Abstract
Rationale:
Ovarian yolk sac tumors (YSTs) are the second most common histologic type of ovarian germ cell tumors. Most patients are adolescent and young women, while cases in postmenopausal women were rarely reported. Due to its rarity, we know little about the treatment and prognosis of postmenopausal patients with ovarian YSTs. We reported 3 cases of mixed ovarian YST in postmenopausal females reviewed the related current English literature.
Patient concerns:
The ages of the three patients were 61, 58 and 77 respectively. The three patients came to the hospital because of the abdominal discomfort or tenderness, and the third patient also has vaginal bleeding.
Diagnoses:
Imaging examination revealed pelvic mass with cystic and solid components. The elevated serum AFP level and pathologcial examination confirmed mixed ovarian YST.
Interventions:
All patients received surgery and chemotherapy. Two patients received PEB (cisplatin, etoposide, and bleomycin) chemotherapy initially and one patient received TC (paclitaxel carboplatin) chemotherapy.
Outcomes:
One patient relapsed 8 months after diagnosis and underwent re-cytoreductive surgery. The three patients all survived at last follow-up.
Lessons:
The diagnosis of postmenopausal ovarian YST is relatively difficult and it can coexist with other germ cell or epithelial tumors. Postmenopausal ovarian YSTs are aggressive, and may have a worse prognosis compared with those in young patients. More aggressive treatment is needed. When YST mixed with epithelial cancer components, adjuvant chemotherapy regimen should include platinum-based chemotherapy aiming at both epithelial ovarian cancer and germ cell tumors.
Keywords: ovarian germ cell, postmenopausal, prognosis, yolk sac tumor
1. Introduction
Ovarian germ-cell tumors (OGCTs) comprise about 15% to 20% of all ovarian tumors and 2% to 5% of all ovarian malignancies.[1] Ovarian yolk sac tumors (YSTs) account for 14% to 20% of all malignant OGCTs. The age distribution of patients reported with YST ranges from 16 months to 86 years, but two-thirds of them are under 20 years of age, occasionally in postmenopausal women.[2] Postmenopausal patients may have different characteristics and prognosis from those of child-bearing age patients. The majority of YSTs in postmenopausal patients are associated with epithelial ovarian carcinoma and appear to be associated with a poorer outcome.[3] There is little knowledge concerning the development, treatment, and outcome of postmenopausal YSTs. To provide additional knowledge to this rare disease, we present 3 cases of ovarian YST in postmenopausal females diagnosed and treated in our hospital from 2000 to 2017. Based on a review of these cases and the related current literature on this topic, we attempted to enhance the understanding of this rare entity.
2. Methods
This study retrospectively analyzed the data of postmenopausal patients with ovarian YST who were diagnosed and treated from 2000 and 2017 in Peking Union Medical College Hospital. The study protocol was performed in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Institutional Ethical Committee of Peking Union Medical College Hospital. All patients signed an informed consent.
The information of postmenopausal patients with ovarian YST, including patient's age at diagnosis, chief complaint, clinical features, tumor markers, imaging findings, surgical records, pathology, treatment modality, and follow-up were recorded. All surgical specimens were re-evaluated by 2 specialized gynecologic pathologists.
3. Results
A review of our database revealed 3 postmenopausal patients diagnosed with ovarian YST (Table 1) and their pathology were confirmed by 2 gynecologic pathologists.
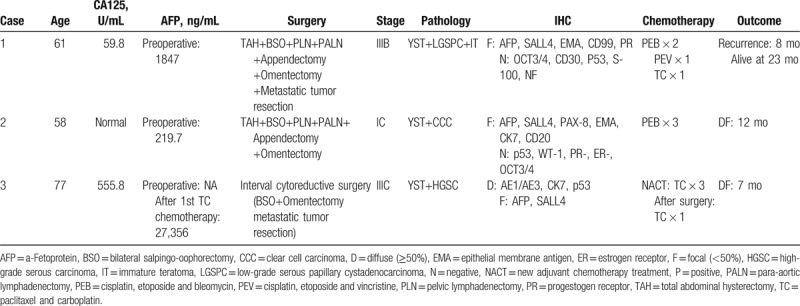
Table 1.
Clinicopathologic details of cases in present study.
3.1. Case 1
A 61-year-old woman presented with a 2-week history of lower abdominal discomfort when urinating, and an abdominal mass was palpable on examination. No family history of cancer. The computed tomographic (CT) imaging demonstrated a pelvic mass disease with mixed density (Fig. 1). Preoperatively, cancer antigen 125 (Ca125) was 59.8 U/mL (normal, 0–35 U/mL), and a-fetoprotein (AFP) was 1847 ng/mL (normal, 0–20 ng/mL) (Fig. 2). The patient underwent primary cytoreductive surgery with total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), pelvic and para-aortic lymphadenectomy, appendectomy, and omentectomy and metastatic tumor resection without macroscopic residual disease. Surgery reviewed a mass arising from the right ovary with involvement of the surface of left ovary, transverse colon surface, the omentum, the right paracolic sulcus, mesentery of the small intestine, uterosacral ligament, right pelvic peritoneum, anterior rectal wall, ileocecal mesangial, and mesoappendix. The FIGO stage was IIIB.
Figure 1.
