Abstract
Characterizing endogenous protein expression, interaction and function, this study identifies in vivo interactions and competitive balance between N-cadherin and E-cadherin in developing avian (Gallus gallus) neural and neural crest cells. Numerous cadherin proteins, including neural cadherin (Ncad) and epithelial cadherin (Ecad), are expressed in the developing neural plate as well as in neural crest cells as they delaminate from the newly closed neural tube. To clarify independent or coordinate function during development, we examined their expression in the cranial region. The results revealed surprising overlap and distinct localization of Ecad and Ncad in the neural tube. Using a proximity ligation assay and co-immunoprecipitation, we found that Ncad and Ecad formed heterotypic complexes in the developing neural tube, and that modulation of Ncad levels led to reciprocal gain or reduction of Ecad protein, which then alters ectodermal cell fate. Here, we demonstrate that the balance of Ecad and Ncad is dependent upon the availability of β-catenin proteins, and that alteration of either classical cadherin modifies the proportions of the neural crest and neuroectodermal cells that are specified.
Keywords: E-cadherin, N-cadherin, neural crest, neuroectoderm, specification, proliferation, cell fate, β-catenin
1. Introduction
Cadherin proteins are homophilic cell-cell adhesion molecules important for epithelial integrity and whose changes in expression are linked to the epithelial to mesenchymal transition (EMT) during embryonic development (Schafer et al., 2014, Kerosuo and Bronner-Fraser, 2012) and cancer metastasis (Kang and Massague, 2004, Thiery et al., 2009). Neural cadherin (Ncad) and epithelial cadherin (Ecad) are the archetypical type I cadherins that function in a calcium-dependent manner, have five extracellular domains, a transmembrane domain and bind to intracellular components such as α-, δ- (p120) and β- catenin to link to the actin cytoskeleton and intracellular signaling pathways (Koch et al., 1999). Although cadherins have been well studied in cancer cell lines and amphibian tissues (Gheldof and Berx, 2013, Kashef et al., 2009, Scarpa et al., 2015), far less is known about their in vivo roles during ectodermal cell fate specification in amniotes.
Ectodermal cells respond to instructive signals early in development to form neural tissue (Gaur et al., 2016, Lamb et al., 1993, Rogers et al., 2008), non-neural ectoderm (NNE), epidermal/placodal tissue (Schlosser, 2014, Nordin and LaBonne, 2014), or neural crest (NC) tissue (Mayor et al., 1995, Selleck and Bronner-Fraser, 2000). The epigenetic and molecular specification of each of these tissues is followed by morphogenetic events such as neural tube closure (NTC) and the epithelial to mesenchymal transition (EMT) of NC cells. Recent studies in chick, amphibian and mouse embryos have identified transcription factors that regulate ectodermal derivative fate specification (Buitrago-Delgado et al., 2015, Bouzas et al., 2016, Mach et al., 2016, Riddiford and Schlosser, 2016, Acloque et al., 2017, Simoes-Costa et al., 2015), NTC (Ray and Niswander, 2016a, Ray and Niswander, 2016b) and NC EMT (Rogers et al., 2013, Schiffmacher et al., 2014, Strobl-Mazzulla and Bronner, 2012). Comparative analysis of early transcriptional regulators shows that many have overlapping expression and directly or indirectly regulate the expression of specific adhesion molecules to control these processes (Ray and Niswander, 2016a, Fairchild et al., 2014, Fairchild and Gammill, 2013, Strobl-Mazzulla and Bronner, 2012). Many transcription factors expressed in early development regulate the expression of cell adhesion molecules, specifically cadherin proteins, and altering cadherin protein expression or function via perturbation of their upstream regulators leads to abnormal embryonic development (Tien et al., 2015, Rogers et al., 2013, Matsumata et al., 2005, Lin et al., 2016). The question remains however, whether the cadherin proteins have independent functions during ectodermal fate specification in addition to their roles in regulating cell movement.
To resolve these issues, we characterize and compare the expression and localization of two cadherin proteins during the separation of neural ectoderm, NNE and NC cells in avian embryos. Here, we show that perturbation of Ncad protein directly alters the expression of proteins thought previously as upstream transcriptional regulators, and leads to defects in ectodermal-derivative specification and NC migration. Our results suggest that there are molecular steps downstream of cadherin proteins that regulate fate specification leading to migration defects. The results show that Ecad protein is expressed in the early epiblast, and its expression is maintained in all three ectodermal derivatives, while Ncad protein appears limited to the neural plate and neural tube region and is absent from premigratory and most migrating NC cells, though expressed at high levels in the neural tube, notochord and cranial mesenchyme. Given the overlap of different cadherins in some tissues, we examined their ability to interact in vivo and report that in addition to homotypic interactions, Ncad and Ecad can form heterotypic complexes with each other in the neural tube. In addition, altering levels of Ncad leads to compensation in the levels of Ecad in a β-catenin protein dependent manner, and leads to defects in the proper specification of neural, NNE and NC tissues.
2. Experimental Procedures
2.1. Embryos
Fertilized chicken eggs were obtained from local commercial sources (McIntyre Farms, San Diego, CA, AA Farms, CA, and Sunstate Ranch, CA) and incubated at 37°C to the desired stages according to the criteria of Hamburger and Hamilton (HH). All use of embryos was approved by the California State University Northridge IACUC protocol: 1516–012a.
2.2. Electroporation of antisense morpholinos and vectors
A translation blocking antisense fluorescein -labeled morpholino to Ncad (NcadMO) was designed (5’-GCGTTCCCGCTATCCGGCACATGGA-3′), as well as a non-specific control morpholino (ContMO) (5′-CCTCTTACCTCAGTTACAATTTATA-3′). Injections of the fluorescein-tagged morpholinos (0.75–1 mM plus 0.5–1.5 mg/ml of PCI carrier plasmid DNA; as in Voiculescu et al., 2008) were performed by air pressure using a glass micropipette targeted to the presumptive neural plate region at HH stages 4–5. DNA plasmids pCS2-Ncad-GFP (Ncad-GFP)(Shiau and Bronner-Fraser, 2009), Ncad-YFP-Δp120 (AAA- Ncad-YFP) (Chen et al., 2003), and truncated mouse β-catenin (β-cateninΔ90) (Wrobel et al., 2007) plasmids were used (1 mg/ml) and were introduced in a similar manner to morpholinos described above. HH stage 4–5 electroporations were conducted on whole chick embryo explants placed ventral side up on filter paper rings. The Ncad morpholino and vectors were injected on the right side of the embryo and where indicated, controls were injected on the left side of the same embryo. Platinum electrodes were placed vertically across the chick embryos and electroporated with five pulses of 6.3–6.8 V in 50 ms at 100-ms intervals.
2.3. Immunohistochemistry
Immunohistochemistry (IHC) for Pax7 (Developmental Studies Hybridoma Bank (DSHB), Pax7), Ncad (DSHB, MNCD2; DSHB, 6B3; Abcam, ab18203), Cad6B (DSHB, CCD6B-1), Ecad (BD Transduction Laboratories, 610181; DSHB, 8C2 (Choi and Gumbiner, 1989); DSHB, 7D6 (Gallin et al., 1983)), β-catenin (Abcam, ab6301), and p120-catenin (Cell Signaling, 4989S) was performed as follows: Embryos were fixed in 4% paraformaldehyde made in phosphate buffer for 15–40 minutes at room temperature. All washes were performed in TBST + Ca2++ with 0.5% triton x-100. Blocking was performed with 10% donkey serum in the same buffer. The primary antibodies (1:5–1:10 for all hybridoma antibodies and 1:200–1:1000 for all others, see Table 1) were incubated in the TBST buffer from overnight to two days at 4°C and secondary antibodies (Alexa Fluor, ThermoFisher Scientific 1:500 to 1:1000) were applied in the same buffer for either three hours at room temperature or overnight at 4°C.
Table 1.
Antibodies used in study.
| Name | Isotype | Species | Dilution | Immunogen | Binding region | Source |
|---|---|---|---|---|---|---|
| ECAD Antibodies | ||||||
| 7D6 (Lcam) | mouse IgG1 |
Chicken | 1:10 | Ft1 fraqment | N-terminus (Extracellular) |
DSHB |
| 610181 (Anti-E- cadherin) | mouse IgG2a |
Human | 1:1000 | AA 735–883 | C-terminus (Cytoplasmic) |
BD Transduction Laboratories |
| 07–697 (Anti-E- Cadherin) | rabbit IgG |
Human | 1:1000 | AA 859–874 | C-terminus (Cytoplasmic) |
EMD Millipore |
| 8C2 (cadherin, E) | mouse IgG1 |
Xenopus | 1:10 | Tryptic fraqment | N-terminus (Extracellular) |
DSHB |
| NCAD Antibodies | ||||||
| MNCD2 (Anti-N- Cadherin) | rat IgG2a | Mouse | 1:10 | AA 308–597 | N-terminus (Extracellular) |
DSHB |
| 6B3 (cadherin, N) | mouse IgG1 |
Chicken | 1:10 | Ncad-α-catenin complex |
N-terminus (Extracellular) |
DSHB |
| ab18203 (Anti-N- cadherin) | rabbit IgG |
Human | 1:1000 | AA 800–900 | C-terminus (Cytoplasmic) |
Abcam |
| CAD7 Antibodies | ||||||
| CCD7–1 (cadherin-7) |
mouse IgG1 |
Chicken | 1:10 | N-term 597 AA and the human immunoglobulin Fc region. |
N-terminus (Extracellular) |
DSHB |
| ab71412 (Anti- Cadherin 7 antibody - N- terminal) |
rabbit IgG |
Human | 1:50 | N-terminal region of human Cadherin 7 conjugated to KLH |
N-terminus (Extracellular) |
Abcam |
| CAD6B Antibodies | ||||||
| ab64917 (Anti-K) | rabbit IgG |
Human | 1:50 | C-terminal region of human K Cadherin | C-terminus (Cytoplasmic) |
Abcam |
| CCD6B-1 (cadherin-6B) |
mouse IgG1 |
Chicken | 1:10 | N-term 605 AA and the human immunoglobulin Fc region. |
N-terminus (Extracellular) |
DSHB |
| Catenin complex antibodies | ||||||
| Ab6301 (anti-beta catenin) | Mouse IgG1 |
Chicken | 1:500 | Recombinant full length protein | N/A | Abcam |
| 4989S (catenin δ- 1 antibody) | Rabbit IgG |
Human | 1:200 | Synthetic peptide | N/A | Cell Signaling Technology |
2.4. Imaging and Fluorescence Quantification
Fluorescence images were taken using Zeiss ImagerM2 with Apotome.2 and Zen software or Axioskop 2 Plus with AxioVision software (Karl Zeiss). Fluorescence was quantified using NIH ImageJ64 by averaging the relative intensity of 1–6 images per embryo. Background was subtracted uniformly across the images using the background subtraction function in NIH ImageJ64 with a rolling-ball radius of 50.00 pixels before quantitation (Hutchins and Szaro, 2013). Half embryos injected with morpholino or DNA plasmid were compared to either the uninjected or control side or embryos injected with control morpholino or GFP.
