Abstract
Introduction:
Platelet dysfunction following traumatic brain injury (TBI) is associated with worse outcomes. The efficacy of platelet transfusion to reverse antiplatelet medication (APM) remains unknown. Thrombelastography platelet mapping (TEG-PM) assesses platelet function. We hypothesize that platelet transfusion can reverse the effects of APM but does not improve outcomes following TBI.
Methods:
An observational study at 6 US trauma centers was performed. Adult patients on APM with CT evident TBI after blunt injury were enrolled. Demographics, brain CT and TEG-PM results before/after platelet transfusion, length of stay (LOS), and injury severity score (ISS) were abstracted.
Results:
66 patients were enrolled (89% aspirin, 50% clopidogrel, 23% dual APM) with 23 patients undergoing platelet transfusion. Transfused patients had significantly higher ISS and admission CT scores. Platelet transfusion significantly reduced platelet inhibition due to aspirin (76.0 ± 30.2% to 52.7 ± 31.5%, p < 0.01), but had a non-significant impact on clopidogrel-associated inhibition (p = 0.07). Platelet transfusion was associated with longer length of stay (7.8 v 3.5 days, p<0.01), but there were no differences in mortality.
Conclusion:
Platelet transfusion significantly decreases platelet inhibition due to aspirin but is not associated with change in outcomes in patients on APM following TBI.
Keywords: traumatic brain injury, brain injury, closed head injuryseth
Introduction
In 2010, the Centers for Disease Control estimated that traumatic brain injury (TBI) accounted for approximately 2.5 million patient emergency department visits, hospitalizations, and deaths, and remains the most common cause of death following trauma [1]. Within this population, patients taking antiplatelet medications (APM) present a challenge, as both APM and trauma cause platelet dysfunction and potentially worsen hemorrhage and the severity of the TBI [2, 3]. Studies on the effects of pre-injury antiplatelet therapy and progressive hemorrhage in patients with TBI have yielded conflicting results with some literature that suggests platelet transfusion provides no clear clinical advantage [4–17].
Quantifying the degree of platelet dysfunction in patients with TBI offers an enticing endpoint for goal-orientated resuscitation with regards to platelet transfusions. Determining the degree of platelet inhibition, however, requires the utilization of assays beyond conventional coagulation tests [18, 19]. Thrombelastography (TEG) allows for the evaluation of the humoral and cellular components of hemostasis. Additionally, the platelet-mapping assay (TEG-PM) quantifies the degree of inhibition caused by inhibition of the arachidonic acid pathway (e.g. aspirin-mediated inhibition) as well as the adenosine diphosphate pathway (e.g. clopidogrel-mediated inhibition) of platelet stimulation [3].
The purpose of this study was to evaluate the effect of platelet transfusion on changes in CT scores and clinical outcome in patients with confirmed use of APM and blunt TBI. We hypothesize that platelet transfusion can reverse the effects of APM on platelet function, demonstrated by TEG-PM, but does not improve clinical outcomes following TBI.
Methods
Patients
After obtaining institutional board review approval from each site, a retrospective multicenter cohort study of prospectively collected data was performed at four level 1 and three level 2 trauma centers in the United States from 8/1/2015 – 12/31/2016. Only facilities that routinely used TEG-PM were included. One of the level two centers was not able to enroll patients during the trial period thereby leaving four level 1 and two level 2 centers as the study sites. Patients 18 years and older who were taking an antiplatelet pharmacologic agent and were noted to have radiographically evident, blunt traumatic brain injury were enrolled. Exclusion criteria included patients less than 18 years old, penetrating brain injury, prisoners, pregnancy, patients transferred from outside hospitals to the trauma center, patients who did not undergo TEG-PM testing on arrival to the trauma center, inability to verify whether a patient was on an antiplatelet agent prior to injury, use of warfarin or a direct oral anticoagulant prior to injury, and platelet transfusion prior to obtaining a TEG-PM.
