Abstract
The sexual behaviors of 15- to 24-year-olds increase the risk of this population to acquire sexually transmitted infections (STIs). The present study aimed to describe the sexual behavior in the transition to adulthood Brazilian population and its association with STI history.
We analyzed cross-sectional data collected from 8562 sexually active women and men who participated in the National Survey of Human Papillomavirus Prevalence (POP-Brazil). This large-scale survey enrolled participants from 26 Brazilian capitals and the Federal District. Professionals from primary care facilities were trained to collect data utilizing a standardized questionnaire with questions on sociodemographic, sexual behavior, and drug use. We constructed a Poisson model with robust variance for both crude and adjusted analysis to investigate the associations between the variables. To adjust the distribution of the sample to the study population, we weighted the measures by the population size in each city and by gender.
There were differences in several aspects from sexual behavior between genders. The majority of men reported an early sexual initiation, more sexual partners, and a different practice in sexual positions when compared with women. Women reported use of contraception more frequently than men (P < .001). The use of alcohol and drugs and the use of drugs before sexual intercourse impact in STIs equally between the genders. Exclusive for women, the presence of any STI was associated with the practice of vaginal sex and other types of intercourse (adjusted prevalence ratio [APR] 1.43, 95% CI 1.08–1.88). For men, the number of sexual partners in the last year (APR 1.02, 95% CI 1.01–1.04), not having vaginal sex (APR 3.25, 95% CI 1.78–5.92) and sexual experience with someone of the same sex (APR 4.05, 95% CI, 2.88–5.70) were associated with a higher presence of STIs.
This is the first report regarding sexual behavior in a nationally representative population sample in Brazil. This study provides more valid estimates of sexual behavior and associated STIs, identifying important differences in sexual behavior and identifying predictors for referred STIs among females and males.
Keywords: epidemiology, sexual behavior, sexually transmitted diseases, young adult
1. Introduction
The age from 15 to 24 years old, which define the transition to adulthood, is a time of exploration, experimentation, and instability in many areas of life, particularly in relation to sexual behavior.[1,2] Adolescents and youth are one of the populations most impacted by sexually transmitted infections (STIs), including human immunodeficiency virus (HIV) and papillomavirus (HPV).[3] Young adults represent 27% of the sexually active population but constitute 50% of the individuals who are diagnosed with any sexual infections.[3,4]
Sexuality is a normative and physiological component of adolescent development[5] and usually this group engage in risky sexual practices such as early sexual intercourse, multiple sexual partners, unprotected sexual intercourse, and casual sex.[6,7] Through diverse sexual experiencing, this population can learn about what they like in a partner and come to understand their own sexual identity, corroborating to the fact that emerging adults are more likely to have multiple partners during the past 12 months compared to any other age group.[8]
Sociocultural differences are determinant of sexual behaviors, influencing the age of first intercourse, number of partners, coercive sexual culture and it can affect the probability of being engaged in risky sex.[9–11] Sexual activities as oral and anal sex differs according to race/ethnicity.[12] A meta-analysis found that the use of condom is lower in younger population, but just in non-African countries, suggesting that socioeconomic status is directly associated with risky-sexual behavior.[13] The intention to use condom was also determined by subjective norms, taboo on discussing sex and factors such as machist behaviors, who change substantially between cultures and between specific populations.[14] Although some evidences reported differences in sexual behaviors according to gender, such as males having higher rates of risky sexual relationships (82.3%) compared to females (63.0%),[15] Petersen and Hyde[16] reported in a meta-analysis that differences in sexual attitudes and behaviors are small and decrease with age.
Social behavior as smoking, alcohol, and drugs use also increase the probability of having a sexual behavior associated with STIs, as well as having an early sexual debut and several sexual partners.[11,17,18] STIs diagnosis was higher in young people reporting recent illicit drug use, but only in men and the odds of using drugs is higher in some sexual behaviors as paying for sex.[19]
The Centers for Disease Control and Prevention (CDC) has reported increasing rates of STIs in adolescents and persisting disparities in STI prevalence, with higher rates in minority groups.[20] Surveillance data show higher rates of reported STIs among some racial or ethnic minority groups compared with these rates among whites.[20–23] Black students usually report having more sexual partners than Hispanic and white students as well as a higher proportion of sex before the age of 13 years old.[20]
Because the rates of infection from HPV and other STIs increase soon after the first intercourse and facing the important role of cultural differences in sexual behavior, knowledge of country-specific data are critical to understand patterns of sexual behavior and associated STIs. This study will provide important information for planning and optimize prevention strategies for HPV and other STIs. Therefore, we aim to describe sexual behavior in the transition to adulthood in a young Brazilian population and its association with STI history.
