Abstract
The antimicrobial effect of nitric oxide (NO) is an essential part of innate immunity. The vigorous host response to the human gastric pathogen Helicobacter pylori fails to eradicate the organism, despite up-regulation of inducible NO synthase (iNOS) in the gastric mucosa. Here we report that wild-type strains of H. pylori inhibit NO production by activated macrophages at physiologic concentrations of l-arginine, the common substrate for iNOS and arginase. Inactivation of the gene rocF, encoding constitutively expressed arginase in H. pylori, restored high-output NO production by macrophages. By using HPLC analysis, we show that l-arginine is effectively consumed in the culture medium by wild-type but not arginase-deficient H. pylori. The substantially higher levels of NO generated by macrophages cocultured with rocF-deficient H. pylori resulted in efficient killing of the bacteria, whereas wild-type H. pylori exhibited no loss of survival under these conditions. Killing of the arginase-deficient H. pylori was NO-dependent, because peritoneal macrophages from iNOS−/− mice failed to affect the survival of the rocF mutant. Thus, bacterial arginase allows H. pylori to evade the immune response by down-regulating eukaryotic NO production.
Helicobacter pylori is a Gram-negative microaerophilic bacterium, which selectively colonizes the human stomach. Current prevalence of H. pylori is ≈40% of the population in the U.S. (1) and substantially higher in underdeveloped regions. H. pylori causes chronic gastritis, peptic ulcers, and gastric carcinoma and lymphoma, leading to its classification as a Class I carcinogen (2). Despite inciting substantial acute and chronic immune and inflammatory responses, H. pylori infection generally persists for the life of the host. Understanding how the bacterium evades the host response remains a critical issue in managing the public health burden of this infection.
Nitric oxide (NO) is a central component of innate immunity and an effective antimicrobial agent (3). This activity is especially marked for intracellular pathogens such as Mycobacterium tuberculosis (4) and Leishmania major (5), which are killed by an NO-dependent mechanism. Reactive nitrogen intermediates can also effectively kill extracellular parasites (6, 7) and bacteria such as Escherichia coli (8). Chemical sources of NO and peroxynitrite have a direct toxic effect on H. pylori (9, 10). However, the effect of cell-derived NO on H. pylori has not been investigated. The survival of H. pylori, despite marked induction of inducible NO synthase (iNOS) in macrophages (11) and gastric tissues (12), suggests that the bacterium has developed mechanisms to avoid NO-dependent killing.
Arginases are a primordial enzyme family, which are highly conserved across kingdoms (13). Mammalian arginases compete with NO synthases for the common substrate l-arginine (14), hydrolyzing the amino acid to urea and l-ornithine. Therefore, arginases can regulate cellular NO production (15, 16) and counteract the biological effects of NO (7, 17). H. pylori possesses the gene rocF, which encodes an arginase (18). Our previous work showed that H. pylori added to macrophages under culture conditions of high l-arginine availability resulted in abundant levels of NO production (11). Here we report the effect of H. pylori arginase on macrophage NO production under conditions of physiologic l-arginine concentration. Although wild-type (WT) H. pylori and strains deficient in rocF stimulate similar levels of iNOS mRNA expression, significantly greater levels of NO are released by macrophages stimulated with rocF-deficient strains. This finding was attributable to l-arginine consumption by the WT but not by the arginase-deficient bacteria. Loss of arginase activity results in marked NO-dependent killing of H. pylori, indicating that the bacterial arginase has evolved as a survival mechanism that may contribute to the ability of H. pylori to successfully colonize the human stomach for the life of the host. To our knowledge, our data constitute the first description of the modulation of eukaryotic NO production by a prokaryotic arginase.
Materials and Methods
Bacteria.
