Abstract
BACKGROUND
A foreign body reaction (FBR) is a typical tissue response to a biomaterial that has been injected or implanted in human body tissue. There has been a lack of data on the classification of foreign body reaction to silicone injection, which can describe the pattern of body tissue responses to silicone.
OBJECTIVE
Determine the foreign body reaction to silicone injection.
METHOD
We modified the classification proposed by Duranti and colleagues, which has categorized a FBR to hyaluronic acid injection into a new classification of an FBR to silicone injection. A cohort study of 31 women suffering from silicone-induced granulomas on their chin was conducted. Granulomatous tissue and submental skin were stained with hematoxylin–eosin and evaluated.
RESULTS
Our data revealed that there were at least 7 categories of FBRs to silicone injection that could be developed. Categories 1 to 4 showed inflammatory activity, and categories 5 to 8 showed tissue repair by fibrosis.
CONCLUSION
Using histopathological staining, we are able to sequence the steps of body reactions to silicone injection. Initial inflammatory reaction is then replaced by fibrosis process repairing the damaged tissues. The process depends on the host immune tolerance.
Filling materials are used for reconstruction of body parts with congenital or traumatic damage. Discovery of stable materials deemed to be “inert” by the human body has allowed more extensive use of these filling materials. Nevertheless, almost all medical-grade filling materials can trigger a “foreign body reaction” (FBR) in the human body tissue. Foreign body reactions can be caused by the chemical properties of the materials or inappropriate application.1
Silicone is the most inert and permanent filling material compared with hyaluronic acid, polymethylmethacrylate, and collagen.2 In addition, its stability and nontoxic properties make it an ideal filling material. The hydrophobicity of silicone, however, triggers adsorption of plasma proteins on the surface of silicone, and a “protein matrix” is formed. The latter triggers the immune system: blood clotting, fibrinolysis, and activation of kinin and complement systems. In addition, various cytokines, chemokines, and growth factors in the matrix on the silicone surface cause activation of certain immune cells. This sequence of reactions is called the “Vroman effect.”3 An inflammatory reaction at the silicone surface produces a microenvironment containing tumor necrosis factor (TNF) α, which in turn triggers maturation of extracellular matrix (ECM) proteins within fibroblasts, thereby causing fibrosis.4 When liquid silicone is injected into tissue, the silicone forms “microdroplets” of 20 to 100 μm2 that can migrate to other areas because of gravity. There are 2 types of migration, that is, a large-volume silicone, which migrates along tissue planes, and a small-volume silicone, which will stay in the tissue and will have undergone phagocytosis by macrophages into small microdroplets. Microdroplets of <15 μm may undergo further phagocytosis by macrophages and will be transported through lymphatic vessels. By contrast, large microdroplets cannot be phagocytosed and cause macrophages to become activated on the silicone surface. In addition, the reaction between silicone and the tissue creates fibrous capsules and triggers granuloma formation.2,5,6
An FBR comprises a series of inflammatory reactions followed by fibrosis. The nature of these reactions is highly dependent on the immune system of the host. Thus, to observe this series of reactions and identify the category of the FBR, biopsies of granulomatous tissue should be performed because biopsy is a more reliable evaluation than measuring cytokine levels in blood.
Duranti and colleagues7 divided FBRs into 4 categories. Our research team, however, do not consider their categories to be suitable for assessment of FBRs to injected silicone. We believe that the dynamics of inflammatory cells in body tissue and surface of fibrotic areas can be used to identify a specific category of FBRs at a particular time. When inflammatory reaction caused by injected silicone has started to diminish, fibrosis appears; therefore, it can serve as a marker that tissue repairing is underway. Hence, we assumed that the area of fibrosis could also be a marker for determining FBR categories. In this study, we proposed a category of FBRs based on the severity of inflammatory reactions, degree of tissue repair, and sequence of immune responses.
Materials and Methods
Patients
The participants in our study were 31 Indonesian women aged 20 to 55 years who had received industrial silicone injection on their chin and subsequently had suffered granulomatous reactions. Thirty-seven specimens of normal skin from patients who had been scheduled to undergo facelift surgery served as the control group. The silicone content was analyzed using atomic absorbance spectroscopy (AAS). The analysis was conducted to confirm whether the granulomatous reactions were caused by silicone or by other filling materials. Written informed consent was obtained from all participants before specimen collection.