2. Methods
2.1. Study design and population
We analyzed data from 8562 participants of the Pop-Brazil study, a cross-sectional study that includes sexually active women and men aged of 16 to 25 years from 26 Brazilian state capitals and the Federal District. Briefly, the participants were recruited in primary care units by using different approaches such as a personal invitation during routine healthcare visits, domiciliary visits, and school-based programs as well as patient lists and local media, between September of 2016 and November of 2017. All participants answered a face-to-face interview with questions about sociodemographic factors, alcohol and drug consumption habits, sexual behaviors, and STIs during life.
This study was approved by the Ethics Committee of Hospital Moinhos de Vento (no. 1607032) and the committees from the collaborators centers and all participants provided a written consent after being informed about the study procedures.
2.2. Study variables
The main outcomes are differences in sexual behavior and associated STI infection.
The participants were asked about their age of first intercourse, the number of sexual partners in the last year, the number of sexual partners in the last 5 years, and types of sex: exclusively vaginal, other excluding vaginal (anal and oral sex or other sexual acts), and same-sex relationships. We also asked about use and type of contraceptive methods, condom use during life and its use in the last sexual intercourse, as well as use of drugs and alcohol.
The diagnosis of STIs throughout life was obtained by self-report. We asked if the participants ever had syphilis, gonorrhea, genital herpes, genital warts (condyloma acuminatum), HPV, or other sexual infections. Some sociodemographic characteristics about the population were also investigated.
2.3. Statistical analysis
A descriptive analysis was done using means and confidence intervals for continuous variables and absolute frequencies for categorical data. The differences among the means were assessed by the t-test and the chi-squared test was used to evaluate the differences among categories. A nonparametric test was used when the data were not normally distributed. We investigated the association between the sociodemographic and sexual aspects of alcohol and drug abuse with the presence of STIs through the construction of a Poisson with robust variance model for both crude (PR) and adjusted (APR) analysis. To adjust the distribution of the sample to the study population, we used a weight adjustment population sizes in each capital and by sex. Analysis was performed by using SAS software (Statistical Analysis System, SAS Institute Inc., Cary, NC), version 9.4, and statistical significance was defined as P < .05.
3. Results
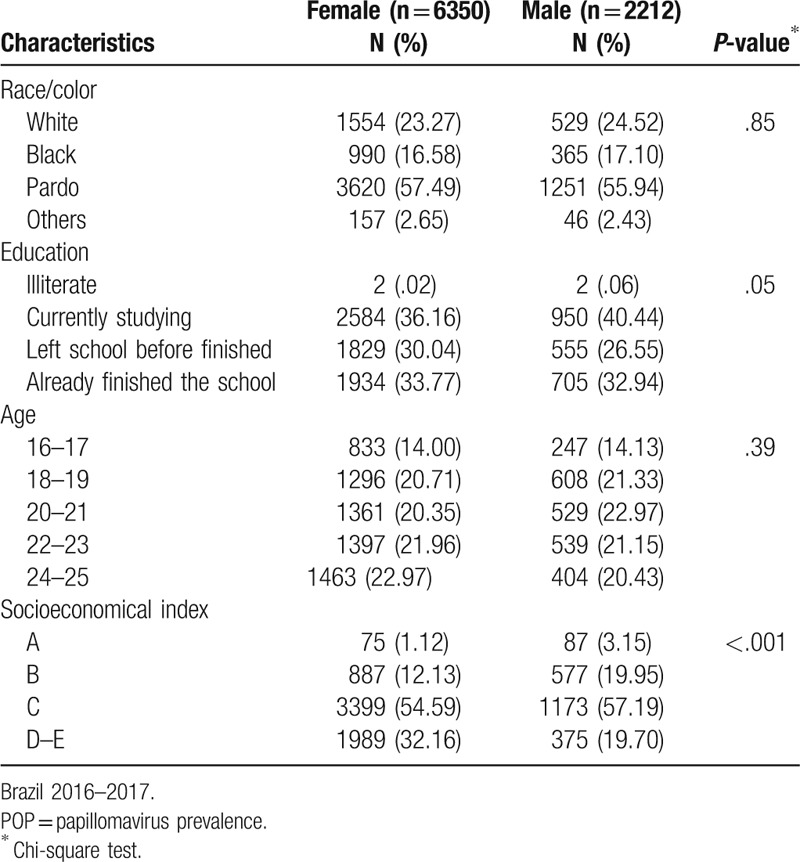
Sociodemographic characteristics were similar in both genders, except for the socioeconomical index (Table 1). The majority of the participants self-declared themselves as pardo, followed by white and black in both genders. There was a higher proportion of young females that left school before finishing it (30.04% females vs 26.55% males; P < .05). There was a significant difference in the socioeconomic index that evaluates the number of goods in a household, according to sex. More than half of the participants were classified as class C, with a higher percentage of women in a lower class (32.16%) compared to men (19.70%) (P < .001).