H. pylori strains SS1 (19) and 26695 (20) were used. In these strains, the rocF gene was disrupted by insertion of an aphA3 cassette, yielding mutants (rocF∷aphA3) completely devoid of arginase activity (18). The aphA3 cassette used to disrupt rocF is unlikely to exert any polar effect on other genes, because the upstream gene (hp1398) is in the opposite orientation to that of rocF, and the 5′ end of the downstream gene (hp1400) is more than 500 bp beyond the 3′ end of rocF. Additionally, rocF is followed by a predicted near-consensus rho-independent transcriptional terminator (CUUUUCAAACCN11GGUUGAAAAAG), followed by an AU-rich region (18). Both WT and rocF mutants were passaged on Brucella agar plates containing 10% sheep blood or cultured in Mueller–Hinton broth with 3% FBS (Life Technologies, Grand Island, NY). For the current experiments, the passage number from the frozen stock was always identical for the WT and mutant strains. H. pylori strains were harvested from growth medium at exponential phase, washed twice, suspended in PBS, and used in the experiments. All cultures of bacteria were grown under microaerobic conditions. Bacterial concentration was determined by spectrophotometry, using OD600 as described (11).
To develop additional preparations of H. pylori, the following manipulations were performed. French press lysates of H. pylori were obtained by passage through a French pressure cell at 20,000 psi (1 psi = 6.89 kPa; ref. 11). Water extracts were obtained by vortexing 109 bacteria in 1 ml of water for 5 min (21). Lysates and water extracts were subjected to centrifugation at 12,000 × g for 10 min, and supernatants were used for the assays. Bacteria were also boiled for 30 min in PBS.
For the H. pylori bactericidal studies, H. pylori colony-forming units were determined before and after cocultures by serial dilution and culture on blood agar plates.
Cells.
The murine macrophage cell line RAW 264.7 was maintained in DMEM (Life Technologies) and supplemented with 10% FBS/100 units/ml penicillin/100 μg/ml streptomycin/1 mM sodium pyruvate/10 mM Hepes in a humidified 5% CO2 atmosphere. For the experiments, RAW 264.7 cells were cocultured with intact or lysed H. pylori, with or without filter supports (0.2 μm pore size; Transwell, Nunc), at a multiplicity of infection (moi) of 10 to 100. To control l-arginine concentrations in the cocultures, serum-free DMEM, devoid of l-arginine and phenol red (Life Technologies) and supplemented with 1 mM sodium pyruvate/10 mM Hepes/4 g/l BSA (Sigma), was used. Macrophages (106 cells per ml) were plated in 24-well plates, and medium was changed after 4 h. The iNOS-specific inhibitor, n-iminoethyl-l-lysine (100 μM; Alexis Biochemicals, San Diego), and various concentrations of l-arginine (Sigma) were then added with the bacteria. In some experiments, macrophages were first activated with 100 units/ml of IFN-γ for 24 h before addition of H. pylori for 6 h.
Peritoneal macrophages were isolated from C57BL/6 WT and iNOS−/− mice (The Jackson Laboratory) as described (22) and cocultured with H. pylori in DMEM-BSA.
Measurement of NO Concentration.
The concentration of the oxidized product of NO, NO2−, was assessed by the Griess reaction, as described elsewhere (11). Concentration of total reactive nitrogen compounds (23) was measured by using an NO analyzer, NOA 280 (Sievers, Boulder, CO), a high-sensitivity detector based on a gas-phase chemiluminescent reaction between NO and ozone as described (24).
Measurement of l-Arginine Concentration.
H. pylori SS1 WT and rocF∷aphA3 (108/ml) were cultured for 24 h in the medium used for the coculture experiments, containing 0.4 mM l-arginine. Amino acid analysis was performed by HPLC on a Beckman Coulter 7300 Amino Acid Analyzer, using anion-exchange chromatography. This system utilizes four lithium chloride elution buffers of increasing pH and ionic strength, postcolumn reaction with ninhydrin, and dual-wavelength detection at 570 and 440 nm. Data capture and integration of peaks were performed by using Beckman Coulter System Gold.
Northern Blot Analysis.
RAW 264.7 macrophages (106/ml) were seeded in 75-cm2 flasks and incubated with H. pylori SS1 WT or rocF∷aphA3 (107/ml). After 6 h, medium was removed and cells were washed twice with PBS. Extraction of total RNA and Northern blot analysis were performed as described (11). For iNOS mRNA detection, a 32P random primer-labeled full-length (3.9 kb) cDNA probe for murine iNOS (obtained from Q. W. Xie and C. Nathan, Cornell University Medical College, New York) was used. The concentration of mRNA was standardized by hybridization with a cDNA probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; CLONTECH).
Results
Analysis of NO Generation by RAW 264.7 Macrophages in Response to WT and Arginase-Deficient H. pylori.