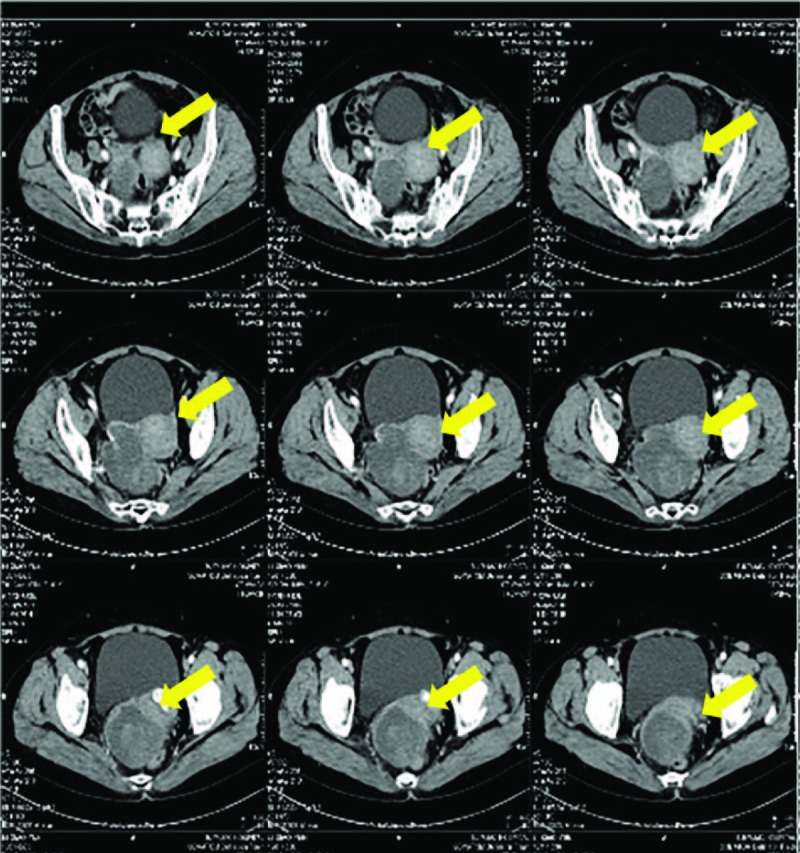
Case 1: Preoperative computed tomography images.
Figure 2.
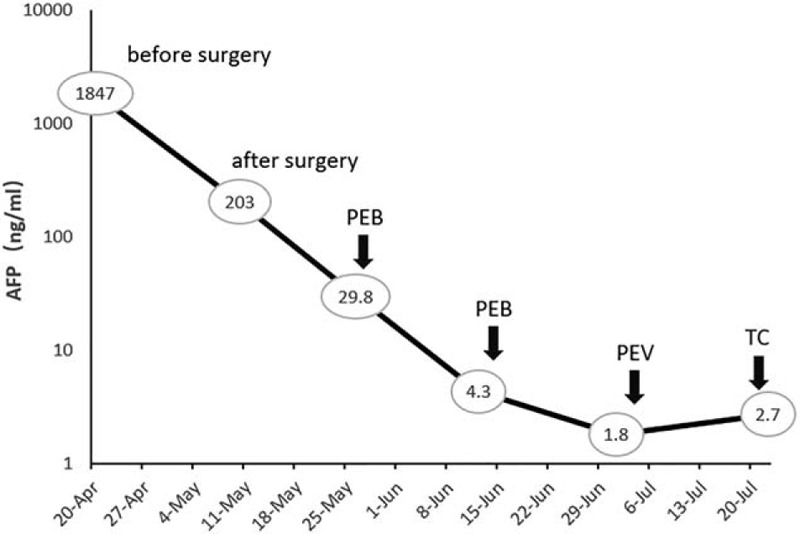
Case1: Changes in serum a-fetoprotein (AFP) levels during chemotherapy. PEB = cisplatin, etoposide and bleomycin, PEV = cisplatin, etoposide and vincristine, TC = paclitaxel and carboplatin.
3.1.1. Histopathologic findings
The postoperative pathology reported this tumor to be a low-grade serous papillary cystadenocarcinoma and mixed germ-cell tumor (immature teratoma and YST) affecting the right ovary. The tumor showed patchy strong positivity for SALL4 and AFP. They also showed focal positivity for epithelial membrane antigen (EMA), cluster of differentiation 99 (CD99), and progestrone receptor (PR). The tumor was negative for octamer 3/4 (OCT3/4), CD30, P53, S-100, and NF.
3.1.2. Treatment and outcome
Postoperative AFP was decreased to be 203.0 ng/mL and CA125 was 71.1 U/mL (Fig. 2). The patient received 2 cycles of PEB (cisplatin, etoposide and bleomycin) chemotherapy. Due to the decline of pulmonary diffuse function, the 3rd cycle regimen was changed to PEV (cisplatin, etoposide, and vincristine). Because of the intolerance of the side effects, the patient received a 4th course of TC (paclitaxel and carboplatin) chemotherapy and stopped treatment. After 8 months of diagnosis, the patient developed intestinal obstruction, suggesting a recurrence of the tumor with normal serum AFP level. The patient received a second cytoreductive surgery (massive resection of small intestine). Because of poor physical condition, she did not receive any chemotherapy. She currently survived for 23 months.
3.2. Case 2
A 58-year-old postmenopausal woman was admitted for lower abdominal tenderness and a pelvic mass. CT scan demonstrated a complex right adnexal mass with cystic and solid components (Fig. 3). The preoperative AFP was 219.7 ng/mL, while carcinoembryonic antigen (CEA), CA125, and CA199 were within the normal range. TAH, BSO, pelvic and para-aortic lymphadenectomy omentectomy and appendectomy were performed.
Figure 3.