Table 1.
Characteristics among participants of POP-Brazil Study aged 16–25 years, by sex.
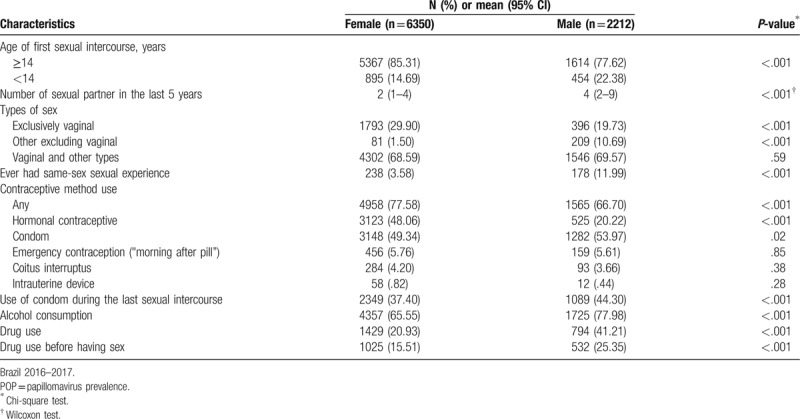
In general, females and males are different regarding sexual behavior, alcohol, and drug use (Table 2). A higher proportion of males had the first intercourse with less than fourteen years old, had more sexual partners in last 5 years, and more same-sex sexual experiences. Vaginal sex exclusively was more frequent in females (29.90%) and males have a higher proportion of other types of sex excluding vaginal penetration (10.69% in males vs. 1.50% in females; P < .001).
Table 2.
Sexual behavior and alcohol and drug consumption in the POP-Brazil Study participants, aged 16–25 years, by sex.
Men reported more oral sex than women (52.67% vs. 47.33%, respectively; P < .001), and more anal sex (34.39% vs. 19.63%, respectively; P < .001) (Fig. 1). Others practices such as ménage à trois, swing, sado-masochistic practices, sex toys, or use of food during sex (honey, chocolate) were reported by 4.87% of the participants, use of sex toys being the most cited (54.00%).
Figure 1.
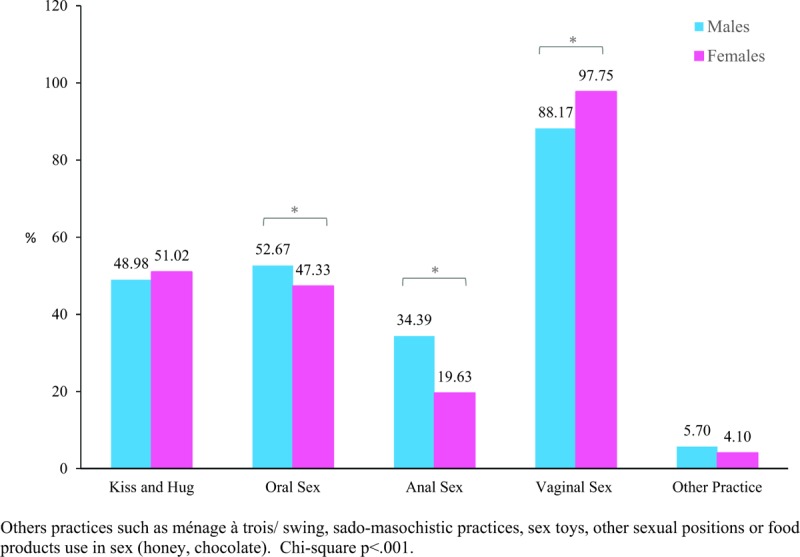
Sexual practices behaviors among participants of POP-Brazil Study aged 16–25 years, by sex. Brazil 2016–2017. POP = papillomavirus prevalence.
