Abstract
Nanoparticles (NPs), introduced into a biological environment, accumulate a coating of biomolecules or biocorona (BC). Although the BC has toxicological and pharmacological consequences, the effects of inter-individual variability and exercise on NP-BC formation are unknown. We hypothesized that NPs incubated in plasma form distinct BCs between individuals, and exercise causes additional intra-individual alterations. 20nm iron oxide (Fe3O4) NPs were incubated in pre- or post-exercise plasma ex vivo, and proteomics was utilized to evaluate BC components. Analysis demonstrated distinct BC formation between individuals, while exercise was found to enhance NP-BC complexity. Abundance differences of NP-BC proteins were determined between individuals and resulting from exercise. Differential human macrophage response was identified due to NP-BC variability. These findings demonstrate that individuals form unique BCs and that exercise influences NP-biomolecule interactions. An understanding of NP-biomolecule interactions is necessary for elucidation of mechanisms responsible for variations in human responses to NP exposures and/or nano-based therapies.
Keywords: Proteomics, Nanotoxicology, Cholesterol, Triglycerides, Exercise, Macrophages
1. Introduction
Nanoparticles (NPs) have the capacity to revolutionize a multitude of technologies across a number of fields, including electronics, consumer products, textiles, biomedicine, and others. Iron oxide (Fe3o4) NPs specifically have the ability to be utilized in the field of biomedicine as MRI contrast agents, treatments for anemia, magnetic sensing probes, and drug delivery agents (Babes et al., 1999; Ghazanfari et al., 2016; Jain et al., 2005; Neuberger et al., 2005). When NPs enter biological environments, such as the circulatory system, a coating of biomolecules forms as proteins, lipids, and other compounds adsorb to the NP surface. This coating or biocorona (BC) imparts a new interactive interface thus altering NP physicochemical properties (hydrodynamic size, ζ-potential, and dissolution), resulting in modified NP functionality as well as cellular and biological responses (Clift et al., 2010; Maiorano et al., 2010; Monopoli et al., 2012; Shannahan et al., 2015b; Walczyk et al., 2010). BC-induced variations in cellular interactions will likely impact the use of NPs for biomedical applications by modifying biodistribution, clearance, immune response, and toxicity (Kreuter, 2013; Momet et al., 2004; Tenzer et al., 2013). Specifically, addition of the BC to superparamagnetic Fe3o4 NPs decreases their effectiveness as MRI contrast agents and drug delivery vehicles (Amiri et al., 2013; Gupta and Gupta, 2005). In order to fully utilize NP-based therapeutics, it is necessary to first understand these initial NP- biomolecule interactions and their biological implications.
The formation of the NP-BC is governed by NP physicochemical properties, time, and the biological environment. To date, the majority of investigations have examined specific NP properties (composition, charge, size, surface coating, defects, etc.) and their role in NP-BC formation (Jedlovszky-Hajdu et al., 2012; Lundqvist et al., 2008; Monopoli et al., 2011; Raghavendra et al., 2017; Walkey and Chan, 2012). Few studies, however, have evaluated the impact of variations in the biological environment (Raghavendra et al., 2017; Shannahan, 2017). Due to their many biomedical applications, NPs will be utilized in highly variable biological environments, which may impact their functionality. Specifically, individual variations in the biomolecular composition within the circulation are of concern for Fe3o4 NPs. Numerous conditions and factors can contribute to variability in circulating macromolecules, including underlying disease state, gender, diet, and others (Gordon et al., 1977; Jenkins et al., 1993; Murphy, 2014; Tran et al., 1983). Exercise, which is one of the focuses of our current study, is known to alter circulating biomolecules differently based on the type, duration, and intensity of the exercise (Balfoussia et al., 2014; Dreyer et al., 2006; Kraus et al., 2002; Tran et al., 1983). Ultimately, the variability of biological environments present within our population, as well as activity-induced alterations in circulating biomolecules, could modify BC formation and result in differential biological interactions.
In our current study, we examined differences in the formation of the Fe3o4 NP-BC due to interindividual variations as well as exercise-induced intra-individual variability by utilizing human plasma samples. Further, a human macrophage cell line was utilized to assess the toxicological consequences (cellular association, cytotoxicity, and inflammatory activation) of distinct Fe3o4 NP-BCs. In order to perform this investigation, 10 human subjects who did not exercise regularly were recruited and blood samples were collected prior to and following a 7-day exercise regimen. Overall, this descriptive study was designed to demonstrate potential differences between individuals’ NP-BCs, as well as the influence of exercise. By elucidating possible variability in the formation of the NP-BC and consequential alterations in immune cell interactions, individual susceptibility to NP-induced health effects may be identified and mitigated.
2. Materials and Methods
2.1. Blood Collection.
Fasting plasma samples were isolated from blood extracted from human subjects (seven male, three female) between the ages of 21–32 y who, according to a self-survey, did not smoke or exercise regularly (Table 1). Subjects were exercised once a day at 70% of maximum for 45 minutes on a stationary exercise bicycle for 7 consecutive days. On days 2, 4, and 6, subjects also performed 3 sets (8–12 repetitions at 80% of maximum) of leg press resistance exercise. Subjects were instructed to not modify any additional aspects of their lifestyle, including diet, for the duration of the study. Fasting blood was again collected from each subject on day 8 at least 12–14 hours after the final exercise session. After each collection, blood was treated with heparin to prevent clotting. The Biomedical Institutional Review Board at Purdue University approved the study protocol, recruitment materials, and consent forms. All study participants gave informed consent and received monetary compensation for their participation.
Table 1.
Subject Blood Parameters Pre- and Post-Exercise
A. Pre-Exercise Blood Results
| Subject # | Sex | Age | BMI | Glucose (mg/dl) |
Insulin (uIU/ml) |
Triglycerides (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
Total Cholesterol (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 27 | 24.5 | 86 | 5 | 65 | 40 | 90 | 143 |
| 2 | M | 21 | 30.8 | 99 | 12 | 128 | 38 | 90 | 154 |
| 3 | M | 21 | 23.3 | 96 | 11 | 88 | 37 | 101 | 156 |
| 4 | F | 29 | 24.1 | 90 | 8 | 63 | 56 | 100 | 169 |
| 5 | F | 27 | 33.7 | 82 | 52 | 176 | 42 | 101 | 178 |
| 6 | F | 30 | 21.8 | 92 | 9 | 90 | 52 | 118 | 188 |
| 7 | M | 24 | 22.0 | 81 | 9 | 143 | 59 | 105 | 193 |
| 8 | M | 32 | 25.9 | 96 | 8 | 111 | 34 | 161 | 217 |
| 9 | F | 21 | 23.3 | 75 | 12 | 131 | 54 | 152 | 232 |
| 10 | M | 32 | 34.1 | 89 | 27 | 194 | 33 | 196 | 268 |
2.2. Plasma Sample Characterization.
An aliquot of each blood sample was analyzed by Mid America Laboratories to determine glucose, insulin, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol levels. Subjects were ordered 1–10 based on total cholesterol content, with 1 having the lowest total pre-exercise cholesterol and 10 having the highest. Each subject retained the same number pre- to post-exercise. Plasma was isolated from another aliquot of blood by centrifugation at 1300 ref for 15 minutes at 4° C and stored at −80° C. Plasma samples from the 10 subjects collected prior to exercise were used to determine inter-individual differences in NP-BC formation. Plasma samples collected from 6 subjects post-exercise were used to evaluate exercise-induced differences in BC formation. This was accomplished by comparing the NP-BC produced following incubation in post-exercise plasma to that produced from pre-exercise plasma from the same human subject. These 6 postexercise plasma samples were selected based on alterations in triglyceride levels following exercise. Specifically, 3 subjects demonstrated minor alterations in triglyceride levels following exercise, whereas 3 other subjects exhibited greater alterations in triglycerides levels (Table 1).
