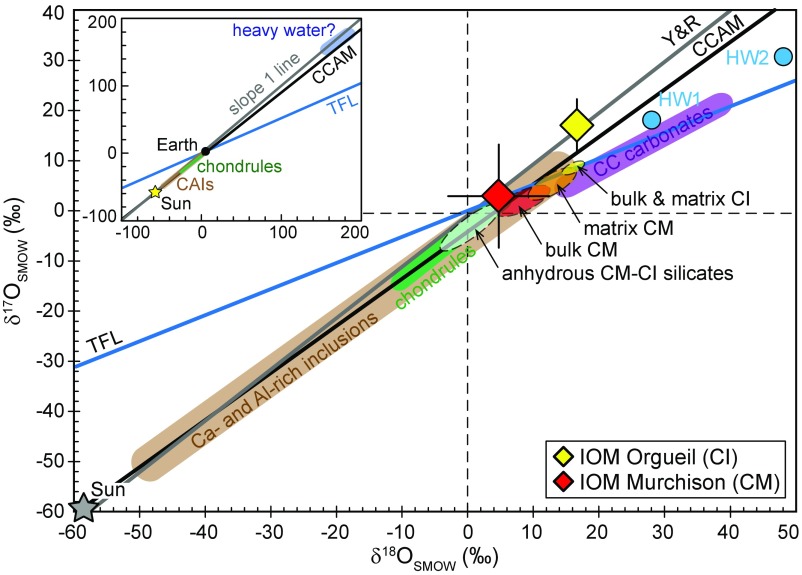
The O isotope compositions estimated for Murchison IOM (δ17,18O = approximately +3–5‰) and Orgueil IOM (δ17,18O = approximately +17‰) fall on the slope 1 line in a δ17O vs. δ18O diagram (Fig. 5). As highlighted by Alexander et al. (11), δ18O values of the CI and CM chondrite IOM are similar to those of their matrix component (Fig. 5). However, the bulk and matrix δ17O values obtained in CI chondrites appear to be lower compared with the δ17O of Orgueil IOM (Fig. 5). These results, combined with the O isotope composition of CI–CM chondrite original anhydrous silicates, of matrix silicates, and of primordial alteration waters (which are thought to be similar for CI and CM chondrite parent bodies; refs. 21 and 26–29), thus seem to rule out scenarios in which the O isotope composition of O-bearing functional groups in CI chondrite IOM resulted from O isotope exchange between organic precursors and silicate components during aqueous alteration on the CI chondrite parent body (Fig. 5). On the other hand, because of the larger uncertainty associated with the δ17,18O estimates for Murchison IOM, its formation during hydrothermal alteration on the CM parent body cannot be totally excluded.
Fig. 5.
O isotope compositions measured by L-SIMS on IOM residues isolated from the Orgueil and Murchison carbonaceous chondrites. The terrestrial fractionation line (TFL), the CCAM line, and the Y&R line are also represented, together with the O isotope compositions of the Sun (17), of CI and CM chondrite components (bulk, matrix, and anhydrous silicates; refs. 21, 22, 26, and 27), of carbonaceous chondrite Ca- and Al-rich inclusions (30–32), chondrules (33–37), and carbonates (38). Estimates for the O isotope composition of CM chondrite primordial water (HW1 and HW2) are from ref. 27 for HW1 and ref. 38 for HW2.