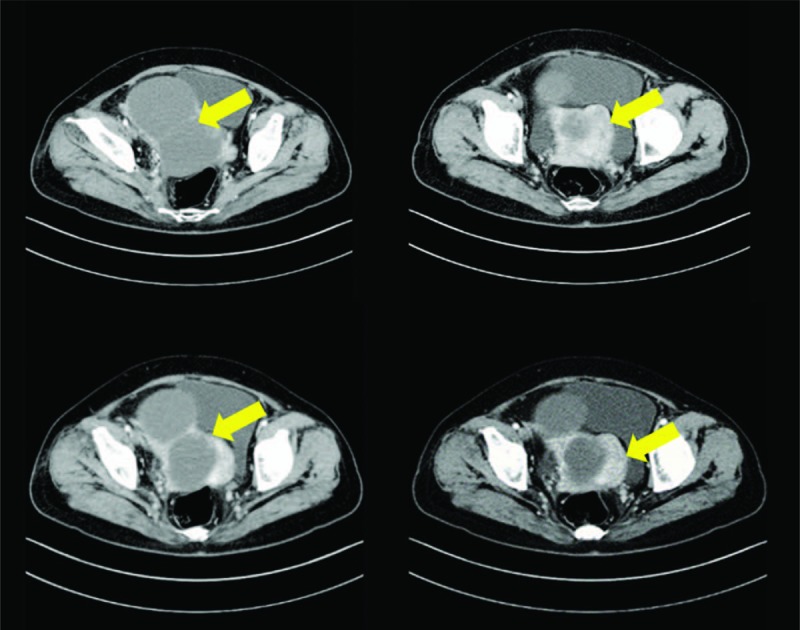
Case 2: Preoperative computed tomography images.
3.2.1. Histopathologic findings
Pathology revealed a clear cell carcinoma arising from the right ovary with YST component (Fig. 4). There are multiple leiomyoma and adenomyosis in uterus. The malignant tumor was confined to the right ovary; no metastases were found. Her FIGO stage was IC. Immunohistochemical staining showed germ cells were positive for SALL4 and AFP, and were negative for p53, OCT3/4, Napsin A, progestrone receptor (PR), and estrogen receptor (ER). In clear cell carcinoma component, CK7 and EMA were diffusely positive and SALL-4 was focally positive.
Figure 4.
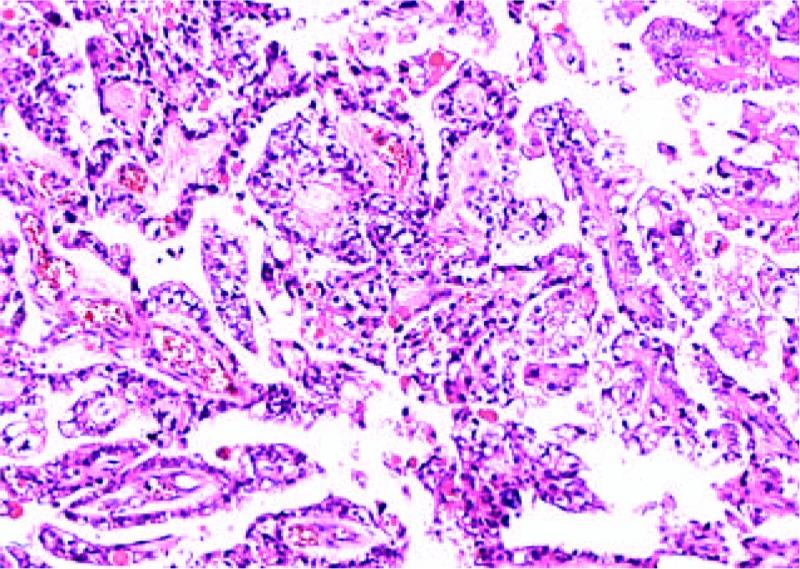
Case 2: Clear cell carcinoma component with yolk sac tumor (hematoxylin and eosin, ×100).
3.2.2. Treatment and outcome
After surgery, she received 3 courses of chemotherapy with PEB regimen. Changes in serum AFP levels during chemotherapy are shown in Figure 5. Due to the severe bone marrow suppression, the patient terminated chemotherapy after 3 cycles of PEB chemotherapy. At the current follow-up, there were no signs of tumor recurrence.
Figure 5.
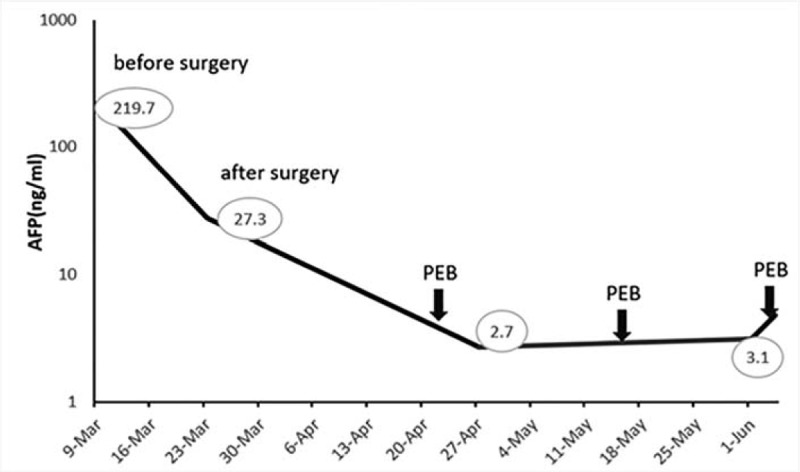
Case 2: Changes in serum a-fetoprotein (AFP) levels during chemotherapy. PEB = cisplatin, etoposide and bleomycin.
3.3. Case 3
A 77-year-old multiparous woman with a 1-month history of lower abdominal discomfort and vaginal bleeding. CT imaging showed multiple solid and cystic pelvic mass (Fig. 6). Positron emission tomography-computed tomography (PET-CT) revealed complex solid and cystic pelvic mass located in the bilateral adnexa and posterior of uterus. There were multiple metastases on mesentery and peritoneum. A preoperative serum CA-125 was 555.8 U/mL and CA199 was 58.2 U/mL. Serum AFP was not performed preoperatively. The gastrointestinal endoscopy examination was negative. The patient subsequently underwent a laparoscopic exploration, patches of biopsies, and curettage. Intraoperatively, multiple metastatic nodules scattered in the omentum and the diaphragm (Fig. 7A). The bilateral annexa was wrapped by omentum, revealing a small part of the mass (Fig. 7B). Postoperative pathology revealed undifferentiated carcinoma of pelvic mass and high-grade serous carcinoma of endometrium. After 3 cycles of TC, she underwent interval cytoreductive surgery. The second postoperative recovery was good, and she received the third TC chemotherapy. Changes in serum AFP levels during chemotherapy and surgery are shown in Figure 8.