2.3. Fe304NP Characterization.
Spherical Fe3o4 NPs with a diameter of 20 nm and suspended in poly-N-vinylpyrrolidone (PVP) at a concentration of 20 mg/ml were purchased from Nanocomposix (San Diego, California) and characterized to verify manufacturer specifications. The hydrodynamic size and ζ-potential (ZetaSizer Nano, Malvern) were assessed in DI water with Fe304 NPs at a concentration of 25 μg/ml (n=6). The number of particles per μg of NP was determined by nanoparticle tracking software (Nanosight, Malvern) (n=3). Shape and size were characterized via transmission electron microscopy (FEI Tecnai G2 20) (Appendix Figure A.l).
2.4. Formation of the Fe304 NP-BC.
BCs were formed on Fe3o4 NPs as described in our recent publications (Shannahan et al., 2016; Shannahan et al., 2013b; Shannahan et al., 2015b). Briefly, Fe3o4 NPs were incubated ex vivo in 10% plasma for 8 h at 4° C while being constantly mixed. Specifically, 125 μl of Fe3o4 NPs (lmg/ml), 325 μl of deionized water, and 50 μl of plasma were combined in a 1.5ml tube. Following incubation, Fe3o4NPs were pelleted via centrifugation at 20,817 ref for 10 min and washed with PBS. A total of 3 washes with PBS were performed to remove free proteins not associated with the Fe3o4 NPs. Following addition of the BC, NPs were reassessed for alterations in hydrodynamic size and ζ-potential (n=6/plasma sample).
2.5. Assessment of the protein components of the Fe3o4 NP-BC.
Protein components of the Fe3o4 NP-BC were evaluated utilizing a label-free proteomics approach to determine the effects of individual variation and the influence of exercise on the formation of the NP-BC. Three replicates of each Fe3o4 NP-BC were produced for proteomic analysis (n=3/plasma sample). The proteomics approach used was similar to our previous studies investigating the NP-BC (Shannahan et al., 2013a; Shannahan et al., 2015b). Further details regarding the proteomics approach including peptide isolation, mass spectrometry, and data analysis can be found in Appendix B.l. Following isolation, peptides were analyzed by reverse-phase HPLC-ESI-MS/MS using a Dionex UltiMate 3000 RSLC Nano System (Thermo Scientific, Waltham, MA) which was directly connected to a Q-Exactive™ HF Hybrid Quadrupole-Orbitrap MS (Thermo Scientific) and a Nanospray Flex™ Ion Source (Thermo Scientific). The files from the mass spectrometer were processed using the MaxQuant computational proteomics platform version 1.6.0.1 (Cox and Mann, 2008). The peak list generated was searched against the Homo sapiens sequences from UNIPROT retrieved on 02/22/2017 and a common contaminants database. Statistical analyses were performed in the R environment (www.cran.r-project.org). A t-test was performed on the LFQ intensities and only proteins with p-value < 0.05 were used in all analyses.
2.6. Proteomic Data Analysis - Identified Proteins.
Identified proteins from each subject were compared and Venn diagrams were produced to determine commonalities and differences in the identities of proteins that associated with Fe3o4 NPs forming the BC.
2.7. Proteomic Data Analysis - Relative Abundance.
To compare differences in abundance between individual subjects’ samples, label-free quantification (LFQ) intensity values were utilized to quantify fold change differences between proteins found to associate in common with Fe3o4 NPs following incubation in individual plasma samples, t-tests were utilized to determine if fold changes were statistically significant, p < 0.05. A similar assessment was performed to evaluate differences in protein abundance between Fe3o4 NPs incubated in pre-exercise samples and post-exercise samples. To examine how the quantities of specific proteins on Fe3o4 NPs were modified following incubation in individual plasma samples, the LFQ intensity was compared. Graphs and analyses were performed using GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA). LFQ intensities from replicates were averaged and used to produce a standard error of the means (n=3/group). Protein LFQs from all subjects’ samples were then statistically compared to subject 1 using a one-way ANOVA with a Dunnett’s multiple comparisons test. Statistical significance was determined by p < 0.05. A Pearson’s correlation analysis was performed to determine relationships between the mean abundance of specific proteins within the NP-BC and measured subject parameters, significance was determined by p < 0.05.
2.8. Cells and Cell culture.
Human monocytes (U937, ATCC, Manassas, VA) were maintained and grown at 37°C in a Galaxy 170 S incubator (New Brunswick Scientific, Edison, NJ) using RPMI1640 media (VWR Life Science, Radnor, PA) supplemented with 10% fetal bovine essence and 1% penicillin/streptomycin. Monocytes were then differentiated into macrophages using 100 ng/ml of phorbol 12-myristate 13-acetate for 72 h. Following differentiation, macrophages were exposed to Fe3o4 NPs with BCs and evaluated for BC-induced alterations in macrophage viability, uptake, and inflammatory response.
2.9. Viability, Uptake, and Inflammation.
Cells were differentiated in 96 well plates in 100 μl cell media to a confluency of 90%. After removal of the cell media, suspensions of Fe304NP-BCs at 25 μg/ml in serum free media were added to the wells. The concentration utilized for this cell response assessment was selected based on previous in vitro experimentation of Fe3o4 NPs and other NPs as well as a pilot study investigating concentration-dependent cytotoxicity in human macrophages (Shannahan et al., 2015a; Xia et al., 2013) (Appendix Figure A.2). The cells were exposed to the NP-BC suspensions for a total of 24 h before being assessed for cell viability via the MTT assay. To analyze NP-cellular association, cells were washed with PBS and collected for BCA assay (Thermo Scientific), followed by digestion in nitric acid. The cellular-associated Fe content was then quantified using atomic absorption spectroscopy (AAS) (Agilent Technologies, Santa Clara, CA). The amount of iron in each sample was normalized to the protein content. The ability of distinct BCs to activate macrophages was evaluated by measuring TNF-a cellular mRNA expression and supernatant protein levels. Additional details regarding the evaluation of inflammatory endpoints can be found in Appendix B.2. Quantitative real-time RT-PCR was performed for TNF-α and beta actin (ACTB) (control). Relative mRNA fold changes were calculated considering serum-free media exposed cells as control and normalized to the housekeeping gene ACTB. Supernatants were also collected at 24 h following exposure and examined by a human-specific TNF-α enzyme-linked immunosorbent assay kit via manufacturer’s instructions (R&D Systems, Inc., Minneapolis, MN, USA). Statistical analysis was performed using Prism software. All data (cytotoxicity, uptake, and inflammation) are presented as mean +/− standard error of the mean (n=6/group). Significant differences between exposures were determined by one-way ANOVA with a Tukey’s posthoc test (p<0.05).
3. Results
3.1. Characterization of Experimental Materials
3.1.1. Human Blood Characterization.
Blood samples were collected from 10 human subjects who, according to a self-survey, did not smoke or exercise regularly. Subjects were then exercised once a day for 7 consecutive days. Subjects were requested to not change their daily routines outside of the assigned exercise. At the conclusion of the 7 days, subjects’ blood was drawn a second time. The pre- and post-exercise blood samples from each subject were then characterized on a number of parameters, including triglycerides, LDL, HDL, and total cholesterol. Body mass index was calculated for each subject based on height and weight (Table 1). Subjects demonstrated variation in total cholesterol, with pre-exercise levels ranging from 143 mg/dL to 268 mg/dL in subjects 1 and 10, respectively (Table 1A). All individuals demonstrated decreased triglyceride levels following exercise, with changes up to 99 mg/dl observed (Subject 5, Table IB). Specifically, exercise was determined to only result in statistically significant reductions in triglyceride levels between pre-exercise (118.9 +/− 14) and post-exercise (82.3 +/− 9.9) groups (p = 0.0467) (data not shown). Six subjects were selected for analysis of exercise-related modifications in the NP-BC based on changes in triglyceride levels that occurred after completion of the exercise plan ( Table IB, bolded). Subjects selected were those with the highest changes in triglycerides (2, 5, 7) and the lowest (1, 6, 8) (Table 1).