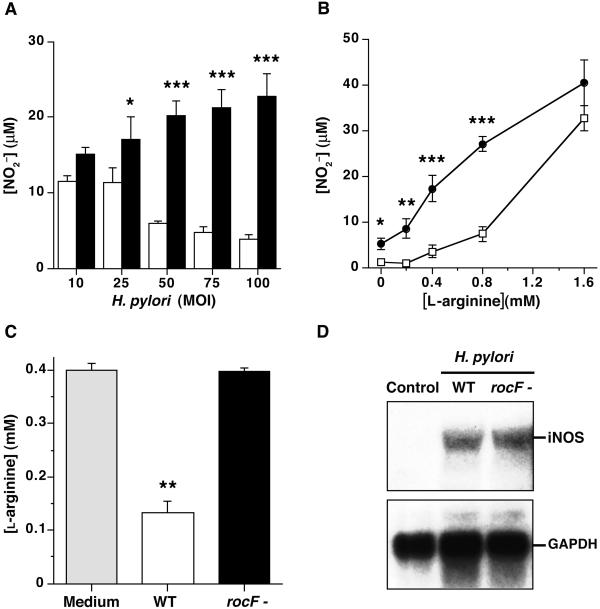
Because H. pylori possesses the gene rocF, which encodes a bacterial arginase, we used isogenic rocF mutant strains to investigate the role of H. pylori-derived arginase on NO production by host cells. At an l-arginine concentration of 0.4 mM, H. pylori SS1 WT inhibited production of NO2− by RAW 264.7 macrophages (Fig. 1A). In contrast, arginase-deficient H. pylori stimulated concentration-dependent increases in NO2− levels. Differences in stimulated macrophage NO2− generation between the WT and rocF∷aphA3 strains occurred at l-arginine concentrations of 0.8 mM or lower, but not at higher levels (Fig. 1B), indicating that the competitive inhibition between bacterial arginase and macrophage iNOS can be overwhelmed by addition of excess substrate. Very similar results were obtained by using WT and rocF∷aphA3 mutant strains of H. pylori 26695 (not shown).
Figure 1.
H. pylori arginase inhibits NO2− generation by RAW 264.7 cells. (A) H. pylori SS1 WT (open bars) or rocF∷aphA3 (solid bars) was cocultured with macrophages in DMEM-BSA containing 0.4 mM l-arginine at an moi between 10 and 100. Production of NO2− was determined in supernatants after 24 h by the Griess reaction. Values are expressed as mean ± SEM of four experiments performed in duplicate. (B) H. pylori SS1 WT (□) or the rocF∷aphA3 (●) strains were added to RAW 264.7 macrophages at an moi of 100 and varying l-arginine concentrations. [NO2−] was measured after 24-h incubation. Data represent mean ± SEM of three experiments performed in duplicate. For A and B, *, P < 0.05; **, P < 0.01; ***, P < 0.001 for H. pylori WT vs. rocF∷aphA3 mutant strain by unpaired t test. (C) H. pylori SS1 WT (open bar) or rocF∷aphA3 (rocF−; solid bar) was incubated in the presence of 0.4 mM l-arginine. After 24 h, [l-arginine] was assessed by HPLC analysis. Data represent mean ± SEM of two separate experiments performed in triplicate. **, P < 0.01 compared with medium alone and rocF∷aphA3 strain by Student–Newman–Keuls multiple analysis comparison. (D) Northern blot analysis of RNA isolated from RAW 264.7 macrophages incubated with H. pylori SS1 WT and rocF∷aphA3 mutant (rocF−) at an moi of 100. After 6 h, total RNA from macrophages was harvested, and 10 μg was loaded per lane. Blots were hybridized sequentially with murine iNOS and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes.
By using an NO analyzer (NOA 280), we verified that total NO metabolites were decreased by 3.1 ± 0.1-fold (P < 0.05, n = 6) with H. pylori SS1 WT compared with the arginase-deficient strain, in studies conducted at 0.4 mM l-arginine and an moi of 100.
Macrophages alone did not produce detectable levels of NO2−, and the iNOS-selective inhibitor, n-iminoethyl-l-lysine, completely inhibited macrophage NO2− production in response to both mutant and WT H. pylori strains (not shown). The differences in NO2− generation by macrophages exposed to WT or rocF∷aphA3 are not attributable to variation in macrophage survival, as determined by cell counts using Trypan blue exclusion or by analysis of macrophage apoptosis using histone-DNA fragmentation ELISA.