Figure 6.
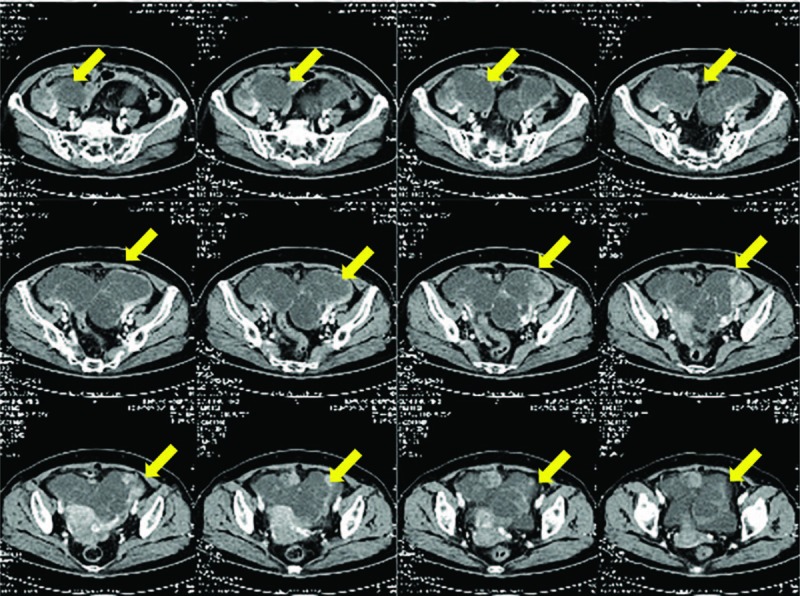
Case 3: Preoperative computed tomography images.
Figure 7.
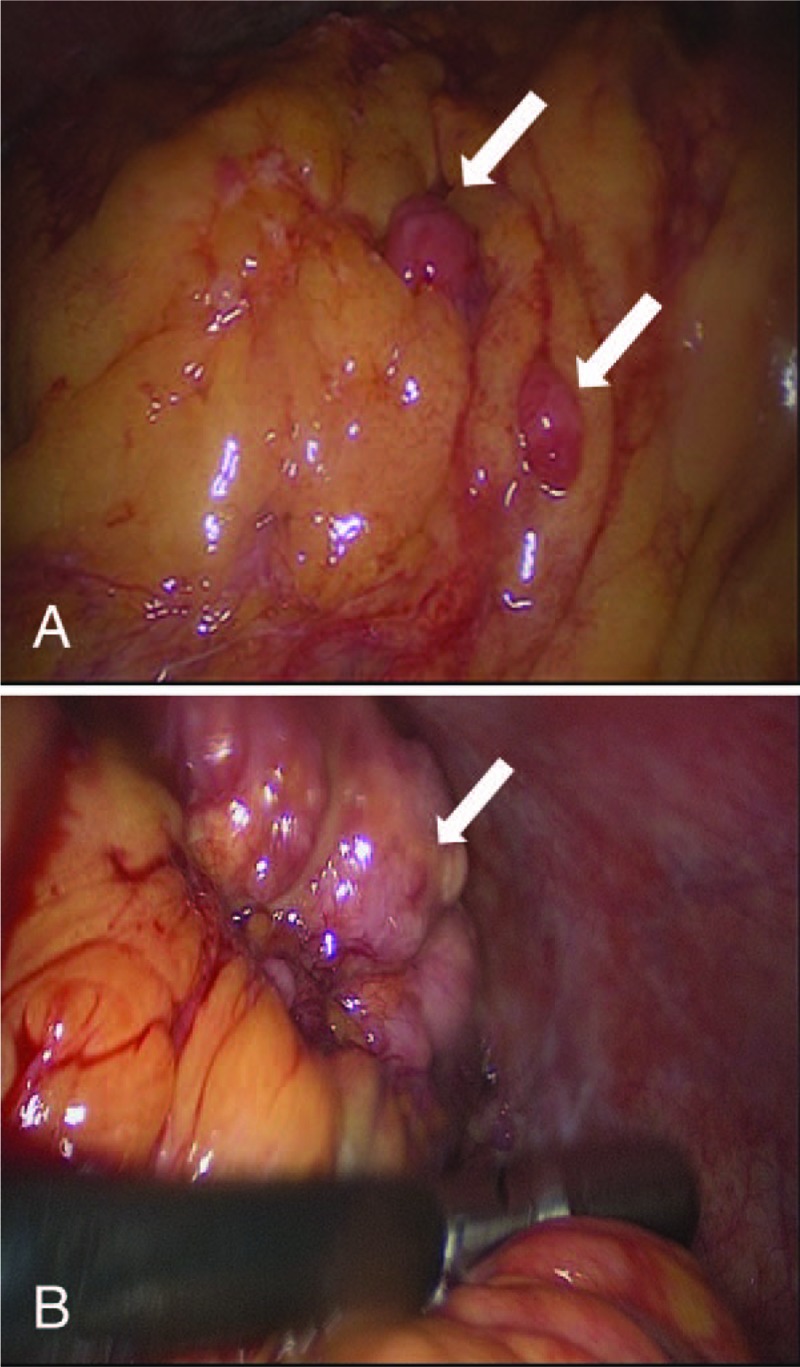
Images in laparoscopic exploration. (A) Metastatic nodules scattered in the omentum. (B) The right annexa was wrapped by omentum.
Figure 8.