Safe sex behavior and contraceptive use also differ between females and males (Table 2). Although men reported more frequent use of condom in the last intercourse (44.81% males vs. 37.17% females P < .001) the frequency of regular use of any contraceptive is lower among men. The use of coitus interruptus as a contraceptive method was reported by around 4.00% of the participants and <1.00% of women report the use of intrauterine devices.
There were also differences in the alcohol and drug consumption between the genders (Table 2). Men use more addictive substances than women (41.21% vs. 20.93%, respectively P < .001) and the most frequent used drug was Marijuana (10.50% of men reported daily use in the last year). Participants who have same-sex sexual experiences reported higher rates of drug use (55.65% comparing to heterosexual ones 26.25%; P < .001). Regarding sexual behavior associated with drugs, men also reported more frequently having sex after using drugs (P < .001).
The frequency of participants who reported ever had a STI was 12.38% (95% CI 11.10–13.67). The overall frequency and Gonorrhea were higher in males than females (Fig. 2). The frequency of other STIs is similar among sexes. The prevalence ratio of STIs varies according with independent behavioral characteristics (Table 3). STI was more frequent reported by people who referred drug and alcohol intake. For women, having other types of sex along with vaginal sex increase the prevalence ratio of ever had an STI (APR 1.43, 95% CI 1.08–1.88). For men, number of sexual partners in the last year (APR 1.02, 95% CI 1.01–1.04), not having vaginal sex (APR 3.25, 95% CI 1.78–5.92) and had same sex experience (APR 4.05, 95% CI 2.88–5.70), were associated with ever had a STI.
Figure 2.
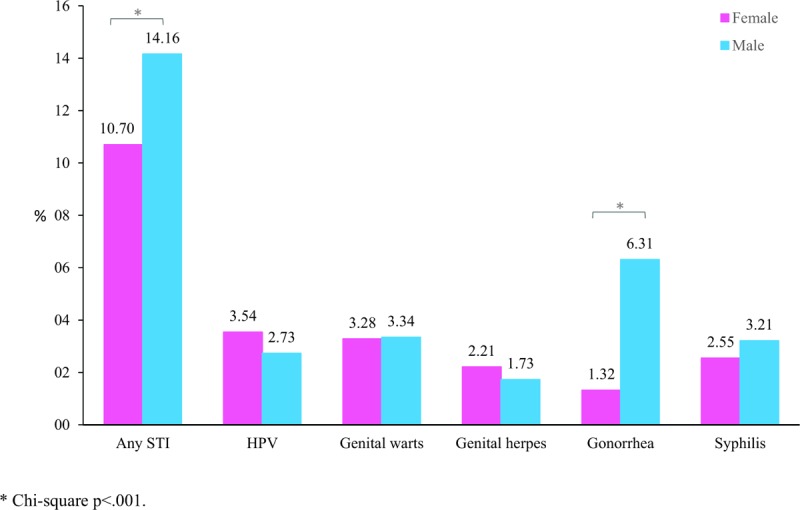
Prevalence of sexually transmitted infections (STI) positivity through the lifetime (self-reported) in participants aged 16–25 years, by sex. Brazil 2016–2017. STI = sexually transmitted infections.
Table 3.
Crude (PR) and adjusted prevalence ratio (APR) of self-reported sexually transmitted infections according with each sexual and social behaviors characteristics in the POP-Brazil Study participants, aged 16–25 years, by sex.