B. Post-Exercise Blood Results
| Subject # | Sex | Age | BMI | Glucose (mg/dl) |
Insulin (uIU/ml) |
Triglycerides (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
Total Cholesterol (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|
| 1* | M | 27 | 24.5 | 93 | 5 | 54 | 44 | 86 | 141 |
| 2* | M | 21 | 30.8 | 92 | 5 | 65 | 48 | 118 | 179 |
| 3 | M | 21 | 23.3 | 96 | 11 | 62 | 36 | 80 | 128 |
| 4 | F | 29 | 24.1 | 94 | 7 | 46 | 61 | 96 | 166 |
| 5* | F | 27 | 33.4 | 92 | 12 | 77 | 45 | 109 | 162 |
| 6* | F | 30 | 21.8 | 87 | 11 | 80 | 49 | 84 | 149 |
| 7* | M | 24 | 22.0 | 84 | 7 | 76 | 51 | 88 | 154 |
| 8* | M | 32 | 26 | 96 | 7 | 104 | 36 | 151 | 208 |
| 9 | M | 21 | 23.5 | 84 | 10 | 107 | 58 | 135 | 214 |
| 10 | M | 32 | 33 | 87 | 25 | 152 | 29 | 210 | 269 |
Table 1. Subject Blood Parameters Pre- and Post-Exercise. After subjects’ blood was drawn, aliquots were analyzed by Mid America Laboratories. A. Blood parameters prior to completion of 7-day exercise regimen. B. Blood parameters following completion of 7-day exercise regimen.
and bold indicate subjects selected for further analysis due to large (2, 5, 7) or small (1, 6, 8) change in triglycerides pre- to post-exercise.
3.1.2. Nanoparticle Characterization.
Fe3o4 NPs were found to have 2.6 ± 0.3 × 109 NPs/μg (Mean ± SEM) as determined by NanoSight (Malvern, Westborough, MA)). Transmission electron microscopy verified the manufacturer’s stated initial particle size and spherical morphology (Appendix Fig A.l). Nanoparticles were characterized by assessment of hydrodynamic size and ζ-potential both with and without a BC present. Addition of all BCs increased NP hydrodynamic size and decreased ζ-potential (Fig 1). Incubation in individual plasma samples and addition of BCs to the Fe3o4 NP surface resulted in non-uniform alterations in hydrodynamic size (Fig 1A). NPs incubated in plasma from subjects 1, 6, and 7 demonstrated increases in Fe3o4 NP hydrodynamic size post-exercise (red bars) as compared to pre-exercise (blue bars) (Fig 1A). Polydispersion index of NPs without a BC was 0.108 +/− 0.002 (mean +/− SEM), while addition of BCs resulted in an average polydispersion index of 0.269 +/− 0.028 (mean +/− SEM), supporting limited polydispersion of Fe3o4 NPs (data not shown). NP ζ- potential was decreased uniformly between subjects, and no exercise-related changes were observed (Fig IB).
Figure 1.
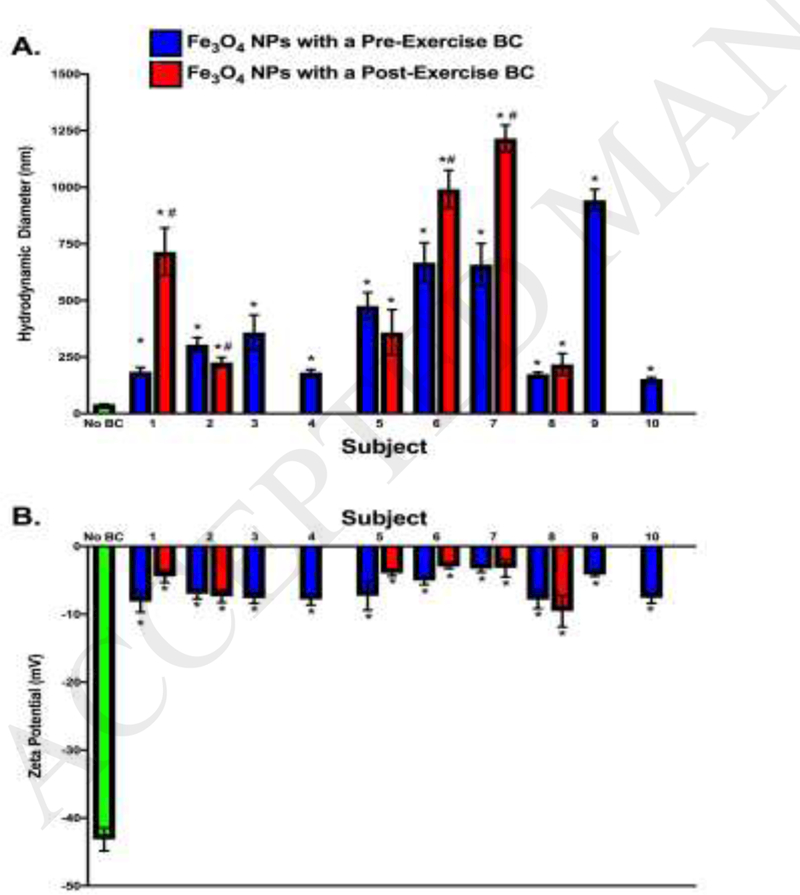
NP Characterization Prior to and Following the Addition of a BC. NPs were diluted in and incubated for 8 h at 4° C in 10% subject plasma. NPs were characterized with and without BCs via assessment of A) hydrodynamic size and B) ζ-potential. * denotes statistical significance from Fe3o4 NPs without a BC, # denotes statistical significance between preexercise NP-BC and post-exercise NP-BC (n=6/group; two-way ANOVA with Tukey post hoc analysis; p<0.05).
3.2. Inter-individual Variability within the Biocorona
3.2.1. Evaluation of the Effect of Individual Variability on the Pre-Exercise BC - Protein Identities.
A label-free mass spectrometry approach was used to identify proteins present in the BC of Fe3o4 NP following ex vivo incubation in plasma from subjects pre- and post-exercise. (Appendix Table C.l). Identified proteins were compared between the pre-exercise plasma of all subjects. A total of 99 proteins were found in common between all 10 subjects pre-exercise, including hemopexin; thrombospondin-1; coagulation factors V, IX, and XI; immunoglobulin κ variables 1–5, 1–8, 1–17, 2–30, 3–15, 3–20, 4–1, and 3D-11; and apolipoproteins A, Al, A2, A4, B, Cl, C2, C3, E, and H (Appendix Table C.2A). Subject 3’s pre-exercise BC was the most unique, containing 32 proteins specific to that subject (Appendix Table C.2A). To demonstrate interindividual differences in the formation of the NP-BC, the protein composition of each preexercise BC was compared to the protein composition of subject l’s pre-exercise BC (Fig. 2, Appendix Table C.2B). Although many proteins were found to bind in common to Fe3o4 NPs, unique proteins were determined to associate for each subject. The BC of subject 3 was determined to be the most unique compared to subject 1, with 41 distinct proteins. Subject 5 was the most similar, demonstrating the presence of only 7 unique proteins compared to subject 1 (Fig. 2). Although the utilized plasma samples were from both males and females, only one protein, immunoglobulin lambda variable 4–69, was found exclusively in male subjects (Appendix Table C.2A).
Figure 2.

Comparison of Pre-Exercise Fe3O4 NP-BC Composition: Number of Proteins shared with Subject 1. Proteins identified from each subject’s BC were compared to proteins identified from subject 1’s BC. Subject 1 was selected as a baseline due to it being the subject with lowest pre-exercise cholesterol levels. Venn diagrams were created to illustrate each comparison. Appendix Table C.l contains a comprehensive list comparing identified proteins between all the pre-exercise subjects. List of specific proteins used to generate Venn diagrams in Figure 2 are in Appendix Table C.2B.
3.2.2. Evaluation of the Effect of Individual Variability on the Pre-Exercise BC - Protein Quantification.
Although most proteins were shared between individual BCs, there were quantifiable differences (Appendix Table C.3). Figure 3 shows the top ten most significantly altered proteins as compared to subject 1 for all other subjects (2–10) (p<0.05). Several proteins were commonly found in this list of top ten most significantly different proteins, such as complement C4-B and cytoplasmic actin 1. However, it should be noted that the directionality of these proteins was not always uniform between subjects. Complement C4-B had a positive fold change (indicating that the protein was less abundant in the BC of subject 1) in subjects 5, 7, and 9, but a negative fold change (indicating that the protein was more abundant in the BC of subject 1) in subjects 2, 6, and 8. Cytoplasmic actin 1 had a positive fold change in all subjects, meaning it was less abundant on Fe3o4 NPs incubated in plasma from subject 1 compared to plasma from all other subjects. In four subjects, cytoplasmic actin 1 had the greatest positive fold change of significance among all proteins, indicating that it was less abundant in the BC of subject 1 compared to other subjects.