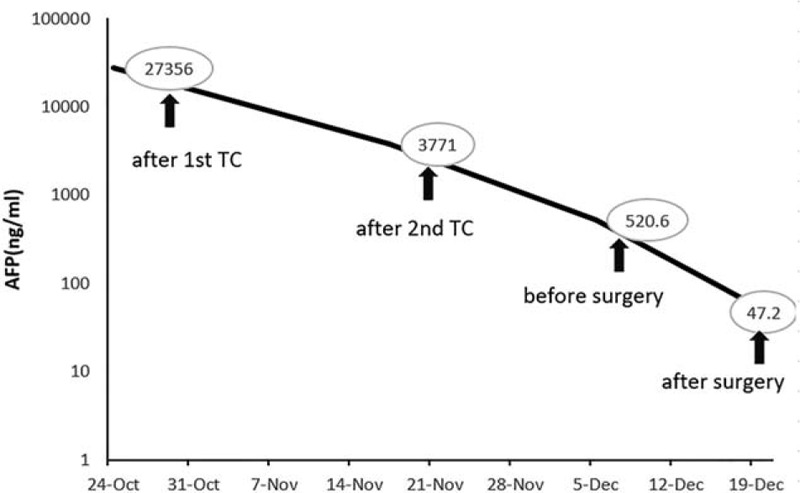
Case3: Changes in serum a-fetoprotein (AFP) levels during chemotherapy and surgery.
3.3.1. Histopathologic findings
Pathology revealed bilateral ovarian high-grade serous carcinomas with YST differentiation involving the surface of omentum, bladder, and rectum. The tumor displayed focal-positive staining for AFP and SALL4. AE1/AE3, CK7, and p53 were diffusely positive. There was negative staining for PAX-8, ER, PR, OCT3/4, and WT-1.
3.3.2. Treatment and outcome
The patient refused to continue chemotherapy after receiving 1 course of TC chemotherapy postoperatively. There are no signs of tumor recurrence at a follow-up of 7 months.
4. Discussion
The YSTs are the second most common ovarian germ cell malignancy, following dysgerminoma. They usually occur in childhood, adolescence, and early adult life, and are extremely rare in perimenopausal and postmenopausal female. Older patients may have different clinicopathologic features and prognosis compared with younger patients. Here, we reported 3 cases diagnosed and treated in Peking Union Medical College Hospital and reviewed the previous cases reported in English literature.
There are 55 cases reported in the previous literature. Their clinical features are listed in Table 2 . The average age of onset was 62.5 years (range: 48–86). Because serum AFP was not routinely tested in postmenopausal women, the preoperative diagnosis of YST component in this population was quite difficult. The preoperative level of serum AFP was elevated in 27 cases,[3,8,10–18,20–22,24,26–28,30] only 4 cases of AFP was normal,[6,11,26] the remaining was unknown.[4,5,7,9,11,19,23,25,26,29–31] Among 3 cases we reported, available serum AFP levels of 2 patients were both elevated, and the serum AFP level of the 3rd case was elevated significantly when testing after 1 cycle of chemotherapy.
Table 2.
Summary of 55 postmenopausal patients with ovarian YSTs.
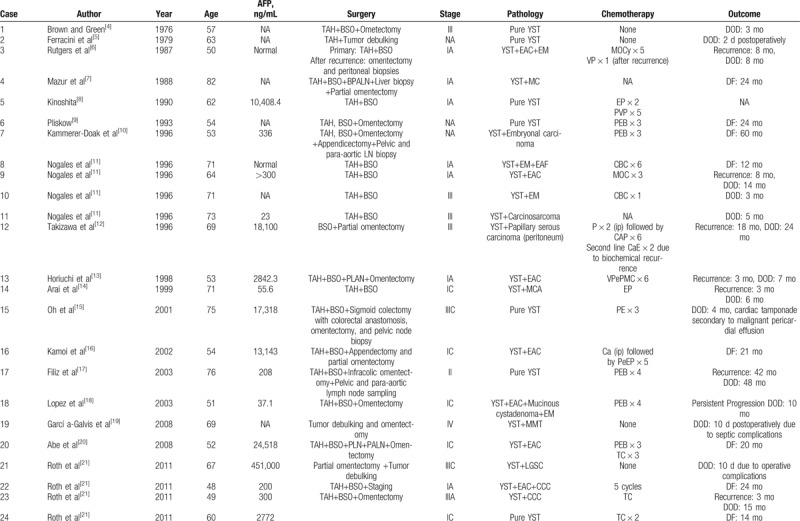
Table 2 (Continued).
Summary of 55 postmenopausal patients with ovarian YSTs.
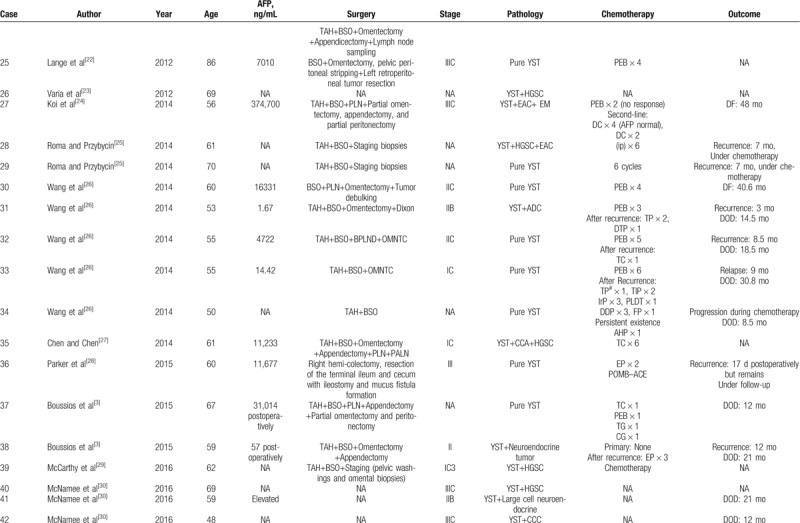
Table 2 (Continued).