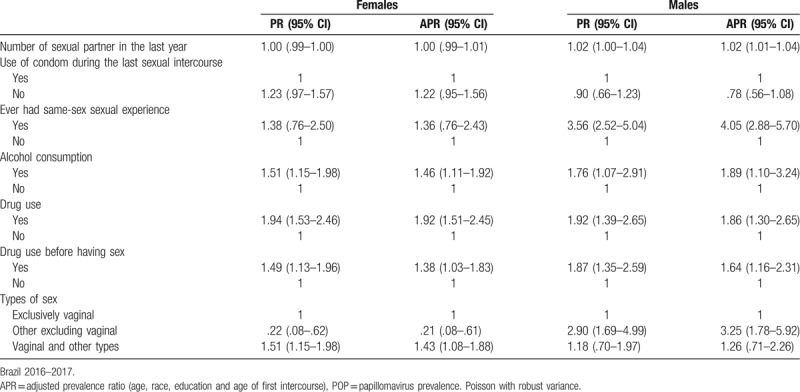
In the full model for women, adjusted by all sexual and behavioral characteristics, age (1.07, 95% CI 1.02–1.12) and drug use (1.80, 95% CI 1.35–2.40) were associate with higher prevalence ratios. In opposite, higher age in the first intercourse (0.93, 95% CI 0.88–0.99) and not having vaginal sex (0.19, 95% CI .07–-.53) was associated with lower prevalence ratio of reporting ever had a STI. In men, the full model shows that age (1.15, 95% CI 1.07–1.23), same-sex sexual relations (3.15, 95% CI 1.93–5.13) and lower education (1.56, 95% CI 1.00–2.45) was associated with higher prevalence ratios (data not shown).
4. Discussion
We evaluated the sexual behavior and the reporting of STIs across the transition to adulthood in a Brazilian population. Although many studies have explored the sexual attitudes in adolescents[3,5,6,24–28] or in specific groups,[29,30] this is the first nationwide Brazilian study that assesses its association with STIs in a broad young population. Besides age, the factors associated with reported STIs are different according with gender. STIs prevalence ratio is associated to drug use and inversely related to older age of the first intercourse or absence of vaginal sex in women. Same-sex intercourse was insignificant in women, but it is the behavior that leads to the higher prevalence ratio in men along with lower education.
To our knowledge, we present the first nationwide study to evaluate sex behavior and its association with referred STI in adolescent and young adults. This study has several limitations. The data collected are from the areas of Primary Care Units. We use a convenience sample, and, although we invited the participants in the community or school, only the ones that attended the Primary Care Unit were included and we could not avoid selection bias. Although we used weight adjustments to incorporate differential probabilities in the patient selection, the representativeness of this study is restricted to the population living in the Brazilian capitals. We collected data on a wide range of sexual and social behaviors, but we do not have information about the time of the referred STIs or serological data support this information. Furthermore, the information collected may be limited to the widely known or easily recognized STIs; therefore, this diagnosis could be subjected to a misclassification bias. For example, we did not ask any information regarding Chlamydia spp, which is not screened or notified in Brazil, and some STIs are not easily recognized. Because of the sensitivity of the topic, the participants may have had the tendency to provide more “desirable” answers than truthful answers; thus, social desirability bias cannot be excluded. Therefore, it is possible that the true proportion of infected people can be higher than estimated by our study.
This young population experiences a diversity of sexual behaviors and there are clear differences between male and female. Most of the participants reported having other types of sexual intercourses in addition to vaginal, similar to the sexual behavior of the adult population in the United States.[31] One in 10 man reported same-sex intercourse, which is higher than frequencies reported in previous studies done in Brazil,[32] but similar to the proportion reported by other Latin American countries.[22] The differences with previous study could be due to the increasing proportion of men who engage in same-sex relationships[33] or due to differences in the sample population.[32]
The age of sexual debut varies among different cultures. Our results confirmed previous studies showing that Brazilians engage in their first intercourse at younger ages than people who live in Great Britain[18,19,38] and other populations[35,36] or in studies conducted more than 10 years ago (18 years)[37] in Brazil. The age of first intercourse has decreased over time in many countries,[38–40] and the same behavior is also observed in Brazil.[41]
Almost half of the participants do not use condom and a smaller proportion reported using one during their last intercourse. This rate is much higher than in some previous studies (25%)[25] conducted in Brazil.[34] This proportion is similar to the young population of Australia, Germany, Spain, and the United States.[15,42,43] In addition, not using condom was associated with STI in different studies,[44,45] but not in ours. The lack of association could be due to the lower use of condom in the overall study population, leading to an incapacity of showing differences between groups.