Utilization of l-Arginine by WT and Arginase-Deficient Strains of H. pylori.
HPLC analysis was used to assess l-arginine concentration in cell culture medium containing H. pylori. After 24 h, l-arginine concentration was dramatically decreased in the culture of WT H. pylori, but not in the culture of the rocF mutant strain (Fig. 1C). These data support the hypothesis that H. pylori arginase effectively decreases l-arginine in the medium, leading to the decrease of macrophage NO production by means of loss of substrate availability for iNOS.
Induction of iNOS mRNA Expression in Macrophages by H. pylori WT and rocF∷aphA3.
iNOS Northern blot analysis was performed to exclude a difference in macrophage activation by the WT vs. the rocF∷aphA3 mutant of H. pylori. Stimulation of macrophages by H. pylori SS1 WT and rocF∷aphA3 resulted in comparable increases in iNOS mRNA levels, compared with undetectable basal iNOS levels in unstimulated macrophages (Fig. 1D). Because we have demonstrated that iNOS mRNA levels correlate directly with iNOS enzymatic activity in response to H. pylori stimulation (11), these data indicate that bacterial arginase regulates macrophage NO production independently of effects on iNOS expression.
Effect of H. pylori Arginase on NO2− Production by IFN-γ-Activated Macrophages.
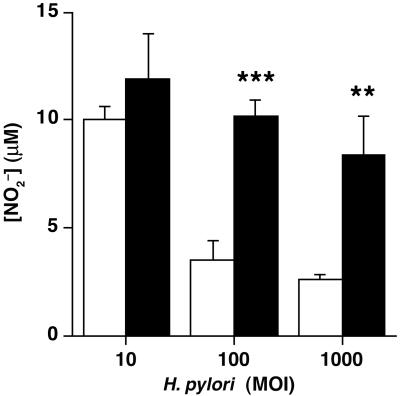
To establish whether macrophage NO inhibition by bacterial arginase can occur regardless of the macrophage stimulation process, we pretreated RAW 264.7 cells with IFN-γ, followed by the addition of H. pylori. In a concentration-dependent manner, H. pylori SS1 WT inhibited macrophage NO2− production by the preactivated cells (Fig. 2). In contrast, the arginase-deficient H. pylori rocF∷aphA3 did not affect NO2− levels. These data strongly suggest that bacterial arginase acts to inhibit NO release by preformed iNOS.
Figure 2.
H. pylori arginase inhibits NO2− production by preactivated macrophages. RAW 264.7 macrophages were stimulated with IFN-γ (100 units/ml) for 24 h. Cells were washed and H. pylori SS1 WT (open bars) or rocF∷aphA3 (solid bars) was added in medium containing 0.4 mM l-arginine at an moi of 10, 100, or 1,000. [NO2−] was assessed in the supernatant after 6 h. Values are expressed as mean ± SEM of six experiments performed in duplicate. **, P < 0.01; ***, P < 0.001, for H. pylori SS1 rocF∷aphA3 compared with WT by unpaired t test.
Live H. pylori-Containing Arginase Is Required to Inhibit Macrophage NO2− Generation.
To further assess the ability of H. pylori to prevent NO production, we stimulated macrophages with different H. pylori preparations. Addition of heat-killed bacteria, water extracts, or French press lysates of H. pylori resulted in loss of the inhibitory effect on NO production by the WT H. pylori (Table 1). Prevention of NO2− release occurred with intact H. pylori or with bacteria separated from the host cells by a filter support. These data suggest that live bacteria, but not contact with the macrophages, are required for functional inhibition of iNOS. Thus, viable bacteria efficiently consume l-arginine available in the extracellular environment, resulting in effective inhibition of NO production.
Table 1.
