Abstract
Atypical antipsychotic drugs (APDs) increase dopamine (DA) release in prefrontal cortex (PFC), an effect probably mediated by the direct or indirect activation of the 5-HT1A receptor (5-HT1AR). Given the very low in-vitro affinity of most APDs for 5-HT1ARs and the large co-expression of 5-HT1ARs and 5-HT2A receptors (5-HT2ARs) in the PFC, this effect might result from the imbalance of 5-HT1AR and 5-HT2AR activation after blockade of these receptors by APDs, for which they show high affinity. Here we tested this hypothesis by examining the dependence of the APD-induced DA release in medial PFC (mPFC) on each receptor by using in-vivo microdialysis in wild-type (WT) and 5-HT1AR and 5-HT2AR knockout (KO) mice. Local APDs (clozapine, olanzapine, risperidone) administered by reverse dialysis induced a dose-dependent increase in mPFC DA output equally in WT and 5-HT2AR KO mice whereas the DA increase was absent in 5-HT1AR KO mice. To examine the relative contribution of both receptors to the clozapine-induced DA release in rat mPFC, we silenced G-protein-coupled receptors (GPCRs) in vivo with N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) while 5-HT1ARs or 5-HT2A/2CRs in the mPFC were selectively protected with the respective antagonists WAY-100635 or ritanserin. The inactivation of GPCRs while preserving ∼70% of 5-HT2A/2CRs prevented the clozapine-induced DA rise in mPFC. In contrast, clozapine increased DA in mPFC of EEDQ-treated rats whose 5-HT1ARs were protected (∼50% of control rats). These results indicate that (1) 5-HT1ARs are necessary for the APDs-induced elevation in cortical DA transmission, and (2) this effect does not require 5-HT2AR blockade by APDs.
Keywords: Antipsychotic drugs, dopamine, prefrontal cortex, 5-HT1A receptor, 5-HT2A receptor
Introduction
Cognitive impairment, including attention disorders, deficits in working memory and executive functions, is a central feature of schizophrenia lacking adequate treatment (Braff, 1993; Elvevag & Goldberg, 2000; Green et al. 2000). Negative symptoms are also poorly treated with current medications (Weickert et al. 2000), which, by themselves, induce this type of symptomatology (Artaloytia et al. 2006). In particular, atypical antipsychotic drugs (APDs) improve negative symptoms and quality of life more than classical antipsychotics (Sumiyoshi et al. 2006; Woodward et al. 2005). Moreover, some APDs (amisulpride, clozapine, olanzapine, risperidone) proved superior to first-generation drugs when considering their overall efficacy (Leucht et al. 2009).
The ability of some APDs to improve negative/cognitive symptoms (Bubenikova-Valesova et al. 2008; Grayson et al. 2007; Harvey et al. 2008) has been attributed to their capacity to increase dopamine (DA)-mediated transmission in the medial prefrontal cortex (mPFC) and hippocampus of experimental animals (Assié et al. 2005; Bortolozzi et al. 2007b; Chung et al. 2004; Díaz-Mataix et al. 2005; Elsworth et al. 2008; Ichikawa et al. 2001; Kuroki et al. 1999; Li et al. 2009; Millan, 2000; Rollema et al. 1997, 2000; Youngren et al. 1999). Indeed, an optimal DA transmission is fundamental for the execution of PFC-dependent cognitive tasks (Vijayraghavan et al. 2007; Williams & Goldman-Rakic, 1995) and, among other anatomical and neurochemical abnormalities in PFC, schizophrenia patients show a reduced dopaminergic innervation (Akil et al. 1999; Lewis & Lieberman, 2000).
APDs share a higher in-vitro affinity and in-vivo occupancy of 5-HT2A receptors (5-HT2ARs) vs. DA D2 receptors (Meltzer et al. 1989; Nyberg et al. 1998; Stockmeier et al. 1993). 5-HT2ARs are densely expressed in PFC, mainly in projection pyramidal neurons (Amargós-Bosch et al. 2004; López-Giménez et al. 1997; Pazos et al. 1985; Santana et al. 2004), including those projecting to the ventral tegmental area (VTA) (Vázquez-Borsetti et al. 2009). 5-HT2AR stimulation in PFC enhances the activity of pyramidal neurons projecting to the VTA (Puig et al. 2003, 2005) and of VTA dopaminergic neurons (Bortolozzi et al. 2005), leading to an increased mesocortical DA release (Bortolozzi et al. 2005; Gobert & Millan, 1999). Further, the 5-HT2AR antagonist M100907 reduced the firing of DA neurons and DA release in mPFC (Bortolozzi et al. 2005; Minabe et al. 2001; Pehek et al. 2001). Overall, these observations are consistent with the above anatomical finding showing the existence of (a) closed mPFC-VTA loops (Carr & Sesack, 2000) and (b) the expression of 5-HT2ARs in mPFC pyramidal neurons projecting to the VTA (Vázquez-Borsetti et al. 2009). However, 5-HT2AR blockade has been suggested to be necessary for APDs to enhance DA release in the mPFC (Bonaccorso et al. 2002; Ichikawa et al. 2001; Liégeois et al. 2002).
In addition to 5-HT2AR blockade, APDs display variable, but often high affinity for other monoamine receptors (see http://kidb.case.edu/pdsp.php; Roth et al. 2003). Agonist activity at 5-HT1A receptors (5-HT1ARs) by APDs appears to contribute to their superior efficacy in treating non-psychotic symptoms (Bantick et al. 2001; Meltzer & Sumiyoshi, 2008; Millan, 2000; Sumiyoshi et al. 2001a, b; but see Rënyi et al. 2001; Yasuno et al. 2003). Hence, although APDs show little or no in-vitro affinity for 5-HT1ARs (Ki = 770 nm for clozapine, >1000 nm for olanzapine and 490 nm for risperidone; Arnt & Skarsfeldt, 1998; Bymaster et al. 1996), these agents increase cortical DA release through 5-HT1AR activation (Díaz-Mataix et al. 2005; Rollema et al. 1997).
Given the large co-expression of 5-HT1A and 5-HT2A receptors in PFC (Amargós-Bosch et al. 2004) and their opposite role in modulating pyramidal neuron activity (Aghajanian & Marek, 1997; Amargós-Bosch et al. 2004; Araneda & Andrade, 1991; Ashby et al. 1994; Puig et al. 2005), the apparent in-vivo action of APDs at 5-HT1ARs might be due to blockade of 5-HT2ARs in cells co-expressing both receptors, thus enhancing 5-HT1AR-mediated neurotransmission. Here we examined this possibility using control mice and mice lacking 5-HT1A or 5-HT2A receptors. We also used an in-vivo rat model consisting in the inactivation of G-protein-coupled receptors (GPCRs) with the alkylating agent N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquino-line (EEDQ), while selectively protecting 5-HT1A or 5-HT2A/2C receptors in the mPFC (Amargós-Bosch et al. 2004).
Materials and methods
Animals
Male albino Wistar rats (250–320 g) were from Iffa Credo (France). Male homozygous 5-HT1AR knockout (KO) mice were generated at Princeton University) (Parks et al. 1998) and 5-HT2AR KO mice were generated at Columbia University) (Fiorica-Howells et al. 2002). Both genotypes were gradually backcrossed to the C57BL/6 background. From these initial sources, some 5-HT1AR KO and 5-HT2AR KO mice were transferred to develop a stable colony in our animal facilities. Wild-type (WT) mice of the same genetic background (C57BL/6) were also used. Mice were aged 10–15 wk at the time of experiments. Animals were maintained in a temperature-controlled room with a 12-h light/dark cycle (lights on 08:00 hours). Food and water were available ad libitum. Animal care followed the European Union regulations (O.J. of E.C. L358/1 18/12/1986) and was approved by the Institutional Animal Care and Use Committee of the School of Medicine, University of Barcelona.
