Abstract
The aim of this study was to evaluate the effect of laparoscopic radical hysterectomy on expressions of circulating tumor cells (CTCs) of cytokeratin 19 (CK19), cytokeratin 20 (CK20), and squamous cell carcinoma antigen (SCC-Ag) mRNA.
We collect 78 patients with stage IA2-IIA1 cervical cancer who underwent radical hysterectomy by laparotomy or laparoscopy in our study, and 34 uterine fibroids patients and 32 healthy subjects were recruited as the positive control group and negative control group, respectively. Blood samples were taken from early-stage primary cervical squamous cell carcinoma patients. Real-time reverse transcription polymerase chain reaction (RT-PCR) was used to amplify peripheral blood CK19, CK20 and SCC-Ag from total RNA. We measured the expression of CK19, CK20, and SCC-Ag before laparoscopic radical hysterectomy, 24 hours and 30 days after surgery. Meanwhile, the expression of these markers was compared between laparoscopic and laparotomy groups.
The expressions of CK19, CK20, and SCC-Ag in the experimental group before surgery were (0.0035 ± 0.0018), (1.06 ± 0.49), and (1.48 ± 0.46), respectively, and the positive rates were 32.1%, 33.3%, and 35.9%, respectively. The expression levels of CK19, CK20, and SCC-Ag in the experimental group before surgery was significantly higher than the positive and negative control groups, and there were no significant differences between the positive and negative control groups. The expressions and positive rates of CK19, CK20, and SCC-Ag before laparoscopic radical hysterectomy were significantly lower than the stage at 24 hours after surgery (P < .05), but higher than the stage at 30 days after surgery (P > .05). There were no significant differences in CK19, CK20, and SCC-Ag expressions before surgery, 24 hours and 30 days after surgery between laparoscopic group and laparotomy group (P > .05).
Both laparotomy and laparoscopic radical mastectomy tend to increase the expression of CTCs in peripheral blood, and the expressions have no differences between these 2 groups. So, the use of CK19, CK20, and SCC-Ag expression levels from peripheral blood from early stage cervical cancer radical patients before hysterectomy can aid to overcome the lack of radiographic examination and tumor markers measurement, and provide clues for postoperative treatment and prognosis determination.
Keywords: cervical cancer, circulating tumor cells, cytokeratin 19, cytokeratin 20, laparoscopic radical hysterectomy, real-time reverse transcription polymerase chain reaction (qRT-PCR), squamous cell carcinoma antigen
1. Introduction
In the past decade, the onset age of cervical squamous cell carcinoma shows younger average age trend gradually, which request better treatment to prolong the survival time of the patients and emphasizes to improve the patient's life quality at the same time.[1] Laparoscopic surgery have these with unique advantages of small trauma, quicker recovery, shorten hospital treatment time, do not delay chemotherapy time, reduce postoperative abdominal adhesions, and lower the complications of postoperative radiotherapy, which get rapid development in the field of surgery.[2,3]
Nowadays, the relapse and metastasis rates of cervical squamous cell carcinoma have increased significantly, and become major causes of cervical cancer-related deaths.[4] Whether invasive operation influences the hematogenous dissemination of tumor cells becomes a hot topic in clinical practice. Animal experiments by Nishizaki et al[5] have shown that surgical procedures can cause tumor cells to spread into the bloodstream, which increases the incidence of postoperative metastases. Nishida et al employed real-time reverse transcription–polymerase chain reaction (RT–PCR) to detect the carcinoembryonic antigen (CEA) mRNA in peripheral blood of patients with gastric cancer, and found that the postoperative positive rate was 33% in 36 patients (22% preoperatively), and draw conclusion that the operation procedure may promote the tumor cells to enter the blood circulation.[6] There is no clear answer on how laparoscopic surgery and laparotomy influence on circulating tumor cells (CTCs) in squamous cervical carcinoma. Blood-borne cancer micrometastases usually happened before clinical metastasis, which can be detect by laboratory test, such as microscopic and submicroscopic residual tumor cells in various body tissues, body fluids, and cell grafts before any clinical manifestations.[7–9] As the micro-metastases have no significant clinical signs, it very hard detect by current imaging technologies, such as computed tomography (CT), magnetic resonance imaging (MRI), and pathological examination.[7–9] The presence of micrometastases directly affects tumor prognosis, and the early detection of micrometastases holds great values for tumor diagnosis, staging, recurrence, prognosis, and the choice of comprehensive postoperative treatment.[10,11] Therefore, the detection of micrometastasis has become another hot spot in the field of clinical medicine in recent years. In this study, we compared the effect of laparoscopic surgery and laparotomy on the changes of micrometastasis biomarkers in tumor cells, and explored the safety of laparoscopic radical hysterectomy for cervical cancer.
Cytokeratin (CK) is the main skeleton protein in Keratinocyte, which is mainly distributed in epithelial cells, and its function is to maintain the integrity and continuity of epithelial epithelium. Because of its missing expression in the interstitial tissue (such as blood, bone marrow, etc.), it becomes a more sensitive circulating tumor biomarker of epithelial cell tumor.[12] Wang et al[13] employed RT-PCR and IHC to detect the metastasis of sentinel lymph node (SLN) in early cervical cancer, the results showed that the positive detection rate of qRT-PCR test CK19 was 20%, and its sensitivity was significantly higher than that of immunohistochemistry. Squamous cell carcinoma antigen (SCC-Ag) is a cancer-related antigen, and the serine protease inhibits the family of albumin subpopulations, which is related to the development of cervical squamous carcinoma. Expression level of SCC-Ag is related to tumor activity, the condition progresses when the expression level increased. So detect the expression of SCC-Ag can be used as the evaluation index of cervical squamous cell carcinoma, and can be applied to guide treatment and monitor recurrence in clinic.[14] Li et al[15] through continuous monitoring of SCC-Ag values before and after radiotherapy and chemotherapy in patients with advanced cervical squamous cell carcinoma, which found that the positive rate of SCC-Ag increased with the increase of clinical stage and was closely related to the degree of tumor differentiation.