2.5. Biochemistry
Embryo lysate was isolated from 30–80 manually dissected chicken heads from stage HH8–10 embryos for co-immunoprecipitation or 10–20 manually dissected stage HH9–10 neural tubes with associated tissues (some ectoderm and mesenchyme) for Western blot analysis after morpholino knockdown. Lysate was isolated using lysis buffer (50 mM Tris-HCL pH 7.4 with 150 mM NaCl plus 1.0% NP-40 and EDTA-free protease inhibitor (Roche complete, # 11697498001). Co-immunoprecipitation was performed on never-frozen protein samples. Protein agarose G beads (Sigma, # P3296–5ML) were incubated for 12–24 hours with antibody prior to incubation with lysate. Tissue lysate was pre-cleared using naked beads and then incubated with prepared beads for 1.5 hours at room temperature. Beads were washed with lysis buffer multiple times and spun down at 5000 × G. Beads with bound protein complexes was resuspended in 8 M urea with 5% SDS, boiled for 30 minutes with intermittent shaking, and frozen at −80° prior to SDS page gel. The co-immunoprecipication was performed in both directions. Ten μg protein lysate was loaded on the gel (Lysate), 5% (0.75 μl) volume of the lysate that was cleared using naked beads (Input) was loaded, and 15 μl of the immunoprecipitation product (product of Co-IP from beads incubated with either Rb-α-Ncad, Ms-α-Ecad, Rb-IgG or Ms-IgG) was loaded. SDS page was run on precast 8–12% bis-tris gel (Invitrogen, # NP0321BOX) for 3 hours at 48 V, gel was transferred to nitrocellulose at 90 V for 1 hour. Nitrocellulose membranes were washed in TBST + Calcium with 0.5% Triton X-100, blocked and incubated with primary antibody in TBST + Calcium with 0.5% Triton X-10 with 5.0% BSA, incubated in (5%) milk protein in (TBST + Calcium) with secondary antibody, and visualized using ECL kit (GE Healthcare Lifesciences, # RPN2232) and exposed to film (GeneMate, #F-9024–8×10).
2.6. Proximity Ligation Assay (Duolink)
The proximity ligation assay was performed on previously cryosectioned embryos on glass slides. The methods were performed as described in the instructions in the Duolink Assay (#DUO92101) by Sigma Aldrich (St. Louis, MO). Embryos were fixed, sectioned and incubated with primary antibodies (see IHC). After primary antibody incubation, sections were washed with TBST+ Ca++ and subsequently incubated with the PLA probe set at 37°C for 1 hour. They were then washed in buffer A, placed in ligation mix for 30 min-1 hour at 37°C. Next, sections were washed in buffer A then incubated with polymerase mix for 110–200 min at 37°C. Finally, sections were washed in 1X and 0.1X buffer B, and mounted with Duolink mounting media with DAPI. Results are shown in native form or with false-colored dots to demonstrate putative interactions.
3. Results
3.1. Ecad and Ncad are co-expressed in the developing neural tube but not neural crest
Ecad and Ncad proteins are well-studied markers of EMT in cancer (reviewed in (Campbell and Casanova, 2016)), and have been associated with the process of gastrulation (Yang et al., 2008) and EMT in cranial NC cells (Rogers et al., 2013, Kuriyama et al., 2014, Scarpa et al., 2015). To characterize two major classical cadherin proteins expressed during ectodermal fate decision stages, we performed immunohistochemistry (IHC) using multiple antibodies (Figure 1, Table 1, Figure S1A-1P) on various stages of avian embryos and compared the localization of type I cadherin proteins (Ncad and Ecad) in neural and NC cells. To mark NC cells, we used Pax7, which labels both premigratory and migratory NC and HNK1, which marks migratory NC cells. Although expression of these proteins has previously been characterized in chicken tissues (Jourdeuil and Taneyhill, 2018, Dady et al., 2012, Dady and Duband, 2017), there is an absence of continuous expression data throughout the stages of ectodermal derivative specification. Rather, their expression has been compared directly in studies during gastrulation or later in neural crest EMT/migration stages until recently by our lab and others (Dady and Duband, 2017). At Hamburger Hamilton (HH) stage 4, Ecad and Ncad are expressed in distinct tissues (Figure 1A–1A’’’). Ecad is expressed in the epiblast (Figure 1A’, 1A’’’), while Ncad expression is limited to the hypoblast as has similarly been reported in zebrafish embryos (Figure 1A”, 1A’’’) (Warga and Kane, 2007). As the neural folds rise at HH7 (Figure 1B–1B’’’) and close at HH8 (Figure 1C–1C’’’), Ecad expression is maintained in the non-neural ectoderm, neural ectoderm and neural plate border (Figure 1B’, 1B’’’, 1C’, 1C’’’), while Ncad is expressed in the neural groove, cranial mesenchyme and endoderm (Figure 1B”, 1B’’’, 1C”, 1C’’’), but is absent from the neural plate border and non-neural ectoderm (Figure 1B”, 1C’’, arrow). As NC cells begin to delaminate at HH9, Ecad protein is expressed on the apical surface of the neuroepithelium where it overlaps with Ncad expression except in the dorsal-most portion of the neural tube (premigratory NC cells) that lacks Ncad (Figure 1D- 1D’’’, arrow, Figure S1M-1P). Additionally, at HH10, (Fig. 1E–1J), Ecad overlaps with premigratory and migratory NC cells, identified by Pax7 staining (pink) (Figure 1G–1J). At this stage, Ncad also is absent from the premigratory and early emigrating NC cells (Figure 1F’, 1H arrow, Figure S1M- 1P). Overall, our results demonstrate that in avian ectodermal derivative cells, Ncad remains absent from most of the cells specified to become NC cells, while Ecad is expressed in a pan-ectodermal manner, suggesting specific roles for Ncad in the developing neural
Figure 1. Ecad and Ncad are co-expressed in the developing neural tube but not neural crest.
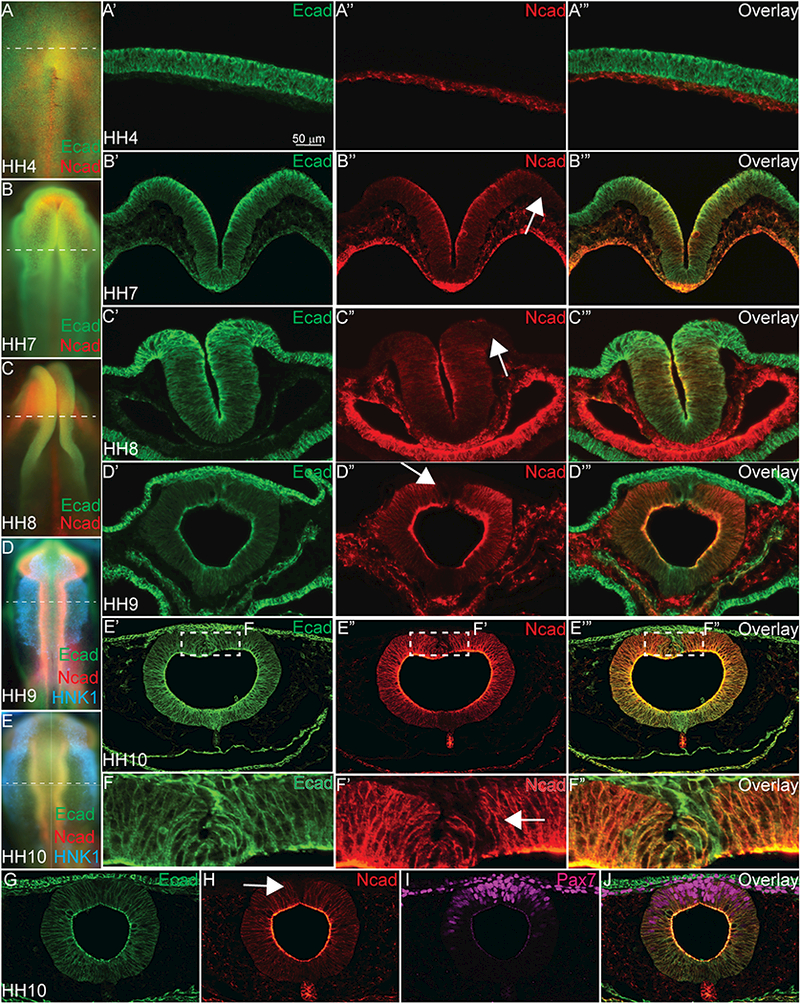
Immunohistochemistry (IHC) on whole embryos or 16 μm cryosections stained with antibodies to Ecad (green), Ncad (red), HNK1 (blue) and Pax7 (magenta). (A-A’’’) In an HH4 embryo (A is wholemount, A’- A’’’ are transverse sections), Ecad is expressed in the epiblast/ectoderm and Ncad is expressed in the hypoblast. (B-B’’’) In an HH7 embryo (B is wholemount, B’-B’’’ are transverse sections), Ecad is expressed in the non-neural ectoderm, the neural folds and the endoderm. Ncad is expressed in the neural plate, cranial mesenchyme and endoderm, but is absent from the neural plate border (white arrow). (C- C’’’) In an HH8, embryo (C is wholemount, C’-C’’’ are transverse sections), Ecad is expressed in the non-neural ectoderm, the neural tube and the neural plate border. Ncad is expressed in the neural tube, cranial mesenchyme and developing gut, but is absent from the dorsal neural tube (white arrow). (D- D’’’) In an HH9 embryo (D is wholemount, D’-D’’’ are transverse sections) Ecad is expressed in the ectoderm, neural tube, migratory NC cells, and developing gut. Ncad protein is localized to the neural tube, cranial mesenchyme, notochord, developing gut, and is absent in the dorsal neural tube (white arrow). (E- E’’’) In an HH10 embryo (E is wholemount, E’-E’’’ are transverse sections), Ecad and Ncad are both expressed similarly to HH9 (D-D’’’), but the dorsal neural tube is magnified (F-F’’) to demonstrate that Ecad is highly expressed in the premigratory NC cells, while Ncad is absent from these specific cells. G-J show coexpression of Ecad (G, J) and Pax7 (I, J) in the migratory NC cells, while Ncad is absent from the most dorsal Pax7+/Ecad+ cells (H, J) Scale bar is as marked.
