Abstract
Urinary tract infections due to the presence of a urinary catheter represent a real problem for patients who have to carry such an invasive device for a long time.
Our aim was to identify the susceptibility of extended spectrum beta lactamases (ESBL) versus non-ESBL bacteria to antibiotics in urinary tract infections in patients who are chronic carriers of urinary catheters.
The retrospective study included a period of 5 years, a total of 405 patients who are chronic carriers of urinary catheters, admitted to rehabilitation and palliative care units.
Escherichia coli (E coli) was isolated in 41.2% of patients, Klebsiella pneumoniae (K pneumoniae) in 24.7%, and Proteus mirabilis (P mirabilis) in 15.3%. E coli microbial resistance rates ranged from a minimum of 7.5% (nitrofurantoin) to a maximum of 77.1% (ampicillin). In the case of K pneumoniae, microbial resistance ranged from 34.2% (netilmicin) to 73.2% (ceftriaxone). Resistance rates of P mirabilis ranged from 11.1% (cefepim) to 89.5% (ampicillin). Positivity of ESBL bacteria was identified in 47.4% of patients. Resistance rates of ESBL-positive E coli ranged from 50.0% (ceftriaxone) to 88.1% (cefepime), and ESBL-negative E coli rates ranged from 3.4% (cefepime) to 64.4% (amikacin). Resistance rates of ESBL-positive K pneumoniae ranged between 39.1% (netilmicin) and 85.1% (ceftriaxone), and ESBL-negative K pneumoniae between 7.1% (cefepime) and 53.3% (amikacin). In cases of ESBL-positive P mirabilis, rates ranged from 13.3% (cefepime) to 90.3% (ceftriaxone), whereas in cases of ESBL-negative P mirabilis, rates ranged between 8.3% (cefepime) and 80.0% (trimetroprim).
Bacteriuria and asymptomatic catheter infection in chronic carriers is an important public health concern due to the frequent presence of multidrug-resistant bacteria. Our study highlights the need to develop control programs of catheter infections to minimize the risk of infections associated with these medical devices, and also the need for treatment of the infection rather than catheter colonization or contamination.
Keywords: antibiotic resistance, chronic carriers of urinary catheter, urinary catheter infections
1. Introduction
Catheter-associated urinary tract infections (CAUTIs) are a real problem for patients who have to carry such an invasive device over an extended period of time. Nearly 80% of the infections due to medical care are urinary catheter infections,[1,2] 1 of the most common nosocomially acquired infections.[3] This patient population includes patients in emergency services, surgical units, and also chronic patients, who, due to related conditions, are required to wear a urinary catheter for a long time. The duration of catheterization is the most important risk factor for the development of infection, and the risk increases by an estimated 5% to 10% per catheterization day. After 4 weeks of continuous presence of the urinary catheter, all patients develop bacteriuria subsequent to catheter infection.[4–6] Approximately 5% of patients attending medical services for chronic patients are carriers of urinary catheter.[7] In these services, these infections are the most common source of bacteria (bacteriuria), and asymptomatic urinary tract infections (UTIs) are the most commonly reported in immobilized patients with spinal cord injuries.[2,8]
Urinary catheters are used in patients who have problems associated with obstructions that prevent the discharge of urine,[9] but often times an inappropriate continuous use may occur, which increases the risk of infectious and noninfectious complications in these patients.[10,11]
As defined by the Infectious Diseases Society of America, urinary catheter infection is: “the presence of symptoms or signs compatible with UTI with no other identified source of infection along with 103 colony-forming units/ mL,” with symptoms and signs including “new onset or worsening of fever, rigors, altered mental status, malaise, or lethargy with no other identified cause; flank pain; costovertebral angle tenderness; acute hematuria; pelvic discomfort.”[2]
Permanent presence of an indwelling urinary catheter is considered to be short-term if the catheter is present for less than 30 days, and long-term if present for more than 30 days.[2]
The question arises whether the presence of such a medical device is necessary, and why it is a risk for infections. Most microorganisms found in the urine collected from urinary catheter come either from commensal skin flora, external genitalia, perianal flora of the patient, or from the healthcare staff who handled the catheter during insertion.[12] In patients with short-term use of a urinary catheter, the infection is usually determined by a single microorganism, the most common being Escherichia coli (E coli), or other enterobacteriaceae.[13,14] Other species such as Enteroccocus sp., Pseudomonas aeruginosa (P aeruginosa), or Candida sp. are less commonly encountered.[15] A characteristic of the isolated bacteria in chronic patients with indwelling urinary catheters is the resistance to antibiotic treatment, sometimes even to the new-generation antibiotics (third and fourth-generation cephalosporins, carbapenems). This resistance may be caused by frequent and repeated treatments with antibiotics, either for the UTI or for other infections. Recent studies and everyday medical practice demonstrate a paradigm—bacteria, and also infections, are more frequent and increasingly difficult to treat. Our study focused on extended spectrum beta lactamases (ESBL) infections because they are associated with increased morbidity and mortality, prolonged hospitalization, and, thus, increased hospital costs.
According to Paterson and Bonomo,[16] ESBL bacteria produce a group of enzymes capable of hydrolyzing, and thus, inactivating the antibiotics in the cephalosporin and penicillin class—the most common genetic variant of ESBL being CTX-M.