Our previous study of CK19 and SCC-Ag showed that the expression of 2 markers in the peripheral blood of patients with early cervical cancer with qRT-PCR technique could improve the positive detection rate.[16] The positive detection rate of CK19 and SCCA-g in peripheral blood of patients with advanced cervical cancer was higher than that of patients with early cervical cancer,[17] and this is the reason for the selection of CK19, CK20, and SCC-Ag mRNA as biomarker for detecting the expression of CTCs in peripheral blood of patients with early cervical squamous cell carcinoma.
RT-PCR technology, with high sensitivity, sensitivity, and specificity, has been widely used in the detection of CTCs. Currently, RT-PCR is the most efficient method for detecting CTCs in peripheral blood. To investigate whether CK19, CK20, and SCC-Ag mRNA can be used as the biomarkers for early cervical squamous cell carcinoma CTCs, we employed RT-PCR to measure the expression of multiple genes (CK19, CK20, and SCC-Ag mRNA) in peripheral blood of early cervical squamous cell carcinoma patients, which aim to detect the relative expression and positive detection rate of CTCs of patient and overcome the shortage of low sensitivity and specificity of CTCs single-gene detection.
2. Patients and methods
2.1. Study population
Experimental group: a total of 78 cases of IA2-IIA1 stage cervical squamous cell carcinoma patients who treated in the Department of Gynecologic Oncology, Third Affiliated Hospital of Kunming Medical University from October 2010 to September 2013, were selected. All cases collected here were diagnosed by pathological examination and were examined by 2 deputy chief physicians before the operation, and related clinical stage was defined by the International Union of International Federation of Gynecology and Obstetrics (FIGO was revised in 2009[18]). The age ranged is 29 to 65 years, with a median age of 42.5 years. The average age of laparoscopic radical hysterectomy (LRH) and abdominal radical hysterectomy (ARH) group was (42.4 ± 5.7) and (43.1 ± 8.9) years with P > .05, and the average weight was (67.9 ± 5.6) kg and (68.9 ± 5.8) kg with P > .05, respectively. All cases were diagnosed as cervical squamous cell carcinoma by cervical biopsy before operation. Preoperative radiotherapy and chemotherapy were not included. It was divided into group LRH and group ARH according to the operation. The age, weight, clinical stage, and histopathologic grade of the 2 groups were comparable, and the difference was not statistically significant (P > .05) (Table 1).
Table 1.
Comparison of clinical data of 2 cases.
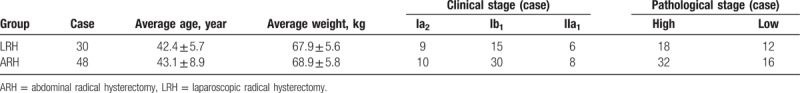
Histological grading: high-middle differentiation in 40 cases, low differentiation in 38 cases. These patients were divided into laparoscopic surgery group (n = 30) and laparotomy group (n = 48) according to the surgical approaches. All the patients did not receive chemoradiotherapy before operation, and these patients with other medical and surgical complications and a secondary tumor were excluded. All patients received surgery by the same surgery group, and received pathological examination after operation.
Positive control group: a total of 34 cases of uterine fibroids patients who treated in the Department of Gynecologic Oncology, Third Affiliated Hospital of Kunming Medical University, were selected with the average age of (43.1 ± 8.9) years and the average weight of (68.9 ± 5.3) kg. These patients with other complex diseases were excluded.
Negative control group: 32 healthy volunteers were selected with the average of (43.4 ± 6.7) years and the average weight of (65.6 ± 6.4) kg, and with no other medical and surgical diseases.
Postoperative follow-up: postoperative follow-up is done by outpatient reexamination, follow-up time varied from 15 to 51 months. Patient will take reexamination every quarterly in first 2 year after surgical, every 6 month the coming 3 to 5 years, and annually after 5 years. The reexamination included gynecological examination, Thinprep cytologic test (TCT, Colposcope), chest film, pelvic cavity B-ultrasound, and CT or MRI examination in case of need. These reexamination aims to observe weather the experimental patient has recurrence or metastasis or not.
The Ethics Committee of Third Affiliated Hospital of Kunming Medical University has approved this study. All the included patients have signed the informed consent.
3. Methods
3.1. Main reagents and instruments
Main instruments are total RNA extraction kit purchased from Beijing Tiangen Biotech Co. Ltd.; cDNA synthesis kit, SYBRGreen real-time PCR kits, general PCR instrument purchased from Bio-Rad Corporation, 7500 real-time PCR instrument purchased from US ABI Company.