3.2. Classical cadherins interact heterotypically in the neural tube
Cadherins are well known to interact homophilically to maintain cell-cell adhesion, critical for cell sorting behavior and tissue integrity (Katsamba et al., 2009). Previous in vitro studies have suggested that Ncad is unable to interact with the type II cadherins, Cad6B and Cad7 (Katsamba et al., 2009, Nakagawa and Takeichi, 1995, Dufour et al., 1999, Friedlander et al., 1989). However, other studies suggested the possibility of heterophilic interactions between different cadherins. In Chinese Hamster Ovary (CHO) cells, Ncad preferentially forms homophilic interactions with itself, but cells over-expressing Ncad and Ecad formed heterophilic aggregates as well (Katsamba et al., 2009), albeit the affinity of Ncad to itself is higher than to Ecad. Additionally, in endoderm-derived tissues and tumors, Ecad and Ncad interact to create heterotypic adherens junctions (Straub et al., 2011). Therefore, based on studies demonstrating that these proteins can interact when co-expressed, we performed experiments to determine if they were functionally interacting in the neural ectoderm.
To visualize where and when two proteins might interact in the intact embryo, we utilized the proximity ligation assay (PLA), which enables detection of protein interactions in situ with high sensitivity and specificity. Using specific antibodies against Ncad and Ecad (Table 1), the PLA allows us to visualize complexes if and when they form between these cadherins. First, we tested for homophilic interactions in early avian embryos using two distinct antibodies to Ncad (Rb-α-human Ncad and Rt-α-chicken Ncad)(Figure 2A–2C). The PLA results are shown in pink (Figure 2A, 2B, 2D, 2E, 2G, 2H) or in inverse images created by ImageJ in black (Figure 2C, 2F, 2I). For the Ncad-Ncad assay, positive PLA signals were identified in the developing neural tube (70.97%), cranial mesenchyme and notochord of an 11 ss embryo. In contrast, Ncad interactions were lower in the dorsal neural tube, and were virtually absent from migrating NC and NNE (Figure 2A, 2B, asterisk, 2C, 2J, dashed lines). We also tested interactions of Ecad with Ecad in a 9 ss embryo (Rb α human Ecad and Ms α human Ecad) and found that Ecad forms homotypic complexes (Figure 2D- 2F) in the NNE, the developing neural tube (62.76%) and importantly, in the early migrating NC cells (Figure 2D, arrow, 2E, dashed box, 2K dashed line). Ecad- Ecad interactions are also visible in the developing gut and some cranial mesenchyme regions. Next, we analyzed heterotypic interactions between Ecad and Ncad at 10 ss. Compared to the homotypic interaction between Ecad proteins (Figure 2D–2F), the strongest PLA signals between Ncad and Ecad were limited and remain localized to the apical side of the neural tube (Figure 2G–2I, 65.29%). They were all but absent from the migratory NC cells (Figure 2H, 2L, box/dashed lines). We performed IHC for Ncad and Ecad on adjacent sections to verify antibody specificity and as a positive control for the assay (Figure 2M–2O). We also tested the possibility that Ncad or Ecad might form heterotypic complexes with heterologous type II cadherins. However, we were unable to detect interactions between either Ncad or Ecad with Cad6B or Cad11 using PLA (Rogers, 2018) at the stages tested, consistent with previous in vitro data suggesting that these proteins are unable to interact (Katsamba et al., 2009).
Figure 2. Ncad and Ecad interact heterotypically in the neural tube.
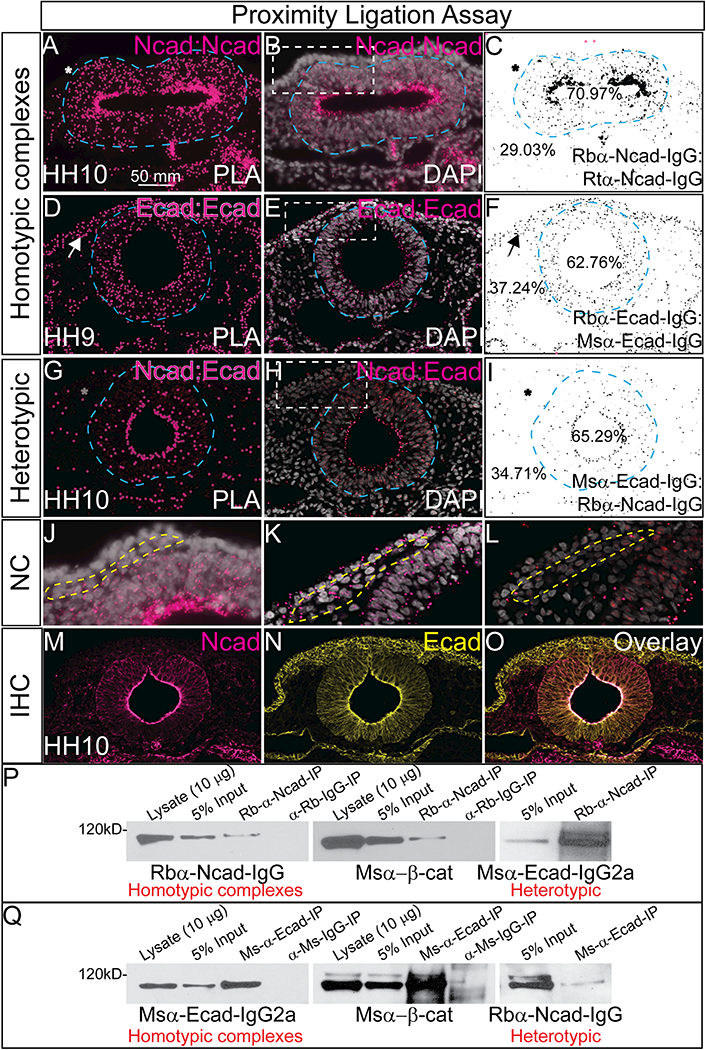
(A-C) Proximity ligation assay (PLA) in an HH10 embryo using antibodies for rabbit αNcad with rat αNcad. (D-F) PLA in a HH9 embryo using antibodies for rabbit αEcad with mouse αEcad. (G-I) PLA in a HH10 embryo using antibodies for rabbit αNcad: mouse αEcad. Each PLA was repeated 3–7 times. (A, B, D, E, G, H) Show the positive PLA signal in pink (A, D, G have larger false colored dots over PLA signal) and (C, F, I) shows inverse image created in NIH ImageJ. Asterisks denote lack of signal, arrows show positive signal. (B, E, H) are PLA signals overlayed with DAPI to show nuclei (white) and (J, K, L) are high mag images focusing on NC region (dashed line). For co-immunoprecipitation assays (P, Q), chicken heads were dissected and lysate was prepared. Western blotting of this endogenous lysate showed that (P) Ncad can pull down Ncad, β-catenin and Ecad and that (Q) Ecad can pull down Ecad, β-catenin and Ncad. Lysate is uncleared, input is 3.0%−5.0% volume of lysate after incubation with naked Protein G Agarose beads, 15 μl of IP samples was loaded. Each co-immunoprecipitation experiment was repeated at least three times using between 24–80 embryo heads each time. Scale bar is as marked.