Generally, ESBLs are inhibited by clavulanic acid and tazobactam. ESBLs are found in Gram-negative bacteria, especially in enterobacteriacea and P aeruginosa.[17,18]
Extended spectrum beta lactamases bacteria compared with non-ESBL ones are often the cause of prolonged hospitalization, and the anti-infective therapy leads to the use of several classes of antibiotics, which, in turn, prove to be ineffective. Our aim was to identify the resistance and susceptibility to antibiotics of ESBL versus non-ESBL bacteria in UTIs in chronic patients with indwelling urinary catheters.
2. Material and methods
We conducted a retrospective study on a group of immobile or hardly mobile patients, admitted to rehabilitation and palliative care units. The patients had a history of chronic diseases (sequelae of stroke, cancers in various stages, job accidents, dementia) chronic carriers of bladder catheter who were hospitalized in our service for neurophysiological recovery or palliative care.
All patients included in the study were, by definition, chronic urinary tract carriers and who were not hospitalized in the past 30 days before admission to our department. From this study, patients who had treatments that might have interfered with the urine summary (patients with cytostatic treatments, diuretics, antibiotics, corticoids) were excluded.
The study was conducted by retrospective data collection from medical records for a period of 5 years (2011–2015), and was approved by the ethical committee of our hospital. A structured form was used to collect data, including demographic characteristics, urine analysis, laboratory results, pathogen susceptibility results, risk factors, diagnoses, and underlying diseases. Blood samples were collected from all the patients for blood counts, and biochemical analyses, urinalysis, and urine sediment analysis were also performed. The recommendation to perform urine culture was based on the results summary and urine sediment analysis. For normal values, erythrocytes should not exceed 2 units/field (2 red blood cells [RBCs]/hpf), leukocytes 2 to 5 items/field (2–5 white blood cells [WBCs]/hpf), nitrites should be negative, and bacteria absent.[19] Because most bacteriuria are accompanied by pyuria, we considered the increased number of leukocytes in the urine sediment, and/ or positive nitrite, and/or the presence of urinary cylinders, respectively. In patients with pelvic stasis, the presence of bacteria induces bacteremia without pyuria. Pyuria is considered at cut off level by the presence of 10 or more white cells per cubic millimeter in a urine specimen, 3 or more white cells per high-power field of unspun urine.
For a properly collected urine sample, a significant bacteriuria means the presence of bacteria over 100,000 CFUs/mL that is suggestive of a UTI correlated with clinical symptoms and leucocyturia. Presence between 10,000 and 100,000 CFUs/mL may indicate a UTI, especially if the urine sample was collected by an invasive procedure (newly inserted catheter) or in a patient undergoing antibiotic treatment, hyperhydrated or with acidosis.[19] In case of a significant bacteriuria, we aimed at microorganism identification and antibiotic susceptibility testing.
2.1. Collection of urine
To avoid any mistakes in the collection of urine, aseptic rules were strictly followed by the medical staff involved, according to the instructions booklet that our hospital uses for collecting urine via newly inserted catheters. To collect urine from patients with indwelling catheter, installed even at the hospital admission, antisepsis of external genitalia was performed by washing with soap and water, rinsing with saline solution, and drying with sterile bandages on inserting the new catheter. After the insertion of the catheter, urine was allowed to run for about 2 to 3 hours, the catheter was clipped as close as possible to the opening of the urethra for about 30 to 60 minutes to allow the bladder to fill, the catheter was disinfected with iodine solution above the clipping, urine was aspirated into a sterile syringe of about 2 to 3 mL which was subsequently placed in a sterile urine collector. The collection of urine for analysis was done manually.
2.2. Antimicrobial susceptibility
Antibiotic susceptibility was performed by disc diffusion method on Mueller-Hinton agar medium (bioMérieux, Marnes-la-Coquette, France) for Gram-negative bacilli, staphylococci, and sometimes for enterococci. The following microtablets were used: ampicillin (10 μg), amoxicillin/clavulanic acid (20 μg/10 μg), amikacin (30 μg), cefuroxime (30 μg), cefepime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), norfloxacin (10 μg), gentamicin (10 μg), netilmicin (30 μg), nalidixic acid (30 μg), nitrofurantoin (300 μg), ertapenem (10 μg), imipenem (10 μg), meropenem (10 μg), penicillin 10 IU, cefoxitin (30 μg), piperacillin/tazobactam (100 μg/10 μg), tetracycline (30 μg), doxycycline (30 μg), trimethoprim/ sulfamethoxazole (11.25 μg/23.75 μg). The interpretation of susceptibility results was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.[20] Quality control was conducted with ATCC reference strains, using ATCC 25923 cards—Staphylococcus aureus control, ATCC 25922; E coli control, ATCC 27153; P aeruginosa control, ATCC 29212; Enterococcus faecalis control.
Extended spectrum beta lactamases testing involved a test with an indicator cephalosporin, looking for resistance or susceptibility, identifying isolates that could contain ESBLs. ESBL is tested on the basis of synergism between amoxiclav + clavulanic acid (beta-lactamase inhibitor), and third-generation cephalosporins and fourth-generation cephalosporin. In addition, through a screening can be determined susceptibility to cefoxitin and resistance to cefodoxin. Organisms that are susceptible to tetracycline are also considered susceptible to doxycycline and minocycline. However, some organisms that are intermediate or resistant to tetracycline may be susceptible to doxicycline, minocycline, or both.