Variables and Statistical Analysis
Data fields abstracted included patient demographics, injury severity score (ISS) and the abbreviated injury score (AIS) for all injuries, type of traumatic brain injury, Marshall CT classification, complete blood count and TEG-PM test results, and timing and number of platelets transfused within the first 24 hours following arrival. Patients underwent initial and follow up CT scan imaging, TEG-PM testing, and platelet transfusion at the discretion of the treating team. As such, there was no standardization in transfusion practice or the decision to obtain repeat imaging or laboratory tests between centers. When present, the results of repeat CT scan of the brain as well as the laboratory tests and TEG-PM were recorded. CT scores were calculated by each author at their respective institutions without the aid of radiologists. Patients were divided into those who did and did not undergo a platelet transfusion. Univariate analysis between groups was carried out using Chi-square or Fisher’s exact test for categorical variables. The Student’s t-tests and Kruskal-Wallis test were used for continuous variables depending on the normalcy of distribution of data. Due to inadequate power, a predictive model of change in Marshall scoring over time was not possible. Therefore, Spearman correlation coefficients (r), which identify correlations in non-linear data distributions, were examined to identify variables that may have correlated with changes in Marshall scores. Variables for consideration included age, sex, initial temperature, INR, platelet count, percent inhibition in the arachidonic acid pathway (% Inh(AA)), percent inhibition in the adenosine diphosphate pathway (% Inh(ADP)), and severity of head injury defined as AIS head. A Spearman r > 0.20 was considered possibly clinically relevant. All data were entered into the Research Electronic Data Capture (REDCap) database by each individual center. All statistical analysis was performed using SAS version 9.3 (Cary, NC).
Outcomes
The primary outcomes were change in TEG-PM parameters following platelet transfusion and change in Marshall CT score over time. Secondary outcomes included need for operative intervention, ICU and hospital length of stay, and mortality.
Results
Sixty-six patients met inclusion criteria, 23 (35%) of whom received platelet a transfusion (table 1). Demographic variables were similar between transfusion and non-transfusion cohorts, including age (76.0 ± 9.3 years vs 78.0 ± 11.6 years, p = 0.48), female sex (65.2% vs 46.5%, p = 0.15), admission INR (1.1 ± 0.1 vs 1.1 ± 0.3, p = 0.34), and admission platelet count (246.7 ± 77.1 vs 213.4 ± 79.2, p = 0.11). Only 4% of patients in each cohort were on an APM agent other than aspirin or clopidogrel. Platelet transfusion was significantly more common in patients on clopidogrel (47.8% vs 27.9%, p < 0.01) or dual APM (39.1% vs 14.0%, p = 0.03) therapy. The average number of unit of platelets transfused was 1.2 ± 0.39 units with only 4 of 23 patients being transfused 2 units of platelets. There was significant heterogeneity (p < 0.001) in transfusion practices between centers (figure 1). Patients within the non-transfused group had a significantly higher percentage of subdural hemorrhage compared to transfused patients (46.5% vs 13.0%, p < 0.01). Transfused patients had a significantly higher mean ISS (23 ± 9 vs 17 ± 10, p = 0.02) and a significantly higher mean AIS TBI score than non-transfused patients (4.0 ± 0.37 vs 3.5 ± 0.67, p < 0.01). This trend was reflected on admission head CTs with transfused patients also having a higher mean Marshall score than non-transfused patients (2.4 ± 0.72 vs 2.1 ± 0.44, p = 0.02).