The PLA assay shows that these proteins are close enough to form a complex, and to verify the physical interaction we utilized a co-immunoprecipitation assay. Embryo heads were lysed and we performed pulldowns in both directions to confirm interaction. First, Ncad (Figure 2P) was pulled down using Rb α Ncad (Table 1), the resulting lysate was then subjected to western blot using antibodies against Ncad to confirm homophillic interactions, β-catenin to confirm the positive association with Ncad and its intracellular partner, and finally Ecad to verify heterotypic interaction. We performed the reverse pulldown with Ecad (Figure 2Q) using Ms α Ecad (Table 1) bound to protein G agarose beads. We additionally used IgG controls to verify specificity. Ncad pull down and subsequent western blot showed that Ncad not only pulled down itself and ß-catenin as expected, but also Ecad (Figure 2P). Reciprocally, Ecad pulled down Ecad, ß-catenin, and Ncad (Figure 2Q). These data demonstrate that in addition to being co-expressed in the developing neural tube, Ecad and Ncad also interact suggesting that they may either function in concert, or regulate each other during development.
3.3. Ncad gain and loss of function leads to compensatory changes in Ecad expression
Ncad and Ecad can interact in the developing neural tube, but they maintain distinct expression in the other ectodermal derivatives. We hypothesized that even when co-expressed, the balance of cadherin proteins is tightly regulated as previous studies have demonstrated in the process of EMT. To test this hypothesis, we first performed loss of function experiments with Ncad by injecting a translation blocking FITC-labeled morpholino oligomer to Ncad (NcadMO) at gastrula stages and comparing the resulting phenotype to the uninjected side of the embryo as well as to separate embryos injected with a non-specific FITC-labeled control morpholino (ContMO). Using IHC and Western blot, we verified that Ncad morpholino efficiently depleted Ncad protein levels in a cell autonomous manner by more than 70% (Figure 3A, 3A’, arrowhead, 3D, p<0.01, 3E, 3F, Figure S2) when compared to the uninjected side or ContMO-injected embryos (Figure 3C–3E). In embryos with depleted Ncad protein, we observed a dramatic increase in Ecad protein on the membrane of the cells lacking Ncad compared to the uninjected (Figure 3B, 3B’) side or ContMO-injected embryos (Figure 3C, 3C’). Ecad immunofluorescence intensity increased to 145% compared to levels in the uninjected side of embryos in both the neural tube and migrating NC cells (Figure 3B, 3B’, dashed box, and 3D, p<0.01). Surprisingly, western blot analysis suggested that the actual levels of Ecad remained the unchanged in the samples that have decreased levels of Ncad (Figure 3E, 3F), however, we believe that due to the mosaic injection of the morpholino, and the ubiquitous expression of Ecad in ectodermal derivatives, the increased Ecad expression may be diluted by the tissues assayed (half heads rather than sorted cells). Additionally, the effect of Ncad knockdown on Ecad expression is specific. Altering levels of Ncad (gain or loss) had no effect on the expression of type II cadherin proteins, Cad6B or Cad7 (Rogers, 2018).
Figure 3. Ncad gain and loss of function leads to compensatory changes in Ecad expression.
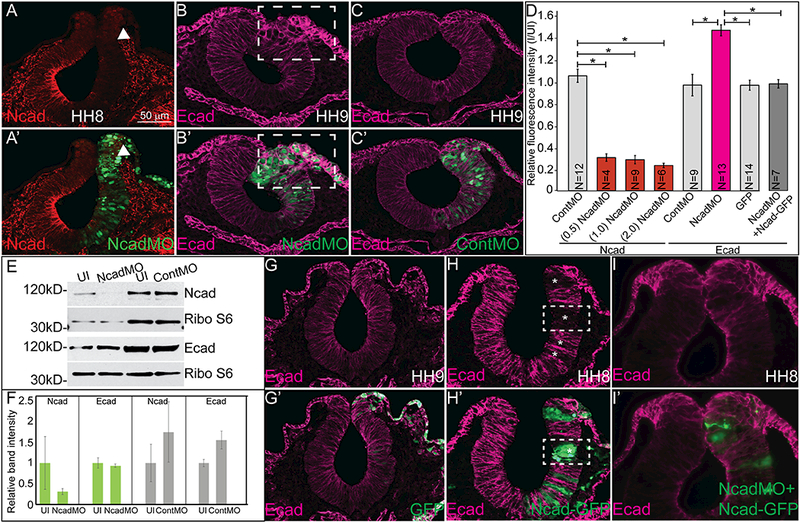
IHC and Western blot show that a translation blocking morpholino for Ncad reduces Ncad (A, A’, D, E, N= 27/27) and increases Ecad localization in the membrane (B, B’, D, N= 21/24) compared to ContMO (C, C’ 9/11). (D) Relative fluorescence intensity calculated using NIH ImageJ64. One-way ANOVA with Tukey’s test was performed to determine significance. Number of embryo sections analyzed for fluorescence intensity was less than total number injected and analyzed in some cases. Error bars are standard error. Asterisks indicate p<0.01. (E) Western blot analysis using antibodies against Ncad and Ribosomal protein S6 as a loading control and Ecad and Ribo S6 as a loading control. (F) Graphs showing Western blot bands normalized by loading controls comparing uninjected to Ncad and Control morpholino injected. Differences were not statistically significant due to variation between experiments. (G, G’) GFP-injected embryos show no change in Ecad, but (Η, H’) overexpression of full-length Ncad reduces Ecad expression (N= 25/26) and dual injection of Ncad-GFP and NcadMO can partially rescue both phenotypes (I, I’, N= 7/7). Scale bar is as marked. Each experiment was repeated at least 3 times.
Next, we performed gain of function experiments by introducing full length Ncad-GFP DNA unilaterally into gastrula stage embryos and comparing its effect to overexpression of GFP alone. Ncad- GFP injection induces excess and ectopic Ncad expression (Figure S2G, S2H). GFP expression had no effect on Ecad expression (Figure 3G, 3G’). Overexpression of the cadherin construct was mosaic, such that cells positive for Ncad-GFP lacked Ecad protein whereas Ncad-GFP negative cells were Ecad positive (Figure 3H, 3H’, dashed boxes, asterisks). However, concomitant overexpression of Ncad-GFP plus NcadMO resulted in partial rescue (Figure 3I, 3I’) of Ecad expression. In these dual-injected embryos, the Ecad protein immunofluorescence intensity was not significantly different from embryos injected with either ContMO or GFP suggesting that altering the levels of Ncad directly affects the localization of Ecad. These data represent that a tenuous relationship exists between the expression of type I cadherin proteins in the neural tube, and altering Ncad drastically changes the expression of Ecad.
3.4. Catenin family proteins control Ecad response to changes in Ncad
Analysis of cadherin protein function would be incomplete without assessing their interacting and functional partners from the catenin family. We have so far demonstrated in vivo that type I cadherin proteins interact in the developing neural tube and that loss of Ncad increases Ecad and gain of Ncad reduces Ecad in a cell-autonomous manner (Figure 2 and Figure 3). To gain insight into the mechanism involved in these changes, we analyzed the response of two proteins from the catenin family, β-catenin and p120-catenin, after Ncad perturbation. Embryos injected with an NcadMO demonstrated an increased Ecad (Figure 4A, 4B), and therefore we tested whether the lack of Ncad and increased Ecad also changed catenin protein expression. After co-injection with ContMO (left) and NcadMO (right), we measured β-catenin protein expression using IHC (Figure 4C–4E) and Western blot analysis (Figure 4E- 4G). Beta-catenin expression was not significantly different on either side (Figure 4C–4E) measured by IHC or Western blot analysis. For Western blot, embryos were injected on one side with either NcadMO or ContMO, neural tubes and associated tissues were dissected, and we performed comparative analysis on the protein lysate from morpholino-injected embryos and their uninjected sides. Normalized to the loading control, the levels of β-catenin were not significantly changed in ContMO-injected samples versus the NcadMO-injected samples (Figure 4E, 4F). In addition to β-catenin, cadherin proteins interact with α- catenin and p120 (δ)-catenin (reviewed in (McCrea and Gottardi, 2016)) both for cell adhesion purposes as well as intracellular signaling (Schiffmacher et al., 2016, Abbruzzese et al., 2016). Therefore, we performed a Western blot for p120-catenin after Ncad perturbation, and we found that the putative Ncad- associated p120 isoform (~120 kD) was decreased in the NcadMO-sample, while a smaller isoform, possibly which may associate with Ecad (~89 kD), remained unchanged (Figure 4E, 4G).
Figure 4. Changes in Ncad and Ecad in response to perturbations are due to competition for β- catenin.