Specimen Collection and Foreign Body Reaction Classification
Specimens were collected from the patients who had been scheduled to undergo granuloma removal. Specimens were collected from the granuloma site, skin surrounding the granuloma, and normal skin in the same patient. Specimens of normal skin were collected from patients who had been scheduled to have a facelift surgery. From each chin, specimens of granulomatous tissue due to silicone injection and submental skin tissues surrounding the granuloma were obtained. Thirty-one specimens from the granuloma site and 31 specimens from submental skin in the same patients were obtained. A total of 62 specimens were collected. For control samples, 37 specimens of normal skin were collected from the skin below the ear after facelift surgeries. Specimens were fixed in buffered formalin and embedded in paraffin.
The specimens were then stained using hematoxylin–eosin, which were conducted at the Department of Pathology of our institution. Observations were made on 5 fields of view on each slide at 400× magnification. Histologic assessments included extravasation of inflammatory cells around silicone droplets, the number of giant cells, and areas of fibrosis. We used a double-blind method with 2 examiners for each specimen to ensure that there was an agreement regarding the category of FBRs to silicone injection. If the 2 examiners disagreed, a third examiner was consulted to make a final decision.
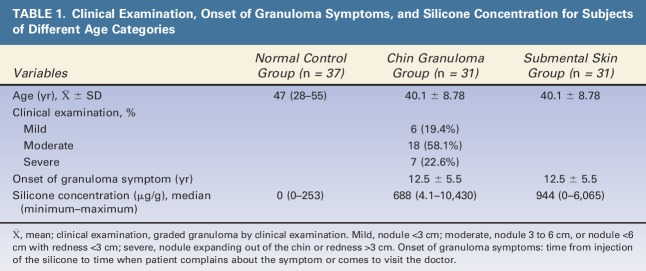
For this study, we proposed 8 categories of FBRs based on the severity of inflammatory reaction and the extent of tissue repair because of immune responses. The following categories were used for statistical analyses (Figure 1): Category 1, no visible reaction; Category 2, mild reaction with few inflammatory cells; Category 3, inflammatory cells with one or 2 giant cells; Category 4, inflammatory cells with more than 2 giant cells and <50% of the area undergoing fibrosis; Category 5, inflammatory cells with more than 2 giant cells and >50% of the area undergoing fibrosis; Category 6, inflammatory cells with one giant cell and >50% of the area undergoing fibrosis; Category 7, <50% of the areas are fibrotic with no giant cells; and Category 8, >50% of the areas are fibrotic with no giant cells. Categories 2 to 4 were determined when FBRs were dominated by inflammatory activity. Categories 5 to 8 were determined when FBRs had been led to fibrosis and tissue repair.
Figure 1.
Categories of foreign body reactions to injected silicone. Category 1: No visible reaction. Category 2: Mild reaction with a few inflammatory cells. Category 3: Inflammatory cells and one or 2 giant cells. Category 4: Inflammatory cells and more than 2 giant cells. Less than 50% of the area is fibrotic. Category 5: Inflammatory cells and more than 2 giant cells. Less than 50% of the area is fibrotic. Category 6: Inflammatory cells and one giant cell. Less than 50% of the area is fibrotic. Category 7: Less than 50% of the area is fibrotic. No giant cells. Category 8: Less than 50% of the area is fibrotic. No giant cells.