Table 1:
Baseline Patient Characteristics by Transfusion Status
| All cases (n = 66) |
Transfusion (n = 23) |
No transfusion (n = 43) |
p-value | |
|---|---|---|---|---|
| Age (years) | 77.3 ± 10.9 | 76.0 ± 9.3 | 78.0 ± 11.6 | 0.48 |
| Sex (female) | 35 (53.0%) | 15 (65.2%) | 20 (46.5%) | 0.15 |
| INR | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.3 | 0.34 |
| Platelet count | 225.0 ± 79.5 | 246.7 ± 77.1 | 213.4 ± 79.2 | 0.11 |
| APM | ||||
| Aspirin | 59 (89.4%) | 21 (91.3%) | 38 (88.3%) | 1 |
| Clopidogrel | 23 (50%) | 11(47.8%) | 12 (27.9%) | <0.01 |
| Dual APM | 15 (22.7%) | 9 (39.1%) | 6 (14.0%) | 0.03 |
| Other | 3 (4.5%) | 1 (4.3%) | 2 (4.7%) | 1.0 |
| Type of TBI | ||||
| SDH | 23 (50.0%) | 3 (13.0%) | 20 (46.5%) | <0.01 |
| EDH | 3 (4.5%) | 1 (4.3%) | 3 (7.0%) | 1.00 |
| IVH/SAH | 30 (45.5%) | 9 (39.1%) | 21 (48.8%) | 0.61 |
| IPH | 46 (63.7%) | 15 (65.2%) | 31 (72.1%) | 0.58 |
| ISS | 19.5 ± 10.4 | 23.4 ± 9.4 | 17.4 ± 10.44 | 0.02 |
| AIS | ||||
| Head | 0.59 ± 1.01 | 0.61 ± 1.16 | 0.58 ± 0.93 | 0.88 |
| TBI | 3.7 ± 0.64 | 4.0 ± 0.37 | 3.5 ± 0.67 | <0.01 |
| CT Score | 2.2 ± 0.57 | 2.4 ± 0.72 | 2.1 ± 0.44 | 0.02 |
| Hospital LOS (days) | 5.0 ± 4.2 | 7.8 ± 4.8 | 3.5 ± 2.8 | <0.01 |
| ICU LOS (days) | 1.8 ± 2.3 | 3.9 ± 2.6 | 0.73 ± 1.1 | <0.01 |
| Craniectomy / -otomy | 6 (9.1%) | 6 (26.1%) | 0 (0%) | <0.01 |
| Mortality | 3 (4.5%) | 2 (8.7%) | 1 (2.3%) | 0.28 |
N (column %) or mean ± sd are shown, INR = international normalized ratio, APM = antiplatelet medication, TBI = traumatic brain injury, SDH = subdural hematoma, EDH = epidural hematoma, IVH = intraventricular hemorrhage, SAH = subarachnoid hemorrhage, IPH = intraparenchymal hemorrhage or cerebral contusion, ISS = injury severity score, AIS = abbreviated injury score, CT Score = Marshall score from computed tomography, LOS = length of stay, ICU = intensive care unit.
Figure 1:
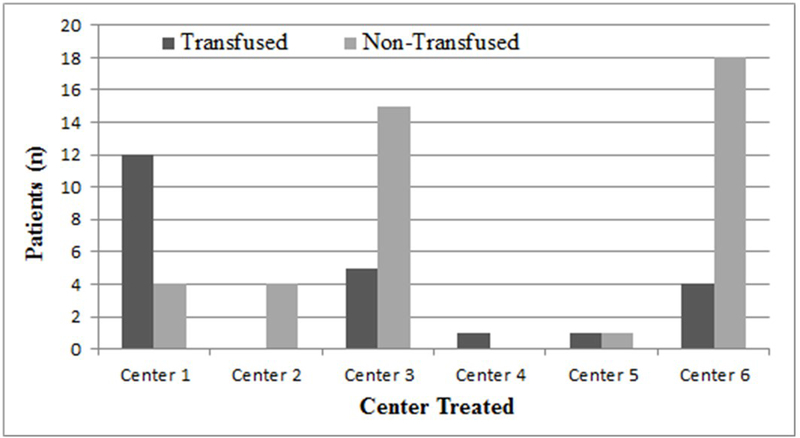
Transfusion status by center treated
When comparing the patients with major injury, defined by an ISS greater than or equal to 15, 22 patients remained in the transfusion group and 24 patients in the non-transfusion group (table 2). Similar to all-comers denoted in table 1, age, gender, admission INR and platelet count were not statistically different between groups. However, admission CT scores, AIS head, and ISS were no longer significantly different between groups, a notable difference compared to all-comers.