3.2. Primer design
Primer requirements: high primer specificity, upstream and downstream primers were around 20 bp; all the primers were designed to cross intron (upstream and downstream primers were located in 2 exons separately) to ensure the amplification of cDNA product, not genomic DNA product. We followed basic principles of primer design, the primer sequences of CK19, CK20, SCC-Ag, and reference gene β-microglobulin were downloaded from the GeneBank, and the Primer5.0 was used for primer design and purification, and synthesized by Shanghai Invitrogen Corporation. The primers were diluted to 100 μmol/L, and preserved at −20°C in the refrigerator.
Primer sequences: upstream of CK19 was CTACAGCCACTACTACACGAC, and downstream was CAGAGCCTGTTCCGTCTCAAA with amplified length of 148 bp; upstream of CK20 was AGGAGACCAAGGCCCGTTA, downstream was ATCAGTTGGGCCTCCAGAGA with amplified length of 76 bp; upstream of SCC-Ag was TGAGGTTAAGGCGGCTAGGA, the downstream was CATGGCGTACTCATCCCCATC with amplified length of 136 bp; upstream of internal reference β-microglobulin was GAGGCTATCCAGCGTACTCCA, downstream was CGGCAGGCATACTCATCTTTT with amplified length of 248 bp.
3.3. Peripheral blood collection
We take 5-mL fasting elbow venous blood from experimental group and benign group at 30 minutes preoperative, 24 hours after operation, 30-day fasting elbow vein blood, and 5 mL from healthy volunteer elbow vein Blood. The initial 1-mL blood sample was discharged to avoid the contamination of skin epithelial cells on the tip, and placed into 5-mL anticoagulant tube (sodium citrate anticoagulant) for blending, and then added into refrigerator and transferred to the laboratory. To prevent RNA degradation, the RNA was extracted within half an hour after blood collection, and the hemolysis and lipid blood samples should not be used.
3.4. Peripheral blood total RNA extraction
Total RNA extraction process was according to the instrument of RNA extraction kit by Beijing Tiangen Biotech Co. Ltd. Two microliters total RNA from sample was collected and diluted with 1 mL by water, zeroed with distilled water, and recorded the absorbance value of the sample at 280 nm and 260 nm, measured the absorption peak at 260 nm and 280 nm by ultraviolet spectrophotometer, then determined RNA purity. The optical density (OD) value was 1.9∼2.0; the next-step experiments were conducted. One microliter RNA sample was takenand the total RNA was calculated as formula. Total RNA (μg) = 40 × OD260 × dilution factor (μg/mL) × total volume (mL). The total 1-μL RNA was taken and detected in 1% agarose gel electrophoresis, taking photos under gel electrophoresis ultraviolet light to determine RNA integrity.
3.5. Reverse transcription synthesis
cDNA synthesis kit from Bio-Rad Company was used, and 1-μg cDNA was synthesized based on the kit instructions; meanwhile, the blank control, negative control, and positive control groups were set. The reaction system and the reaction conditions could be found on the kit instructions. The cDNA was preserved at −80°C in the refrigerator.
3.6. Real-time fluorescent quantitative PCR amplification
The SYBRGreen real-time fluorescent quantitative PCR kit purchased from Bio-Rad Company was used and real-time fluorescent quantitative PCR amplification was conducted based on the kit instructions. Reaction system: 2 × SYBR Green PCR Mastermix 25 μL, 20 μmol/L upstream primer was 1.5 μL, and 20 μmol/L downstream primer was 1.5 μL, RNA enzyme-free water 17 μL, and cDNA 2 μL (50 ng). PCR conditions: 95°C denaturation for 3 minutes; 95°C denaturation for 10 seconds, 58°C annealing for 15 seconds, and 72°C extension for 5 seconds, a total of 40 cycles.
3.7. Preparation of standard curve and determination of linear range
The simple and accurate quantitative real-time PCR method is the standard curve method.[19] The cervical cancer Hela cell cDNA (provided by the Yunnan Tumor Institute) was used as relative quantitative standards, and diluted by 1:10 times to get standard templates with different concentrations. The natural logarithm of the initial copy number was the horizontal axis, and the Ct value was the vertical axis, the real-time quantitative PCR amplification efficiency was calculated according to the formula E = 10[−1/slope]. Real-time PCR instrument can process and analyze the data automatically, and generate real-time PCR standard curves of CK19, CK20, SCC-Ag, and β-microglobulin protein and gene.[20] The literatures suggest that slope about −3.3 is best results, and results with slope of −3.0 to 3.9 are considered feasible results.[21] When the amplification curve correlation coefficient (R2) >0.98, and then 2-ΔCt method can be used to analyze the mRNA relative expression.
3.8. Quantitative PCR amplification data processing
Cervical cancer Hela cell cDNA was used as positive control template, the peripheral blood cDNA of healthy volunteers was used as negative control template, and double-distilled water was used as the blank control, each specimen sets 3 wells. Internal reference gene β-microglobulin protein was used to correct the differences of the RNA quality and reverse transcription efficiency between different specimens. The 2-ΔCt was used to analyze and calculate the mRNA expression levels.[22] ΔCt = average Ct values (sample group) of the target gene, the average Ct value (sample group) of internal reference. The cutoff threshold value was set to the mean value of the corresponding biomarkers (CK19, CK20, and SCC-Ag) in the healthy population of the negative control group and the standard deviation,[23] which is higher than the positive expression.