Summary of 55 postmenopausal patients with ovarian YSTs.
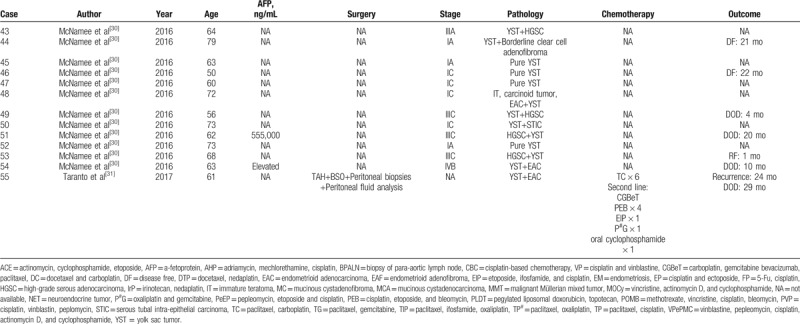
The YST can be pure or mixed with other germ-cell or epithelial tumors. As was seen in our cases, 3 postmenopausal women with YSTs all presented with a coexisting epithelial component and 1 case possessed 2 types of germ-cell tumors and epithelial components. In all previous cases, 20 were histologically classified as pure YST and 35 were mixed with other histologic components. The most common mixed component were endometrioid adenocarcinoma[6,11,13,16,18,20,21,24,25,30,31] and serous adenocarcinoma,[12,21,23,25,27,29,30] and other components included clear cell carcinoma,[21,27,30] carcinosarcoma,[11] embryonal carcinoma,[10] malignant mixed Müllerian tumor,[19] and so on. The differential diagnosis of ovarian YST in elderly patients is difficult, and the presence of mixed components adds difficulty to it. Especially when the YST component is limited, it can be overlooked. Due to the existence of the inherent heterogeneity of this tumor, adequate sampling is very important for a comprehensive and accurate diagnosis.
Although OGCTs are characterized by high malignancy, they are curable by surgery and combination chemotherapy. PEB chemotherapy is the most commonly recommended first-line chemotherapy regimen. For patients with malignant OGCTs receiving cisplatin-based first-line chemotherapy, the 5-year survival rate is close to 90%.[32] The cure rates for patients with early stage approach 100%, and even in patients with advanced stage, the cure rates are reportedly at least 75%.[33]
Interestingly, the simultaneous presence of YST and ovarian malignant epithelial tumor may herald a more aggressive behavior regardless of the stage of presentation.[21] The exact explanation of this biologic behavior is not yet clear. When malignant epithelial components and germ-cell tumors co-exists, the choice of which chemotherapy regimen is problematic. No ideal regimen has yet been established for this type of tumor. It has been hypothesized that the chemosensitivity of these tumor cells will differ from that of pure or mixed germ-cell tumors, since the yolk sac components of these tumors may originate from a different molecular pathway than do germ cell-tumors in younger patients.[21] Although the exact explanation for the pathogenesis of these tumor is unknown, 4 theories including the teratoma theory, retrodifferentiation, collision theory, and neometaplasia theory have been proposed.[6,16,18,30] Review of previous cases, 22 patients did not receive any chemotherapy or had no record for adjuvant therapy postoperatively. Among the 33 patients who received chemotherapy, 28 patients received platinum-based chemotherapy. Among 13 patients receiving PEB chemotherapy regimen, 2 patients[18,24] experienced disease progression during chemotherapy and 1 patient[24] experienced complete remission after changing to a second-line chemotherapy (docetaxel and carboplatin). She had been followed up for 48 months without sign of tumor recurrence.
Studies have shown that prognostic factors of ovarian YST includes tumor stage,[34] amount of ascites,[35] serum AFP decline rate,[35] residual tumor,[36] and chemotherapy regimen and chemotherapy course.[36,37] In premenopausal women, multivariate analysis showed that age was not a significant prognostic factor.[35] Due to the rarity of YST in postmenopausal women, whether age affects the prognosis has not yet been clear. Among the 55 patients reported in the literature, 28 were diagnosed as stage I or II, 16 stage III, and 2 stage IV. The stage of 9 patients were not available. The prognosis of OGCT in postmenopausal women is poor, even for patients with early-stage disease. Twenty-two of 46 patients with available staging were diagnosed with stage I disease,[6–8,11,13,14,16,18,20,21,26,27,29,30] but only 7 of them (32%) were alive for more than 20 months[7,16,20,21,26] with 8 cases’ survival information unavailable. Twenty-four died of the disease at a median time of 12.8 months after diagnosis and 13 patients still survived during follow-up. The longest follow-up time documented was 60 months.[10] Fourteen patients died of the disease within 1 year, although 5 patients had stage I or II disease with no residual tumor, and 3 of them received adjuvant chemotherapy with a PEB regimen. The recurrence time was documented in only 15 patients (median relapse time: 10.4 months [range: 1–42]), and only 3 of 15 patients with recurrence records were still under follow-up, and the others were all died (median additional survival time after recurrence: 7.3 months). After recurrence, no salvage surgery was reported before and most patients accepted platinum-based chemotherapy. The longest survival time after recurrence was 21.8 months.[26] The above data suggested that postmenopausal ovarian YST patients may have a poor prognosis compared with young patients.