Drugs and reagents
All reagents used were of analytical grade and were obtained from Merck (Germany). 5-HT oxalate, clozapine, 1-[2,5-dimethoxy-4-iodophenyl-2-amino-propane] (DOI), dopamine hydrochloride, EEDQ, risperidone, ritanserin, 6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy) pyridin-3-yl carbamoyl] indoline (SB-242084), spiperone and N-[2-(4-(2-meth-oxyphenyl)-1-piperazinyl)ethyl]-N-(2-pyridyl) cyclohexane carboxamide 3 HCl (WAY-100635) were from Sigma/RBI (Spain). BAY × 3792, citalopram HBr and olanzapine were from Bayer, Lundbeck A/S (Denmark) and Eli Lilly (USA), respectively. To assess local effects in microdialysis experiments, drugs were dissolved in perfusion fluid [aCSF (artificial cerebrospinal fluid, mM): NaCl, 125; KCl, 2.5; CaCl2, 1.26 and MgCl2, 1.18] and administered by reverse dialysis at the stated concentrations (uncorrected for membrane recovery). Clozapine, olanzapine and risperidone were initially dissolved in a drop of acetic acid and diluted to appropriate concentrations in aCSF. All other drugs were dissolved in distilled water, saline or aCSF, as required. Concentrated solutions (1 mM; pH adjusted to 6.5–7 with NaHCO3 when necessary) were stored at −80 °C and working solutions were prepared daily by dilution in aCSF. Control mice and rats were perfused with aCSF. Bars in the figures show the period of drug administration, corrected for the void volume of the system.
Microdialysis procedures
Microdialysis experiments in rats and mice were conducted as described previously (Amargós-Bosch et al. 2004; Bortolozzi et al. 2003). Rats were anaesthetized with sodium pentobarbital (60 mg/kg i.p.) and implanted with 4-mm concentric dialysis probes (Cuprophan) in the mPFC [coordinates in mm: AP +3.2, L −0.8, DV −6.0 (Paxinos & Watson, 2005)]. Experiments were performed in awake animals ∼20 h after surgery. Probes were perfused with aCSF at 1.5 μl/min. After an initial 100 min stabilization period, four baseline samples were collected (20 min each) before local drug administration, and successive dialysate samples were collected. For mice, the surgical and microdialysis procedures were identical to those described for rats, except for the dose of anaesthesia (40 mg/kg i.p.), length of dialysis membrane (2 mm), and the brain coordinates (in mm) of the mPFC: AP +2.2, L −0.2, DV −3.4 (Franklin & Paxinos, 1997).
Monoamine concentration in dialysate samples was determined by HPLC with electrochemical detection (Hewlett Packard 1049; +0.75 for DA, +0.6 V for 5-HT) as described previously (Bortolozzi et al. 2003; Díaz-Mataix et al. 2005). Detection limits were 2–3 fmol for DA and 5-HT.
At the end of the experiments, animals were killed by an overdose of anaesthetic. Brains were quickly removed and frozen in dry ice before being sectioned (40 μm) with a cryostat (HM500-Om Microm, Germany) in coronal planes. Brain sections were stained with Neutral Red to verify the correct placement of probes.
Silencing of GPCRs in vivo with selective protection of 5-HT1ARs or 5-HT2A/2CRs in mPFC
We used a previously described strategy to selectively protect 5-HT1ARs or 5-HT2A/2CRs in mPFC from the overall inactivating effect of EEDQ on GPCRs (Amargós-Bosch et al. 2004). EEDQ was systemically administered to inactivate GPCRs while selectively protecting one or other receptor by the local perfusion of selective antagonists (WAY-100635 for 5-HT1ARs, ritanserin for 5-HT2A/2CRs) through the microdialysis probes. EEDQ alkylates several GPCRs and inactivates their function (Gozlan et al. 1994), except those whose binding pockets are occupied. Thus, the perfusion of WAY-100635 or ritanserin in mPFC confers a selective protection of 5-HT1ARs or 5-HT2A/2CRs in mPFC, respectively, during EEDQ treatment. These two experimental groups are designated as GPCR-silenced+5-HT1AR-protected and GPCR-silenced+ 5-HT2A/2CR-protected, respectively.
Three to four hours after implantation, microdialysis probes were perfused with WAY-100635 (300 μm) for 3 h at 1.5 μl/min (5-HT1AR protection). One hour after starting the perfusion, EEDQ (dissolved in ethanol/water 1:1) was administered at 6 mg/kg i.p. The same procedure was applied to protect 5-HT2A/2CRs using the 5-HT2A/2CR antagonist ritanserin (300 μm). Control rats received vehicle intraperitoneally and aCSF through the dialysis probes. On the following day, histological or microdialysis experiments were performed. For autoradiographic studies, 14-μm-thick coronal sections were cut, thawmounted onto 3-aminopropyltriethoxysilane (APTS; Sigma/RBI, Spain) coated slides, and kept at −20 °C until required. All experiments with control and EEDQ-treated rats were run in parallel.
Receptor autoradiography
To determine the extent of regional 5-HT1AR or 5-HT2A/2CR protection in EEDQ-treated rats we performed receptor autoradiography for 5-HT1A and 5-HT2A/2C receptors using the ligands [3H]8-OH-DPAT (227.0 Ci/mmol) and [3H]mesulergine (83.0 Ci/mmol), respectively, from Amersham (GE Healthcare, Spain). Fresh frozen coronal sections of PFC from control and EEDQ-treated rats were used. Incubation conditions for [3H]8-OH-DPAT were as previously described (Mengod et al. 1996). Non-specific binding was defined as that remaining in presence of 10−5 m 5-HT. Incubation conditions for [3H]mesulergine were as previously described (López-Giménez et al. 2002; Pazos et al. 1985). Non-specific binding was defined as that remaining in the presence of 10−5 m mianserin. After incubation and washing, tissue sections were dipped in distilled, ice-cold water and dried rapidly under a cold air stream. Tissues were exposed to tritium-sensitive film (Kodak Biomax MR; Kodak, USA) together with plastic 3H standards for 60 d at 4 °C. All tissue sections used for quantification of receptor sites were processed simultaneously under the same conditions.
5-HT1A, 5-HT2A and 5-HT2C receptors were examined in mice brain by receptor autoradiography as previously described (López-Giménez et al. 2002; Mengod et al. 1996; Pazos et al. 1985) using (a) [3H]8-OH-DPAT for 5-HT1ARs, (b) [3H]mesulergine (plus 10−7 m of the selective 5-HT2CR antagonist SB242084) for 5-HT2ARs, and (c) [3H]mesulergine (plus 10−7 m of the 5-HT2AR antagonist spiperone) for 5-HT2CRs. Quantitative analysis of the autoradiograms was done with AIS computerized image analysis system (Imaging Research Inc., Canada).
Data and statistical analysis
Microdialysis results are expressed as fmol/30-μl fraction for DA and 5-HT and shown in the Figures as percentages of baseline (individual means of four predrug fractions). Area under the curve (AUC) of selected time periods (fractions 6–16) was also calculated. Statistical analysis was performed using one- or two-way ANOVAs for repeated measures or AUC of DA or 5-HT values followed by Newman–Keuls post-hoc test.
Quantitative autoradiographic measurements obtained from the different radioligands were analysed using one-way ANOVA followed by Newman–Keuls post-hoc test or Student's t test, as appropriate. Data are expressed as means±s.e.m. Statistical significance has been set at the 95% confidence level (two tailed).
Results
Basal values of DA and 5-HT in mPFC dialysates
Basal extracellular levels of DA and 5-HT in dialysates from mPFC of mice and rats are shown in Table 1. Non-significant differences were found between mice genotype or between control and EEDQ-pretreated rats.