3.9. Statistical analysis
All data analyses were processed by SPSS17.0 software, the gene expression levels were expressed with the mean ± standard deviation (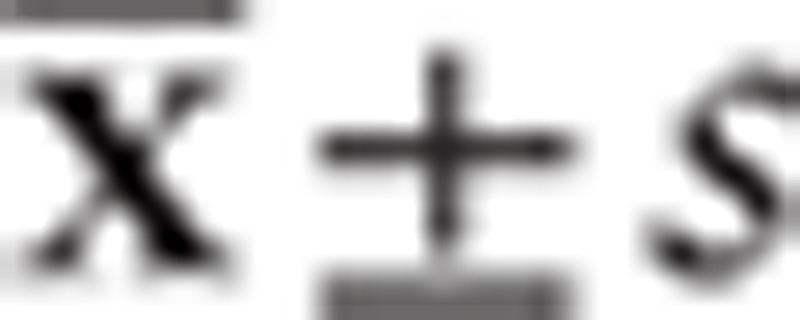 ), and t test was used for the comparison between 2 groups, and variance analysis was used for the comparison among groups; the comparison of positive rate of gene expression was analyzed with χ2 test; correlation analysis between 2 variables was computed by Pearson and Spearman analysis. A P value <.05 indicates statistical significance; P < .01 indicates significant difference.
), and t test was used for the comparison between 2 groups, and variance analysis was used for the comparison among groups; the comparison of positive rate of gene expression was analyzed with χ2 test; correlation analysis between 2 variables was computed by Pearson and Spearman analysis. A P value <.05 indicates statistical significance; P < .01 indicates significant difference.
4. Results
4.1. Comparison of 3 genes expression levels and positive rates between groups
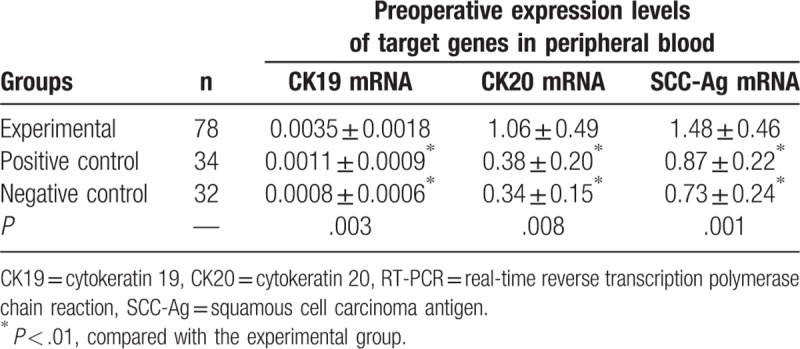
The expression levels of CK19 in the experimental, positive, and negative control groups were (0.0035 ± 0.0018), (0.0011 ± 0.0009), and (0.0008 ± 0.0006), respectively, and there was statistical significance (P < .01). The expressions levels of CK19 in the experimental, positive, and negative control groups were (1.06 ± 0.49), (0.38 ± 0.20), and (0.34 ± 0.15), respectively, and there was statistical significance (P < .01). The expression levels of CK19 mRNA -Ag mRNA in the experimental, positive, and negative control groups were (1.48 ± 0.46), (0.87 ± 0.22), and (0.73 ± 0.24), respectively, and there was statistical significance (P < .01). There were no significant differences in gene expressions between positive control and negative control groups (P > .05, Table 2).
Table 2.
The expression levels of CK19, CK20, and SCC-Ag mRNA in peripheral blood from experimental group and control groups detected by qRT-PCR (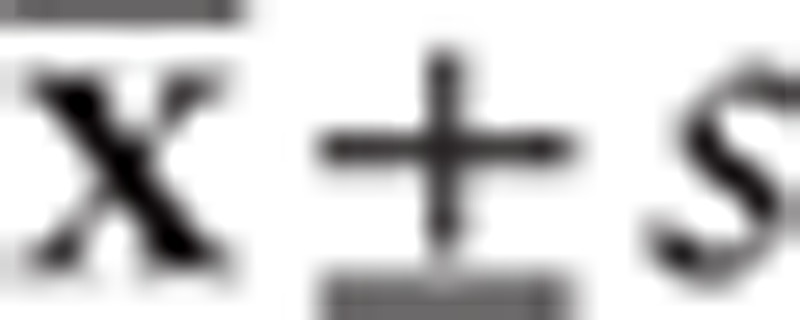 ).
).
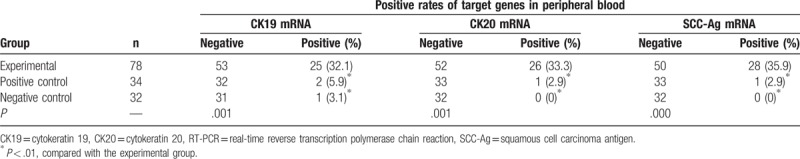
The positive rates of CK19, CK19, and SCC-Ag in the experimental group were 32.1% (25/78), 33.3% (26/78), and 35.9% (28/78), respectively. The positive rates of CK19, CK19, and SCC-Ag in the positive control group were 5.9% (2/34), 2.9% (1/34), and 2.9% (1/34), respectively. In the negative control group, only CK19 showed positive expression with the positive rate of 3.1% (1/32); the positive rates of CK19 and SCC-Ag were both 0 (0/30). The positive rates of CK19, CK20, and SCC-Ag in the experimental group were significantly higher than both in the positive and negative control groups respectively (P < .01, Table 3). However, there were no significant differences between positive and negative control groups (P < .05, Table 3).
Table 3.
The positive rates of CK19, CK20, and SCC-Ag mRNA in peripheral blood from experimental group and control groups detected by qRT-PCR.