2.3. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 20.0). Continuous data were presented as medians (interquartile ranges [IQRs]) or means (±standard deviation) and categorical data as proportions. The Mann-Whitney U test and Student t test were used for the remaining continuous variables. Statistical differences between groups were assessed with the chi-square test using Yates correction or Fisher exact test, when appropriate, for categorical variables. We interpreted all tests against a P = .05 significance threshold and statistical significance was considered for P values below the significance threshold.
3. Results
3.1. Personal data of the subjects
The mean age of patients was 56.3 ± 17.9 years (minimum 18, maximum 93 years); 51.8% of patients were male. Gram-negative bacteria were found in 362 (89.4%) patients and Gram-positive bacteria in 43 (10.6%) patients.
Extended spectrum beta lactamases bacteria positivity was detected in 192 (47.4%) patients, most of whom were male (55.2%).
Escherichia coli was isolated in 167 (41.2%) patients, K pneumoniae in 100 (24.7%), and P mirabilis in 62 (15.3%) patients. Enterobacter cloacae, P aeruginosa, and E faecalis were isolated each in about 3% and 4% of patients. Among other bacteria were: Seratia marcescens, Providencia stuarti, Morganella morgani, Klebsiella oxytoca, and Enterococcus sp. About 23% (94 patients) had a second bacterium (Table 1).
Table 1.
Characteristics of the patients.
Nitrites were positive in 69.1% of patients. Mean values (minimum-to-maximum) for elements in the urinary sediment were as follows: erythrocytes: 368.3 (0–27, 901.3); leucocytes: 1573.1 (0–25, 445.2); epithelial cells: 13.2 (0–493.5); cylinders: 18.5 (0–2951.6); bacteria: 23,062.6 (0–154, 537.4).
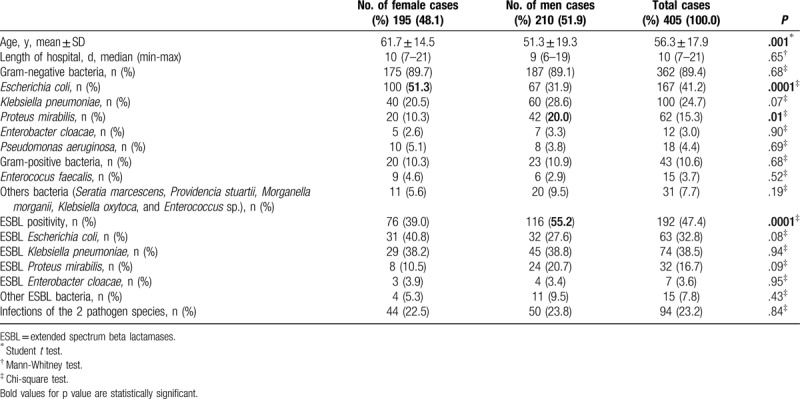
Table 2 shows rates of resistance/susceptibility to antibiotics of the most common pathogen identified in frequent UTIs in our patients. Microbial resistance rates for E coli ranged from 1.4% to ertapenem up to 77.2% to ampicillin. In the case of K pneumonia, microbial resistance ranged from 34.2% (netilmicin) up to 73.2% (ceftriaxone). Resistance rates of P mirabilis ranged from 11.1% (cefepime) up to 89.5% (ampicillin). Insufficient data for P aeruginosa prevented us from expressing our opinion about antibiotic resistance.
Table 2.
Results of antimicrobial agents producing uropathogenic. Escherichia coli (E coli), Klebsiella pneumonia (K pneumonia), Proteus mirabilis (P mirabilis), or Pseudomonas Aeruginosa (P aeruginosa).
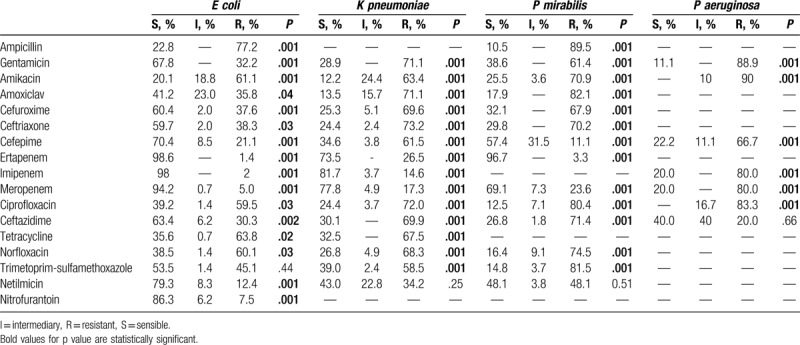
Resistance rates were determined for the 192 cases of infection with ESBL-positive bacteria. These rates ranged from 50.6% (cefepime) up to 87.9% (ceftriaxone). A total of 213 (52.6%) cases of infection with ESBL-negative bacteria were identified, for which resistance rates were also established. These rates ranged from 8.5% (cefepime) to 64.4% (amikacin) (Fig. 1).