Effect of different preparations of H. pylori SS1 and SS1 rocF∷aphA3 on NO2− generation by RAW 264.7 macrophages
| Preparation | [NO2−], μM
|
Fold increase | P | |
|---|---|---|---|---|
| SS1 | SS1 rocF∷aphA3 | |||
| Bacteria, untreated | 2.5 ± 1.1 | 17.3 ± 1.8 | 6.8 | <0.0001 |
| Bacteria in Transwell | 5.9 ± 1.9* | 19.1 ± 1.5 | 3.2 | 0.0002 |
| Boiled bacteria | 26.3 ± 4.6 | 30 ± 4.8 | 1.1 | NS |
| Water extract | 19.6 ± 1.2 | 17 ± 2.1 | 1.1 | NS |
| French press lysate | 22.3 ± 3.3 | 21.3 ± 3.8 | 0.9 | NS |
RAW 264.7 macrophages were cocultured with intact H. pylori SS1 WT or rocF∷aphA3 or with the preparations of bacteria listed. H. pylori were also separated from macrophages by a filter support. Experiments were performed with an moi of 100, at 0.4 mM l-arginine in the medium. [NO2−] in culture supernatants was measured after 24 h. Results are the mean ± SEM of three separate experiments performed in duplicate. Statistical comparison of values for SS1 WT vs. SS1 rocF∷aphA3 were performed by the unpaired t test.
No significant difference vs. untreated bacteria by unpaired t test. NS, not significant.
Regulation of Host Cell NO Production by Bacterial Arginase Is an H. pylori Survival Mechanism.
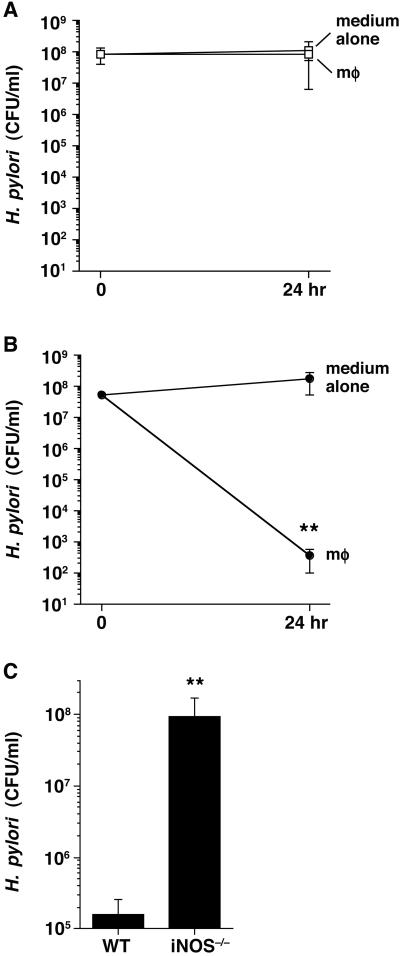
The ability of NO to kill microbes makes it an important part of primordial host defense. Because we found that only live H. pylori can modulate l-arginine substrate availability for iNOS, we reasoned that bacterial arginase contributes to the viability of H. pylori. We tested the effect of H. pylori arginase on NO-mediated killing of H. pylori by macrophages. After coculture with RAW 264.7 macrophages for 24 h, H. pylori SS1 WT did not have any loss of survival (Fig. 3A), whereas the arginase-deficient mutant strain had more than a 5-log-order decrease in colony-forming units (Fig. 3B). To demonstrate that killing of H. pylori was NO-dependent, we exposed the rocF∷aphA3 strain to resident peritoneal macrophages obtained from WT or iNOS−/− mice. Although there was more than a 450-fold decrease in survival with the WT macrophages (Fig. 3C), there was no killing of the arginase-deficient H. pylori after coculture with the iNOS−/− macrophages.
Figure 3.
H. pylori escapes the effect of macrophage-derived NO by its arginase activity. H. pylori WT (A) or rocF∷aphA3 (B) strains were cultured alone or above filter supports in the presence of macrophages (mφ) at an moi of 100 with 0.4 mM l-arginine in the medium. Colony-forming unit and NO2− concentrations were determined before and after 24 h of coculture. Results from four independent experiments performed in quadruplicate are represented by mean values ± SEM. **, P < 0.01 compared with medium alone by Mann–Whitney U test. NO2− levels were 5.8 ± 0.7 μM for SS1WT and 20.3 ± 1.2 μM for SS1 rocF∷aphA3. (C) H. pylori SS1 rocF∷aphA3 were added above filter supports to peritoneal macrophages from WT or iNOS−/− mice at an moi of 100 with 0.4 mM l-arginine in the medium. **, P < 0.01 by Mann–Whitney U test. NO2− levels were 16.3 ± 2.5 μM and 2.1 ± 0.1 μM for WT and iNOS−/− peritoneal macrophages, respectively.