Figure 3.
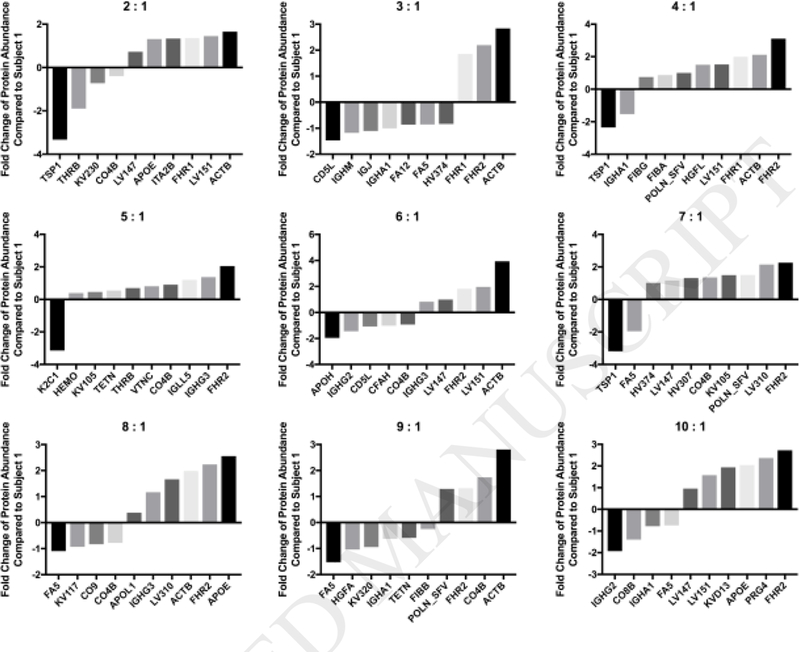
Fold Change of Selected Proteins Compared to Sample 1. Quantities of shared proteins between each subject and subject 1 are depicted as fold changes. Negative values indicate that the subject was more abundant in subject 1, while positive values indicate that the subject was less abundant in subject 1. Individual graphs depict the 10 most significantly altered proteins in terms of abundance between subjects and subject 1 (n=3/group; one-way ANOVA with Tukey post hoc analysis; p<0.05). Appendix Table C.3 includes a comprehensive list of all protein fold changes compared to subject 1.
Assessing the ten most changed proteins allows for a global perspective, however; by examining quantities of individual proteins (Fig 4, Appendix Table C.3), specific information may be gained regarding the effect of individual variability. Protein levels vary across subjects, demonstrating the impact of individual variability on the NP-BC composition (Fig. 4). For several of these proteins, changes in relative quantity are observed between subject 1 and others. A statistically significantly higher relative level of APOC1 was observed in 3 subjects, APOE in 7 subjects, and C04B in 3 subjects compared to subject 1. All nine comparisons showed a lower relative quantity of FINC as compared to subject 1, with all comparisons being statistically significant, with the exception of subject 6. No statistically significant changes were observed across subjects in the levels of APOC2, PLMN, or TTHY. APOA1 was statistically elevated in only one individual; subject 2. TSP1 had both statistically significant increases and decreases as compared to subject 1, with six of the nine comparisons showing levels far lower than those in subject 1, and two of the subjects showing levels far higher than those in subject 1.
Figure 4.
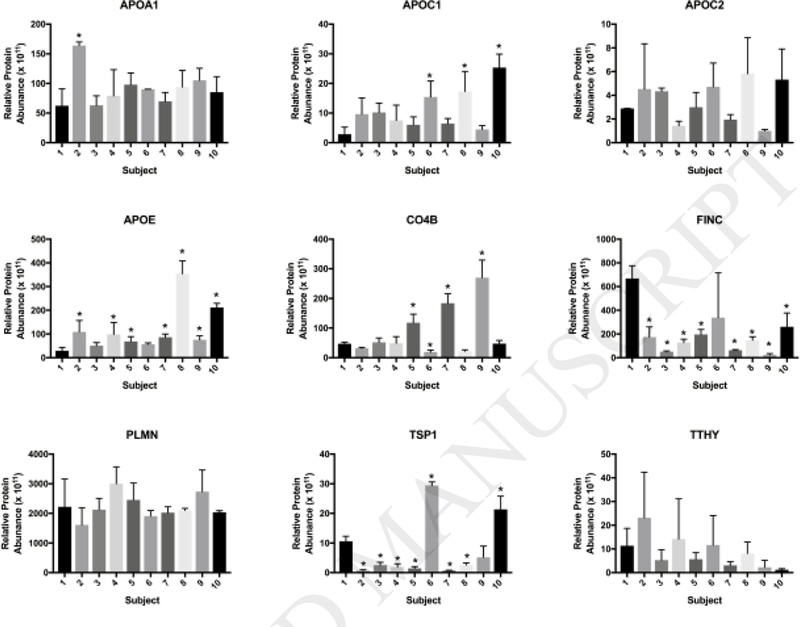
Relative Abundances of Specific Proteins across All Individual Pre-Exercise Subjects. Relative quantities of proteins shared between all 10 pre-exercise subjects were plotted to make global comparisons between all samples. * = statistical significance p<0.05 compared to subject 1 by one-way ANOVA with Tukey post hoc analysis (n=3/group). Appendix Table C.3 includes a comprehensive list of the calculated abundances for all proteins forming NP-BC in all subjects.
Relationships between the abundance of distinct proteins in the NP-BC (Appendix Table C.3) and subject characteristics (Total Cholesterol, LDL, HDL, Triglycerides, Glucose, Insulin, and BMI) (Table 1) were evaluated to determine correlations between typically measured medical parameters and nanoparticle-protein interactions (Appendix Table C.4). Certain proteins tended to increase in abundance within the NP-BC as total cholesterol increased, including apolipoprotein Cl (R2=0.42, p=0.04) and proteoglycan 4 (R2=0.44, p=0.04), whereas proteins such as transthyretin (R2=0.4, p=0.049) and immunoglobulin κ constant (R2=0.6, p=0.01) were decreased. Hepatocyte growth factor-like protein (R2=0.61, p=0.001), immunoglobulin κ variable 4–1 (R2=0.51, p=0.02), and insulin-like growth factor-binding protein 4 (R2=0.41, p=0.04), appear to increase directly with HDL, while apolipoprotein C3 (R2=0.41, p=0.04), and insulin-like growth factors 2 (R2=0.72, p=0.002) and 3 (R2=0.57, p=0.01) appear to decrease within the BC as HDL increases. The BC associated proteins apolipoprotein Cl (R2=0.55, p=0.01), proteoglycan 4 (R2=0.43, p=0.04), and platelet basic protein (R2=0.42, p=0.04) increased in abundance on the NP surface as LDL levels increased, whereas immunoglobulin κ constant (R2=0.50, p=0.02), and immunoglobulin κ variable 4–1 (R2=0.45, p=0.03) decreased. As plasma triglyceride levels increased, proteoglycan 4 (R2=0.48, p=0.03) and immunoglobulin heavy constant γ 3 (R2=0.43, p=0.04) increased within the NP-BC. Conversely, kinnogen-1 (R2=0.44, p=0.04) abundance decreased as triglyceride levels increased. As plasma glucose levels increased, so did insulin-like growth factor-2 (R2=0.64, p=0.01), apolipoprotein C2 (R2=0.59, p=0.01), and transferrin (R2=0.48, p=0.03) while prothrombin (R2=0.50, p=0.02), insulin-like growth factor-binding protein complex acid labile subunit (R2=0.56, p=0.01), and immunoglobulin heavy constant γ 1 (R2=0.49, p=0.02) decreased. Complement C3 (R2=0.81, p=0.0004), prothrombin (R2=0.43, p=0.04), and properdin (R2=0.4, p=0.048) increased within the BC with increased individual levels of insulin. Proteoglycan 4 (R2=0.56, p=0.01), and vitronectin (R2=0.44, p=0.04) within the BC tended to increase as BMI increased. To evaluate sex-related differences in NP-BC formation in terms of protein abundance, mean relative abundances of individual proteins were compared between male and female subjects (Appendix Table C.4). Male subjects were determined to have significantly less immunoglobulin κ variable 3–15, complement Clq subcomponent subunit A and B compared to female subjects (Appendix Table C.4). BCs formed following incubation in female plasma samples were determined to have significantly greater amounts of inter-a-trypsin inhibitor heavy chain H4, coagulation factor XII, and plasminogen (Appendix Table C.4).