A high proportion of STIs were described for this population, especially between men. There is an increase in the incidence of STIs in US[20] and other countries[46,47] and also an increase in the reported diagnosis of syphilis in Brazil in recent years.[47] Yavorsky et al[48] showed that the agreement between self-reported and tested samples is higher than 90% for HIV, gonorrhoea, and syphilis.
This study provides valid estimates of sexual behavior and associated STIs, identifying important differences in sexual behavior and also in the predictors of prevalence ratio of referred STIs during life among females and males. Condom use is extremely low in this population and do not differ between genders. An improved understanding of factors associated with STIs among genders and differences in sexual attitudes will lead to improved intervention policy frameworks and programing, ultimately increasing safe sex practices and reducing STIs during life. In addition, future research is necessary to access the ways in which safe sex and STI awareness can be enhanced in males and females.
Acknowledgments
We want to thank all the health professionals from more than 100 health units who participated in the data acquisition and Dr Daniela Riva Knauth for her valuable comments on the construction of the questionnaire.
Author contributions
Conceptualization: Adele Schwartz Benzaken.
Formal analysis: Marina Bessel.
Investigation: Eliana Marcia Wendland, Jaqueline Driemeyer C. Horvath, Natália Luiza Kops, Juliana Caierão, Glaucia Fragoso Hohenberger.
Methodology: Eliana Marcia Wendland.
Project administration: Eliana Marcia Wendland.
Supervision: Eliana Marcia Wendland.
Validation: Carla Magda Domingues, Ana Goretti Kalume Maranhão, Flavia Moreno Alves de Souza, Adele Schwartz Benzaken.
Visualization: Carla Magda Domingues, Ana Goretti Kalume Maranhão, Flavia Moreno Alves de Souza, Adele Schwartz Benzaken.
Writing – original draft: Eliana Marcia Wendland, Juliana Caierão.
Writing – review & editing: Jaqueline Driemeyer C. Horvath, Natália Luiza Kops, Glaucia Fragoso Hohenberger.
Footnotes
Abbreviations: ABEP = Brazilian Market Research Association, APR = adjusted prevalence ratio, CDC = Centers for Disease Control and Prevention, HIV = human immunodeficiency virus, HPV = papillomavirus, PR = crude prevalence ratio, STIs = sexually transmitted infections.
Funding: This study was supported by the Support Program for Institutional Development of the Brazilian Unified Health System (PROADI-SUS).
Conflicts of interest: CD, AGKM, FMAS, and ASB work for the Ministry of Health of Brazil. The other authors declare no conflicts of interest.
References
- [1].Vasilenko SA, Linden-Carmichael A, Lanza ST, et al. Sexual behavior and heavy episodic drinking across the transition to adulthood: differences by college attendance. J Res Adolesc 2017;28:473–87. Available at: http://doi.wiley.com/10.1111/jora.12348. Access in April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wong CF, Schrager SM, Chou C-P, et al. Changes in developmental contexts as predictors of transitions in HIV-risk behaviors among young men who have sex with men (YMSM). Am J Community Psychol 2013;51:439–50. [DOI] [PubMed] [Google Scholar]
- [3].Wilson CM, Wright PF, Safrit JT, et al. Epidemiology of HIV infection and risk in adolescents and youth. J Acquir Immune Defic Syndr 2010;54:S5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siracusano S, Silvestri T, Casotto D. Sexually transmitted diseases: epidemiological and clinical aspects in adults. Riv Urol 2014;81:200–8. [DOI] [PubMed] [Google Scholar]
- [5].Gambadauro P, Carli V, Hadlaczky G. Correlates of sexual initiation among European adolescents. PLoS One 2018;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gebresllasie F, Tsadik M, Berhane E. Potential predictors of risk sexual behavior among private college students in Mekelle City, North Ethiopia. Pan Afr Med J 2017;28:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].WHO | Sexually transmitted infections among adolescents: the need for adequate health services [Internet]. WHO. Available at: http://www.who.int/reproductivehealth/publications/adolescence/9241562889/en/. [DOI] [PubMed] [Google Scholar]