Results
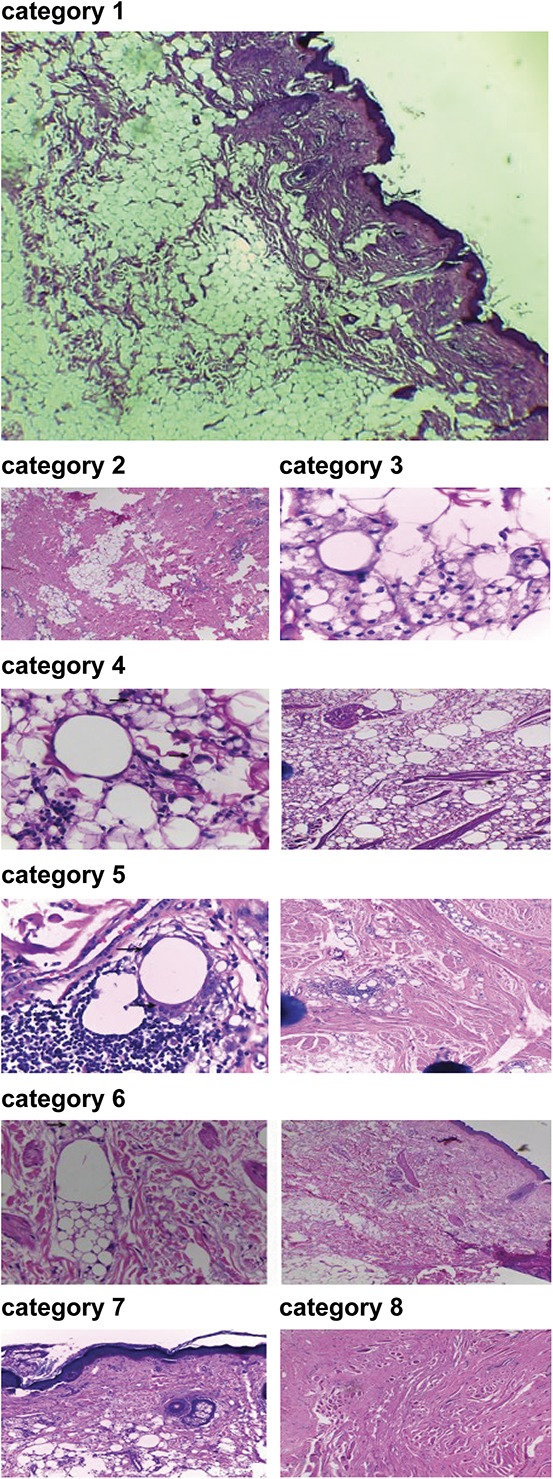
Atomic absorbance spectroscopy results showed that silicone levels were 4.1 to 10,430 μg/g in granulomatous tissues and 0 to 6,065 μg/g in submental skin tissue for all FBR categories. These findings are surprising because the mean level of silicone in normal skin is 44.07 μg/g. Specimens were obtained from patients who had received silicone injections 3 to 25 years previously. Mean age of onset for granuloma symptoms was 12.5 years (Table 1).
TABLE 1.
Clinical Examination, Onset of Granuloma Symptoms, and Silicone Concentration for Subjects of Different Age Categories
An inflammatory process was found as the initial FBR response (Figure 2). Category 4 of FBR reflected the peak inflammatory activity in tissues, which was characterized by the large number of giant cells. Fibrotic tissue was not yet widespread. Category 4 (i.e., inflammatory activity) seemed to peak at 10 to 19 years. Hence, the mean age of onset of granuloma-associated symptoms was 12.5 years (i.e., the time at which the patient came to us because of granuloma-associated symptoms). It seemed that inflammation tended to decrease after 19 years of age.
Figure 2.
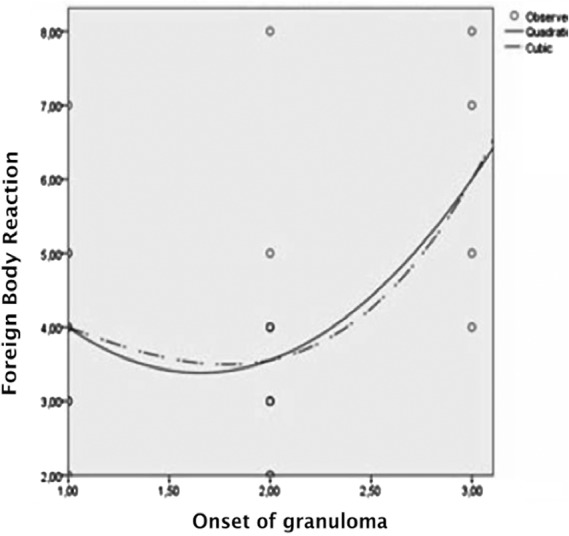
Association of 8 foreign body reaction categories and the onset of granuloma in the granuloma group. The onset of granuloma symptom can be divided into 3 Categories 1, 0 to 9 years; 2, 10 to 19 years; 3, >19 years. cCurve estimation quadratic and cubic test; *p ≤ .05; R2: Coefficient of determination for histopathology examination and the 8 foreign body reaction categories. Foreign body reactions due to injected silicone show inflammatory response followed by fibrosis. The inflammatory response peaks at 10 to 19 years, followed by decreasing activity until 25 years. Decreasing inflammatory activity was followed by increasing fibrotic activity to initiate tissue repair.