Immunohistochemical staining of AFP is valuable for the histologic diagnosis of YST, while serum AFP levels contribute to the clinical diagnosis of YST and can help monitoring disease activity and chemotherapy response. Newer markers for YST including glypican-3 and SALL-like protein 4 (SALL4) may be useful in the identification of the YST component histologically. Glypican-3 (GLP3), which is an oncofetal protein expressed in fetal liver and malignant tumors of hepatocytic lineage, is more sensitive than AFP but not as specific.[11] Only AFP and GLP3 are used as YST characteristic immunopathologic markers that can be correlated with their corresponding serum levels.[38] SALL4 may be useful in differentiating YSTs areas originating in somatic tumors and also in the differential diagnosis with ovarian clear cell carcinoma.[39] All of our 3 cases showed diffuse strong expression of AFP and SALL4 in the YST component, supporting the diagnosis of YST. OCT3/4 is positive in dysgerminoma and embryonal carcinoma but negative in YST, as it was in our 3 cases. It should be noted that SALL4, AFP, and GLP3 co-expression in some gastric clear cell and hepatoid carcinomas.[40]
5. Conclusion
We reported 3 cases of mixed YST in postmenopausal females and as far as we know, we reported the first case of YST combined with both low-grade serous carcinoma and immature teratoma. Ovarian YSTs in postmenopausal women are characterized by high malignancy and may have a worse prognosis compared with those in younger patients. More active treatment is needed especially when mixed with epithelial cancer components. When YST and epithelial cancer components co-exist, YST components may be less responsive to traditional germ-cell tumor chemotherapy because of possible differentiation of epithelial components. Adjuvant chemotherapy should be selected to simultaneously target epithelial ovarian tumors and germ-cell tumors. Platinum-based chemotherapy is recommended.
Acknowledgments
The authors thank all the patients and their family members for their contribution to the study. The authors are also grateful to the colleagues in the Medical record department for providing us with case record support.
Author contributions
Conceptualization: Yao Wang, Mei Yu.
Data curation: Yao Wang, Jiaxin Yang, Dongyan Cao, Ying Zhang, Xuan Zong, Keng Shen.
Formal analysis: Yao Wang.
Resources: Ying Zhang.
Validation: Mei Yu, Jiaxin Yang, Dongyan Cao, Keng Shen.
Writing – original draft: Yao Wang, Xuan Zong.
Writing – review & editing: Yao Wang, Mei Yu, Jiaxin Yang, Dongyan Cao, Ying Zhang, Keng Shen.
Footnotes
Abbreviations: AFP = a-fetoprotein, BSO = bilateral salpingo-oophorectomy, CA125 = cancer antigen 125, CEA = carcinoembryonic antigen, OGCT = ovarian germ-cell tumor, PEB = cisplatin, etoposide and bleomycin, PEV = cisplatin, etoposide and vincristine, TAH = total abdominal hysterectomy, TC = paclitaxel-carboplatin, YST = yolk sac tumor.
This work was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-1-002).
The authors have no conflicts of interest to disclose.
References
- [1].Tewari K, Cappuccini F, Disaia PJ, et al. Malignant germ cell tumors of the ovary. Obstet Gynecol 2000;95:128–33. [DOI] [PubMed] [Google Scholar]
- [2].Shah JP, Kumar S, Bryant CS, et al. A population-based analysis of 788 cases of yolk sac tumors: a comparison of males and females. Int J Cancer 2008;123:2671–5. [DOI] [PubMed] [Google Scholar]
- [3].Boussios S, Attygalle A, Hazell S, et al. Malignant ovarian germ cell tumors in postmenopausal patients: the Royal Marsden Experience and Literature Review. Anticancer Res 2015;35:6713–22. [PubMed] [Google Scholar]
- [4].Brown JR, Green JD. Yolk sac carcinoma. South Med J 1976;69:728–31. [DOI] [PubMed] [Google Scholar]
- [5].Ferracini R, Gardini G, Lanzanova G, et al. Endodermal sinus tumor in a 63 year old female. Pathologica 1979;71:885–7. [PubMed] [Google Scholar]
- [6].Rutgers JL, Young RH, Scully RE. Ovarian yolk sac tumor arising from an endometrioid carcinoma. Hum Pathol 1987;18:1296–9. [DOI] [PubMed] [Google Scholar]
- [7].Mazur MT, Talbot WH, Jr, Talerman A. Endodermal sinus tumor and mucinous cystadenofibroma of the ovary. Occurrence in an 82-year-old woman. Cancer 1988;62:2011–5. [DOI] [PubMed] [Google Scholar]
- [8].Kinoshita K. A 62-year-old woman with endodermal sinus tumor of the ovary. Am J Obstet Gynecol 1990;162:760–2. [DOI] [PubMed] [Google Scholar]
- [9].Pliskow S. Endodermal sinus tumor of the ovary: review of 10 cases. South Med J 1993;86:187–9. [DOI] [PubMed] [Google Scholar]
- [10].Kammerer-Doak D, Baurick K, Black W, et al. Endodermal sinus tumor and embryonal carcinoma of the ovary in a 53-year-old woman. Gynecol Oncol 1996;63:133–7. [DOI] [PubMed] [Google Scholar]
- [11].Nogales FF, Bergeron C, Carvia RE, et al. Ovarian endometrioid tumors with yolk sac tumor component, an unusual form of ovarian neoplasm. Analysis of six cases. Am J Surg Pathol 1996;20:1056–66. [DOI] [PubMed] [Google Scholar]
- [12].Takizawa Ken, Kawana Takashi, Kakinoki Shigeko, et al. Case report of a 69-year-old woman with double cancers: primary yolk sac tumor of the right ovary and primary serous surface papillary carcinoma of the peritoneum. Int J Clin Oncol 1996;1:190–4. [Google Scholar]