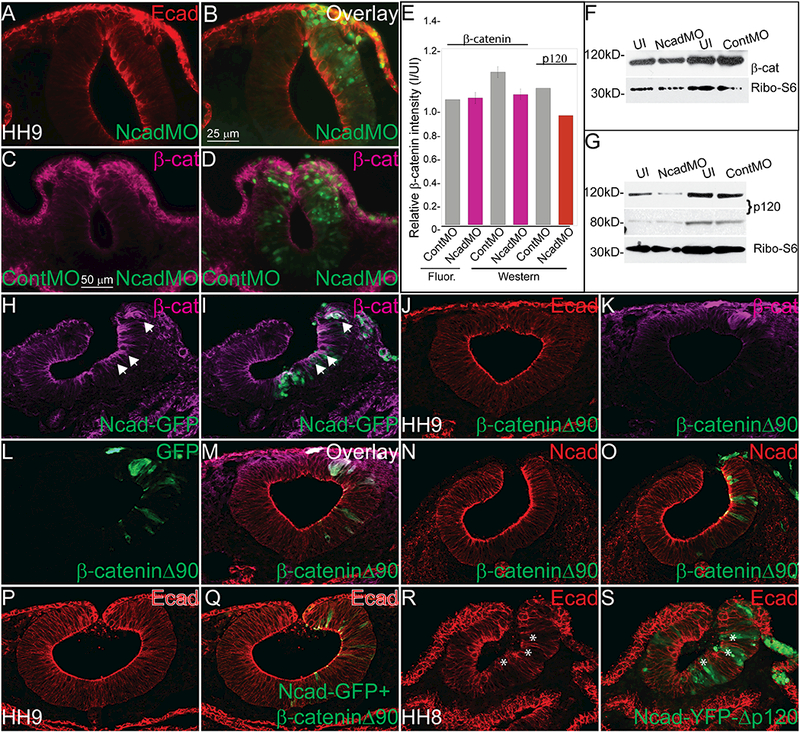
IHC in an HH10 embryo for (A, B) Ecad (N= 21/24) and (C, D) β-catenin (N= 15/20) after injection with NcadMO unilaterally (A-D) or NcadMO on the right side and ContMO on the left (C, D). (E) Relative fluorescence or band intensity of (C, D) β-catenin and (G) p120 catenin in ContMO and NcadMO-injected embryos compared to uninjected calculated using NIH ImageJ64. One-way ANOVA with Tukey’s test was performed to determine significance. Error bars are standard error. There were no statistically significant differences between the treatments. Western blot showing (F) β-catenin (G) p120 levels after NcadMO-injection. IHC for (H, I) β-catenin after injection with Ncad-GFP (N= 11/13). IHC for (J) Ecad (N= 7/9), (K) β-catenin, (L) GFP, and M (overlay) or (N) Ncad (N= 14/14) and (O) overlay after overexpression of dominant active β-catenin (β-cateninΔ90). IHC for (P, Q) Ecad in embryo injected with Ncad-GFP+ β-cateninΔ90 (N= 10/12) or (R, S) Ncad-YFP-Δp120 showing that the p120 interaction site is not required for reduction in Ecad after Ncad overexpression (N= 15/15, asterisks). Scale bar is as marked. Each experiment was repeated at least 3 times.
Previous studies have demonstrated that cadherin proteins require an association with β-catenin for transport to the cell membrane (Chen et al., 1999, Kurth et al., 1999, Wahl et al., 2003). Therefore, we hypothesized that there may be a limited pool of β-catenin available for interaction with cadherins in the endoplasmic reticulum, and altering levels of one cadherin would either limit or increase the pool of ß-catenin for the other. Overexpression of Ncad drastically reduces Ecad expression (Figure 3H), yet in cells with excess Ncad, β-catenin expression appears increased at the cell membrane (Figure 4H, 4I). To test whether increasing the available pool of β-catenin might rescue the decrease of Ecad phenotype observed after overexpression of Ncad-GFP (see Figure 3H, 3H’), we injected a stabilized form of β- catenin (Wrobel et al., 2007). Surprisingly, overexpression of the stabilized β-catenin alone had little effect on either Ecad (Figure 4J-M) or Ncad (Figure 4N, 4O) expression. However, injection of the construct in the presence of exogenous Ncad, rescued the loss of Ecad expression (Figure 4P, 4Q). Additionally, to determine if the interaction with p120 was necessary for Ecad phenotype caused by Ncad overexpression, we injected a mutated form of mouse Ncad (Ncad-YFP-Δp120) that is unable to bind to p120-catenin (Chen et al., 2003), and even in the absence of an interaction with p120 catenin, Ncad- YFP-Δp120 decreased Ecad expression cell autonomously, suggesting that an interaction with β-catenin is sufficient to allow Ncad to outcompete Ecad (Figure 4R, 4S). These results show that, in avian embryos, Ncad and Ecad proteins appear to compete for a constant limited pool of β-catenin, and when levels of Ncad are altered; Ecad is either outcompeted for the available β-catenin, or it can utilize the excess β-catenin in the absence of Ncad and be transported to the cell membrane. These changes can happen even in the absence of an interaction with p120.
3.5. Perturbation Ncad leads to aberrant ectodermal cell fate specification
To understand the effect of excess Ncad on ectodermal specification, we overexpressed Ncad- GFP and performed IHC for NC and neural tube markers. Compared to the normal patterning exhibited by HH8 embryos injected with GFP, where Pax7 is strongly expressed in the dorsal neural tube cells and Sox2 absent from the premigratory NC cells (Figure 5A- 5B’), cells with excess Ncad have abnormal ectodermal derivative specification. As demonstrated previously, Ncad overexpression reduces Ecad cell autonomously (Figure 3), and in the same cells with excess Ncad, there is a complete loss of Pax7- positive cells (Figure 5C, 5C’, asterisks, 5H) at the onset of NC specification (HH8-). However, the Pax7- positive NC cells recover by HH10, and although there is a slightly reduced population, they are able to migrate out of the neural tube (Figure 5D, 5D’, 5H). By HH11, cells marked by Snai2, a definitive NC marker, are expanded compared to the uninjected side (Figure 5E, 5E’, arrows). At HH10 when NC cells are rebounding, there is a concurrent decrease in Sox2-positive cells (Figure 5F, 5F’, asterisk, 5H) compared to the uninjected side. Additionally, excess Ncad had no significant effect on cell proliferation marked by PH3 (Figure 5G–5G’, 5H p>0.5).
Figure 5. Exogenous Ncad affects the timing of NC specification.
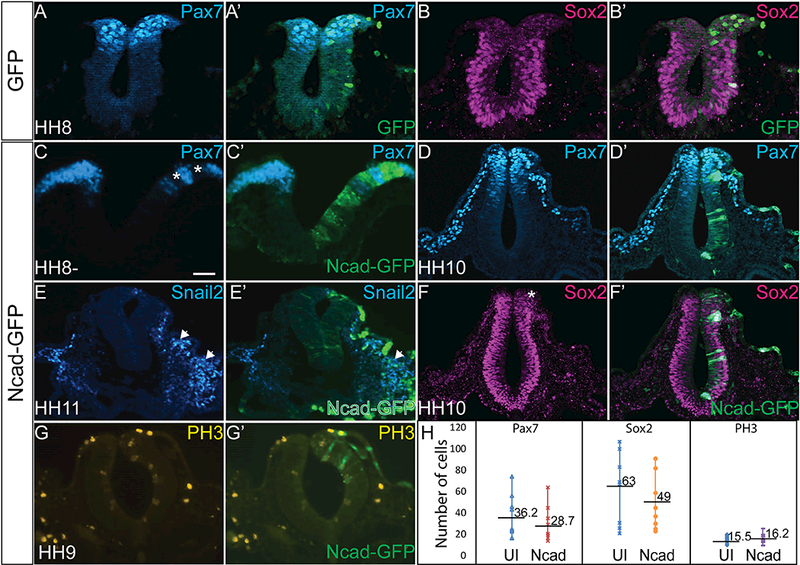
IHC for (A, A’) Pax7 (N= 8/8) and (B, B’) Sox2 (N= 8/8) in a GFP-injected embryo demonstrating normal regions of NC and neural progenitors. IHC showing Pax7 in an (C, C’, H) HH8- embryo (D, D’) in an HH10 embryo, (E, E’) Snai2 in an HH11 embryo, and (F, F’, H) Sox2 in an HH10 embryo after Ncad-GFP electroporation shows that exogenous Ncad reduces the expression of both NC and neural progenitor markers at early stages, even though NC cells rebound at later stages demonstrated by Snai2 (N= 7/8). Pax7 (N= 48/53), Sox2 (N= 10/11). (G, G’, H) PH3 expression is unaffected in embryos injected with Ncad-GFP compared with uninjected sides (N= 17). Numbers marked on graph represent average number of cells expressing indicated marker. Scale bar is 50 μm.
Given previous observations that NC development requires a balance between Ncad and Ecad (Rogers et al., 2013, Scarpa et al., 2015, Huang et al., 2016), and that perturbation of Ncad affects the expression of Ecad in vivo (Figure 3, 4), we examined the consequences of modulating Ncad expression on the ectodermal derivatives that come from the tissues expressing these two cadherins. First, we knocked down Ncad expression with the NcadMO to identify if Ncad is necessary for normal formation of ectodermal derivatives (Figure 6A-6M). Loss of Ncad at gastrula stage reduces the population of cells marked by Pax7 (Figure 6B, 6E), and concurrently expands the Sox2-expressing cells into the NC and NNE territories (Figure 6C-6E) at HH8, the onset of bonafide NC marker expression. The population of NC cells remains reduced as the cells migrate at HH9 (Figure 6E, 6G) and the Sox2-positive cells remain expanded into NC and NNE territory (Figure 6H). In contrast to the expansion of Sox2, loss of Ncad drastically reduces the cells expressing the definitive neural marker, Pax2 in the neural tube (Figure 6J- 6L, asterisks). Next, we identified that the Sox2+, Ecad+ cells are also marked by a 2.0 fold increase in the expression of phosphorylated histone H3 (PH3) (Figure 6L-6Q) suggesting that these cells have maintained a proliferative progenitor fate, or that there is some effect on cell cycle progression. Based on the specification phenotypes we hypothesize that Ncad is required for the progression of neural progenitor cells to definitive neural tube and NC cells (Figure 6E). ContMO did not have a significant effect on Ncad (Figure 6N, 6O), Pax7 (Figure 6P, 6Q), Sox2 (Figure 6R, 6S), Pax2 (Figure 6T, 6U) or PH3 (Figure 6V, 6W) expression.
Figure 6. Loss of Ncad affects ectodermal derivative specification.