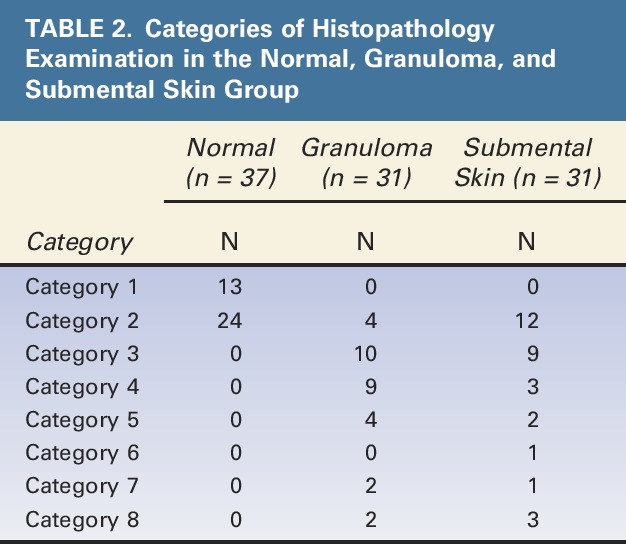
Histopathological examination showed that samples from the normal group were in Categories 1 and 2 only. Most samples in the granuloma group were included in FBR Categories 3 and 4, whereas those in the submental skin group had Categories 2 and 3 (Table 2). Therefore, peak inflammatory reaction was found in the granuloma group, and in the submental group (i.e., those with sample skin near the granuloma), the inflammatory reactions increased and reached the level of inflammation as high as those observed in the granuloma group.
TABLE 2.
Categories of Histopathology Examination in the Normal, Granuloma, and Submental Skin Group
Moreover, there was a correlation between the granuloma group and submental skin group (r = 0.507, p = .004) (Table 3). Also, there was a correlation between the onset of granuloma-associated symptoms and histopathological findings in the granuloma group (p = .020) (Figure 2) as well as in the submental skin group (p = .046) (Figure 3).
TABLE 3.
Comparison of Histopathology Between the Granuloma and Submental Skin Group
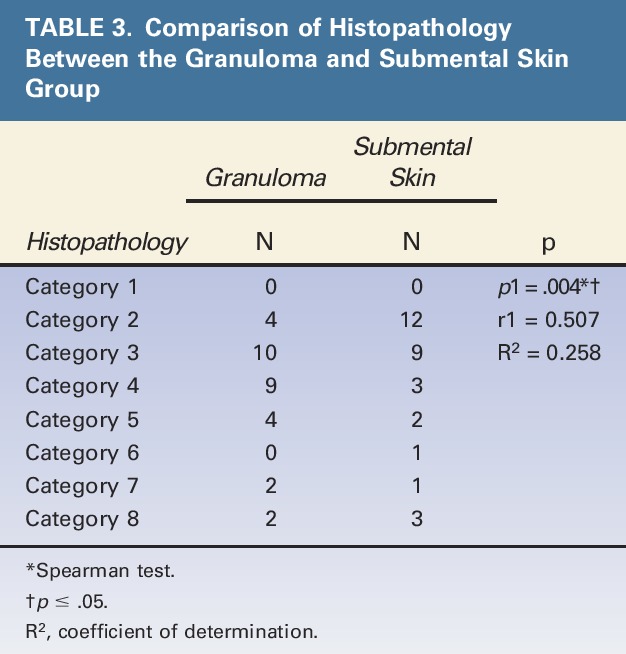
Figure 3.