Discussion
iNOS-derived NO is a central effector molecule in the innate immune response to pathogens. A major finding of our current study is that extracellular H. pylori can be killed by NO released from activated macrophages. We show that H. pylori arginase competes with host cell iNOS for the common substrate l-arginine. Thus, WT H. pylori prevents NO production by host cells. Arginase-deficient H. pylori cannot limit macrophage NO production and are efficiently killed by macrophage-derived NO. These observations are independent of any effect on iNOS transcriptional regulation. We suggest that our results represent the description of a unique pathway of immune escape by extracellular-infecting bacteria. The protozoan, Leishmania, possesses arginase activity; however, this is an obligate intracellular parasite and the biological importance of the arginase is unrelated to modulation of NO synthesis (25). Mammalian arginases have the ability to modulate NO production in host–pathogen interactions as well, but our data now indicate that a prokaryotic arginase can have the same effect.
The inhibition of macrophage NO production by WT H. pylori occurred at physiologic concentrations of l-arginine (7) and at mois that mimic conditions documented in vivo (26), supporting the likelihood that the data we have observed have direct relevance in vivo. In fact, the SS1 rocF mutant strain used in the current study has attenuated ability to colonize mice (18). This finding raises the question of the role of iNOS-derived NO in H. pylori infection in vivo. Because we have previously shown that iNOS expression is up-regulated in human H. pylori gastritis (12), we have infected iNOS−/− mice and WT C57BL/6 mice with WT SS1. We found no significant difference in colonization levels or histologic injury scores, despite consistent expression of iNOS in the WT mouse stomach (K.T.W., unpublished observations). Based on the in vitro data presented here, we hypothesize that the lack of difference between iNOS-deficient and WT mice in H. pylori infection could be related to the ability of H. pylori arginase to modulate mucosal NO synthesis by iNOS in WT mice. With this unique insight, experiments have been initiated in our lab to compare the rocF∷aphA3 to WT SS1 strains in iNOS−/− vs. WT mice, which are expected to determine the relative importance of host iNOS induction and H. pylori arginase in vivo. H. pylori are located in close proximity to the gastric epithelium in the mucous layer of the stomach, which results in iNOS expression in the epithelium and lamina propria (12). Thus, it is likely that the H. pylori arginase could directly modulate the iNOS-derived NO levels because of this close interaction between bacteria and the iNOS-expressing cells. There is also nonenzymatically formed NO in the gastric juice caused by acidification of salivary nitrate and nitrite, but this is unlikely to play any significant role in the H. pylori survival as a result of the location of the bacterium in the mucous layer.
Amino acid metabolism is essential for H. pylori growth (27). l-Arginine is not synthesized by H. pylori and therefore the bacterium must obtain this amino acid from extracellular sources (28). In fact, l-arginine is one of the amino acids completely consumed by H. pylori in broth culture (29, 30). By direct analysis of l-arginine utilization, we found that H. pylori consumed ≈70% of the l-arginine available in the cell culture medium over 24 h. The incomplete utilization of the l-arginine is most likely the result of the reduced metabolism of H. pylori in the minimal medium of serum-free DMEM. Nonetheless, our data indicate that l-arginine consumption by WT H. pylori strains explains the inhibition of host NO production. The lack of significant NO production by macrophage iNOS, despite the residual level of 0.1 mM l-arginine in the medium, may be caused by factors such as altered l-arginine uptake by the macrophages or metabolism of l-arginine by other biochemical pathways. The loss of the inhibitory effect on NO production when WT H. pylori are heat-killed is consistent with denaturation of arginase. Lack of effects on NO production by water extracts or French press lysates is consistent with the loss of active l-arginine transport (31) for subsequent metabolism by arginase or with the loss of bacterial arginase after centrifugation of lysates, because arginase is associated with the cell envelope (32). Our direct analysis of l-arginine levels in media exposed to H. pylori indicates that viable bacteria efficiently consume l-arginine available in the extracellular environment, providing a means to decrease macrophage NO production.