Table 1. Basal DA and 5-HT dialysate values in the mPFC of mice and rats.
| Group | Baseline DA (fmol/20-min fraction) | Baseline 5-HT (fmol/20-min fraction) |
|---|---|---|
| WT mice | 6.3±0.8 (n=54) | 17.2±1.9 (n=25) |
| 5-HT1AR KO mice | 6.6±0.8 (n=30) | 19.7±1.9 (n=9) |
| 5-HT2AR KO mice | 5.7±0.6 (n=49) | 16.5±1.4 (n=24) |
| Control rats | 8.9±1.0 (n=27) | n.e. |
| EEDQ pretreated rats | 11.2±1.6 (n=17) | n.e. |
n.e., Not examined.
Data are means±s.e.m. of the number of animals shown in parentheses.
5-HT1AR and 5-HT2AR KO mice: receptor autoradiography and neurochemical analysis
The lack of 5-HT1ARs in these KO mice was previously assessed by autoradiography, electrophysiology and microdialysis procedures (Amargós-Bosch et al. 2004). Here, we extended previous autoradiographic observations. [3H]8-OH-DPAT binding to 5-HT1ARs showed a high density in PFC, hippocampus and raphe nuclei of WT mice (Fig. 1, panels A1–A3). Homozygous 5-HT1AR KO mice showed no specific binding in either region (Fig. 1, panels A4–A6).
Fig. 1.
Representative autoradiograms of [3H]8-OH-DPAT binding in the brains of wild-type (WT) (A1–A3, upper panels) and 5-HT1AR knockout (KO) mice (A4–A6, lower panels). In WT mice, note the high density for 5-HT1ARs in (A1) prefrontal cortex [cingulated (Cg1), prelimbic (PrL), infralimbic (IL); AP: 1.78 mm), (A2) hippocampus (AP: −2.06 mm) and (A3) dorsal raphe nuclei (DR) and entorhinal cortex (Ent) (AP: −4.36 mm). Parallel sections of the corresponding null mutant mice (KO) show a lack of [3H]8-OH-DPAT binding (A4–A6). Scale bar, 1 mm.
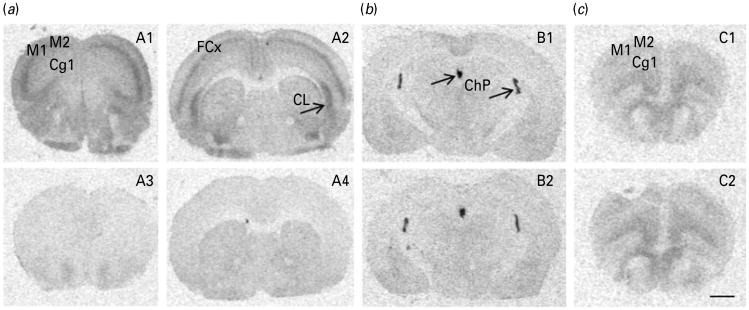
The absence of 5-HT2ARs in these KO mice was evaluated by receptor autoradiography and by the neurochemical response to the preferential 5-HT2AR agonist DOI. Autoradiographic analysis of 5-HT2ARs revealed the presence of a strong signal in the frontal cortex and claustrum of WT mice (Fig. 2, panels A1-A2). Homozygous 5-HT2AR KO mice showed no specific binding in either region (Fig. 2, panels A3–A4). Quantitative assessments of 5-HT1AR and 5-HT2CR density are shown in Table 2. No genotype differences between the densities of 5-HT2CRs and 5-HT1ARs were found in receptor-rich areas such as the choroid plexus and PFC, respectively (Fig. 2b, c).
Fig. 2.
Representative autoradiograms of [3H]mesulergine (a, b) and [3H]8-OH-DPAT (c) binding in the brains of wild-type (WT) (upper panels) and 5-HT2AR knockout (KO) mice (lower panels). In WT mice, note the expected high density for 5-HT2ARs as visualized with [3H]mesulergine plus 10−7 m SB242084 in (A1) prefrontal cortex [cingulated (Cg1). Motor (M1, M2), AP: 2.10 mm] and, (A2) frontal cortex (FCx) and claustrum (CL) (AP: 0.74 mm). Parallel sections of the corresponding null mutant mice (KO) show a conspicuous lack of binding (A3-A4). No differences between genotypes were noted for 5-HT2CRs as visualized with [3H]mesulergine plus 10−7 m spiperone in the choroid plexus (ChP) (B1-B2) nor for 5-HT1ARs, as visualized with [3H]8-OH-DPAT in prefrontal cortex (C1–C2) in WT and 5-HT2AR KO mice, respectively. Scale bar, 1 mm.
Table 2. 5-HT1AR and 5-HT2CR labelling in different brain regions from WT and 5-HT2AR KO mice.
| 5-HT1AR [3H]8- OHDPAT (fmol/mg tissue) | 5-HT2CR [3H]M esulergine +10−7 m spiperone (fmol/mg tissue) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Prefrontal cortex | Frontal cortex | Frontal cortex | Claustrum | Choroid plexus | |
| WT | 34.1±0.5 | 23.1±1.1 | 18.4±3.2 | 18.0±4.0 | 127.5±4.2 |
| 5-HT2AR KO | 32.5±1.0 | 23.2±1.7 | 13.6±4.7 | 21.3±4.6 | 128.6±9.0 |
5-HT1AR labelling by [3H]8-OHDPAT and 5-HT2CR labelling by [3H]mesulergine +10−7 m spiperone were measured in different brain regions including prefrontal cortex (AP: 2.1 mm), frontal cortex and claustrum (AP: ∼0.74 mm) and lateral and medial choroid plexuses (AP: about −1.58 mm). Results, expressed as fmol/mg tissue, are the means±s.e.m. of 4–8 observations per mouse (n = 4) (one or two observations for each hemisphere of two consecutive sections per animal and four animals per group). Non-significant differences were observed between both genotypes (Student's t test).
Perfusion of aCSF did not significantly alter DA and 5-HT output in the mPFC of WT and 5-HT2AR KO mice (DA, n = 6 and 5, respectively; 5-HT, n = 5 for each genotype) (Fig. 3a, b). Local administration of DOI (100 μm for 5-HT and 300 μm for DA; see Bortolozzi et al. 2003, 2005) enhanced 5-HT and DA output in the mPFC of WT mice (Fig. 3c, d). DOI induced a maximal elevation of the 5-HT output to 205±19% of baseline (n = 10) [F(15, 135) = 7.71, p <0.001]. Similarly, DOI elevated DA output to 192±23% of baseline (n = 10) [F(15, 135) = 6.46, p<0.001]. Neither of these effects was observed when DOI was perfused in the mPFC of 5-HT2AR KO mice (Fig. 3c, d).
Fig. 3.
Local effect of the 5-HT2A/2CR agonist DOI (100–300 μm) on the output of 5-HT (c) and DA (d) in the mPFC of wild-type (WT) and 5-HT2AR knockout (KO) mice. The perfusion of DOI increased 5-HT and DA levels in mPFC of WT mice (n = 10). Both effects were absent in 5-HT2AR KO mice (n = 7–10). The administration of aCSF did not alter prefrontal 5-HT (a) and DA (b) in either genotype (n = 5–6). Data are expressed as mean±s.e.m. See Results section for statistical analysis.