Table 2:
Patient Characteristics by Transfusion Status for ISS >15
| Transfusion (n = 22) |
No transfusion (n = 24) |
p-value | |
|---|---|---|---|
| Age (years) | 76.5 ± 9.2 | 77.9 ± 11.1 | 0.65 |
| Sex (female) | 13 (59.1%) | 15 (62.5%) | 0.06 |
| INR | 1.1 ± 0.1 | 1.1 ± 0.32 | 0.88 |
| Platelet count | 245.1 ± 78.5 | 208.8 ± 78.5 | 0.12 |
| APM | |||
| Aspirin | 20 (90.9%) | 19 (79.2%) | 0.06 |
| Clopidogrel | 9 (40.9%) | 8 (33.3%) | 0.92 |
| Dual APM | 8 (36.4%) | 4 (16.7%) | 0.16 |
| Other | 1 (4.5%) | 1 (4.3%) | 0.95 |
| Type of TBI | |||
| SDH | 3 (13.6%) | 10 (41.7%) | 0.04 |
| EDH | 1 (4.5%) | 1 (4.2%) | 0.95 |
| IVH/SAH | 8 (63.6%) | 10 (41.7%) | 0.71 |
| IPH | 14 (63.6%) | 16 (66.7%) | 0.83 |
| ISS | 23.9 ± 9.4 | 22.2 ± 11.8 | 0.59 |
| AIS | |||
| Head | 0.64 ± 1.2 | 0.92 ± 1.1 | 0.41 |
| TBI | 4.1 ± 0.29 | 3.9 ± 0.58 | 0.21 |
| CT Score | 2.41 ± 0.73 | 2.09 ± 0.43 | 0.08 |
| Hospital LOS (days) | 7.6 ± 4.9 | 4.1 ± 3.3 | <0.01 |
| ICU LOS (days) | 4.1 ± 2.5 | 1.3 ± 1.2 | <0.01 |
| Craniectomy / -otomy | 6 (27.3%) | 0 (0%) | <0.01 |
| Mortality | 2 (9.1%) | 1 (4.2%) | 0.50 |
N (column %) or mean ± sd are shown, INR = international normalized ratio, APM = antiplatelet medication, TBI = traumatic brain injury, SDH = subdural hematoma, EDH = epidural hematoma, IVH = intraventricular hemorrhage, SAH = subarachnoid hemorrhage, IPH = intraparenchymal hemorrhage or cerebral contusion, ISS = injury severity score, AIS = abbreviated injury score, CT Score = Marshall score from computed tomography, LOS = length of stay, ICU = intensive care unit.
There were no significant differences in baseline % Inh(AA) or % Inh(ADP) (table 3). However, patients who received a platelet transfusion had a significant reduction in % Inh(AA) following transfusion (76.0 ± 30.2 vs 52.7 ± 31.5, p < 0.01). Similarly, % Inh(ADP) also decreased following transfusion, but the change was only significant at a trend level (64.5 ± 28.0 vs 48.4 ± 31.1, p = 0.07).
Table 3:
TEG variables before and after platelet transfusion
| Pre-Platelet Transfusion | ||||
|---|---|---|---|---|
|
All cases (63) |
Transfusion (n=23) |
No transfusion (n=43) |
p-value | |
| % Inh(AA) pre | 74.2 ± 27.6 | 76.0 ± 30.2 | 73.3 ± 26.4 | 0.71 |
| % Inh(ADP) pre | 59.9 ± 27.9 | 64.5 ± 28.0 | 57.5 ± 27.9 | 0.34 |
| MA pre | 66.3 ± 5.8 | 66.6 ± 6.2 | 66.2 ± 5.6 | 0.83 |
| Pre-Transfusion | Post-Transfusion | Δ | p-value | |
| %Inh(AA) | 76.0 ± 30.2 | 52.7 ± 31.5 (10) | 23.3 ± 1.3 | <0.01 |
| %Inh(ADP) | 64.5 ± 28.0 | 48.4 ± 31.1 (10) | 16.1 ± 3.1 | 0.07 |
| MA | 66.6 ± 6.2 | 67.3 ± 5.7 (5) | 0.7 ± 0.5 | 0.38 |
Inh = inhibition, MA = maximal amplitude, AA = arachidonic acid, ADP = adenosine diphosphate
Changes in Marshall scoring over time were not significant in either cohort (figure 2). Among transfused patients, change in mean Marshall score was 0.21 ± 0.60 (pre-transfusion 2.4 ± 0.72, post-transfusion 2.6 ± 0.94, p = 0.10), while change in mean Marshall score in non-transfused patients who had both admission and repeat head CT was 0.07 ± 0.37 (initial CT 2.1 ± 0.37 vs follow-up CT 2.0 ± 0.0, p = 0.32).