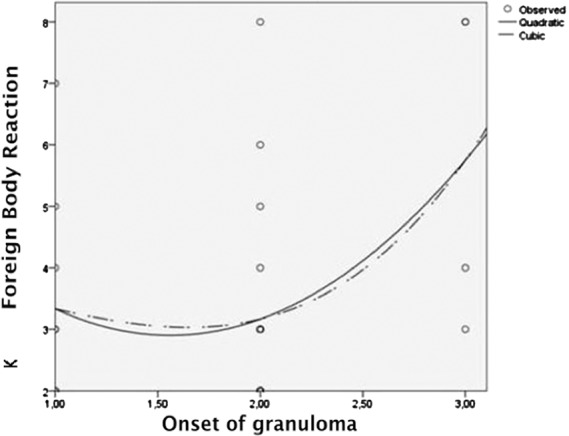
Association of 8 foreign body reaction categories and the onset of granuloma in the submental skin group. The onset of granuloma symptom can be divided into 3 Categories: 1, 0 to 9 years; 2, 10 to 19; years; 3, >19 years. cCurve estimation quadratic and cubic test; *p ≤ .05, R2: Coefficient of determination for histopathology examination and the 8 foreign body reaction categories.
Discussion
Silicone levels were 4.1 to 10,430 μg/g in granulomatous tissues and 0 to 6,065 μg/g in submental skin tissue for all FBR categories, whereas the mean level of silicone in normal skin is 44.07 μg/g (Table 1). It has been known that silicone has been widely used in everyday life, such as for households, microchips, computers, electronics, and cosmetics, including for various medications starting from antacids, syringes to mineral waters. Although we have not known how much silicone has been absorbed by our body, silicone is an essential mineral for our bone strength and elasticity. The human body needs silica about 24 to 33 mg/d. Some foods are high in silica content, such as grist, sugar cane, strawberry, and cereal.8,9
Our histopathological findings showed that samples in the normal group shared characteristics in Categories 1 and 2 only. The characteristics of the sample in Category 1 were those with no visible reaction, whereas in Category 2, there were mild reactions with few inflammatory cells. It can occur because female patients might have used skin care or other facial treatment before they decided to have the face lift. Although those samples had inflammatory cells, the process of macrophage changes into datia cells was not initiated. Datia cells are important characteristic signs of FBR.
At first, we used Duranti's FBR classification for histopathological on granulomatous tissues; however, we subsequently found 7 of 8 categories (Table 2). It seemed that the granulomatous histopathological features caused by the silicone injections were started from Category 2, which was then followed by greater inflammation and reached the greatest in Category 4. In Category 5, there was also great inflammation; however, dominant fibrosis process had taken place and the inflammation subsided, and ultimately, there was >50% fibrosis without any inflammation in Category 8 (Figure 1). It is also consistent with Figure 2, which shows the highest onset of the patients' visit due to the complaints of granuloma at Category 4, that is, between 10 and 19 years. It was consistent with the history taking, that is, the patients came with granuloma complaints with mean age of onset of 12.5 years (Table 1). In Table 2, it can be seen that the skin adjacent to the granuloma, that is, the submental skin, seemed to be more likely to have inflammatory changes similar to the granulomatous areas. Most cases of submental skin were found in Categories 2 and 3, whereas most cases of granuloma were found in Categories 3 and 4, and there was a correlation between the granuloma FBR in the chin and submental skin (Table 3, p = .004).
Specimens were obtained from patients who had received silicone injections 3 to 25 years previously. Mean age of onset of granuloma symptoms was 12.5 years (Table 1). We presume that the onset of granuloma symptoms depends on the host's immune system. Interaction between silicone antigen with or without infection and immune tolerance status can induce delayed hypersensitivity, which may cause granuloma symptoms.
Silicone is composed of nonbiodegradable molecules; therefore, it can persist in tissue long after it has been injected. Its persistence is due to its large molecular size and physical–chemical stability. Large-volume silicone after the injection will migrate along tissue planes, and small volume will stay in the tissue and will be phagocytosed by macrophages into small microdroplets (Figure 4). Microspheres of <15 μm in size are generally phagocytosed and transported to local lymph nodes. However, some larger microspheres from nonresorbable polymers with a smooth surface are encapsulated by fibrous tissue and escape phagocytosis.2
Figure 4.
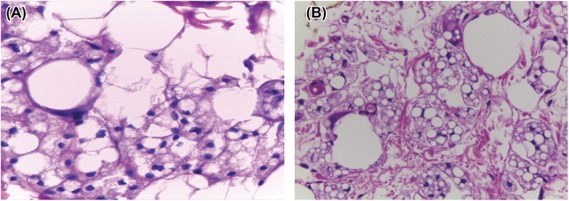
Process of phagocytosis. (A) Fusion of a macrophage with a giant cell. The giant cell is phagocytizing silicone, reducing its overall size. (B) An area with more giant cells and a reduced amount of silicone.