4.2. Effect of surgery on CTCs
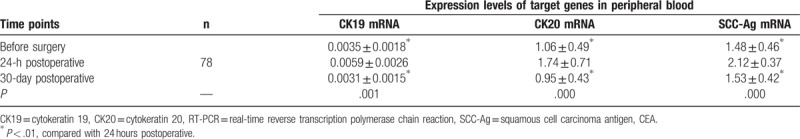
The expression levels of CK19, CK20, and SCC-Ag in peripheral blood circulation of the experimental group were increased rapidly at 24 hours after surgery, and the expression levels were (0.0059 ± 0.0026), (1.74 ± 0.71), and (2.12 ± 0.37), respectively, which were higher than (0.0035 ± 0.0018), (1.06 ± 0.49), and (1.48 ± 0.46) before surgery; the difference was significant (P < .01). The expression levels of (0.0031 ± 0.0015), (0.95 ± 0.43), and (1.53 ± 0.42) at 30 days after surgery were decreased to the levels before surgery or even lower than those (P > .05, Table 4).
Table 4.
Comparison of expression levels of CK19, CK20, and SCC-Ag mRNA in peripheral blood at different time points in the experimental group detected by qRT-PCR ( ).
).
4.3. Effect of different surgical procedures on CTCs
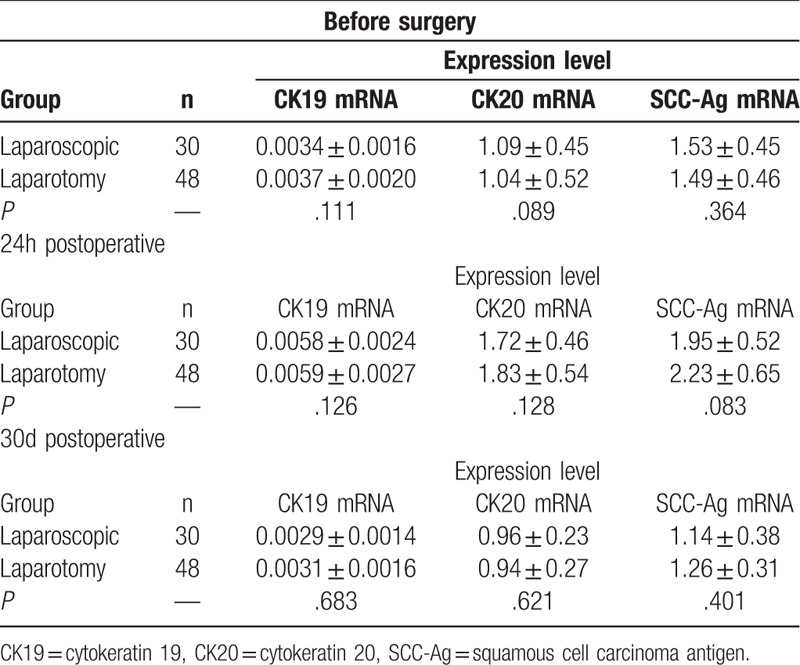
The preoperative expression levels of peripheral blood CK19, CK19, and SCC-Ag in the laparoscopic group were (0.0034 ± 0.0016), (1.09 ± 0.45), and (1.53 ± 0.45), respectively, whereas the expression levels in the laparotomy group were (0.0037 ± 0.0020), (1.04 ± 0.52), and (1.49 ± 0.46), respectively, and there were no statistical significant differences between groups (P > .05). There were no significant differences in the expression levels at 24 hours and 30 days after operation between laparoscopic and laparotomy groups (Table 5).
Table 5.
Comparison of CK19, CK20, and SCC-Ag mRNA expression levels in peripheral blood at different time points between laparoscopic group and laparotomy group ( ).
).
4.4. Analysis on the positive rates of peripheral blood CK19, CK20, and SCC-Ag and the prognosis of the patients in the experimental group
The early cervical squamous cell carcinoma patients were followed up to December 2014, the longest follow-up time was 51 months, and 9 cases were lost to follow-up (loss to follow-up rate was 9.4%). One case who received laparotomy radical hysterectomy suffered from left supraclavicular lymph node metastasis at 10 months after surgery, and the expressions of CK20 and SCC-Ag were positive at 30 days after surgery. One case who was treated with laparoscopic radical hysterectomy had vaginal top local recurrence at 15 months after surgery, and the expression level of peripheral blood SCC-Ag was increased at 30 days after surgery; postoperative pathologic examination in 2 cases showed no pathological risk factor, and no recurrence or metastasis was observed in other patients.
5. Discussion
In 1869, Ashworth[8] proposed the concept of CTCs. In recent years, the study on CTCs has become a hot spot topic in biomedical research. Now that CTCs are the tumor cells that separate from the primary foci and enter into peripheral blood circulation system,[9] most of them will be recognized and killed by the immune system, but few of them can escape from the immune surveillance to survival in a dormant state. And then these survival CTCs divided and proliferated in a suitable environment, and infiltrated in the tissue interstitial and substance, results in news metastases.[24] Therefore, the detection of CTCs in peripheral blood indicates the increased possibility of tumor metastasis, and it has great significance on the detection of tumor recurrence and metastasis, assessing and prognosis, and the development of more effective individualized programs, thus guiding clinical treatment.