- [8].Lyons HA. Heterosexual casual sex and STI diagnosis: a latent class analysis. Int J Sex Health 2017;29:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heinemann J, Atallah S, Rosenbaum T. The impact of culture and ethnicity on sexuality and sexual function. Curr Sex Health Rep 2016;8:144–50. [Google Scholar]
- [10].Odimegwu C, Somefun OD. Ethnicity, gender and risky sexual behaviour among Nigerian youth: an alternative explanation. Reprod Health 2017;14(16). Available at: http://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-017-0284-7. Access in March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agardh A, Odberg-Pettersson K, Östergren P-O. Experience of sexual coercion and risky sexual behavior among Ugandan university students. BMC Public Health 2011;11:52711(1). Available at: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-11-527. Access in March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thomas TL, Yarandi HN, Dalmida SG, et al. Cross-cultural differences and sexual risk behavior of emerging adults. J Transcult Nurs 2015;26:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berhan Y, Berhan A. A meta-analysis of risky sexual behaviour among male youth in developing countries. AIDS Res Treat 2015;2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kocken P. The relevance of cultural factors in predicting condom-use intentions among immigrants from the Netherlands Antilles. Health Educ Res 2005;21:230–8. [DOI] [PubMed] [Google Scholar]
- [15].Puente D, Zabaleta E, Rodríguez-Blanco T, et al. Gender differences in sexual risk behaviour among adolescents in Catalonia, Spain. Gac Sanit 2011;25:13–9. [DOI] [PubMed] [Google Scholar]
- [16].Petersen JL, Hyde JS. A meta-analytic review of research on gender differences in sexuality. Psychol Bull 2010;136:21–38. Access in March 2018. [DOI] [PubMed] [Google Scholar]
- [17].Patra S. Socio-cultural correlates and risky sexual behaviour influencing prevalence of HIV/AIDS and STIs in Uganda: a gender perspective. Cogent Soc Sci 2018;2: Available at: https://www.cogentoa.com/article/10.1080/23311886.2016.1166472. Access in March 2018. Access in March 2018. [Google Scholar]
- [18].Edelman N, Cassell JA, de Visser R, et al. Can psychosocial and socio-demographic questions help identify sexual risk among heterosexually-active women of reproductive age? Evidence from Britain's third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). BMC Public Health 2017;17:517(1). Available at: http://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-3918-8. Access in April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paquette R, Tanton C, Burns F, et al. Illicit drug use and its association with key sexual risk behaviours and outcomes: Findings from Britain's third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). PLos One 2017;12:e0177922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kann L. Youth Risk Behavior Surveillance—United States, 2015. MMWR Surveill Summ 2016;65: Available at: https://www.cdc.gov/mmwr/volumes/65/ss/ss6506a1.htm. Access in March 2018. [DOI] [PubMed] [Google Scholar]
- [21].Newman LM, Berman SM. Epidemiology of STD disparities in African American communities. Sex Transm Dis 2008;35supplement:S4–12. [DOI] [PubMed] [Google Scholar]
- [22].Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis 2008;35supplement:S13–8. [DOI] [PubMed] [Google Scholar]
- [23].STDs in Racial and Ethnic Minorities—2014 STD Surveillance [Internet]. Available at: https://www.cdc.gov/std/stats14/minorities.htm Access in March 2018. [Google Scholar]
- [24].Oliveira-Campos M, Giatti L, Malta D, et al. Contextual factors associated with sexual behavior among Brazilian adolescents. Ann Epidemiol 2013;23:629–35. [DOI] [PubMed] [Google Scholar]
- [25].Oliveira-Campos M, Nunes ML, Madeira F, et al. Sexual behavior among Brazilian adolescents, National Adolescent School-based Health Survey (PeNSE 2012). Rev Bras Epidemiol 2014;17suppl 1:116–30. [DOI] [PubMed] [Google Scholar]
- [26].Sanchez Z, Nappo S, Cruz J, et al. Sexual behavior among high school students in Brazil: alcohol consumption and legal and illegal drug use associated with unprotected sex. Clinics 2013;68:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brasil Fundação Oswaldo Cruz. Heilborn ML, ed. O aprendizado da sexualidade: reprodução e trajetórias sociais de jovens brasileiros. Rio de Janeiro, R. Editora: Garamond. 1ª Edition; 496 pages. [Google Scholar]