The limited existing O isotope dataset obtained on a handful of CC meteorites so far suggests that IOM in the CI chondrite Orgueil tends to be enriched in 17,18O compared with IOM in the CM chondrite Murchison (Fig. 5). Interestingly, this relationship is consistent with the variations of average H and N isotope compositions in CM and CI IOM, where CI IOM is enriched in D and 15N compared with CM IOM (11, 12). The D and 15N enrichments commonly observed in CC IOM have generally been attributed to low-temperature processes (<150 K) such as ion–molecule reactions taking place in dense interstellar media or at the surface of the PSN. However, recent experimental studies focused on the IOM molecular structure (9, 39), its bulk D/H (40) and the occurrence of D/H hot spots (41), and its noble gas isotope signatures (9), have argued that CC IOM could be produced by photochemical reactions involving organic radicals and taking place in the PSN regions where solar UV irradiation would have induced dissociation of CxHy molecules. Experiments have shown that photochemical reactions can produce mass-independent O isotope anomalies (e.g., refs. 42–44). One could thus postulate that the mass-independent isotopic fractionation of oxygen isotopes in CC IOM also resulted from chemical reactions involving radical chemistry of CHON-bearing species in the PSN. If correct, such an effect now remains to be experimentally documented in a setting relevant to organics formation.
Cosmochemical Implications.
The O isotope compositions estimated for CI–CM chondrite IOM fall on a slope 1 line in a δ17O vs. δ18O diagram, which, at a larger scale, describes the O isotope variations of most Solar System objects such as the Sun, high-temperature phases (i.e., CAI and chondrules) formed during the first few million years of Solar System evolution, and terrestrial planets for example (Fig. 5). However, the origin of this slope 1 line in planetary materials is still unknown. A possible mechanism involves self-shielding of 16O-rich CO gas by UV light during photodissociation (e.g., ref. 42), but whether this occurred in the presolar molecular cloud (45) or in the PSN (46) remains debated. O isotope compositions of CC IOM appear to fall on a slope 1 line; it could thus be argued that oxygen contained in CC IOM derived from a combination of that found in 16O-rich CO and 17,18O-rich H2O molecules formed as a result of self-shielding. If the different CC parent bodies accreted IOM sourced from a common carbonaceous reservoir, formed in the presolar molecular cloud, one may expect the various asteroidal parent bodies to have accreted presolar IOM grains characterized by similar O isotope compositions. The different triple O isotope compositions for Orgueil and Murchison IOM, dispersed along the slope 1 line, do not seem to favor such a scenario. Alternatively, and considering that Murchison and Orgueil IOM O isotope compositions are representative of those of CM and CI IOM in general, the observation that δ17,18OCM IOM < δ17,18OCI IOM, consistently with what has been measured for H and N isotope data (δDCM IOM < δDCI IOM and δ15NCM IOM < δ15NCI IOM; refs. 11 and 12), may indicate that carbonaceous asteroids accreted IOM that formed locally in the PSN through photochemical radical chemistry involving CHON-bearing species (9, 41). Because of its elevated δD and δ15N values, it has been proposed that CR IOM could represent the least processed IOM component accreted in carbonaceous asteroids (see discussion in ref. 2). Based on the observed relationship between H, N, and O isotope compositions in CI and CM chondrite IOM, we would expect CR chondrite IOM to have δ17,18O values higher than those of CI chondrite IOM. Determining with high precision the triple O isotope composition of CR chondrite IOM would thus provide important constraints to further explore the formation mechanism(s) of CC IOM.
Materials and Methods
Organic Matter Isolation.
IOM was isolated from the Orgueil, Murchison, and Cold Bokkeveld carbonaceous chondrite meteorites through successive demineralization using a HF/HCl acidic treatment (47). Powdered meteorite samples were first stirred at room temperature in water, followed by CH2Cl2/MeOH [2/1 (vol/vol)], to remove soluble organic compounds. Carbonates were then removed at room temperature using 6 M HCl to minimize the formation of fluorides during HF/HCl maceration. Samples were then centrifuged and washed with distilled water until reaching neutrality. Isolation of IOM was achieved through acid maceration at room temperature in a HF/HCl mixture [2/1 (vol/vol)]. Samples were further centrifuged and washed with distilled water to reach neutrality. Neoformed fluorides were then degraded using 6 M HCl at 60 °C for 24 h. After HCl hot-acid maceration, IOM residues were washed with distilled water until reaching neutrality and thoroughly dried. For SIMS investigations, a few milligrams of IOM samples were pressed into high-purity indium (99.999%) and carbon coated.