Table 2 shows that the relative expression levels of target genes in the peripheral blood in the experimental group were significantly higher than those in the negative control group (P < .01), and there were no significant differences in gene expression levels between negative and positive control groups (P > .05). By the statistical analysis of the positive rate of peripheral blood target genes in the experimental, positive control, and the negative control groups, the author found that the positive rates of target genes in the experimental group were significantly higher than those in the positive and the negative control groups (P < .01), and there were no significant differences in genes between positive and negative control groups (P > .05). The experimental results showed that no high expression of the target genes was detected in the peripheral blood of normal and benign tumor patients, and there was a significant difference compared with cervical squamous cell carcinoma patients, indicating that the CK19, CK20, SCC-Ag in peripheral blood circulation can be used as the CTCs markers of early cervical squamous cell carcinoma micrometastases, which is shown in Table 3.
The expression of CK19, CK20 has already been used to assess the presence of CTCs from various types of cancer in many previous studies,[25–27] however, the presence of its pseudogenes may lead to decreased specificity sensitivity of the assay. Table 2 shows that the expression of CK19 in peripheral blood is lower than that of CK20 and SCC-Ag (P < .05), and the positive rate of CK19 is lower. During the course of the experiment, the project team found that the peak of amplification curve of CK19 in early stage of cervical squamous cell carcinoma was peaked late, the dissolution curve was not smooth enough and the peak was low, and the fragment of RT-PCR product of target gene had impurity band development; thus, we doubt that the CK19 fragment amplification had background impurity interference, and then its specificity remains to be verified. In contrast, the amplification curves of CK20 and SCC-Ag peaked early, the dissolution curves were relatively smooth, and the peak was high without impurity peak. The RT-PCR product electrophoresis fragments of CK20 and SCC-Ag were single and bright, which proved that the amplification quality of the target genes was high. Therefore, CK20 and SCC-Ag are more suitable for the study of CTCs micrometastasis in early cervical squamous cell carcinoma than CK19, but this may be related to the small sample size. The results need to be further studied in larger sample to verify.
Our research found that the expression levels of CK19, CK20 and SCC-Ag in peripheral blood circulation of the cervical squamous cell carcinoma patients were increased rapidly at 24 hours after surgery, whereas the expression levels at 30 days after surgery were decreased to preoperative levels or even lower than the preoperative level (P > .05). Wound healing and tumors are characterized by cell proliferation, remodeling of extracellular matrix, invasion and migration of cancer cells, neovascularization, and coagulation regulation, such as the involvement of the STAT3 signaling pathway in tumorigenesis and tissue damage by regulating a common set of genes involved in wound healing and cancer.[28] Postoperative gene regulation may be one of the reasons for the homing phenomenon. Surgical interventions, such as intraoperative squeeze of tumor, shedding of tumor cells, opening of vascular beds, and postoperative immunosuppression, affect certain signaling pathways involved in wound healing; these include cell invasion and migration, angiogenesis, coagulation regulation, and suppression of interferon-inducing genes. Similarly, a study by Dauer et al[29] found increased activity of STAT3 signaling pathways, which is associated with wound healing in mice with chronic inflammatory lung cancer, and was invading the paraneoplastic tissue stroma expression, which explained that cancers provided molecular basis for the relaxation of normal wound healing process.
Surgical trauma may cause a sudden increase in the number of tumor cells released into the circulation.[30,31] As CTCs are passively squeezed into the peripheral circulation, the viability and the ability of evading from immune surveillance may be weakened. A certain period of immune system recognizing can kill most TCSs, leading to the rapid increase in CK19, CK20, and SCC-Ag expression levels in peripheral blood circulation at 24 hours after surgery, and then decrease to preoperative level or even lower than the preoperative level at 30 days after surgery.[32,33] But this mechanism should be verified with more time and more clinical trials.
Recently, the average age of onset of cervical cancer showed a younger trend, which requests higher requirements for treatment, while prolonging the survival of patients at the same time, emphasizing the improvement of postoperative quality of life of patients. With the development of minimally invasive surgery, as the representative, the laparoscopic surgery has been widely used in pelvic and abdominal surgery. Laparoscopic surgery has been rapidly developed in the field of minimally invasive surgery with its advantages of small trauma and rapid recovery, but because of the wide range and big difficulty in radical hysterectomy, it is prone to complications, especially when the operation under microscope is not familiar, and the accident is more likely to occur, which makes the laparoscopic radical hysterectomy develop slowly in a very long period. There is no common view on this topic, and major debate issue is that whether laparoscopic radical hysterectomy can achieve the same radical effect with traditional laparotomy? Whether it will increase the blood and lymphatic spread of tumor cells? Some studies suggested that laparoscopic surgery needs CO2 pneumoperitoneum to create enough space for surgery, and CO2 causes acidic environment which can accelerate tumor cells entering into the blood, suggesting that laparoscopic surgery may accelerate the tumor cells entering into the blood and leading to the increase of occurrence rate of micrometastases. In this study, we compared the expression levels of 3 target genes before surgery, at 24 hours and 30 days after surgery between laparoscopic group and laparotomy group, and the results showed that there were no significant differences in CK19, CK20, and SCC-Ag expression levels in peripheral blood of patients between the laparoscopic group and the laparotomy group (P > .05); the expression levels of the rest data are showed in Table 4. Our results showed that laparoscopic radical hysterectomy did not cause any more CTCs than traditional abdominal radical hysterectomy, and suggested that it did not increase the risk of metastases. Furthermore, laparoscopic radical hysterectomy has some advantages vs. traditional abdominal radical hysterectomy. For example, it could reduce the number of patients developing complications, shorten their hospital stay, and not delay the time interval between surgery and postoperative radiation therapy.