Giant cell formation is the result of fusion of several local macrophages. Such formation suggests that there is a chronic inflammation caused by persistence of a foreign material (e.g., silicone) in tissue.10 Macrophages phagocytose silicone, and thereby, reducing the size of the silicone deposits (Figure 4). Macrophages release cytokines, such as TNFα. Tumor necrosis factor α activity may have been dominant in Categories 2 to 4 and may have been a key factor in initiation of fibrosis (categories 5–8).
Macrophages are major producers of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases. The balance in the level of these proteins determines whether collagen from the ECM of local fibroblasts is formed or degraded. Initially, interleukin (IL)-6 is also involved in acute inflammation. Then, IL-6 changes its characteristics and helps T-helper (Th) lymphocytes, making Th1 lymphocytes the predominant cell type (in the inflammatory categories). Afterward, they are polarized into Th2 lymphocytes (during anti-inflammatory categories). In this category, IL-13 (a product of Th2 lymphocytes) activity increases and triggers fibroblast proliferation, which causes fibrosis.4 The phenomenon is shown in Figure 1, that is, the inflammatory response increased from Category 2 to Category 4, and the fibrotic process was developed during Categories 5 to 8. It seems that there is a shift of Type-1 lymphocytes in Categories 2 to 4 to Type-2 lymphocytes in Categories 5 to 8.
In the FBR classification described by Duranti and colleagues,7 the peak of Stage 4 is reached when the implant is encapsulated with an obvious FBR. Nonetheless, the value of Stage 4 FBR in their study when applied to injected silicone was not confirmed. It seemed that there is a difference; therefore, we modified the sequential categories for FBRs.
Eight categories were proposed to describe FBRs regarding injected silicone, which assessed inflammatory and tissue repair responses over a period. Categories 2 to 4 describe the dynamics of the inflammatory process with a peak activity marked by numerous giant cells and fibrosis of <50% in tissue, whereas in Category 5, there are still great inflammation with numerous datia cells, but the fibrosis are also abundant, which is >50%. Categories 6 to 8 covers the sequential reactions of fibrotic response, which is marked by the decrease (or absence) of inflammatory cells in the tissue and increased fibrosis up to ≥50%.
The pathologic FBR to a foreign body is dependent on the characteristics of the immune system, which is under genetic control.11 Therefore, tissue responses to silicone injections vary among patients even during the same period of injection. Th1 lymphocytes have roles during inflammation (Categories 2–4), whereas Th2 lymphocytes have roles in tissue repair during fibrosis (Categories 5–8). Fibrosis is supported by cytokines, which was produced by Th2 lymphocytes, and it is also supported by regulatory T (Treg) lymphocytes, which release transforming growth factor (TGF) β and IL-10. TGFβ1 is known to support fibrosis.
Activity of Treg lymphocytes increases to control the inflammation occurring in the tissue. Persistence of Treg lymphocytes in tissue can be affected by ECM proteins, such as hyaluronan. The presence of hyaluronan is a sign that the tissue is no longer damaged, signaling that Treg lymphocytes should stop contributing to an anti-inflammatory response.12 Cellular activity of Treg lymphocytes (e.g., TGFβ1 secretion) is in an autocrine manner for differentiation of Treg lymphocytes in peripheral circulation.13 However, TGFβ1 is a potent cytokine that supports fibrosis. Hence, Treg lymphocytes may also have a role in the fibrosis caused by injected silicone.
The fibrotic process is often interpreted in the context of tissue repair, and this fibrotic activity is persistent. Excessive fibrosis causes dysfunctional tissue at physiological level; however, fibrosis remains an important stage in repair of damaged tissue. Collagen proteoglycans and some ECM proteins are essential components for filling damaged tissue. Their production is stopped automatically when damaged tissue has been replenished by ECM proteins.14,15 An FBR is dependent on T-helper lymphocytes. Th1 lymphocytes predominate in the inflammation stage, and Th2 lymphocytes predominate in the fibrotic stage. Other cells, such as Treg lymphocytes, are involved in immune tolerance; however, their roles in FBRs necessitate further investigation.