Bacteria have developed various mechanisms to escape the effect of the immune system. For example, the intracellular pathogen, Burkholderia cepacia, which causes lung infection in cystic fibrosis patients, seems to enhance its own survival after macrophage apoptosis by inhibiting host NO production, but the mechanism has not been established (33). In response to NO, E. coli defends itself by up-regulating expression of soxR, a gene essential for the resistance to oxidative stress (34). H. pylori have similarly developed strategies for defense against oxidative stress, such as constitutively expressing superoxide dismutase and catalase, which detoxify superoxide anion and hydrogen peroxide, respectively (35), and alkylhydroperoxide reductase (36), which detoxifies peroxynitrite. Thus, H. pylori arginase is not the only potential strategy for enhancement of bacterial survival, but it has several unusual characteristics: Rather than defending against a toxic radical, it prevents NO production, and it does not require de novo gene transcription. Contrary to inducible arginases from other bacteria (37, 38) and yeast (39), H. pylori arginase is constitutively expressed (29). Its activity is present in all strains tested to date (18, 32), suggesting that arginase has evolved in H. pylori as an important enzyme.
Inhibition of host NO production by H. pylori not only protects the bacterium, but may also prevent mucosal injury. NO and peroxynitrite at high levels can kill eukaryotic cells and cause DNA damage (40, 41) and lipid peroxidation (42). Limiting host NO production by consuming l-arginine may be an evolutionary advantage in the successful adaptation of H. pylori to its ecologic niche of the gastric mucosa. A chronic infection for the life of the host would benefit from limitation of host mucosal destruction, which has been likened to a form of parasitism (43) in the case of H. pylori.
Acknowledgments
This work was supported by National Institutes of Health Grants DK53620 (to K.T.W.), DK02469 (to K.T.W.), DK56938 (to K.T.W), AI25567 (to H.L.T.M.), and AI10098-01 (to D.J.M.); and by the Office of Medical Research, Department of Veterans Affairs (K.T.W.).
Abbreviations
- iNOS
inducible NO synthase
- WT
wild-type strains of H. pylori
- moi
multiplicity of infection
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Varanasi R V, Fantry G T, Wilson K T. Helicobacter. 1998;3:188–194. doi: 10.1046/j.1523-5378.1998.08001.x. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. J Am Med Assoc. 1994;272:65–69. [PubMed] [Google Scholar]
- 3.Nathan C, Shiloh M U. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekker L G, Freeman S, Murray P J, Ryffel B, Kaplan G. J Immunol. 2001;166:6728–6734. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]
- 5.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Becherel P, Debre P, Moncada S, Mossalayi M D. Proc Natl Acad Sci USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mnaimneh S, Geffard M, Veyret B, Vincendeau P. J Immunol. 1997;158:308–314. [PubMed] [Google Scholar]
- 7.Gobert A P, Daulouede S, Lepoivre M, Boucher J L, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P. Infect Immun. 2000;68:4653–4657. doi: 10.1128/iai.68.8.4653-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacelli R, Wink D A, Cook J A, Krishna M C, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell J B. J Exp Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dykhuizen R S, Fraser A, McKenzie H, Golden M, Leifert C, Benjamin N. Gut. 1998;42:334–337. doi: 10.1136/gut.42.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, Okamoto S, Maeda H. Infect Immun. 2000;68:4378–4383. doi: 10.1128/iai.68.8.4378-4383.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson K T, Ramanujam K S, Mobley H L T, Musselman R F, James S P, Meltzer S J. Gastroenterology. 1996;111:1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- 12.Fu S, Ramanujam K S, Wong A, Fantry G T, Drachenberg C B, James S P, Meltzer S J, Wilson K T. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 13.Ouzounis C A, Kyrpides N C. J Mol Evol. 1994;39:101–104. doi: 10.1007/BF00178255. [DOI] [PubMed] [Google Scholar]