Figure 2:
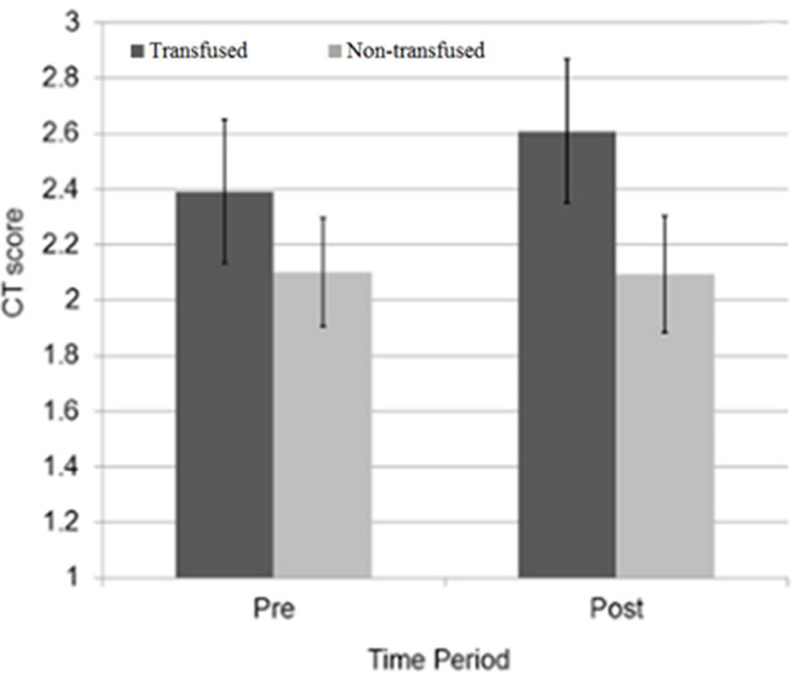
Mean CT score over time by transfusion status. Error bars show the 95% confidence interval.
Compared to non-transfused patients, transfused patients were more likely to undergo craniectomy/craniotomy (26.1% vs 0%, p < 0.01) and had a significantly longer ICU and overall hospital length of stay in the ICU (3.9 ± 2.6 days vs 0.73 ± 1.1 days, p < 0.01 and 7.8 ± 4.8 days vs 3.5 ± 2.8 days, p < 0.01, respectively). In-hospital mortality was four times higher in the transfused group, but this difference was not statistically significant (8.7% vs 2.3%, p = 0.28).
In patients with ISS > 15 (table 2), transfused patients were twice as likely to die as non-transfused patients, but, as in the overall cohort, this difference did not reach statistical significance (9.1% vs 4.2%, p = 0.50). Similar to all-comers, however, transfused patients had higher rates of craniectomy/craniotomy (27.3% vs 0%, p < 0.01), longer hospital length of stay (7.6 ± 4.9 days vs. 4.1 ± 3.3 days, p < 0.01), and longer ICU length of stay (4.1 ± 2.5 days vs. 1.3 ± 1.2 days, p < 0.01).
Creating a predictive, multivariate model was not possible due to small sample size; however, we did examine potential correlations between admission variables and changes in Marshall score over time (table 4). These correlations can serve as a guide to help define which patients may be at risk for worsening hemorrhage and could guide future studies. None of our admission variables, however, showed significant correlation with changes in Marshall score. Moreover, our highest Spearman correlation coefficient was r = 0.23 for AIS head, which signified a weakly positive correlation accounting for changes in CT scores.