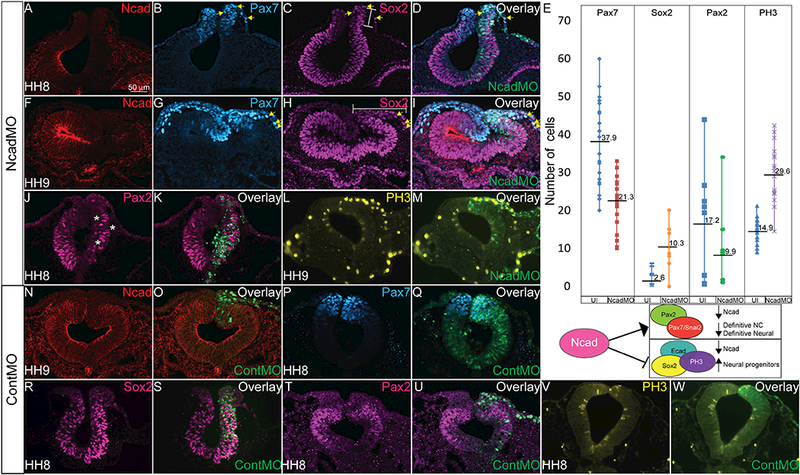
IHC in an HH8 embryo for (A, F, D, I, N= 35/35) Ncad, (B, G, D, I, N= 38/38) Pax7, and (C, H, D, I, N= 25/47) Sox2 in cells electroporated with NcadMO show decreased expression of Ncad and Pax7 while Sox2 is expanded into the dorsal neural tube and NNE. Bars indicate expanded regions of Sox2 expression. (E) Graph showing actual cell numbers marked by indicated markers in uninjected (UI) and NcadMO-injected embryos. Numbers marked on graph represent average number of cells expressing indicated marker. Diagram below graph shows hypothetical role of Ncad in driving neural cell determination. (J, L) Pax2 expression in NcadMO- injected embryos demonstrates reduction in Pax2 (N= 10/10, asterisks). IHC for (L, M) PH3 expression after NcadMO-electroporation. NcadMO increases the number of PH3 positive cells 2.0 fold as demonstrated by number of PH3 positive cells in uninjected sides of embryos compared with NcadMO (N= 25/25). IHC for (P, Q, N= 15/17) Pax7, (R, S, N= 9/11) Sox2, (T, U, N= 6/9) Pax2 and (V, W, N= 23/23) PH3 in embryos injected with ContMO alone demonstrating specificity of NcadMO phenotypes.
In contrast to the clear-cut results demonstrating reciprocal responses by Ecad when Ncad is perturbed, both gain and loss of Ncad results in a smaller population of early NC cells, while loss of Ncad increases Sox2 (and decreases Pax2) expression and gain of Ncad reduces Sox2 compared to uninjected contralateral sides as well as GFP-injected control embryos. These data demonstrate that there is tight regulation of the amounts and localization of type I cadherin proteins required to allow for proper ectodermal derivative specification. We additionally tested whether alterations in Ncad had any effect on the major signaling pathways (Wnt, Notch, TGF-β) to explain the resulting neural and neural crest phenotypes. However, loss of Ncad had little effect on these pathways as marked by the expression of downstream reporters, direct targets and intracellular effectors (Rogers, 2018) suggesting the effects on ectodermal derivatives may be a direct response to cadherin intracellular signaling, or possibly, there is another pathway involved in the phenotypes that has yet been identified.
4. Conclusions
Our data reveal a previously unrealized relationship between the classical cadherins in the developing neural tube and NC cells of avian embryos. We find that Ecad is strongly expressed throughout all ectodermal derivatives, in contrast to early studies that showed it was reduced in the neural ectoderm (Thiery et al., 1984), but consistent with more recent reports (Dady et al., 2012, Lee et al., 2013, Dady and Duband, 2017, Hardy et al., 2011). It is then retained in the dorsal neural tube and in the migratory NC at stages when down-regulation of Ncad in NC precursors is required for their emigration (Figure 1, Figure S1). Our data suggest that Ecad, but not Ncad protein, co-localizes with Pax7 in migratory cranial NC cells, a finding that differs from that observed in anamniotes like Xenopus (Kuriyama et al., 2014) and zebrafish (Tuttle et al., 2014).
By carefully examining cadherin distribution as a function of time, this study resolves some conflicting interpretations in the literature regarding Ncad protein localization during neural and NC cell development. Whereas early studies failed to detect Ncad protein in migratory cranial NC cells in vivo (Nakagawa and Takeichi, 1995), others suggested that a cleaved-version of the protein is maintained in migratory NC cells derived in vitro from explanted trunk neural tubes (Shoval et al., 2007). Contrasting with type I cadherin expression reported in Xenopus embryos, here we show that Ncad is not expressed at significant levels in cranial NC cells prior to or during EMT, although it may be re-expressed in later migratory cells as our analyses stopped at 12 ss. Multiple studies have demonstrated that Ncad protein is expressed in many migratory cranial NC cells, and Ecad appears to be lacking (Kuriyama et al., 2014, Scarpa et al., 2015), while other Xenopus studies show that similar to our data, Ecad is required for NC cell development (Huang et al., 2016). In NC cells and other cell types, Ncad is removed from the membrane by proteolytic cleavage, suggesting that if it is expressed in some of the migratory NC cells, it may be stored in vesicles rather than on the membrane (Shoval et al., 2007, Reiss et al., 2005, Marambaud et al., 2003). Taken together, these results suggest some species-specific differences in terms of expression and function of Ncad and Ecad, while maintaining the existence of a reciprocal relationship between these two cadherins. Further comparative analysis of cadherin proteins between amniotes and anamniotes is necessary to identify the ancestral versus derived expression and function of cadherin proteins in vertebrate embryos.
The general dogma is that cadherin proteins function as homodimers both intra- and extracellularly. However, our data suggest the possible existence of heterologous cadherin pairs in an endogenous context in some tissues. In support of this hypothesis, in vitro studies using cell lines demonstrated that Ncad and Ecad can aggregate together even though they preferentially bound via homophilic interactions (Katsamba et al., 2009, Shimoyama et al., 2000, Shan et al., 2000, Inuzuka et al., 1991), and that in endodermally-derived cell types, Ecad and Ncad interact to form adherens junctions (Straub et al., 2011). Our co-immunoprecipitation and proximity ligation assays demonstrate that, in addition to forming homotypic or homophilic complexes, Ncad and Ecad can bind to each other heterotypically in the neuroepithelium. However, we were unable to detect similar heterophilic interactions between Ncad or Ecad with Cad6B or Cad11 (Rogers, 2018). There are several possible explanations for the apparent lack of interactions between these specific cadherins. First, since Ncad and Ecad are type I cadherin proteins and use an HAV interaction domain to bind to each other, Cad6B and Cad11, which are a type II cadherins, may lack appropriate domains to interact with Ncad and Ecad. As this is a negative result, we cannot rule out the possibility that these cadherins interact but not within the resolution of the PLA technique. An additional complication is that cadherin proteins can form both cis- and trans- homophilic interactions (Koch et al., 1999). Our observations cannot discriminate between whether the complexes formed by Ncad and Ecad are cell-cell interactions (trans-) or are within a single cell (cis-). We believe that Ncad can form both cis- and trans- complexes with Ecad, but that the cis-complexes are more prevalent based on the localization of these proteins on the apical side of the neural tube, and the weaker PLA signal on the lateral and basal sides of the neuroepithelium where both proteins are also expressed.
In the past, adhesion molecules were thought to adhere only to the same subtype of cadherin molecules during development (Kemler, 1992). This hypothesis was the basis for the mechanism by which cell-type and tissue-types segregated during development; different tissues express different cadherins and therefore stick together. Recent analysis has shown that in fact, tissue segregation is a more flexible process and the levels of specific cadherins that are expressed in each tissue allows them to bind to each other with more or less force (Duguay et al., 2003, Steinberg and Takeichi, 1994, Wu et al., 2015). Our data, along with in vitro studies (Leckband and Sivasankar, 2012, Katsamba et al., 2009, Duguay et al., 2003, Straub et al., 2011), show that cadherins can interact heterotypically in the developing neural tube. The in vivo interactions between Ncad and Ecad in the developing tissues support the Differential Adhesion Hypothesis (DAH), which states that tissues maintain different surface tensions due to cell-cell adhesion (Foty and Steinberg, 2013, Steinberg, 2007). We postulate that the developing neural tube, which is epithelial in nature but must maintain the ability to mesenchymalize to allow for NC migration, expresses Ecad, Ncad, Cad6B and Cad7 (Figure 1, S1, and data not shown)(Rogers, 2018). As NC cells begin to undergo EMT, Ncad and Cad6B are downregulated and Cad7 and Cad11 are maintained or upregulated allowing for more flexibility and fluidity of tissues due to the three cadherins that are expressed and may interact during migration, (Ecad, Cad11 and Cad7). Other tissues that are epithelial only in nature such as the non-NC neural tube, NNE and developing gut maintain high levels of Ncad and/or Ecad to maintain the high surface tension and remain tightly bound.
Many in vitro studies have demonstrated the importance of the interactions between the catenin proteins and type I cadherins for normal cadherin function. P120-catenin and β-catenin have both been reported as required for the transport of Ncad and Ecad to intercellular adherens junctions where they functions in adhesion complexes (Chen et al., 2003, Wehrendt et al., 2016, Chen et al., 1999, Wahl et al., 2003). In support of the previous studies, our data showed that reducing levels of Ncad does not affect levels of β-catenin, but that increasing Ncad increases the membrane-associated β-catenin. We believe that these data suggest that cadherin proteins are not required for the expression of β-catenin, but that when cadherins are expressed, β-catenin is necessary for their membrane localization. In essence, β-catenin is a permissive molecule that is in limited supply. In contrast, our results demonstrate in a decrease of one isoform of p120 (Figure 4) after Ncad knockdown, yet the interaction with p120 is not necessary for exogenous Ncad to outcompete Ecad for localization to the membrane. Our data demonstrates that adding exogenous constitutively active β-catenin can rescue the loss of Ecad caused by exogenous Ncad, but that an interaction with p120 catenin is not required for Ncad overexpression to reduce Ecad levels. Both of these results support the hypothesis that our phenotypes are due to alterations in membrane-bound cadherin proteins rather than cleaved intracellular isoforms, however, further studies are required to clarify the downstream mechanism involved.