For a long time, we used routine pathological examination to judge whether patients need sufficient treatment after surgery. The routine imaging examination was used to determine tumor progress, but these tests are not enough to find the tumor cells that entered early into the circulatory system caused by surgical intervention. Some studies showed that at least 105 tumor cells in peripheral blood circulation could cause disease recurrence,[34] and tumor with the diameter >2 mm can be detected by routine pathological examination, and this time, the tiny tumor cells may have entered into the peripheral blood circulation and evaded from the immune surveillance and searching for a new transfer sites, implanted and found a micro-environment to protect it from apoptosis, thus gradually expanding and forming metastases.[35] Our postoperative follow-up results showed that 1 case with positivity for expressions of CK20 and SCC-Ag mRNA in peripheral blood 30 days after surgery was found to the occurrence of supraclavicular lymph node metastasis at 10 months after surgery. Moreover, another 1 case with positivity for expressions of SCC-Ag mRNA in peripheral blood 30 days after surgery was found to occur vaginal local recurrence at 15 months after surgery. Interestingly, the 2 cases had no pathological risk factors by postoperative pathologic examination. Thus, our results indicated CK19, CK20, and SCC-Ag mRNA were suitable biomarker for detecting the expression of CTCs in peripheral blood of patients with early cervical squamous cell carcinoma.
In current study we found, for the first time, that CK19, CK20, and SCC-Ag mRNA could be promising novel CTCs biomarkers to detect early cervical squamous cell carcinoma micrometastases. However, compared with CK19, CK20 and SCC-Ag are more suitable for the study of micrometastases of CTCs in early stage of cervical squamous cell carcinoma (this may be related to the small sample size. This sample size needs to be further expanded to confirm that this result is reliable). Third, we need larger patients to systematically develop a nationally recognized statistical cut-point threshold to better guide clinical staging, prognosis assessment, and develop a more effective individualized treatment plan to guide clinical treatment. Fourth, laparoscopic radical hysterectomy does not increase circulating tumor cells into the blood, and the method is safe and feasible in the treatment of early cervical squamous cell carcinoma, and it is worthy of clinical promotion.
Acknowledgement
The authors thank Chun-Xiu Wu for her help in pre-experiment work, and Dr. Yupeng Cun for his suggestions and proof reading of the manuscript.
Author contributions
Conceptualization: XiangQun Wei.
Data curation: XiangQun Wei, Yuan Ma, Min Zhao.
Formal analysis: XiangQun Wei, Yan Chen, Xin Liu, Min Zhao, LiWen Zhou.
Funding acquisition: XiangQun Wei.
Methodology: XiangQun Wei, Yuan Ma, Xin Liu, Min Zhao, LiWen Zhou.
Validation: XiangQun Wei, Yuan Ma, Yan Chen, Xin Liu, Min Zhao, LiWen Zhou.
Writing – original draft: Yuan Ma, Yan Chen, Xin Liu, Min Zhao, LiWen Zhou.
Writing – review & editing: XiangQun Wei.
Footnotes
Abbreviations: ARH = abdominal radical hysterectomy, CEA = carcinoembryonic antigen, CK19 = cytokeratin 19, CK20 = cytokeratin 20, CT = computed tomography, CTCs = circulating tumor cells, LRH = laparoscopic radical hysterectomy, MRI = magnetic resonance imaging, OD = optical density, RT-PCR = real-time reverse transcription polymerase chain reaction, SCC-Ag = squamous cell carcinoma antigen, TCT = Thinprep cytologic test, TSCs = tumor stem cells.
X-QW and YM contributed equally to this article, they are co-first authors.
This study was funded by grants from the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University (No. 2010DC182, 2014FZ036), the National Natural Science Foundation of China (No. 81560380), and the Yunnan Provincial
Technology Project of Health (No. 2014NS024).
The authors report no conflict of interest.