3.3. Intra-individual Variability within the Biocorona
3.3.1. Evaluation of the Effect of Exercise on Variations in the BC - Protein Identities.
Six subjects were selected for pre- to post- exercise comparisons based on changes in triglyceride levels (Table 1). Subjects 1, 6, and 8 were selected because they demonstrated the smallest changes in triglyceride levels pre- to post-exercise, while subjects 2, 5, and 7 were selected because they exhibited the largest triglyceride changes (Table 1, Bolded). A label-free mass spectrometry approach was used to identify proteins present in all subjects’ post-exercise BCs. (Appendix Table C.l). Protein components of the post-exercise Fe3o4 NP BCs were initially compared to pre-exercise BCs based on the presence of identified proteins (Appendix Table C.5A). A total of 104 proteins were found in be in common between all subjects pre- and postexercise (Appendix Table C.5A). Protein IDs were also compared within individual subjects and differences were observed due to exercise (Fig. 5, Appendix Table C.5B). In general, the postexercise BC contained a larger number of unique proteins than the pre-exercise BC, with the exception of subject 8. Subject 2 had the largest number of unique post-exercise proteins, with 22 proteins present in the post-exercise BC that were not present in the pre-exercise BC. Subject 8 had the smallest number, at 10. In general, the post-exercise BCs from individuals with larger changes in triglyceride levels (subjects 2, 5, 7) contained increased numbers of unique proteins (as compared to the corresponding pre-exercise BC) than the post-exercise BCs of those with smaller changes in triglycerides (subjects 1, 6, 8) (Fig. 5, Appendix Table C.5B). Certain proteins were only present in pre-exercise NP-BCs (matrix gla protein, inter-α-trypsin inhibitor heavy chain HI) or only present in post-exercise NP-BCs (lipopolysaccharide binding protein, immunoglobulin heavy variable 2–5, immunoglobulin heavy variable 3–33).
Figure 5.
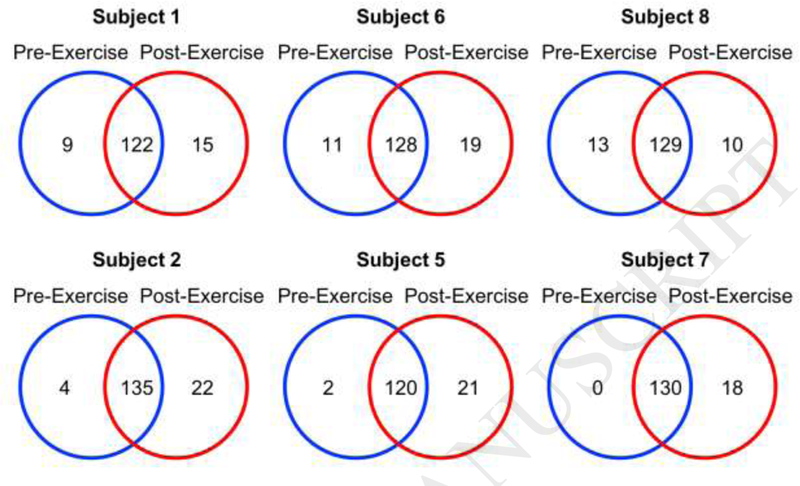
Comparison of Pre-Exercise to Post-Exercise Biocorona Composition within the Same Subject: Number of Proteins Shared Between Pre- and Post-Exercise Subjects. Proteins identified from each pre-exercise BC were compared to proteins identified from its corresponding post-exercise BC. Subjects were selected due their low (Subjects 1, 6, 8) or high (Subjects 2, 5, 7) changes in triglyceride levels pre- to post- exercise. Venn diagrams were created to illustrate each comparison. Appendix Table C.5A contains a comprehensive list comparing all identified proteins in pre- and post-exercise subjects. List of specific proteins used to generate Venn diagrams in figure 5 specifically are found in Appendix Table C.5B.
3.3.2. Evaluation of the Effect of Exercise on Variations in the BC - Protein Quantification.
Despite the many commonalties in protein identities in subjects’ BCs pre- to post-exercise, quantities of these shared proteins differed due to exercise. Figure 6 shows the top ten most significantly altered proteins as compared to the corresponding pre-exercise BC (p<0.05) (Appendix Table C.6). Few commonalities existed between these top ten most significantly altered proteins. Exercise was determined to increase the quantity of most of these shared proteins. In some subjects, increases were observed post-exercise in both non-structural polyprotein and immunoglobulin κ variable 1–16. In subjects 5 and 7, the post-exercise increase in immunoglobulin κ variable 1–16 was the largest positive fold change among all shared proteins. Non-structural polyprotein experienced the largest positive fold change of significance following exercise among all proteins in subjects 2 and 6. Five of the six subjects experienced a fold change above 4 for insulin-like growth factor-binding protein 2 following exercise (Appendix Table C.6). Apolipoprotein C3 and gelsolin were decreased in all subjects’ BCs following exercise (Appendix Table C.6).
Figure 6.
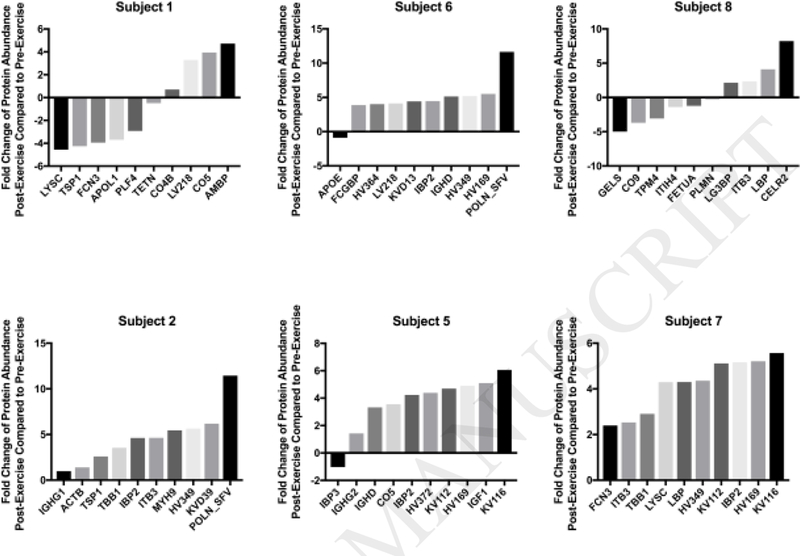
Fold Change of Specific Proteins in the Pre- and Post-Exercise Fe3O4 NP-BC. Quantities of shared proteins between each pre-exercise and post-exercise BC are depicted as fold changes. Appendix Table C.6 contains a comprehensive list of all protein quantity comparisons. Individual graphs depict the 10 most significantly different proteins in terms of fold change between pre- and post-exercise subjects (n=3/group; one-way ANOVA with Tukey post hoc analysis; p<0.05). Negative values indicate that the subject was more abundant prior to exercise, while positive values indicate that the subject was more abundant after exercise.
3.4. Assessment of Immune Cells Response to NP exposure
Cellular Viability, NP Association, and Inflammatory Response.