- 14.Boucher J L, Moali C, Tenu J P. Cell Mol Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buga G M, Singh R, Pervin S, Rogers N E, Schmitz D A, Jenkinson C P, Cederbaum S D, Ignarro L J. Am J Physiol. 1996;271:H1988–H1998. doi: 10.1152/ajpheart.1996.271.5.H1988. [DOI] [PubMed] [Google Scholar]
- 16.Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M. J Biol Chem. 1997;272:3689–3693. doi: 10.1074/jbc.272.6.3689. [DOI] [PubMed] [Google Scholar]
- 17.Chang C I, Liao J C, Kuo L. Cancer Res. 2001;61:1100–1106. [PubMed] [Google Scholar]
- 18.McGee D J, Radcliff F J, Mendz G L, Ferrero R L, Mobley H L. J Bacteriol. 1999;181:7314–7322. doi: 10.1128/jb.181.23.7314-7322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 20.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, et al. Nature (London) 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 21.Mai U E, Perez-Perez G I, Wahl L M, Wahl S M, Blaser M J, Smith P D. J Clin Invest. 1991;87:894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert A P, Semballa S, Daulouede S, Lesthelle S, Taxile M, Veyret B, Vincendeau P. Infect Immun. 1998;66:4068–4072. doi: 10.1128/iai.66.9.4068-4072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamler J S, Singel D J, Loscalzo J. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 24.Binion D G, Fu S, Ramanujam K S, Chai Y C, Dweik R A, Drazba J A, Wade J G, Ziats N P, Erzurum S C, Wilson K T. Am J Physiol. 1998;275:G592–G603. doi: 10.1152/ajpgi.1998.275.3.G592. [DOI] [PubMed] [Google Scholar]
- 25.Iniesta V, Gomez-Nieto L C, Corraliza I. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khulusi S, Mendall M A, Patel P, Levy J, Badve S, Northfield T C. Gut. 1995;37:319–324. doi: 10.1136/gut.37.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais A, Mendz G L, Hazell S L, Megraud F. Microbiol Mol Biol Rev. 1999;63:642–674. doi: 10.1128/mmbr.63.3.642-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds D J, Penn C W. Microbiology. 1994;140:2649–2656. doi: 10.1099/00221287-140-10-2649. [DOI] [PubMed] [Google Scholar]
- 29.Mendz G L, Hazell S L. Microbiology. 1996;142:2959–2967. doi: 10.1099/13500872-142-10-2959. [DOI] [PubMed] [Google Scholar]
- 30.Stark R M, Suleiman M S, Hassan I J, Greenman J, Millar M R. J Med Microbiol. 1997;46:793–800. doi: 10.1099/00222615-46-9-793. [DOI] [PubMed] [Google Scholar]
- 31.Mendz G L, Hazell S L. Int J Biochem Cell Biol. 1995;27:1085–1093. doi: 10.1016/1357-2725(95)00069-2. [DOI] [PubMed] [Google Scholar]
- 32.Mendz G L, Holmes E M, Ferrero R L. Biochim Biophys Acta. 1998;1388:465–477. doi: 10.1016/s0167-4838(98)00207-6. [DOI] [PubMed] [Google Scholar]
- 33.Saini L S, Galsworthy S B, John M A, Valvano M A. Microbiology. 1999;145:3465–3475. doi: 10.1099/00221287-145-12-3465. [DOI] [PubMed] [Google Scholar]
- 34.Nunoshiba T, deRojas-Walker T, Wishnok J S, Tannenbaum S R, Demple B. Proc Natl Acad Sci USA. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramarao N, Gray-Owen S D, Meyer T F. Mol Microbiol. 2000;38:103–113. doi: 10.1046/j.1365-2958.2000.02114.x. [DOI] [PubMed] [Google Scholar]
- 36.Bryk R, Griffin P, Nathan C. Nature (London) 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 37.Gardan R, Rapoport G, Debarbouille M. Mol Microbiol. 1997;24:825–837. doi: 10.1046/j.1365-2958.1997.3881754.x. [DOI] [PubMed] [Google Scholar]
- 38.Kanda M, Ohgishi K, Hanawa T, Saito Y. Arch Biochem Biophys. 1997;344:37–42. doi: 10.1006/abbi.1997.0174. [DOI] [PubMed] [Google Scholar]
- 39.Smart W C, Coffman J A, Cooper T G. Mol Cell Biol. 1996;16:5876–5887. doi: 10.1128/mcb.16.10.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wink D A, Kasprzak K S, Maragos C M, Elespuru R K, Misra M, Dunams T M, Cebula T A, Koch W H, Andrews A W, Allen J S, Keefer L K. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen T, Brunson D, Crespi C L, Penman B W, Wishnok J S, Tannenbaum S R. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 43.Blaser M J, Parsonnet J. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]