IMS 1270/80 Secondary Ion Mass Spectrometry.
Triple O isotope compositions of the IOM samples were measured using the CAMECA IMS 1270 E7 and 1280 HR2 ion probe instruments at the Centre de Recherches Pétrographiques et Géochimiques in Nancy, France, over several analytical sessions, using identical analytical protocols. Negative 16O−, 17O−, and 18O− secondary ions produced using a ∼10-nA Cs+ primary beam, accelerated at 10 kV and rastered over ∼20-μm diameter areas, were measured in multicollection mode with one Faraday cup (FC) on the L′2 trolley for 16O− and two electron multipliers (EMs) for 17O− (central EM) and 18O− (H2 EM). To maximize peak flatness, entrance and exit slits were adjusted to achieve a mass resolving power of ∼8,000 for 17O− on the central EM and ∼2,500 on the off-axis L′2 FC and H2 EM (using slit #1 of the off-axis collectors). OM samples contain significant amounts of OH (average 16OH−/17O−, ∼97 ± 17, ∼120 ± 31, and ∼121 ± 20 in Orgueil IOM, Murchison IOM, and Cold Bokkeveld IOM, respectively), and the protocol used did not completely eliminate contribution from the 16OH− tail on the 17O− peaks. To quantify this contribution and adequately correct the measured 17O/16O ratios, we assumed that the 16OH− peak was symmetrical, calculated the mass difference between the center of the 17O− (16.9991 amu) and 16OH− (17.0027 amu) peaks, and counted the 16OH− tail intensity at mass 17.0063 amu (mass 16OH− + [mass 16OH− − mass 17O−]) for 50 s before and after each analysis. The 16OH− tail/peak ratios were ∼1.5 ± 0.5 × 10−5 in Murchison IOM, ∼1.1 ± 0.1 × 10−5 in Cold Bokkeveld, and ∼1.9 ± 0.5 × 10−5 in Orgueil IOM. This resulted in correction of the measured δ17O values by 0.7–2.8‰ in Murchison IOM, 1.3–1.8‰ in Cold Bokkeveld IOM, and 0.8–2.4‰ in Orgueil IOM (SI Appendix, Table S1). For each analysis, the FC background was measured during presputtering. Dead time of the EMs was also calibrated once per analytical session. The total analysis time was 260 s (60-s presputtering and 40 cycles of 5 s each measurement time). Instrumental mass fractionation (IMF) for O isotope measurements in IOM samples was corrected by repeated analyses of our Clarno kerogen standard (δ18Obulk = 14.3 ± 0.1‰; ref. 48), for which we assumed a δ17Obulk of 7.4‰, that is, a terrestrial O isotope composition. Count rates obtained on the Clarno kerogen standard were 0.3–1.2 × 107 cps⋅nA−1 for 16O−, 1.1–4.5 × 103 cps⋅nA−1 for 17O− and 0.6–2.3 × 104 cps⋅nA−1 for 18O−, similar to those obtained on the IOM samples (0.3–3.1 × 107 cps⋅nA−1 for 16O−, 0.1–1.1 × 104 cps⋅nA−1 for 17O− and 0.5–5.5 × 104 cps⋅nA−1 for 18O−). The final uncertainties for individual δ17,18O values, reported in SI Appendix, Table S1 at the 2σ level, include uncertainties related to counting statistics associated with each individual analysis and the external reproducibility measured for δ17,18O values on the Clarno kerogen standard. Over three analytical sessions in February 2016, July 2016, and December 2016, we obtained a weighted average Δ17O of −0.1 ± 0.4‰ (95% confidence level, n = 66, mean square weighted deviation = 3.0) (SI Appendix, Fig. S1). We further tested our analytical protocol on the Silurian Zdanow terrestrial kerogen and obtained an average δ18OSIMS of 12.4 ± 4.6‰ (2 SD; n = 9), which is consistent with its bulk δ18O of 13.4 ± 0.2‰ (48). The Δ17O measured on Zdanow was 0.2 ± 1.8‰ (2 SD; n = 9), indicating that Zdanow sits on the terrestrial fractionation line (TFL), which shows that our L-SIMS protocol accurately measures the triple O isotope composition of organic residues.