Figure 1.
Antibiotic susceptibility testing of ESBL versus non-ESBL bacteria. ESBL = extended spectrum beta lactamases.
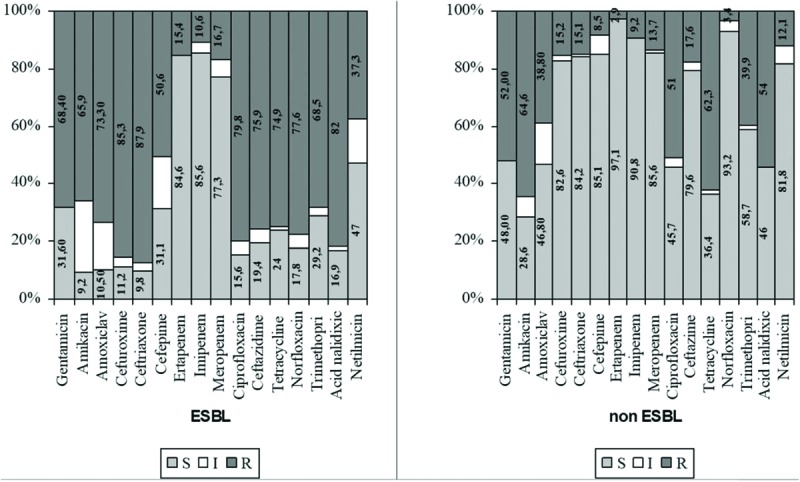
Depending on the ESBL-positive or negative bacteria, we noted the resistance rates and susceptibility to antibiotics of the most common bacteria found in the urine of patients included in our study. Resistance rates of ESBL-positive E coli ranged from 50.0% to 88.1% (ceftriaxone), whereas those of ESBL-negative E coli ranged between 3.4% (cefepime) and 65.6% (ampicillin) (Fig. 2).
Figure 2.
Antibiotic susceptibility testing of ESBL E coli versus non-ESBL E coli. E coli = Escherichia coli, ESBL = extended spectrum beta lactamases.
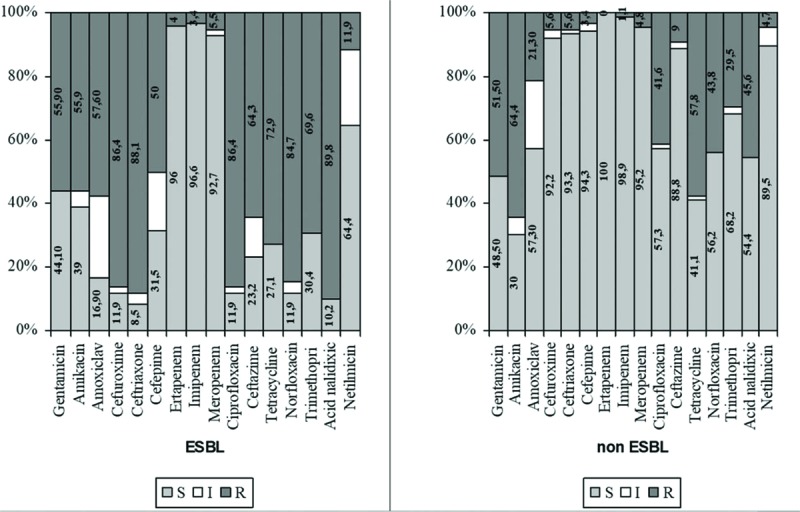
In ESBL-positive K pneumoniae, resistance rates ranged between 39.1% (netilmicin) and 85.1% (ceftriaxone), whereas those for ESBL-negative K pneumoniae ranged from 7.1% (cefepime) to 53.3% (amikacin and ciprofloxacin) (Fig. 3).
Figure 3.
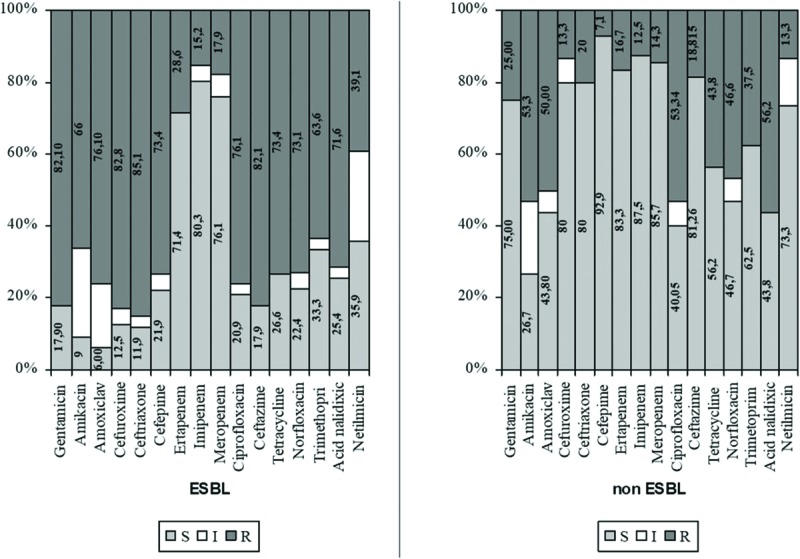
Antibiotic susceptibility testing of ESBL K pneumoniae versus non-ESBL K pneumoniae., K ESBL = extended spectrum beta lactamasespneumoniae = Klebsiella pneumoniae.