A concentration of 25 μg/ml was selected based on preliminary dose-response experiments in which human macrophages were exposed to Fe3o4 NPs, both with and without a BC formed from a representative human serum sample, at concentrations of 6.25, 12.5, 25, and 50 μg/mL for 24 h (Appendix Fig A.2). None of the exposures at these concentrations demonstrated overt toxicity following a 24 h exposure (Appendix Fig A.2). Exposure to Fe3o4 NPs with a pre-exercise BC caused no significant differences in cell viability compared to the control (Fig 7A). While macrophages associated NPs with the BC, no statistically significant differences were observed between NPs coated with any of the pre-exercise BCs (Fig 7B). Inflammation was assessed by analysis of alterations in mRNA expression of TNF-a and macrophage release of TNF-α into the supernatant (Figure 7C and 7D). TNF-a mRNA expression was significantly increased due to exposure to NPs with BCs formed from pre-exercise plasma from subjects 4, 5, 6, and 7 compared to controls (Fig 7C). A decrease in protein levels of TNF-α was observed in the supernatant of all samples exposed to subjects’ pre-exercise BCs. This decrease was statistically significant compared to the control for eight of the ten subjects, with the exceptions being subjects 1 and 8 (Fig 7D).
Figure 7.
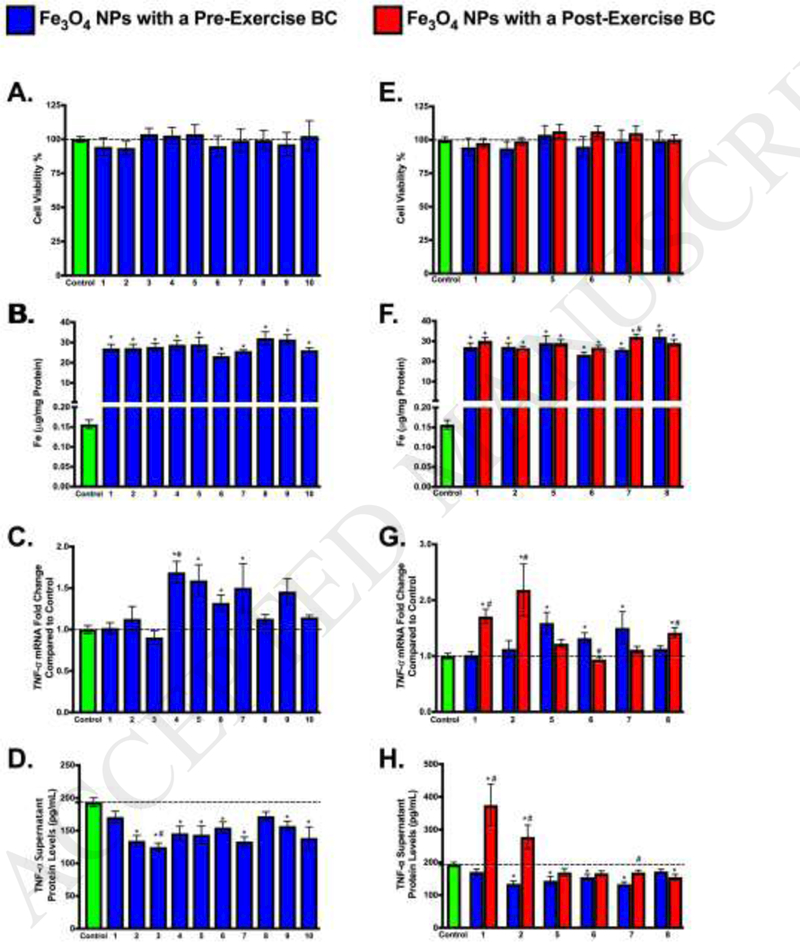
Macrophage response following exposure to Individual Fe3o4 NP-BCs. Differentiated human macrophages were exposed to Fe3o4 NPs with a distinct BC at a concentration of 25 μg/ml for 24 h. Blue bars indicate Fe3o4 NPs with a BC formed following incubation in plasma collected from individuals prior to the exercise regimen. Red bars indicate Fe3o4 NPs with a BC formed following incubation in plasma collected from individuals following completion of the exercise regimen. A, E. Cytotoxicity after a 24 h exposure to Fe304 NPs with BCs. Dotted line indicates controls with a cell viability of 100%. B. F. Macrophage association of Fe3o4 NPs with BCs after a 24 h exposure. C, G. TNF-a mRNA expression relative to serum-free media control cells following 24 h exposure to 25 μg/ml Fe3o4 NPs with BCs. D, H. Protein levels of TNF-α in supernatant following 24 h exposure to 25 μg/ml Fe3o4 NPs with BCs. A-D Utilized pre-exercise BCs, while E-H utilized pre and post-exercise BCs. * denotes statistical significance compared to controls (n=6/group; one-way ANOVA with Tukey post hoc analysis; p<0.05). # denotes statistical significance between Fe3O4 NPs with pre- and post-exercise BCs (n=6/group; two-way ANOVA with Tukey post hoc analysis; p<0.05).
Post-exercise BCs were also evaluated and compared to their corresponding pre-exercise BCs to determine differences in immune cell response. As with the pre-exercise BCs, no change in cell viability was observed after 24 h exposure (Fig 7E). In general, cellular association of Fe3o4 NPs was similar between post-exercise and pre-exercise BCs (Fig 7F). Subject 7 was the only exception to this; the cellular association of the NP with the post-exercise BC was significantly higher than the association of the pre-exercise BC. (Figure 7F). TNF-a mRNA was increased post-exercise (as compared to pre-exercise) for three of the six subject BC exposures tested (1,2, and 8) and decreased in the other three (5, 6, and 7), though this difference was only statistically significant for subjects 1, 2, 6 and 8 (Fig 7G). Upon examination of supernatant TNF-α protein levels following exposure to NPs with pre-exercise or post-exercise BCs, post-exercise subject 8 had significantly lower levels of TNF-α (Fig 7H). In exposures to post-exercise BCs of subjects 1, 2, and 7, supernatant levels of TNF-α were significantly higher than the subjects’ corresponding pre-exercise BCs, and for subjects 1 and 2, the level of post-exercise TNF-α was significantly higher than that of the control.
4. Discussion
In order to efficiently and safely utilize NPs in biomedicine, it is essential to understand the formation of the BC and its impact on subsequent NP-cellular interactions. Presently, our knowledge is lacking regarding the influences of individual variability and exercise in relation to the composition of the BC, as well as the possible subsequent toxicological consequences. Our findings demonstrate variability between individuals, as well as variability due to exercise, in NP-BC protein composition. These compositional alterations in the BC were determined to impact NP hydrodynamic size and resulted in differential macrophage interactions and inflammatory response. Ultimately, this variability in NP-BC composition may contribute to inconsistencies in response to exposures and therapeutics observed within the general population.
4.1. NP Properties Modified Following Addition of BCs
NPs can be designed with an array of diverse physicochemical properties (size, surface coating, charge, composition, etc.) allowing for their utilization in a variety of technologies and applications. Addition of the BC can impact NP properties, therefore disrupting their intended function (Amiri et al., 2013; Salvati et al., 2013). Our current study demonstrated that NP hydrodynamic size increased as cholesterol levels of the pre-exercise subjects increased, suggesting enhanced agglomeration of NPs. These changes in agglomeration and the observed variability are likely due to compositional differences in the BC. Evaluation of protein compositional differences did not demonstrate clear proteins associated with altered agglomeration between individuals. Past research has demonstrated that NPs in a hyperlipidemic environment associate large amounts of cholesterol, which may initiate alterations in NP agglomeration (Shannahan et al., 2016). Our findings demonstrate that NP properties such as agglomeration may be distinctively modified between individuals, resulting in differential functionality in their use as MRI contrast agents.
4.2. Interindividual Variations in the NP-BC
NPs were determined to form unique BCs following incubation in each of the 10 pre-exercise plasma samples. While many proteins were shared between NP-BCs, 8 of the 10 pre-exercise BCs contained at least one unique protein that was not found in the other BCs, underling the impact of inter-individual variability. Proteins that were shared between individuals differed in the quantity in which they were present within the NP-BCs. Proteins frequently appearing in the list of those most quantifiably changed as compared to subject 1 included thrombospondin1, cytoplasmic 1 actin, complement factor H-related protein 2, and complement C4. The BCs of female subjects had higher levels of plasminogen and coagulation factor XII than those of male subjects, possibly due to elevated levels in the blood caused by the menstrual cycle and/or the use of birth control (Casslen and Ohlsson, 1981; Gordon et al., 1980; Lackner and Javid, 1973). In summary, correlation analysis demonstrated that association of specific proteins with the NP surface could only be partial explained by the measured subject parameters. This suggests that interactions are complex and likely dependent on multiple physiological factors.