Table 4:
Spearman correlations with changes in Marshall Scoring
| Spearman r with Δ Marshall Score |
p-value | |
|---|---|---|
| Age | 0.09 | 0.52 |
| Sex | −0.01 | 0.95 |
| % Inh(AA) | −0.10 | 0.48 |
| % Inh(ADP) | −0.19 | 0.18 |
| MA | 0.00 | 0.98 |
| Temperature | −0.11 | 0.43 |
| INR | 0.17 | 0.28 |
| Platelet count | 0.14 | 0.32 |
| AIS head | 0.23 | 0.11 |
| AIS TBI | 0.09 | 0.53 |
Δ = change, Inh = inhibition, AA = arachidonic acid, ADP = adenosine diphosphate, MA = maximal amplitude, INR = international normalized ratio, AIS = abbreviated injury score, TBI = traumatic brain injury
Discussion
The integration of TEG into clinical decision making for patients with trauma has been an evolving process with multiple studies describing its usefulness in goal directed resuscitation [20–22]. Within the TBI population, however, there are notable differences in patient demographics and the pathophysiology of brain injury that make the usefulness of TEG-PM more difficult to establish. Specifically, patients on APMs constitute a large subgroup at increased risk for progressive intracranial hemorrhage and for whom the TEG-PM assay may allow for targeted intervention with platelet transfusion. In this study, patients with TBI who received platelet transfusion had significant reductions in the degree of platelet inhibition in the arachidonic acid pathway. The difference in severity of intracranial hemorrhage as assessed using the CT Marshall score over time was not significantly different between the groups despite a significantly higher admission Marshall score in patients who received transfusions. Additionally, there were no significant correlations between admission variables and changes in CT scores over time between transfused and non-transfused patients.
Classically, patients with TBI who are taking APMs and those whose intracranial hemorrhage progress are transfused platelets [23], but to date there have been no validated or standardized transfusion protocols to justify this practice. Consistent with this, our study found marked variability in transfusion practice amongst enrolling centers. There was no standardization of transfusion protocols between centers, and the decision to administer platelets was therefore based upon clinician discretion These institutions represent level 1 and 2 trauma centers that are diverse both in the patient populations encountered and geographic region. As such, although the lack of a protocolized approach to transfusion resulted in variability in practice, the study design is generalizable in that it is reflective of real-world practice. This study also establishes equipoise for future randomized clinical trials that attempt to establish a standardized transfusion protocol.
Our data demonstrated significant differences in need for operative intervention, duration of hospitalization, and days spent within the intensive care unit for transfused versus non-transfused patients. However, the average ISS, AIS head, and mean admission CT score for transfused patients were significantly higher compared to the non-transfused cohort, indicating more severe injury. To determine if there was benefit from platelet transfusion in patients with severe injuries, we performed a subgroup analysis of patients with major injury denoted by an ISS greater than or equal to 15. Within this subgroup, differences between mean ISS, AIS TBI, and admission CT scores were no longer statistically significant between transfused and non-transfused patients, but differences in need for operative intervention, duration of hospitalization, and ICU days remained significantly higher in the transfused cohort. The increased length of stay in the transfused cohort subgroup is likely influenced by the increased need for operative intervention in that same group; however, just as the absence of standardized clinical practice guidelines results in variability in transfusion practices between centers, differences in clinical decision-making may likely result in similar variability towards craniectomy. Therefore, there is likely a confounding relation between the decision to transfuse platelets and surgeon discretion to pursue operative intervention which manifested in different outcomes between cohorts despite similar injury characteristics.
Interestingly, in all comers despite higher admission Marshall scores and AIS TBI, there were no significant admission variables that were correlated with worsening Marshall score over time. Specifically, a significant decrease in % Inh(AA) did not result in meaningful correlations with changes in CT scores. These findings mirror prior work where no correlation was found between TEG-PM parameters and clinical outcomes including ISS, length of stay, or mortality [3]. Moreover, the effect of platelet administration on clinical outcomes following TBI has come into question with several studies suggesting equivocal short and long-term results [9, 17, 23]. This underscores the need for large, randomized clinical trials assessing the efficacy of platelet transfusion in augmenting outcomes following traumatic brain injury.
Our decision to analyze Spearman correlations between variables of interest was multifactorial. On the one hand, our study was limited in power to find statistically significant relationships between admission variables of interest. On the other hand, identifying pertinent variables that were highly correlated with changes in Marshall score might be clinically relevant in predicting injury progression and thereby may help guide transfusion practices. At a minimum, such correlation may delineate potential areas of inquiry for subsequent studies. Among all variables outlined, only the AIS head score had a weak correlation (r = 0.23) while all other admission variables demonstrated no correlation. There are several reasons that can account for the lack of correlation. First, the incidence of delayed hemorrhage following injury is low, and so correlations between admission variables and worsening CT scores may be difficult to establish [6]. Second, previous studies have identified elevated INR as a risk factor for delayed hemorrhage, usually in the setting of concomitant warfarin use [24]. However, patients taking warfarin were excluded in our study and the mean INR in both groups was less than 1.2. Lastly, the predictive value of TEG-PM parameters for progression in intracranial hemorrhage has not yet been validated [3].