Rates for ESBL-positive P mirabilis varied from 13.3% (cefepime) to 90.3% (ceftriaxone), whereas for ESBL-negative P mirabilis, rates ranged between 8.3% (cefepime) and 88.0% (tetracycline) (Fig. 4).
Figure 4.
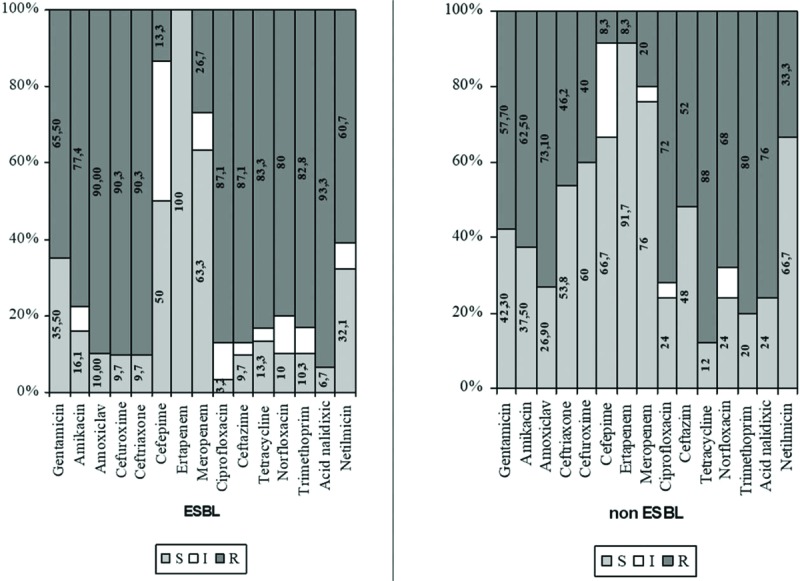
Antibiotic susceptibility testing of ESBL P mirabilis versus non-ESBL P mirabilis. ESBL = extended spectrum beta lactamases, P mirabilis = Proteus mirabilis.
4. Discussion
The presence of a urinary catheter for a longer period of time presents a risk for UTIs.
In terms of pathogenesis, the development of a biofilm along the urinary catheter is the most important cause of bacteriuria.[21] The biofilm is an organic material composed of bacteria growing in colonies supported by the mucopolysaccharides they produce, it contains kidney-specific proteins (Tamm-Horsfall protein), and the change of local pH by alkalifying calcium and magnesium phosphate precipitates in the biofilm.[22] This biofilm is an inevitable consequence of the presence of the urinary catheter, because its insertion immediately produces a body-specific protein adherence on the internal surface of the catheter forming a layer that favors[2] the adhesion of microorganisms which normally colonize the urethra or which are derived from a foreign flora due to urinary catheter manipulation. Once attached to this favoring layer, the bacterium produces a polysaccharide that forms the propitious environment for the development and persistence of bacterial colonies. An important factor in the variation of microorganisms in patients who are carriers of urinary catheters is that this biofilm is dynamic. The longer the urinary catheter is present, the biofilm embeds new bodies that are present in the bladder, substituting other already present microorganisms. Some of the urease-producing bacteria such as P mirabilis, K pneumonia, or Providencia stuartii (30% of the strains) produce and favor the formation of a large amount of biofilm which quickly goes into the bladder. Urea hydrolysis produced by these bacteria alkalifies urine which favors the precipitation of calcium and magnesium ions producing a material similar to kidney stones, which may lead to catheter obstruction.[23] In the case of chronic patients with indwelling urinary catheters, several different species can be isolated from urine which can include a wide variety of enterobacteriaceae and other microorganisms. The most common microorganisms producing urease are K pneumoniae, Klebsiella oxytoca, P mirabilis, P aeruginosa (11%–89% of strains), Morganella morganii, or Providencia stuartii (30% of strains).[15,24] Gram-positive bacteria such as coagulase-negative staphylococci and enterococci can be isolated from the urine of such patients, but these bacteria are commonly found in patients without symptoms of UTI.[15]
We have identified studies that have been monitored acute UTI with the patients who have not been exposed to catheter. Those studies have confirmed that a history of UTI was 1 of the main independent risk factors for antimicrobial resistance and found that only age over 85 years increased this risk. Factors associated with increased antimicrobial resistances were age over 50 years, the presence of complicated UTI, the use of antibiotics in the past 3 months before the onset of UTI, and the use of quinolones in the past 6 months. Fluoroquinolones, co-trimoxazole, and beta-lactams are frequently involved in the increase in antibiotic resistances.[25–27]
Our study conducted on patients who are chronic carriers of urine catheters admitted to neurological recovery and palliative care units between 2011 and 2015 contributes to a better understanding of circulating microbial flora and resistance to antibiotics in a patient population with a risk of UTI in our region.