Effect of atypical antipsychotics on mPFC DA output in 5-HT1AR and 5-HT2AR KO mice
These experiments were conducted to examine whether the increase in DA output induced by APDs in mPFC is primarily associated with activation of 5-HT1ARs or with blockade of 5-HT2ARs. Local administration of clozapine (300 μm), olanzapine (100 μm) (Díaz-Mataix et al. 2005) and risperidone (100 μm) by reverse dialysis increased the DA concentration similarly in the mPFC of WT and 5-HT2AR KO mice. Two-way ANOVAs revealed a significant effect of time and non-significant effects of genotype and time×genotype interaction (Fig. 4a–c). The maximal effect induced by clozapine on mPFC DA output was 428±52% of baseline in WT mice (n = 9) and 442±54% of baseline in 5-HT2AR KO mice (n = 9) [time effect: F(15, 240) = 37.22, p<0.0001]. Olanzapine perfusion increased DA output to 363±61% of basal values in mPFC of WT mice (n = 6) and to 348±75% in 5-HT2AR KO mice (n = 7) [time effect: F(15, 165) = 10.84, p<0.0001], and the maximal elevation of mPFC DA release produced by risperidone was 283±71% of baseline in WT mice (n = 5) and 310 ± 68% of baseline in 5-HT2AR KO mice (n = 5) [time effect: F(15,120) = 10.85, p <0.0001].
Fig. 4.
The local administration of (a) clozapine (300 μm, n = 9), (b) olanzapine (100 μm, n = 6–7) and (c) risperidone (100 μm, n = 5) increased similarly DA levels in mPFC of wild-type (WT) and 5-HT2AR knockout (KO) mice. This effect was not observed when APDs were infused in the mPFC of 5-HT1AR KO mice (n = 4–6, a–c). Data are expressed as mean±s.e.m. See Results section for statistical analysis.
However, clozapine (300 μm), olanzapine (100 μm) and risperidone (100 μm) were unable to increase DA output in the mPFC of 5-HT1AR KO mice (n = 4–6) (Fig. 4a–c). Two-way ANOVAs revealed significant differences in the effects of APDs between the strains of mice: (a) clozapine [genotype effect: F(2, 19) = 7.83, p<0.01; time effect: F(15, 285) = 21.88, p< 0.0001; time × genotype interaction: F(30, 285) = 3.79, p<0.0001], (b) olanzapine [genotype effect: F(2, 14) = 5.65, p<0.05; time effect: F(15, 210) = 7.42, p<0.0001; time × genotype interaction: F(30, 210) = 2.05, p < 0.001] and (c) risperidone [genotype effect: F(2, 13) = 8.66, p < 0.01; time effect: F(15, 195) = 10.41, p<0.0001; time × genotype interaction: F(30, 195) = 3.73, p <0.0001].
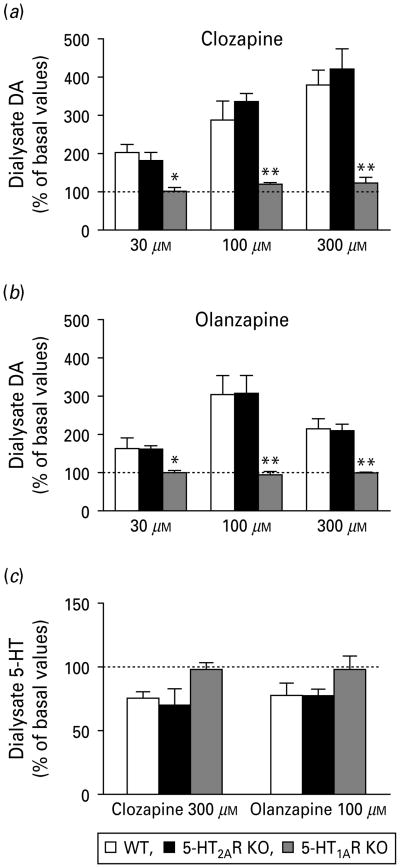
In addition, the local perfusion of clozapine and olanzapine at increasing concentrations (30– 100–300 μm) significantly raised DA concentration in mPFC of both WT and 5-HT2AR KO, but not in 5-HT1AR KO mice in a concentration-dependent manner (Fig. 5a, b). Two-way ANOVAs of AUC revealed significant differences in the effects of APDs on the different genotypes: (a) clozapine [concentration effect: F(2, 40) = 8.42, p<0.001; genotype effect: F(2, 40) = 16.02, p<0.0001] and (b) olanzapine [concentration effect: F(2, 31) = 5.40, p<0.01; genotype effect: F(2, 31) = 11.60, p<0.001]. Unlike DA, the concentration of 5-HT in mPFC dialysates was similarly affected by clozapine (300 μm) and olanzapine (100 μm) in the three genotypes. A marginal reduction was noted in WT and 5-HT2AR KO, but not in 5-HT1AR KO mice (p = 0.09 for clozapine; p = 0.17 for olanzapine; one-way ANOVA of AUCs) (Fig. 5c).
Fig. 5.
Local administration of (a) clozapine and (b) olanzapine at increasing concentrations (30–100–300 μm) raised a similar DA output in mPFC of wild-type (WT) and 5-HT2AR KO mice. This effect was not observed when APDs were perfused in the mPFC of 5-HT1AR KO mice. (c) Local effect of clozapine (300 μm) and olanzapine (100 μm) on 5-HT output in mPFC of WT, 5-HT2AR KO and 5-HT1AR KO mice. Data are AUCs (fractions 6–16) expressed as percentage of baseline. N = 4–9 mice for all groups, except for 300 μm olanzapine in mPFC of 5-HT2AR KO mice, where n = 3. See Results section for statistical analysis. * p <0.05, ** p <0.01 vs. WT and 5-HT2AR KO mice.
Effect of clozapine on mPFC DA output after GPCR silencing with selective protection of 5-HT1A or 5-HT2A/2C receptors in rats
To assess the involvement of 5-HT1A and 5-HT2A receptors in the clozapine-induced increase of DA output in rat PFC, we examined its effect in control rats and in rats whose GPCRs were previously inactivated by EEDQ together with a selective protection of 5-HT1A or 5-HT2A/2C receptors in mPFC (Amargos-Bosch et al. 2004, see Methods section).
Clozapine perfusion (300 μm) increased the DA output in control rats (n = 10) to 170 ±14% of baseline but to a much lower extent (a transient increase to 121 ± 24% of baseline) in rats with preserved 5-HT2A/2CRs (n = 5) (Fig. 6a). Two-way ANOVAs revealed a significant group effect [F(1, 13) = 9.41, p< 0.009], time effect [F(15, 195) = 3.98, p < 0.0001] and time × group interaction [F(15, 195) = 3.23, p <0.0001].
Fig. 6.
(a) The perfusion of 300 μm clozapine in mPFC of control rats increased local DA output (n = 10). This effect was absent in the mPFC of rats whose GPCRs were silenced by a prior EEDQ injection (6 mg/kg i.p.) and their prefrontal 5-HT2A/2CRs had been protected by ritanserin (300 μm) administration through the microdialysis probe (n = 5) (see Methods section). (b) Conversely, clozapine administration (300 μm) elicited a significant DA elevation in mPFC of rats treated with EEDQ whose 5-HT1ARs were preserved by prior WAY-100635 (300 μm) administration (n = 6). This effect was significantly greater than in control rats (n = 10). Data are expressed as mean ± s.e.m. See Results section for statistical analysis.
We also tested the role of mPFC 5-HT1ARs in the clozapine-induced DA release in rats using the EEDQ model. 5-HT1ARs were unilaterally protected in rat mPFC by local administration of WAY-100635 (300 μm) during EEDQ treatment (see Methods section). In this group of rats, clozapine (300 μm) elicited a significant DA elevation (299 ± 57% of baseline, n = 6) which was greater than in control rats (170 ± 14% of baseline, n = 10; see above) (Fig. 6b). Two-way ANOVAs revealed a significant effect of group [F(1, 14) = 8.17, p <0.01], time [F(15, 210) = 14.08, p< 0.0001] and time × group interaction [F(15, 210) = 3.51, p< 0.0001].