The secondary species 12C1H, 16O, 28Si, 32S, and 56Fe16O were collected following O isotope analyses on the same analytical spots using the magnetic peak switching mode and a ∼10-nA Cs+ beam to identify and filter the IOM data largely affected by contamination by residual silicate and oxide phases (see details in refs. 48 and 49).
Nanoscale Secondary Ion Mass Spectrometry.
The triple O isotope composition of the Orgueil and Murchison IOM residues was also measured using the CAMECA NanoSIMS 50L ion probe instrument at The University of Manchester. Negative 16O−, 17O−, 18O−, 12C2−, 12C214N−, 28Si−, and 56Fe16O− secondary ion species produced using a ∼15-pA Cs+ primary beam, accelerated at 16 kV and rastered over 40 μm × 40-μm areas, were measured in multicollection mode on seven EMs. Before analysis, a ∼100-pA Cs+ primary beam was rastered over 50 × 50-μm areas for 5 min to clean the sample surface and reach sputtering equilibrium. To limit the 16OH− interference on the 17O− peak, a 10-μm-wide entrance slit (ES5) was used at the entrance of the mass analyzer, and a 150-μm-wide aperture slit (AS3) reduced the beam divergence, resulting in a mass resolving power of ∼8,000. An electron gun was used for charge compensation. Using these conditions, the count rates were 15,000–35,000 cps for 16O, 500–1,800 cps for 12C2, 3,000–10,000 cps for 12C14N, and 5–20 cps for 56Fe16O, ensuring no detector aging over the week-long analytical session. During the session, the vacuum in the analysis chamber remained constant at ∼3 × 10−10 mbar. For data acquisition, the 40-μm2 areas were divided in 256 × 256 pixels and between 320 and 600 frames were acquired at 1,000 μs/px, resulting in a total analysis time of 6–11 h per analysis. Automatic alignment of the secondary beam (EOS, Cy, and P2/P3) and of the peak positions was performed every 50 frames during each analysis based on scanning the 16O− peak. IMF for O isotope measurements in IOM samples was corrected by analyzing the same Clarno kerogen standard used for the L-SIMS analyses (see above). The final uncertainties for individual δ17,18O values, reported in SI Appendix, Table S2 at the 2σ level, include those related to counting statistics for each individual analysis and the external reproducibility measured for δ17,18O on the Clarno kerogen standard (±11.1‰ and ±11.9‰ for δ18O and δ17O, respectively; 2 SE, n = 3).
The NanoSIMS data were processed off-line using the l’Image software package (L. Nittler, Carnegie Institution of Washington, Washington, DC). A 44-ns dead time was applied to all EMs, and individual frames were binned into packages of 6–10 frames for each analysis to handle these large datasets more easily. ROIs were defined using lower and upper thresholds for the different species and comprised, for each analysis, the whole analyzed area, an area with intermediate 16O intensity, and a 16O-rich area corresponding to hot spots. A ROI of “pure IOM” was then defined by subtracting the ROI corresponding to the area with intermediate 16O intensity to the ROI corresponding to the whole analyzed area. The 17O/16O, 18O/16O, 12C14N/12C2, and 16O/12C2 ratios were calculated using l’Image.
All processed data are given in SI Appendix, Tables S1 and S2.