This study has several limitations which must be addressed. Though many of the results are generalizable due to the multicenter design of the study, the observational nature of our study design and lack of standardization in the indications for platelet transfusion and operative intervention contributed to a wide variability in practice. Likewise, the decision to repeat CT scans at the discretion of the treating physician may have led to some patients having progression in their CT findings that would not have been captured, and therefore bias our results. The calculation of Marshall scores from each head CT was performed by the researchers at their respective institutions and not by neuroradiologists, and internal or external validation of the CT scoring process within or between institutions was not performed. Our patient selection criteria excluded many higher severity injuries due to the inherent difficulty of confirming home medication regimens. This selection for less severe injuries created a narrower range of patient presentations included within the study which may have further blunted our ability to discern meaningful clinical differences between patient populations. This likely contributed to our small sample size and limited our ability to perform multivariate analyses that could adjust for confounders or include meaningful clinical variables. Lastly, the small sample sizes leave this study underpowered, thereby limiting our ability to pursue multivariable regression to account for differences between groups produced by discordant transfusion protocols, and, in turn, impede our ability to draw stronger conclusions regarding differences in outcomes.
We hope that future studies can utilize our findings to further study the need for and efficacy of platelet transfusion in patients with TBI. Moreover, future studies should focus specifically on outcomes in patients with intraparenchymal hemorrhage alone given that there are very limited surgical options in this cohort.
Conclusion
In this observational multicenter pilot study, platelet transfusion significantly decreases the degree of platelet inhibition in the arachidonic acid pathway but does not improve outcomes in patients on APM following TBI.
Acknowledgments
Funding:
NCATS UL1TR000445 for REDCap (all authors), Vanderbilt Faculty Research Scholars Program (mbp); National Institutes of Health NHLBI R01HL111111 (mbp) and NIGMS R01GM120484 (mbp)
Funding was provided by NCATS UL1TR000445 for REDCap (all authors), Vanderbilt Faculty Research Scholars Program (mbp); National Institutes of Health NHLBI R01HL111111 (mbp) and NIGMS R01GM120484 (mbp).
Footnotes
Disclosures: Dr. Sarani serves as an advisor to Haemonetics, Corps.
Dr. Sarani serves as an advisor to Haemonetics, Corps.
Declaration of Interest: Authors report no conflicts of interest.
References
- 1.Kauvar DS, Lefering R, Wade CE: Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. The Journal of trauma 2006, 60(6):S3–11. [DOI] [PubMed] [Google Scholar]
- 2.Briggs A, Gates JD, Kaufman RM, Calahan C, Gormley WB, Havens JM: Platelet dysfunction and platelet transfusion in traumatic brain injury. The Journal of surgical research 2015, 193(2):802–806. [DOI] [PubMed] [Google Scholar]
- 3.Sirajuddin S, Valdez C, DePalma L, Maluso P, Singhal R, Schroeder M, Sarani B: Inhibition of platelet function is common following even minor injury. The journal of trauma and acute care surgery 2016, 81(2):328–332. [DOI] [PubMed] [Google Scholar]
- 4.Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ: Intracranial complications of preinjury anticoagulation in trauma patients with head injury. The Journal of trauma 2002, 53(4):668–672. [DOI] [PubMed] [Google Scholar]
- 5.Narum S, Brors O, Stokland O, Kringen MK: Mortality among head trauma patients taking preinjury antithrombotic agents: a retrospective cohort analysis from a Level 1 trauma centre. BMC emergency medicine 2016, 16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishijima DK, Offerman SR, Ballard DW, Vinson DR, Chettipally UK, Rauchwerger AS, Reed ME, Holmes JF, Clinical Research in Emergency S, Treatment N: Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Annals of emergency medicine 2012, 59(6):460–468 e461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonville DJ, Ata A, Jahraus CB, Arnold-Lloyd T, Salem L, Rosati C, Stain SC: Impact of preinjury warfarin and antiplatelet agents on outcomes of trauma patients. Surgery 2011, 150(4):861–868. [DOI] [PubMed] [Google Scholar]