We included 405 patients who had positive biological samples in the presence of bacteria in the urine. Of the patients included in this study, 25% showed leukocytosis which includes them in the category of patients with asymptomatic urinary catheter infection.[28] A percentage of 76.8% had leucocyturia, whereas 73.1% had urinary cylinders. We suspected UTI if leukocyturia was present.[19] The presence of urinary cylinder indicates the existence of a UTI in the renal parenchyma of these patients.[29] These simple tests can guide the clinician towards the decision either to administer antibiotic treatment immediately or to wait for antibiotic test result if the urinary catheter infection is a medical emergency.
Increasing resistance among pathogens in UTIs is indicated, especially by the growing number of antibiotic-resistant strains. Multiple drug resistance (MDR) was defined as acquired nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories.[30]
Of all the identified bacteria, E coli was the most commonly encountered in 51.3% of the female group. E coli was isolated in 167 (41.2%) patients; the same percentage was recorded in patients admitted to urology units who are carriers of urinary catheters.[31] Of the total number of ESBL bacteria, E coli was found in 32.8% of cases.
A high resistance of E coli strains to aminopenicillins (tested on ampicillin) was noted, reaching a rate of 77.2%. This percentage lists us among countries with the highest resistance to these antibiotics along with Bulgaria, Lithuania, and Luxemburg.[32] Another growing trend revealed by our study, which should draw the attention of the attending physician to prescribing antibiotics, is resistance to cephalosporins. Strains of E coli showed good susceptibility to cephalosporins which ranged from 59.7% (ceftriaxone) to 70.4% (cefepime). ESBL E coli strains still show a low resistance to netilmicin (11.9%), but a high resistance to tetracycline (72.9%). In the tested aminoglycosides, resistance was identical to gentamicin (55.9%) and to amikacin (55.9%), the new-generation antibiotic.
Our study identified 100 cases of K pneumoniae. We noted a high resistance to cephalosporins (between 73.2% resistance to first and second-generation cephalosporins and 61.5% to fourth-generation cephalosporins). K pneumoniae also had a high resistance to aminoglycosides (71.1% gentamicin and 63.4% amikacin). Of the ESBL-positive strains, a percentage of 38.5% was noted for K pneumoniae. The highest susceptibility was noted in ESBL K pneumoniae strains to netilmycine; only a percentage of 39.1% was resistant to them. Resistance to fluoroquinolones was high in ESBL K pneumoniae strains: 71.6% (nolicin) and 76.1% (ciprofloxacin). ESBL K pneumoniae showed less resistance to trimethoprim sulfamethoxazole (63.6%) compared with other antibiotics commonly used in the treatment of UTIs, whereas tetracyclines had a rate of 73.4% resistance.
Compared with other strains, P mirabilis was more rarely identified: it was positive in 10.3% of women, ESBL strains representing 16.7%. ESBL-positive strains had a high resistance to aminoglycosides (60.7% netilmicin, 64.5% gentamicin, and 77.4% amikacin). The 90.3% resistance rate to first and second-generation cephalosporins, and 13.3% to fourth-generation cephalosporins was similar to that of K pneumoniae and E coli. A high resistance was recorded to fluoroquinolone: 93.3% to nolicin and 87.1% to ciprofloxacin. Unlike E coli and K pneumoniae, P mirabilis showed positive ESBL resistance to ciprofloxacin (80.4%), amoxiclav (82.1%), and to trimethoprim-sulfamethoxazole (82.8%).
A more particular case, in terms of resistance to antibiotics, was revealed by P aeruginosa strains. Our study highlights the great resistance of P aeruginosa to aminoglycosides, which varied: 88.9% (gentamicin), 90.0% (amikacin), and 100% (netilmicin). These percentages are higher than the 63.4% reported for Romania.[32] Regarding cephalosporins, we identified 66.7% resistance to fourth-generation cephalosporins, and 20% to ceftazidime. This percentage is lower than the 59.1% reported for Romania.[32] We found a high resistance to fluoroquinolones. Resistance rate percentages ranged from 20.0% (ceftazidime) to 100% in the case of netilmicin, a higher percentage compared with the 55.4% reported for Romania.[32] A high resistance was found in the case of carbapenem, 80.0% compared with 58.5% reported for Romania.[32]
The epidemiology of infections with ESBL bacteria is quite complicated because it varies by geographic location, region, country, hospital, community, or host.
For example, according to some studies, resistance rates of E coli in UTIs had different values: 16.7% in Portugal, 34.0% in California, up to 76.5% in India.[33–35]
The Health Ministry of Romania started to send reports to the European Center for Disease Prevention and Control in 2003, but during 2011 to 2014, the reports included more complete data collected from several national laboratories. These reports are quantitatively small compared with Western European countries which acknowledged the impact of antibiotic resistance earlier.[32]
Higher resistance rates of E coli bacteria to aminopenicillins or fluoroquinolones (antibiotic commonly used in Romania for UTIs) were recorded in Romania, and there is an increasing trend of these rates. This becomes more evident when our study relates to other European countries regarding resistance to fluoroquinolones.[32]
There are significant differences between European countries in the case of K pneumoniae resistance rates by testing against fluoroquinolones, aminoglycosides, and third-generation cephalosporins.[32] According to the same report, 3 countries (Greece, Italy, and Romania) reported high resistance rates to carbapenems, considerably higher than any other country (62.3%, 32.9%, and 31.5%).