Supplementary Material
Acknowledgments
We thank S. Derenne for providing the Murchison IOM sample, N. Bouden and J. Villeneuve for their help with L-SIMS analyses in Nancy, and I. C. Lyon for his help with NanoSIMS analyses in Manchester. We also thank the anonymous referees for their constructive reviews. This work was supported by European Research Council Grant PaleoNanoLife [290861; to principal investigator (PI) F.R.], Agence Nationale de la Recherche Grant CRADLE (ANR-15-CE31-0004-01; to PI M.C.), and UK Science and Technology Facilities Council Grants ST/M001253/1 (to project co-investigator I. C. Lyon) and ST/P005225/1 (to PI R.T.). The NanoSIMS at The University of Manchester was funded by UK Research Partnership Investment Funding Manchester Research Partnership Investment Funding Round 2. This is Institut de Physique du Globe de Paris Contribution 3964 and Centre de Recherches Pétrographiques et Géochimiques Contribution 2598.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Raw data from this manuscript have been deposited in the Mendeley Data repository (dx.doi.org/10.17632/hwwsdz8997.1).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808101115/-/DCSupplemental.
References
- 1.Hayes JM. Organic constituents of meteorites—a review. Geochim Cosmochim Acta. 1967;31:1395–1440. [Google Scholar]
- 2.Alexander CMO’D, Cody GD, De Gregorio BT, Nittler LR, Stroud RM. The nature, origin and modification of insoluble organic matter in chondrites, the major source of Earth’s C and N. Chem Erde Geochem. 2017;77:227–256. doi: 10.1016/j.chemer.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert F, Epstein S. The concentration and isotopic composition of hydrogen, carbon and nitrogen in carbonaceous meteorites. Geochim Cosmochim Acta. 1982;46:81–95. [Google Scholar]
- 4.Yang J, Epstein S. Interstellar organic matter in meteorites. Geochim Cosmochim Acta. 1983;47:2199–2216. [Google Scholar]
- 5.Busemann H, et al. Interstellar chemistry recorded in organic matter from primitive meteorites. Science. 2006;312:727–730. doi: 10.1126/science.1123878. [DOI] [PubMed] [Google Scholar]
- 6.Remusat L, Palhol F, Robert F, Derenne S, France-Lanord C. Enrichment of deuterium in insoluble organic matter from primitive meteorites: A solar system origin? Earth Planet Sci Lett. 2006;243:15–25. [Google Scholar]
- 7.Gourier D, et al. Extreme deuterium enrichment of organic radicals in the Orgueil meteorite: Revisiting the interstellar interpretation? Geochim Cosmochim Acta. 2008;72:1914–1923. [Google Scholar]
- 8.Derenne S, Robert F. Model of molecular structure of the insoluble organic matter isolated from Murchison meteorite. Meteorit Planet Sci. 2010;45:1461–1475. [Google Scholar]
- 9.Kuga M, Marty B, Marrocchi Y, Tissandier L. Synthesis of refractory organic matter in the ionized gas phase of the solar nebula. Proc Natl Acad Sci USA. 2015;112:7129–7134. doi: 10.1073/pnas.1502796112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cody GD, et al. Establishing a molecular relationship between chondritic and cometary organic solids. Proc Natl Acad Sci USA. 2011;108:19171–19176. doi: 10.1073/pnas.1015913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander CMO’D, Fogel M, Yabuta H, Cody GD. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim Cosmochim Acta. 2007;71:4380–4403. [Google Scholar]
- 12.Alexander CMO’D, et al. Deuterium enrichments in chondritic macromolecular material—implications for the origin and evolution of organics, water and asteroids. Geochim Cosmochim Acta. 2010;74:4417–4437. [Google Scholar]