- 8.Spektor S, Agus S, Merkin V, Constantini S: Low-dose aspirin prophylaxis and risk of intracranial hemorrhage in patients older than 60 years of age with mild or moderate head injury: a prospective study. Journal of neurosurgery 2003, 99(4):661–665. [DOI] [PubMed] [Google Scholar]
- 9.Ohm C, Mina A, Howells G, Bair H, Bendick P: Effects of antiplatelet agents on outcomes for elderly patients with traumatic intracranial hemorrhage. The Journal of trauma 2005, 58(3):518–522. [DOI] [PubMed] [Google Scholar]
- 10.Jones K, Sharp C, Mangram AJ, Dunn EL: The effects of preinjury clopidogrel use on older trauma patients with head injuries. American journal of surgery 2006, 192(6):743–745. [DOI] [PubMed] [Google Scholar]
- 11.Major J, Reed MJ: A retrospective review of patients with head injury with coexistent anticoagulant and antiplatelet use admitted from a UK emergency department. Emergency medicine journal: EMJ 2009, 26(12):871–876. [DOI] [PubMed] [Google Scholar]
- 12.Beynon C, Hertle DN, Unterberg AW, Sakowitz OW: Clinical review: Traumatic brain injury in patients receiving antiplatelet medication. Critical care 2012, 16(4):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishijima DK, Zehtabchi S, Berrong J, Legome E: Utility of platelet transfusion with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. J Trauma Acute Care Surg 2012, 72(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducruet AF, Hickman ZL, Zacharia BE, Grobelny BT, DeRosa PA, Landes E, LeiS, Khandji J, Gutbrod S, Connolly ES Jr.: Impact of platelet transfusion on hematoma expansion in patients receiving antiplatelet agents before intracerebral hemorrhage. Neurological research 2010, 32(7):706–710. [DOI] [PubMed] [Google Scholar]
- 15.Downey DM, Monson B, Butler KL, Fortuna GR Jr Saxe JM, Dolan JP, Markert RJ, McCarthy MC: Does platelet administration affect mortality in elderly head-injured patients taking antiplatelet medications? The American surgeon 2009, 75(11):1100–1103. [DOI] [PubMed] [Google Scholar]
- 16.Washington CW, Schuerer DJ, Grubb RL Jr: Platelet transfusion: an unnecessary risk for mild traumatic brain injury patients on antiplatelet therapy. The Journal of trauma 2011, 71(2):358–363. [DOI] [PubMed] [Google Scholar]
- 17.Naidech AM, Liebling SM, Rosenberg NF, Lindholm PF, Bernstein RA, Batjer HH, Alberts MJ, Kwaan HC: Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocritical care 2012, 16(1): 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL: Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Seminars in thrombosis and hemostasis 2010, 36(7):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nekludov M, Bellander BM, Blomback M, Wallen HN: Platelet dysfunction in patients with severe traumatic brain injury. Journal of neurotrauma 2007, 24(11):1699–1706. [DOI] [PubMed] [Google Scholar]
- 20.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC et al. : Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Annals of surgery 2010, 251(4):604–614. [DOI] [PubMed] [Google Scholar]
- 21.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D: Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. Journal of the American College of Surgeons 2012, 215(4):496–502. [DOI] [PubMed] [Google Scholar]
- 22.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S et al. : Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of surgery 2012, 256(3):476–486. [DOI] [PubMed] [Google Scholar]
- 23.McMillian WD, Rogers FB: Management of prehospital antiplatelet and anticoagulant therapy in traumatic head injury: a review. The Journal of trauma 2009, 66(3):942–950. [DOI] [PubMed] [Google Scholar]
- 24.Peck KA, Sise CB, Shackford SR, Sise MJ, Calvo RY, Sack DI, Walker SB, Schechter MS: Delayed intracranial hemorrhage after blunt trauma: are patients on preinjury anticoagulants and prescription antiplatelet agents at risk? The Journal of trauma 2011, 71(6):1600–1604. [DOI] [PubMed] [Google Scholar]