High resistance rates to P aeruginosa were reported especially in eastern and southeastern Europe in 2014. Resistance to carbapenems increased significantly from 16.8% in 2011 to 18.3% in 2014, and if we refer to the national percentages in 2014, they ranged from 4.4% (The Netherlands) and 58.5% (Romania). In countries where resistance rates of P aeruginosa to carbapenems were high, resistance rates of E coli, K pneumonia, and Acinetobacter sp. were also high.
The prevalence and high resistance of ESBL-producing enterobacteria was demonstrated by other studies.[36–45]
Statistics from our study should be an alert regarding the resistance to the antimicrobial action of antibiotics of the microbial flora in patients who are chronic carriers of urinary catheters. Our study emphasizes the need to administer antibiotic treatment (if necessary) strictly after the results of the antibiogram. For the clinician, ESBL strains, in particular, present a real problem because of the reduced options for antibiotic treatment. This study shows the increasing trend of antibiotic resistance by flora responsible for UTIs in Romania. Moreover, our study is intended to help the clinician in making a decision over the prescription of empiric antibiotic treatments. However, this empirical antibiotic therapy should not be administered in case of urinary catheter infections unless it is a medical emergency.[46]
For our study, we can mention some limitations like in other studies. First of all, our data were obtained from a single hospital. We used a minimal exclusion criterion (patients who had treatments that might have interfered with the urine summary) which resulted in a diverse and probably representative population for patients in other hospitals. Second, we considered readmission only for our hospital, and maybe not have detected readmitted patients who were hospitalized in other hospitals. Third, our study is retrospective and with the risk to introduce certain levels of bias.
5. Conclusions
Asymptomatic bacteriuria and catheter infections in chronic carriers of catheters are an important public health issue due to the frequent presence of multidrug-resistant bacteria. E coli was detected most frequently, followed by K pneumoniae and P mirabilis, almost half of these being ESBL bacteria. Our study highlights the need to develop control programs of catheter infections to minimize the risk of infection associated with these medical devices and to differentiate and treat the infection and not the catheter colonization or contamination.
Author contributions
SV projected the study design, prepared the material and methods used, evaluated the data statistically and interpreted the results. AS coordinated and monitored the study, and the collection of data, critically revised the article. MG reviewed the material and the final article, and designed the Discussion chapter. The microbiologists contributed by collecting data in the field, introducing data in the database and prepared the Material and methods section. All the authors read and approved the final manuscript.
Conceptualization: Septimiu Voidazan.
Formal analysis: Septimiu Voidazan.
Investigation: Doina Bilca, Monica Badiu, Andreea Truţă, Marian Ciorea, Geanina Moldovan.
Methodology: Sorin Albu, Septimiu Voidazan, Monica Badiu, Andreea Truţă, Marian Ciorea.
Resources: Alin Ichim, Diana Luca.
Supervision: Sorin Albu, Geanina Moldovan.
Validation: Doina Bilca, Geanina Moldovan.
Writing – original draft: Septimiu Voidazan.
Writing – review & editing: Sorin Albu.
Footnotes
Abbreviations: CAUTIs = catheter-associated urinary tract infections, CFU = colony-forming unit, CLSI = Clinical and Laboratory Standards Institute, ESBL = extended spectrum beta lactamases, MDR = multiple drug resistance, RBC = red blood cells, UTI = urinary tract infection, WBC = white blood cells.
The author(s) of this work have nothing to disclose.
References
- [1].Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:S41–50. [DOI] [PubMed] [Google Scholar]
- [2].Hooton TM, Bradley SE, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625–63. [DOI] [PubMed] [Google Scholar]
- [3].Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29suppl 1:S41–50. [DOI] [PubMed] [Google Scholar]
- [5].Mulhall AB, Chapman RG, Crow RA. Bacteriuria during indwelling urethral catheterization. J Hosp Infect 1988;11:253–62. [DOI] [PubMed] [Google Scholar]
- [6].Nicolle LE. The chronic indwelling catheter and urinary tract infection in long-term-care facility residents. Infect Control Hosp Epidemiol 2001;22:316–21. [DOI] [PubMed] [Google Scholar]
- [7].Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: infection prevention and control in the long term care facility. Infect Control Hosp Epidemiol 2008;29:785–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am 2012;26:13–27. [DOI] [PubMed] [Google Scholar]
- [9].Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010;31:319–26. [DOI] [PubMed] [Google Scholar]
- [10].Fakih MG, Shemes SP, Pena ME, et al. Urinary catheters in the emergency department: very elderly women are at high risk for unnecessary utilization. Am J Infect Control 2010;38:683–8. [DOI] [PubMed] [Google Scholar]
- [11].Jain P, Parada JP, David A, et al. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med 1995;155:1425–9. [PubMed] [Google Scholar]
- [12].Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001;7:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bakke A, Vollset SE. Risk factors for bacteriuria and clinical urinary tract infection in patients treated with clean intermittent catheterization. J Urol 1993;149:527–31. [DOI] [PubMed] [Google Scholar]