The ability of NPs to differentially associate proteins due to variations in the physiological environment has potential use in fields of both pharmacology and toxicology. Specifically, if NPs are able to reduce levels of specific proteins via preferential adsorption, this association could assist in the targeted removal of proteins from the circulation, contributing to disease treatments. Further, the association of specific proteins could assist in the targeting of nano- based theragnostic approaches. Previous research has shown that apolipoprotein-rich BCs enhance interactions between NPs and LDL receptors, as well as increasing transport across the blood-brain barrier (Kreuter, 2004, 2013). From a toxicological perspective, differential binding of proteins in disease environments could interfere with normal biological processes or enhance inflammatory responses, resulting in adverse outcomes. Ultimately, our current data supports that NPs introduced intravenously will not form consistent BCs between individuals. This variation may result in differential effectiveness of nano-based therapeutics between individuals, as well as disruption of biological systems by association of specific circulating biomolecules.
4.3. Intraindividual Differences in the NP-BC Related to Exercise
An exercise model was utilized to evaluate the potential for intra-individual differences in the formation of the NP-BC. Specifically, a mixed exercise model including both endurance and resistance components was used, as it represents the ideal exercise paradigm according to the United States Department of Health and Human Services (2009). Exercise resulted in unique BC formation on Fe3O4 NPs following ex vivo incubation in collected subject plasma pre- and postexercise. The post-exercise BC tended to be more diverse than the pre-exercise BC in terms of the number of unique proteins that were present. Interestingly, triglycerides were decreased following exercise for all subjects (Table 1). High intensity exercise has been shown to elevate triglycerides (Kraus et al., 2002) therefore this effect was most likely due to the resistance exercise component. These exercise-induced changes in triglyceride levels were found to influence BC formation, as subjects that demonstrated greater changes in triglyceride levels were found to have greater protein diversity within the BC.
Specific proteins were found to exclusively associate with NPs based on pre- or post-exercise conditions. Inter-alpha-trypsin inhibitor heavy chain H1 and matrix Gla protein were found only in the pre-exercise BCs, while immunoglobulin heavy variable 2–5, immunoglobulin heavy variable 3–33, immunoglobulin heavy variable 1–69, and lipopolysaccharide-binding protein were found only in the post-exercise BCs. Lipopolysaccharide-binding protein, which was only seen post-exercise, has been shown to be associated with stress, inflammation, and obesity. Because the recruited subjects within our study typically did not exercise, they likely responded to the stress of exercise with an acute phase response, leading to induction of lipopolysaccharide- binding protein (Branescu et al., 2012; Stehle et al., 2012). Conversely, matrix Gla protein was only found in pre-exercise BCs. Matrix Gla protein has been shown to be increased in individuals with hyperlipidemia compared to controls (Kullich et al., 2003). Further, matrix Gla protein correlates with cholesterol, triglyceride, and low-density lipoprotein levels in the circulation (Kullich et al., 2003). Decreased abundance of matrix Gla protein on the NP surface suggests exercise-induced reductions within the circulation. It is likely that exercise resulted in reductions of certain circulating biomolecules, thus allowing lower abundance proteins an opportunity to associate with the NP surface. Further, certain proteins were likely increased within the NP-BC due to their increased availability resulting from exercise-induced synthesis. Other proteins, while not unique to the pre- or post-exercise conditions, were found to exhibit quantifiable differences following exercise, further demonstrating the dynamic nature of nano- based pharmaceutical interactions within a physiological environment. A decrease in the abundance of gelsolin on the NP surface following exercise was observed post-exercise. Gelsolin has been shown to be increased following acute eccentric exercise-induced muscle damage (Tekus et al., 2017). It is likely that muscle damage occurred early in our exercise regimen as a result of the resistance exercise component, causing an increase in gelsolin, and that the observed decrease in NP association of gelsolin was a result of the ongoing recovery process occurring at the time point we collected the plasma sample. The amount of insulin-like growth factor-binding protein 2 within the NP-BC was increased following exercise. Endurance exercise, such as the cycling performed in our study, is known to increase the circulating levels of insulin-like growth factor binding proteins in humans which may have increased NP interactions (Suikkari et al., 1989).
Overall, these quantifiable changes suggest that exercise induces alterations in circulating biomolecule content, thereby altering BC formation. Due to the subjects’ sedentary lifestyles, it is likely that exercise induced a general stress response and that the observed changes in the NP- BC are not all exercise-specific. However, our study indicates that minor or major lifestyle variations which impact the composition of the biological environment, such as exercise, diet, disease states, or stress, could also affect the composition of the BC, thereby resulting in altered NP efficacy and/or toxicity.
4.4. Immune Cell Response to NP-BCs
As expected, exposure to all Fe3o4 NPs with a BC was not found to induce cytotoxicity in human macrophages at the concentrations and time points assessed in this study. Macrophages were selected due to their distribution at sites of NP deposition, role in the induction of the immune response, and ability to phagocytize foreign materials such as NPs (Amida et al., 2011; Walkey et al., 2012; Zhang et al., 2002). TNF-α mRNA and protein levels in cells exposed to Fe304 NPs with BCs varied both between individuals and following exercise. NPs incubated in post-exercise serum were found to be more likely to increase the inflammatory response than decrease it. Alterations observed in NP properties following addition of the BC appeared to have no impact on the cellular response, suggesting that the changes in immune cell response were due to the presence of specific biomolecules on the NP surface. Together, these findings demonstrate that alterations in NP-biomolecule interactions may result in differential immune cell activation dependent upon an individual’s characteristics.
5. Study Limitations and Conclusions
A few limitations should be considered in regards to this study. First, although study participants were encouraged to maintain their normal lifestyles, it is unknown if individuals modified their diet or other aspects, which may have further altered circulating biomolecules. Additionally, only protein components of the BC were evaluated, however, it is likely that lipids and other biomolecules absorbed to the NP surface were also altered. Finally, our evaluation was performed on ten recruited individuals. For more patterns to be evident in regards to NP- biomolecule interactions, this study would have to be expanded to include a larger sample size.
In conclusion, these data substantiate that NP-biomolecule interactions are variable between individuals and can vary due to changes in an individual’s activity. Further, this variability in the formation of the NP-BC was determined to modify NP toxicity, which may correspond to the differential toxicity which is often observed with pharmaceuticals in the general population. This study provides foundational knowledge and rationale to proceed with mechanistic examinations of the BC and its biological implications for nano-based precision therapeutics. Future investigation should incorporate other factors which may alter the BC, such as disease states, diet, longer-term exercise, drug/alcohol use, stress, etc. Ultimately, a thorough understanding of initial interactions between NPs and biomolecules will allow for safer utilization of NP-enabled theragnostics in the complex biological environments that exist within our population.
Supplementary Material
Highlights.
Nanoparticles interact with serum biomolecules differentially between individuals
Exercise alters an individual’s nanoparticle-biocorona composition
Variability in the biocorona results in differential macrophage responses
Acknowledgements
The Purdue Proteomics Facility, Dr. Uma Aryal, Vicki Hedrick, and Dr. Tiago Sobreira are thanked for their assistance.