Previous studies have shown that clozapine reversed the increase of cortical 5-HT release induced by DOI (Bortolozzi et al. 2003). Since DOI also elevates DA release in mPFC by a 5-HT2AR-dependent mechanism (Bortolozzi et al. 2005), we examined whether clozapine was able to counteract the increase in DA output induced by DOI, despite its ability to increase DA output by itself. DOI (300 μm) was locally administered in the mPFC of control rats and of EEDQ-treated rats with protected 5-HT2A/2CRs (n = 6 and 8, respectively). DOI increased DA output in control rats and in those treated with EEDQ (with protected 5-HT2A/2CRs). Two-way ANOVAs revealed a significant effect of time [time effect: F(9, 108) = 18.88, p<0.0001; nonsignificant effects of the group or time × group interactions; fractions 1–10) (Fig. 7a). The co-perfusion of clozapine (300 μm) reversed the effect of DOI on DA output in the mPFC of control rats but not of those treated with EEDQ with protected 5-HT2A/2CRs [group effect: F(1, 12) = 11.58, p <0.005; time effect: F(15, 180) = 8.87, p<0.0001; time × group interaction: F(15, 180) = 3.69, p<0.0001; fractions 1–16] suggesting that this effect does not involve blockade of 5-HT2ARs.
Fig. 7.
(a) The perfusion of 300 μm DOI (5-HT2A/2C agonist) increased DA output in the mPFC of control rats and of rats treated with EEDQ and whose mPFC 5-HT2A/2CRs were unilaterally preserved by prior ritanserin administration. The perfusion of clozapine (300 μm) reversed the effect of DOI only in control rats (n = 6–8) but not in those with protected 5-HT2A/2CR. (b) In control rats, the local perfusion of BAY × 3702 (30 μm) antagonized the increase of DA output in mPFC induced by the local administration of DOI (300 μm) (n = 5). Data are expressed as mean ± s.e.m. See Results section for statistical analysis.
To examine the involvement of 5-HT1ARs in the clozapine-mediated reversal of DOI action on PFC DA output, we conducted additional experiments in which we evaluated the ability of the 5-HT1A agonist BAY × 3702 to antagonize the DOI-mediated DA increase. The local perfusion of 30 μm BAY × 3702 reversed the DA elevation in PFC induced by local DOI administration [group effect: F(1, 7) = 31.60, p<0.0008; time effect: F(15, 105) = 7.26, p<0.0001; time×group interactions: F(15, 105) = 3.04, p <0.0001] (Fig. 7b).
Autoradiographic examination of5-HT1A and 5-HT2A/2C receptors in the GPCR-silencing model
We performed additional autoradiographic experiments in rats not subjected to drug infusion (except for WAY-100635 or ritanserin administration during EEDQ treatment) to determine the site and extent of 5-HT1AR and 5-HT2A/2CR protection in the EEDQ-treated rats. EEDQ evoked a massive reduction of 5-HT1AR and 5-HT2A/2CR density (Figs. 8 and 9).
Fig. 8.
(a) Representative autoradiograms showing the density of 5-HT2A/2CRs labelled with [3H]mesulergine in coronal sections of PFC (AP in mm: 3.70–3.20) from different pretreatment groups: (A1) controls (rats received the EEDQ vehicle i.p. and aCSF through the dialysis probe), (A2) GPCR-silenced rats (injected with 6 mg/kg i.p EEDQ and perfused with aCSF through the dialysis probe), (A3) GPCR-silenced+5-HT1AR-protected rats (treated with 6 mg/kg i.p. EEDQ while 300 μm WAY-100635 was perfused through the dialysis probe), and (A4) GPCR-silenced+5-HT2A/2CR-protected rats (treated with 6 mg/kg i.p. EEDQ while 300 μm ritanserin was perfused through the dialysis probe). A5 shows non-specific binding. Panels A1a–A4a are photomicrographs showing enlargements of the marked area in panels A1–A4. Note the higher 5-HT2A/2CR binding in ipsilateral (protected) mPFC with respect to the contralateral (unprotected) side of panel A4 and the very low occupancy for 5-HT2A/2CRs in both hemispheres of panels A2 and A3. Scale bars, 2 mm (A1–A4) and 500 μm (A1a–A4a). (b) Densitometric quantification of 5-HT2A/2CR binding in mPFC including cingulate, prelimbic and infralimbic cortices of the different group of rats (B1–B4). Bars represent mean 5-HT2A/2CR fmol/mg tissue ±s.e.m. of 4–8 observations (two or four observations at left and right hemispheres of two consecutive sections per animal and two to four animals per group). ** p<0.001 significantly different from corresponding contralateral (C) and ipsilateral (I) mPFC of control rats, ++p<0.001 significantly different from contralateral mPFC of GPCR-silenced+5-HT2A/2CR-protected rats, using one-way ANOVA followed by Newman–Keuls post-hoc test.
Fig. 9.
(a) Representative autoradiograms showing the density of 5-HT1ARs labelled with [3H]8-OH-DPAT in coronal sections of PFC (AP in mm: 3.70–3.20) from different pretreatment groups: (A1) controls (rats received the EEDQ vehicle i.p. and aCSF through the dialysis probe), (A2) GPCR-silenced rats (injected with 6 mg/kg i.p. EEDQ and perfused with aCSF through the dialysis probe), (A3) GPCR-silenced + 5-HT2A/2CR-protected rats (injected with 6 mg/kg i.p. EEDQ while 300 μm ritanserin was perfused through the dialysis probe), and (A4) GPCR-silenced + 5-HT1AR-protected rats (injected with 6 mg/kg i.p. EEDQ while 300 μm WAY-100635 was perfused through the dialysis probe). A5 shows non-specific binding. Panels A1a–A4a are photomicrographs showing enlargements of the marked area in panels A1–A4. Note the higher 5-HT1AR binding in ipsilateral (protected) mPFC with respect to the contralateral (unprotected) side of A4 and the very low occupancy for 5-HT1ARs in both hemispheres of panels A2 and A3. Scale bars, 2 mm (A1–A4) and 500 μm (A1a–A4a). (b) Densitometric quantification of 5-HT1AR binding in mPFC including cingulate, prelimbic and infralimbic cortices of the different group of rats (B1–B4). Bars represent mean 5-HT1AR fmol/mg tissue ±s.e.m. of 4–8 observations (two or four observations at left and right hemispheres of two consecutive sections per animal and two to four animals per group). ** p<0.001 significantly different from corresponding contralateral (C) and ipsilateral (I) mPFC of control rats, ++p<0.001 significantly different from contralateral mPFC of GPCR-silenced + 5-HT1AR-protected rats, using one-way ANOVA followed by Newman–Keuls post-hoc test.
Local, unilateral ritanserin perfusion by reverse dialysis in the mPFC partially avoided 5-HT2A/2CR inactivation by EEDQ. Densities of 5-HT2A/2CR-binding sites in the ipsilateral (protected) mPFC at AP coordinates (in mm) 3.20–3.70 ranged from 61% to 83% (mean 71±4%, n = 4 rats) relative to mPFC of control rats in the same hemisphere (n = 2). In the contralateral (unprotected) side, 5-HT2A/2CR-binding sites were 31 ± 2% (n = 4 rats) relative to the mPFC in the same hemisphere of control rats (n = 2). One-way ANOVA indicated that [3H]mesulergine binding in ipsilateral mPFC of the GPCR-silenced + 5-HT2A/2CR-protected group was significantly different from contralateral mPFC (p <0.001) (Fig. 8a, b). Coronal sections from an AP coordinate distant from local ritanserin administration (e.g. 4.20 mm), revealed a marginally significant difference between ipsilateral and contralateral sides (68±3 vs. 43±3% relative to PFC in the same hemispheres of control rats, respectively, p = 0.058).