Previous studies showed that Ncad protein is down regulated in the dorsal neural tube to allow for EMT, likely regulated by bone morphogenetic protein (BMP) (Shoval et al., 2007). Reciprocally, overexpression of Ncad using adenoviral vectors prevented NC migration and led to NC cells in the lumen of the neural tube in the trunk (Nakagawa and Takeichi, 1998). Here, we postulate that it is possible that the proper balance of adhesion molecules, specifically cadherins, is critical for ectodermal derivative specification, but that the players and their affinities may vary somewhat from organism to organism. In breast cancer, melanoma and in avian embryos, Ncad and Ecad levels are tightly controlled by the available pool of β-catenin (Figure 4). When Ncad levels are changed, Ecad responds in a reciprocal manner (Figure 3). These changes lead to rearrangements in proportions of ectodermal derivatives (Figure 5, 6). Although we have yet to resolve the mechanism that lies downstream of cadherin proteins regulating cell fate, it is clear that the expression of cadherins must be maintained at specific levels to get proper timing and specification of neural tube cells as well as normal NC specification, EMT, and migration. NC cells appear to be the most sensitive to changes, as they are lost or reduced at early stages whether Ncad is knocked down or in excess, while Sox2-positive cells respond in the opposite manner, where less Ncad leads to more Sox2 and vice versa. Along with the expression data, our gain and loss of function assays suggest that epiblast cells express Ecad prior to Ncad, and that the expression of Ncad in the neural plate at the right time and in the right cells is crucial to allow formation of the appropriate proportions of neural tube and NC cells. However, our data also suggests that cells that have lost Ncad express excess Ecad and Sox2 and they proliferate, but are absent of Ncad and Pax2, two major markers of definitive neural cells. We hypothesize that our Ncad-deficient cells maintain either an ectodermal or neural tube progenitor state rather than differentiating into definitive neuronal or glial cells. Early studies of SoxB1 expression in avian embryos demonstrated that although Sox3 is expressed in the early epiblast, Sox2 is only activated in the cells that are specified to become neural tube at late gastrula stage and it is expressed prior to Ncad (Rex et al., 1997). Therefore, we do not believe that our Ncad deficient cells are primitive epiblast, but rather have been specified to become neural plate suggesting that Ncad is not required for neural induction, but neural determination.
Significant questions remain about the differences in the role of adhesion molecules in neural and NC cells between organisms. In frog embryos, for example, Ecad may not be expressed in the developing neural tube, and loss of Ncad prevents completion of NC EMT (Kuriyama et al., 2014). Additionally, Ncad is maintained in migratory NC cells although it must undergo endocytosis to allow for normal cranial NC migration (Scarpa et al., 2015). Unlike avian embryos, cranial NC cells in the frog may not express Ecad, however, a recent study identified a role for Ecad in cranial NC cell migration, and therefore the jury is still out (Huang et al., 2016). We suggest that the contrasting results may be due to a functional switch of orthologous proteins that may have occurred during a genome duplication event similar to the differences between Pax3 and Pax7 expression and function in Xenopus embryos versus avian embryos (Maczkowiak et al., 2010). In this case, it is possible that Ncad functions as a mesenchymal cadherin in Xenopus and an epithelial cadherin in chicken, and Ecad functions in a reciprocal manner in chicks versus frogs. However, in both cases, it is clear that the localization and levels of cadherin proteins are tightly regulated during embryonic development to allow for normal neural and NC development. Future studies will focus on identifying the cadherin downstream signaling pathways that regulate ectodermal specification events.
Supplementary Material
Highlights.
N-cadherin and E-Cadherin proteins have overlapping and distinct localization
N-cadherin and E-cadherin interact heterotypically
Type I cadherins compete for ß-catenin and space on the cell membrane
Alterations in N-cadherin levels lead to reciprocal changes in E-cadherin
Changing type I cadherin levels alters ectodermal derivative specification
Acknowledgements
We would like to thank the Mary-Pat Stein at CSUN and the Bronner lab at Caltech for helpful discussions, the CSUN biology department for funding and assistance, and the NIH NICHD for funding.
Funding
This work was supported by a National Institute of Health, NICHD P01 grant HD037105 to MEB, a Caltech Baxter Fellowship to CDR, Startup Funding from CSUN to CDR, and a National Institute of Health, NICHD R15 grant R15HD092170 to CDR.
Footnotes
Competing interests
No competing interests declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABBRUZZESE G, BECKER SF, KASHEF J & ALFANDARI D 2016. ADAM13 cleavage of cadherin-11 promotes CNC migration independently of the homophilic binding site. Dev Biol, 415, 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACLOQUE H, OCANA OH, ABAD D, STERN CD & NIETO MA 2017. Snail2 and Zeb2 repress P-cadherin to define embryonic territories in the chick embryo. Development, 144, 649–656. [DOI] [PubMed] [Google Scholar]
- BOUZAS SO, MARINI MS, TORRES ZELADA E, BUZZI AL, MORALES VICENTE DA & STROBL-MAZZULLA PH 2016. Epigenetic activation of Sox2 gene in the developing vertebrate neural plate. Mol Biol Cell, 27, 1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUITRAGO-DELGADO E, NORDIN K, RAO A, GEARY L & LABONNE C 2015. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science, 348, 1332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL K & CASANOVA J 2016. A common framework for EMT and collective cell migration. Development, 143, 4291–4300. [DOI] [PubMed] [Google Scholar]
- CHEN X, KOJIMA S, BORISY GG & GREEN KJ 2003. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol, 163, 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN YT, STEWART DB & NELSON WJ 1999. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol, 144, 687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI YS & GUMBINER B 1989. Expression of cell adhesion molecule E-cadherin in Xenopus embryos begins at gastrulation and predominates in the ectoderm. J Cell Biol, 108, 2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DADY A, BLAVET C & DUBAND JL 2012. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn, 241, 1333–49. [DOI] [PubMed] [Google Scholar]
- DADY A & DUBAND JL 2017. Cadherin interplay during neural crest segregation from the non-neural ectoderm and neural tube in the early chick embryo. Dev Dyn, 246, 550–565. [DOI] [PubMed] [Google Scholar]
- DUFOUR S, BEAUVAIS-JOUNEAU A, DELOUVEE A & THIERY JP 1999. Differential function of N-cadherin and cadherin-7 in the control of embryonic cell motility. J Cell Biol, 146, 501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUAY D, FOTY RA & STEINBERG MS 2003. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol, 253, 309–23. [DOI] [PubMed] [Google Scholar]
- FAIRCHILD CL, CONWAY JP, SCHIFFMACHER AT, TANEYHILL LA & GAMMILL LS 2014. FoxD3 regulates cranial neural crest EMT via downregulation of tetraspanin18 independent of its functions during neural crest formation. Mech Dev, 132, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIRCHILD CL & GAMMILL LS 2013. Tetraspanin18 is a FoxD3-responsive antagonist of cranial neural crest epithelial-to-mesenchymal transition that maintains cadherin-6B protein. J Cell Sci, 126, 1464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOTY RA & STEINBERG MS 2013. Differential adhesion in model systems. Wiley Interdiscip Rev Dev Biol, 2, 631–45. [DOI] [PubMed] [Google Scholar]
- FRIEDLANDER DR, MEGE RM, CUNNINGHAM BA & EDELMAN GM 1989. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci U S A, 86, 7043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIN WJ, EDELMAN GM & CUNNINGHAM BA 1983. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A, 80, 1038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUR S, MANDELBAUM M, HEROLD M, MAJUMDAR HD, NEILSON KM, MAYNARD TM, MOOD K, DAAR IO & MOODY SA 2016. Neural transcription factors bias cleavage stage blastomeres to give rise to neural ectoderm. Genesis, 54, 334–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHELDOF A & BERX G 2013. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci, 116, 317–36. [DOI] [PubMed] [Google Scholar]
- HARDY KM, YATSKIEVYCH TA, KONIECZKA J, BOBBS AS & ANTIN PB 2011. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol, 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C, KRATZER MC, WEDLICH D & KASHEF J 2016. E-cadherin is required for cranial neural crest migration in Xenopus laevis. Dev Biol. [DOI] [PubMed] [Google Scholar]
- HUTCHINS EJ & SZARO BG 2013. c-Jun N-terminal kinase phosphorylation of heterogeneous nuclear ribonucleoprotein K regulates vertebrate axon outgrowth via a posttranscriptional mechanism. J Neurosci, 33, 14666–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INUZUKA H, MIYATANI S & TAKEICHI M 1991. R-cadherin: a novel Ca(2+)-dependent cell-cell adhesion molecule expressed in the retina. Neuron, 7, 69–79. [DOI] [PubMed] [Google Scholar]
- JOURDEUIL K & TANEYHILL LA 2018. Spatiotemporal expression pattern of Connexin 43 during early chick embryogenesis. Gene Expr Patterns, 27, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG Y & MASSAGUE J 2004. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell, 118, 277–9. [DOI] [PubMed] [Google Scholar]