- 13.Orthous-Daunay FR, et al. Mid-infrared study of the molecular structure variability of insoluble organic matter from primitive chondrites. Icarus. 2013;223:534–543. [Google Scholar]
- 14.Quirico E, et al. Origin of insoluble organic matter in type 1 and 2 chondrites: New clues, new questions. Geochim Cosmochim Acta. 2014;136:80–99. [Google Scholar]
- 15.Hashiguchi M, Kobayashi S, Yurimoto H. Deuterium- and 15N-signatures of organic globules in Murchison and Northwest Africa 801 meteorites. Geochem J. 2015;49:377–391. [Google Scholar]
- 16.Clayton RN. Oxygen isotopes in meteorites. Annu Rev Earth Planet Sci. 1993;21:115–149. [Google Scholar]
- 17.McKeegan KD, et al. The oxygen isotopic composition of the Sun inferred from captured solar wind. Science. 2011;332:1528–1532. doi: 10.1126/science.1204636. [DOI] [PubMed] [Google Scholar]
- 18.Le Guillou C, Bernard S, Brearley AJ, Remusat L. Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim Cosmochim Acta. 2014;131:368–392. [Google Scholar]
- 19.Clayton RN, Onuma N, Grossman L, Mayeda TK. Distribution of the presolar component in Allende and other carbonaceous chondrites. Earth Planet Sci Lett. 1977;34:209–224. [Google Scholar]
- 20.Young ED, Russell SS. Oxygen reservoirs in the early solar nebula inferred from an Allende CAI. Science. 1998;282:452–455. [PubMed] [Google Scholar]
- 21.Clayton RN, Mayeda TK. The oxygen isotope record in Murchison and other carbonaceous chondrites. Earth Planet Sci Lett. 1984;67:151–161. [Google Scholar]
- 22.Leshin LA, Rubin AE, McKeegan KD. The oxygen isotopic composition of olivine and pyroxene from CI chondrites. Geochim Cosmochim Acta. 1997;61:835–845. [Google Scholar]
- 23.Halbout J, Robert F, Javoy M. Hydrogen and oxygen isotope compositions in kerogen from the Orgueil meteorite: Clues to a solar origin. Geochim Cosmochim Acta. 1990;54:1453–1462. [Google Scholar]
- 24.Kroopnick P, Craig H. Atmospheric oxygen: Isotopic composition and solubility fractionation. Science. 1972;175:54–55. doi: 10.1126/science.175.4017.54. [DOI] [PubMed] [Google Scholar]
- 25.Luz B, Barkan E. The isotopic composition of atmospheric oxygen. Global Biogeochem Cycles. 2011;25:GB3001. [Google Scholar]
- 26.Rowe MW, Clayton RN, Mayeda TK. Oxygen isotopes in separated components of CI and CM meteorites. Geochim Cosmochim Acta. 1994;58:5341–5347. [Google Scholar]
- 27.Clayton RN, Mayeda TK. Oxygen isotope studies of carbonaceous chondrites. Geochim Cosmochim Acta. 1999;63:2089–2104. [Google Scholar]
- 28.Fujiya W. Oxygen isotopic ratios of primordial water in carbonaceous chondrites. Earth Planet Sci Lett. 2018;481:264–272. [Google Scholar]
- 29.Marrocchi Y, Bekaert DV, Piani L. Origin and abundance of water in carbonaceous asteroids. Earth Planet Sci Lett. 2018;482:23–32. [Google Scholar]
- 30.Aléon J, El Goresy A, Zinner E. Oxygen isotope heterogeneities in the earliest protosolar gas recorded in a meteoritic calcium–aluminum-rich inclusion. Earth Planet Sci Lett. 2007;263:114–127. [Google Scholar]
- 31.Krot AN, et al. Oxygen isotopic compositions of Allende type C CAIs: Evidence for isotopic exchange during nebular melting and asteroidal metamorphism. Geochim Cosmochim Acta. 2008;72:2534–2555. [Google Scholar]
- 32.Bodénan JD, Starkey NA, Russell SS, Wright IP, Franchi IA. An oxygen isotope study of Wark-Lovering rims on type A CAIs in primitive carbonaceous chondrites. Earth Planet Sci Lett. 2014;401:327–336. [Google Scholar]