Figure 9a shows autoradiograms of 5-HT1ARs in PFC at AP 3.20–3.70 mm from different groups of rats: control (n = 2), GPCR-silenced (n = 2), GPCR-silenced + 5-HT2A/2CR-protected (n = 4) and GPCR-silenced + 5-HT1AR-protected (n = 4). WAY-100635 perfusion partially protected 5-HT1ARs from inactivation by EEDQ. One-way ANOVA indicated that [3H]8-OH-DPAT binding in ipsilateral PFC of the GPCR-silenced + 5-HT1AR-protected group was significantly different from contralateral PFC (51±7 vs. 17±4% relative to ipsilateral and contralateral cortices of control rats, respectively, p <0.001) (Fig. 9b). In this case, 5-HT1AR density was also significantly different between both ipsilateral and contralateral mPFC at AP ∼4.20 mm of GPCR-silenced + 5-HT1AR-protected rats (data not shown).
Discussion
The main finding of the present study is that APDs such as clozapine, olanzapine and risperidone do not require interaction with 5-HT2ARs to elevate DA release in rodent mPFC. This observation is relevant to understanding the neurobiological basis of the superior therapeutic action of these APDs in schizophrenia (Leucht et al. 2009) and may help to develop new drugs overcoming the limitations of existing treatments.
Methodological considerations
Two experimental models have been used in the present study: (a) mice lacking 5-HT1A or 5-HT2A receptors, and (b) rats, whose GPCRs were inactivated by EEDQ using selective protection of 5-HT1A or 5-HT2A/2C receptors.
The lack of 5-HT1ARs in KO mice (Parks et al. 1998) was assessed by receptor autoradiography, electro-physiology and microdialysis (Amargós-Bosch et al. 2004; present study). Here we extend these observations to 5-HT2AR KO mice (Fiorica-Howells et al. 2002). A preliminary account of these data has been presented previously (Bortolozzi et al. 2007a). We show the absence of compensatory changes of 5-HT1AR and 5-HT2CR proteins in mice lacking 5-HT2ARs, similarly to Popa et al. (2005) who reported an unaltered 5-HT2CR mRNA expression in 5-HT2AR KO mice. Consistent with the autoradiographic data, the preferential5-HT2AR agonist DOI did not increase 5-HT and DA release in the mPFC of 5-HT2AR KO mice, an effect requiring the activation of post-synaptic 5-HT2ARs on pyramidal cells projecting to the midbrain monoaminergic nuclei (Bortolozzi et al. 2005; Martín-Ruiz et al. 2001; Vázquez-Borsetti et al. 2009).
To examine the role of 5-HT1A and 5-HT2A/2C receptors in rat PFC, we used a previously described model (Amargós-Bosch et al. 2004), consisting in the selective protection of one or other receptor from the inactivating action of EEDQ (Battaglia et al. 1987; Gozlan et al. 1994; Keck & Lakoski, 2000) through the local administration of antagonists (WAY-100635 or ritanserin, respectively) to occupy 5-HT1A or 5-HT2A/2C receptors in mPFC during EEDQ treatment. This model is far from the specificity of KO mice yet it allows for a preliminary examination of the involvement of 5-HT1A and 5-HT2A receptors on the effects of APDs in the rat brain.
The present autoradiographic data indicate that (1) EEDQ produces a massive loss of 5-HT1A and 5-HT2A/2C receptors in vivo, and (2) the local protection of 5-HT1A or 5-HT2A/2C receptors by the respective antagonists was relatively successful, as shown by differences in receptor density between (a) ipsilateral (protected) and contralateral (unprotected) mPFCs, and (b) the ipsilateral side in EEDQ-treated and control rats. The fact that receptor densities in protected sides were lower than in control rats may be partly due to the damage caused by the dialysis probe, which forced us to use coronal sections relatively distant from the administration site, and thus, with lower antagonist occupancy than sites close to the microdialysis probes receiving a higher antagonist concentration.
5-HT1ARs have a great sensitivity to EEDQ in vitro (Gozlan et al. 1994). The present in-vivo data are consistent with this view, since EEDQ reduced 5-HT1A and 5-HT2A/2C receptor densities to 15% and 30% of controls, respectively. Similar differences have been noted for DA D1 and D2 receptors (see Cox & Waszczak, 1993; Hemsley & Crocker, 2001 and references therein). Interestingly, despite measured receptor densities in protected sides being lower than 100% of controls, DOI increased DA release to the same extent in the mPFC of control rats and of those receiving EEDQ + ritanserin, indicating that local 5-HT2A/2CRs remained entirely functional using this experimental paradigm. Similar results have been reported for 5-HT1AR agonists (Amargós-Bosch et al. 2004).
Role of 5-HT1A and 5-HT2A receptors in the APD-induced DA release
Despite the diverse pharmacological profiles of APDs (Arnt & Skarsfeldt, 1998), they share the ability to increase DA release in rodent mPFC through 5-HT1AR activation (Bortolozzi et al. 2007b; Díaz-Mataix et al. 2005; Ichikawa et al. 2001; Li et al. 2009; Rollema et al. 1997, 2000). This effect was attributed to simultaneous blockade of 5-HT2A and D2 receptors (Ichikawa et al. 2001) yet it seems to depend exclusively on the activation of 5-HT1ARs in mPFC (Bortolozzi et al. 2007b; Díaz-Mataix et al. 2005). In the present study, we further confirm these previous observations in 5-HT1AR KO mice and show that 5-HT2AR blockade is not a requirement. Further, we extend these observations to rat mPFC, where clozapine increased local DA release in presence of ∼50% of mPFC 5-HT1ARs but not in rats whose 5-HT1ARs were inactivated to 15% of controls by EEDQ treatment. The greater DA increase induced by clozapine in rats whose GPCRs were silenced by EEDQ – yet with preserved 5-HT1ARs – suggests an additional regulatory role of other receptors in the clozapine-evoked DA release (e.g. DA D2, α2-adrenoceptors) once the DA increase has been induced by 5-HT1AR stimulation. It has been suggested that WAY-100635 may also bind to DA D4 receptors in addition to 5-HT1ARs (Chemel et al. 2006; Martel et al. 2007) and therefore, some protection for D4 receptors may exist in the EEDQ + WAY-100635 model. Thus, it cannot be excluded that D4 receptors play a role in the clozapine-induced cortical DA release using this model in rat mPFC despite its effects being totally absent in 5-HT1AR KO mice (Díaz-Mataix et al. 2005; present study).
The apparent bell-shaped dose–effect relationship of olanzapine on DA release (Fig. 5b) suggests the involvement of other prefrontal monoaminergic receptors (e.g. α1-adrenoceptors; Amargós-Bosch et al. 2003) for which olanzapine shows nm affinity (Arnt & Skarsfeldt, 1998).
The similar in-vivo DA increases in PFC produced by several APDs (Díaz-Mataix et al. 2005; Ichikawa et al. 2001), does not bear a relationship with their in-vitro affinities for 5-HT1ARs, e.g. high for ziprasidone, low for clozapine and risperidone (yet clozapine occupies 5-HT1ARs in vivo; Chou et al. 2003) or negligible for olanzapine (Arnt & Skarsfeldt, 1998; Bymaster et al. 1996; Newman-Tancredi et al. 1998). The exact way by which APDs lacking in-vitro affinity interact in vivo with 5-HT1AR-mediated neurotransmission is unclear. The DA output induced by APDs and 5-HT1A agonists was cancelled by co-perfusion with the GABAA antagonist bicuculline, suggesting the involvement of 5-HT1ARs in GABA interneurons (Díaz-Mataix et al. 2005). Given the inhibitory nature of 5-HT1ARs, a preferential action of APDs on 5-HT1ARs located on GABA interneurons would eventually result in an increased excitatory cortical output to the VTA to enhance DA neuron activity (Gessa et al. 2000), an effect qualitatively similar to that of selective 5-HT1A agonists (Díaz-Mataix et al. 2005, 2006).