References
- [1].Pelkofski E, Stine J, Wages NA, et al. Cervical cancer in women aged 35 years and younger. Clin Ther 2016;38:459–66. [DOI] [PubMed] [Google Scholar]
- [2].Vieira MA, Rendon GJ, Munsell M, et al. Radical trachelectomy in early-stage cervical cancer: a comparison of laparotomy and minimally invasive surgery. Gynecol Oncol 2015;138:585–9. [DOI] [PubMed] [Google Scholar]
- [3].Wang YZ, Deng L, Xu HC, et al. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer 2015;15:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peiretti M, Zapardiel I, Zanagnolo V, et al. Management of recurrent cervical cancer: a review of the literature. Surg Oncol 2012;21:e59–66. [DOI] [PubMed] [Google Scholar]
- [5].Nishizaki T, Matsumata T, Kanematsu T, et al. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res 1990;49:92–7. [DOI] [PubMed] [Google Scholar]
- [6].Nishida S, Kitamura K, Ichikawa D, et al. Molecular detection of disseminated cancer cells in the peripheral blood of patients with gastric cancer. Anticancer Res 2000;20:2155–9. [PubMed] [Google Scholar]
- [7].Lloyd JM, McIver CM, Stephenson SA, et al. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res 2006;12:417–23. [DOI] [PubMed] [Google Scholar]
- [8].Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869;14:146–9. [Google Scholar]
- [9].Dittmar T, Heyder C, Gloria-Maercker E, et al. Adhesion molecules and chemokines: the navigation system for circulating tumor (stem) cells to metastasize in an organ-specific manner. Clin Exp Metastasis 2008;25:11–32. [DOI] [PubMed] [Google Scholar]
- [10].Schindlbeck C, Andergassen U, Jueckstock J, et al. Disseminated and circulating tumor cells in bone marrow and blood of breast cancer patients: properties, enrichment, and potential targets. J Cancer Res Clin Oncol 2016;29:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor dna as liquid biopsy. Cancer Discov 2016;6:479–91. [DOI] [PubMed] [Google Scholar]
- [12].Zhang HW, Yu XF, Wang XJ. The detection of cytokeratin 19 mRNA in peripheral blood of patients with non-small cell lung cancer using RT-PCR. Chinese J Clin Oncol 2005;32:1042–4. [Google Scholar]
- [13].Wang HY, Sun JM, Lu HF, et al. Micrometastases detected by cytokerat in 19 expression in sentinel lymph nodes of patients with early-stage cervical cancer. Int J Gynecol Cancer 2006;16:643–8. [DOI] [PubMed] [Google Scholar]
- [14].Sandri MT, Salvatici M, Mauro C, et al. Detection of squamous cell carcinoma antigen with two systems in the follow-up of patients with cervical cancer. Int J Biol Markers 2013;28:313–7. [DOI] [PubMed] [Google Scholar]
- [15].Li GL, Zhao GL. Clinical significance of squamous cell carcinoma antigen monitoring in the treatment of cervical squamous cell carcinoma. Chinese J Clin Oncol 2010;37:313–7. [Google Scholar]
- [16].Wei XQ, Chen Y, Li RM. The diagnostic value of combined detection of peripheral blood CK19 and SCCAg mRNA expression in the early cervical cancer micrometastasis. Journal of Kunming Medical University 2013;7–12. [Google Scholar]
- [17].Wei XQ, Li RM, Chen Y. The influence of radical hysterectomy in the expression of CK19 and SCCAg mRNA of cervical cancer. Prog Obstet Gynecol 2014;23:274–7. [Google Scholar]
- [18].Pecorelli S. Revised FIGO staging for carcinoma of the vulva cervix and endometrium. Int J Gynaecol Obstet 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- [19].Molloy TJ, Devriese LA, Helgason HH, et al. A multimarker QPCR-based platform for the detection of circulating tumor cells in patients with early-stage breast cancer. Br J Cancer 2011;104:1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biermann JC, Holzscheiter L, Kotzsch M, et al. Quantitative RT-PCR assays for the determination of urokinase-type plasminogen activator and plasminogen activator inhibitor type 1 mRNA in primary tumor tissue of breast cancer patients: comparison to antigen quantification by ELISA. Int J Mol Med 2008;21:251–9. [PubMed] [Google Scholar]
- [21].Kolenova A, Hikkel I, Ilencikova D, et al. Minimal residual disease detection using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the non-MRD-based ALL IC-BFM 2002 protocol for childhood ALL: Slovak experience. Neoplasma 2010;57:552–61. [DOI] [PubMed] [Google Scholar]
- [22].Sadikovic B, Thorner P, Chilton-Macneill S, et al. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer 2010;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mikhitarian K, Martin RH, Ruppel MB, et al. Detection of mammaglobin mRNA in peripheral blood is associated with high grade breast cancer: interim results of a prospective cohort study. BMC Cancer 2008;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou QJ, Yang JM, Gastroenterology DO. Advances in research on circulating tumor cells. World Chinese Journal of Digestology 2010;18:1081–7. [Google Scholar]
- [25].Vaiopoulos AG, Kostakis ID, Gkioka E, et al. Detection of circulating tumor cells in colorectal and gastric cancer using a multiplex PCR assay. Anticancer Res 2014;34:3083–92. [PubMed] [Google Scholar]
- [26].Tunca B, Egeli U, Cecener G, et al. CK19, CK20, EGFR and HER2 status of circulating tumor cells in patients with breast cancer. Tumori 2012;98:243–51. [DOI] [PubMed] [Google Scholar]
- [27].Gervasoni A, Monasterio Munoz RM, Wengler GS, et al. Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett 2008;263:267–79. [DOI] [PubMed] [Google Scholar]
- [28].Liu RJ. Tumor metastasis-homing of tumor stem cells. Journal of Shanghai Jiaotong University (Medical Science) 2008;28:209–11. [Google Scholar]
- [29].Dauer Daniel J, Ferraro Bernadette, Song Lanxi, et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene 2005;24:3397–408. [DOI] [PubMed] [Google Scholar]
- [30].O’Leary D, O’Leary E, Foley N, et al. Effects of surgery on the cancer stem cell niche. Eur J Surg Oncol 2016;42:319–25. [DOI] [PubMed] [Google Scholar]
- [31].Bi Y, Meng Y, Wu H, et al. Expression of the potential cancer stem cell markers CD133 and CD44 in medullary thyroid carcinoma: A ten-year follow-up and prognostic analysis. J Surg Oncol 2016;113:144–51. [DOI] [PubMed] [Google Scholar]
- [32].Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 2007;8:444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schmidt-Kittler O, Ragg T, Daskalakis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci 2003;100:7737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wyld DK, Selby P, Perren TJ, et al. Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer 1998;79:288–93. [DOI] [PubMed] [Google Scholar]
- [35].Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol 2010;222:1–5. [DOI] [PubMed] [Google Scholar]