All granulomas can be considered as dynamic structures, which are composed of multiple cell types. They commonly share a general characteristic, that is, the presence of a central accumulation of mononuclear cells, which is surrounded by activated T cells and cytokine release. The inflammatory granulomatous response depends on antigen involved in the granuloma formation, which is also associated with the predominant Th1 and Th2 as well as the pattern of cytokine production. It explains the specific pathological features found in granulomas.16
Similar to granuloma associated with sarcoidosis or mycobacteria infection, the dominant cytokine is IL2, which tends to indicate Type-1 granuloma,16 whereas in the FBR caused by silicone, there is fibrosis, which suggests a Type-2 granuloma.
We observed that there were at least 2 types of FBR during tissue repair. First, there was an inflammatory response followed by mild fibrosis of Categories 2 to 4 and 7. These responses resulted from attempts to repair damaged tissue after inflammatory process. Second, the inflammatory response was followed by development of severe fibrosis of Categories 2 to 6 and 8. Thus, it indicates that the physiological function of tissue was diminished after the fibrotic process was complete. It can be assumed that when the immune response of an individual is good, the inflammatory process can be suppressed, and there will be less fibrosis resulting in less tissue damage as found in patients with FBR Categories 2 to 4 and 7. Further studies on immune system and the interaction between silicone antigen and immune tolerance that contribute to delayed hypersensitivity and tissue repair should be investigated in the future.
In terms of clinical application, our study has revealed FBR categories for the ongoing process. If a granuloma is still in the inflammation stage, we suggest that it should be removed as soon as possible to avoid an extended period of inflammation, which would enlarge the granulomatous nodule. Tissue repair will continue, but the development of fibrosis could be kept to a minimum level and thereby improve cosmetic appearance. If fibrosis has been started, we suggest that the granuloma is removed as early as possible for the same reason, that is, to reach better cosmetic effect. In this study, the patients sought treatment because of their granulomas.
When the granuloma cannot be removed, other modalities can be given after wound healing period, such as using intralesion corticosteroid injection, which is 15- to 20-mg/mL triamcinolone every 2 months as well as topical treatment of pimetacrolimus b.i.d. for 3 months and topical imiquimod for 8 weeks. Other treatments include minocycline, allopurinol, and oral prednisone of 30 mg/d. However, when the results of those treatments are still not satisfying, intralesional injection of triamsinolone is more significant to overcome the occurring inflammation.17,18
Etanercept, which works on TNF-α receptor and binds the Fc domain of IgG isotypes, has been reported to have a good effect on silicone-associated granuloma. A subcutaneous administration of 50 mg twice a week or 25 mg twice weekly has provided a quite satisfying result.19–21 Further studies are necessary to discover therapy or medications that can suppress TNF-α cytokines in patients with granuloma because of silicone injection or on therapy that can increase the body tolerance.
The limitation of our study was that we evaluated the FBR to injected silicone-only histopathological examination. More studies need to be performed on how the inflammation disappears and fibrosis starts, which may depend on the subject's immune tolerance.
Our study only used unpurified industrial silicone, and therefore, it may contain other substances that may cause immune response. The impurity signifies its difference with the medical-grade liquid silicone; however, it has the same basic ingredient of liquid silicone, that is, dimethylpolysiloxane, which still has similar properties. The key reason of developing the reaction is started when the liquid silicone is being localized in the tissue, which slowly will cause adsorption of plasma protein on the surface of the silicone, forming a protein matrix and inducing the recruitment of immune cells to the matrix area resulting in nonspecific immune response in the matrix area (Vromann effect).3,22 Although for the unpurified industrial silicone, the immune reactions will develop faster than those with medical-grade silicone.
Conclusion
Foreign body reactions to injected silicone can be classified into at least 2 main categories, that is, reaction of inflammation and tissue repair/fibrosis. These 2 main categories can be broken down into 8 more definitive categories. Categories 2 to 4 reflect inflammatory activity, whereas Categories 5 to 8 reflect fibrosis, which may lead to tissue repair.