The increase in mPFC DA release produced by APDs might theoretically result from an interaction between 5-HT1A and 5-HT2A receptors in neurons co-expressing these receptors (Amargós-Bosch et al. 2004). Thus, 5-HT2AR blockade by APDs might alter the physiological balance between 5-HT1A and 5-HT2A receptors, resulting in an increase of 5-HT1AR-mediated neurotransmission. However, the present data do not support this possibility, since the APDs clozapine, olanzapine and risperidone increased a similar DA release in the mPFC of mice lacking 5-HT2ARs (with no alteration of 5-HT1AR density) and in WT controls. These results in mice were confirmed by rat data showing that clozapine was ineffective in enhancing DA output in the mPFC of rats whose 5-HT1ARs were inactivated (∼15% of controls), yet whose 5-HT2A/2CRs were protected (∼70% of controls).
Interestingly, and despite its ability to stimulate DA release when given alone, clozapine counteracted the increase in PFC DA output induced by DOI, suggesting a different effect in PFC in basal or stimulated conditions. This pattern is similar to that observed in some electrophysiological studies, where clozapine displays a state-dependent action, reducing neuronal hyperactivity (e.g. Homayoun & Moghaddam, 2007; Kargieman et al. 2007; Schwieler & Erhardt, 2003). Thus, clozapine would activate the mesocortical DA system from basal conditions but would dampen cortical hyperactivity.
Clozapine could not reverse the effect of DOI in GPCR-silenced + 5-HT2A/2CR-protected rats. In this group, 5-HT2ARs were entirely functional, as indicated by the local effect of DOI on DA output, comparable to that in controls. The inability of clozapine to counteract DOI's effect in these rats suggests the involvement of other receptors, different from 5-HT2ARs, to reverse the action of DOI in control rats. Although we could not perform a systematic study, the comparable effect of clozapine and the selective 5-HT1AR agonist BAY × 3702 suggests the involvement of 5-HT1ARs.
Overall, these findings indicate that the stimulation of mesocortical DA release by APDs does not require the presence of 5-HT2ARs and suggest that these drugs activate 5-HT1ARs to enhance cortical DA neurotransmission. Alternatively, it is conceivable that other receptor–receptor interactions might explain this marked discrepancy between in-vitro and in-vivo actions of APDs at 5-HT1ARs.
Functional consequences
The present data, obtained in mice and rats using two different experimental models (permanent 5-HT receptor KO mice and GPCR inactivation with selective protection of 5-HT receptors) indicate that blockade of 5-HT2ARs by APDs is not a necessary step to elevate DA release and that this effect is mediated by indirect activation of 5-HT1ARs in PFC. The molecular/cellular basis of the present in-vivo results is not known.
These observations do not preclude at all that 5-HT2AR blockade by APDs participates in their therapeutic action. Our conclusions are restricted to the role of 5-HT receptors required to enhance mesocortical DA, an effect potentially important for the actions of APDs on negative symptoms and cognitive deficits of schizophrenia patients. Given the lack of adequate treatment of these problems, further detailed studies are required to examine the ability of APDs – and in particular, of clozapine – to stimulate cortical 5-HT1AR-mediated neurotransmission despite their low or negligible in-vitro affinity.
Acknowledgments
This work was supported by grants SAF 2007-62378 and SENY Fundació. A.B. is recipient of a Ramón y Cajal contract from MICINN-IDIBAPS. M.M. is a recipient of a predoctoral fellowship from CSIC (I3P program). We thank the pharmaceutical companies for drug supply. We also thank Leticia Campa for her skilful maintenance of HPLC equipment and dialysate analyses. We acknowledge the skilful technical assistance of Mrs Verónica Paz. We thank Dr M. Hamon and Dr L. Lanfumey (Paris, France) for their help with 5-HT2AR KO mice.
Footnotes
Statement of Interest: None.
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. American Journal of Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M, Bortolozzi A, Adell A, Artigas F. Stimulation of α1-adrenoceptors in the rat medial prefrontal cortex increases the local in vivo 5-hydroxytryptamine release. Reversal by antipsychotics drugs. Journal of Neurochemistry. 2003;87:831–842. doi: 10.1046/j.1471-4159.2003.02044.x. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine-2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Artaloytia JF, Arango C, Lahti A, Sanz J, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. American Journal of Psychiatry. 2006;163:488–493. doi: 10.1176/appi.ajp.163.3.488. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Assié MB, Ravailhe V, Faucillon V, Newman-Tancredi A. Contrasting contribution of 5-hydroxytryptamine1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. Journal of Pharmacology and Experimental Therapeutics. 2005;315:265–272. doi: 10.1124/jpet.105.087163. [DOI] [PubMed] [Google Scholar]
- Bantick RA, Deakin JF, Grasby PM. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics? Journal of Psychopharmacology. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Norman AB, Creese I. Differential serotonin2 receptor recovery in mature and senescent rat brain after irreversible receptor modification: effect of chronic reserpine treatment. Journal of Pharmacology and Experimental Therapeutics. 1987;243:69–75. [PubMed] [Google Scholar]
- Bonaccorso S, Meltzer HY, Li Z, Dai J, et al. SR46349-B, a 5-HT2A/2C receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology. 2002;27:430–441. doi: 10.1016/S0893-133X(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargós-Bosch M, Adell A, Díaz-Mataix L, et al. In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT2A receptors: effect of antipsychotic drugs. European Journal of Neuroscience. 2003;18:1235–1246. doi: 10.1046/j.1460-9568.2003.02829.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Díaz-Mataix L, Cortés R, Scorza C, et al. Clozapine increases dopamine output in medial prefrontal cortex by a 5-HT2A receptor-independent mechanism. Society for Neuroscience. 2007a Abstract 499.12. [Google Scholar]
- Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, et al. The activation of 5-HT2A receptors in prefrontal cortex enhances dopaminergic activity. Journal of Neurochemistry. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Díaz-Mataix L, Toth M, Celada P, et al. In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology (Berlin) 2007b;191:745–758. doi: 10.1007/s00213-007-0698-y. [DOI] [PubMed] [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophrenia Bulletin. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Stuchlik A, Svoboda J, Bures J, et al. Risperidone and ritanserin but not haloperidol block effect of dizocilpine on the active allothetic place avoidance task. Proceedings of National Academic of Sciences USA. 2008;105:1061–1066. doi: 10.1073/pnas.0711273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. Journal of Neuroscience. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, et al. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berlin) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Chou YH, Halldin C, Farde L. Occupancy of 5-HT1A receptors by clozapine in the primate brain: a PET study. Psychopharmacology (Berlin) 2003;166:234–240. doi: 10.1007/s00213-002-1256-2. [DOI] [PubMed] [Google Scholar]
- Chung YC, Li Z, Dai J, Meltzer HY, et al. Clozapine increases both acetylcholine and dopamine release in rat ventral hippocampus: role of 5-HT1A receptor agonism. Brain Research. 2004;1023:54–63. doi: 10.1016/j.brainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Cox RF, Waszczak BL. Inhibition of substantia nigra dopamine cell firing by R(-)-N-n-propylnorapomorphine: electrophysiological and autoradiographic studies after regional inactivation of dopamine receptors by microinjection of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. Brain Research. 1993;613:32–42. doi: 10.1016/0006-8993(93)90450-2. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L, Artigas F, Celada P. Activation of pyramidal cells in rat medial prefrontal cortex projecting to ventral tegmental area by a 5-HT1A receptor agonist. European of Neuropsychopharmacology. 2006;16:288–296. doi: 10.1016/j.euroneuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, et al. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. Journal of Neuroscience. 2005;25:10831–10843. doi: 10.1523/JNEUROSCI.2999-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Morrow BA, Redmond DE, Jr, et al. Clozapine normalizes prefrontal cortex dopamine transmission in monkeys subchronically exposed to phencyclidine. Neuropsychopharmacology. 2008;33:491–496. doi: 10.1038/sj.npp.1301448. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14:1–21. [PubMed] [Google Scholar]