Funding
This work was supported by the National Institute of Environmental Health Sciences Grant number ES024392.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2009. 2008 Physical Activity Guidelines for Americans. J Cardiovasc Nurs 24, 2–3. [Google Scholar]
- Amiri H, Bordonali L, Lascialfari A, Wan S, Monopoli MP, Lynch I, Laurent S, Mahmoudi M, 2013. Protein corona affects the relaxivity and MRI contrast efficiency of magnetic nanoparticles. Nanoscale 5, 8656–8665. [DOI] [PubMed] [Google Scholar]
- Arnida Janat-Amsbury, Ray MM, Peterson A, Ghandehari CM, H., 2011. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm 77, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P, 1999. Synthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric study. J Colloid Interf Sci 212, 474–482. [DOI] [PubMed] [Google Scholar]
- Balfoussia E, Skenderi K, Tsironi M, Anagnostopoulos AK, Parthimos N, Vougas K, Papassotiriou I, Tsangaris GT, Chrousos GP, 2014. A proteomic study of plasma protein changes under extreme physical stress. J Proteomics 98, 1–14. [DOI] [PubMed] [Google Scholar]
- Branescu C, Serban D, Savlovschi C, Dascalu AM, Kraft A, 2012. Lipopolysaccharide binding protein (L.B.P.)--an inflammatory marker of prognosis in the acute appendicitis. J Med Life 5, 342–347. [PMC free article] [PubMed] [Google Scholar]
- Casslen B, Ohlsson K, 1981. Cyclic Variation of Plasminogen Activation in Human Uterine Fluid and the Influence of an Intrauterine-Device. Acta Obstet Gyn Scan 60, 97–101. [PubMed] [Google Scholar]
- Clift MJD, Bhattacharjee S, Brown DM, Stone V, 2010. The effects of serum on the toxicity of manufactured nanoparticles. Toxicol Lett 198, 358–365. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB, 2006. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. The Journal of physiology 576, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfari MR, Kashefi M, Shams SF, Jaafari MR, 2016. Perspective of Fe304 Nanoparticles Role in Biomedical Applications. Biochem Res Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Ratnoff OD, Saito H, Donaldson VH, Pensky J, Jones PK, 1980. Rapid Fibrinolysis, Augmented Hageman-Factor (Factor-Xii) Titers, and Decreased Cl-Esterase Inhibitor Titers in Women Taking Oral-Contraceptives. J Lab Clin Med 96, 762–769. [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR, 1977. Diabetes, blood lipids, and the role of obesity in coronary heart disease risk for women: the Framingham Study. Annals of internal medicine 87, 393–397. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gupta M, 2005. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021. [DOI] [PubMed] [Google Scholar]
- Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V, 2005. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharmaceut 2, 194–205. [DOI] [PubMed] [Google Scholar]
- Jedlovszky-Hajdu A, Bombelli FB, Monopoli MP, Tombacz E, Dawson KA, 2012. Surface Coatings Shape the Protein Corona of SPIONs with Relevance to Their Application in Vivo. Langmuir 28, 14983–14991. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Rao AV, Hegele RA, Mitchell SJ, Ransom TPP, Boctor DL, Spadafora PJ, Jenkins AL, Mehling C, Relle LK, Connelly PW, Story JA, Furumoto EJ, Corey P, Wursch P, 1993. Effect on Blood-Lipids of Very High Intakes of Fiber in Diets Low in Saturated Fat and Cholesterol. New Engl J Med 329, 21–26. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA, 2002. Effects of the amount and intensity of exercise on plasma lipoproteins. New Engl J Med 347, 1483–1492. [DOI] [PubMed] [Google Scholar]
- Kreuter J, 2004. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J Nanosci Nanotechno 4, 484–488. [DOI] [PubMed] [Google Scholar]
- Kreuter J, 2013. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J Microencapsul 30, 49–54. [DOI] [PubMed] [Google Scholar]
- Kullich W, Machreich K, Hawa G, Eichinger B, Klein G, 2003. [Calcification marker matrix G1a protein in patients with hyperlipidemia]. Wien Med Wochenschr 153, 360–364. [DOI] [PubMed] [Google Scholar]
- Lackner H, Javid JP, 1973. The clinical significance of the plasminogen level. Am J Clin Pathol 60, 175–181. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA, 2008. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. P Natl Acad Sci USA 105, 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano G, Sabella S, Sorce B, Brunetti V, Malvindi MA, Cingolani R, Pompa PP, 2010. Effects of Cell Culture Media on the Dynamic Formation of Protein-Nanoparticle Complexes and Influence on the Cellular Response. Acs Nano 4, 7481–7491. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Aberg C, Salvati A, Dawson KA, 2012. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7, 779–786. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA, 2011. Physical- Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J Am Chem Soc 133, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Mornet S, Vasseur S, Grasset F, Duguet E, 2004. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem 14, 2161–2175. [Google Scholar]
- Murphy WG, 2014. The sex difference in haemoglobin levels in adults - Mechanisms, causes, and consequences. Blood Rev 28, 41–47. [DOI] [PubMed] [Google Scholar]
- Neuberger T, Schopf B, Hofmann H, Hofmann M, von Rechenberg B, 2005. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293, 483–496. [Google Scholar]
- Raghavendra AJ, Fritz K, Fu S, Brown JM, Podila R, Shannahan JH, 2017. Variations in biocorona formation related to defects in the structure of single walled carbon nanotubes and the hyperlipidemic disease state. Sci Rep-Uk 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA, 2013. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 8, 137–143. [DOI] [PubMed] [Google Scholar]
- Shannahan J, 2017. The biocorona: a challenge for the biomedical application of nanoparticles. Nanotechnol Rev 6, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Brown JM, Chen R, Ke PC, Lai XY, Mitra S, Witzmann FA, 2013a. Comparison of Nanotube-Protein Corona Composition in Cell Culture Media. Small 9, 2171–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Fritz KS, Raghavendra AJ, Podila R, Persaud I, Brown JM, 2016. Disease-Induced Disparities in Formation of the Nanoparticle-Biocorona and the Toxicological Consequences. Toxicol Sci 152, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Lai XY, Ke PC, Podila R, Brown JM, Witzmann FA, 2013b. Silver Nanoparticle Protein Corona Composition in Cell Culture Media. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Podila R, Aldossari AA, Emerson H, Powell BA, Ke PC, Rao AM, Brown JM, 2015a. Formation of a Protein Corona on Silver Nanoparticles Mediates Cellular Toxicity via Scavenger Receptors. Toxicol Sci 143, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Podila R, Brown JM, 2015b. A hyperspectral and toxicological analysis of protein corona impact on silver nanoparticle properties, intracellular modifications, and macrophage activation. Int J Nanomed 10, 6509–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JR, Leng XY, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP, 2012. Lipopolysaccharide-Binding Protein, a Surrogate Marker of Microbial Translocation, Is Associated With Physical Function in Healthy Older Adults. J Gerontol a-Biol 67, 1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suikkari AM, Sane T, Seppala M, Ykijarvinen H, Karonen SL, Koivisto VA, 1989. Prolonged Exercise Increases Serum Insulin-Like Growth Factor-Binding Protein Concentrations. J Clin Endocr Metab 68, 141–144. [DOI] [PubMed] [Google Scholar]
- Tekus E, Vaczi M, Horvath-Szalai Z, Ludany A, Koszegi T, Wilhelm M, 2017. Plasma Actin, Gelsolin and Orosomucoid Levels after Eccentric Exercise. J Hum Kinet 56, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK, Stauber RH, 2013. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol 8, 772–U1000. [DOI] [PubMed] [Google Scholar]
- Tran ZV, Weltman A, Glass GV, Mood DP, 1983. The Effects of Exercise on Blood-Lipids and Lipoproteins - a Meta-Analysis of Studies. Med Sci Sport Exer 15, 393–402. [PubMed] [Google Scholar]
- Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA, 2010. What the Cell “Sees” in Bionanoscience. J Am Chem Soc 132, 5761–5768. [DOI] [PubMed] [Google Scholar]
- Walkey CD, Chan WCW, 2012. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev 41, 2780–2799. [DOI] [PubMed] [Google Scholar]
- Walkey CD, Olsen JB, Guo HB, Emili A, Chan WCW, 2012. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J Am Chem Soc 134, 2139–2147. [DOI] [PubMed] [Google Scholar]
- Xia T, Hamilton RF, Bonner JC, Crandall ED, Elder A, Fazlollahi F, Girtsman TA, Kim K, Mitra S, Ntim SA, Orr G, Tagmount M, Taylor AJ, Telesca D, Tolic A, Vulpe CD, Walker AJ, Wang X, Witzmann FA, Wu NQ, Xie YM, Zink JI, Nel A, Holian A, 2013. Interlaboratory Evaluation of in Vitro Cytotoxicity and Inflammatory Responses to Engineered Nanomaterials: The NIEHS Nano GO Consortium. Environ Health Persp 121, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kohler N, Zhang MQ, 2002. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 23, 1553–1561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