Histopathological examination showed that 8 categories of FBRs because of silicone injection could be created. Those categories could be split further into the following 7 stages without Category 1: Stage 1 (Category 2), mild reaction with few inflammatory cells; Stage 2 (Category 3), inflammatory cells with one or 2 giant cells; Stage 3 (Category 4), inflammatory cells with more than 2 giant cells and <50% of the area undergoing fibrosis; Stage 4 (Category 5), inflammatory cells with more than 2 giant cells and >50% of the area undergoing fibrosis; Stage 5 (Category 6), inflammatory cells with one giant cell and >50% of the area undergoing fibrosis; and Stage 6 (Category 7), <50% of the area now fibrotic with no giant cells; Stage 7 (Category 8), >50% of the area now fibrotic with no giant cells.
Footnotes
The authors have indicated no significant interest with commercial supporters.
References
- 1.Senet P, Bachelez H, Ollivaud L, Vignon-Pennamen D, et al. Minocycline for treatment of cutaneous silicone granulomas. Br J Dermatol 1999;140:985. [DOI] [PubMed] [Google Scholar]
- 2.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesth Plast Surg 2003;27:354–66. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Rodriguez A, Chang DT. Foreign body reactions to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wick G, Grundtman C, Mayerl C, Wimpissinger T, et al. The immunology of fibrosis. Annu Rev Immunol 2013;31:107–35. [DOI] [PubMed] [Google Scholar]
- 5.Tomazic-Jezic VJ, Merritt K, Umbreit TH. Significance of type and the size of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res 2001;55:523. [DOI] [PubMed] [Google Scholar]
- 6.Rapaport MJ, Vinnik C, Zarem H. Injectable silicone: cause of facial nodules, cellulites, ulceration, and migration. Aesth Plast Surg 1996;20:267. [DOI] [PubMed] [Google Scholar]
- 7.Duranti F, Salti G, Bovani B, Calandra M, et al. Injectable hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 1998;24:1317. [DOI] [PubMed] [Google Scholar]
- 8.Jugdaohsingh R. Silicone and bone health. J Nutr Health Aging 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- 9.Jungdaohsingh R, Anderson S, Tucker KL, Elliot H, et al. Dietary silicone intake and absorption. Am J Clin Nutr 2002;75:887–93. [DOI] [PubMed] [Google Scholar]
- 10.Cakmak O, Turkoz HK, Polat S, Serin GM, et al. Histopathologic response to highly purified liquid silicone injected intradermally in rats' skin. Aesth Plast Surg 2011;35:538–44. [DOI] [PubMed] [Google Scholar]
- 11.Noone RB. A review of possible health implications of silicone breast implants. Cancer 1997;79:174–56. [DOI] [PubMed] [Google Scholar]
- 12.Bollyky PL, Falk BA, Wu RP, Buckner JH, et al. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol 2009;86:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol 2009;27:485–17. [DOI] [PubMed] [Google Scholar]
- 14.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 15.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implication for tissue repair and fibrosis. J Pathol 2013;229:298–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agustini C, Semenzato G. Biology and immunology of granuloma. In: James DG, Zumla A, editors. Granulomatous Disorders. Cambridge, United Kindom: Cambridge University Press; 1999; p. 3–16. [Google Scholar]
- 17.Ellis LZ, Cohen JL, High W. Granulomatous reaction to silicone injection. J Clin Aesthet Deramatol.2012;5;44–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Arin MJ, Bate J, Krieg T, Hunzelmann N. Silicone granuloma of face treated with minocyline. JAAD 2005;52:S53–6. [DOI] [PubMed] [Google Scholar]
- 19.Pasternack FR, Fox LP, Engler DE. Silicone granulomas treated with etanercept. Arch Dermatol 2005;141:13–5. [DOI] [PubMed] [Google Scholar]
- 20.Desai AM, Browning J, Rosen T. Etanercept therapy for silicone granuloma. J Drugs Dermatol 2006;5:894–6. [PubMed] [Google Scholar]
- 21.Styperek A, Bayers S, Beer K. Nonmedical-grade injections of permanent fillers medical and medico-legal considerations. J Clin Aesthet Dermatol 2013;6:20–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Battiston KG, Labow RS, Santerre JP. Protein binding mediation of biomaterial-dependent monocyte activation on degradable polar hydrophobic ionic polyurethane. Biomaterials 2012;33:8316–28. [DOI] [PubMed] [Google Scholar]