- [14].Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med 2000;160:678–82. [DOI] [PubMed] [Google Scholar]
- [15].Nicolle LE. Catheter-related urinary tract infection. Drugs Aging 2005;22:627–39. [DOI] [PubMed] [Google Scholar]
- [16].Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005;18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bradford PA. Extended spectrum beta-lactamases in the 21st century: characterization, epidemiology and the detection of this important resistance threat. Clin Microbiol Rev 2001;14:933–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nordmann P, Guibert M. Extended spectrum beta-lactamases in Pseudomonas aeruginosa. J Antimicrob Chemother 1998;42:128–31. [DOI] [PubMed] [Google Scholar]
- [19].Available at: http://emedicine.medscape.com/article/2074001-overview Accessed June 7, 2016. [Google Scholar]
- [20].Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; Twenty-fourth informational supplement. CLSI document M100-S24. CLSI, Wayne, PA; 2014. [Google Scholar]
- [21].Stickler DJ. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol 2008;5:598–608. [DOI] [PubMed] [Google Scholar]
- [22].Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med 2014;276:120–9. [DOI] [PubMed] [Google Scholar]
- [23].Stickler DJ, Feneley RC. The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 2010;48:784–90. [DOI] [PubMed] [Google Scholar]
- [24].Warren JW, Tenney JH, Hoopes HM, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982;146:719–23. [DOI] [PubMed] [Google Scholar]
- [25].Wright SW, Wrenn KD, Haynes M, et al. Prevalence and risk factors formultidrug resistant uropathogens in ED patients. Am J Emerg Med 2000;18:143–6. [DOI] [PubMed] [Google Scholar]
- [26].Shilo S, Assous MV, Lachish T, et al. Risk factors for bacteriuria with carbapenem resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection 2013;41:503–9. [DOI] [PubMed] [Google Scholar]
- [27].Hayakawa K, Gattu S, Marchaim D, et al. Epidemiology and risk factors for isolation of Escherichia coli producing CTX-M-type extended-spectrum (-lactamase in a large U.S. Medical Center. Antimicrob Agents Chemother 2013;57:4010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Available at: https://www.cdph.ca.gov/programs/hai/Documents/6CAUTI_RevisedFinal5.11.15.pdf. [Google Scholar]
- [29].Available at: http://www.med.illinois.edu/depts_programs/sciences/clinical/internal_med/residency/edmod/mod1/casts.htm. [Google Scholar]
- [30].Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [31].Michael F. Ksycki Nicholas Namias. Nosocomial urinary tract infection. Surg Clin N Am 2009;89:475–81. [DOI] [PubMed] [Google Scholar]
- [32].European Centre for Disease Prevention and Control: “Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net)”, Stockholm. Available at: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2014.pdf. [Google Scholar]
- [33].Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO·SENS study revisited. Int J Antimicrob Agents 2012;39:45–51. [DOI] [PubMed] [Google Scholar]
- [34].Smith SP, Manges AR, Riley LW. Temporal changes in the prevalence of community-acquired antimicrobial-resistant urinary tract infection affected by Escherichia coli clonal group composition. Clin Infect Dis 2008;46:689–95. [DOI] [PubMed] [Google Scholar]
- [35].Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res 2014;139:945–8. [PMC free article] [PubMed] [Google Scholar]
- [36].Stefaniuk E, Suchocka U, Bosacka K, et al. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur J Clin Microbiol Infect Dis 2016;35:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu YH, Chen PL, Hung YP, et al. Risk factors and clinical impact of levofloxacin or cefazolin nonsusceptibility or ESBL production among uropathogens in adults with communityonset urinary tract infections. J Microbiol Immunol Infect 2014;47:197–203. [DOI] [PubMed] [Google Scholar]
- [38].Maraki S, Mantadakis E, Michailidis L, et al. Changing antibiotic susceptibilities of community-acquired uropathogens in Greece, 2005–2010. J Microbiol Immunol Infect 2013;46:202–9. [DOI] [PubMed] [Google Scholar]
- [39].Roxo I, Magalhães S, Ramalheira E, et al. Epidemiology of ESBL-producing isolates causing UTI in the elderly (Aveiro, Portugal). In: Proceedings of the 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark, 25–28th April 2015, poster PO974. [Google Scholar]
- [40].Guevara N, Guzmán M, Merentes A, et al. Antimicrobial susceptibility patterns of Gram-negative bacteria isolated in urinary tract infections in Venezuela: results of the SMART study 2009–2012. Rev Chilena Infectol 2015;32:639–48. [DOI] [PubMed] [Google Scholar]
- [41].Gopal Rao G, Batura D, Batura N, et al. Key demographic characteristics of patients with bacteriuria due to extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in a multiethnic community, in North West London. Infect Dis (Lond) 2015;47:719–24. [DOI] [PubMed] [Google Scholar]
- [42].Shaikh S, Fatima J, Shakil S, et al. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci 2015;22:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wagenlehner FME, Cek Mete, Naber Kurt G, et al. Epidemiology, treatment and prevention of healthcare-associated urinary tract infections. World J Urol 2012;30:59–67. [DOI] [PubMed] [Google Scholar]
- [44].Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013;34:1–4. [DOI] [PubMed] [Google Scholar]
- [45].Qiao LD, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ 2013;3:e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Milan PB, Ivan IM. Catheter-associated and nosocomial urinary tract infections: antibiotic resistance and influence on commonly used antimicrobial therapy. Int Urol Nephrol 2009;1:461–4. [DOI] [PubMed] [Google Scholar]