- 33.Clayton RN, et al. Oxygen isotopic compositions of chondrules in Allende and ordinary chondrites. In: King EA, editor. Chondrules and Their Origins. Lunar and Planetary Institute; Houston: 1983. pp. 37–43. [Google Scholar]
- 34.Rubin AE, Wasson JT, Clayton RN, Mayeda TK. Oxygen isotopes in chondrules and coarse-grained chondrule rims from the Allende meteorite. Earth Planet Sci Lett. 1990;96:247–255. [Google Scholar]
- 35.Weisberg MK, Prinz M, Clayton RN, Mayeda TK. The CR (Renazzo-type) carbonaceous chondrite group and its implications. Geochim Cosmochim Acta. 1993;57:1567–1586. [Google Scholar]
- 36.Jones RH, et al. Oxygen isotope heterogeneity in chondrules from the Mokoia CV3 carbonaceous chondrite. Geochim Cosmochim Acta. 2004;68:3423–3438. [Google Scholar]
- 37.Jenniskens P, et al. Sutter’s Mill Meteorite Consortium Radar-enabled recovery of the Sutter’s Mill meteorite, a carbonaceous chondrite regolith breccia. Science. 2012;338:1583–1587. doi: 10.1126/science.1227163. [DOI] [PubMed] [Google Scholar]
- 38.Verdier-Paoletti MJ, et al. Oxygen isotope constraints on the alteration temperatures of CM chondrites. Earth Planet Sci Lett. 2017;458:273–281. [Google Scholar]
- 39.Biron K, Derenne S, Robert F, Rouzaud JN. Toward an experimental synthesis of the chondritic insoluble organic matter. Meteorit Planet Sci. 2015;50:1408–1422. [Google Scholar]
- 40.Laurent B, et al. The deuterium/hydrogen distribution in chondritic organic matter attests to early ionizing irradiation. Nat Commun. 2015;6:8567. doi: 10.1038/ncomms9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert F, et al. Hydrogen isotope fractionation in methane plasma. Proc Natl Acad Sci USA. 2017;114:870–874. doi: 10.1073/pnas.1615767114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiemens MH, Heidenreich JE., 3rd The mass-independent fractionation of oxygen: A novel isotope effect and its possible cosmochemical implications. Science. 1983;219:1073–1075. doi: 10.1126/science.219.4588.1073. [DOI] [PubMed] [Google Scholar]
- 43.Chakraborty S, Ahmed M, Jackson TL, Thiemens MH. Experimental test of self-shielding in vacuum ultraviolet photodissociation of CO. Science. 2008;321:1328–1331. doi: 10.1126/science.1159178. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty S, Yanchulova P, Thiemens MH. Mass-independent oxygen isotopic partitioning during gas-phase SiO2 formation. Science. 2013;342:463–466. doi: 10.1126/science.1242237. [DOI] [PubMed] [Google Scholar]
- 45.Yurimoto H, Kuramoto K. Molecular cloud origin for the oxygen isotope heterogeneity in the solar system. Science. 2004;305:1763–1766. doi: 10.1126/science.1100989. [DOI] [PubMed] [Google Scholar]
- 46.Lyons JR, Young ED. CO self-shielding as the origin of oxygen isotope anomalies in the early solar nebula. Nature. 2005;435:317–320. doi: 10.1038/nature03557. [DOI] [PubMed] [Google Scholar]
- 47.Durand B, Nicaise G. Procedures for kerogen isolation. In: Durand B, editor. Kerogen: Insoluble Organic Matter from Sedimentary Rocks. Technip; Paris: 1980. pp. 35–54. [Google Scholar]
- 48.Tartèse R, Chaussidon M, Gurenko A, Delarue F, Robert F. In situ oxygen isotope analysis of fossil organic matter. Geochim Cosmochim Acta. 2016;182:24–39. [Google Scholar]
- 49.Tartèse R, Chaussidon M, Gurenko A, Delarue F, Robert F. Warm Archean oceans reconstructed from oxygen isotope composition of early-life remnants. Geochem Perspect Lett. 2017;3:55–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.