- [28].Vasilenko SA, Linden-Carmichael A, Lanza ST, et al. Sexual behavior and heavy episodic drinking across the transition to adulthood: differences by college attendance. J Res Adolesc 2018;28:473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Silveira MF, Beria JU, Horta BL, et al. Factors associated with risk behaviors for sexually transmitted disease/AIDS among urban Brazilian women: a population-based study. Sex Transm Dis 2002;29:536–41. [DOI] [PubMed] [Google Scholar]
- [30].Pinto VM, Tancredi MV, Golub JE, et al. Prior history of sexually transmitted diseases in women living with AIDS in São Paulo, Brazil. Braz J Infect Dis 2012;16:226–31. [PMC free article] [PubMed] [Google Scholar]
- [31].Herbenick D, Bowling J, Fu TJ, et al. Sexual diversity in the United States: Results from a nationally representative probability sample of adult women and men. PLoS One 2017;12:e0181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Pesquisa de conhecimento, atitudes e práticas na população brasileira. Ministério da Saúde; 2016. 166 p. (Série G. Estatística e Informação em Saúde). Avaiable at: http://www.aids.gov.br/pt-br/pub/2016/pesquisa-de-conhecimentos-atitudes-e-praticas-na-populacao-brasileira-pcap-2013 Access in March 2018. [Google Scholar]
- [33].Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J 2012;6:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hugo TD, de O, Maier VT, et al. Fatores associados à idade da primeira relação sexual em jovens: estudo de base populacional. Cad Saúde Pública 2011;27:2207–14. [DOI] [PubMed] [Google Scholar]
- [35].Guttmacher Institute American teens’ sexual and reproductive health. 2014. Available at: http://www.guttmacher.org/pubs Access in March 2018. [Google Scholar]
- [36].Ribeiro AA, Costa MC, Alves RRF, et al. HPV infection and cervical neoplasia: associated risk factors. Infect Agent Cancer 2015;10:1610(1). Available at: http://www.infectagentscancer.com/content/10/1/16. Access in April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet Lond Engl 2006;368:1706–28. [DOI] [PubMed] [Google Scholar]
- [38].Lewis R, Tanton C, Mercer CH, et al. Heterosexual practices among young people in Britain: evidence from three national surveys of sexual attitudes and lifestyles. J Adolesc Health Off Publ Soc Adolesc Med 2017;61:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013;208:385–93. [DOI] [PubMed] [Google Scholar]
- [40].Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382:1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Paiva V, Calazans G, Venturi G, et al. Idade e uso de preservativo na iniciação sexual de adolescentes brasileiros. Rev Saúde Pública 2008;42suppl 1:45–53. [DOI] [PubMed] [Google Scholar]
- [42].Lim MSC, Bowring AL, Gold J, et al. Trends in sexual behavior, testing, and knowledge in young people; 2006–2011. Sex Transm Dis 2012;39:831–4. [DOI] [PubMed] [Google Scholar]
- [43].Remschmidt C, Fesenfeld M, Kaufmann AM, et al. Sexual behavior and factors associated with young age at first intercourse and HPV vaccine uptake among young women in Germany: implications for HPV vaccination policies. BMC Public Health 2014;14:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Casalino E, Choquet C, Leleu A, et al. Trends in condom use and risk behaviours after sexual exposure to HIV: a seven-year observational study. PLoS One 2011;9:e104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ostergren JE, Rosser BRS, Horvath KJ. Reasons for non-use of condoms among men who have sex with men: a comparison of receptive and insertive role in sex and online and offline meeting venue. Cult Health Sex 2011;13:123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].NewMan L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015;10:e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ministério da Saúde. Secretaria de Vigilância em Saúde. Sifilis. Ministério da Saúde; 2017. (Boletim Epidemiológico). Avaiable at: http://portalarquivos.saude.gov.br/images/pdf/2017/novembro/13/BE-2017-038-Boletim-Sifilis-11-2017-publicacao-.pdf Access in March 2018. [Google Scholar]
- [48].Yavorsky RL, Hollman D, Steever J, et al. Prevalence of sexually transmitted infections in at-risk adolescent females at a comprehensive, stand-alone adolescent health center in New York City. Clin Pediatr (Phila) 2014;53:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