- Fiorica-Howells E, Hen R, Gingrich J, Li Z, et al. 5-HT2A receptors: location and functional analysis in intestines of wild-type and 5-HT2A knockout mice. American Journal of Physiology, Gastrointestinal and Liver Physiology. 2002;282:G877–993. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, USA: Academic Press; 1997. [Google Scholar]
- Gessa GL, Devoto P, Diana M, Flore G, et al. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology. 2000;22:642–649. doi: 10.1016/S0893-133X(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Laporte AM, Thibault S, Schechter LE, et al. Differential effects of N-ethoxycarbonyl-2-ethoxy-12-dihydroquinoline (EEDQ) on various 5-HT receptor binding sites in the rat brain. Neuropharmacology. 1994;33:423–431. doi: 10.1016/0028-3908(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural Brain Research. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Sacchetti E, Galluzzo A, Romeo F, et al. A randomized double-blind comparison of ziprasidone vs. clozapine for cognition in patients with schizophrenia selected for resistance or intolerance to previous treatment. Schizophrenia Research. 2008;105:138–143. doi: 10.1016/j.schres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Crocker AD. Changes in muscle tone are regulated by D1 and D2 dopamine receptors in the ventral striatum and D1 receptors in the substantia nigra. Neuropsychopharmacology. 2001;25:514–526. doi: 10.1016/S0893-133X(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Fine-tuning of awake prefrontal cortex neurons by clozapine: comparison with haloperidol and N-desmethylclozapine. Biological Psychiatry. 2007;61:679–687. doi: 10.1016/j.biopsych.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, O'Laughlin IA, et al. 5-HT2A and D2 receptor blockade increases cortical DA release via of 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. Journal of Neurochemistry. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, et al. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proceeding of National Academic of Science USA. 2007;104:14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck BJ, Lakoski JM. Regional heterogeneity of serotonin(1A) receptor inactivation and turnover in the aging female rat brain following EEDQ. Neuropharmacology. 2000;39:1237–1246. doi: 10.1016/s0028-3908(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 1999;288:774–781. [PubMed] [Google Scholar]
- Leucht S, Corves C, Arbter D, Engel RR, et al. Second-generation versus first generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Li Z, Prus AJ, Dai J, Meltzer HY. Differential effects of M1 and 5-HT1A receptors on atypical antipsychotics drug-induced dopamine efflux in the medial prefrontal cortex. Journal of Pharmacology and Experimental Therapeutics. 2009;330:948–955. doi: 10.1124/jpet.109.155663. [DOI] [PubMed] [Google Scholar]
- Liégeois JF, Ichikawa J, Meltzer HY. 5-HT2A receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Research. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Archives of Pharmacology. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Tecott LH, Palacios JM, Mengod G, et al. Serotonin 5-HT2C receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. Journal of Neuroscience Research. 2002;67:69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormière AM, Faucillon V, et al. WAY-100635 has high selectivity for serotonin 5-HT1A versus dopamine D(4) receptors. European Journal of Pharmacology. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Martín-Ruiz R, Puig MV, Celada P, Shapiro DA, et al. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate dependent mechanism. Journal of Neuroscience. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D1, D2 and serotonin2 pKi values. Journal of Pharmacology and Experimental Therapeutics. 1989;251:238–246. [PubMed] [Google Scholar]
- Meltzer HY, Sumiyoshi T. Does stimulation of 5-HT1A receptors improve cognition in schizophrenia. Behavioral Brain Research. 2008;195:98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Mengod G, Vilaró MT, Raurich A, López-Giménez JF, et al. 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochemical Journal. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Improving the treatment of schizophrenia: focus on serotonin 5-HT1A receptors. Journal of Pharmacology and Experimental Therapeutics. 2000;295:853–861. [PubMed] [Google Scholar]
- Minabe Y, Hashimoto K, Watanabe KI, Ashby CR., Jr Acute and repeated administration of the selective 5-HT2A receptor antagonist M100907 significantly alters the activity of midbrain dopamine neurons: an in vivo electrophysiological study. Synapse. 2001;40:102–112. doi: 10.1002/syn.1031. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, et al. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. European Journal of Pharmacology. 1998;355:245–256. doi: 10.1016/s0014-2999(98)00483-x. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Dencker SJ, Malm U, Dahl ML, et al. D2 and 5-HT2 receptor occupancy in high-dose neuroleptic-treated patients. International Journal of Neuropsychopharmacology. 1998;1:95–101. doi: 10.1017/S1461145798001229. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, et al. Increased anxiety of mice lacking the 5-HT1A receptor. Proceedings of National Academic of Sciences USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Research. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th. Sydney, Australia: Academic Press; 2005. [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, et al. M100,907, a selective 5-HT2A antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Research. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Popa D, Léna C, Fabre V, Prenat C, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. Journal of Neuroscience. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cerebral Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Díaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cerebral Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Rënyi L, Evenden JL, Fowler CJ, Jerning E, et al. The pharmacological profile of (R)-3,4-dihydro-N-isopropyl-3-(N-isopropyl-Npropylamino)-2H-1-benzopyran arboxamide, a selective 5-hydroxytryptamine(1A) receptor agonist. Journal of Pharmacology and Experimental Therapeutics. 2001;299:883–893. [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, et al. 5-HT1A receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biological Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. European Journal of Pharmacology. 1997;338:R3–R5. doi: 10.1016/s0014-2999(97)81951-6. [DOI] [PubMed] [Google Scholar]
- Roth BL, Shefflerb D, Potkin SG. Atypical antipsychotic drug actions: unitary or multiple mechanisms for ‘atypicality’? Clinical Neuroscience Research. 2003;3:108–117. [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, et al. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S. Inhibitory action of clozapine on rat ventral tegmental area dopamine neurons following increased levels of endogenous kynurenic acid. Neuropsychopharmacology. 2003;28:1770–1777. doi: 10.1038/sj.npp.1300255. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, et al. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. Journal of Pharmacology and Experimental Therapeutic. 1993;266:1374–1384. [PubMed] [Google Scholar]
- Sumiyoshi C, Sumiyoshi T, Roy A, Jayathilake K, et al. Atypical antipsychotic drugs and organization of long-term semantic memory: multidimensional scaling and cluster analyses of category fluency performance in schizophrenia. International Journal of Neuropsychopharmacology. 2006;9:677–683. doi: 10.1017/S1461145705006310. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, et al. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. American Journal of Psychiatry. 2001a;158:1722–1725. doi: 10.1176/appi.ajp.158.10.1722. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Yamashita I, Nohara S, et al. The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biological Psychiatry. 2001b;49:861–868. doi: 10.1016/s0006-3223(00)01025-8. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Nakayama T, Ichimiya T, et al. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. American Journal of Psychiatry. 2003;160:334–340. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]
- Youngren KD, Inglis FM, Pivirotto PJ, Jedema HP, et al. Clozapine preferentially increases dopamine release in the rhesus monkey prefrontal cortex compared with the caudate nucleus. Neuropsychopharmacology. 1999;20:403–412. doi: 10.1016/S0893-133X(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Vázquez-Borsetti P, Cortés R, Artigas F. Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cerebral Cortex. 2009;19:1678–1686. doi: 10.1093/cercor/bhn204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Archives of General Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zal DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. International Journal of Neuropsychopharmacology. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]