- [13].Horiuchi A, Osada R, Nakayama K, et al. Ovarian yolk sac tumor with endometrioid carcinoma arising from endometriosis in a postmenopausal woman, with special reference to expression of alpha-fetoprotein, sex steroid receptors, and p53. Gynecol Oncol 1998;70:295–9. [DOI] [PubMed] [Google Scholar]
- [14].Arai T, Kitayama Y, Koda K. Ovarian mucinous cystadenocarcinoma with yolk sac tumor in a 71-year-old woman. Int J Gynecol Pathol 1999;18:277–80. [DOI] [PubMed] [Google Scholar]
- [15].Oh C, Kendler A, Hernandez E. Ovarian endodermal sinus tumor in a postmenopausal woman. Gynecol Oncol 2001;82:392–4. [DOI] [PubMed] [Google Scholar]
- [16].Kamoi S, Ohaki Y, Mori O, et al. A case of ovarian endometrioid adenocarcinoma with yolk sac tumor component in a postmenopausal woman. APMIS 2002;110:508–14. [DOI] [PubMed] [Google Scholar]
- [17].Filiz G, Ozuysal S, Bilgin T. Ovarian endodermal sinus tumor in a 76-year-old woman. J Obstet Gynaecol Res 2003;29:309–11. [DOI] [PubMed] [Google Scholar]
- [18].Lopez JM, Malpica A, Deavers MT, et al. Ovarian yolk sac tumor associated with endometrioid carcinoma and mucinous cystadenoma of the ovary. Ann Diagn Pathol 2003;7:300–5. [DOI] [PubMed] [Google Scholar]
- [19].García-Galvis OF, Cabrera-Ozoria C, Fernandez JA, et al. Malignant Mullerian mixed tumor of the ovary associated with yolk sac tumor, neuroepithelial and trophoblastic differentiation (teratoid carcinosarcoma). Int J Gynecol Pathol 2008;27:515–20. [DOI] [PubMed] [Google Scholar]
- [20].Abe A, Furumoto H, Yoshida K, et al. A case of ovarian endometrioid adenocarcinoma with a yolk sac tumor component. Int J Gynecol Cancer 2008;18:168–72. [DOI] [PubMed] [Google Scholar]
- [21].Roth LM, Talerman A, Levy T, et al. Ovarian yolk sac tumors in older women arising from epithelial ovarian tumors or with no detectable epithelial component. Int J Gynecol Pathol 2011;30:442–51. [DOI] [PubMed] [Google Scholar]
- [22].Lange S, Livasy C, Tait DL. Endodermal sinus tumor of the ovary in an 86 year old woman. Gynecol Oncol Case Rep 2012;2:65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Varia M, McCluggage WG, Oommen R. High grade serous carcinoma of the ovary with a yolk sac tumour component in a postmenopausal woman: report of an extremely rare phenomenon. J Clin Pathol 2012;65:853–4. [DOI] [PubMed] [Google Scholar]
- [24].Koi C, Kurita T, Kagami S, et al. A case of ovarian yolk sac tumor associated with endometrioid adenocarcinoma. Gynecol Oncol Case Rep 2014;9:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Roma AA, Przybycin CG. Yolk sac tumor in postmenopausal patients: pure or associated with adenocarcinoma, a rare phenomenon. Int J Gynecol Pathol 2014;33:477–82. [DOI] [PubMed] [Google Scholar]
- [26].Wang X, He J, Li Y. Ovarian yolk sac tumor in postmenopausal females: a report of five cases and a literature review. Eur J Gynaecol Oncol 2016;37:374–9. [PubMed] [Google Scholar]
- [27].Chen Q, Chen X. Bilateral ovarian mixed epithelial adenocarcinoma in a postmenopausal woman with unilateral ovarian yolk sac tumor component. Int J Clin Exp Pathol 2014;7:8259–65. [PMC free article] [PubMed] [Google Scholar]
- [28].Parker VL, Sanderson P, Naik V, et al. Post-menopausal presentation of yolk sac germ cell tumour. Gynecol Oncol Rep 2015;11:16–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McCarthy WA, Masand RP. Ovarian yolk sac tumor with high-grade serous carcinoma in a 62-year-old woman. Int J Surg Pathol 2016;24:360–5. [DOI] [PubMed] [Google Scholar]
- [30].McNamee T, Damato S, McCluggage WG. Yolk sac tumours of the female genital tract in older adults derive commonly from somatic epithelial neoplasms: somatically derived yolk sac tumours. Histopathology 2016;69:739–51. [DOI] [PubMed] [Google Scholar]
- [31].Taranto P, Carvalho FM, Roithmann S, et al. Ovarian yolk sac tumor coexisting with epithelial ovarian cancer: an aggressive rare entity. Gynecol Oncol Rep 2017;22:37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Solheim O, Gershenson DM, Trope CG, et al. Prognostic factors in malignant ovarian germ cell tumours (The Surveillance, Epidemiology and End Results experience 1978–2010). Eur J Cancer 2014;50:1942–50. [DOI] [PubMed] [Google Scholar]
- [33].Gershenson DM. Management of ovarian germ cell tumors. J Clin Oncol 2007;25:2938–43. [DOI] [PubMed] [Google Scholar]
- [34].Dallenbach P, Bonnefoi H, Pelte MF, et al. Yolk sac tumours of the ovary: an update. Eur J Surg Oncol 2006;32:1063–75. [DOI] [PubMed] [Google Scholar]
- [35].de La Motte Rouge T, Pautier P, Rey A, et al. Prognostic factors in women treated for ovarian yolk sac tumour: a retrospective analysis of 84 cases. Eur J Cancer 2011;47:175–82. [DOI] [PubMed] [Google Scholar]
- [36].Nawa A, Obata N, Kikkawa F, et al. Prognostic factors of patients with yolk sac tumors of the ovary. Am J Obstet Gynecol 2001;184:1182–8. [DOI] [PubMed] [Google Scholar]
- [37].Tong X, You Q, Li L, et al. Prognostic factors of patients with ovarian yolk sac tumors: a study in Chinese patients. Onkologie 2008;31:679–84. [DOI] [PubMed] [Google Scholar]
- [38].Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol 2010;16:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cao D, Guo S, Allan RW, et al. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am J Surg Pathol 2009;33:894–904. [DOI] [PubMed] [Google Scholar]
- [40].Ushiku T, Shinozaki A, Shibahara J, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol 2010;34:533–40. [DOI] [PubMed] [Google Scholar]