- KASHEF J, KOHLER A, KURIYAMA S, ALFANDARI D, MAYOR R & WEDLICH D 2009. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev, 23, 1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATSAMBA P, CARROLL K, AHLSEN G, BAHNA F, VENDOME J, POSY S, RAJEBHOSALE M, PRICE S, JESSELL TM, BEN-SHAUL A, SHAPIRO L & HONIG BH 2009. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci U S A, 106, 11594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMLER R 1992. Classical cadherins. Semin Cell Biol, 3, 149–55. [DOI] [PubMed] [Google Scholar]
- KEROSUO L & BRONNER-FRASER M 2012. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin Cell Dev Biol, 23, 320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH AW, BOZIC D, PERTZ O & ENGEL J 1999. Homophilic adhesion by cadherins. Curr Opin Struct Biol, 9, 275–81. [DOI] [PubMed] [Google Scholar]
- KURIYAMA S, THEVENEAU E, BENEDETTO A, PARSONS M, TANAKA M, CHARRAS G, KABLA A & MAYOR R 2014. In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J Cell Biol, 206, 113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURTH T, FESENKO IV, SCHNEIDER S, MUNCHBERG FE, JOOS TO, SPIEKER TP & HAUSEN P 1999. Immunocytochemical studies of the interactions of cadherins and catenins in the early Xenopus embryo. Dev Dyn, 215, 155–69. [DOI] [PubMed] [Google Scholar]
- LAMB TM, KNECHT AK, SMITH WC, STACHEL SE, ECONOMIDES AN, STAHL N, YANCOPOLOUS GD & HARLAND RM 1993. Neural induction by the secreted polypeptide noggin. Science, 262, 713–8. [DOI] [PubMed] [Google Scholar]
- LECKBAND D & SIVASANKAR S 2012. Cadherin recognition and adhesion. Curr Opin Cell Biol, 24, 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE RT, NAGAI H, NAKAYA Y, SHENG G, TRAINOR PA, WESTON JA & THIERY JP 2013. Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme. Development, 140, 4890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN J, WANG C, YANG C, FU S & REDIES C 2016. Pax3 and Pax7 interact reciprocally and regulate the expression of cadherin-7 through inducing neuron differentiation in the developing chicken spinal cord. J Comp Neurol, 524, 940–62. [DOI] [PubMed] [Google Scholar]
- MACRI S, SIMULA L, PELLARIN I, PEGORARO S, ONORATI M, SGARRA R, MANFIOLETTI G & VIGNALI R 2016. Hmga2 is required for neural crest cell specification in Xenopus laevis. Dev Biol, 411, 25–37. [DOI] [PubMed] [Google Scholar]
- MACZKOWIAK F, MATEOS S, WANG E, ROCHE D, HARLAND R & MONSORO-BURQ AH 2010. The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev Biol, 340, 381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARAMBAUD P, WEN PH, DUTT A, SHIOI J, TAKASHIMA A, SIMAN R & ROBAKIS NK 2003. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell, 114, 635–45. [DOI] [PubMed] [Google Scholar]
- MATSUMATA M, UCHIKAWA M, KAMACHI Y & KONDOH H 2005. Multiple N-cadherin enhancers identified by systematic functional screening indicate its Group B1 SOX-dependent regulation in neural and placodal development. Dev Biol, 286, 601–17. [DOI] [PubMed] [Google Scholar]
- MAYOR R, MORGAN R & SARGENT MG 1995. Induction of the prospective neural crest of Xenopus. Development, 121, 767–77. [DOI] [PubMed] [Google Scholar]
- MCCREA PD & GOTTARDI CJ 2016. Beyond beta-catenin: prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol, 17, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAGAWA S & TAKEICHI M 1995. Neural crest cell-cell adhesion controlled by sequential and subpopulation- specific expression of novel cadherins. Development, 121, 1321–32. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA S & TAKEICHI M 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development, 125, 2963–71. [DOI] [PubMed] [Google Scholar]
- NORDIN K & LABONNE C 2014. Sox5 Is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm. Dev Cell, 31, 374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY HJ & NISWANDER LA 2016a. Dynamic behaviors of the non-neural ectoderm during mammalian cranial neural tube closure. Dev Biol, 416, 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY HJ & NISWANDER LA 2016b. Grainyhead-like 2 downstream targets act to suppress epithelial-to- mesenchymal transition during neural tube closure. Development, 143, 1192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISS K, MARETZKY T, LUDWIG A, TOUSSEYN T, DE STROOPER B, HARTMANN D & SAFTIG P 2005. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J, 24, 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REX M, ORME A, UWANOGHO D, TOINTON K, WIGMORE PM, SHARPE PT & SCOTTING PJ 1997. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn, 209, 323–32. [DOI] [PubMed] [Google Scholar]
- RIDDIFORD N & SCHLOSSER G 2016. Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS CD 2018. The effects of N-cadherin perturbation on type II cadherin proteins and major signaling pathways Data in Brief, Submitted. [DOI] [PMC free article] [PubMed]
- ROGERS CD, ARCHER TC, CUNNINGHAM DD, GRAMMER TC & CASEY EM 2008. Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev Biol, 313, 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS CD, SAXENA A & BRONNER ME 2013. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol, 203, 835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCARPA E, SZABO A, BIBONNE A, THEVENEAU E, PARSONS M & MAYOR R 2015. Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. Dev Cell, 34, 421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFER G, NARASIMHA M, VOGELSANG E & LEPTIN M 2014. Cadherin switching during the formation and differentiation of the Drosophila mesoderm - implications for epithelial-to-mesenchymal transitions. J Cell Sci, 127, 1511–22. [DOI] [PubMed] [Google Scholar]
- SCHIFFMACHER AT, PADMANABHAN R, JHINGORY S & TANEYHILL LA 2014. Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol Biol Cell, 25, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFMACHER AT, XIE V & TANEYHILL LA 2016. Cadherin-6B proteolysis promotes the neural crest cell epithelial-to-mesenchymal transition through transcriptional regulation. J Cell Biol, 215, 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLOSSER G 2014. Early embryonic specification of vertebrate cranial placodes. Wiley Interdiscip Rev Dev Biol, 3, 349–63. [DOI] [PubMed] [Google Scholar]
- SELLECK MA & BRONNER-FRASER M 2000. Avian neural crest cell fate decisions: a diffusible signal mediates induction of neural crest by the ectoderm. Int J Dev Neurosci, 18, 621–7. [DOI] [PubMed] [Google Scholar]
- SHAN WS, TANAKA H, PHILLIPS GR, ARNDT K, YOSHIDA M, COLMAN DR & SHAPIRO L 2000. Functional cis-heterodimers of N- and R-cadherins. J Cell Biol, 148, 579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIAU CE & BRONNER-FRASER M 2009. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development, 136, 4155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOYAMA Y, TSUJIMOTO G, KITAJIMA M & NATORI M 2000. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J, 349, 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOVAL I, LUDWIG A & KALCHEIM C 2007. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development, 134, 491–501. [DOI] [PubMed] [Google Scholar]
- SIMOES-COSTA M, STONE M & BRONNER ME 2015. Axud1 Integrates Wnt Signaling and Transcriptional Inputs to Drive Neural Crest Formation. Dev Cell, 34, 544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG MS 2007. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev, 17, 281–6. [DOI] [PubMed] [Google Scholar]
- STEINBERG MS & TAKEICHI M 1994. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A, 91, 206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUB BK, RICKELT S, ZIMBELMANN R, GRUND C, KUHN C, IKEN M, OTT M, SCHIRMACHER P & FRANKE WW 2011. E-N-cadherin heterodimers define novel adherens junctions connecting endoderm- derived cells. J Cell Biol, 195, 873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROBL-MAZZULLA PH & BRONNER ME 2012. A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. J Cell Biol, 198, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIERY JP, ACLOQUE H, HUANG RY & NIETO MA 2009. Epithelial-mesenchymal transitions in development and disease. Cell, 139, 871–90. [DOI] [PubMed] [Google Scholar]
- THIERY JP, DELOUVEE A, GALLIN WJ, CUNNINGHAM BA & EDELMAN GM 1984. Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev Biol, 102, 61–78. [DOI] [PubMed] [Google Scholar]
- TIEN CL, JONES A, WANG H, GERIGK M, NOZELL S & CHANG C 2015. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development, 142, 722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUTTLE AM, HOFFMAN TL & SCHILLING TF 2014. Rabconnectin-3a regulates vesicle endocytosis and canonical Wnt signaling in zebrafish neural crest migration. PLoS Biol, 12, e1001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHL JK 3RD, KIM YJ, CULLEN JM, JOHNSON KR & WHEELOCK MJ 2003. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem, 278, 17269–76. [DOI] [PubMed] [Google Scholar]
- WARGA RM & KANE DA 2007. A role for N-cadherin in mesodermal morphogenesis during gastrulation. Dev Biol, 310, 211–25. [DOI] [PubMed] [Google Scholar]
- WEHRENDT DP, CARMONA F, GONZALEZ WUSENER,AE, GONZALEZ A, MARTINEZ JM & ARREGUI CO 2016. P120-Catenin Regulates Early Trafficking Stages of the N-Cadherin Precursor Complex. PLoS One, 11, e0156758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBEL CN, MUTCH CA, SWAMINATHAN S, TAKETO MM & CHENN A 2007. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol, 309, 285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU F, KUMAR P, LU C, EL MARJOU A, QIU W, LIM CT, THIERY JP & LIU R 2015. Homophilic interaction and deformation of E-cadherin and cadherin 7 probed by single molecule force spectroscopy. Arch Biochem Biophys, 587, 38–47. [DOI] [PubMed] [Google Scholar]
- YANG X, CHRISMAN H & WEIJER CJ 2008. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development, 135, 3521–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


